Abstract
Burn wound conversion describes the process by which superficial partial thickness burns convert into deeper burns necessitating surgical intervention. Fully understanding and thus controlling this phenomenon continues to defy burn surgeons. However, potentially guiding burn wound progression so as to obviate the need for surgery while still bringing about healing with limited scarring is the major unmet challenge. Comprehending the pathophysiologic background contributing to deeper progression of these burns is an essential prerequisite to planning any intervention. In this study, a review of articles examining burn wound progression over the last five years was conducted to analyze trends in recent burn progression research, determine changes in understanding of the pathogenesis of burn conversion, and subsequently examine the direction for future research in developing therapies. The majority of recent research focuses on applying therapies from other disease processes to common underlying pathogenic mechanisms in burn conversion. While ischemia, inflammation, and free oxygen radicals continue to demonstrate a critical role in secondary necrosis, novel mechanisms such as autophagy have also been shown to contribute affect significantly burn progression significantly. Further research will have to determine whether multiple mechanisms should be targeted when developing clinical therapies.
Keywords: Burn wound conversion, Secondary burn progression, Autophagy
INTRODUCTION
Over the last ten years, more than 190,000 patients have been admitted for acute burns in the United States with 24,591 patients admitted in 2013 alone [1]. The majority of these cases involved a small total body surface area (TBSA). However, those patients with higher percent TBSA burns had significantly increased mortality in all age groups [1]. While the direct damage to the tissue area caused by the primary injury is often irreversible, the secondary insult caused by progression of the burn wound is amenable to therapeutic intervention.
Burn wound conversion can be defined as the progression of superficial partial-thickness burns to deep partial-thickness and full-thickness burns [2]. This concept originates in the traditional description of burn injury zones by Jackson, in which three different zones of tissue damage of varying degrees occurred after burn injury [2]. The central irreversibly damaged zone of coagulation, the surrounding damaged but threatened zone of stasis, and the outermost recoverable zone of hyperemia. The middle zone of stasis was noted to be viable by Jackson [3] and has since been deemed a therapeutically critical section of burn surface area to address as it loses perfusion in its natural course, dies, and regresses into the inner zone of coagulation that cannot be salvaged [2]. The issue of progression of injury in the zone of stasis is pivotal since burn wound conversion often contributes to both greater burn surface area and burn depth. This larger, deeper wound has multiple local and systemic consequences that increase complications and morbidity [4–6].
As halting the progression of damage in the zone of stasis is a logical point of medical intervention in burn treatment, much research has been devoted to better understanding the process of burn conversion, and halting it. A recent review found 29 studies in 2012 to 2013 investigating experimental burn conversion treatments [7]. While several advances have been made in elucidating new mechanisms of burn progression, some of the leading theories behind secondary burn damage have remained the same for many years. Microthrombosis was noted in burn wounds in 1949 [8] and shortly thereafter found to be reversible to prevent necrosis of otherwise viable burn tissue [9]. The importance of reactive oxygen species (ROS), such as hydrogen peroxide and hydroxyl radicals, as mediators of tissue injury post-burn has also been known for some time [10]. On the other hand, more recent data has recognized the importance of other mechanisms, such as autophagy, in contribution to burn wound progression [11]. Though our understanding of burn wound progression pathogenesis is changing, the principles and applications of burn treatment have remained fairly stable.
Current therapies in medical burn wound treatment after stabilization and prior to reconstruction are aimed at treating the complications of burns, promoting healing, and preventing further complications. Fluid resuscitation serves as the mainstay of systemic treatment in moderate and severe burns to maintain organ and tissue perfusion. Local wound management includes topical antibiotics and various biologic and non-biologic dressing as means of protection from the environment, drainage absorption, pain control, and providing a moist environment for wound healing [5, 12]. Though such treatments may have secondary effects on wound conversion [13], as the pathogenesis of burn wound progression is still not well understood, few clinical therapies are designed to directly address the issue of wound progression.
The purpose of this study is to investigate the recent progress in understanding the pathophysiology of burn wound conversion. Elucidating the trends in burn wound progression research may help provide a better understanding of whether therapeutic interventions for halting burn progression will continue to be targeted towards conventional therapies or whether new treatments might offer increased efficacy.
MATERIALS AND METHODS
A review of the current literature was conducted to identify recent studies on the pathogenesis of burn wound conversion. A literature search was performed by querying the MEDLINE database for full-text articles over the last five years (2010 – 2014) using the keywords, “burn AND wound AND (conversion OR progression OR expansion)”. A preliminary review of article titles was used to include any basic or clinic science studies on burn progression. Review articles, articles in languages other than English, and abstracts were excluded from the study.
We identified abstracts focusing specifically on the pathophysiology of burn wound conversion, either proposing new theories or expanding on previously known mechanisms of burn progression. Full texts were then categorized according to the particular burn conversion pathophysiology investigated in each article. Studies describing new potential treatments for already defined mechanisms of progression were also addressed.
RESULTS
The initial search criteria yielded a total of 254 potential articles for the time period of 2010 to 2014. After screening titles and abstracts, and applying the inclusion and exclusion criteria, a total of 24 articles were included for review in the study (Table 1). All studies utilized animal models for experiments and the majority (67 percent) used the comb burn model [14] to induce burn injuries and replicate the zone of stasis.
Table 1.
Studies examining burn wound progression from 2010 to 2014
| Mechanism | Author(s) | Year | Animal Model | Burn Model | Conclusions |
|---|---|---|---|---|---|
| Autophagy | Tan et al. | 2013 | Murine | Comb model | Autophagy contributes to cell death in zone of stasis; autophagy and apoptosis peak at different time intervals |
| Xiao et al. | 2013 | Murine | Heated brass rod | Induction of autophagy (with rapamycin) decreases burn wound progression and improves wound healing | |
| Xiao et al. | 2014 | Murine | Heated brass rod | Autophagy is decreased and apoptosis is increased early in the course of burn progression in deep second-degree burns | |
| Inflammation | Begieneman et al. | 2012 | Murine | Heated copper stamp | Long term treatment with a C1 inhibitor post-burn improves wound healing and decreases local inflammation, particularly macrophage infiltration |
| Bohr et al.* | 2013 | Murine | Comb model, scald burn | Resolvin D2 prevents thrombosis of the DDVN, TNF-α secretion and secondary burn progression; Resolvin D2 does not reduce PMN infiltration | |
| Eski et al. | 2011 | Murine | Comb model | Cerium nitrate baths immediately after burn injury prevent progressive tissue necrosis in the zone of stasis | |
| Friedrich et al. | 2013 | Murine | Heated brass disk | TNF-α conjugated to HA more effectively prevent burn progression and decrease inflammatory markers compared to TNF-α alone or mixed with HA | |
| Rizzo et al. | 2013 | Murine | Comb model | Systemic hypothermia after thermal injury decreases levels of tissue remodeling genes, increase expression of skin protective genes and reduce burn conversion | |
| Singer et al.** | 2011 | Murine | Comb model | Curcumin reduces burn wound progression when administered after burn injury | |
| Sun et al. | 2012 | Murine | Heated brass disk | Topical antibodies to TNF-α, but not IL-6, reduce burn wound progression | |
| Ischemia | Bohr et al.† | 2013 | Murine | Scald burn | Alternative EPO-mediated signaling lessens TNF-α secretion, decreases microvascular thrombosis and reduces burn wound conversion |
| Fourman et al. | 2013 | Porcine | Comb burn model | ICG angiography delineates viable versus noviable tissue in the zone of injury up to 1 hour post-burn | |
| Hirth et al. | 2013 | Porcine | Heated aluminum bar | Endothelial cell necrosis at 1 hour post burn is predictive of apoptosis at 24 hours and tissue necrosis at 7 days; the zone of stasis is comprised of two vertically-divided subzones based on endothelial cell necrosis | |
| Tobalem et al. | 2012 | Murine | Comb model | Early (45 minute), but not late (6 hour) EPO treatment post-burn decreases burn progression | |
| Tobalem et al. | 2013 | Murine | Comb model | EPO decreases secondary burn progression in a dose-dependent manner, independent of iNOS expression, anti-inflammatory or angiogenic effects | |
| Tobalem et al. | 2013 | Murine | Comb model | Immediate application of warm water to burn wounds improves early perfusion and decreases zone of stasis necrosis | |
| Yuhua et al.† | 2012 | Murine | Heated brass rod | Systemic treatment with Poloxamer 188 reduces burn wound progression in deep second-degree burns | |
| ROS | Deniz et al. | 2013 | Murine | Comb model | N-acetylcysteine prevents secondary tissue necrosis in the zone of stasis |
| Shalom et al. | 2011 | Murine | Comb model | Prophyalactic treatment with recombinant copper-zinc SOD does not improve zone of stasis survival | |
| Other | Macri et al. | 2013 | Porcine | Comb model | Immediate burn excision does not prevent burn progression in surrounding zone of stasis |
| Oksuz et al. | 2013 | Murine | Comb model | Subcutaneous MSC injections 30 minutes post-burn decrease apoptosis in the zone of stasis and necrosis in the zone of stasis | |
| Singer et al. | 2010 | Porcine | Comb model | Bromelain-based enzymatic debridement of burn eschar reduces tissue necrosis in the zone of stasis compared to controls | |
| Singer et al. | 2013 | Murine | Comb model | Systemic MSC treatment 1 hour after burn injury reduces necrosis in the zone of stasis by around 20% | |
| Turkaslan et al. | 2010 | Murine | Comb model | HBOT increases cells in the proliferative phase at 24 hours post-burn, increases tissue perfusion at 5 days post-burn, and decreases secondary necrosis in the zone of stasis |
PMN, Polymorphonuclear neutrophil; TNF-α, Tumor necrosis factor-alpha; HA, Hyaluronic acid; IL-6, Interleukin-6; ICG, Indocyanine green; EPO, Erythropoietin; iNOS, Inducible nitric oxide synthase; HBOT, Hyperbaric oxygen treatment; ROS, Reactive oxygen species; SOD, Superoxide dismutase; MSC, Mesenchymal stem cell.
Study also addressed *ischemia, **ROS, and †inflammation
Articles were categorized according to the main theory of pathogenesis that was being investigated or treated in the study (Fig. 1). The majority of articles addressed better studied concepts such as ischemia (9 studies) and inflammation (6 studies), whereas more recently discovered mechanisms such as autophagy (3 articles) had less focus, as expected. Articles grouped under “other” included studies on stem cell therapies [15, 16], enzymatic wound debridement [17], burn excision [18] and hyperbaric oxygen treatment (HBOT) [19]. We found that most studies on burn wound progression over the last five years involved experimental treatments in animal models, and very few studies were solely focused on further elucidating the mechanisms behind burn wound conversion (Fig. 2). Studies focusing on pathophysiology addressed autophagy and ischemia while the remainder emphasized novel treatments for secondary ischemia and inflammation.
Fig. 1.
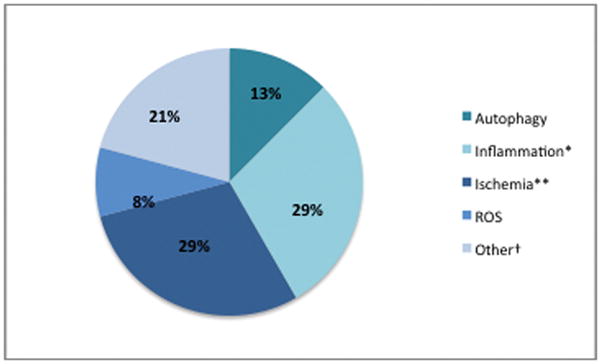
Categorization of studies on burn wound conversion from 2010 – 2014 according to particular pathogenic mechanism examined. ROS, Reactive oxygen species. *One article also addressed ischemia, and another, ROS. **Two articles also addressed inflammation. †Other category includes studies on wound debridement/excision, hyperbaric oxygen treatment, and stem cell therapies.
Fig. 2.
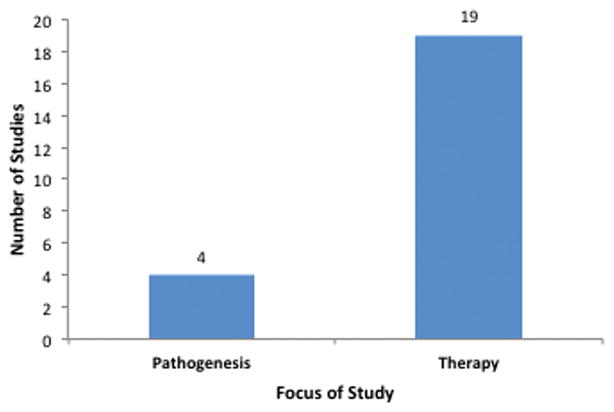
Number of studies addressing pathogenesis (4) versus therapy (19). One study analyzed diagnostic methods for burn wound conversion.
DISCUSSION
The differentiation between salvageable and unsalvageable tissue in burns was a key breakthrough in burn research that has since led to better understanding the pathogenesis of burn wound conversion at the tissue, cellular, and molecular levels. As our understanding of this pathogenesis continues to evolve, most experimental therapies still focus on only a few of the current theories behind burn conversion. The goal of this study was to analyze recent rends in burn progression research to develop a better understanding of new directions for this research, novel mechanisms of burn progression, and new potential therapies.
The majority of studies over the last five years have focused on assessing the efficacy of well-established therapies in other disease processes and applying these to burn wound conversion. The few articles dedicated to exploring pathophysiology were almost solely related to autophagy, or the process of cellular waste degradation, as this phenomenon is a much newer concept in burn progression research. However, the recent studies examining new treatments for well-known mechanisms of secondary burn progression also elucidate further details about the pathogenesis that contributes to burn wound progression. These findings, along with their implications for future trends in research and potential therapies, are reviewed below.
Autophagy
Autophagy is the degradation pathway in which cellular waste is delivered to lysosomes to be broken down and recycled. These recycled substrates are then used by the cells for tissue regeneration [20]. This process appears to work effectively as an enhancement to tissue healing, provided that the system is not overwhelmed with excessive breakdown products from more severe cellular injury. Though not a new concept, autophagy is a new area of research in relation to burn wound progression. The studies over the last several years have focused on defining the role of autophagy in burn wound conversion and better understanding its relation to apoptosis in this process. Apoptosis was suggested to contribute to burn wound progression in 2006 when it was shown that more apoptotic cells were found in deep partial-thickness burns than in normal skin or superficial partial-thickness burns [21, 22]. Later work also demonstrated the contribution of apoptosis as well as oncosis, or ischemic cell death characterized by cell swelling, to tissue death in the zone of stasis [23]. Decreased apoptosis and improved healing in burns have also been reported after treatment with a c-Jun inhibitor, further supporting the importance of apoptosis in the process of secondary tissue loss in the burn wound [24]. The function of autophagy in the post-burn wound environment, however, was not well understood until recently.
In 2013, Tan et al. reported higher autophagy than apoptosis rates in hair follicle epithelium from two to 24 hours after burn injury using the comb burn model [25]. The authors concluded that both processes contribute to cell death in the zone of stasis but with a different time course, suggesting that different treatments may be necessary to target the two processes. The role of autophagy in cell death – specifically whether autophagy is a mediator or preventer of cell death – is controversial. Though autophagy has been referred to as “type II programmed cell death” [26] or “macroautophagy”, autophagy has been shown to protect against apoptosis [27] and is more widely believed to be a degradation pathway that supports cellular homeostasis [28].
Contrary to the results of Tan et al., Xiao et al. reported that autophagy decreases early in the course of burn injury progression and increases later, though autophagy levels always remained below those in normal skin in full-thickness wound tissues [29]. The authors also observed, however, that in the deep dermal layer, which they state may correlate to zone of stasis, staining for autophagy marker LC3 was increased, similar to the observations of Tan et al. Xiao et al. have also reported that augmenting autophagy with the antibiotic rapamycin lessened burn wound progression and improved wound healing, further suggesting that autophagy may have a beneficial role in preventing burn wound progression [30]. These studies are difficult to compare as different burn models are utilized, not all observed markers are similar, and different areas of tissue are analyzed. Autophagy may likely have both protective and detrimental effects on the cell, possibly depending on the degree of cell damage and the timing from initial injury. Further elucidating the role of autophagy in the zone of stasis will be crucial to determining whether potential treatments should be aimed at enhancing or inhibiting this process.
Inflammation
The detrimental effects of the prolonged inflammatory response in burn wounds have been well established and can be attributed to a myriad of different factors including complement activation, cytokine production, delayed inflammatory cell apoptosis, and ROS production [31–37]. Current common methods of reducing inflammation involve wound debridement to remove surface eschar, bacteria, and inflammatory cells, as well as maintaining a favorable wound environment by using appropriate dressings [36]. More recent approaches have focused on targeting cytokines, signaling pathways, and inflammatory cells that contribute to the heightened inflammatory milieu of the burn wound microenvironment. Our laboratory is currently investigating the profile of these inflammatory mediators in the first 48 hours after partial thickness burns to guide the administration of local agents aimed at controlling and directing the initial immune response to reduce burn wound progression. In a similar approach, several specific mediators in the inflammatory signaling cascade have been targeted for potentially reducing excess inflammation.
Our review showed that recent studies have investigated novel uses for well-known anti-inflammatory agents or have focused on specific points in inflammatory signaling pathways that may be amenable to intervention. Singer et al. take a broader approach by investigating the efficacy of curcumin, a powerful anti-inflammatory and antioxidant agent [38–40]. The authors demonstrate that treatment of burn wounds with intravenous curcumin in a rat comb burn model decreased burn wound progression and that these effects were bimodal, suggesting more than one mechanism of action [41]. Eski et al. report that treatment with cerium nitrate baths immediately after thermal injury prevented progressive tissue necrosis in the zone of stasis in both short-term (3 day) and long-term (21 day) follow-up [42]. Cerium nitrate has been shown to reduce tumor necrosis factor alpha (TNF-α) levels in burns[43], decrease transmigration of leukocytes [44], and denature the lipid protein complex (LPC) in burn eschars, which plays a role in phagocyte activation [45, 46]. As inflammation, particularly with regards to phagocyte activation, is inherently associated with the production of ROS [47](discussed below), such treatment paradigms may reduce cell death in the zone of stasis through multiple different mechanisms.
Other studies have focused on the infiltration of inflammatory cells into the burn wound, particularly polymorphonuclear neutrophils (PMNs), which are known to occlude vasculature leading to ischemia [35, 36] as well as secrete a variety of deleterious proinflammatory mediators [48, 49]. Bohr et al. investigate the use of Resolvin D2 (RvD2), a lipid mediator shown to block secretion of pro-inflammatory mediators such as TNF-α and interleukin-1 beta (IL-1β) and reduce PMN adhesion and infiltration into tissues [50], to treat burn wound progression [51, 52]. The authors observed that treatment prevented secondary thrombosis of the deep dermal vascular network (DDVN) and subsequently decreased burn wound progression. RvD2, however, did not reduce the number of PMNs in the wounds, but instead decreased levels of TNF-α as well as the expression of inflammatory cell adhesion molecules, indicating that the activation and secretion of inflammatory mediators by PMNs may be more important in burn wound conversion than their physical obstruction of vasculature..
Efforts to reduce global inflammation in the burn wound have also utilized non-pharmacological means, such as hypothermia. Hypothermia has been shown to limit cellular injury after an insult [53] and downregulate inflammatory genes [54]. Rizzo et al. demonstrate that moderate systemic hypothermia after burn injury decreases burn depth progression [55]. Interestingly, some inflammatory genes including those for certain chemokines were upregulated after exposure to hypothermia. These genes were noted to be different than those upregulated by burns, suggesting that targeting of specific inflammatory mediators may be a more efficacious strategy than attempting to broadly limit inflammation.
One such target is TNF-α, a cell signaling protein that regulates inflammation and is observed at high levels in the burn wound microenvironment [56]. Sun et al. demonstrate that topical antibodies to TNF-α conjugated with hyaluronic acid (HA) reduce levels of downstream inflammatory mediators, decrease macrophage infiltration, and lessen secondary necrotic expansion of tissues [57]. In a later study, the authors demonstrate that anti-TNF-α conjugated to HA more effectively prevents necrosis of tissue and decreases inflammatory markers than anti-TNF-α alone or anti-TNF-α mixed with HA [58], further suggesting the need to better understand how to control the interactions of TNF-α in the post-burn environment.
Interleukin-6 (IL-6), another pro-inflammatory cytokine, is also a target in potential burn wound progression therapies. IL-6 is elevated in burn patients [59], and plays a role in cytotoxic T-cell differentiation and T-cell proliferation [56]. Antibodies to IL-6, however, did not reduce burn progression compared to controls [10, 36, 60], suggesting that TNF-α, a more upstream cytokine, may play a more significant role in mediating inflammation in the burn wound environment. Moreover, the success of a topical therapy suggests that immunomodulatory agents may be able to be utilized without the risk systemic administration.
The complement cascade has also served as a target for anti-inflammatory therapies since complement contributes to wound edema, vascular thrombosis, production of oxygen radicals, and reactive lysis of viable cells in the burn wound [37]. C1 inhibitors have previously been shown to reduce neutrophil adhesion and decrease the progression of wound depth in porcine deep partial-thickness burn models [61]. The only study focusing on complement in burn wound conversion over the last five years is a study by Begieneman et al. in 2012 that investigates the effects of long-term (14 day) C1 inhibitor treatment on burn progression [62]. Similar to prior studies, the authors demonstrate improved wound healing and decreased local inflammation – particularly fewer infiltrating macrophages – in animals treated with the C1 inhibitor. As the critical intervention for burn wound progression must occur within the first 72 hours post burn injury, it is unclear whether the extended treatment seen in this study would be more clinically efficacious compared to treatment reserved to the critical window.
Taken together, these studies reinforce not only the multifactorial nature of burn wound conversion, but also the complex nature of the specific inflammatory interactions that contribute to burn progression. As multiple pathways converge on multiple different effector mechanisms, combined local therapies may potentially offer the most efficacious results while lessening the risks associated with immunomodulation. Furthermore, while most studies focus on reducing signaling cytokines in inflammatory cascades, certain factors, such as interleukin-4 (IL-4), are anti-inflammatory mediators and are beneficial in wound healing [36]. Therefore, therapy should be aimed at controlling the exaggerated inflammatory response in burns, while maintaining an environment of “balanced inflammation” in the burn wound.
Ischemia
Ischemia is a well-known cause of cell and tissue death in a number of different disease processes and has been implicated in contributing to burn conversion as well [63]. The etiology of ischemia in the zone of stasis is multifactorial, and includes damage to the vascular endothelium from ROS and PMN aggregates as well as thrombosis, vasoconstriction, and edema [8, 64].
Prior studies have demonstrated the contribution of microthrombosis to postburn dermal ischemia [65] and have identified certain factors such as bradykinin, an inflammatory mediator, as potential therapeutic targets to prevent thrombosis [66]. Vasoconstriction [67] as well as local loss of fluid secondary to increased vascular permeability [68] cause impaired circulation and decreased tissue perfusion that can lead to tissue necrosis and progression of injury in the zone of stasis [13]. The systemic consequences of burns, such as sepsis and shock, can result in widespread lack of organ perfusion, including at the wound bed. The current mainstay of treatment for addressing these sequelae is aggressive fluid resuscitation, the extent of which has indeed been shown to influence burn wound progression [69]. Such treatments, however, do not address the cause of ischemia and are only methods of salvage. By better understanding the pathophysiology behind burn wound ischemia and targeting treatments toward causal factors for decreased perfusion, future therapies will help to prevent the unwanted effects of ischemia.
In 2013, Hirth et al. demonstrated that burn progression occurs within 24 hours of the initial burn, and that endothelial cell necrosis at one hour post-burn was predictive of the level of apoptosis at 24 hours post-burn as well as histologic tissue necrosis at 7 days post-burn [8, 64]. Traditionally, dermal ischemia has been implicated as a causal factor of burn wound progression [70], which is supported by these findings of initial endothelial cell necrosis prior to interstitial and adnexal cell damage in this study. The authors also suggest dividing Jackson’s zone of stasis into two subzones depending on the viability of endothelial cells at 1 hour: (1) the upper zone with necrotic endothelial cells but spared alternate cell types which inevitably progresses to full necrosis secondary to ischemia and (2) the lower subzone with spared endothelial cells initially, which only sometimes progresses to necrosis. These findings reinforce the importance of ischemia and programmed cell death in burn wound progression while further elucidating the cellular mechanisms of cell death in a spatiotemporal-dependent manner in this critical area.
Fourman et al. attempted to predict necrosis in the zone of stasis by analyzing real-time perfusion using quantitative indocyanine green (ICG) angiography to delineate viable and non-viable areas up to one hour after the initial tissue insult [71–73]. However, this technique relies on how ICG dye angiography functions in the burn wound environment where perfusion patterns complicated by capillary leakage, microthrombosis, vasoconstriction, and vasodilation make it difficult to interpret ICG signals.
In our review, several studies over the last five years examined the role of erythropoietin (EPO), a hormone with known anti-inflammatory, angiogenic, and vasodilatory properties [74–76], in the prevention and treatment of burn wound progression [75]. Tobalem et al. demonstrate that treatment with EPO limits interspace necrosis and burn depth extension in a dose-dependent manner in a comb burn model [76]. Interestingly, while both high and low-dose EPO upregulated inducible nitric oxide synthase (iNOS) and decreased inflammation, only low-dose EPO treatment prevented burn progression. Additionally, neoangiogenesis was noted only after necrotic tissue had already demarcated. These results indicate that alterations in microperfusion during the early post-burn period may be more important than inflammatory effects or changes in angiogenesis in contributing to burn wound progression, and that factors other than iNOS may contribute to EPO’s beneficial effects on the microcirculation. Tobalem et al. also show that the efficacy of EPO treatment is time-dependent, and though iNOS mediated vasodilation and anti-inflammatory effects occur independent of timing, only early (45 minutes post-burn) and not late (6 hours post-burn) treatment decreases burn progression [74].
The mechanisms behind the beneficial effects of EPO are further elucidated in a study by Bohr et al. who examined a secondary EPO signaling pathway not responsible for the traditional roles of hematopoiesis, platelet stimulation, and endothelial cell activation [63, 77, 78]. They demonstrate that activation of this alternative pathway using the peptide ARA290 lessens TNF-α secretion by immune cells, decreases microvascular thrombosis, and preserves the deep dermal vascular network in burn wounds, subsequently reducing burn wound conversion. A recent study by Yuhua et al. similarly showed reduced burn wound progression when secondary burns were treated with Poloxamer 188 (P188), a copolymer suggested to reduce microvascular stasis and decrease PMN ROS generation [79]. Both of these studies emphasize the effects of poor perfusion, microthrombosis, and pro-inflammatory mediators in burn wound conversion.
Other studies have taken different approaches to local tissue ischemia. Tobalem et al. demonstrated that topical application of warm water immediately after burn injury in an effort to induce vasodilation and decrease ischemia decreased burn wound conversion [80, 81]. This lies in contradiction to the mainstay of burn wound treatment which has been cold water exposure as a means to reduce heat as well as inflammation and edema [10]. While cooling treatments may reduce initial injury, subsequent heat-induced vasodilation may improve perfusion, suggesting the need for possible temporally variable therapies.
Reactive Oxygen Species
Free radicals have long been considered as mediators of progressive tissue damage after initial burn injury [82]. The occurrence of ROS in the burn wound can be attributed to direct production by the thermal energy of burns [83] as well as the increased activity of xanthine oxidase and NADPH oxidase [84, 85]. ROS such hydrogen peroxide, superoxide radical, and hydroxyl radicals contribute to cell death in the zone of stasis through lipid peroxidation and denaturation of protein [86]. In addition, decreases in antioxidants and free radical scavengers in the burn wound further oxidative stress and subsequent tissue damage [87].
Much recent research is dedicated to investigating the efficacy of different antioxidant agents in minimizing free radical damage and burn wound progression. Deniz et al. demonstrated that prevention of burn wound progression could be achieved by treatment with N-acetylcysteine (NAC) one hour after burns. NAC is a precursor to reduced glutathione, which has previously been shown to prevent necrosis in the zone of stasis [41]. Other antioxidant agents investigated included curcumin, which was shown to reduce burn wound progression in a rat comb burn model [88, 89]. Copper-zinc superoxide dismutase, (CuZnSOD), another free radical scavenger, has improved zone of stasis survival and wound healing in topical formulations [90]. Other studies, however, have failed to show any benefit when CuZnSOD was administered after burn injury or as a prophylactic intravenous treatment [17]. These discrepancies may be due to different routes of introduction of CuZnSOD as well different burn models, and suggest further investigation into optimal utilization of this agent is necessary.
These studies demonstrate that the recent free radical species research focus has been dedicated to refining current therapies and exploring alternative interventions rather that trying to better understand the production and interaction of ROS in the zone of stasis. While our understanding of the pathophysiology of these events has not changed significantly over the last several years, novel therapeutic interventions have shown promising results.
Other Mechanisms
The majority of studies over the last five years have focused on applying well-known treatments for traditional mechanisms of burn wound progression pathogenesis. That being said, several studies in this review investigated alternative methods to prevent secondary necrosis. Two studies examined the adaption of conventional methods of local treatment of burn wounds, particularly wound debridement [18] and burn excision [17], in the context of burn conversion. Singer et al. evaluate the ability of a novel bromelin-based enzymatic preparation in salvaging tissue in the zone of stasis after immediately applying the compound to exposed dermis in burn wounds [18]. While animals with enzymatic debridement of eschar had significantly less interspace tissue necrosis in the burn comb model, two-thirds of the experimental group still exhibited partial-thickness necrosis of the zone of stasis and the remaining one-third had full-thickness necrosis. Similarly, a study by Macri et al. showed that excision of burn wounds immediately after thermal injury did not limit burn progression of surrounding zone of stasis [42]. Though removal of eschar is critical for healing of deep burn wounds and studies have suggested that eschar may contribute to phagocyte activation [91], the inability to prevent necrosis in the zone of stasis in these studies indicates that the other pathophysiologic mechanisms described in this study may play a more important role in wound conversion.
Hyperbaric oxygen treatment has been another approach that has previously been used to treat burn wounds [92] and has been shown to improve wound healing in human burn wound models [19]. Turkaslan et al. investigated the effects of HBOT in the zone of stasis and reported increased cells in the synthesis stage by 24 hours post-burn, increased radioactive uptake by 5 days post-burn, and decreased necrosis overall in the treatment group [75]. Similar to the study by Tobalem et al. [93, 94], new vessel formation was not significantly different between the treatment and control group in the early critical stages of burn wound progression, suggesting a less important role for neoangiogenesis.
Cellular therapies have also emerged as potential treatments for burn wound progression. Mesenchymal stem cells (MSCs) are known for their ability to secrete numerous cytokines and growth factors that are important in wound healing [95, 96] and have been shown to improve wound healing in different environments [97, 98]. MSCs have also have an anti-inflammatory and immunosuppressive effect [16], which may be beneficial in the exaggerated inflammatory environment of the burn wound. With regards to burn wound progression, two studies by Singer et al. [15] and Oksuz et al. [16] have examined the effects of systemic and local administration of MSCs on the zone of stasis. Singer et al. report that systemic administration of MSCs 1 hour after burn injury reduced necrosis of the interspaces in a burn comb model by approximately 20 percent [69]. As necrosis of endothelial cells has been demonstrated to occur by 1 hour post-injury, earlier administration of systemic stem cell therapy [15], allowing time for localization to the wound bed, may improve results. Oksuz et al. similarly investigate the potential benefits of MSCs in the burn progression but utilize subcutaneous MSC injections 30 minutes post-burn, which may be a more favorable route of delivery for translational applications. In the treatment group, the authors reported lower apoptosis counts, increased perfusion, and higher percentage of vital tissue in the zone of stasis, reaffirming the multifactorial nature of progression pathophysiology and indicating possible mechanisms for the therapeutic effects of stem cell treatments. Again, earlier administration of therapy may possibly augment results based on the known timing of pathophysiologic events in the zone of stasis.
CONCLUSIONS
Burn wound conversion continues to remain one of the least understood and poorly treated aspects of burn care. However, recent research has made strides in both better understanding of the contributing factors in the pathogenesis of wound conversion as well as applying novel treatments to well-known mechanisms of secondary progression. The majority of studies over the last five years have focused on adaption of therapies for other diseases to serve as potential interventions targeting the zone of stasis. Numerous experimental treatments aimed at reducing prolonged inflammation, decreasing free oxygen radicals, and improving perfusion have all shown promising results.
While ischemia and cell necrosis secondary to inflammation, ROS, and numerous other factors are important, the roles of apoptosis and autophagy have been each shown to be critical and must be included when considering potential therapies. The need for rapid treatment is also crucial, regardless of the mode of intervention, as cell necrosis has been shown to occur at the one-hour time point after initial insult. The wide variety of mechanisms studied and the varying degrees of success with targeted therapies for each mechanism further lend credence to the multifactorial and complex nature of burn wound progression. Moving forward, future research will have to determine the weight of each of these factors in burn wound progression in order to decide what combination of mechanisms need to be addressed to develop optimal clinical therapies.
Highlights.
we review recent progress in the pathophysiology of burn wound conversion.
halting burn progression will impact on the scar outcome and healing trajectory
ischemia, inflammation, oxygen radicals are well accepted - autophagy is a new concept
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Burn Association. National burn repository. 2014. [Google Scholar]
- 2.Jackson DM. The diagnosis of the depth of burning. The British journal of surgery. 1953;40:588–96. doi: 10.1002/bjs.18004016413. [DOI] [PubMed] [Google Scholar]
- 3.Jackson DM. Second thoughts on the burn wound. The Journal of trauma. 1969;9:839–62. doi: 10.1097/00005373-196910000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Shakespeare P. Burn wound healing and skin substitutes. Burns: journal of the International Society for Burn Injuries. 2001;27:517–22. [PubMed] [Google Scholar]
- 5.Johnson RM, Richard R. Partial-thickness burns: identification and management. Adv Skin Wound Care. 2003;16:178–87. doi: 10.1097/00129334-200307000-00010. quiz 88–9. [DOI] [PubMed] [Google Scholar]
- 6.Grunwald TB, Garner WL. Acute burns. Plastic and reconstructive surgery. 2008;121:311e–9e. doi: 10.1097/PRS.0b013e318172ae1f. [DOI] [PubMed] [Google Scholar]
- 7.Schmauss D, Rezaeian F, Finck T, Machens HG, Wettstein R, Harder Y. Treatment of secondary burn wound progression in contact burns-a systematic review of experimental approaches. Journal of burn care & research: official publication of the American Burn Association. 2015;36:e176–89. doi: 10.1097/BCR.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 8.Sevitt S. Local blood-flow changes in experimental burns. J Pathol. 1949;61:427. [Google Scholar]
- 9.Zawacki BE. Reversal of capillary stasis and prevention of necrosis in burns. Annals of surgery. 1974;180:98–102. doi: 10.1097/00000658-197407000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward PA, Till GO. Pathophysiologic events related to thermal injury of skin. The Journal of trauma. 1990;30:S75–9. doi: 10.1097/00005373-199012001-00018. [DOI] [PubMed] [Google Scholar]
- 11.Tan JQ, Zhang HH, Lei ZJ, Ren P, Deng C, Li XY, et al. The roles of autophagy and apoptosis in burn wound progression in rats. Burns: journal of the International Society for Burn Injuries. 2013;39:1551–6. doi: 10.1016/j.burns.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 12.Hartford C, Kealey G. Care of Outpatient burns. In: Herndon D, editor. Total Burn Care. 3. Elsevier, Inc; 2007. [Google Scholar]
- 13.Kim DE, Phillips TM, Jeng JC, Rizzo AG, Roth RT, Stanford JL, et al. Microvascular assessment of burn depth conversion during varying resuscitation conditions. The Journal of burn care & rehabilitation. 2001;22:406–16. doi: 10.1097/00004630-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Regas FC, Ehrlich HP. Elucidating the vascular response to burns with a new rat model. The Journal of trauma. 1992;32:557–63. doi: 10.1097/00005373-199205000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Oksuz S, Ulkur E, Oncul O, Kose GT, Kucukodaci Z, Urhan M. The effect of subcutaneous mesenchymal stem cell injection on statis zone and apoptosis in an experimental burn model. Plastic and reconstructive surgery. 2013;131:463–71. doi: 10.1097/PRS.0b013e31827c6d6f. [DOI] [PubMed] [Google Scholar]
- 16.Singer DD, Singer AJ, Gordon C, Brink P. The effects of rat mesenchymal stem cells on injury progression in a rat model. Academic emergency medicine: official journal of the Society for Academic Emergency Medicine. 2013;20:398–402. doi: 10.1111/acem.12116. [DOI] [PubMed] [Google Scholar]
- 17.Singer AJ, McClain SA, Taira BR, Rooney J, Steinhauff N, Rosenberg L. Rapid and selective enzymatic debridement of porcine comb burns with bromelain-derived Debrase: acute-phase preservation of noninjured tissue and zone of stasis. Journal of burn care & research: official publication of the American Burn Association. 2010;31:304–9. doi: 10.1097/BCR.0b013e3181d0f4d4. [DOI] [PubMed] [Google Scholar]
- 18.Macri LK, Singer AJ, Taira BR, McClain SA, Rosenberg L, Clark RA. Immediate burn excision fails to reduce injury progression. Journal of burn care & research: official publication of the American Burn Association. 2013;34:e153–60. doi: 10.1097/BCR.0b013e31828fc8cd. [DOI] [PubMed] [Google Scholar]
- 19.Turkaslan T, Yogun N, Cimsit M, Solakoglu S, Ozdemir C, Ozsoy Z. Is HBOT treatment effective in recovering zone of stasis? An experimental immunohistochemical study. Burns: journal of the International Society for Burn Injuries. 2010;36:539–44. doi: 10.1016/j.burns.2009.06.210. [DOI] [PubMed] [Google Scholar]
- 20.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gravante G, Filingeri V, Delogu D, Santeusanio G, Palmieri MB, Esposito G, et al. Apoptotic cell death in deep partial thickness burns by coexpression analysis of TUNEL and Fas. Surgery. 2006;139:854–5. doi: 10.1016/j.surg.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 22.Gravante G, Palmieri MB, Esposito G, Delogu D, Santeusanio G, Filingeri V, et al. Apoptotic death in deep partial thickness burns vs. normal skin of burned patients. The Journal of surgical research. 2007;141:141–5. doi: 10.1016/j.jss.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 23.Singer AJ, McClain SA, Taira BR, Guerriero JL, Zong W. Apoptosis and necrosis in the ischemic zone adjacent to third degree burns. Academic emergency medicine: official journal of the Society for Academic Emergency Medicine. 2008;15:549–54. doi: 10.1111/j.1553-2712.2008.00115.x. [DOI] [PubMed] [Google Scholar]
- 24.Giles N, Rea S, Beer T, Wood FM, Fear MW. A peptide inhibitor of c-Jun promotes wound healing in a mouse full-thickness burn model. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2008;16:58–64. doi: 10.1111/j.1524-475X.2007.00331.x. [DOI] [PubMed] [Google Scholar]
- 25.Tan SH, Shui G, Zhou J, Shi Y, Huang J, Xia D, et al. Critical role of SCD1 in autophagy regulation via lipogenesis and lipid rafts-coupled AKT-FOXO1 signaling pathway. Autophagy. 2014;10:226–42. doi: 10.4161/auto.27003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clarke PG. Developmental cell death: morphological diversity and multiple mechanisms. Anatomy and embryology. 1990;181:195–213. doi: 10.1007/BF00174615. [DOI] [PubMed] [Google Scholar]
- 27.Ding Y, Kim JK, Kim SI, Na HJ, Jun SY, Lee SJ, et al. TGF-{beta}1 protects against mesangial cell apoptosis via induction of autophagy. The Journal of biological chemistry. 2010;285:37909–19. doi: 10.1074/jbc.M109.093724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reggiori F, Klionsky DJ. Autophagy in the eukaryotic cell. Eukaryotic cell. 2002;1:11–21. doi: 10.1128/EC.01.1.11-21.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao M, Li L, Li C, Zhang P, Hu Q, Ma L, et al. Role of autophagy and apoptosis in wound tissue of deep second-degree burn in rats. Academic emergency medicine: official journal of the Society for Academic Emergency Medicine. 2014;21:383–91. doi: 10.1111/acem.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao M, Li L, Hu Q, Ma L, Liu L, Chu W, et al. Rapamycin reduces burn wound progression by enhancing autophagy in deep second-degree burn in rats. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2013;21:852–9. doi: 10.1111/wrr.12090. [DOI] [PubMed] [Google Scholar]
- 31.Bucky LP, Vedder NB, Hong HZ, Ehrlich HP, Winn RK, Harlan JM, et al. Reduction of burn injury by inhibiting CD18-mediated leukocyte adherence in rabbits. Plastic and reconstructive surgery. 1994;93:1473–80. doi: 10.1097/00006534-199406000-00021. [DOI] [PubMed] [Google Scholar]
- 32.Cetinkale O, Demir M, Sayman HB, Ayan F, Onsel C. Effects of allopurinol, ibuprofen and cyclosporin A on local microcirculatory disturbance due to burn injuries. Burns: journal of the International Society for Burn Injuries. 1997;23:43–9. doi: 10.1016/s0305-4179(96)00079-4. [DOI] [PubMed] [Google Scholar]
- 33.Nagane NS, Bhagwat VR, Subramanium M. Increased free radical activity in burns. Indian journal of medical sciences. 2003;57:7–11. [PubMed] [Google Scholar]
- 34.Mileski W, Borgstrom D, Lightfoot E, Rothlein R, Faanes R, Lipsky P, et al. Inhibition of leukocyte-endothelial adherence following thermal injury. The Journal of surgical research. 1992;52:334–9. doi: 10.1016/0022-4804(92)90112-d. [DOI] [PubMed] [Google Scholar]
- 35.Moore FD, Jr, Davis C, Rodrick M, Mannick JA, Fearon DT. Neutrophil activation in thermal injury as assessed by increased expression of complement receptors. The New England journal of medicine. 1986;314:948–53. doi: 10.1056/NEJM198604103141503. [DOI] [PubMed] [Google Scholar]
- 36.Singh V, Devgan L, Bhat S, Milner SM. The pathogenesis of burn wound conversion. Annals of plastic surgery. 2007;59:109–15. doi: 10.1097/01.sap.0000252065.90759.e6. [DOI] [PubMed] [Google Scholar]
- 37.Henze U, Lennartz A, Hafemann B, Goldmann C, Kirkpatrick CJ, Klosterhalfen B. The influence of the C1-inhibitor BERINERT and the protein-free haemodialysate ACTIHAEMYL20% on the evolution of the depth of scald burns in a porcine model. Burns: journal of the International Society for Burn Injuries. 1997;23:473–7. doi: 10.1016/s0305-4179(97)00019-3. [DOI] [PubMed] [Google Scholar]
- 38.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochemical pharmacology. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Dairam A, Fogel R, Daya S, Limson JL. Antioxidant and iron-binding properties of curcumin, capsaicin, and S-allylcysteine reduce oxidative stress in rat brain homogenate. Journal of agricultural and food chemistry. 2008;56:3350–6. doi: 10.1021/jf0734931. [DOI] [PubMed] [Google Scholar]
- 40.Phan TT, See P, Lee ST, Chan SY. Protective effects of curcumin against oxidative damage on skin cells in vitro: its implication for wound healing. The Journal of trauma. 2001;51:927–31. doi: 10.1097/00005373-200111000-00017. [DOI] [PubMed] [Google Scholar]
- 41.Singer AJ, Taira BR, Lin F, Lim T, Anderson R, McClain SA, et al. Curcumin reduces injury progression in a rat comb burn model. Journal of burn care & research: official publication of the American Burn Association. 2011;32:135–42. doi: 10.1097/BCR.0b013e318203337b. [DOI] [PubMed] [Google Scholar]
- 42.Eski M, Ozer F, Firat C, Alhan D, Arslan N, Senturk T, et al. Cerium nitrate treatment prevents progressive tissue necrosis in the zone of stasis following burn. Burns: journal of the International Society for Burn Injuries. 2012;38:283–9. doi: 10.1016/j.burns.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 43.Deveci M, Eski M, Sengezer M, Kisa U. Effects of cerium nitrate bathing and prompt burn wound excision on IL-6 and TNF-alpha levels in burned rats. Burns: journal of the International Society for Burn Injuries. 2000;26:41–5. doi: 10.1016/s0305-4179(99)00107-2. [DOI] [PubMed] [Google Scholar]
- 44.Eski M, Deveci M, Celikoz B, Nisanci M, Turegun M. Treatment with cerium nitrate bathing modulate systemic leukocyte activation following burn injury: an experimental study in rat cremaster muscle flap. Burns: journal of the International Society for Burn Injuries. 2001;27:739–46. doi: 10.1016/s0305-4179(01)00038-9. [DOI] [PubMed] [Google Scholar]
- 45.Allgower M, Schoenenberger GA, Sparkes BG. Burning the largest immune organ. Burns: journal of the International Society for Burn Injuries. 1995;21(Suppl 1):S7–47. doi: 10.1016/0305-4179(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 46.Kremer B, Allgower M, Graf M, Schmidt KH, Schoelmerich J, Schoenenberger GA. The present status of research in burn toxins. Intensive care medicine. 1981;7:77–87. doi: 10.1007/BF01687264. [DOI] [PubMed] [Google Scholar]
- 47.Robinson JM. Reactive oxygen species in phagocytic leukocytes. Histochemistry and cell biology. 2008;130:281–97. doi: 10.1007/s00418-008-0461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serhan CN, Gotlinger K, Hong S, Arita M. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their aspirin-triggered endogenous epimers: an overview of their protective roles in catabasis. Prostaglandins & other lipid mediators. 2004;73:155–72. doi: 10.1016/j.prostaglandins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 49.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, et al. Resolvin D2 is apotent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–91. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bohr S, Patel SJ, Sarin D, Irimia D, Yarmush ML, Berthiaume F. Resolvin D2 prevents secondary thrombosis and necrosis in a mouse burn wound model. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2013;21:35–43. doi: 10.1111/j.1524-475X.2012.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maier CM, Sun GH, Kunis D, Yenari MA, Steinberg GK. Delayed induction and long-term effects of mild hypothermia in a focal model of transient cerebral ischemia: neurological outcome and infarct size. Journal of neurosurgery. 2001;94:90–6. doi: 10.3171/jns.2001.94.1.0090. [DOI] [PubMed] [Google Scholar]
- 52.Kochanek PM, Drabek T, Tisherman SA. Therapeutic hypothermia: the Safar vision. Journal of neurotrauma. 2009;26:417–20. doi: 10.1089/neu.2008.0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alam HB, Hashmi S, Finkelstein RA, Shuja F, Fukudome EY, Li Y, et al. Alterations in gene expression after induction of profound hypothermia for the treatment of lethal hemorrhage. The Journal of trauma. 2010;68:1084–98. doi: 10.1097/TA.0b013e3181d76bd1. [DOI] [PubMed] [Google Scholar]
- 54.Rizzo JA, Burgess P, Cartie RJ, Prasad BM. Moderate systemic hypothermia decreases burn depth progression. Burns: journal of the International Society for Burn Injuries. 2013;39:436–44. doi: 10.1016/j.burns.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 55.Schwacha MG, Thobe BM, Daniel T, Hubbard WJ. Impact of thermal injury on wound infiltration and the dermal inflammatory response. The Journal of surgical research. 2010;158:112–20. doi: 10.1016/j.jss.2008.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun LT, Friedrich E, Heuslein JL, Pferdehirt RE, Dangelo NM, Natesan S, et al. Reduction of burn progression with topical delivery of (antitumor necrosis factor-alpha)-hyaluronic acid conjugates. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2012;20:563–72. doi: 10.1111/j.1524-475X.2012.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Friedrich EE, Sun LT, Natesan S, Zamora DO, Christy RJ, Washburn NR. Effects of hyaluronic acid conjugation on anti-TNF-alpha inhibition of inflammation in burns. Journal of biomedical materials research Part A. 2014;102:1527–36. doi: 10.1002/jbm.a.34829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Finnerty CC, Herndon DN, Przkora R, Pereira CT, Oliveira HM, Queiroz DM, et al. Cytokine expression profile over time in severely burned pediatric patients. Shock. 2006;26:13–9. doi: 10.1097/01.shk.0000223120.26394.7d. [DOI] [PubMed] [Google Scholar]
- 59.Zhou H, Tu JJ, Huang Y, Chen XG, Deng YJ. Changes in serum contents of interleukin-6 and interleukin-10 and their relation with occurrence of sepsis and prognosis of severely burned patients. Zhonghua shao shang za zhi = Zhonghua shaoshang zazhi = Chinese journal of burns. 2012;28:111–5. [PubMed] [Google Scholar]
- 60.Heideman M, Bengtsson A. The immunologic response to thermal injury. World journal of surgery. 1992;16:53–6. doi: 10.1007/BF02067115. [DOI] [PubMed] [Google Scholar]
- 61.Begieneman MP, Kubat B, Ulrich MM, Hahn NE, Stumpf-Stolker Y, Tempelaars M, et al. Prolonged C1 inhibitor administration improves local healing of burn wounds and reduces myocardial inflammation in a rat burn wound model. Journal of burn care & research: official publication of the American Burn Association. 2012;33:544–51. doi: 10.1097/BCR.0b013e31823bc2fc. [DOI] [PubMed] [Google Scholar]
- 62.Salmon-Ehr V, Ramont L, Godeau G, Birembaut P, Guenounou M, Bernard P, et al. Implication of interleukin-4 in wound healing. Laboratory investigation; a journal of technical methods and pathology. 2000;80:1337–43. doi: 10.1038/labinvest.3780141. [DOI] [PubMed] [Google Scholar]
- 63.Baskaran H, Toner M, Yarmush ML, Berthiaume F. Poloxamer-188 improves capillary blood flow and tissue viability in a cutaneous burn wound. The Journal of surgical research. 2001;101:56–61. doi: 10.1006/jsre.2001.6262. [DOI] [PubMed] [Google Scholar]
- 64.Boykin JV, Eriksson E, Pittman RN. In vivo microcirculation of a scald burn and the progression of postburn dermal ischemia. Plastic and reconstructive surgery. 1980;66:191–8. doi: 10.1097/00006534-198008000-00002. [DOI] [PubMed] [Google Scholar]
- 65.Nwariaku FE, Sikes PJ, Lightfoot E, Mileski WJ, Baxter C. Effect of a bradykinin antagonist on the local inflammatory response following thermal injury. Burns: journal of the International Society for Burn Injuries. 1996;22:324–7. doi: 10.1016/0305-4179(95)00130-1. [DOI] [PubMed] [Google Scholar]
- 66.Robson MC, Del Beccaro EJ, Heggers JP. The effect of prostaglandins on the dermal microcirculation after burning, and the inhibition of the effect by specific pharmacological agents. Plastic and reconstructive surgery. 1979;63:781–7. [PubMed] [Google Scholar]
- 67.Lund T, Onarheim H, Reed RK. Pathogenesis of edema formation in burn injuries. World journal of surgery. 1992;16:2–9. doi: 10.1007/BF02067107. [DOI] [PubMed] [Google Scholar]
- 68.Kao CC, Garner WL. Acute burns. Plastic and reconstructive surgery. 2000;105:2482–92. quiz 93; discussion 94. [PubMed] [Google Scholar]
- 69.Hirth D, McClain SA, Singer AJ, Clark RA. Endothelial necrosis at 1 hour postburn predicts progression of tissue injury. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2013;21:563–70. doi: 10.1111/wrr.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fourman MS, Phillips BT, Crawford L, McClain SA, Lin F, Thode HC, Jr, et al. Indocyanine green dye angiography accurately predicts survival in the zone of ischemia in a burn comb model. Burns: journal of the International Society for Burn Injuries. 2014;40:940–6. doi: 10.1016/j.burns.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 71.Ribatti D, Presta M, Vacca A, Ria R, Giuliani R, Dell’Era P, et al. Human erythropoietin induces a pro-angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Blood. 1999;93:2627–36. [PubMed] [Google Scholar]
- 72.Haroon ZA, Amin K, Jiang X, Arcasoy MO. A novel role for erythropoietin during fibrin-induced wound-healing response. The American journal of pathology. 2003;163:993–1000. doi: 10.1016/S0002-9440(10)63459-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bader A, Lorenz K, Richter A, Scheffler K, Kern L, Ebert S, et al. Interactive role of trauma cytokines and erythropoietin and their therapeutic potential for acute and chronic wounds. Rejuvenation research. 2011;14:57–66. doi: 10.1089/rej.2010.1050. [DOI] [PubMed] [Google Scholar]
- 74.Bohr S, Patel SJ, Shen K, Vitalo AG, Brines M, Cerami A, et al. Alternative erythropoietin-mediated signaling prevents secondary microvascular thrombosis and inflammation within cutaneous burns. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:3513–8. doi: 10.1073/pnas.1214099110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tobalem M, Harder Y, Rezaeian F, Wettstein R. Secondary burn progression decreased by erythropoietin. Critical care medicine. 2013;41:963–71. doi: 10.1097/CCM.0b013e318275cee7. [DOI] [PubMed] [Google Scholar]
- 76.Tobalem M, Harder Y, Schuster T, Rezaeian F, Wettstein R. Erythropoietin in the prevention of experimental burn progression. The British journal of surgery. 2012;99:1295–303. doi: 10.1002/bjs.8847. [DOI] [PubMed] [Google Scholar]
- 77.Harting MT, Jimenez F, Kozar RA, Moore FA, Mercer DW, Hunter RL, et al. Effects of poloxamer 188 on human PMN cells. Surgery. 2008;144:198–203. doi: 10.1016/j.surg.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yuhua S, Ligen L, Jiake C, Tongzhu S. Effect of Poloxamer 188 on deepening of deep second-degree burn wounds in the early stage. Burns: journal of the International Society for Burn Injuries. 2012;38:95–101. doi: 10.1016/j.burns.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 79.Tobalem M, Harder Y, Tschanz E, Speidel V, Pittet-Cuenod B, Wettstein R. First-aid with warm water delays burn progression and increases skin survival. Journal of plastic, reconstructive & aesthetic surgery: JPRAS. 2013;66:260–6. doi: 10.1016/j.bjps.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 80.Boykin JV, Jr, Eriksson E, Sholley MM, Pittman RN. Cold-water treatment of scald injury and inhibition of histamine-mediated burn edema. The Journal of surgical research. 1981;31:111–23. doi: 10.1016/0022-4804(81)90038-x. [DOI] [PubMed] [Google Scholar]
- 81.Schaser KD, Stover JF, Melcher I, Lauffer A, Haas NP, Bail HJ, et al. Local cooling restores microcirculatory hemodynamics after closed soft-tissue trauma in rats. The Journal of trauma. 2006;61:642–9. doi: 10.1097/01.ta.0000174922.08781.2f. [DOI] [PubMed] [Google Scholar]
- 82.Slater TF. Free-radical mechanisms in tissue injury. The Biochemical journal. 1984;222:1–15. doi: 10.1042/bj2220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fazal N, Knaus UG, Sabeh F, Gamelli RL, McNulty JA, Sayeed MM. Enhanced expression of neutrophil NADPH oxidase components in intestine of rats after burn injury. Shock. 1999;12:438–42. doi: 10.1097/00024382-199912000-00004. [DOI] [PubMed] [Google Scholar]
- 84.Latha B, Babu M. The involvement of free radicals in burn injury: a review. Burns: journal of the International Society for Burn Injuries. 2001;27:309–17. doi: 10.1016/s0305-4179(00)00127-3. [DOI] [PubMed] [Google Scholar]
- 85.Parihar A, Parihar MS, Milner S, Bhat S. Oxidative stress and anti-oxidative mobilization in burn injury. Burns: journal of the International Society for Burn Injuries. 2008;34:6–17. doi: 10.1016/j.burns.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 86.Horton JW. Free radicals and lipid peroxidation mediated injury in burn trauma: therole of antioxidant therapy. Toxicology. 2003;189:75–88. doi: 10.1016/s0300-483x(03)00154-9. [DOI] [PubMed] [Google Scholar]
- 87.Zor F, Ozturk S, Deveci M, Karacalioglu O, Sengezer M. Saving the zone of stasis: is glutathione effective? Burns: journal of the International Society for Burn Injuries. 2005;31:972–6. doi: 10.1016/j.burns.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 88.Vorauer-Uhl K, Furnschlief E, Wagner A, Ferko B, Katinger H. Reepithelialization of experimental scalds effected by topically applied superoxide dismutase: controlled animal studies. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2002;10:366–71. doi: 10.1046/j.1524-475x.2002.t01-1-10605.x. [DOI] [PubMed] [Google Scholar]
- 89.Vorauer-Uhl K, Furnschlief E, Wagner A, Ferko B, Katinger H. Topically applied liposome encapsulated superoxide dismutase reduces postburn wound size and edema formation. European journal of pharmaceutical sciences: official journal of the European Federation for Pharmaceutical Sciences. 2001;14:63–7. doi: 10.1016/s0928-0987(01)00149-x. [DOI] [PubMed] [Google Scholar]
- 90.Shalom A, Kramer E, Westreich M. Protective effect of human recombinant copper-zinc superoxide dismutase on zone of stasis survival in burns in rats. Annals of plastic surgery. 2011;66:607–9. doi: 10.1097/SAP.0b013e3181fc04e1. [DOI] [PubMed] [Google Scholar]
- 91.Wasiak J, Bennett M, Cleland HJ. Hyperbaric oxygen as adjuvant therapy in the management of burns: can evidence guide clinical practice? Burns: journal of the International Society for Burn Injuries. 2006;32:650–2. doi: 10.1016/j.burns.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 92.Niezgoda JA, Cianci P, Folden BW, Ortega RL, Slade JB, Storrow AB. The effect of hyperbaric oxygen therapy on a burn wound model in human volunteers. Plastic and reconstructive surgery. 1997;99:1620–5. [PubMed] [Google Scholar]
- 93.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–8. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 94.Wang M, Crisostomo PR, Herring C, Meldrum KK, Meldrum DR. Human progenitor cells from bone marrow or adipose tissue produce VEGF, HGF, and IGF-I in response to TNF by a p38 MAPK-dependent mechanism. American journal of physiology Regulatory, integrative and comparative physiology. 2006;291:R880–4. doi: 10.1152/ajpregu.00280.2006. [DOI] [PubMed] [Google Scholar]
- 95.Ebrahimian TG, Pouzoulet F, Squiban C, Buard V, Andre M, Cousin B, et al. Cell therapy based on adipose tissue-derived stromal cells promotes physiological and pathological wound healing. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:503–10. doi: 10.1161/ATVBAHA.108.178962. [DOI] [PubMed] [Google Scholar]
- 96.Rigotti G, Marchi A, Galie M, Baroni G, Benati D, Krampera M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plastic and reconstructive surgery. 2007;119:1409–22. doi: 10.1097/01.prs.0000256047.47909.71. discussion 23–4. [DOI] [PubMed] [Google Scholar]
- 97.Gonzalez MA, Gonzalez-Rey E, Rico L, Buscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978–89. doi: 10.1053/j.gastro.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 98.Pinheiro CH, de Queiroz JC, Guimaraes-Ferreira L, Vitzel KF, Nachbar RT, de Sousa LG, et al. Local injections of adipose-derived mesenchymal stem cells modulate inflammation and increase angiogenesis ameliorating the dystrophic phenotype in dystrophin-deficient skeletal muscle. Stem cell reviews. 2012;8:363–74. doi: 10.1007/s12015-011-9304-0. [DOI] [PubMed] [Google Scholar]


