Abstract
The post-oral actions of glucose stimulate intake and condition flavor preferences in rodents. Hypothalamic melanin-concentrating hormone (MCH) neurons are implicated in sugar reward, and this study investigated their involvement in glucose preference conditioning in mice. In Exp. 1 MCH receptor 1 knockout (KO) and C57BL/6 wildtype (WT) mice learned to prefer 8% glucose over an initially more-preferred non-nutritive 0.1% sucralose + saccharin (S+S) solution. In contrast, the KO and WT mice preferred S+S to 8% fructose, which is consistent with this sugar’s weak post-oral reinforcing action. In Exp. 2 KO and WT mice were trained to drink a flavored solution (CS+) paired with intragastric (IG) infusion of 16% glucose and a different flavored solution (CS−) paired with IG water. Both groups drank more CS+ than CS− in training and preferred the CS+ to CS− in a 2-bottle test. These results indicate that MCH receptor signaling is not required for flavor preferences conditioned by the post-oral actions of glucose. This contrasts with other findings implicating MCH signaling in other types of sugar reward processing.
Keywords: Melanin-concentrating hormone, flavor conditioning, glucose, fructose, non-nutritive sweetener, intragastric
1. Introduction
It is well documented that sugar appetite in rodents is determined by both the oral and post-oral actions of sugars [21]. In the mouth, sugars stimulate the T1R2/T1R3 sweet receptors that, via gustatory nerve connections to the brain, activate brain dopamine and opioid systems that mediate sweet taste reward [6]. Once ingested, sugars also stimulate post-oral sensors that enhance the reward value of the nutrient in part by activating the brain dopamine system [6,27]. Recent findings indicate that intestinal sodium-glucose transporters (SGLTs), which also function as glucose sensors (“transceptors”), are critically involved in post-oral stimulation of sugar appetite, a process referred to as appetition [19,25,40]. Other evidence suggests that glucose sensors in the hepatic-portal region contribute to sugar reward [16]. How stimulation of these pre- and post-absorptive sugar glucose sensors signal the brain circuits that modulate sugar reward is not clear. A recent study implicates melanin-concentrating hormone (MCH) neurons in the lateral hypothalamus as a critical link in the activation of the brain dopamine reward system by the post-oral actions of sucrose [7]. In this study, mice were given choice tests with 0.4 M sucrose, which has post-oral reward effects, and 1.5 mM sucralose, a non-nutritive sweetener which has a less preferred taste at the concentration selected and no post-oral reward actions. The mice strongly preferred sucrose to sucralose in two-bottle tests. However, when sucralose consumption was linked to optogenetic stimulation of MCH neurons, which stimulated striatal DA release, the mice preferred sucralose to sucrose. This was taken as evidence that optogenetic activation of MCH neurons mimics the post-oral actions of sucrose on the dopamine reward system. Conversely, mice with targeted lesions of MCH neurons did not prefer sucrose to sucralose which, according to Domingos et al. [7], occurred because post-oral sucrose reward was blocked. These findings are consistent with other results implicating the MCH system in sugar reward [3,4,9,13,15,18,38]. However, as noted by Domingos et al. [7], their findings obtained with MCH neuron activation or ablation do not establish whether it was the MCH peptide per se or other neurotransmitters (e.g., GABA [12], glutamate [5], or other peptides such as CART or Nesfatin [36]) released by MCH neurons that modulate post-oral sugar reward effects.
The present study investigated the role of MCH peptide signaling in post-oral sugar appetition using MCH-R1 knockout mice (KO) which are missing the MCH-R1 receptor, the only functional MCH receptor in rodents [33]. MCH-R1 KO mice are deficient in learning a conditioned reinforcement response to a sucrose-paired stimulus and fail to overeat in response to a sucrose-paired cue [30,31]. These deficits, however, do not necessarily indicate that the KO mice are insensitive to the post-oral reinforcement actions of sugar. To address this issue, we compared the ability of MCH-R1 KO mice and wildtype (WT) mice to acquire a preference for glucose or a glucose-paired flavor using two different conditioning paradigms. Glucose rather than sucrose was used in these studies because it is primarily responsible for the post-oral reward actions of sucrose, which is a glucose + fructose disaccharide.
2. Experiment 1. Learned preference for nutritive sugar over non-nutritive sweetener
Like Domingo et al. (2013), we have investigated post-oral sugar appetition by comparing the preferences for nutritive sugars vs. non-nutritive sweeteners [26,28,29]. In our studies, however, mice were offered an initially preferred non-nutritive sweetener solution and a less preferred sugar solution, and experience-induced changes in sweetener preference were assessed. We observed that naive C57BL/6J (B6) mice significantly preferred a mixture of 0.1% sucralose and 0.1% saccharin (S+S) to 8% sucrose, glucose, and fructose solutions in brief two-bottle taste tests [29]. However, after separate 2-day tests with the S+S and sugar solutions, the mice displayed strong preferences for sucrose and glucose over S+S but they continued to prefer S+S to fructose [14,28,29]. These findings are consistent with the ability of intragastric (IG) sucrose and glucose infusions, but not fructose infusions, to condition significant preferences for a flavored saccharin solution [20,23,24,26]. In the present experiment, we compared the preferences of MCH-R1 KO and WT mice for S+S over glucose and fructose solutions. If MCH-R1 receptor signaling is critical for post-oral glucose appetition, then KO mice, unlike WT mice, should prefer S+S to glucose as well as to fructose after experience with the different sweeteners.
2.1. Methods
2.1.1. Animals
Adult MCH-R1 KO mice (10 male, 10 female) and B6 WT mice (10 male, 10 female) were singly housed in tub cages in a room with a 12 h light-dark cycle. The generation of the MCH-R1 KO mice on a C57BL/6 background has been previously described [2,31]. The genotype status of the MCH-R1 KO and WT mice was confirmed by real-time PCR of ear biopsies (Transnetyx, Cordova, TN). The animals were given ad libitum access to chow (LabDiet Standard Laboratory Rodent Diet #5001, PMI Nutrition International, Brentwood, MO) and water except where noted.
2.1.2. Test Solutions
Sugar solutions (8%) were prepared using food-grade glucose and fructose (Tate and Lyle, Honeyville Food Products, Rancho Cucamonga, CA) dissolved in deionized water. The noncaloric sweetener solution (S+S) was prepared using 0.1% sucralose (Tate & Lyle, Dayton, OH) and 0.1% sodium saccharin (Sigma-Aldrich, St. Louis, MO). B6 mice significantly prefer the S+S solution to 8% glucose and fructose in 1-min two-bottle tests, suggesting that it has a “sweeter” taste than the sugar solutions [29]. The solutions were available through stainless steel sipper spouts attached to 50-ml plastic tubes that were placed on the grid top of the cage and fixed in place with clips. Fluid intakes were measured to the nearest 0.1 g by weighing the drinking bottles on an electronic balance. Intakes were corrected for spillage, which was estimated by recording the change in weight of two bottles that were placed on an empty cage.
2.1.3. Procedure
The mice were adapted to home cages with two water bottles and ad libitum chow for 1 week. Two groups each of KO and WT mice (5 males and 5 females in each group) were tested with 8% glucose and fructose. They were given a series of 2-day, two-bottle choice tests as follows: Test 1 (days 1–2) sugar vs. 0.1% S+S, Test 2 (days 3–4) sugar vs. water, Test 3 (days 5–6) S+S vs. water, and Test 4 (days 8–9) sugar vs. S+S. The mice were given water only on day 7 between Tests 3 and 4. Water was available in Tests 2 and 3 so that the animals were not forced to drink the sweetener, but they consumed little or no water in these tests. The left–right position of the sweetener and water bottles were switched from the first to second day of each test.
2.1.4. Data Analysis
Daily solution intakes were averaged over the 2 days of each test, and sweetener preferences were expressed as percent solution intakes (e.g., glucose intake/total intake x 100). Intakes were analyzed using a mixed model analysis of variance (ANOVA) with test and solution as repeated factors. A second ANOVA included results from Tests 2 and 3, and compared the intakes of each sweetener vs. water within groups. Percent sweetener intakes within groups were analyzed with t-tests. Additional between group ANOVAs were performed as described below.
2.2. Results
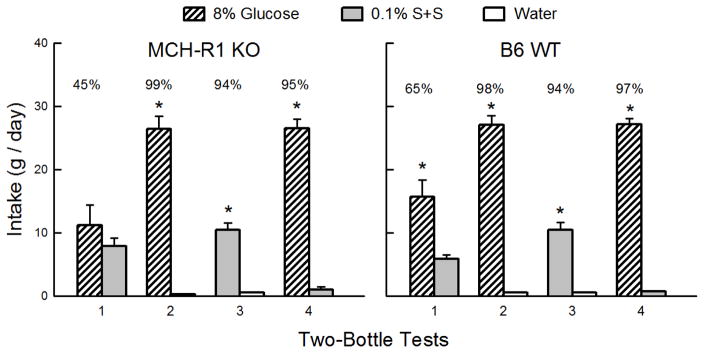
The KO and WT Glucose groups consumed somewhat more sugar than S+S in Test 1 but substantially more glucose in Test 4 (Sweetener x Test interaction, F(1,18) = 72.9, P < 0.001) (Figure 1). The groups did not differ significantly in their sweetener intakes or preferences, although the WT but not the KO group consumed more (P < 0.05) glucose than S+S in Test 1. Overall, glucose preferences increased from 55% in Test 1 to 96% in Test 4 (F(1,18) = 33.0, P < 0.001). A closer analysis of Test 1 revealed that, overall, the mice increased their percent glucose intakes from 36% on day 1 of the test to 67% on day 2 and the KO and WT mice showed parallel increases in sugar preference (F(1,18) = 19.4, P < 0.001). This suggests that during the 2-day test the mice were learning to prefer glucose to S+S based on the sugar’s post-oral reinforcing actions. In the sweetener vs. water Tests 2 and 3, the KO and WT glucose groups consumed substantially more sugar and S+S than water (F(1,18) = 554.4, P < 0.001) and consumed more than twice as much glucose as S+S (F(1,18) = 134.5, P < 0.001). Percent glucose intake also exceeded percent S+S intake (98% vs. 94%, F(1,18) = 30.3, P < 0.001).
Figure 1.
Experiment 1. Glucose and non-nutritive sweetener preferences in MCH-R1 KO and B6 WT mice. Mean (+SEM) intakes of 8% glucose and 0.1% sucralose + 0.1% saccharin (S+S) in 2-day 2-bottle Tests 1–4. The MCH-R1 KO mice (left panel) and B6 WT mice (right panel) were given the choice of glucose versus S+S in Tests 1 and 4, and glucose versus water and S+S versus water in Tests 2 and 3. The tests were conducted in the order indicated. Numbers atop bars represent mean percent preference for that solution. Significant (P < 0.05) differences within each test are indicated by an asterisk (*).
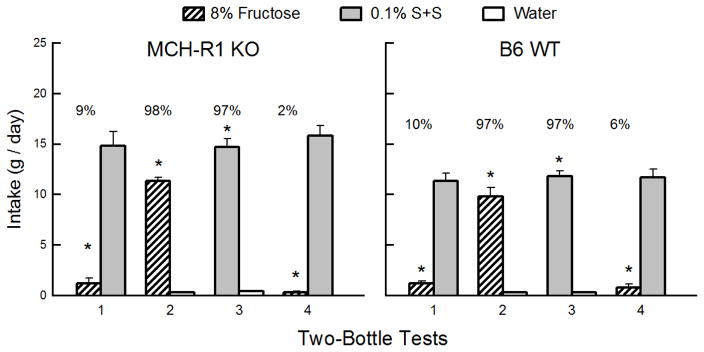
Overall, the KO and WT Fructose groups consumed substantially more S+S than fructose in both Tests 1 and 4 (F(1,18) = 279.7, P < 0.001) and their intake of the two solutions did not change from the first to last test (Figure 2). The percent fructose intakes decreased from Test 1 to 4 (F(1,18) = 6.8, P < 0.05). Overall, the KO mice consumed more S+S than did the WT mice in the two tests (F(1,18) = 7.4, P < 0.05). In Tests 2 and 3, the KO and WT mice consumed more fructose and S+S than water (F(1,18) = 819.4, P < 0.001) and more S+S than fructose (F(1,18) = 22.4, P < 0.01). Percent fructose and S+S intakes were similar, however (97–98%). The KO mice consumed more of both sweeteners than did the WT mice in Tests 2 and 3 (F(1,18) = 7.2, P < 0.05).
Figure 2.
Experiment 1. Fructose and non-nutritive sweetener preferences in MCH-R1 KO and B6 WT mice. Mean (+SEM) intakes of 8% fructose and 0.1% sucralose + 0.1% saccharin (S+S) in 2-day 2-bottle Tests 1–4. The MCH-R1 KO mice (left panel) and B6 WT mice (right panel) were given the choice of fructose versus S+S in Tests 1 and 4, and fructose versus water and S+S versus water in Tests 2 and 3. The tests were conducted in the order indicated. Numbers atop bars represent mean percent preference for that solution. Significant (P < 0.05) differences within each test are indicated by an asterisk (*).
These findings confirm prior results with B6 mice, and indicate that deletion of the MCH-R1 receptor does not alter the learned preference for glucose over S+S or the persistent preference for S+S over fructose.
3. Experiment 2. Flavor conditioning by IG glucose infusions
The strong glucose preference displayed by the KO mice in Experiment 1 following separate experience with glucose and S+S indicates that MCH receptor signaling is not required for post-oral glucose appetition. Experiment 2 sought direct evidence for this interpretation using the IG sugar conditioning procedure. Our prior studies demonstrate that B6 mice trained to drink a flavored saccharin solution (CS+) paired with IG self-infusions of 16% glucose increased their CS+ intake and acquired a significant preference for the CS+ flavor over an alternate flavor (CS−) paired with IG water infusions [26,39,41]. In Experiment 2 we compared the flavor conditioning response of MCH-R1 KO and B6 WT mice to IG self-infusions of 16% glucose. The IG glucose infusion was diluted in the stomach by the orally consumed CS+ solution to an 8% solution, the sugar concentration studied in the first experiment.
3.1. Method
3.1.1. Animals
Adult MCH-R1 KO mice (7 male, 5 female) and B6 WT mice (7 male, 5 female) were housed as in Experiment 1. They were fed lab chow (5001) or, when food restricted, fixed-size chow pellets (0.5 or 1 g, F0171, F0173; Bio-Serv, Frenchtown, NJ) that allowed for precise adjustment of daily food rations.
3.1.2. Surgery
The mice were anesthetized with 2% isoflurane inhalation and fitted with a gastric catheter, as described previously [23]. Two weeks after surgery, the animals were briefly (5 min) anesthetized with isoflurane, fitted with a harness and a spring tether (CIH62; Instech Laboratories, Plymouth Meeting, PA), and transferred to infusion test cages.
3.1.2. Apparatus
IG infusion training and testing were conducted in plastic test cages fitted with two sipper spouts attached to glass drinking tubes [23]. The sipper spouts were interfaced via electronic lickometers to a computer that operated a syringe pump which infused liquid into the gastric catheters as the animals licked; the oral-to-infusion intake ratio was maintained at ~1:1. The pump rate was nominally 0.5 ml/min, but the overall infusion rate and volume was controlled by the animal’s licking behavior. Daily oral fluid intakes were measured to the nearest 0.1 g, and IG infusions were recorded to the nearest 0.5 ml.
3.1.3. Test solutions
The mice were initially trained to drink in the infusion cages using unflavored saccharin solutions; initially the saccharin concentration was 0.025% but it was increased to 0.04% on the last pre-training session to stimulate intakes adequate for conditioning (see Table 1). The mice were then trained for the remainder of the experiment with flavored solutions (CS) containing 0.04% saccharin and distinctive odor cues (0.02% ethyl acetate or propyl acetate; Sigma) [32,42]. During training, the CS− solution was paired with IG infusion of water while the CS+ solution was paired with IG infusion of 16% glucose (Honeyville Food Products). For half the animals the CS− solution contained ethyl acetate and the CS+ solution contained propyl acetate; the flavors were reversed for the remaining animals.
Table 1.
Training and Test Sessions in Experiment 2.
| Days | Oral Solution | IG Infusate |
|---|---|---|
| Pre-Training Sessions
| ||
| 1–2 | 0.025% saccharin | water |
| 3–5 | 0.025% saccharin | water |
| 6 | 0.04% saccharin | water |
|
| ||
| CS+ and CS− Training and Test Sessions
| ||
| 1–3 | CS− | water |
| 4–6 | CS+ | glucose |
| 7 | CS− | water |
| 8 | CS+ | glucose |
| 9 | CS− vs. water | water |
| 10 | CS+ vs. water | glucose |
| 11–12 | CS+ vs. CS− | none |
Mice were water restricted in pre-training sessions
1–2 and food restricted in all subsequent sessions.
CS− and CS+ solutions contained 0.02% ethyl acetate or propyl acetate flavors in 0.04% saccharin solutions.
A water tube (not paired with infusions) was available in the last CS− and CS+ training sessions (Days 9–10).
3.1.4. Procedure
Two weeks after surgery the mice were water restricted (1 h/day) and trained in the infusion cages to drink unflavored saccharin paired with matched IG infusions of water for two 1 h/day sessions. They were then switched to a food restriction schedule that maintained them at ~90% body weight by giving them limited food rations daily. Another four 1/h day sessions were run with unflavored saccharin paired with IG water infusions (Table 1).
The mice were given one-bottle training sessions (1 h/day) with the CS− flavored saccharin solution paired with IG water (days 1–3) and CS+ flavored saccharin solution paired with IG glucose (days 4–6). This was followed by four alternating 1-h/day sessions with the CS− (days 7 and 9) and CS+ (days 8 and 10) paired with their respective IG infusions (Table 1). These alternating sessions were designed to enhance the ability of the mice to discriminate between the CS− and CS+ flavors in the two-bottle test sessions. In the final CS− and CS+ training sessions a second sipper tube containing water, not paired with IG infusions, was available to familiarize the mice to the presence of two sipper tubes in the subsequent two-bottle test. A two-bottle choice test was conducted on days 11 and 12 (1 h/day) with the CS+ vs. CS− solutions without IG infusions. The left-right positions of the CS+ and CS− solutions were counterbalanced throughout training and testing. This training/test series was based on our prior studies [26,28,40,41].
3.1.5. Data analysis
Licks and total intakes (oral + IG infusate) during the last two CS− sessions were averaged. The data from these two sessions, referred to as CS− Test 0, and the licks and intakes during the subsequent three CS+ sessions (Tests 1–3) were analyzed using a mixed-model ANOVA with a group factor (KO, WT) and repeated-measures factor (Tests 0–3). The mean CS− and CS+ licks during the alternating one-bottle sessions and the two-bottle test were compared in separate ANOVAs.
3.2. Results and Discussion
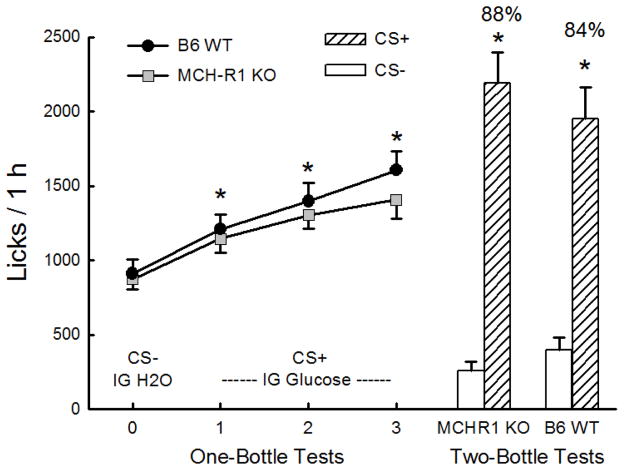
During CS− Test 0 the licks of the KO and WT mice were similar (Figure 3). When switched to the CS+ flavor paired with IG glucose infusions in Tests 1–3, both groups significantly increased their 1-h licks above the level of that of CS− Test 0 (F(3,66) = 52.4, P < 0.001) and there were no group differences in CS+ licks. Overall, licks increased (P < 0.05) in each successive CS+ test. The mice also increased their solution intakes (oral + IG) from CS− Test 0 to CS+ Test 3 and the groups did not differ on this measure (KO: 2.3 to 3.2 g/h, WT: 2.5 to 3.7 g/h; F(3,66) = 40.0, P < 0.001). In the alternating CS training sessions, the KO and WT mice licked more for CS+ than CS− (1458 vs. 1276 licks/h; F(1,22) = 41.5, P < 0.001) and did not differ on this measure. In the two-bottle choice test, both groups licked significantly more for the CS+ than CS− (F(1,22) = 120.1, P < 0.001) and there were no group differences (Figure 3). The KO and WT mice were also similar in their percent CS+ preferences (88% vs. 84%).
Figure 3.
Experiment 2: Glucose stimulation of licking and flavor preference conditioning in MCH-R1 KO and B6 WT mice. The mice drank (1 h/day) a CS− flavored saccharin solution paired with IG water infusions in Test 0 before being switched to a CS+ flavored saccharin solution paired with IG 16% glucose infusions in Tests 1–3. Left: 1-h licks (means ±SEM) are plotted for 1-bottle Tests 0–3. Right: 1-h licks (means +SEM) are plotted for CS+ and CS− flavored saccharin solutions during the 2-bottle preference test. CS+ and CS− intakes were not paired with IG infusions in the 2-bottle test. Number above bar represents mean percent preference for the CS+ solution. Significant (P < 0.05) differences between Test 0 and Tests 1–3 licks and between CS+ and CS− licks are indicated by an asterisk (*).
In agreement with prior results [39,40,41], IG glucose self-infusions increased the licking for the CS+ from the very first training session and conditioned a significant preference for the CS+ over the CS−. The new finding here is that MCH-R1 KO mice displayed similar CS+ appetition responses to the IG glucose infusions to those of the WT mice.
4. General Discussion
The present study investigated the role of MCH-R1 receptor signaling in the post-oral appetite stimulating actions of glucose. The results were quite clear: MCH-R1 KO mice were similar to WT mice in acquiring a strong preference for glucose over an initially preferred non-nutritive sweetener solution (S+S) in Experiment 1 and in acquiring a preference for a flavored non-nutritive solution (CS+) paired with IG infusions of glucose in Experiment 2. In contrast, the KO and WT mice strongly preferred the S+S solution to fructose, which confirms prior results with B6 mice [14,28,29]. These findings contrast with the report of Domingos et al. [7] indicating that hypothalamic MCH neurons mediate the post-oral reinforcing effects of sucrose, which is attributed to the glucose component of the sugar. As noted by Domingos et al., however, their results did not demonstrate whether it is the MCH peptide or other neurotransmitters released by MCH neurons that are responsible for the observed behavioral effects of MCH neuron activation or ablation. The present findings indicate that MCH peptide signaling at MCH-R1 receptors is not required for post-oral glucose appetition. It is possible, however, that acute, central blockade of MCH-R1 receptors may have a different effect on post-oral glucose conditioning than does genetic deletion of the MCH-R1 receptors. This remains to be determined. Sherwood et al. [31], however, reported both MCH-R1 KO mice and WT mice treated with an MCH-R1 antagonist showed deficits in conditioned reinforcement performance for sucrose reinforcement (see below).
A close examination of the Domingos et al. [7] study revealed that only some of their findings specifically implicated MCH neuron involvement in post-oral sucrose reinforcement. In particular, the observation that optogenetic stimulation of MCH neurons induced a preference for sucralose over sucrose does not necessarily mean that MCH activation mimicked post-oral sugar reinforcement. It is possible that MCH neuron activation enhanced the sweet taste reward value of the sucralose. Although the authors state the sucralose is not preferred to sucrose unless it is “supplemented by a proxy” for post-oral sugar reward, we have reported that there are concentrations of sucralose (0.8%) or sucralose+saccharin mixtures (0.1%) that are preferred to at least some sucrose (8%) or glucose (8–16%) solutions [29](Sclafani unpublished findings). Interestingly, MCH optogenetic stimulation did not enhance the intake of or preference for plain water, which implied that MCH reward activation is specific to a sweet solution. However, IG glucose infusions increase the licking of a “tasteless” dry sipper tube [10,22], the intake of plain water (Sclafani, unpublished findings) and of bitter and sour solutions [8,17]. Thus, the failure of MCH neuron activation to increase water intake and preference suggests that it does not fully mimic the post-oral actions of glucose.
Domingos et al. [7] also investigated the effects of selective ablation of MCH neurons on sugar reward. MCH-ablated B6 mice consumed less sucrose than did control animals, which was attributed to a lack of post-oral sucrose reinforcement, but this could have been due to a reduction in sweet taste reward value. Arguing against this interpretation, MCH-ablated mice displayed normal preferences for sucrose and sucralose over water. Yet ablated mice failed to prefer sucrose to sucralose in a short-term choice test, which is unexpected because at the concentrations used (0.4 M and 1.5 mM, respectively) sucrose should have been preferred to sucralose based on its taste alone. Thus, the interpretation of the MCH neuron ablation findings is not straightforward and requires further investigation.
Sweet “tasteless” TRPM5 knockout mice were also used to evaluate the impact of MCH ablation on post-oral sucrose reinforcement [7]. Ablated and control KO mice were trained to drink sucrose and water from sipper tubes at specific locations. In a subsequent choice test with both sipper tubes containing water, the control KO mice licked more from the sucrose-paired sipper tube, which was attributed to a learned response reinforced by the sugar’s post-oral actions. The MCH ablated KO mice, however, did not prefer the sucrose-paired sipper tube, which supports the view that MCH neurons mediate post-oral sucrose reinforcement. Sipper position preferences have also been conditioned in rats by hepatic-portal glucose infusions [16], whereas hepatic-portal glucose infusions did not condition a preference for a CS+ flavored saccharin solution [1]. Thus, glucose-conditioned sipper position and flavor preferences may not involve the same post-oral reinforcement processes. It will be of interest to determine if MCH neuron ablation impairs flavor conditioning by post-oral sugar infusions and, if so, to identify the neurotransmitter signaling process involved.
In contrast to the normal glucose preference conditioning displayed by the KO mice in the present study, other experiments reported selective disruptions in other types of sugar conditioning in MCH-R1 KO mice [30,31]. In particular, KO and WT were similar in acquiring a food cup approach response to an auditory stimulus (CS+) paired with presentation of a sucrose solution. However, presentation of the CS+ alone failed to reinforce a new response (nose poke) in KO mice, although it was effective in WT mice [31]. In contrast, the sucrose-paired CS+ facilitated responding in a Pavlovian-instrumental transfer task in KO and WT mice [31]. In another experiment, the presentation of a sucrose-paired auditory CS+ stimulated overdrinking of a sucrose solution in WT mice but not in KO mice [30]. These findings indicated that deletion of the MCH-R1 receptor impairs incentive motivation conditioning to a sucrose-paired cue. The present experiments imply that this learning deficit is independent of the ability of KO mice to learn flavor preferences based on post-oral nutrient reinforcement. Presumably, KO mice would show similar impairments in incentive motivation learning when trained with sweet solutions (fructose, S+S) that do not support post-oral flavor conditioning.
An important feature of MCH neurons is that they are activated by increases in blood glucose, which led Domingos et al. to suggest that direct glucose sensing by these neurons may modulate sugar reward [7,37]. Other investigators have also assumed that brain glucose sensing regulates sugar seeking in animals [35]. This is an intriguing possibility although there is as yet no direct evidence that post-oral sugar reinforcement involves brain glucose sensing. Intestinal glucose sensors are implicated in glucose appetition by the findings that gastric and upper intestinal infusions but not lower intestinal, hepatic-portal or intraperitoneal glucose infusions condition preferences for flavored saccharin solutions [1,41]. In addition, IG infusions of α-methyl-D-glucopyranoside, a non-metabolizable glucose analog that binds to intestinal glucose sensors but does not elevate blood glucose, conditioned flavor preferences in mice [40]. Other studies reported that hepatic-portal glucose infusions conditioned preferences for flavored food or for a sipper tube position more effectively than intravenous glucose infusions, which implicated hepatic-portal glucose sensors rather than brain sensors in this form of sugar conditioning [16,34]. In addition, hepatic-portal glucose infusions are more effective than jugular infusions in stimulating striatal dopamine release [11,16]. Thus, while a role of brain glucose sensors in sugar appetite cannot be ruled out, the available evidence suggests that sugar sensors in the mouth (sweet taste receptors), intestinal tract and hepatic portal region are primarily responsible for driving sugar consumption.
Highlights.
Flavor preferences are conditioned by sugars in mice.
MCH neurons are implicated in sugar reward processing.
MCH receptor deletion did not impair glucose-conditioned flavor preferences.
MCH receptor signaling is not essential for sugar conditioned preferences.
Acknowledgments
This research was supported by grant DK-31135 from the National Institute of Diabetes and Digestive and Kidney Diseases to A.S. and K.A. A.A. is supported by the Human Frontier Science Program (RGY0076/2012), the Swiss National Science Foundation, the Inselspital and the University of Bern. We thank Martin Zartarian and Kwame McCartney for their expert technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ackroff K, Yiin YM, Sclafani A. Post-oral infusion sites that support glucose-conditioned flavor preferences in rats. Physiol Behav. 2010;99:402–411. doi: 10.1016/j.physbeh.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamantidis A, Thomas E, Foidart A, Tyhon A, Coumans B, Minet A, Tirelli E, Seutin V, Grisar T, Lakaye B. Disrupting the melanin-concentrating hormone receptor 1 in mice leads to cognitive deficits and alterations of NMDA receptor function. Eur J Neurosci. 2005;21:2837–2844. doi: 10.1111/j.1460-9568.2005.04100.x. [DOI] [PubMed] [Google Scholar]
- 3.Baird JP, Rios C, Gray NE, Walsh CE, Fischer SG, Pecora AL. Effects of melanin-concentrating hormone on licking microstructure and brief-access taste responses. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1265–R1274. doi: 10.1152/ajpregu.00143.2006. [DOI] [PubMed] [Google Scholar]
- 4.Benoit SC, Clegg DJ, Woods SC, Seeley RJ. The role of previous exposure in the appetitive and consummatory effects of orexigenic neuropeptides. Peptides. 2005;26:751–757. doi: 10.1016/j.peptides.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Chee MJS, Arrigoni E, Maratos-Flier E. Melanin-concentrating hormone neurons release glutamate for feedforward inhibition of the lateral septum. J Neurosci. 2015;35:3644–3651. doi: 10.1523/JNEUROSCI.4187-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Araujo IE. Sweet taste signaling and the formation of memories of energy sources. Front Syst Neurosci. 2011;5:99. doi: 10.3389/fnsys.2011.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Domingos AI, Sordillo A, Dietrich MO, Liu ZW, Tellez LA, Vaynshteyn J, Ferreira JG, Ekstrand MI, Horvath TL, de Araujo IE, Friedman JM, Nathans J. Hypothalamic melanin concentrating hormone neurons communicate the nutrient value of sugar. eLife. 2013;2:e01462. doi: 10.7554/eLife.01462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drucker DB, Ackroff K, Sclafani A. Nutrient-conditioned flavor preference and acceptance in rats: Effects of deprivation state and nonreinforcement. Physiol Behav. 1994;56:701–707. doi: 10.1016/0031-9384(94)90230-5. [DOI] [PubMed] [Google Scholar]
- 9.Duncan EA, Proulx K, Woods SC. Central administration of melanin-concentrating hormone increases alcohol and sucrose/quinine intake in rats. Alcohol Clin Exp Res. 2005;29:958–964. doi: 10.1097/01.alc.0000167741.42353.10. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira JG, Tellez LA, Ren X, Yeckel CW, de Araujo IE. Regulation of fat intake in the absence of flavor signaling. J Physiol. 2012;590:953–972. doi: 10.1113/jphysiol.2011.218289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han W, Tellez L, Niu J, Medina S, Ferreira T, Zhang X, Su J, Tong J, Schwartz GJ, van den Pol A, de Araujo IE. Striatal dopamine links gastrointestinal rerouting to altered sweet appetite. Cell Metab. 2015;23:103–112. doi: 10.1016/j.cmet.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jego S, Glasgow SD, Herrera CG, Ekstrand M, Reed SJ, Boyce R, Friedman J, Burdakov D, Adamantidis AR. Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat Neurosci. 2013;16:1637–1643. doi: 10.1038/nn.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karlsson C, Zook M, Ciccocioppo R, Gehlert DR, Thorsell A, Heilig M, Cippitelli A. Melanin-concentrating hormone receptor 1 (MCH1-R) antagonism: Reduced appetite for calories and suppression of addictive-like behaviors. Pharmacol Biochem Behav. 2012;102:400–406. doi: 10.1016/j.pbb.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Kraft TT, Huang D, Lolier M, Warshaw D, Lamagna S, Natanova E, Sclafani A, Bodnar RJ. BALB/c and SWR inbred mice differ in post-oral fructose appetition as revealed by sugar versus non-nutritive sweetener tests. Physiol Behav. 2016;153:64–69. doi: 10.1016/j.physbeh.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez CA, Guesdon B, Baraboi ED, Roffarello BM, Hétu M, Richard D. Involvement of the opioid system in the orexigenic and hedonic effects of melanin-concentrating hormone. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1105–R1111. doi: 10.1152/ajpregu.00076.2011. [DOI] [PubMed] [Google Scholar]
- 16.Oliveira-Maia AJ, Roberts CD, Walker QD, Luo B, Kuhn C, Simon SA, Nicolelis MA. Intravascular food reward. PLoS One. 2011;6:e24992. doi: 10.1371/journal.pone.0024992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pérez C, Lucas F, Sclafani A. Increased flavor preference and acceptance conditioned by the postingestive actions of glucose. Physiol Behav. 1998;64:483–492. doi: 10.1016/s0031-9384(98)00104-8. [DOI] [PubMed] [Google Scholar]
- 18.Sakamaki R, Uemoto M, Inui A, Asakawa A, Ueno N, Ishibashi C, Hirono S, Yukioka H, Kato A, Shinfuku N, Kasuga M, Katsuura G. Melanin-concentrating hormone enhances sucrose intake. Int J Mol Med. 2005;15:1033–1039. [PubMed] [Google Scholar]
- 19.Sclafani A. Gut-brain nutrient signaling: appetition vs. satiation. Appetite. 2013;71:454–458. doi: 10.1016/j.appet.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sclafani A, Ackroff K. Flavor preferences conditioned by intragastric glucose but not fructose or galactose in C57BL/6J mice. Physiol Behav. 2012;106:457–461. doi: 10.1016/j.physbeh.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sclafani A, Ackroff K. The role of gut nutrient sensing in stimulating appetite and conditioning food preferences. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1119–R1133. doi: 10.1152/ajpregu.00038.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sclafani A, Ackroff K. Operant licking for intragastric sugar infusions: Differential reinforcing actions of glucose, sucrose and fructose in mice. Physiol Behav. 2016;153:115–124. doi: 10.1016/j.physbeh.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sclafani A, Glass DS, Margolskee RF, Glendinning JI. Gut T1R3 sweet taste receptors do not mediate sucrose-conditioned flavor preferences in mice. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1643–R1650. doi: 10.1152/ajpregu.00495.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sclafani A, Glendinning JI. Sugar and fat conditioned flavor preferences in C57BL/6J and 129 mice: Oral and postoral interactions. Am J Physiol Regul Integr Comp Physiol. 2005;289:R712–R720. doi: 10.1152/ajpregu.00176.2005. [DOI] [PubMed] [Google Scholar]
- 25.Sclafani A, Koepsell H, Ackroff K. SGLT1 sugar transporter/sensor is required for post-oral glucose appetition. Am J Physiol Regul Integr Comp Physiol. 2016 doi: 10.1152/ajpregu.00432.2015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sclafani A, Touzani K, Ackroff K. Ghrelin signaling is not essential for sugar or fat conditioned flavor preferences in mice. Physiol Behav. 2015;149:14–22. doi: 10.1016/j.physbeh.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sclafani A, Touzani K, Bodnar RJ. Dopamine and learned food preferences. Physiol Behav. 2011;104:64–68. doi: 10.1016/j.physbeh.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sclafani A, Zukerman S, Ackroff K. Fructose and glucose conditioned preferences in FVB mice: Strain differences in post-oral sugar appetition. Am J Physiol Regul Integr Comp Physiol. 2014;307:R1448–R1457. doi: 10.1152/ajpregu.00312.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sclafani A, Zukerman S, Ackroff K. Post-oral glucose sensing, not caloric content, determines sugar reward in C57BL/6J mice. Chem Senses. 2015;40:245–258. doi: 10.1093/chemse/bjv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherwood A, Holland PC, Adamantidis A, Johnson AW. Deletion of melanin concentrating hormone receptor-1 disrupts overeating in the presence of food cues. Physiol Behav. 2015;152:402–407. doi: 10.1016/j.physbeh.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 31.Sherwood A, Wosiski-Kuhn M, Nguyen T, Holland PC, Lakaye B, Adamantidis A, Johnson AW. The role of melanin-concentrating hormone in conditioned reward learning. Eur J Neurosci. 2012;36:3126–3133. doi: 10.1111/j.1460-9568.2012.08207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slotnick B. Olfactory performance of rats after selective deafferentation of the olfactory bulb by 3-methyl indole. Chem Senses. 2007;32:173–181. doi: 10.1093/chemse/bjl046. [DOI] [PubMed] [Google Scholar]
- 33.Tan CP, Sano H, Iwaasa H, Pan J, Sailer AW, Hreniuk DL, Feighner SD, Palyha OC, Pong SS, Figueroa DJ, Austin CP, Jiang MM, Yu H, Ito J, Ito M, Ito M, Guan XM, MacNeil DJ, Kanatani A, Van der Ploeg LH, Howard AD. Melanin-concentrating hormone receptor subtypes 1 and 2: species-specific gene expression. Genomics. 2002;79:785–792. doi: 10.1006/geno.2002.6771. [DOI] [PubMed] [Google Scholar]
- 34.Tordoff MG, Friedman MI. Hepatic-portal glucose infusions decrease food intake and increase food preference. Am J Physiol. 1986;251:R192–R196. doi: 10.1152/ajpregu.1986.251.1.R192. [DOI] [PubMed] [Google Scholar]
- 35.Wakabayashi KT, Kiyatkin EA. Behavior-associated and post-consumption glucose entry into the nucleus accumbens extracellular space during glucose free-drinking in trained rats. Front Behav Neurosci. 2015;9:173. doi: 10.3389/fnbeh.2015.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whiddon BB, Palmiter RD. Ablation of neurons expressing melanin-concentrating hormone (MCH) in adult mice improves glucose tolerance independent of MCH signaling. J Neurosci. 2013;33:2009–2016. doi: 10.1523/JNEUROSCI.3921-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yapici N, Zimmer M, Domingos AI. Cellular and molecular basis of decision-making. EMBO reports. 2014;10:1023–1035. doi: 10.15252/embr.201438993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yen HH, Roseberry AG. Decreased consumption of rewarding sucrose solutions after injection of melanocortins into the ventral tegmental area of rats. Psychopharmacology. 2015;232:285–294. doi: 10.1007/s00213-014-3663-6. [DOI] [PubMed] [Google Scholar]
- 39.Zukerman S, Ackroff K, Sclafani A. Rapid post-oral stimulation of intake and flavor conditioning by glucose and fat in the mouse. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1635–R1647. doi: 10.1152/ajpregu.00425.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zukerman S, Ackroff K, Sclafani A. Post-oral appetite stimulation by sugars and non-metabolizable sugar analogs. Am J Physiol Regul Integr Comp Physiol. 2013;305:R840–R853. doi: 10.1152/ajpregu.00297.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zukerman S, Ackroff K, Sclafani A. Post-oral glucose stimulation of intake and conditioned flavor preference in C57BL/6J mice: A concentration-response study. Physiol Behav. 2013;109:33–41. doi: 10.1016/j.physbeh.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zukerman S, Touzani K, Margolskee RF, Sclafani A. Role of olfaction in the conditioned sucrose preference of sweet-ageusic T1R3 knockout mice. Chem Senses. 2009;34:685–694. doi: 10.1093/chemse/bjp055. [DOI] [PMC free article] [PubMed] [Google Scholar]