Abstract
Motion-based therapies have been applied to promote healing of arthritic joints. The goal of the current study was to determine the early molecular events that are responsible for the beneficial actions of motion-based therapies on meniscal fibrocartilage. Rabbit knees with Antigen-Induced-Arthritis (AIA) were exposed to continuous passive motion (CPM) for 24 or 48 h and compared to immobilized knees. The menisci were harvested and glycosaminoglycans (GAG), interleukin-1β (IL-1β), matrix metalloproteinase-1 (MMP-1), cyclooxygenase-2 (COX-2), and interleukin-10 (IL-10) were determined by histochemical analysis.
Within 24 h, immobilized knees exhibited marked GAG degradation. The expression of proinflammatory mediators MMP-1, COX-2, and IL-1β was notably increased within 24 h and continued to increase during the next 24 h in immobilized knees. Knees subjected to CPM revealed a rapid and sustained decrease in GAG degradation and the expression of all proinflammatory mediators during the entire period of CPM treatment. More importantly, CPM induced synthesis of the anti-inflammatory cytokine IL-10. The results demonstrate that mechanical signals generated by CPM exert potent anti-inflammatory signals on meniscal fibrochondrocytes. Furthermore, these studies explain the molecular basis of the beneficial effects of CPM observed on articular cartilage and suggest that CPM suppresses the inflammatory process of arthritis more efficiently than immobilization.
Keywords: Rheumatoid arthritis, Interleukin-1, Continuous passive motion, Meniscus, Fibrocartilage
Introduction
The meniscal fibrocartilage of the knee joint is important for load bearing, load distribution, shock absorption, and stability of the knee [5]. The functions of the menisci are facilitated by proteoglycans and collagen type I and type II [4,5]. Proteoglycans enable the matrix to absorb shock, whereas collagens provide the rigidity [4]. Joints afflicted by rheumatoid arthritis (RA) exhibit meniscal matrix degradation [11]. Evidences suggest that RA is initiated by a sustained antigenic stimulus that induces perpetual activation of inflammatory and resident cells of the joint and results in the production of proinflammatory mediators that initiates progressive erosion of cartilage [21]. Interleukin-1 (IL-1), a major mediator, induces proinflammatory mediators, cyclooxygenase-2 (COX-2), and matrix metalloproteinases (MMPs) and triggers the pathologies of the cartilage and fibrocartilage associated with RA [1,13]. Both chondrocytes and fibrochondrocytes from inflamed joints are known to produce proinflammatory mediators in response to IL-1 in vitro [1,18] and in vivo [11].
The beneficial effects of CPM on the healing of articular tissue are well recognized [10,16,17]. However, to date, little is known about the molecular mechanisms responsible for the actions of motion-based therapies on the cartilage or the fibrocartilage of the joint. In light of the fact that motion can exert beneficial effects on the arthritic cartilage of the joints, it was our hypothesis that (i) CPM promotes healing by inhibiting the expression of proinflammatory mediators in the meniscus, as compared to immobilization, which leads to cartilage degradation via induction of proinflammatory mediators, and (ii) the effects of mechanical signals are rapid and can be observed within 24–48 h. Therefore, in this report we have examined the early biochemical events induced by mobilization or immobilization on meniscus from an AIA joint to understand the mechanisms of actions of motion-based therapies in vivo.
Material and methods
Reagents
Anti-COX-2, anti-MMP-1, and anti-IL-1β immunoglobulins were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), and their respective fluorescein isothiocyanate (FITC) labeled secondary antibodies were purchased from Jackson Immuno Research (West Grove, PA). Monoclonal rat anti-mouse IL-10 antibody and FITC labeled monoclonal mouse anti-rat IgG were purchased from BD Pharmingen (San Diego, CA). Protein blocking agent was from Thermo Electron Corp. (Pittsburgh, PA) and mounting solution Vectashield from Vector Labs (Burlingame, CA). All other reagents were purchased from Sigma-Aldrich (Saint Louis, MO).
Induction of antigen-induced arthritis
All protocols were approved by the Institutional Animal Care Committee at the University of Pittsburgh and University of Toronto. Male New Zealand white rabbits (18–20 weeks old) were sensitized with 5 mg of Bovine Serum Albumin (BSA) in 0.5 ml saline emulsified with 0.5 ml of Freund’s Complete Adjuvant. After 20 days, hypersensitivity to BSA was examined by subcutaneous injection of 0.1 mg BSA in 0.5 ml saline in 0.5 ml Freund’s Incomplete Adjuvant. Five days later, rabbits exhibiting hypersensitivity reactions were anesthetized, right knees shaved, and 2.5 mg BSA in 0.5 ml saline was injected intra-articularly to induce AIA (10).
Immobilization or CPM treatment of the knees
Following intra-articular injection, the right knee of the rabbits (n = 5 rabbits/group for each time point) was immediately placed on a CPM device kindly provided by Orthomotion Inc, Pickering, Ontario, Canada. The angle of flexion of the joint was 70° with movement between 40° and 110° at a rate of 45 s per cycle. For immobilization, the right knee of rabbits (n = 5/group for each time point) was wrapped with bandages immediately after intra-articular injection with BSA. In both groups, the left limbs of the rabbits were not subjected to any treatment. To examine the early molecular events induced by motion, the rabbit knees were immobilized or exposed to CPM for 24 or 48 h and the rabbits were sacrificed to harvest the tissue.
Tissue preparation and immunohistochemical analysis
After harvesting, menisci were cleaned with saline and fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned at 5 μm thickness. The GAG content was analyzed by 1.5% safranin-O staining [9]. During examination, each meniscus was divided into two parts: the outer 25% zone (zone A), and the inner 75% fibrocartilage (zone B).
For immunohistochemistry, sections were deparaffinized, hydrated, and treated with 0.1M sodium citrate buffer-pH 6.0 at 70 °C for antigen retrieval. Subsequently, sections were blocked in 5% pre-immune serum diluted in Protein Blocking Agent and incubated in 1:400 dilution of primary antibodies at 4 °C overnight. Thereafter, sections were washed with phosphate buffered saline (PBS) containing 1% BSA and 0.02% Tween-20 and incubated with 1:200 dilution of FITC-conjugated secondary antibody for 1 h. The primary antibodies used were goat polyclonal anti-mouse IL-1β, goat polyclonal anti-human MMP-1, and goat polyclonal anti-rat COX-2. Secondary antibodies for the above primary antibodies were FITC conjugated donkey anti-goat IgG. To detect IL-10, monoclonal rat anti-mouse IL-10 antibody and FITC-conjugated monoclonal mouse anti-rat IgG1 were used. The slides were washed 5 times with PBS at each step, mounted with Vectashield and observed under UV light in an Zeiss Axioplan-2 epifluorescence microscope equipped with Axiovision image capturing software. The control slides were also stained with secondary antibody alone to assure specificity of primary antibodies.
Data analysis
Sections obtained from knees subjected to CPM or immobilization in each group (n = 5) were analyzed after histochemical and immunofluorescence staining. In all sections four 500 μm2 areas were enumerated for COX-2, MMP-1, IL-1, and IL-10 positive cells and presented as mean ± error of the mean. The statistical significance was calculated by students t test and considered significant at a value of p = 0.05. The analysis of slides was carried out by two investigators, one blinded to rabbit group assignments and the other was aware of the rabbit group assignments. The data collected by both investigators was found to be similar in all cases.
Results
Menisci from joints afflicted with AIA exhibit lesser GAG loss following CPM treatment
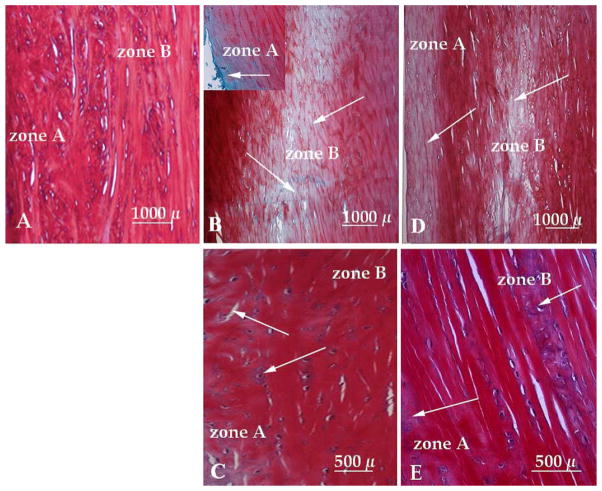
Safranin-O stained cross sections of menisci from healthy knees exhibited the presence of GAGs in zones A and B (Fig. 1A). The immobilized knees revealed that 48% of zone A and 26% of the total zone B lacked GAGs within 24 h as compared to the sections from healthy knees (Fig. 1A and B). Further immobilization induced greater reduction in GAGs, i.e., 37% of the zone A and 26% of the zone B exhibited loss of GAGs after 48 h (Fig. 1D). This degradation in Zone B was localized in the central area of the meniscus (Fig. 1B and D). On the contrary, exposure of the AIA knee to CPM for the initial 24 h resulted in a 12% reduction of GAGs in zone A and 6% reduction of GAGs in zone B as compared to healthy controls (Fig. 1C). Menisci from knees subjected to CPM showed a sustained retention of GAGs, in comparison to immobilized menisci. Only an 8% reduction of GAGs in zone A and a 3% reduction of GAGs in zone B was observed after 48 h exposure to CPM (Fig. 1E). However, some matrix disorganization was apparent at 24 and 48 h in the CPM treated knees (Fig. 1C and E).
Fig. 1.
Cross sections of meniscus showing (A) normal GAG distribution in a meniscus from non-arthritic knee, (B) GAG loss in zones A (inset) and B in an AIA meniscus immobilized for 24 h, (C) GAG contents in zones A and B in AIA meniscus subjected to CPM for 24 h, (D) GAG loss in zones A and B in AIA meniscus immobilized for 48 h, and (E) GAG contents in AIA meniscus subjected to CPM for 48 h. Arrows indicate regions devoid of GAGs.
CPM treatment results in lower cyclooxygenase-2 synthesis in AIA afflicted menisci
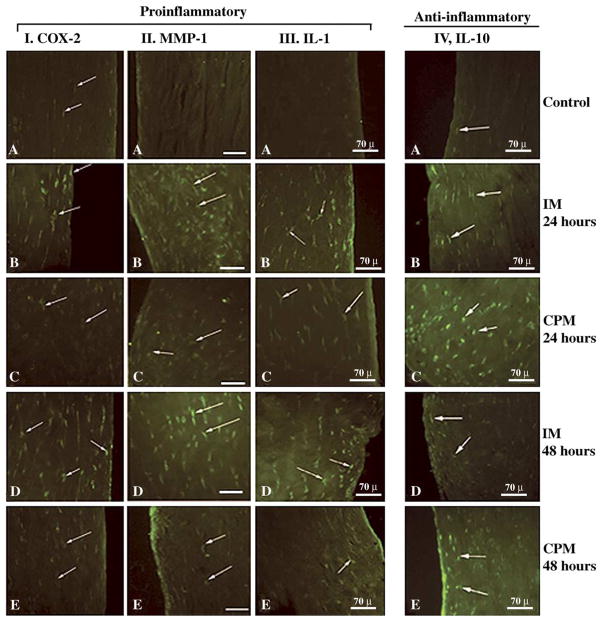
We next examined whether CPM affects the synthesis of COX-2, responsible for prostaglandin-E2 production [22]. AIA knees immobilized for 24 or 48 h exhibited a marked expression of COX-2 in meniscal cells as compared to those observed in healthy control fibrochondrocytes (Fig. 2 Column II A, B and D). However, fibrochondrocytes in AIA knees were subjected to CPM for 24 or 48 h exhibited a significantly (p = 0.05) lower intensity of fluorescence (Fig. 2, Column II C and D; Table 1). This reduction in COX-2 was apparent in the number of cells producing COX-2, as well as in the intensity of fluorescence in each cell of the fibrocartilage (Fig. 2 Column II B–E).
Fig. 2.
Cross sections of menisci exhibiting synthesis of proinflammatory and anti-inflammatory molecules as a result of CPM and IM treatment of AIA knees. Sections from rabbit knees subjected to CPM or IM were immunostained to examine COX-2 (Column I), MMP-1 (Column II), IL-1 (Column III), or IL-10 (Column IV). In all columns sections from untreated control knees are A, sections from AIA knees subjected to various treatments are: (B) IM for 24 h, (C) CPM for 24 h, (D) IM for 48 h, and (E) CPM for 48 h. Each photograph represents one out of five sections obtained from different animals receiving identical treatments. Arrows point to cells positive for each mediator.
Table 1.
Expression of inflammatory mediators or anti-inflammatory cytokine IL-10 in AIA knees subjected to IMM or CPM
| Expression of molecule | Control knee | IM 24 h | IM 48 h | CPM 24 h | CPM 48 h |
|---|---|---|---|---|---|
| COX-2 | 2 3 | 83 ± 11 | 86 ± 19 | 22 ± 6* | 21 ± 9* |
| MMP-1 | 4–7 | 79 ± 17 | 84 ± 22 | 26 ± 11* | 24 ± 16* |
| IL-1 | 1–3 | 103 ± 23 | 121 ± 31 | 18 ± 9* | 13 ± 6* |
| IL-10 | 9–16 | 39 ± 18 | 51 ± 22 | 111 ± 24* | 122 ± 27* |
| Faint fluorescence | Faint fluorescence | Strong fluorescence | Strong fluorescence |
Untreated control or AIA knees subjected to IMM or CPM were analyzed by immunohistochemical analysis. The percentage of cells positive for each mediator were counted in four 500 μm2 areas in each slide. Data represent mean number of cells positive in sections obtained from 5 different rabbits in each group.
Denote p < 0.05 as compared to number of fluorescence positive cells in IM group at a specific time point.
CPM treatment of AIA afflicted knees results in lower MMP-1 expression in menisci
MMP-1, the major matrix degrading enzyme, is increased in inflamed fibrocartilage [8,11]. Therefore, we next determined the effects of CPM on MMP-1 synthesis. Meniscal fibrochondrocytes from healthy knees did not exhibit the presence of MMP-1 (Fig. 2 Column II A). Immobilization of AIA knees for 24 or 48 h resulted in high MMP-1 expression which was apparent in cells as well as matrix surrounding the cells. In comparison, knees exposed to CPM for 24 or 48 h exhibited significantly (p ≤ 0.05) fewer MMP-1 positive cells. In these menisci, the majority of cells producing MMP-1 were localized in the superficial layers of zone A (Fig. 2 Column II C and E; Table 1).
CPM treatment of AIA afflicted knees results in lower IL-1β expression
Since IL-1β is the major mediator responsible for the induction of MMPs and COX-2 in joints [1,21], we next determined whether the expression of IL-1β is differentially regulated by CPM or immobilization. A marked induction of IL-1β was observed in fibrochondrocytes from the immobilized knees as compared to non-arthritic menisci (Fig. 2 Column III A, B and D). Meniscal fibrochondrocytes from knees subjected to CPM for 24 or 48 h exhibited significantly lower number of IL-1 positive cells. Furthermore, the cells in CPM group revealed much lower intensity of fluorescence than those present in immobilized menisci (Fig. 2 Column III C and E; Table 1). The IL-1β-producing cells in CPM treated menisci were predominantly observed in zone A. However, CPM did not completely inhibit IL-1β production.
Anti-inflammatory actions of CPM include induction of IL-10 in AIA afflicted joints
IL-10 is one of the major anti-inflammatory cytokines that is expressed in cells during inflammation [14]. Whether CPM also induces expression of IL-10 was examined in menisci. Twenty-four and 48 h immobilization of knees caused some induction of IL-10 in zones A and B (Fig. 2 Column IV A, B and D; Table 1). Densitometric measurements of the relative amounts of IL-10 expression in the fibrochondrocytes from the menisci subjected to CPM revealed a significantly greater expression of IL-10 than those immobilized for 24 or 48 h (Fig. 2, column IV A–E; Table 1). It is important to note that while fibrochondrocytes from the menisci of IM knees also exhibited IL-10 production, it was much less than that induced by CPM. Since IL-10 is secreted by the cells, its presence was visible in cells as well as in the matrix around the cells in all sections.
Discussion
In this study we have examined the early actions of CPM therapy on meniscal fibrochondrocytes. Although in earlier studies CPM therapies were shown to provide long-term beneficial effects on the cartilage [10,16,17], neither the effects of CPM therapies on meniscal cells are completely understood, nor are the mechanisms of its action clear. Our findings reveal that the differences between CPM treated and immobilized meniscal cells can be as early as 24 h. In these experiments we have compared the effects of CPM with those of immobilization because the experimental conditions for both immobilization and CPM can be controlled precisely. Additionally, mobilization and immobilization are two frequently used modalities for the treatment of meniscal pathologies [19,20].
In order to elucidate the early actions of CPM and immobilization, we have induced AIA with an intraarticular injection of 2.5 mg of BSA in sensitized animals to initiate a strong immune reaction in the knee. This resulted in the activation and migration of immune cells into the synovium, as well as in the activation of fibrochondrocytes of the meniscus. In the immobilized AIA knee, the disruption of the matrix organization and GAG loss was much greater during the first 24 h and was sustained for 48 h. However, in AIA knees subjected to CPM, the GAG degraded to a lesser extent. GAG degradation is one of the major events in AIA and is shown to alter the collagen–chondroitin sulfate ratio and lead to weakening of the meniscus and subsequently predispose meniscus to tears [6,10]. Our observations suggest that the immobilized knees exhibit markedly greater proteoglycan degradation. This is similar to earlier studies where acceleration of inflammation and thus progression of the arthritic condition have been described as a consequence of immobilization [3,10,17]. These effects of immobilization are believed to be mediated via inhibition of blood flow [2]. Since our results demonstrate that the greatest GAG loss is observed in the central avascularized area of the immobilized meniscus, it appears that the lack of mechanical signals on the fibrocartilage may be sufficient for the initiation of its degradation. CPM, on the other hand, is shown to increase proteoglycan contents in the meniscus, but not shown to facilitate increased flow of synovial fluid to the meniscus [6]. These observations further substantiate the importance of the fact that mechanical signals act on cells of the meniscal fibrocartilage to inhibit inflammation and GAG loss.
The effects of CPM, on the other hand, demonstrate that preventing matrix degradation facilitates the capacity of meniscus to transmit load and consequently avoids lesions. CPM is not shown to facilitate increased flow of synovial fluid to the meniscus, but increases the proteoglycan contents in the meniscus [6]. Here we demonstrate that mechanical signals generated by CPM act on cells of the meniscal fibrocartilage to inhibit inflammation and GAG loss.
In addition to the degradation of GAGs, collagen proteolysis is another key factor in cartilage degradation. It is well established that MMP-1 mediates cartilage destruction by augmenting collagen degradation in RA [8]. Our results show that this increase in MMP-1 is an early and sustained event and can be observed within 24 h in the immobilized knee. Furthermore, CPM can counteract the inflammation by suppressing MMP-1 synthesis during the initial phases of AIA-induced cartilage degradation. These results suggest that the increase in MMP-1 in the immobilized knees may correlate with the observed disorganization of extracellular matrix in menisci. By the same token, the preserved integrity of extracellular matrix in the menisci of knees exposed to CPM may be a result of the suppression of MMP-1 expression.
COX-2 is directly linked to inflammatory processes and associated pain in the inflamed knee [22]. In fact, in humans it has been noted that CPM regimens ameliorate pain as suggested by the fact that patients on CPM therapy ask for fewer pain killers than those not being treated by CPM [16]. Since zone A of the menisci is innervated with nerve fibers and nociceptors, the perception of pain realized during inflammation of the knee joint may be in part attributed to the increased COX-2 levels in the fibrochondrocytes [15]. We have observed that CPM limits expression of COX-2 in menisci, which provides mechanistic evidence for the observed effects of CPM therapy on pain.
RA is associated with an increased production of a range of cytokines including IL-1β [21]. Our results reveal that IL-1β is highly expressed in the immobilized AIA knees. The relative decrease in expression of IL-1β in knees subjected to CPM in the fibrochondrocytes of menisci again suggests that the mechanical signals generated by motion may exert anti-inflammatory effects in vivo. This is evident by the fact that CPM inhibited inflammation by direct inhibition of IL-1 induction, and consequent inhibition of IL-1-mediated induction of MMP-1 and COX-2. Thus, these studies explain the molecular basis of the beneficial effects of CPM observed on articular cartilage, and suggest that the effects of CPM are experienced by the cells of the fibrocartilage in the initial stages of inflammation.
IL-10, an important anti-inflammatory cytokine, has been shown to be beneficial to arthritic joints [14]. Intraarticular administration of IL-10 has been shown to inhibit the development of joint inflammation in the animal models [14]. Therefore, we examined the effects of CPM and immobilization on the synthesis of IL-10. In our study, IL-10 positive cells were found in both the vascular and avascular zones of the menisci. Interestingly, IL-10, albeit low, was present in immobilized menisci. This is not surprising as human chondrocytes synthesize IL-10 and the IL-10 mRNA expression is higher in osteoarthritic than normal chondrocytes [7]. It is noteworthy that fibrochondrocytes in knees subjected to CPM exhibited approximately six fold greater IL-10 production, than that produced by fibrochondrocytes in immobilized knees. These studies suggest that CPM not only inhibits induction of proinflammatory genes, but also augments induction of anti-inflammatory cytokines like IL-10, to minimize inflammation. Induction of IL-10 in turn may inhibit expression of IL-1β-induced production of COX-2 and MMPs and thus ameliorate the effects of proinflammatory mediators [1,12].
The observations that cells of the deeper areas of zones A and B exhibit suppression of the synthesis of proinflammatory mediators rather than the superficial areas of zone A suggest that mechanical signals may initiate repair by acting directly on the cells of the fibrocartilage to inhibit inflammation. It is not clear why the superficial layers of meniscus show the presence of inflammatory mediators even after 48 h of CPM treatment. Possibly, the proximity to the synovial membrane or the extent of vascularization may play a part in the inflammation of the superficial zones. Interestingly, dynamic tensile and compressive forces of low physiological magnitudes inhibit mRNA expression and synthesis of MMP-1, COX-2, inducible nitric oxide synthase, and IL-1β in chondrocytes in vitro [12,13]. Thus present studies utilizing CPM in an animal model of AIA support the in vitro findings that biomechanical signals inhibit not only proinflammatory events but also antagonize inflammation by inducing anti-inflammatory cytokines and thus preventing matrix destruction.
Acknowledgments
This publication was supported by Grants, R21HD40939 from National Institute of Child Health and Human Development, and RO1AR48781 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NICHHD or NIAMS or the National Institutes of Health.
References
- 1.Agarwal S, Long P, Gassner R, et al. Cyclic tensile strain suppresses catabolic effects of interleukin-1beta in fibrochondrocytes from the temporomandibular joint. Arthritis Rheum. 2001;44(3):608–17. doi: 10.1002/1529-0131(200103)44:3<608::AID-ANR109>3.0.CO;2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray RC, Smith JA, Eng MK, et al. Vascular response of the meniscus to injury: effects of immobilization. J Orthop Res. 2001;19(3):384–90. doi: 10.1016/S0736-0266(00)00037-1. [DOI] [PubMed] [Google Scholar]
- 3.Djurasovic M, Aldridge JW, Grumbles R, et al. Knee joint immobilization decreases aggrecan gene expression in the meniscus. Am J Sports Med. 1998;26(3):460–6. doi: 10.1177/03635465980260032101. [DOI] [PubMed] [Google Scholar]
- 4.Ehrlich S, Wolff N, Schneiderman R, et al. The osmotic pressure of chondroitin sulphate solutions: experimental measurements and theoretical analysis. Biorheology. 1998;35(6):383–97. doi: 10.1016/s0006-355x(99)80018-3. [DOI] [PubMed] [Google Scholar]
- 5.Fithian DC, Kelly MA, Mow VC. Material properties and structure-function relationships in the menisci. Clin Orthop. 1990:19–31. [PubMed] [Google Scholar]
- 6.Gershuni DH, Hargens AR, Danzig LA. Regional nutrition and cellularity of the meniscus. Implications for tear and repair. Sports Med. 1988;5(5):322–7. doi: 10.2165/00007256-198805050-00004. [DOI] [PubMed] [Google Scholar]
- 7.Iannone F, DeBari C, Dell’Accio F, et al. Interleukin-10 and interleukin-10 receptor in human osteoarthritic and healthy chondrocytes. Clin Exp Rheumatol. 2000;19(2):139–45. [PubMed] [Google Scholar]
- 8.Kane D, Jensen LE, Grehan S, et al. Quantitation of metalloproteinase gene expression in rheumatoid and psoriatic arthritis synovial tissue distal and proximal to the cartilage-pannus junction. J Rheumatol. 2004;31(7):1274–80. [PubMed] [Google Scholar]
- 9.Kang QK, LaBreck C, Gruber HE, An YH, An YH, Martin KL. Handbook of histological methods for bone and cartilage. 1. Totowa: Humana Press Inc; 2003. Histological techniques for decalcified bone and cartilage. [Google Scholar]
- 10.Kim HK, Kerr RG, Cruz TF, Salter RB. Effects of continuous passive motion and immobilization on synovitis and cartilage degradation in antigen induced arthritis. J Rheumatol. 1995;22(9):1714–21. [PubMed] [Google Scholar]
- 11.Lindy S, Turto H, Sorsa T, et al. Increased collagenase activity in human rheumatoid meniscus. Scand J Rheumatol. 1986;15(3):237–42. doi: 10.3109/03009748609092585. [DOI] [PubMed] [Google Scholar]
- 12.Long P, Hu J, Piesco N, et al. Low magnitude of tensile strain inhibits IL-1beta-dependent induction of pro-inflammatory cytokines and induces synthesis of IL-10 in human periodontal ligament cells in vitro. J Dent Res. 2001;80(5):1416–20. doi: 10.1177/00220345010800050601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Long P, Liu F, Piesco NP, et al. Signaling by mechanical strain involves transcriptional regulation of proinflammatory genes in human periodontal ligament cells in vitro. Bone. 2002;30(4):547–52. doi: 10.1016/s8756-3282(02)00673-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lubberts E, Joosten LA, Van Den Bersselaar L, et al. Intraarticular IL-10 gene transfer regulates the expression of collagen-induced arthritis (CIA) in the knee and ipsilateral paw. Clin Exp Immunol. 2000;120(2):375–83. doi: 10.1046/j.1365-2249.2000.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mine T, Kimura M, Sakka A, Kawai S. Innervation of nociceptors in the menisci of the knee joint: an immunohistochemical study. Arch Orthop Trauma Surg. 2000;120(3–4):201–4. doi: 10.1007/s004020050044. [DOI] [PubMed] [Google Scholar]
- 16.Salter RB. The physiological basis of continuous passive motion for articular cartilage healing and regeneration. Hand Clinics. 1994;10(2):211–9. [PubMed] [Google Scholar]
- 17.Salter RB, Simmonds DF, Malcolm BW, et al. The biological effect of continuous passive motion on the healing of full-thickness defects in articular cartilage. An experimental investigation in the rabbit. J Bone Joint Surg Am. 1980;62(8):1232–51. [PubMed] [Google Scholar]
- 18.Shin SJ, Fermor B, Weinberg JB, et al. Regulation of matrix turnover in meniscal explants: role of mechanical stress, interleukin-1, and nitric oxide. J Appl Physiol. 2003;95(1):308–13. doi: 10.1152/japplphysiol.00131.2003. [DOI] [PubMed] [Google Scholar]
- 19.Spindler KP, McCarty EC, Warren TA, et al. Prospective comparison of arthroscopic medial meniscal repair technique: inside-out suture versus entirely arthroscopic arrows. Am J Sports Med. 2003;31(6):929–34. doi: 10.1177/03635465030310063101. [DOI] [PubMed] [Google Scholar]
- 20.Steenbrugge F, Verdonk R, Hurel C, Verstraete K. Arthroscopic meniscus repair: inside-out technique vs. Biofix meniscus arrow. Knee Surg Sports Traumatol Arthrosc. 2004;12(1):43–9. doi: 10.1007/s00167-003-0446-8. [DOI] [PubMed] [Google Scholar]
- 21.Vervoordeldonk MJ, Tak PP. Cytokines in rheumatoid arthritis. Curr Rheumatol Rep. 2002;4(3):208–17. doi: 10.1007/s11926-002-0067-0. [DOI] [PubMed] [Google Scholar]
- 22.Warner TD, Mitchell JA. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. FASEB J. 2004;18(7):790–804. doi: 10.1096/fj.03-0645rev. [DOI] [PubMed] [Google Scholar]