Dear Editor,
In sub-Saharan Africa (SSA), approximately 3 million HIV-infected individuals have chronic hepatitis B virus (HBV) co-infection [1]. HIV–HBV-co-infected patients experience increased mortality, reduced immune recovery and increased risk of hepatotoxicity during antiretroviral therapy (ART) compared to those with HIV alone [2–4]. The World Health Organization (WHO) recommends HBV screening with a hepatitis B surface antigen (HBsAg) test for all HIV-infected individuals at the time of linkage to care and/or prior to ART initiation [5]. According to these guidelines, HIV–HBV patients with advanced liver disease should initiate ART regardless of CD4+ count. Despite its recommendation by the majority of national ART guidelines, HBV screening has not been widely implemented in most ART programmes in SSA; as a result, the epidemiology and outcomes of HIV–HBV co-infection remain poorly characterized in the region. In 2010, the Zambian Ministry of Health (MOH) [6] HIV treatment policy shifted from targeted HBsAg testing to routine baseline testing at enrolment. HBsAg-positive patients with ALT > 2.5 times normal were ART eligible regardless of CD4+ count and WHO stage. In this report, we describe HBV screening and initial treatment practices among public sector HIV clinics in Zambia's capital city Lusaka during 2008–2012.
In each calendar quarter (Q), we determined the proportion of newly enrolled patients who received an HBsAg test at baseline (defined as within 6 months of enrolment and prior to ART initiation) or during follow-up and compared these proportions over time using a Jonckheere–Terpstra test for trend. In the period after dissemination of the 2010 guidelines, using multivariable logistic regression, we identified factors associated with baseline testing including age, sex, WHO clinical stage, ALT, CD4+ count and facility volume, which we defined as the number of new patient enrolments per year. We also modelled patient demographic and clinical correlates of HBsAg positivity. We used Stata version 12 (Statacorp, College Station, TX, USA) for analysis. The ethics committees of the University of Zambia (Lusaka, Zambia) and the University of North Carolina at Chapel Hill (North Carolina, USA) approved the study.
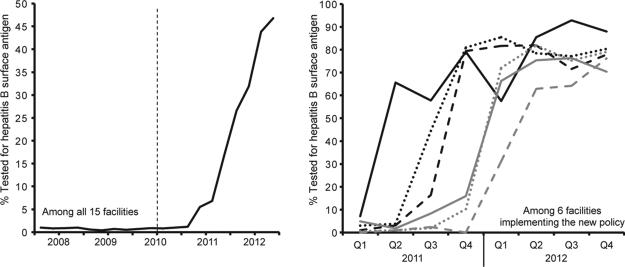
From 1 January 2008 to 31 December 2012, 60 060 HIV-infected patients enrolled across 15 treatment facilities in Lusaka district. There was a rapid and substantial increase in HBsAg testing following dissemination of the MOH's 2010 HIV treatment guidelines (Fig. 1). The overall percentage of patients tested increased from 1.0% in Q1 of 2008 to 46.8% in Q4 of 2012 (P for trend < 0.001). During this time, the percentage of HBsAg tests that occurred at baseline increased from 16.1% to 99.7% (P for trend < 0.001).
Fig. 1.
Rapid increase in the percent of HIV-infected individuals screened for hepatitis B surface antigen in Lusaka district following release of the Zambian Ministry of Health 2010 HIV guidelines.
At the facility level, there was a wide variation in HBsAg testing in the 24 months following the guideline change, with only six of 15 facilities increasing testing rates (Fig. 1). Among the six facilities that increased baseline testing, by Q4 of 2012, nearly 80% of newly enrolled patients were HBsAg tested. The nine sites that did not increase HBsAg testing had similar patient volumes to those in the sites that increased testing. Among facilities that increased testing, during 2011–2012, adults [16+ years old; adjusted odds ratio (AOR) 6.09; 95% confidence interval (CI), 4.55–8.14] and males (AOR 1.13; 95% CI, 1.02–1.25) were more likely to be HBsAg tested, whereas patients with tuberculosis (AOR 0.60; 95% CI, 0.49–0.72) and/or WHO stage 3 or 4 (AOR 0.87; CI, 0.78–0.97) had reduced odds of testing.
The percentage of positive HBsAg tests decreased (16.4% in 2008–2010 vs 11.8% in 2011–2012, P < 0.001) as routine testing increased. Adults were more likely than children to be HBsAg positive (12.2% vs 8.5%, P = 0.01). Among the 4147 HBsAg-tested patients in 2011–2012, HIV–HBV-co-infected patients were more likely to be male (AOR 1.45; 95% CI, 1.18–1.78) and have WHO stage 3 or 4 (AOR 1.69; 95% CI, 1.37–2.09), ALT >40 U/L (AOR 2.35; 95% CI, 1.87–2.96) and CD4+ count <200 cells/mm3 (AOR 1.45; 95% CI, 1.18–1.78).
HIV–HBV patients were more likely to be ART eligible (85.7% vs 72.4%, P < 0.001) and to initiate ART (76.0% vs 66.4%, P < 0.001) compared to those with HIV alone. Among HIV–HBV patients, only 5 (0.9%) became ART eligible on the basis of a positive HBsAg test and grade 2 or more ALT elevation. Regardless of HBV status, the majority of patients were prescribed a regimen containing two HBV-active drugs (92.0% in HIV alone vs 90.4% in HIV–HBV).
This report highlights the importance of health policy dissemination and its monitoring in HIV treatment programmes in settings such as ours. In one of the first reports of public sector HBV screening in Africa, we observed rapid but variable increases in HBsAg testing across clinics in Lusaka following the MOH's revised testing policy. Among HBsAg-positive patients, approximately 10% did not receive recommended tenofovir disoproxil fumarate (TDF)-containing regimens.
Implementation of routine HBV testing was associated with a reduction in the proportion of positive HBsAg tests because those tested prior to 2011 were a selected group that were likely sicker than other patients. HBsAg positivity in 2011–2012 (11.8%) is likely a good estimate of HBV co-infection prevalence in the programme and is similar to the 9.9% reported in one prior Zambian study [7] and 4.8–19.7% reported in South African studies [8,9]. In our programme, adult males were most likely to be screened; however, additional information is needed to identify groups that may lag behind in access to HBsAg testing.
HBsAg testing at baseline did not substantially affect the decision to initiate ART as most HIV–HBV patients were ART eligible prior to HBsAg testing. In our programme, very few HIV–HBV patients with WHO stage 1 or 2 and high CD4+ counts had grade 2 ALT elevation. However, ALT is a poor surrogate for true liver disease, and better tests for liver disease staging are needed to fully understand the proportion of HIV–HBV patients who need ART. Regardless of HBsAg result, 90% of those on ART initiated a TDF-containing regimen, as TDF was a component of the preferred first-line ART regimen during the period of our analysis. In settings where TDF is not a predominant first-line drug, knowledge of HBsAg status would have a larger impact on ART regimen selection.
In summary, monitoring the implementation of HBsAg testing is an important programme component that can optimize the care of HIV–HBV patients. New strategies in dissemination and implementation are critically needed, if we are to maximize the effectiveness of health programmes and close the gap between policy and practice.
ACKNOWLEDGEMENTS
M.J.V. received support from the Fogarty International Center of the National Institutes of Health (R25TW009340 and K01TW009998). K.M. received a scholarship from HIV Research Trust (Cheshire, United Kingdom). G.W. was supported by an Ambizione-PROSPER fellowship from the Swiss National Science Foundation (PZ00P3_154730).
Footnotes
CONFLICT OF INTEREST
All authors report no conflict of interests.
REFERENCES
- 1.Puoti M, Manno D, Nasta P, Carosi G. Hepatitis B virus and HIV coinfection in low-income countries: unmet needs. Clin Infect Dis. 2008;46(3):367–369. doi: 10.1086/525532. [DOI] [PubMed] [Google Scholar]
- 2.Hawkins C, Christian B, Ye J, et al. Prevalence of hepatitis B co-infection and response to antiretroviral therapy among HIV-infected patients in Tanzania. AIDS. 2013;27:919–927. doi: 10.1097/QAD.0b013e32835cb9c8. [DOI] [PubMed] [Google Scholar]
- 3.Wandeler G, Gsponer T, Bihl F, et al. Hepatitis B virus infection is associated with impaired immune recovery during antiretroviral therapy in the Swiss HIV cohort study. J Infect Dis. 2013;208(9):1454–1458. doi: 10.1093/infdis/jit351. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann CJ, Charalambous S, Thio CL, et al. Hepatotoxicity in an African antiretroviral therapy cohort: the effect of tuberculosis and hepatitis B. AIDS. 2007;21(10):1301–1308. doi: 10.1097/QAD.0b013e32814e6b08. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . Consolidated Guidelines on the Use of Anti-retroviral Drugs for Treating and Preventing HIV Infection. WHO; Geneva: 2013. [PubMed] [Google Scholar]
- 6.Zambian Ministry of Health . Guidelines for the Care of Individuals with HIV/AIDS. Zambian Ministry of Health; Lusaka, Zambia: 2010. [Google Scholar]
- 7.Kapembwa KC, Goldman JD, Lakhi S, et al. HIV, hepatitis B, and hepatitis C in Zambia. J Glob Infect Dis. 2011;3(3):269–274. doi: 10.4103/0974-777X.83534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann CJ, Charalambous S, Martin DJ, et al. Hepatitis B virus infection and response to antiretroviral therapy (ART) in a South African ART program. Clin Infect Dis. 2008;47(11):1479–1485. doi: 10.1086/593104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Firhaber C, Reyneke A, Schulz D, et al. The prevalence of hepatitis B co infection in a South African (SA) urban government HIV clinic. S Afr Med J. 2008;98(7):541–544. [PMC free article] [PubMed] [Google Scholar]