Abstract
Background:
Angiotensin receptor blockers (ARBs) are preferred antihypertensive therapies in patients with type 2 diabetes mellitus (T2DM). Azilsartan medoxomil (AZL-M) is a potent ARB for the treatment of stages 1-2 hypertension. We compared the efficacy, safety, and metabolic effects of AZL-M to both valsartan (VAL) and olmesartan (OLM), separately in patients with impaired fasting glucose (prediabetes mellitus) and T2DM.
Methods:
A pooled analysis of 3821 patients from three separate randomized placebo-controlled trials comparing the effects of AZL-M (40 and 80 mg), OLM (40 mg), VAL (320 mg), and placebo on changes in ambulatory and clinic blood pressure (BP) among patients with hypertension and prediabetes mellitus or T2DM was performed. Two analysis pools were created to facilitate comparisons: Pool A included patients who received placebo, AZL-M or OLM and Pool B included those who received AZL-M or VAL. Within each pool, patients were stratified by glycemic subgroups (normoglycemic, prediabetes mellitus, or T2DM) based on hemoglobin A1c values. Changes from baseline in both 24-h and clinic SBP were the primary efficacy assessments.
Results:
Baseline 24-h mean SBPs were approximately 145 and 146 mmHg in the prediabetes mellitus and T2DM subgroups, respectively; corresponding clinic SBPs were approximately 158 and 159 mmHg. Baseline hemoglobin A1c values for each subgroup (both pools) were normoglycemic, 5.3%; prediabetes mellitus, 6.0%; and T2DM, 6.9%. Changes from baseline in 24-h or clinic SBP were significantly greater with AZL-M, 80 mg compared with either OLM 40 mg or VAL 320 mg in all subgroups in each pool. Safety and tolerability were similar among the active treatment and placebo subgroups.
Conclusion:
These analyses indicate that AZL-M, 80 mg/day lowers SBP by a greater magnitude than OLM or VAL at maximally approved doses in patients with prediabetes mellitus and T2DM. These findings have important clinical implications for this high-risk patient group.
Keywords: ambulatory blood pressure, angiotensin receptor blockers, azilsartan medoxomil, prediabetes, type 2 diabetes
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is estimated to affect nearly 400 million people worldwide, and more than 60% with T2DM have systemic hypertension [1,2]. An even larger number of people have prediabetes, defined as a fasting glucose of 100–125 mg/dl or a hemoglobin A1c (HbA1c) of 5.7–6.4%. In the US alone, more than 25 million people have T2DM, and a further 80 million are estimated to have prediabetes and at risk of progression to T2DM [1]. When both T2DM and hypertension are present, elevated blood pressure (BP) appears to be the more important factor driving cardiovascular outcomes [3,4]. In fact, T2DM confers approximately a two-fold increase of cardiovascular events in men and three-fold increase in women; with concurrent hypertension, there is an approximately four-fold increase in cardiovascular risk [5,6]. The importance of lowering BP in patients with T2DM to prevent cardiovascular events is seen in several studies [7–9]. A recent meta-analysis of BP reduction in T2DM demonstrated that a 10 mmHg reduction of SBP lowered all-cause mortality [relative risk (RR) 0.87], cardiovascular (RR 0.89) events, stroke (RR 0.73), as well as risk of retinopathy (RR 0.87) and albuminuria (RR 0.83) [10].
Azilsartan medoxomil (AZL-M) is the newest angiotensin receptor blocker (ARB) approved for the treatment of hypertension. It is the first to provide 24-h ambulatory blood pressure monitoring data to show sustainability and the only one to do comparative studies prior to approval [11–13]. In the present study, we evaluated the efficacy, safety, and metabolic effects of AZL-M in patients with prediabetes mellitus and T2DM in a pooled analysis of over 3800 patients in randomized, controlled trials [11–13].
METHODS
Overview of included studies
Three randomized, double-blind, placebo and/or active-controlled clinical trials were included in this analysis [11–13]. A total of 3821 patients were randomized to either: AZL-M 40 mg or 80 mg [11–13]; olmesartan (OLM) 40 mg [11,12]; valsartan (VAL) 320 mg [12,13]; or placebo [11,12]. Inclusion criteria common to all trials were men and women at least 18 years, and a diagnosis of hypertension as defined by the following: clinic SBP at least 150 mmHg and 180 mmHg or less, and ambulatory 24-h mean SBP at least 130 mmHg and 170 mmHg or less.
Exclusion criteria for all trials included: known secondary hypertension, severe diastolic hypertension (seated DBP at least 114 mmHg), stage IV chronic kidney disease (GFR ≤ 30 mL/min per 1.73 m2), type 1 or poorly controlled T2DM (HbA1c > 8%), congestive heart failure NYHA classes II-IV, and recent major cardiovascular events (<6 months prior to randomization).
Additional metabolic assessments at baseline included BMI, and laboratory evaluation of HbA1c, fasting plasma glucose (FPG), fasting plasma insulin, homeostasis model assessment of insulin sensitivity [14], plasminogen activator inhibitor-1, adiponectin, serum lipoproteins, and high-sensitivity C-reactive protein.
Two of the three trials used for this analysis [11,12] were of 6 weeks duration, and ambulatory BP monitoring was performed at baseline and following 6 weeks of double-blind therapy. The third trial [13] used data at an 8-week analysis time point when ambulatory BP recordings and other assessments were obtained.
Pooling of clinical trials and glycemic subgroups
Patients in the three clinical trials were separated into two study pools. Pool A (placebo and OLM comparisons with AZL-M) included AZL-M 40 mg, AZL-M 80 mg, OLM 40 mg, and placebo groups. Pool B (VAL comparison with AZL-M) included AZL-M 40 mg, AZL-M 80 mg, and VAL 320 mg.
Within each pool, the populations were further stratified by subgroups based on baseline HbA1c values: prediabetes mellitus was defined as HbA1c at least 5.7% and less than 6.5% and T2DM was defined as HbA1c at least 6.5%. The remainder of patients were defined as normoglycemic (HbA1c < 5.7%).
Analyses
Demographic and baseline characteristics were tabulated by treatment group, and included age, age category (<65, ≥65, and ≥75 years), sex, ethnicity, race, height, weight, BMI, and baseline SBP and DBP. Participants were analyzed according to the treatment they were randomized to, in a modified intention-to-treat analysis. All study participants that received any double-blind medication were included in the safety analysis, under the actual treatment received.
Efficacy evaluation
Efficacy variables for the pooled analysis included ambulatory and clinic BPs. The efficacy analyses were based on the full analysis set (those patients receiving at least one dose of double-blind medication), with the last observation carried forward method for variables with multiple postbaseline measures.
The efficacy analysis was performed based on change from baseline to final on-treatment value. Treatment groups were compared using an analysis of covariance model with baseline values as a covariate and study region, discrete study within the pool, and treatment group as fixed effects.
RESULTS
Participant enrolment and disposition
There were 3821 participants included in the analysis for pool A (placebo and OLM comparison) and pool B (VAL comparison). Pool A consisted of 1998 participants; 793 in the normoglycemic subgroup, 823 in the prediabetes mellitus subgroup, and 382 in the T2DM subgroup. Pool B consisted of 1823 participants; 759 in the normoglycemic subgroup, 694 in the prediabetes mellitus subgroup, and 370 participants in the T2DM subgroup. The disposition of the study participants within the pools is shown in supplemental Figure 1).
Within pool A, 1998 participants were randomized to the following treatments: 561 to AZL-M 40 mg; 568 to AZL-M 80 mg; 572 to OLM 40 mg; and 297 to placebo. Of the 1998 participants within pool A, 1848 completed the study as planned.
Within pool B, 1823 participants were randomized to the following treatments: 607 to AZL-M 40 mg; 613 to AZL-M 80 mg; and 603 to VAL 320 mg. Of the 1823 patients within pool B, 1508 completed the study as planned. The most common reasons for discontinuation in both pools A and B were adverse events, voluntary withdrawal from the study, and lack of efficacy.
Characteristics at baseline
The demographic and baseline characteristics of all patients in the normoglycemic, prediabetes mellitus, and T2DM subgroups in both pooled treatment groups are shown in Table 1. The mean age ranged from 54 to 61 years, and the sex distributions were similar among the subgroups in both pools. The normoglycemic subgroup was younger than the subgroups with prediabetes mellitus and T2DM (55 years versus 58.5 years). The mean baseline BMI ranged from 29 to 34 kg/m2 and was higher in the T2DM subgroups than in the normoglycemic and prediabetes mellitus subgroups. Baseline HbA1c values were as follows for each subgroup (both pools): normoglycemic, 5.3%; prediabetes mellitus, 6.0%; and T2DM, 6.9%. The mean 24-h ambulatory SBPs at baseline were 144–148 mmHg, and were similar in the glycemic subgroups.
TABLE 1.
Demographic and baseline characteristics
| Demographic variable | Normoglycemic subgroup | Prediabetes mellitus subgroup | T2DM subgroup | |||||||||
| Pool A | Placebo | AZL-M 40 mg | AZL-M 80 mg | OLM 40 mg | Placebo | AZL-M 40 mg | AZL-M 80 mg | OLM 40 mg | Placebo | AZL-M 40 mg | AZL-M 80 mg | OLM 40 mg |
| N | 114 | 203 | 243 | 233 | 133 | 231 | 216 | 243 | 50 | 127 | 109 | 96 |
| Sex, M/F (%) | 60/40 | 58/42 | 56/44 | 58/42 | 51/49 | 47/53 | 49/51 | 49/51 | 60/40 | 50/50 | 54/46 | 48/52 |
| Race, Black/White/Other (%) | 15/60/25 | 11/72/16 | 12/68/20 | 13/71/16 | 12/64/24 | 16/63/21 | 13/70/17 | 14/67/20 | 22/66/12 | 17/60/24 | 18/63/18 | 20/62/19 |
| Age (years) | 56 ± 12 | 55 ± 12 | 55 ± 13 | 55 ± 11 | 59 ± 10 | 58 ± 11 | 58 ± 10 | 59 ± 11 | 59 ± 8 | 58 ± 9 | 59 ± 11 | 61 ± 11 |
| BMI (kg/m2) | 30 ± 5 | 30 ± 5 | 29 ± 5 | 30 ± 5 | 30 ± 4 | 31 ± 6 | 31 ± 6 | 30 ± 5 | 33 ± 6 | 33 ± 7 | 32 ± 6 | 33 ± 6 |
| 24-h SBP (mmHg) | 145 ± 12 | 146 ± 10 | 145 ± 10 | 145 ± 10 | 144 ± 11 | 145 ± 9 | 146 ± 10 | 146 ± 10 | 147 ± 11 | 145 ± 11 | 147 ± 10 | 145 ± 9 |
| Clinic SBP (mmHg) | 157 ± 12 | 158 ± 12 | 157 ± 12 | 157 ± 12 | 158 ± 12 | 158 ± 13 | 160 ± 12 | 160 ± 12 | 159 ± 12 | 158 ± 13 | 161 ± 12 | 159 ± 13 |
| GFR < 60 mL/min per 1.73 m2 (%) | 5 | 6 | 5 | 2 | 7 | 4 | 4 | 6 | 2 | 7 | 7 | 2 |
| GFR ≥ 60/<90 ml/min per 1.73 m2 (%) | 49 | 45 | 46 | 49 | 51 | 48 | 51 | 49 | 46 | 41 | 40 | 54 |
| HbA1c (%) | 5.27 ± 0.3 | 5.28 ± 0.3 | 5.34 ± 0.2 | 5.30 ± 0.3 | 5.97 ± 0.2 | 5.98 ± 0.2 | 5.97 ± 0.2 | 5.97 ± 0.2 | 6.96 ± 0.9 | 6.89 ± 0.7 | 6.88 ± 0.8 | 7.00 ± 0.8 |
| Pool B | – | AZL-M 40 mg | AZL-M 80 mg | VAL 320 mg | – | AZL-M 40 mg | AZL-M 80 mg | VAL 320 mg | – | AZL-M 40 mg | AZL-M 80 mg | VAL 320 mg |
| N | – | 248 | 269 | 242 | – | 236 | 220 | 238 | – | 123 | 124 | 123 |
| Sex, M/F (%) | – | 52/48 | 55/45 | 55/45 | – | 53/47 | 52/48 | 53/47 | – | 46/54 | 48/52 | 54/46 |
| Race, Black/White/Other (%) | – | 14/73/13 | 14/73/13 | 13/74/13 | – | 17/67/16 | 18/72/10 | 18/71/11 | – | 20/63/17 | 16/71/13 | 19/68/13 |
| Age (years) | – | 55 ± 13 | 55 ± 12 | 54 ± 11 | – | 58 ± 12 | 57 ± 10 | 57 ± 11 | – | 60 ± 10 | 59 ± 10 | 59 ± 10 |
| BMI (kg/m2) | – | 30 ± 5 | 29 ± 5 | 30 ± 5 | – | 31 ± 5 | 31 ± 6 | 31 ± 6 | – | 34 ± 7 | 33 ± 6 | 32 ± 6 |
| 24-h SBP (mmHg) | – | 146 ± 9 | 145 ± 9 | 145 ± 10 | – | 145 ± 10 | 145 ± 10 | 145 ± 10 | – | 145 ± 10 | 146 ± 10 | 148 ± 11 |
| Clinic SBP (mmHg) | – | 157 ± 13 | 156 ± 13 | 157 ± 14 | – | 158 ± 14 | 157 ± 11 | 157 ± 13 | – | 160 ± 15 | 159 ± 13 | 157 ± 13 |
| GFR < 60 mL/min per 1.73 m2 (%) | – | 4 | 4 | 2 | – | 6 | 1 | 8 | – | 7 | 6 | 2 |
| GFR ≥ 60/<90 mL/min per 1.73 m2 (%) | – | 48 | 46 | 53 | – | 49 | 51 | 50 | – | 46 | 43 | 42 |
| HbA1c | – | 5.29 ± 0.3 | 5.30 ± 0.2 | 5.30 ± 0.3 | – | 5.95 ± 0.2 | 5.98 ± 0.2 | 5.96 ± 0.2 | – | 6.82 ± 0.7 | 6.73 ± 1.0 | 6.71 ± 0.8 |
Data are mean ± SD or median [min, max]. AZL-M, azilsartan medoxomil; GFR, glomerular filtration rate; OLM, olmesartan; VAL, valsartan.
Changes from baseline in 24-h SBP
The effects of the various treatments on 24-h SBP for each pool and the glycemic subgroups within the pools are shown in Table 2 and Fig. 1. Within pool A, all treatments led to a statistically significant decrease in SBP compared with placebo (P ≤ 0.001). There were greater reductions from baseline in the 24-h SBP on AZL-M at 80 mg in the prediabetes mellitus group (P ≤ 0.001) compared with OLM 40 mg. AZL-M 40 mg achieved 24-h SBP reductions ranging from −12.1 mmHg (T2DM group) to −15.1 mmHg (normoglycemic subgroup). At 80 mg of AZL-M, reductions in 24-h SBP ranged from −12.3 mmHg in the T2DM subgroup to −16.8 mmHg (reaching statistical significance compared to OLM) in the prediabetes mellitus subgroup. OLM 40 mg reduced 24-h SBP from −9.8 mmHg (T2DM) to −12.9 mmHg (prediabetes mellitus).
TABLE 2.
Ambulatory blood pressure results by treatment group and pool
| Variable | Normoglycemic subgroup | Prediabetes mellitus subgroup | T2DM subgroup | |||||||||
| Pool A SBP | Placebo | AZL-M 40 mg | AZL-M 80 mg | OLM 40 mg | Placebo | AZL-M 40 mg | AZL-M 80 mg | OLM 40 mg | Placebo | AZL-M 40 mg | AZL-M 80 mg | OLM 40 mg |
| Baseline (mmHg) | 146 | 146 | 145 | 146 | 145 | 145 | 146 | 146 | 147 | 144 | 146 | 144 |
| Δ (mmHg) | −0.8 ± 1.1 | −15.1 ± 0.9* | −14.2 ± 0.8* | −13.2 ± 0.8* | −1.8 ± 1.0 | −13.4 ± 0.8* | −16.8 ± 0.8*,§ | −12.9 ± 0.7* | 0.5 ± 1.7 | −12.1 ± 1.0* | −12.3 ± 1.1* | −9.8 ± 1.2* |
| Pool A DBP | Placebo | AZL-M 40 mg | AZL-M 80 mg | OLM 40 mg | Placebo | AZL-M 40 mg | AZL-M 80 mg | OLM 40 mg | Placebo | AZL-M 40 mg | AZL-M 80 mg | OLM 40 mg |
| Baseline (mmHg) | 90 | 90 | 89 | 89 | 86 | 87 | 88 | 88 | 89 | 85 | 86 | 84 |
| Δ (mmHg) | −0.1 ± 0.7 | −9.7 ± 0.6* | −8.6 ± 0.5* | −8.5 ± 0.5* | −1.1 ± 0.7 | −8.2 ± 0.5* | −10.5 ± 0.5*,§ | −7.9 ± 0.5* | +0.7 ± 1.1 | −7.1 ± 0.7* | −7.3 ± 0.7* | −5.4 ± 0.7* |
| N | 100 | 173 | 200 | 203 | 114 | 200 | 184 | 216 | 40 | 108 | 88 | 85 |
| Pool B SBP | Placebo | AZL-M 40 mg | AZL-M 80 mg | VAL 320 mg | Placebo | AZL-M 40 mg | AZL-M 80 mg | VAL 320 mg | Placebo | AZL-M 40 mg | AZL-M 80 mg | VAL 320 mg |
| Baseline (mmHg) | – | 145 | 145 | 145 | – | 144 | 144 | 145 | – | 144 | 145 | 146 |
| Δ (mmHg) | – | −15.0 ± 0.8§ | −15.4 ± 0.8§ | −11.1 ± 0.8 | – | −13.3 ± 0.8†† | −15.1 ± 0.9§ | −10.9 ± 0.8 | – | −11.8 ± 1.1 | –12.0 ± 1.2 | –10.1 ± 1.2 |
| Pool B DBP | Placebo | AZL-M 40 mg | AZL-M 80 mg | VAL 320 mg | Placebo | AZL-M 40 mg | AZL-M 80 mg | VAL 320 mg | Placebo | AZL-M 40 mg | AZL-M 80 mg | VAL 320 mg |
| Baseline (mmHg) | – | 90 | 89 | 89 | – | 86 | 87 | 87 | – | 83 | 86 | 87 |
| Δ (mmHg) | – | −9.8 ± 0.6‡ | −10.0 ± 0.5† | −7.4 ± 0.6 | – | −8.2 ± 0.6 | −9.7 ± 0.6† | −6.9 ± 0.6 | – | −6.8 ± 0.7 | −7.7 ± 0.8 | −6.0 ± 0.8 |
| N | – | 211 | 215 | 199 | – | 202 | 175 | 201 | – | 102 | 99 | 96 |
Data are LS mean ± standard error (SE). AZL-M, azilsartan medoxomil; OLM, olmesartan; VAL, valsartan. Δ = change from baseline.
*P ≤ 0.001 versus placebo.
†P ≤ 0.01 versus OLM/VAL.
‡P < 0.05 versus OLM/VAL.
§P ≤ 0.001 versus OLM/VAL.
††P = 0.03 versus VAL.
FIGURE 1.
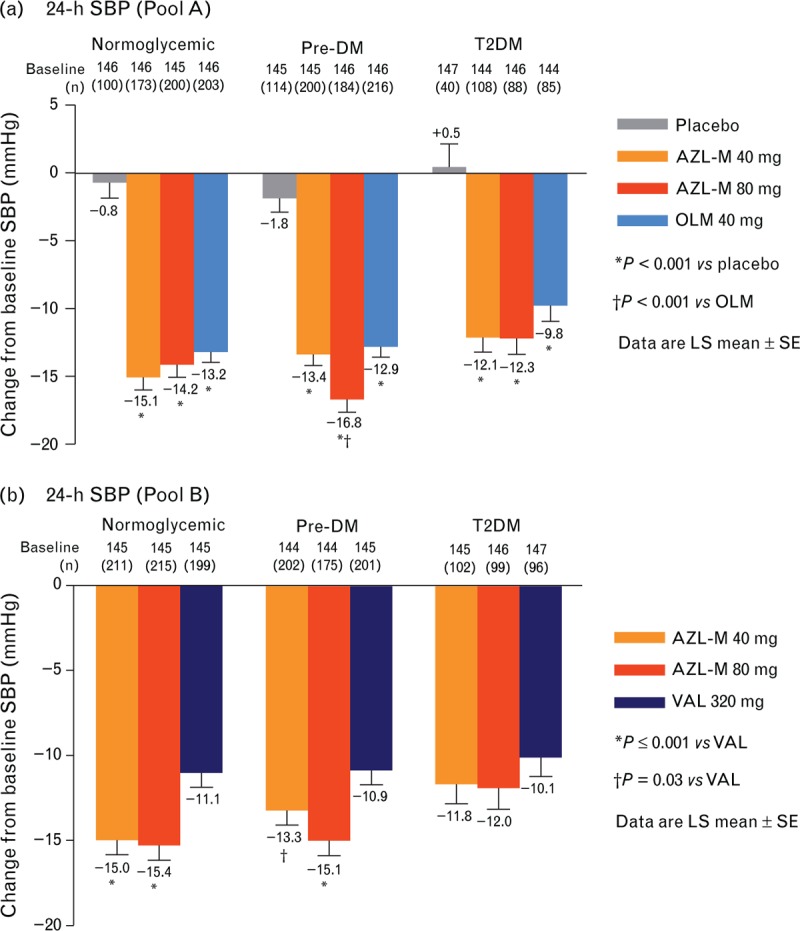
Changes from baseline in 24-h SBP by treatment group and glycemic subgroup. (a) Pool A. (b) Pool B.
In pool B, AZL-M at both 40 and 80 mg achieved greater 24-h SBP reduction than VAL 320 mg in the normoglycemic and prediabetes mellitus subgroups. The changes from baseline in 24-h SBP on AZL-M 40 mg were −15.0 mmHg in the normoglycemic subgroup (P ≤ 0.001 vs. VAL), −13.3 mmHg in the prediabetes mellitus subgroup (P = 0.03 vs. VAL), and −11.8 mmHg in the T2DM subgroup. The changes from baseline in 24-h SBP on AZL-M 80 mg were −15.4 mmHg in the normoglycemic subgroup (P ≤ 0.001 vs. VAL); −15.1 mmHg in the prediabetes mellitus subgroup (P ≤ 0.001 vs. VAL); and −12.0 mmHg in the T2DM subgroup (P = 0.22 vs. VAL).
Changes from baseline in ambulatory DBP
Changes from baseline in the ambulatory DBP pooled results are shown in Table 2. In pool A, all treatments were superior to placebo (P ≤ 0.001). Significant reductions were observed in the prediabetes mellitus group with AZL-M 80 mg (P ≤ 0.01) compared with OLM 40 mg. In pool B, AZL-M 80 mg achieved greater ambulatory DBP reduction than VAL 320 mg in both the normoglycemic and prediabetes mellitus subgroups (P ≤ 0.01). Additionally, AZL-M 40 mg had greater reductions in 24-h DBP than VAL in the normoglycemic subgroup (P < 0.05).
Changes from baseline in the clinic blood pressure
The pooled analyses of clinic BPs for the normoglycemic, prediabetes mellitus, and T2DM groups are shown in Table 3 and Fig. 2. In pool A, all treatments significantly decreased clinic SBP compared with placebo (P < 0.001). Within the prediabetes mellitus subgroup, AZL-M 80 mg reduced clinic SBP to a greater extent than OLM 40 mg (P < 0.001). In the T2DM subgroup, AZL-M 40 mg significantly decreased clinic SBP compared to OLM 40 mg (P = 0.032). In pool B, AZL-M at both 40 and 80 mg in the normoglycemic subgroup, 80 mg in the prediabetes mellitus subgroup, and both 40 and 80 mg in the T2DM subgroup significantly reduced clinic SBP compared with VAL 320 mg (P < 0.001).
TABLE 3.
Changes from baseline in clinic SBP and DBP
| SBP | Normoglycemic subgroup | Prediabetes mellitus subgroup | T2DM subgroup | |||||||||
| Pool A | Placebo | AZL-M 40 mg | AZL-M 80 mg | OLM 40 mg | Placebo | AZL-M 40 mg | AZL-M 80 mg | OLM 40 mg | Placebo | AZL-M 40 mg | AZL-M 80 mg | OLM 40 mg |
| N | 111 | 195 | 232 | 229 | 130 | 226 | 212 | 238 | 47 | 124 | 105 | 96 |
| Baseline (mmHg) | 157 | 158 | 157 | 158 | 158 | 158 | 159 | 160 | 159 | 158 | 161 | 159 |
| Δ (mmHg) | −2.33 ± 1.5 | −16.7 ± 1.2* | −15.8 ± 1.0* | −15.8 ± 1.1* | −3.5 ± 1.3 | −16.1 ± 1.0* | −21.5 ± 1.1*,§ | −14.9 ± 1.0* | −0.3 ± 2.4 | −15.1 ± 1.5*,‡ | −14.2 ± 1.6* | 10.5 ± 1.7* |
| Pool B | − | AZL-M 40 mg | AZL-M 80 mg | VAL 320 mg | − | AZL-M 40 mg | AZL-M 80 mg | VAL 320 mg | − | AZL-M 40 mg | AZL-M 80 mg | VAL 320 mg |
| N | − | 238 | 254 | 236 | − | 234 | 205 | 237 | − | 120 | 121 | 120 |
| Baseline (mmHg) | − | 156 | 156 | 158 | − | 158 | 157 | 157 | − | 159 | 158 | 157 |
| Δ (mmHg) | − | −16.4 ± 1.1§ | −16.5 ± 1.1§ | −11.21 ± 1.1 | − | −13.9 ± 1.1 | −17.3 ± 1.2§ | −11.3 ± 1.1 | − | −14.5 ± 1.5§ | −15.5 ± 1.5§ | −7.8 ± 1.5 |
| DBP | Normoglycemic subgroup | Prediabetes mellitus subgroup | T2DM subgroup | |||||||||
| Pool A | Placebo | AZL-M 40 mg | AZL-M 80 mg | OLM 40 mg | Placebo | AZL-M 40 mg | AZL-M 80 mg | OLM 40 mg | Placebo | AZL-M 40 mg | AZL-M 80 mg | OLM 40 mg |
| N | 111 | 195 | 232 | 229 | 130 | 226 | 212 | 238 | 47 | 124 | 105 | 96 |
| Baseline (mmHg) | 93 | 93 | 92 | 92 | 91 | 92 | 92 | 92 | 93 | 89 | 90 | 88 |
| Δ (mmHg) | −0.6 ± 0.9 | −8.0 ± 0.7* | −8.5 ± 0.6* | −7.8 ± 0.6* | −1.2 ± 0.8 | −6.8 ± 0.6* | −9.6 ± 0.6*,† | −6.9 ± 0.6* | +0.4 ± 1.3 | −7.1 ± 0.8*,‡ | −7.8 ± 0.9*,† | −4.7 ± 0.9* |
| Pool B | Placebo | AZL-M 40 mg | AZL-M 80 mg | VAL 320 mg | Placebo | AZL-M 40 mg | AZL-M 80 mg | VAL 320 mg | Placebo | AZL-M 40 mg | AZL-M 80 mg | VAL 320 mg |
| N | − | 238 | 254 | 236 | − | 234 | 205 | 237 | − | 120 | 121 | 120 |
| Baseline (mmHg) | − | 92 | 91 | 92 | − | 90 | 90 | 90 | − | 87 | 89 | 88 |
| Δ (mmHg) | − | −8.1 ± 0.6§ | −8.6 ± 0.6§ | −5.2 ± 0.6 | − | −6.2 ± 0.7 | −7.7 ± 0.7† | −5.3 ± 0.7 | − | −6.7 ± 0.8§ | −7.0 ± 0.8§ | −2.6 ± 0.8 |
Data are LS mean ± standard error (SE). Δ, change from baseline. AZL-M, azilsartan medoxomil; OLM, olmesartan; VAL, valsartan.
*P ≤ 0.001 versus placebo.
†P ≤ 0.01 versus OLM/VAL.
‡P < 0.05 versus OLM/VAL.
§P ≤ 0.001 versus OLM/VAL.
FIGURE 2.
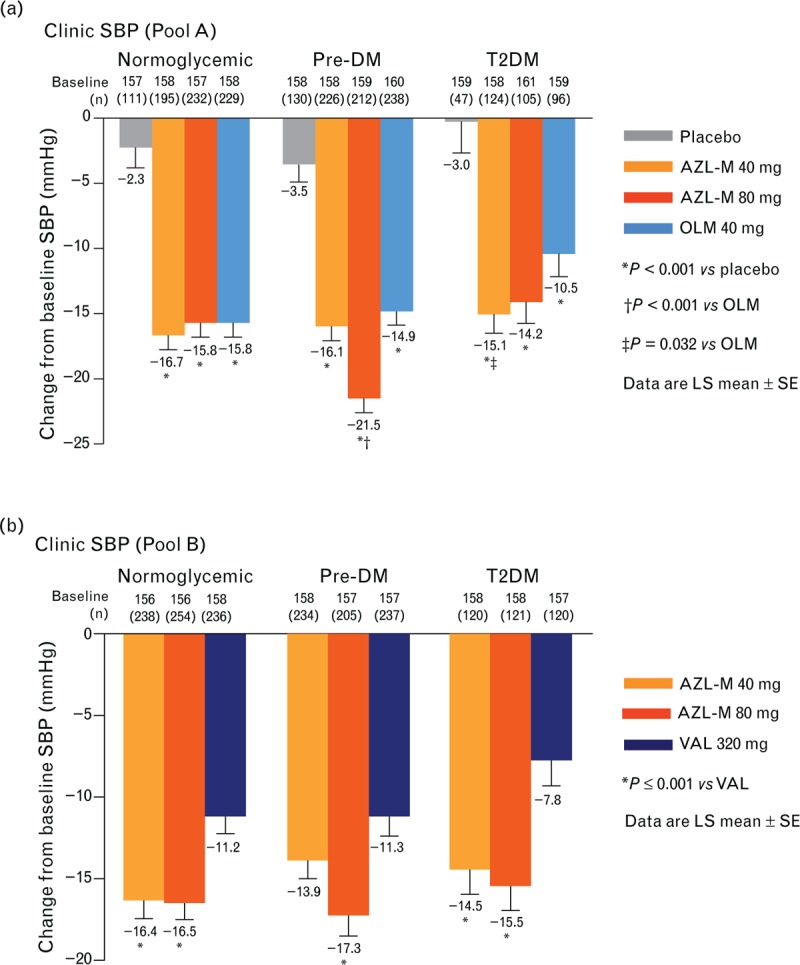
Changes from baseline in clinic SBP by treatment group and glycemic subgroup. (a) Pool A. (b) Pool B.
The results for the changes from baseline in the clinic DBP are also shown in Table 3. In pool A, all treatments demonstrated greater DBP reduction than placebo (P ≤ 0.001). Significantly greater reductions in the clinic DBP were seen in the following subgroups on AZL-M compared to OLM 40 mg: AZL-M 80 mg (P = 0.002) in prediabetes mellitus; and AZL-M at both 40 mg (P < 0.034) and 80 mg (P ≤ 0.01) in T2DM. In pool B, AZL-M at both doses decreased the clinic DBP to a significantly greater extent compared with VAL 320 mg in the normoglycemic and T2DM subgroups (P < 0.001). In the prediabetes mellitus subgroup, AZL-M 80 mg reduced clinic DBP to a greater extent than VAL (P ≤ 0.008).
Metabolic effects
Results of the metabolic parameters are shown in Table 4. Owing to the short-term nature of the trials, HbA1c values were not repeated postrandomization. There were small and significantly greater reductions in the FPG, free insulin levels, and homeostasis model assessment on azilsartan 80 mg versus OLM 40 mg in pool A in the normoglycemic subgroup. In addition, there were small and significantly larger reductions in FPG on azilsartan 80 mg versus OLM 40 mg and placebo in pool A in the T2DM subgroup. There were no other significant differences in the levels of lipoproteins or other biomarkers on AZL-M, OLM, and VAL in pool B (Table 4).
TABLE 4.
Changes in Biomarkers from Baseline to Week 6/8
| Variable | Mean change (baseline) | |||||||||||
| Normoglycemic subgroup | Prediabetes mellitus subgroup | T2DM subgroup | ||||||||||
| Pool A | Placebo | AZL-M 40 mg | AZL-M 80 mg | OLM 40 mg | Placebo | AZL-M 40 mg | AZL-M 80 mg | OLM 40 mg | Placebo | AZL-M 40 mg | AZL-M 80 mg | OLM 40 mg |
| N | 41–95 | 92–171 | 106–200 | 92–198 | 68–113 | 114–190 | 120–181 | 128–200 | 27–42 | 67–113 | 50–95 | 54–83 |
| FPG (mmol/l) | −0.0 (5.3) | +0.0 (5.3) | −0.1 (5.3)† | +0.1 (5.3) | +0.0 (5.6) | +0.0 (5.7) | +0.0 (5.6) | −0.0 (5.5) | +0.5 (7.3) | +0.1 (6.9) | −0.2 (6.9)*,† | +0.4 (7.1) |
| Free insulin (μIU/ml) | −3.1 (9.6) | −0.9 (14) | −2.2 (13)† | +1.8 (13) | −1.0 (15) | +0.3 (13) | +1.8 (15) | −1.2 (13) | +1.8 (13) | +1.5 (19) | +2.7 (16) | −3.2 (20) |
| HOMA | −1.1 (2.4) | −0.2 (3.8) | −0.8 (3.3)† | +0.4 (3.6)* | −0.1 (3.9) | +0.0 (3.6) | +0.7 (4.2) | −0.4 (3.6) | +1.0 (4.3) | +0.8 (5.9) | +1.2 (5.3) | −0.7 (6.9) |
| hsCRP (mg/l) | +0.3 (4.5) | +0.3 (3.8) | −0.3 (3.6) | −0.1 (3.2) | 0.0 (3.0) | +0.2 (4.3) | −0.2 (3.9) | +0.3 (4.9) | −2.0 (6.9) | −0.9 (5.3) | +0.1 (4.9)* | −0.1 (4.8) |
| PAI-1 (ng/ml) | +10 (76) | −4.4 (72)* | +6.2 (74) | +2.7 (77) | +16 (71) | +10 (77) | +12 (82) | +7.5 (70) | +14 (79) | +4.8 (79) | +5.6 (85) | −0.7 (81) |
| Adiponectin (μg/ml) | +0.4 (9.4) | −0.3 (10) | −0.4 (8.7) | +0.1 (9.4) | +0.7 (8.2) | +0.3 (8.3) | +0.7 (9.0) | +0.5 (9.2) | +0.1 (7.4) | +0.4 (8.5) | +0.8 (8.0) | −0.1 (8.3) |
| GFR (ml/min per 1.73 m2) | −5.3 (90) | −1.9 (89) | −1.7 (90) | −0.8 (90) | +1.6 (88) | −1.6 (90) | −2.4 (87) | −2.9 (87) | +3.3 (85) | −4.7 (89) | −3.1 (89) | −3.6 (89) |
| Pool B | – | AZL-M 40 mg | AZL-M 80 mg | VAL 320 mg | – | AZL-M 40 mg | AZL-M 80 mg | VAL 320 mg | – | AZL-M 40 mg | AZL-M 80 mg | VAL 320 mg |
| N | – | 83–126 | 99–119 | 87–107 | 86–106 | 70–109 | 79–126 | 51–58 | 45–61 | 45–60 | ||
| FPG (mmol/l) | – | −0.1 (5.4) | −0.1 (5.3) | +0.0 (5.3) | – | −0.1 (5.7) | −0.1 (5.7) | −0.1 (5.7) | – | +0.1 (6.7) | −0.2 (6.9) | +0.0 (7.4) |
| Free insulin (μIU/ml) | – | −0.2 (15) | −3.3 (13) | −1.7 (12) | – | −1.6 (15) | −1.3 (17) | −4.5 (19) | – | +1.0 (15) | +3.9 (17) | −6.1 (26.7) |
| HOMA | – | −0.1 (4.2) | −1.0 (3.6) | −0.0 (2.8) | – | −0.7 (3.8) | −0.2 (4.8) | −1.5 (5.3) | – | +0.3 (4.7) | +2.2 (5.6) | −1.2 (8.2) |
| hsCRP (mg/l) | – | −0.1 (4.0) | −0.3 (3.8) | −0.3 (3.3) | – | +0.3 (4.3) | −0.9 (3.9) | −0.1 (4.5) | – | −0.2 (5.6) | +1.7 (4.9) | −0.0 (4.7) |
| PAI-1 (ng/ml) | – | +5.9 (72) | +17 (69) | +5.3 (76) | – | +9.9 (80) | +8.6 (81) | +13 (76) | – | +13 (75) | +27 (87) | +18 (81) |
| Adiponectin (μg/mL) | – | −0.5 (10) | −0.1 (8.4) | −0.2 (9.4) | – | +0.7 (8.1) | +1.3 (9.8) | +0.8 (8.2) | – | +0.9 (9.3) | +1.0 (8.3) | −0.5 (7.1) |
| GFR (ml/min per 1.73 m2) | – | −2.2 (89) | −4.3 (90) | −1.4 (91) | – | −3.4 (85) | −4.5 (89) | −1.2 (86) | – | −4.8 (86) | −3.0 (90) | +0.1 (90) |
Data are LS mean, except for GFR (arithmetic mean). AZL-M, azilsartan medoxomil; FPG, fasting plasma glucose; GFR, glomerular filtration rate (calculated using Modification of Diet in Renal Disease (MDRD) method); HOMA, homeostasis model assessment (insulin sensitivity); hsCRP, high-sensitivity C-reactive protein; OLM, olmesartan; PAI-1, plasminogen activator inhibitor-1; VAL, valsartan.
*P < 0.05 versus placebo.
†P ≤ 0.05 versus OLM; no P values available for GFR.
Safety and tolerability
The safety and tolerability findings were similar for AZL-M at both the study doses, OLM, VAL, and placebo in both pools across all three glycemic subgroups (Table 5).
TABLE 5.
Adverse events
| % Patients with event | ||||||||||||
| Adverse events | Normoglycemic subgroup | Prediabetes mellitus subgroup | T2DM subgroup | |||||||||
| Pool A | Placebo (n = 114) | AZL-M 40 mg (n = 203) | AZL-M 80 mg (n = 243) | OLM 40 mg (n = 233) | Placebo (n = 133) | AZL-M 40 mg (n = 231) | AZL-M 80 mg (n = 216) | OLM 40 mg (n = 243) | Placebo (n = 50) | AZL-M 40 mg (n = 127) | AZL-M 80 mg (n = 109) | OLM 40 mg (n = 96) |
| Any adverse events | 43.0 | 41.4 | 46.5 | 51.1 | 41.4 | 42.0 | 47.2 | 40.7 | 42.0 | 42.5 | 43.1 | 41.7 |
| Serious adverse events | 3.5 | 1.0 | 0.4 | 2.1 | 0.8 | 0 | 0.5 | 0.4 | 0 | 0 | 1.8 | 0 |
| Adverse events leading to discontinuation | 4.4 | 1.5 | 3.3 | 2.1 | 1.5 | 2.2 | 2.3 | 2.1 | 4.0 | 1.6 | 0.9 | 0 |
| Adverse events (preferred term) in ≥5% of study participants in any group | ||||||||||||
| Headache | 7.0 | 2.5 | 5.8 | 8.6 | 10.5 | 6.1 | 5.6 | 4.1 | 4.0 | 6.3 | 1.8 | 2.1 |
| Dyslipidemia | 0 | 2.0 | 4.1 | 3.4 | 1.5 | 3.0 | 2.8 | 1.6 | 4.0 | 4.7 | 6.4 | 2.1 |
| Nasopharyngitis | 1.8 | 1.5 | 2.9 | 1.3 | 0.8 | 0.4 | 2.8 | 1.6 | 0 | 3.1 | 1.8 | 5.2 |
| Pool B | Placebo | AZL-M 40 mg (n = 248) | AZL-M 80 mg (n = 269) | VAL 320 mg (n = 242) | Placebo | AZL-M 40 mg (n = 236) | AZL-M 80 mg (n = 220) | VAL 320 mg (n = 238) | Placebo | AZL-M 40 mg (n = 123) | AZL-M 80 mg (n = 124) | VAL 320 mg (n = 123) |
| Any adverse events | − | 60.9 | 57.2 | 53.3 | − | 55.1 | 62.7 | 52.9 | − | 54.5 | 54.8 | 55.3 |
| Serious adverse events | − | 2.0 | 1.1 | 0.8 | − | 0.4 | 0.9 | 2.9 | − | 3.3 | 2.4 | 1.6 |
| Adverse events leading to discontinuation | − | 6.9 | 5.9 | 2.9 | − | 3.8 | 6.8 | 5.5 | − | 3.3 | 3.2 | 5.7 |
| Adverse events (preferred term) in ≥5% of study participants in any group | ||||||||||||
| Headache | − | 8.9 | 6.3 | 7.4 | − | 8.9 | 7.7 | 8.4 | − | 6.5 | 5.6 | 8.9 |
| Dizziness | − | 7.3 | 6.7 | 5.0 | − | 6.4 | 6.8 | 2.9 | − | 3.3 | 4.8 | 0.8 |
| Urinary tract infection | − | 4.8 | 5.2 | 3.7 | − | 5.5 | 4.1 | 2.5 | − | 8.1 | 6.5 | 3.3 |
AZL-M, azilsartan medoxomil; OLM, olmesartan; VAL, valsartan.
DISCUSSION
Principal findings
AZL-M had previously been shown to have superior efficacy to both OLM and VAL in controlled clinical trials using ambulatory BP as the primary endpoint in a general patient population with hypertension [11,12]. The present analyses extend our knowledge on this efficacy of AZL-M in participants with prediabetes and type 2 diabetes, patient subgroups with particularly high cardiovascular risk when BP control is deficient [10]. These findings demonstrate that AZL-M is a highly effective ARB for hypertension control in patients with prediabetes mellitus and T2DM.
Impact of angiotensin II receptor blockers in patients with type 2 diabetes
In patients with T2DM, the ARBs and ACE inhibitors are specifically recommended as initial therapy in patients with diabetes by various management guidelines [15,16]. Previous trials have utilized 24-h BP monitoring to compare the efficacy among the ARBs in patients with hypertension and T2DM, although most were performed in studies of fixed-dose combinations with diuretics or calcium antagonists.
In a small study from Japan that examined effects of losartan 50 mg and telmisartan 40 mg on ambulatory BP variability in 30 patients with diabetic nephropathy, similar changes from baseline in 24-h BP were reported with both drugs [17]. Another study from Japan compared the effects of OLM 20 mg versus telmisartan 40 mg, in 20 patients previously on VAL 80 mg during an 8-week run-in period, on ambulatory blood pressure in Japanese diabetic patients with hypertension [18]. In that study, OLM showed greater blood pressure reduction compared with telmisartan. OLM decreased ambulatory BP by 5/1 mmHg from the VAL-treated baseline, whereas telmisartan decreased ambulatory SBP by 1 mmHg from baseline (P = 0.031). The superior antihypertensive effects of AZL-M may be because of its high potency as well as its slow dissociation (tight binding) from the angiotensin II receptor [19].
In the current analysis, in the pool A (OLM comparison) T2DM subgroup, AZL-M reduced 24-h ambulatory blood pressure by 12.1/7.1 mmHg (40 mg) and 12.3/7.3 mmHg (80 mg), and OLM 40 mg reduced ambulatory BP by 9.8/5.4 mmHg. In the pool B (VAL comparison) T2DM subgroup, AZL-M reduced ABP by 11.8/6.8 mmHg (40 mg) and 12.0/7.7 mmHg (80 mg). Our findings are novel as there are no comparable analyses using ambulatory BP monitoring to assess ARBs in large numbers of patients with hypertension and T2DM or prediabetes.
The comparative effects of AZL-M versus VAL and OLM on ambulatory BP were similar to the comparative effects of these ARBs in all glycemic subgroups. These findings provide clinicians with an effective medication option for patients with comorbid hypertension and T2DM or prediabetes mellitus, in whom the risk of cardiovascular morbidity is high. Therefore, AZL-M, as part of a comprehensive management plan to address cardiovascular risk factors and comorbidities, has the potential to benefit patients with hypertension with prediabetes or T2DM.
Metabolic effects of the angiotensin receptor blockers in the present analysis
Animal models have shown that azilsartan, the active metabolite of AZL-M increased insulin sensitivity and glucose uptake [19], but in-vitro studies failed to show that azilsartan increased transcriptional activity of peroxisome proliferator-activated receptor γ [20]. In the present study, we observed some significant effects of AZL-M on fasting blood glucose and insulin levels in the normoglycemic and type 2 diabetes subgroups relative to other comparator treatment arms, but the effects were neither consistent nor very robust. In addition, there were no significant effects of any of the ARBs on the other biomarkers, including adiponectin and lipoproteins. The short-term nature of the trials used in the present analysis may have played a role in the negative findings on some of these metabolic parameters.
These finding were similar to previous studies, which found no significant changes in similar metabolic parameters among hypertensive, diabetic patients treated with other ARBs, including eprosartan, OLM, and telmisartan [18,21–23].
Strengths and limitations
Our analysis had over 3800 patients who were well characterized by their baseline glycemic status and ambulatory BP data. In each of the three studies, 24-h ambulatory BP monitoring was used as a primary efficacy end point and the inclusion and exclusion criteria and study designs were similar, which allowed the pooling of the ambulatory and clinic BP results. Of note, reductions from baseline in the clinic and ambulatory BP findings were relatively similar and we believe are due, in part, to use of standardized, semi-automated BP monitors at study sites that required the printing and recording of results, which would likely lead to greater reliability and correlation between clinic and ambulatory readings.
The limitation of the analysis relates to the short-term nature of the studies. Although this was an adequate time period to assess changes in clinic and ambulatory BPs, it was not likely to have been enough time to assess the differential effects of the various ARBs on the metabolic parameters. Furthermore, because of follow-up periods of 2 months or less, there were no posttreatment values for the HbA1c.
In conclusion, AZL-M has greater antihypertensive efficacy than OLM or VAL in patients with prediabetes mellitus and T2DM based on both clinic and 24-h BP results. The importance of hypertension control in this population is documented as a means to decrease cardiovascular morbidity and mortality [10]. Hence, having the option of a well-tolerated ARB that provides an additional 3–7 mmHg SBP reduction is an important therapeutic advance for the treatment of patients with hypertension with T2DM.
ACKNOWLEDGEMENTS
This analysis was supported by Takeda Development Center Americas, Inc., Deerfield, Illinois, USA.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Reviewer's Summary Evaluation
Reviewer 1
Strengths: Blood pressure control also depends on the level of blood glucose control.
This paper indicates that azilsartan is a potent antihypertensive agent also in the presence of a poor metabolic control.
Weaknesses: The efficacy of angiotenin II blockers in diabetic hypertensive patients is well known and data derived from a pooled analysis of different studies.
Footnotes
Abbreviations: ARB, angiotensin receptor blocker; AZL-M, azilsartan medoxomil; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; OLM, olmesartan; T2DM, type 2 diabetes mellitus; VAL, valsartan
REFERENCES
- 1.Lorber D. Importance of cardiovascular disease risk management in patients with type 2 diabetes mellitus. Diabetes Metab Syndr Obes 2014; 7:169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rydén L, Grant P, Anker SD, Berne C, Cosentino F, Danchin N, et al. ESC Guidelines on diabetes, prediabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, prediabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J 2013; 34:3035–3087. [DOI] [PubMed] [Google Scholar]
- 3.Chen G, McAlister FA, Walker RL, Hemmelgarn BR, Campbell NR. Cardiovascular outcomes in Framingham participants with diabetes: the importance of blood pressure. Hypertension 2011; 57:891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010; 375:2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mogensen J. New treatment guidelines for a patient with diabetes and hypertension. J Hypertens Suppl 2003; 21:S25–30. [PubMed] [Google Scholar]
- 6.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234. [DOI] [PubMed] [Google Scholar]
- 7.U.K. Prospective Diabetes Study. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 8.Jamerson K, Weber MA, Bakris GL, Dahlöf B, Pitt B, Shi V. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med 2008; 359:2417–2428. [DOI] [PubMed] [Google Scholar]
- 9.Turnbull F, Neal B, Algert C, Chalmers J, Chapman N, Cutler J, et al. Effects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials. Arch Intern Med 2005; 165:1410–1419. [DOI] [PubMed] [Google Scholar]
- 10.Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA 2015; 313:603–615. [DOI] [PubMed] [Google Scholar]
- 11.Bakris GL, Sica D, Weber M, White WB, Roberts A, Perez A, et al. The comparative effects of azilsartan medoxomil and olmesartan on ambulatory and clinic blood pressure. J Clin Hypertens (Greenwich) 2011; 13:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White WB, Weber MA, Sica D, Bakris GL, Perez A, Cao C, et al. Effects of the angiotensin receptor blocker azilsartan medoxomil versus olmesartan and valsartan on ambulatory and clinic blood pressure in patients with stages 1 and 2 hypertension. Hypertension 2011; 57:413–420. [DOI] [PubMed] [Google Scholar]
- 13.Sica D, White WB, Weber MA, Bakris GL, Perez A, Cao C, et al. Comparison of the novel angiotensin II receptor blocker azilsartan medoxomil vs valsartan by ambulatory blood pressure monitoring. J Clin Hypertens (Greenwich) 2011; 13:467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28:412–419. [DOI] [PubMed] [Google Scholar]
- 15.James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in Adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520. [DOI] [PubMed] [Google Scholar]
- 16.Weber MA, Schiffrin EL, White WB, Mann S, Lindholm LH, Kenerson JG, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich) 2014; 16:14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masuda S, Tamura K, Wakui H, Kanaoka T, Ohsawa M, Maeda A, et al. Effects of angiotensin II type 1 receptor blocker on ambulatory blood pressure variability in hypertensive patients with overt diabetic nephropathy. Hypertens Res 2009; 32:950–955. [DOI] [PubMed] [Google Scholar]
- 18.Nakayama S, Watada H, Mita T, Ikeda F, Shimizu T, Uchino H, et al. Comparison of effects of olmesartan and telmisartan on blood pressure and metabolic parameters in Japanese early-stage type-2 diabetics with hypertension. Hypertens Res 2008; 31:7–13. [DOI] [PubMed] [Google Scholar]
- 19.Ojima M, Igata H, Tanaka M, Sakamoto H, Kuroita T, Kohara Y, et al. In vitro antagonistic properties of a new angiotensin type 1 receptor blocker, azilsartan, in receptor binding and function studies. J Pharmacol Exp Ther 2011; 336:801–808. [DOI] [PubMed] [Google Scholar]
- 20.Iwai M, Chen R, Imura Y, Horiuchi M. TAK-536, a new AT1 receptor blocker, improves glucose intolerance and adipocyte differentiation. Am J Hypertens 2007; 20:579–586. [DOI] [PubMed] [Google Scholar]
- 21.Kajiya T, Ho C, Wang J, Vilardi R, Kurtz TW. Molecular and cellular effects of azilsartan: a new generation angiotensin II receptor blocker. J Hypertens 2011; 29:2476–2483. [DOI] [PubMed] [Google Scholar]
- 22.Derosa G, Ragonesi PD, Mugellini A, Ciccarelli L, Fogari R. Effects of telmisartan compared with eprosartan on blood pressure control, glucose metabolism and lipid profile in hypertensive, type 2 diabetic patients: a randomized, double-blind, placebo-controlled 12-month study. Hypertens Res 2004; 27:457–464. [DOI] [PubMed] [Google Scholar]
- 23.Arao T, Okada Y, Mori H, Nishida K, Tanaka Y. Antihypertensive and metabolic effects of high-dose olmesartan and telmisartan in type 2 diabetic patients with hypertension. Endocr J 2013; 60:563–570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


