Abstract
Background:
Arterial stiffness plays a fundamental role in the development of hypertension and is a risk factor for both cardiovascular disease and mortality. The stiffening that occurs with increasing age has, in numerous cross-sectional studies, been shown to be associated with several cardiovascular risk factors. This observational study aims to characterize the predictive and cross-sectional markers focusing on the non-hemodynamic component of arterial stiffness.
Method:
In all, 2679 men and women from Malmö, Sweden, were examined at baseline during 1991–1994, and again at follow-up during 2007–2012 (mean age 72 years, 38% men). Follow-up examination included measurement of arterial stiffness by carotid–femoral pulse wave velocity (c-fPWV), after a mean period of 17 years. The associations between c-fPWV and risk markers were calculated with multiple linear regression.
Results:
The results indicated that for both sexes, waist circumference (β = 0.17, P < 0.001), fasting glucose (β = 0.13, P < 0.001), Homeostatic Model Assessment – Insulin Resistance (β = 0.10, P < 0.001), triglycerides (β = 0.10, P < 0.001), and high-density lipoprotein cholesterol (β = −0.08, P < 0.001) were all predictors of cfPWV adjusted for mean arterial pressure and heart rate, as well as for classical cardiovascular risk factors and drug treatment. There were no associations between baseline or follow-up low-density lipoprotein cholesterol, smoking, or eGFR and c-fPWV.
Conclusion:
The non-hemodynamic cluster of risk markers and predictors of arterial stiffness in a middle-aged population includes abdominal obesity, hyperglycemia, and dyslipidemia, but not smoking and low-density lipoprotein cholesterol. This pattern existed in both sexes.
Keywords: ageing, arterial stiffness, diabetes mellitus, epidemiology, follow-up, glucose, hypertension, pulse wave velocity
BACKGROUND
Stiffening of the large arteries has been shown to be an important risk marker for future cardiovascular events and mortality beyond well known cardiovascular risk factors [1,2]. Measurement of arterial stiffness is preferably performed by use of carotid–femoral pulse wave velocity (c-fPWV) according to a consensus document [3].
Arterial stiffness, or arteriosclerosis, is known to be strongly associated with age and hypertension [4,5] – findings which were also confirmed in a longitudinal study [6]. The arterial ageing is tightly intercorrelated with blood pressure and causes an increase in pulse pressure (PP) seen in aged individuals [4]. In some individuals, the arterial stiffening seen with increasing age is more pronounced and occurs earlier in life – a phenomenon described as early vascular ageing (EVA) [7]. A number of nonhemodynamic components are thought to affect the arterial ageing [7]. Several cross-sectional studies have shown an association between arterial stiffness and diabetes, as well as with markers of impaired glucose metabolism [8–10]. Individuals with end-stage renal disease (ESRD) are also known to exhibit an increased central arterial stiffness, but results from studies investigating the association between arterial stiffness and stages of chronic kidney disease (CKD) have presented conflicting results [11]. Results from a prospective study showed that central obesity predicts arterial stiffness over a period of 16 years [12], whereas a 20-year follow-up study including men indicates that heavy smoking, C-reactive protein (CRP), and PP are predictors of arterial stiffness [13].
The study aims to determine the predictive and cross-sectional nonhemodynamic markers of arterial stiffness (cfPWV), after adjustment for mean blood pressure and heart rate, over 17 years of follow-up in a middle-aged urban population. The investigated predictors include markers of glucose and lipid metabolism, renal function, obesity, and smoking. Furthermore, the study aims to characterize individuals younger than 75 years with an increased arterial stiffness, which is a core feature of EVA.
PATIENTS AND METHODS
Study population
The cohort study includes individuals from the Malmö Diet and Cancer (MDC) study, a large prospective cohort, consisting of male and female participants from the city of Malmö, Sweden [14,15]. From this cohort, in all, 6103 individuals took part in the Cardiovascular Arm of the MDC cohort (MDC-CV), investigated during 1991–1994, that constitutes the baseline in this study [16]. Follow-up data come from the re-examination of 3734 of these individuals performed from May 2007 to September 2012 (76% attendance rate of the surviving baseline population). The reasons for nonparticipation are presented in Fig. 1. This follow-up examination included measurement of c-fPWV in 3056 individuals. There is complete baseline and follow-up laboratory and anthropometric data available on 2679 individuals, constituting the study population described in Table 1.
FIGURE 1.
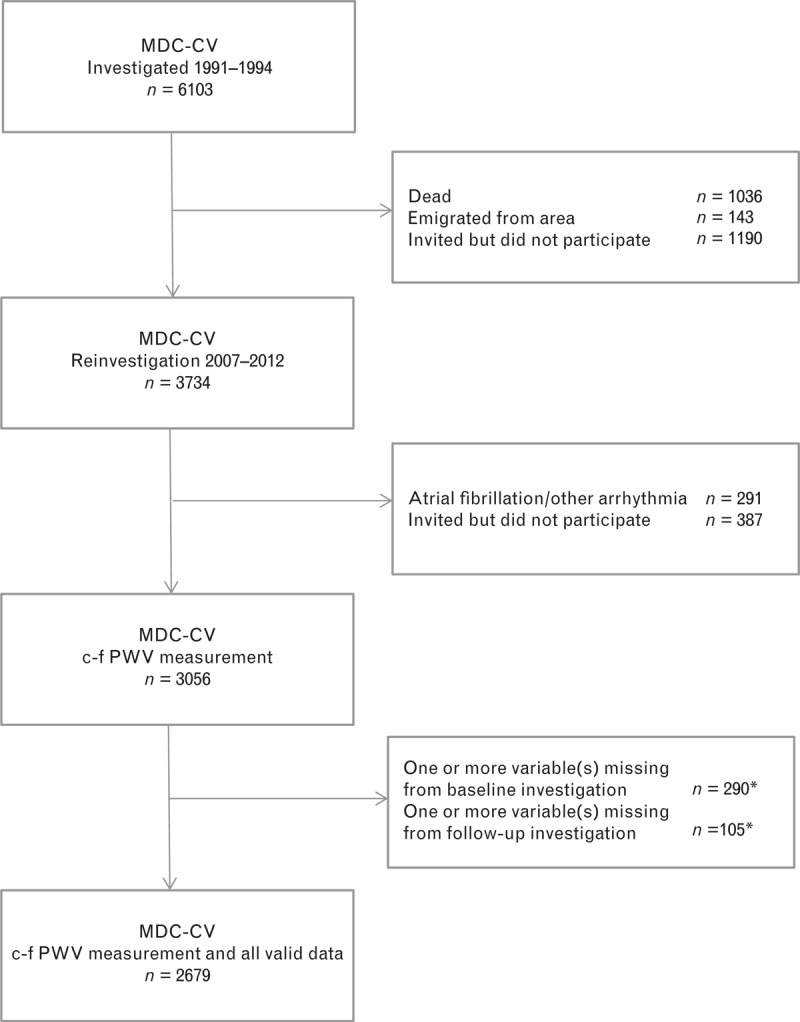
Flow diagram of nonparticipation. (∗) For some individuals, data were missing both from baseline and follow-up investigation, therefore presented in both categories.
TABLE 1.
Characteristics of the study population (n = 2679) at baseline and follow-up
| Characteristics | Baseline | Follow-up | ||
| Men (n = 1007) | Women (n = 1672) | Men (n = 1007) | Women (n = 1672) | |
| Age (years) | 56.0 (5.8) | 56.0 (5.6) | 72.0 (5.7) | 72.2 (5.5) |
| BMI (kg/m2) | 25.7 (3.1) | 25.0 (3.7) | 26.8 (3.7) | 26.5 (4.4) |
| Waist circumference (cm) | 91 (9) | 76 (9) | 98 (10) | 87 (11) |
| SBP (mmHg) | 140 (17) | 137 (18) | 136 (17) | 135 (18) |
| DBP (mmHg) | 88 (9) | 85 (9) | 76 (9) | 75 (9) |
| Fasting glucose (mmol/l) | 5.0a [4.7–5.3] | 4.8a [4.5–5.1] | 6.0b [5.5–6.6] | 5.7b [5.3–6.2] |
| HbA1c (%) | 4.7 [4.5–5.0] | 4.8 [4.5–5.0] | n.a. | n.a. |
| HOMA-IR (n = 2636) | 1.46 [0.98–2.12] | 1.23 [0.82–1.74] | n.a. | n.a. |
| Triglycerides (mmol/l) | 1.23 [0.93–1.69] | 1.02 [0.78–1.38] | 1.00 [0.70–1.30] | 0.90 [0.70–1.30] |
| LDL cholesterol (mmol/l) | 4.12 (0.87) | 4.12 (1.02) | 3.14 (0.88) | 3.46 (0.93) |
| HDL cholesterol (mmol/l) | 1.23 (0.29) | 1.53 (0.36) | 1.25 (0.36) | 1.56 (0.43) |
| eGFR (ml/min per 1.73 m2) (n = 2418) | 95 (13) | 88 (12) | 70 (15) | 66 (14) |
| c-fPWV (m/s) | n.a. | n.a. | 10.4 [9.0–12.1] | 9.9 [8.7–11.5] |
| Diabetes [n (%)] | 72 (7) | 63 (4) | 165 (16) | 189 (11) |
| Current smoking [n (%)] | 229 (23) | 369 (22) | 102 (10) | 146 (9) |
| Former smoking [n (%)] | 439 (44) | 474 (28) | 542 (54) | 653 (39) |
| Lipid-lowering drug therapy, n (%) | 26 (3) | 28 (2) | 336 (33) | 437 (26) |
| Blood pressure-lowering drug therapy [n (%)] | 140 (14) | 193 (12) | 571 (57) | 885 (53) |
For continuous variables, normally distributed characteristics expressed with mean (±SD) and others expressed as median (first quartile–third quartile). c-fPWV, carotid–femoral pulse wave velocity; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; HOMA-IR, Homeostatic Model Assessment – Insulin Resistance; LDL, low-density lipoprotein; n.a., not applicable. Numbers of individuals with valid data are specified between brackets.
aB-glucose.
bP-glucose.
The study was approved by the ethical committee at Lund University, Lund, Sweden (baseline ID LU-5190, follow-up ID 532–2006). A written informed consent was obtained from all participants.
Baseline measurements
Examination included a self-administered questionnaire, a physical examination with blood pressure measurement after 10 min of supine rest, as well as blood samples after an overnight fast. Details on these procedures have been previously reported [16]. High-density lipoprotein (HDL) cholesterol and triglycerides were determined according to standard procedures. Low-density lipoprotein (LDL) cholesterol was calculated by Friedewald's formula. Cystatin C was measured using a particle-enhanced immunonephelometric assay and plasma creatinine was analyzed with the Jaffé method [17]. Estimated glomerular filtration rate (eGFR) was calculated with the combined creatinine–cystatin C described by Inker et al.[18]. The same methods were used in the follow-up measurements.
Diabetes was defined based on a self-reported history of diabetes, ongoing pharmacological treatment against diabetes, or fasting blood glucose at least 6.1 mmol/l. Homeostatic Model Assessment – Insulin Resistance (HOMA-IR) was calculated according to Matthews et al.[19] by using the formula: (fasting insulin × fasting glucose)/22.5, where insulin is expressed as mIU/l and glucose as mmol/l. Due to missing data, the total population in multiple regression with HOMA-IR consists of 2636 individuals.
Follow-up measurements
The examination included a self-administered questionnaire, blood sampling, and a physical examination with blood pressure measurement (OMRON M5–1 IntelliSense) after 5 min of supine rest. Blood samples were taken after an overnight fast and included a capillary plasma glucose (p-glucose) measurement with HemoCue (HemoCue AB, Ängelholm, Sweden) and for nondiabetic patients also an oral glucose tolerance test (OGTT) with repeated plasma glucose measurement 120 min after intake of 75 g of glucose (2-h glucose). A self-reported history of diabetes or ongoing pharmacological treatment against diabetes was defined as diagnosed diabetes. Fasting p-glucose at least 7.0 mmol/l or at least 12.2 mmol/l following OGTT at two separate visits without any previously diagnosed diabetes was defined as undiagnosed (new) diabetes. Smoking habits were divided into three categories: never smoking, former smoking, and current smoking which was defined as regular or occasional smoking. Physical activity level was divided into two categories: sedentary or moderate exercise and regular exercise or hard training.
Measurement of carotid–femoral pulse wave velocity
Carotid–femoral PWV was measured at follow-up only, over a wide time range, due to logistical arrangements and staff availability. On average 261 days after the physical examination and retrieval of blood samples, measurements of c-fPWV were performed using applanation tonometry (SphygmoCor, Atcor Medical, Australia) with patients in supine position after 5 min of rest. The distance was calculated as the suprasternal notch to the umbilicus and the umbilicus to the measuring point at the femoral artery minus the suprasternal notch to the measuring point at the carotid artery. With simultaneous ECG registration, the software calculates the time from the peak of the R-wave on ECG to the foot of the pulse wave at the carotid and femoral arteries, respectively. The number of successful measurements in each individual varied from one to five, with a goal of three measurements (86.7% of cases). Results are based on mean c-fPWV from these assessments. Mean number of measurements of c-fPWV for each individuals were three times and mean coefficient of variation between c-fPWV measurements was 6.3% (±SD 4.4). This examination also included two blood pressure measurements (OMRON M5–1 IntelliSense) after 5 min of supine rest immediately before c-fPWV measurement. We have used these blood pressure measurements throughout the study, except in Table 2, where, instead, blood pressure at follow-up physical examination is presented. Mean arterial pressure (MAP) was calculated as (2 × diastolic pressure + systolic pressure)/3. Hypertension was defined as a self-reported history of hypertension or ongoing blood pressure-lowering treatment, or SBP of at least 140 mmHg or DBP of at least 90 mmHg at c-fPWV measurement.
TABLE 2.
Comparison between characteristics at follow-up of individuals with or without successful carotid–femoral pulse wave velocity measurements
| Characteristics | c-fPWV data | No cfPWV data (n = 678) | |
| Included in multiple regression (n = 2679) | Not included in multiple regression (n = 377) | ||
| Age (years) | 72.1 (5.5) | 71.5 (5.3) | 74.3 (5.5)a |
| BMI (kg/m2) | 26.6 (4.2) | 27.5 (4.5)a | 27.6 (5.2)a |
| Waist circumference (cm) | 91.3 (11.9) | 95.2 (12.9)a | 95.5 (14.0)a |
| SBP (mmHg) | 143 (19) | 144 (18) | 144 (20) |
| DBP (mmHg) | 83 (10) | 83 (10) | 84 (12) |
| c-fPWV | 10.1 [8.8–11.8] | 10.4 [9.0–12.0] | n.a. |
| Fasting glucose (mmol/l) | 5.8 [5.4–6.4] | 5.9 [5.4–6.5] | 6.1 [5.5–5.7]a |
| Triglycerides (mmol/l) | 1.0 [0.7–1.3] | 1.0 [0.8–1.4]a | 1.0 [0.8–1.4]a |
| LDL cholesterol (mmol/l) | 3.34 (0.92) | 3.28 (1.00) | 3.10 (0.97)a |
| HDL cholesterol (mmol/l) | 1.44 (0.43) | 1.37 (0.43) | 1.38 (0.43)a |
| Men [n (%)] | 1007 (37.6) | 201 (53.3)a | 314 (46.3)a |
| Diagnosed diabetes [n (%)] | 240 (9.0) | 60 (15.9)a | 96 (14.2)a |
| Ever smoking [n (%)] | 1443 (53.9) | 178 (47.2) | 348 (51.3) |
| Lipid-lowering drug therapy | 773 (28.9) | 122 (32.4) | 234 (34.5)a |
| Blood pressure-lowering drug therapy | 1456 (54.3) | 199 (52.8) | 472 (69.6)a |
For continuous variables, normally distributed characteristics expressed with mean (±SD) and others expressed as median (first quartile–third quartile). c-fPWV, carotid–femoral pulse wave velocity; HDL, high-density lipoprotein; LDL, low-density lipoprotein; n.a., not applicable. Missing data up to 5.3 percentage of cases in some of the below listed variables.
aSignificant difference compared to group included in multiple regression (P < 0.01).
Statistical analysis
Statistical calculations were carried out using IBM SPSS Statistics, version 21 (IBM Corp., Armonk, New York, USA). The differences between characteristics of individuals without measurement of c-fPWV, individuals missing other data, and individuals included in multiple regression analysis were calculated with analysis of variance (ANOVA) for continuous variables, and in case of significant differences, were further analyzed with Tukey's post-hoc analysis. For categorical variables, chi-square test was used. The coefficient of variation between c-fPWV measurements for each individual was calculated. Multiple regression analysis was performed in two different models: model 1, adjusting for age, heart rate (HR), and MAP at the time of the c-fPWV measurement; and model 2, with further adjustments for sex, waist circumference, smoking, ongoing blood pressure-lowering drug, and lipid-lowering drug therapy. Individuals with missing data were excluded from the analysis. In multiple regression and ANOVA analysis, the natural logarithms of c-fPWV, fasting glucose, HOMA-IR, HbA1c, and triglycerides were used, to achieve normal distributions. Median c-fPWV in men and women were tested for statistical difference with Mann–Whitney U test. Values of c-fPWV between individuals with diagnosed diabetes, undiagnosed diabetes, and nondiabetic individuals, respectively, were tested for statistically significant differences with ANOVA, and in case of significant differences, were further analyzed with Tukey's post-hoc analysis.
We also performed a comparison of two polar groups, with c-fPWV at least 12 m/s and less than 8 m/s. This analysis was restricted to individuals aged below 75 years, since a very high proportion of the elderly have c-fPWV at least 12 m/s. The difference between the means was tested with analysis of covariance (ANCOVA) for continuous variables and logistic regression for categorical variables, after adjustments for age, sex, HR, and MAP. A P value less than 0.01 was considered significant.
RESULTS
Mean follow-up time from baseline examination to c-fPWV measurement was 16.9 years. The characteristics of the study population at baseline and follow-up are presented in Table 1. Characteristics of individuals with and without successful c-fPWV measurements are compared in Table 2. Individuals without available c-fPWV data were older, had a higher BMI, higher fasting glucose, and were more likely to be men and to have known diabetes than individuals with available c-fPWV data.
Median c-fPWV was 10.1 m/s, significantly (P < 0.001) higher in men than in women (10.4 vs. 9.9 m/s). Values of c-fPWV were positively skewed. Figure 2 shows c-fPWV stratified for different age groups and sex.
FIGURE 2.
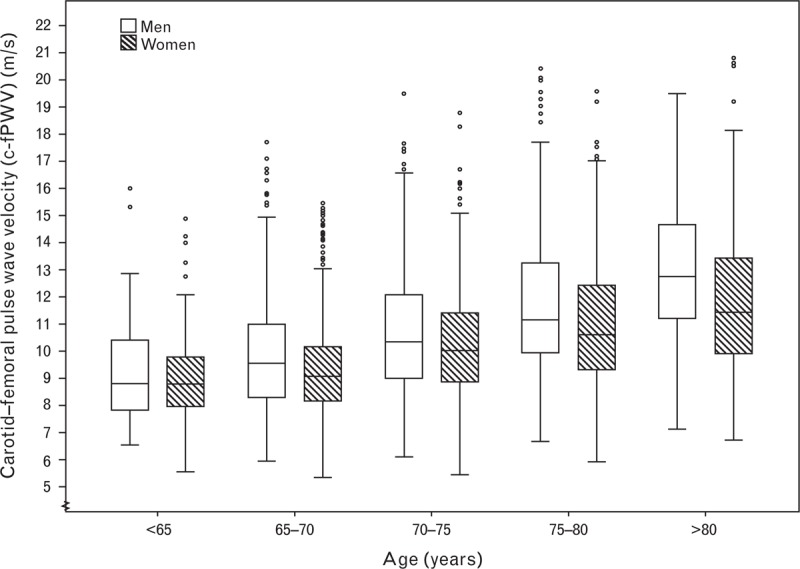
Box-plot of c-fPWV in different age groups, organized by sex. Boxes contain 50% of observations bars contain the remainder. Horizontal lines indicate median value; circles and asterisk indicate outliers and extreme outliers, respectively. c-fPWV, carotid–femoral pulse wave velocity.
Baseline predictive analyses of arterial stiffness at follow-up
Results from multiple regression are presented in Table 3. When adjusting for cardiovascular risk factors (model 2), c-fPWV was significantly and positively associated with the following baseline variables: age (β = 0.38, P < 0.001), male sex (β = 0.10, P < 0.001), BMI (β = 0.08, P < 0.001), waist circumference (β = 0.17, P < 0.001), SBP (β = 0.11, P < 0.001), pulse pressure (β = 0.13, P < 0.001), fasting glucose (β = 0.13, P < 0.001), HOMA-IR (β = 0.10, P < 0.001), HbA1c (β = 0.06, P < 0.001), and triglycerides (β = 0.10, P < 0.001), but negatively associated with HDL cholesterol (β = −0.08, P < 0.001). Except for BMI in men and HbA1c in women, all associations were significant for both sexes when analyzed separately. There was no prediction of c-fPWV from baseline eGFR, LDL cholesterol levels, or smoking after adjustment for the cardiovascular risk factors in model 2. Figure 3 displays mean c-fPWV in different decentiles of fasting glucose, HOMA-IR, and HbA1c.
TABLE 3.
Multiple linear regression analysis for baseline and follow-up determinants of the dependent variable, ln carotid–femoral pulse wave velocity
| Determinant | Model 1 | Model 2 | ||||||
| Both sexes | Both sexes | Men | Women | |||||
| β | P | β | P | β | P | β | P | |
| Baseline | ||||||||
| Age | 0.40 | <0.001 | 0.38 | <0.001 | 0.42 | <0.001 | 0.36 | <0.001 |
| Male sex | 0.11 | <0.001 | 0.10 | <0.001 | ||||
| SBP | 0.15 | <0.001 | 0.11 | <0.001 | 0.13 | <0.001 | 0.11 | <0.001 |
| DBP | 0.08 | <0.001 | 0.02 | NS | 0.05 | NS | 0.01 | NS |
| PP | 0.15 | <0.001 | 0.13 | <0.001 | 0.13 | <0.001 | 0.13 | <0.001 |
| BMI | 0.10 | <0.001 | 0.08 | <0.001 | 0.07 | 0.011 | 0.10 | <0.001 |
| Waist circumference | 0.18 | <0.001 | 0.17 | <0.001 | 0.12 | <0.001 | 0.14 | <0.001 |
| Ln fasting glucose | 0.17 | <0.001 | 0.13 | <0.001 | 0.14 | <0.001 | 0.12 | <0.001 |
| Ln HbA1c | 0.07 | <0.001 | 0.06 | <0.001 | 0.08 | 0.002 | 0.04 | 0.048 |
| Ln HOMA-IR | 0.14 | <0.001 | 0.10 | <0.001 | 0.10 | 0.001 | 0.10 | <0.001 |
| Ln triglycerides | 0.14 | <0.001 | 0.10 | <0.001 | 0.11 | <0.001 | 0.09 | <0.001 |
| LDL cholesterol | 0.04 | NS | 0.03 | NS | 0.04 | NS | 0.03 | NS |
| HDL cholesterol | −0.14 | <0.001 | −0.08 | <0.001 | −0.07 | 0.005 | −0.08 | <0.001 |
| eGFR | 0.05 | 0.006 | 0.03 | NS | 0.05 | NS | 0.02 | NS |
| Current smoking | 0.01 | NS | 0.02 | NS | 0.02 | NS | 0.01 | NS |
| Former smoking | 0.04 | 0.02 | 0.02 | NS | 0.03 | NS | 0.01 | NS |
| Follow-up | ||||||||
| Age | 0.40 | <0.001 | 0.39 | <0.001 | 0.43 | <0.001 | 0.37 | <0.001 |
| Male sex | 0.12 | <0.001 | 0.11 | <0.001 | ||||
| BMI | 0.11 | <0.001 | 0.08 | <0.001 | 0.07 | 0.008 | 0.10 | <0.001 |
| Waist circumference | 0.18 | <0.001 | 0.14 | <0.001 | 0.11 | <0.001 | 0.13 | <0.001 |
| Ln fasting glucose | 0.15 | <0.001 | 0.09 | <0.001 | 0.10 | <0.001 | 0.09 | <0.001 |
| Ln triglycerides | 0.13 | <0.001 | 0.10 | <0.001 | 0.09 | 0.001 | 0.11 | <0.001 |
| LDL cholesterol | −0.10 | <0.001 | −0.02 | NS | −0.04 | NS | 0.00 | NS |
| HDL cholesterol | −0.15 | <0.001 | −0.08 | <0.001 | −0.08 | 0.003 | −0.08 | 0.001 |
| eGFR | −0.02 | NS | 0.00 | NS | −0.01 | NS | 0.01 | NS |
| Current smoking | −0.01 | NS | 0.00 | NS | −0.01 | NS | 0.00 | NS |
| Former smoking | 0.0 | 0.017 | 0.01 | NS | 0.03 | NS | −0.01 | NS |
| MAP | 0.31 | <0.001 | 0.29 | <0.001 | 0.31 | <0.001 | 0.28 | <0.001 |
| HR | 0.18 | <0.001 | 0.20 | <0.001 | 0.24 | <0.001 | 0.18 | <0.001 |
eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; HOMA-IR, Homeostatic Model Assessment – Insulin Resistance; HR, heart rate; LDL, low-density lipoprotein; ln, natural logarithm; MAP, mean arterial pressure; PP, pulse pressure. Model 1: The model includes age, MAP, and HR. The rest of the determinants were individually entered into the model together with covariates included in model 1. Model 2: The model includes age, sex, BMI, current smoking, ongoing blood pressure-lowering drug therapy, ongoing lipid-lowering drug therapy, HR, and MAP. Fasting glucose, HbA1c, HOMA-IR, triglycerides, HDL, LDL, and eGFR were individually entered into the model together with covariates included in model 2. When waist circumference was entered in model 2, BMI was excluded from the model. All β-coefficients are standardized.
FIGURE 3.
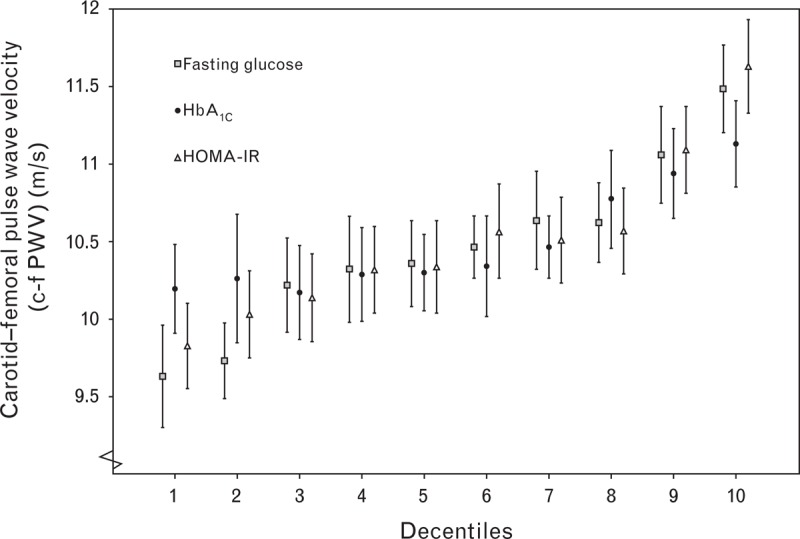
Mean and 95% confidence interval of c-fPWV in different decentiles of fasting glucose, HOMA-IR and HbA1c. c-fPWV, carotid–femoral pulse wave velocity; HOMA-IR, Homeostatic Model Assessment – Insulin Resistance.
Cross-sectional associations with arterial stiffness
The cross-sectional associations are presented in Table 3. c-fPWV was associated with 2-h glucose (β = 0.13, P < 0.001), MAP (β = 0.29, P < 0.001), and HR (β = 0.20, P < 0.001) in model 2. The same variables that were predictive of c-fPWV were also associated with c-fPWV in the cross-sectional analysis except for in model 1, in which a negative association was found for LDL (β = −0.10, P < 0.001) and no association was found for eGFR. When excluding individuals with ongoing lipid-lowering drug therapy at follow-up, there were no significant associations between c-fPWV and LDL cholesterol. The associations between HDL cholesterol and triglycerides, however, remained after this exclusion in model 1 (β = −0.14, P < 0.001; and β = 0.11, P < 0.001, respectively) and in model 2 (β = −0.08, P < 0.001; and β = 0.09, P < 0.001, respectively). Median c-fPWV for individuals without diabetes was 10.0 m/s, with undiagnosed diabetes was 11.7 m/s, and with diagnosed diabetes was 11.1 m/s. These differences were statistically significant when comparing individuals without diabetes with known or newly detected diabetes, but not between the two diabetes groups (Fig. 4).
FIGURE 4.
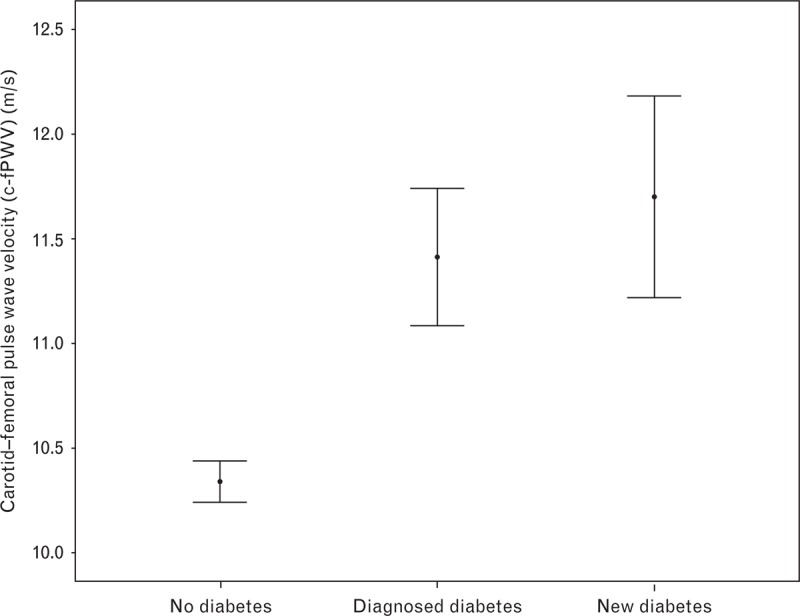
Mean and 95% confidence interval of c-fPWV in different diabetes status. c-fPWV, carotid–femoral pulse wave velocity.
Characteristics of individuals with high or low arterial stiffness
Table 4 shows a comparison of follow-up characteristics between individuals aged below 75 years with high (c-fPWV ≥12 m/s) or low (<8 m/s) arterial stiffness, respectively. After adjustment for age, sex, HR, and MAP, the group with high arterial stiffness (EVA) was characterized by significantly more prevalent diabetes and hypertension compared to the group with low arterial stiffness, whereas there was no difference in smoking habits or level of physical activity.
TABLE 4.
Comparison of follow-up variables for individuals aged below 75 years with high arterial stiffness (carotid–femoral pulse wave velocity ≥12 m/s) and low arterial stiffness (carotid–femoral pulse wave velocity <8 m/s), respectively, with means or percentages
| High arterial stiffness (n = 294) | Low arterial stiffness (n = 320) | |
| c-fPWV (m/s) | 13.8 | 7.3a |
| Age (years) | 70.1 | 67.5a |
| Men (%) | 52 | 37a |
| Diagnosed diabetes (%) | 20 | 3a |
| Diabetes [total (%)] | 31 | 8a |
| Current smoking (%) | 9 | 14 |
| Ever smoking (%) | 56 | 53 |
| Regular exercise/hard training (%) | 14 | 24 |
| Hypertension (%) | 84 | 43a |
Diabetes total: Individuals with diagnosed or undiagnosed (fasting glucose ≥7.0 mmol/l or 2 h glucose ≥12.2 mmol/l) diabetes. c-fPWV, carotid–femoral pulse wave velocity; MAP, mean arterial pressure.
aSignificant difference between groups (P < 0.01) after adjustment for age, sex, hazard ratio, and MAP.
DISCUSSION
This observational study shows that waist circumference, fasting glucose, HOMA-IR, HbA1c, triglycerides, and HDL cholesterol are all predictors of arterial stiffness after a follow-up period of 17 years in a middle-aged population at baseline. The results also indicated higher c-fPWV values in men than in women, which correspond to previous findings [20].
Lipids and obesity
Our findings further show that both triglycerides and HDL cholesterol at both baseline and follow-up were associated with arterial stiffness after adjusting for cardiovascular risk factors including BMI, whereas LDL cholesterol was not. The negative cross-sectional association between LDL cholesterol and arterial stiffness seen before full adjustment was not seen in the sub-analysis of individuals without lipid-lowering treatment and is most likely caused by an over-representation of individuals with cardiovascular disease receiving treatment.
In a systematic review from 2009, low HDL cholesterol and increased triglycerides (dyslipidemia) were significantly associated with arterial stiffness in only four out of 37, and one out of 38 studies, respectively, although many of these studies were small or carried out in specific populations [5]. In general, our findings on lipids are similar to results from a study presenting longitudinal associations between arterial stiffness and waist circumference, HDL cholesterol, and triglycerides [12]. Measures of obesity both at baseline and follow-up are associated with arterial stiffness, where the association to waist circumference tends to be stronger than that to BMI, a previously reported pattern [21]. This indicates that abdominal obesity is the underlying factor behind these results, which in itself is a marker of metabolic changes and chronic inflammation based on other studies [22].
Diabetes and hyperglycemia
Our results show independent associations between baseline fasting glucose, HOMA-IR, and HbA1c in relation to c-fPWV. Fasting glucose and 2-h glucose at follow-up (cross-sectional) were also associated with arterial stiffness. Diabetes is well known to be associated with increased arterial stiffness, and some studies indicate that the impact of diabetes on the elastic arteries takes place early, already during a prediabetic state of insulin resistance [8,9]. This is supported by our results among individuals with no history of diabetes, showing that individuals with undiagnosed diabetes have stiffer arteries than individuals with fasting and 2-h glucose below the reference values for diabetes.
It has been debated how much hyperglycemia increases arterial stiffness directly via, for example, advanced glycosylated end (AGE) products causing cross-linking of collagen in the arterial intima-media and reduction of the elastin content, and how much can be attributed to more complex metabolic changes associated with hyperglycemia and insulin resistance [9,23]. Our data support the hypothesis that hyperglycemia, dyslipidemia, and abdominal obesity accelerates the vascular stiffening seen with increasing chronological age. The mechanisms behind this process are not clear, but insulin resistance might be involved in the pathogenesis. However, baseline HOMA-IR did not show a stronger association to arterial stiffness than fasting glucose. This does not support the hypothesis of insulin resistance as a driving force behind the arterial stiffening process, although HOMA-IR might be regarded as too crude a measure of insulin sensitivity.
Some studies have demonstrated that diabetes should have a higher impact on arterial stiffness in women than in men [9,24,25] – results that could not be confirmed in this study. Longitudinal studies with more precise measurements of glucose metabolism and insulin sensitivity (clamp) are thus needed to shed more light on the pathophysiological mechanisms behind these associations.
Glomerular filtration rate
There were no associations between baseline or follow-up eGFR levels and arterial stiffness after adjustment for other cardiovascular risk factors. Several studies have shown that CKD patients have an increased arterial stiffness, but results regarding the relationship between arterial stiffness and CKD severity have been conflicting [11,26]. In our community-based population, the eGFR is higher than in CKD patients and can, to a greater extent, be explained by age and other cardiovascular risk factors. Thus, there should be fewer individuals exhibiting the phenotype of arterial remodeling with calcifications associated with more severe CKD, which could explain the absence of association between eGFR and c-fPWV.
Smoking
Smoking is known to be an important contributor to atherosclerosis, but the possible role of smoking in arterial stiffening remains much more uncertain. Although some previous studies show an association between smoking and arterial stiffness [27,28], many studies do not [29]. Since nicotine increases HR, adjusting for HR and MAP at the time of the PWV measurement is essential for any analysis investigating this relationship. In this study, there was no association between current or ever-smoking and arterial stiffness. Even in the comparison between individuals with high or low arterial stiffness, the results show no tendency towards smoking being more prevalent among individuals with high arterial stiffness, which is a marker of EVA. This absence of association between smoking and arterial stiffness is, in our opinion, in line with the majority of studies on this subject.
Our interpretation of the findings in general is that the well known cluster of risk factors associated with ‘atherosclerosis’ (smoking, hypertension, LDL cholesterol, diabetes) is only partly associated with ‘arteriosclerosis’ (hypertension, dyslipidemia, hyperglycemia) [4]. As the development of arteriosclerosis is believed to precede the development of atherosclerosis, or at least run in parallel during early stages, our findings also point to the importance of nonhemodynamic metabolic factors for this development.
Strengths and limitations of the study
Strengths of this study include its large sample from a community-based population and the adjustment for hemodynamic factors (MAP, HR, ongoing drug pressure-lowering drug treatment). Its observational design with measurement of arterial stiffness at follow-up only prevents us from drawing firm conclusions regarding causality. Previous studies from MDC have shown that the cohort was fairly representative at the baseline examination, even though mortality rates were higher in nonparticipants [15]. The participant rates at the re-examination was high – about 70% of those who were alive and invited to the re-examination participated. However, as many of the participants from baseline investigation were not alive for the follow-up investigation, because of missing blood samples, and since measurement of c-fPWV cannot be performed in individuals with atrial fibrillation or other cardiac arrhythmias, the total drop-out from MDC baseline is 56% and it can be assumed that the final population was healthier than the general population. Even so, the associations between the risk markers and arterial stiffness (c-fPWV) found in this study should be valid also in the general population in a wider age span. Our risk markers do not, regretfully, include any marker of inflammation, which is unfortunate, as several inflammatory conditions are known to be associated with arterial stiffness [3]. However, inflammatory markers such as CRP are affected to a large degree by different pro-inflammatory conditions such as (abdominal) obesity, a phenotypic variable used in our analyses. Future studies will expand on the influence of chronic inflammation as well as genetic markers on arterial stiffness in our population.
In conclusion, results from this observational study show that hyperglycemia, dyslipidemia (high triglycerides, low HDL cholesterol), and waist circumference are all independent nonhemodynamic long-term predictors of arterial stiffness, following full adjustment, in both sexes. Smoking, LDL cholesterol, and eGFR were not associated with arterial stiffness.
ACKNOWLEDGEMENTS
The study was supported by the Medical Research Council of Sweden (grant K2011–65X-20752–04–6), the Region Skåne County Council, the Ernhold Lundstrom Foundation, and so on.
Conflicts of interest
There are no conflicts of interest.
Reviewers’ summary evaluations
Reviewer 1
The nonhemodynamic cluster of risk factors and predictors of arterial stiffness in a middle-aged population included abdominal obesity, hyperglycemia and dyslipidemia but not smoking and LDL cholesterol. This pattern existed in both sexes. The strength of the article is that it is a large study from a community-based population. The experimental design is observational with measurement of arterial stiffness at follow-up only which prevents firm conclusions regarding causality. The weakness of the study is the high-drop out rate.
Reviewer 2
A strength of this study is the recruitment of a large sample from a community-based population. In addition, the follow-up period is long. A limitation is the fact that complete data were obtained from only 44% of the baseline population. From the data presented in the paper, one may conclude that the subjects not included in the regression analyses had more risk factors than the general population. Therefore, the absence of correlations between risk markers and arterial stiffness should be interpreted with caution.
Footnotes
Abbreviations: c-fPWV, carotid–femoral pulse wave velocity; eGFR, estimated glomerular filtration rate; EVA, early vascular ageing; HOMA-IR, Homeostatic Model Assessment – Insulin Resistance; OGTT, oral glucose tolerance test; PP, pulse pressure
REFERENCES
- 1.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010; 55:1318–1327. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17 635 subjects. J Am Coll Cardiol 2014; 63:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert concencus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006; 27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 4.O’Rourke MF, Safar ME, Dzau V. The Cardiovascular Continuum extended: aging effects on the aorta and microvasculature. Vasc Med 2010; 15:461–468. [DOI] [PubMed] [Google Scholar]
- 5.Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension 2009; 54:1328–1336. [DOI] [PubMed] [Google Scholar]
- 6.AlGhatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, et al. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension 2013; 62:934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nilsson PM, Boutouyrie P, Cunha P, Kotsis V, Narkiewicz K, Parati G, et al. Early vascular ageing in translation: from laboratory investigations to clinical applications in cardiovascular prevention. J Hypertens 2013; 31:1517–1526. [DOI] [PubMed] [Google Scholar]
- 8.Webb DR, Khunti K, Silverman R, Gray LJ, Srinivasan B, Lacy PS, et al. Impact of metabolic indices on central artery stiffness: independent association of insulin resistance and glucose with aortic pulse wave velocity. Diabetologia 2010; 53:1190–1198. [DOI] [PubMed] [Google Scholar]
- 9.Stehouwer CD, Henry RM, Ferreira I. Arterial stiffness in diabetes and the metabolic syndrome: a pathway to cardiovascular disease. Diabetologia 2008; 51:527–539. [DOI] [PubMed] [Google Scholar]
- 10.Scuteri A, Cunha PG, Rosei EA, Badariere J, Bekaert S, Cockcroft JR, et al. Arterial stiffness and influences of the metabolic syndrome: a cross-countries study. Atherosclerosis 2014; 233:654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briet M, Pierre B, Laurent S, London GM. Arterial stiffness and pulse pressure in CKD and ESRD. Kidney Int 2012; 82:388–400. [DOI] [PubMed] [Google Scholar]
- 12.Johansen NB, Vistisen D, Brunner EJ, Tabak AG, Shipley MJ, Wilkinson IB, et al. Determinants of aortic stiffness: 16-year follow-up of the Whitehall II study. PLoS One 2012; 7:e37165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McEniery CM, Spratt M, Munnery M, Yarnell J, Lowe GD, Rumley A, et al. An analysis of prospective risk factors for aortic stiffness in men: 20-year follow-up from the Caerphilly prospective study. Hypertension 2010; 56:36–43. [DOI] [PubMed] [Google Scholar]
- 14.Berglund G, Elmstähl S, Janzon L, Larsson SA. The Malmö Diet and Cancer study. Design and feasibility. J Intern Med 1993; 233:45–51. [DOI] [PubMed] [Google Scholar]
- 15.Manjer J, Carlsson S, Elmstahl S, Gullberg B, Janzon L, Lindstrom M, et al. The Malmo Diet and Cancer Study: representativity, cancer incidence and mortality in participants and nonparticipants. Eur J Cancer Prev 2001; 10:489–499. [DOI] [PubMed] [Google Scholar]
- 16.Hedblad B, Nilsson P, Janzon L, Berglund G. Relation between insulin resistance and carotid intima-media thickness and stenosis in nondiabetic subjects. Results from a cross-sectional study in Malmö, Sweden. Diabet Med 2000; 17:299–307. [DOI] [PubMed] [Google Scholar]
- 17.Svensson-Farbom P, Ohlson Andersson M, Almgren P, Hedblad B, Engstrom G, Persson M, et al. Cystatin C identifies cardiovascular risk better than creatinine-based estimates of glomerular filtration in middle-aged individuals without a history of cardiovascular disease. J Intern Med 2014; 275:506–521. [DOI] [PubMed] [Google Scholar]
- 18.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012; 367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28:412–419. [DOI] [PubMed] [Google Scholar]
- 20.Reference Values for Arterial Stiffness C. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J 2010; 31:2338–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutton-Tyrrell K, Newman A, Simonsick EM, Havlik R, Pahor M, Lakatta E, et al. Aortic stiffness is associated with visceral adiposity in older adults enrolled in the study of health, aging, and body composition. Hypertension 2001; 38:429–433. [DOI] [PubMed] [Google Scholar]
- 22.Fuentes E, Fuentes F, Vilahur G, Badimon L, Palomo I. Mechanisms of chronic state of inflammation as mediators that link obese adipose tissue and metabolic syndrome. Mediators Inflamm 2013; 2013:136584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sell DR, Monnier VM. Molecular basis of arterial stiffening: role of glycation: a mini-review. Gerontology 2012; 58:227–237. [DOI] [PubMed] [Google Scholar]
- 24.De Angelis L, Millasseau SC, Smith A, Viberti G, Jones RH, Ritter JM, et al. Sex differences in age-related stiffening of the aorta in subjects with type 2 diabetes. Hypertension 2004; 44:67–71. [DOI] [PubMed] [Google Scholar]
- 25.Gottsater M, Lanne T, Nilsson PM. Predictive markers of abdominal aortic stiffness measured by echo-tracking in subjects with varying insulin sensitivity. J Hum Hypertens 2014; 28:456–460. [DOI] [PubMed] [Google Scholar]
- 26.Hermans MM, Henry R, Dekker JM, Kooman JP, Kostense PJ, Nijpels G, et al. Estimated glomerular filtration rate and urinary albumin excretion are independently associated with greater arterial stiffness: the Hoorn Study. J Am Soc Nephrol 2007; 18:1942–1952. [DOI] [PubMed] [Google Scholar]
- 27.Jatoi NA, Jerrard-Dunne P, Feely J, Mahmud A. Impact of smoking and smoking cessation on arterial stiffness and aortic wave reflection in hypertension. Hypertension 2007; 49:981–985. [DOI] [PubMed] [Google Scholar]
- 28.van de Laar RJ, Stehouwer CD, Boreham CA, Murray LM, Schalkwijk CG, Prins MH, et al. Continuing smoking between adolescence and young adulthood is associated with higher arterial stiffness in young adults: the Northern Ireland Young Hearts Project. J Hypertens 2011; 29:2201–2209. [DOI] [PubMed] [Google Scholar]
- 29.Doonan RJ, Hausvater A, Scallan C, Mikhailidis DP, Pilote L, Daskalopoulou SS. The effect of smoking on arterial stiffness. Hypertens Res 2010; 33:398–410. [DOI] [PubMed] [Google Scholar]


