Abstract
Objective
Delayed gastric emptying (DGE) in patients with acute pancreatitis (AP) can be caused by gastroparesis or gastric outlet obstruction, which may occur when pancreatic pseudocyst (PP) or walled-off necrosis (WON) compresses the stomach. The aim of the study was to explore a proper surgical treatment.
Methods
From June 2010 to June 2013, 25 of 148 patients with AP suffered DGE. Among them, 12 were caused by gastroparesis, 1 was a result of obstruction from a Candida albicans plug, and 12 were gastric outlet obstruction (GOO) compressed by PP (n = 8) or WON (n = 4), which were treated by percutaneous catheter drainage (PCD).
Results
All 12 cases of compressing GOO achieved resolution by PCD after 6 [1.86] and 37.25 [12.02] days for PP and WON, respectively. Five cases developed intracystic infection, 3 cases had pancreatic fistulae whereas 2 achieved resolution and 1 underwent a pseudocyst jejunostomy.
Conclusions
Gastric outlet obstruction caused by a PP or WON is a major cause of DGE in patients with AP. Percutaneous catheter drainage with multiple sites, large-bore tubing, and lavage may be a good therapy due to high safety and minimal invasiveness.
Key Words: acute pancreatitis, gastric outlet obstruction, percutaneous catheter drainage, walled-off necrosis, pancreatic pseudocyst
Delayed gastric emptying (DGE) is a common complication in patients with acute pancreatitis (AP).1–7 Its early signs are often subtle because of non-peros and may only be observed by increased flow during gastric decompression. Later manifestations include postprandial abdominal fullness, nausea, and vomiting. Many scholars consider DGE an equivalent as gastroparesis8,9 and can be relieved by controlling primary diseases, gastrokinetic agents, and antiemetic agents.10 Some patients, however, may have persistent symptoms despite optimal treatment. Computed tomography (CT) scan often reveals pancreatic pseudocyst (PP) or walled-off necrosis (WON) in these patients.11,12 Gastroscopy examination may indicate an exogenous compression, leading to gastric outlet obstruction (GOO). According to the revision (2012) of the Atlanta Classification of AP,11 WON is defined as necrotic tissue contained within an enhancing wall of reactive tissue. Very few cases of PP-caused GOO are available at the moment.3–5,13 Furthermore, the incidence of GOO as a result of PP or WON and its differential diagnosis with gastroparesis have not been clearly demonstrated.
There is still no consensus on whether surgical drainage is required for AP-induced PP and WON without intraluminal infection. However, given the facts that persistent GOO leads to malnutrition and patients’ condition deteriorates once intraluminal infections occur, most scholars agree that surgical drainage is an effective treatment.13,14 Because of the difficulties in draining WON, the indications for multiple site drainage, large-bore catheters, and surgical repair have not been well characterized. Moreover, clinical studies with larger sample size to investigate AP with secondary compressing GOO have not been conducted. On the basis of the observations of 148 patients with AP, we studied the incidence of compressing GOO, the clinical course, and therapeutic effects, to raise the awareness for this complication. The effectiveness of several different types of percutaneous catheter drainage (PCD) has been explored as well.
MATERIALS AND METHODS
Between June 2010 and June 2013, a total of 148 patients with AP who have been admitted to the intensive care unit were included in the study; among them, 25 patients developed DGE. Twelve cases were successfully treated with intraintestinal nutrition, gastrokinetic agents, and antiemetic agents and were later diagnosed with secondary gastroparesis. Twelve cases failed to improve after the previously mentioned treatments; therefore, abdominal contrast-enhanced CT (CECT) scan, upper gastrointestinal radiography (GI) endoscopy, and upper GI series were performed. The results suggested that compressing GOO was caused by 8 cases of PP (Fig. 1) and 4 cases of WON (Fig. 2). One case was found to be caused by a Candida albicans plug and viscous gastric fluid.
FIGURE 1.
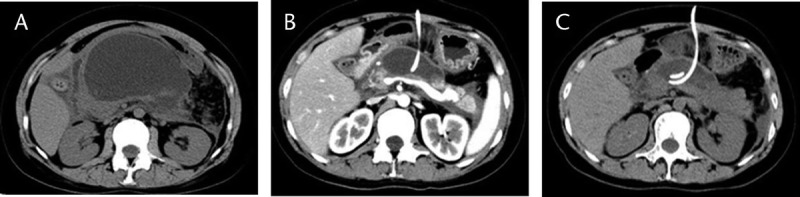
The CT images of the onset and resolution of a GOO case caused by PP. A, 25 days after the onset of AP, a massive PP compressed the stomach, causing a GOO. B, 1 week after PCD, significant reduction in the size of the pseudocyst and relief of obstructive symptoms were achieved. C, 3 weeks after PCD, full resolution of obstructive symptoms with normal food intake. No other complications were observed.
FIGURE 2.
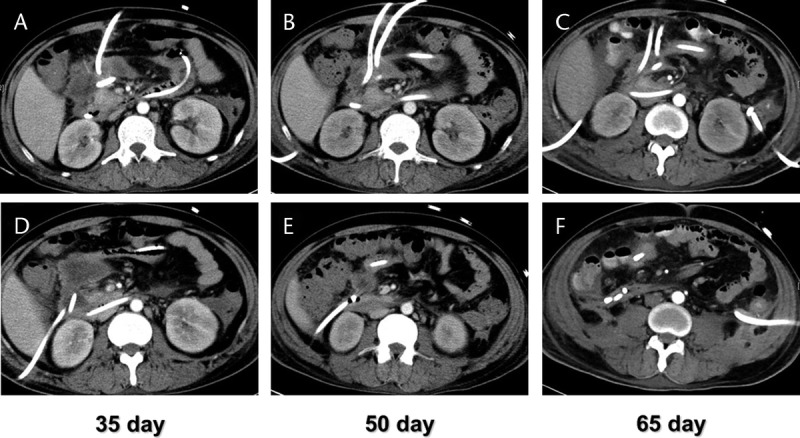
A DGE case caused by AP with WON was treated with multisite drainage. A and D, DGE occurred 35 days after the onset of AP. The CECT indicated gastric outlet was compressed by WON. Double-site PCD was placed at the head of the pancreas. B and E, 50 days after the onset, symptoms of DGE have not improved. The third PCD catheter was placed at the head of the pancreas accompanied by intermittent lavage. C and F, 65 days after the onset, DGE symptoms improved and the size of WON has significantly reduced.
Diagnosis Criteria for DGE, Gastroparesis, and Compressing GOO
Delayed Gastric Emptying
The following are the criteria for DGE: repeated postprandial abdominal fullness, gastroesophageal reflux, nausea, and vomiting; drainage with a nasogastric tube with greater than 800 mL/d or longer than 7 days15–17; contrast fails to pass through the pylorus during an upper GI series; with no obvious water-electrolyte imbalance or acid-base imbalance; no significant underlying diseases such as diabetes or connective tissue diseases; and no recent use of medications affecting smooth muscle activity.
Gastroparesis
Mechanical compression is ruled out by CT and upper GI endoscopy; symptomatic relief is achieved by the use of gastrokinetic agents and antiemetic agents.
Compressing GOO
Delayed gastric emptying symptoms are not relieved by gastrokinetic agents or antiemetic agents; CT and upper GI endoscopy reveal adjacent enlargements such as PP or WON.
Therapy
Twenty-five DGE patients all received endoscopic placement of a jejunal feeding tube, gastrokinetic agents, and antiemetic agents. The catheter tip was 20 to 30 cm beyond the ligament of Treiz to ensure proper delivery. Twelve cases with compressing GOO undergone ultrasound-guided PCD (Figs. 1B, C; 2B–D; 3). Multiple site drainage and/or lavage were chosen for some cases depending on the outcome of drainage. One case of fungal gastritis received antifungal therapy.
FIGURE 3.
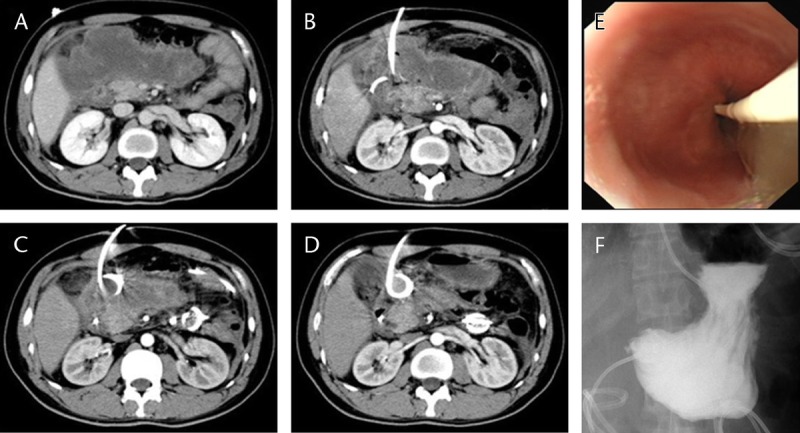
The management of a GOO case caused by WON. A, DGE occurred 6 weeks after the onset of AP. The CECT showed a compressed gastric outlet as a result of a nearby area of WON. B, 1 week after PCD with 1 catheter, WON started to shrink whereas DGE persisted. C, 5 weeks after PCD with 2 catheters plus lavage, WON was significantly reduced in size and DGE improved. D, 8 months after PCD with 2 catheters plus lavage, most of the WON disappeared. There was no recurrence of DGE. E, During the placement of a jejunal feeding tube through endoscopy, signs of compression were observed at the pylorus. F, Upper GI series after the placement of a jejunal feeding tube showed accumulation of contrast at the pylorus. Three hours later, only trace amounts of contrast entered the distal intestine, with most of the contrast left in the stomach.
Observation and Statistical Analysis
The diagnostic algorithm and management process of AP complicated with DGE are depicted in Figure 4. The severity, onset, therapeutic effects, and complications in these patients were evaluated, with emphasis on the incidence and PCD outcomes of compressing GOO caused by PP or WON. Time of onset and resolution in these patients were expressed as mean (SEM) and were analyzed with 1-way analysis of variance (SPSS 17.0). A P value of ≤0.05 is considered statistically significant.
FIGURE 4.
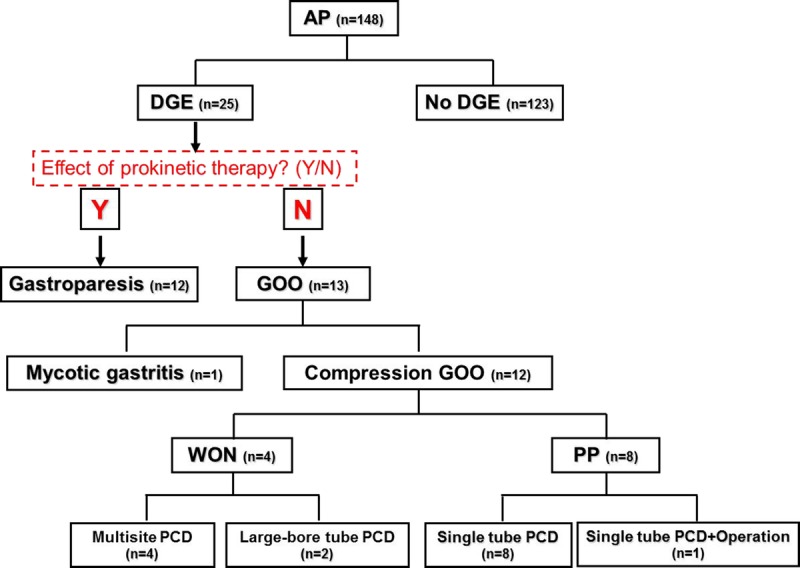
Diagnostic algorithm and management process of AP complicated by DGE.
RESULTS
The Relationship Between Onset of DGE and Severity of AP
In this study, severe AP (SAP) was confirmed according to the 2012 Atlanta Classification of AP. The percentage of SAP patients with DGE induced by gastroparesis (7/12) seemed higher than that caused by compressing GOO (3/12), suggesting more severe conditions in gastroparetic cases. This trend needs to be validated when more cases are added to the study to reach statistical significance. The percentage of SAP patients in DGE cases is higher than that in non-DGE cases (P = 0.024). Compared with patients with gastroparesis, the onset of DGE is later in compressing GOO (PP, P < 0.0001; WON, P = 0.002), and there is no difference between PP and WON (P = 0.2922, Table 1).
TABLE 1.
Clinical Scenarios of the Subjects With AP Complicated by DGE
Management of AP With DGE
Both groups with gastroparesis and compressing GOO achieved resolution by intraintestinal nutrition plus gastrokinetic agents and PCD, respectively. The PP-induced GOO patients treated with PCD had less time to resolve than WON cases, whereas there was no significant difference in the gastroparetic cases (P = 0.0542). The WON-induced GOO patients needed more time to recover when compared with the PP cases (P = 0.0043) and gastroparetic cases (P = 0.0124). Eight cases of PP were treated successfully by single catheter drainage, whereas WON cases required multisite drainage (4 cases, Fig. 2) or large-bore catheter drainage (2 cases, Fig. 5) due to the presence of intraluminal viscous necrotic tissue. See Figure 4 for the detailed management process, and one successful case was shown in Figure 3.
FIGURE 5.
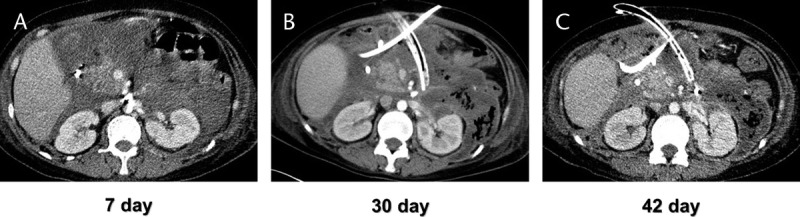
A DGE case caused by AP with WON was treated by lavage with large-bore catheter. A, 7 days after the onset of AP, the CECT showed significant amounts of fluid accumulated inside and around the pancreas. B, 30 days after the onset, both DGE and WON were seen. Two PCD catheters were placed and intermittent lavage was performed. One of the catheters was large bore. C, 42 days after the onset, DGE symptoms have been relieved, and WON at the head of the pancreas shrank considerably.
Complications
Five of 12 cases of compressing GOO developed secondary intraluminal infections, in which 2 cases were PP (25% of all PP cases) and 3 cases were WON (75% of all WON cases, Table 1). All cases were cured with intensified drainage or lavage. Three of 12 cases of compressing GOO (all were PP) had persistent pancreatic leakage (Table 1), in which 2 cases achieved spontaneous resolution and 1 case was cured by pseudocyst jejunostomy 16 months after the onset of AP (Fig. 6).
FIGURE 6.
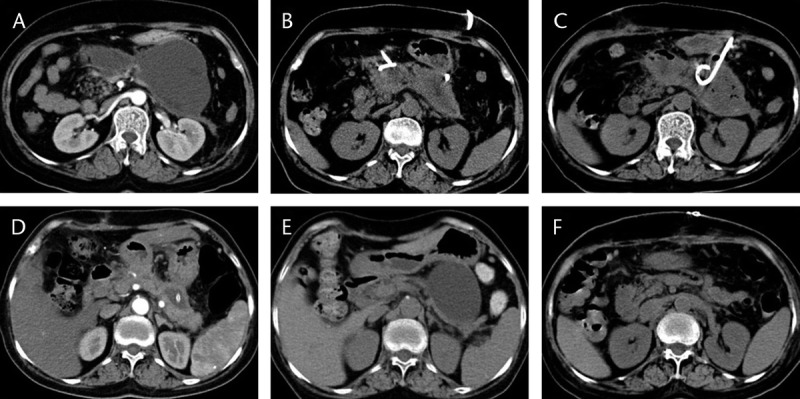
The management of a GOO case caused by a PP and complicated by pancreatic leakage. A, 4 weeks after the onset of AP, the patient developed DGE and the CECT showed GOO caused by a PP. B, 3 days into PCD treatment, both DGE symptoms and pseudocyst size improved significantly. C, 15 days into PCD treatment, a pseudocyst developed secondary infections and the CECT suggested intraluminal gas. D, 3 weeks after intensified drainage and lavage, intraluminal infection improved and cavities shrank. E, 16 months after the onset, the patients experienced back pain. The CECT showed re-enlargement of the PP. Increased amylase in the paracentesis fluid suggested pancreatic leakage. F, 1 week after pseudocyst jejunostomy, the pseudocyst disappeared.
DISCUSSION
Delayed gastric emptying is a common complication in patients with AP. Most DGE cases are functional. The current consensus on its etiology is that gastroparesis is the result of GI vagal dysfunction18 from inflammatory stress caused by AP, which leads to DGE.9,19 Therefore, SAP with extensive inflammation is more likely to cause gastroparesis, which was also found in this study. Some DGE cases are caused by nearby compression of the gastric outlet, such as from a PP (PP) or WON from local complications14 or, in rare cases, pylorus obstruction from excessive viscous gastric content. A case of DGE caused by severe mycotic gastritis-induced viscous gastric fluids was identified in our study. Because DGE cases from different causes may need different treatment regimens, early diagnosis is especially important in patients who have already suffered badly from pancreatitis. Specific signs, however, are hard to find in clinical settings among these patients, delaying the proper treatment.
Both PP and WON are complications of mid to late phase AP and are derived from acute peripancreatic fluid collections and acute necrotic collections, respectively.11,20 Key factors leading to compressing GOO include the vicinity (relative to the gastric outlet), tension, size, and duration. Tension can be increased when pancreatic leakage is present, where pancreatic fluids can flow into PP or WON induced by food ingestion. Our study shows that 20 to 30 days after the onset of AP is the period with the highest incidence of compressing GOO, much later than gastroparesis. Most patients have started oral food intake during that period; therefore, postprandial abdominal fullness, nausea, and vomiting are common symptoms. The 12 cases of compressing GOO all had these manifestations. The other 12 cases of gastroparesis had early DGE symptoms and were in more severe condition overall (7 were SAP), whereas all recovered with conservative treatment. Thus, the patients with SAP had an early onset of unexplained gastric fluid increase, the primary consideration for this is gastroparesis. When signs and symptoms of DGE are not improved after intensive treatment with gastrokinetic agents and intraintestinal nutrition, diagnosis of compressing GOO such as from a PP or WON needs to be explored with CECT, GI series, or upper gastric endoscopy.
It is commonly accepted that asymptomatic and noninfectious PPs or WON does not need interventional treatment.21 Once GOO appears active, measures including surgery may be necessary, because ineffective intraintestinal nutrition may cause malnutrition and the risk of aspiration pneumonia and intraluminal infection can lead to patients’ deterioration. This can lead to a prolonged hospital stay and to increased mortality. During our management of 25 DGE patients, intraintestinal nutrition was employed by the placement of jejunal feeding tubes before resolution,2,22 to meet patients’ energy requirements and restore the normal intestinal barrier function. We adopted a minimally invasive step-up approach23,24 for compressing GOO cases. The DGE symptoms in 12 GOO patients achieved resolution after ultrasound-guided PCD,25–27 followed by drainage and multisite lavage. Only 1 case required pseudocyst jejunostomy because of persistent pancreatic leakage. For GOO patients secondary to PP undergo PCD, symptoms disappear rapidly (6.0 [1.86] days), whereas the resolution of WON has a more prolonged course (37.25 [12.02] days). Compared with PP, the management of WON is more challenging.1,28 Invasive procedures are more difficult to perform in WON because of the presence of a thick-high-tension wall. Intraluminal semisolid necrotic tissue and viscous fluid significantly complicate the clearance efforts, even with intensified measures, such as multisite lavage and continuous drainage with large-bore catheter. Necrotic tissue and delayed resolution unavoidably lead to an increased intraluminal infection after invasive procedures. In this study, 3 of 4 WON cases had secondary intraluminal infections (75%), higher than that of seen in PPs (25%).
In summary, clinicians need to be aware of DGE in patients with AP and the differential diagnosis of compressing GOOs. Once GOO is confirmed, early and intensive treatments are required to minimize further complications. Percutaneous catheter drainage and lavage with multisite, large-bore catheters can be a minimally invasive and safe therapy for compressing GOO, especially WON. Delayed gastric emptying might be difficult to diagnose in some patients with SAP because of serious constitutional and local conditions. In these patients, more research needs to be conducted to aid in the early diagnosis and effective treatment of this condition.
Footnotes
The first two authors contributed equally to this study.
This study was supported by grants from the National Natural Science Foundation of China (No. 81471623, 81130007, 81270446, and 30801188), Key Program of International Cooperation of Science and Technology Department of Zhejiang Province, China (No. 2011C14028), and Natural Science Foundation of Zhejiang Province, China (No. Y2090443 and LY13H030001).
The authors declare no conflict of interest.
REFERENCES
- 1. Ross AS, Irani S, Gan SI, et al. Dual-modality drainage of infected and symptomatic walled-off pancreatic necrosis: long-term clinical outcomes. Gastrointest Endosc. 2014; 79: 929– 935. [DOI] [PubMed] [Google Scholar]
- 2. O’Keefe S, Rolniak S, Raina A, et al. Enteral feeding patients with gastric outlet obstruction. Nutr Clin Pract. 2012; 27: 76– 81. [DOI] [PubMed] [Google Scholar]
- 3. Rheingold OJ, Walker JA, Barkin JS. Gastric outlet obstruction due to a pancreatic pseudocyst: report of two cases. Am J Gastroenterol. 1978; 69: 92– 96. [PubMed] [Google Scholar]
- 4. Bawany MZ, Rafiq E, Ahmad S, et al. Endoscopic therapy for significant gastric outlet obstruction caused by a small pancreatic pseudocyst with a unique shape and location. J Interv Gastroenterol. 2012; 2: 196– 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aranha GV, Prinz RA, Greenlee HB, et al. Gastric outlet and duodenal obstruction from inflammatory pancreatic disease. Arch Surg. 1984; 119: 833– 835. [DOI] [PubMed] [Google Scholar]
- 6. Vitello JM. Gastric intramural pseudocyst with associated gastric outlet obstruction: recognition and management. South Med J. 1996; 89: 534– 537. [DOI] [PubMed] [Google Scholar]
- 7. Burke GW, Binder SC, Barron AM, et al. Heterotopic pancreas: gastric outlet obstruction secondary to pancreatitis and pancreatic pseudocyst. Am J Gastroenterol. 1989; 84: 52– 55. [PubMed] [Google Scholar]
- 8. Parkman HP, Hasler WL, Fisher RS, et al. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004; 127: 1592– 1622. [DOI] [PubMed] [Google Scholar]
- 9. Tang DM, Friedenberg FK. Gastroparesis: approach, diagnostic evaluation, and management. Dis Mon. 2011; 57: 74– 101. [DOI] [PubMed] [Google Scholar]
- 10. Camilleri M, Parkman HP, Shafi MA, et al. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013; 108: 18– 37; quiz 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013; 62: 102– 111. [DOI] [PubMed] [Google Scholar]
- 12. Ramia JM, de la Plaza R, Quiñones-Sampedro JE, et al. Walled-off pancreatic necrosis. Neth J Med. 2012; 70: 168– 171. [PubMed] [Google Scholar]
- 13. Cheruvu CV, Clarke MG, Prentice M, et al. Conservative treatment as an option in the management of pancreatic pseudocyst. Ann R Coll Surg Engl. 2003; 85: 313– 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gluck M, Ross A, Irani S, et al. Endoscopic and percutaneous drainage of symptomatic walled-off pancreatic necrosis reduces hospital stay and radiographic resources. Clin Gastroenterol Hepatol. 2010; 8: 1083– 1088. [DOI] [PubMed] [Google Scholar]
- 15. Horstmann O, Becker H, Post S, et al. Is delayed gastric emptying following pancreaticoduodenectomy related to pylorus preservation? Langenbecks Arch Surg. 1999; 384: 354– 359. [DOI] [PubMed] [Google Scholar]
- 16. Izbicki JR, Bloechle C, Broering DC, et al. Extended drainage versus resection in surgery for chronic pancreatitis: a prospective randomized trial comparing the longitudinal pancreaticojejunostomy combined with local pancreatic head excision with the pylorus-preserving pancreatoduodenectomy. Ann Surg. 1998; 228: 771– 779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Warshaw AL, Torchiana DL. Delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy. Surg Gynecol Obstet. 1985; 160: 1– 4. [PubMed] [Google Scholar]
- 18. Azpiroz F, Feinle-Bisset C, Grundy D, et al. Gastric sensitivity and reflexes: basic mechanisms underlying clinical problems. J Gastroenterol. 2014; 49: 206– 218. [DOI] [PubMed] [Google Scholar]
- 19. Shafi MA, Pasricha PJ. Post-surgical and obstructive gastroparesis. Curr Gastroenterol Rep. 2007; 9: 280– 285. [DOI] [PubMed] [Google Scholar]
- 20. Stamatakos M, Stefanaki C, Kontzoglou K, et al. Walled-off pancreatic necrosis. World J Gastroenterol. 2010; 16: 1707– 1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tenner S, Baillie J, DeWitt J, et al. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013; 108: 1400– 1415. [DOI] [PubMed] [Google Scholar]
- 22. Spanier BW, Bruno MJ, Mathus-Vliegen EM. Enteral nutrition and acute pancreatitis: a review. Gastroenterol Res Pract. 2011; 2011: 857949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Steinberg WM. A step-up approach, or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010; 363: 1286– 1287; author reply 1287. [DOI] [PubMed] [Google Scholar]
- 24. da Costa DW, Boerma D, van Santvoort HC, et al. Staged multidisciplinary step-up management for necrotizing pancreatitis. Br J Surg. 2014; 101: e65– e79. [DOI] [PubMed] [Google Scholar]
- 25. Tang LJ, Wang T, Cui JF, et al. Percutaneous catheter drainage in combination with choledochoscope-guided debridement in treatment of peripancreatic infection. World J Gastroenterol. 2010; 16: 513– 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Freeny PC, Hauptmann E, Althaus SJ, et al. Percutaneous CT-guided catheter drainage of infected acute necrotizing pancreatitis: techniques and results. AJR Am J Roentgenol. 1998; 170: 969– 975. [DOI] [PubMed] [Google Scholar]
- 27. Sleeman D, Levi DM, Cheung MC, et al. Percutaneous lavage as primary treatment for infected pancreatic necrosis. J Am Coll Surg. 2011; 212: 748– 752. [DOI] [PubMed] [Google Scholar]
- 28. Fischer A, Schrag HJ, Keck T, et al. Debridement and drainage of walled-off pancreatic necrosis by a novel laparoendoscopic rendezvous maneuver experience with 6 cases. Gastrointest Endosc. 2008; 67: 871– 878. [DOI] [PubMed] [Google Scholar]


