Abstract
Purpose of review
To discuss recent strategies for boosting the efficacy of noninvasive transcranial brain stimulation to improve human brain function.
Recent findings
Recent research exposed substantial intra- and inter-individual variability in response to plasticity-inducing transcranial brain stimulation. Trait-related and state-related determinants contribute to this variability, challenging the standard approach to apply stimulation in a rigid, one-size-fits-all fashion. Several strategies have been identified to reduce variability and maximize the plasticity-inducing effects of noninvasive transcranial brain stimulation. Priming interventions or paired associative stimulation can be used to ‘standardize’ the brain-state and hereby, homogenize the group response to stimulation. Neuroanatomical and neurochemical profiling based on magnetic resonance imaging and spectroscopy can capture trait-related and state-related variability. Fluctuations in brain-states can be traced online with functional brain imaging and inform the timing or other settings of transcranial brain stimulation. State-informed open-loop stimulation is aligned to the expression of a predefined brain state, according to prespecified rules. In contrast, adaptive closed-loop stimulation dynamically adjusts stimulation settings based on the occurrence of stimulation-induced state changes.
Summary
Approaches that take into account trait-related and state-related determinants of stimulation-induced plasticity bear considerable potential to establish noninvasive transcranial brain stimulation as interventional therapeutic tool.
Keywords: brain plasticity, brain rhythms, closed-loop brain stimulation, noninvasive transcranial brain stimulation (NTBS), open-loop brain stimulation, state dependence
INTRODUCTION
A range of noninvasive transcranial brain stimulation (NTBS) techniques is widely used in neuroscience and clinical settings. Transcranial magnetic stimulation (TMS) relies on ‘inductive’ electrical stimulation of the brain via a strong time-varying electromagnetic field, while transcranial current stimulation (TCS) passes electrical currents directly through the skull to stimulate the brain. Depending on the type of current, TCS is called transcranial direct current stimulation (TDCS) or transcranial alternating current stimulation (TACS). Although TMS induces electrical currents that are sufficiently strong to induce action potentials in axonal structures close to the hemispherical surface, TDCS and TACS generate weaker electrical currents that produce slight shifts in the membrane potential, and hereby modulate intrinsic neural activity without directly inducing action potentials.
TMS, TDCS and TACS can influence brain activity beyond the stimulation period, although their biophysical properties differ substantially in terms of generated current strength, focality and temporal stimulation pattern [1,2]. The physiological after-effects of interventional NTBS are far from being understood, but there is consensus that NTBS can induce long term potentiation (LTP)-like and long term depression (LTD)-like effects [3], homeostatic-like plasticity [4], as well as cause lasting changes in effective connectivity at the brain network level [5]. The plasticity-inducing potential of NTBS attracted considerable clinical interest as a relatively safe and pain-free tool to improve brain function in various brain disorders and prompted substantial efforts in the last two decades to exploit the various NTBS techniques therapeutically [6]. Yet, therapeutic studies have only provided sufficient level-A evidence of definite efficacy for the antidepressant effect of high-frequency repetitive TMS (rTMS) of left dorsolateral prefrontal cortex and the analgesic effect of high-frequency rTMS of the primary motor cortex (M1) contralateral to the affected side in neuropathic pain [6,7]. Currently, a major obstacle for the broad therapeutic use of interventional NTBS is that most after-effects show strong inter-individual and intra-individual variability [8].
Box 1.
no caption available
‘VARIABILITY’ AS AN EMERGING THEME
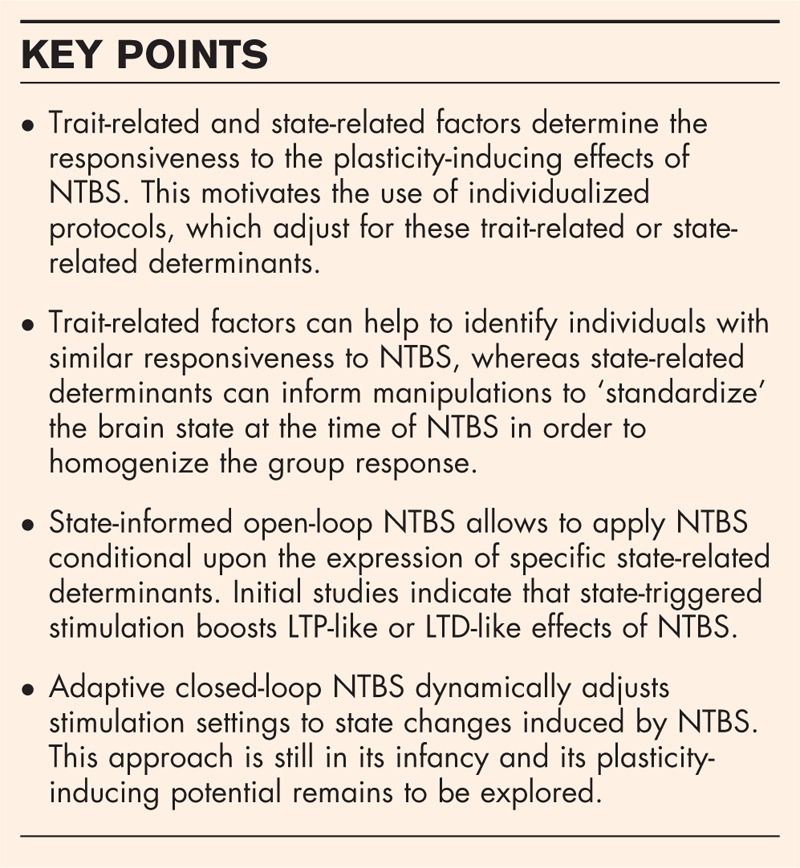
Interventional NTBS protocols are commonly applied in a rigid, ‘one-size-fits-all’ fashion (Fig. 1). With the exception of stimulus intensity in the case of TMS, all NTBS variables are usually preprogrammed and applied in an open-loop manner. The NTBS protocol is kept constant across individuals and throughout the course of stimulation. This rigid approach ignores both intra-individual and inter-individual variations in the neurobiology of the brain and may be the reason for the considerable variability of the brain response to current NTBS protocols [8]. Indeed, recent neurophysiological studies in relatively large cohorts of healthy individuals have consistently shown that inter-individual variability of NTBS after-effects is not the exception but the rule, and the size of inter-individual variability has been considerably underestimated in previous research. A number of studies on the plasticity-inducing effects of NTBS targeting area M1 have shown that the number of responders, showing the expected LTP-like or LTD-like effect can be lower than 50% with similar percentages showing reversed LTP-like or LTD-like effects [9–12].
FIGURE 1.
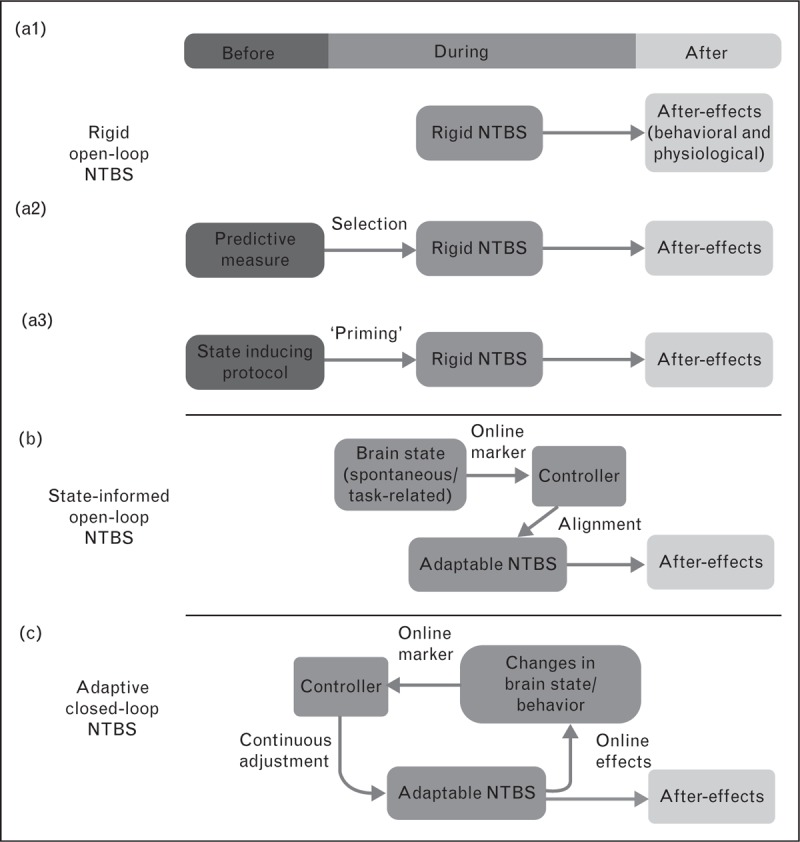
Towards closed-loop NTBS. (a1) Standard application of NTBS. The protocol is selected based on its known average impact on physiological or behavioral variables. It is applied stereotypically in all patients or participants. (a2) The best protocol among some alternatives is selected based on prior measurements of markers, which have been demonstrated to be predictive of individual outcome. (a3) A combination of protocols is used to stabilize outcome. Initially, a protocol is applied which is known to ‘set’ the brain in a state that renders it sensitive to the main NTBS protocol. (b) Markers of a preselected brain state are continuously read out and used to align the application of the NTBS protocol. Electroencephalography (EEG) band activity is a feasible marker with good temporal resolution. This approach cannot only be applied during rest, but also to align the NTBS protocol with task-related activity. (c) Full closed-loop application of an adaptable NTBS protocol. In this setting, neuroimaging (or another readout) is used to assess markers of the immediate effects of the NTBS protocol on brain activity. These markers are then used for on-the-fly adaptations of the protocol.
In good agreement with these results, another recent line of research showed that a wide range of neuroanatomical, neurochemical and neurophysiological factors could determine the after-effects of NTBS [13,14] (Fig. 2). Neuroanatomical determinants include cortical and skull thickness [15,16], differences in gyral shape and white matter structure [17,18]. Neurochemical determinants include factors like neurotransmitter availability and hormone levels [19–22]. Neurophysiological determinants involve attention state [23], activity history [4,24] and endogenous oscillatory patterns [25,26]. These determinants are not mutually independent, can modulate each other [27], and can be modified by other variables such as age [28] or genetic traits [29]. Some plasticity-determining factors exert relatively stable invariant effects on the individual responsiveness to NTBS, including demographic, genetic, and neuroanatomical characteristics, but also stable neurophysiological traits such as the individual alpha rhythm frequency. Because these trait-related determinants contribute to inter-individual variability, it may be possible to reduce inter-individual variability by preselecting participants or adjusting NTBS variables to the individually expressed ‘trait’. For instance, circadian fluctuations in the responsiveness to NTBS can be controlled by applying NTBS at a fixed time of the day [30]. Functional connectivity patterns have also been used to predict the therapeutic effect of TMS. Using resting-state MRI (rs-MRI), several groups reported that rs-connectivity of the subgenual cingulate cortex, measured days before therapy onset, predicted the therapeutic effect of a 5-week high-frequency rTMS intervention in major depressive disorder [30–33].
FIGURE 2.
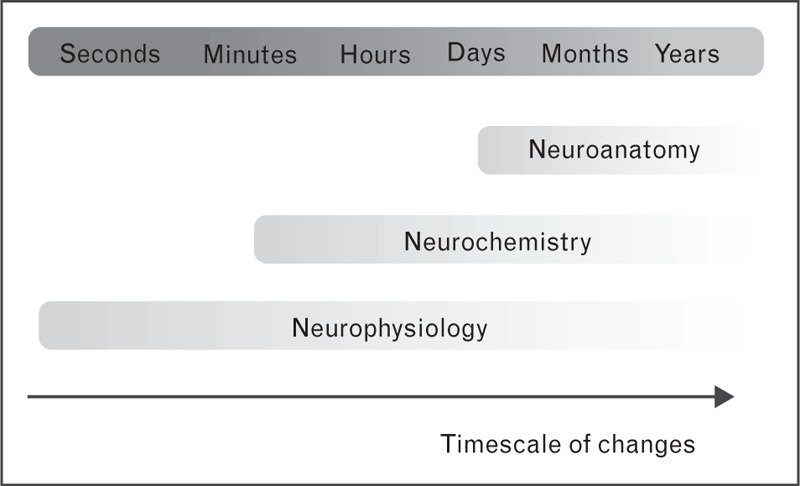
Timescales of changes in neuroanatomy, neurochemistry and neurophysiology determining the ability of NTBS to induce long-term potentiation (LTP)-like or long term depression (LTD)-like plasticity. Neuroanatomical changes on the microscopic level (such as myelination) and the macroscopic level (such as cortical thickness or folding pattern) are slow and it can be assumed that neuroanatomical features remain constant for the duration of a brain stimulation protocol. Neurochemical changes can be faster and can be influenced by the time of the day (which is an easily controllable factor), but also faster acting factors such as motivation. Neurochemical features can undergo changes during the administration of NTBS. Neurophysiological changes can occur on the sub-second time scale. For example, attentional changes or changes of the involvement of the stimulated area in the time course of a behavioral task can be very fast. They lend themselves best for the usage in online control settings based on central and peripheral markers of brain state. NTBS, noninvasive transcranial brain stimulation.
In contrast to ‘trait-related’ determinants, the modifying effects of other plasticity-determining factors are tightly linked to the physiological state expressed by the brain at the time of stimulation, such as the phase or amplitude of oscillatory brain activity [1]. These ‘state-related’ determinants are liable to rapid within-session and between-session changes over the course of the NTBS intervention and thus, contribute to both inter-individual and intra-individual variability in responsiveness to NTBS. Fast-fluctuating neurophysiological determinants have mainly been identified by post-hoc grouping of NTBS trials based on concurrently recorded electroencephalography (EEG) signals, providing correlational evidence for the importance of oscillatory phase and amplitude for NTBS outcome [34–41].
Previous work has often tried to deal with state-associated factors by using rigorous standardization, matching state-related factors like attention, time of day and preintervention motor-activity constant across individuals [13,42]. However, rigorous standardization of state-related determinants may not be applicable in a clinical setting.
Individually adjusted open-loop noninvasive transcranial brain stimulation
Stable trait-related factors or very slowly changing state-related factors that determine plasticity-inducing efficacy of NTBS can be used to individually adjust the variables of rigid NTBS protocols (Fig. 1). In this context, ‘open-loop NTBS’ covers all NTBS protocols consisting of a predefined set of stimulation variables that are kept constant during the administration of NTBS.
Most studies use neurophysiologic traits to individually adjust open-loop NTBS. For M1, the timing of intracortical facilitation can be probed within the sub-millisecond time range using paired-pulse TMS [43]. Adjusting the inter-pulse interval of repetitive TMS to the intrinsic rhythmicity of intracortical facilitation enhances the LTP-inducing capabilities of specific TMS protocols [44]. EEG can also readily provide subject-specific information about endogenous oscillatory patterns. Both repetitive TMS and TACS are often given at a stimulation rate of 10 Hz, a frequency close to the endogenous alpha rhythm (8–12 Hz). Several groups have boosted electrophysiological and behavioral effects when the frequency of rTMS or TACS was adjusted to the preferred individual alpha rhythm [45,46▪,47]. In clinical studies, attempts to specifically target the individual alpha frequency (IAF) yielded mixed results. In patients with schizophrenia, two studies compared the effectiveness of IAF-stimulation to rigid-state, 10 Hz stimulation and found a significantly greater therapeutic effect for IAF-stimulation [48,49], whereas two trials on treatment of major depression could not find a significant advantage of IAF-stimulation [50,51].
We are not aware of any study, which prospectively used neuroanatomical or neurochemical determinants to individually adjust the variables of rigid NTBS protocols. Profiling neuroanatomical and neurochemical factors often exceeds the scope of a short ‘calibration’ experiment. This hampers the integration of these factors into an individual adjustment routine. At least with respect to neuroanatomical profiling, individualization of NTBS is within reach in the foreseeable future. Macroscopic neuroanatomical features are readily revealed by neuroimaging. Additionally, software solutions become increasingly available which can use the neuroimaging data to model the impact of variations in the shape and volume of the targeted cortical gyrus on the distribution of the induced electrical fields in the cortical target and surrounding cortical areas [52,53].
State control of open-loop noninvasive transcranial brain stimulation
Preselection of individuals or individually adjusted rigid NTBS is not an option, if one wants to control the influence of state-related factors that vary on a time scale of minutes or shorter. One strategy to ‘homogenize’ the individual response to the NTBS intervention is to secure that all participants express a comparable neurophysiological or neurochemical state at the time of NTBS [4]. For instance, a priming intervention may be applied to achieve ‘state control’, including a preceding NTBS [54], pharmacological manipulation [55] or a preceding period of physical (in)activity [24] (Fig. 1).
Another mean of ‘standardizing’ the brain state at the time of stimulation is associative pairing. Associative pairing exploits Hebbian principles of spike-time dependent-like neural plasticity. Pairing electrical nerve stimulation with single-pulse TMS, paired associative stimulation (PAS), has long been used to induce spike-timing dependent-like plasticity in human M1 [56]. Recently, the concept of peripheral-cortical PAS was successfully transferred to other NTBS techniques by pairing brief trains of peripheral afferent stimulation with short bursts of high frequency (≥80 Hz) TACS over contralateral M1 [57▪]. In addition to peripheral-cortical PAS, dual-site cortico-cortical PAS has been used to induce spike-time dependent-like neural plasticity, for instance by associative pairing of TMS over the left and right hand area of M1 [58] or over pairing of TMS to parietal or frontal areas and M1 [59,60]. Associative cortico-cortical pairing of NTBS has also been demonstrated outside of M1. Polania et al.[61▪] showed that antiphasic pairing of frontal and parietal TACS influenced behavior in a value-based choice task, whereas in-phase pairing was not different from sham-stimulation. Whether in-phase dual-site TACS also can be used to induce Hebbian LTP-like after-effects in the stimulated areas and whether this is related to Hebbian principles of plasticity remains to be addressed in future studies. An innovative extension of the PAS approach was recently introduced in patients with Parkinson's disease, in which deep brain stimulation (DBS) of the subthalamic nucleus (STN) was paired with single-pulse TMS of M1 [62▪▪].
STATE-INFORMED OPEN-LOOP NONINVASIVE TRANSCRANIAL BRAIN STIMULATION
Another possibility to deal with time-varying state-related determinants of NTBS-induced plasticity is the ‘temporal neuronavigation’ of NTBS by adjusting the timing of NTBS to the expression of a given cortical state like the phase or power of cortical oscillatory activity (Fig. 1). This requires that endogenous fluctuations of relevant state-markers are continuously read out and are used to trigger NTBS in a feed-forward manner. The feasibility of activity-dependent stimulation has been illustrated in a proof-of-principle study [63] in which EEG-derived phase information was exploited to temporally navigate single TMS pulses into the up-state and down-state of slow-wave oscillations during non-rapid eye movement sleep. Using this state-informed NTBS approach, the authors showed that the motor evoked potential, triggered by TMS was significantly larger when the pulse was given in the up-phase than when the pulse was given in the down phase of slow-wave sleep oscillations. Although this study showed the feasibility of ‘on-demand’ state-informed NTBS, it remains to be clarified whether such state-triggered stimulation can more reliably induce LTP-like plasticity than state-naive NTBS.
An encouraging proof-of-concept study has demonstrated that state-triggered NTBS can induce LTP-like changes in corticospinal excitability [64▪]. In that study, on-line EEG triggered the administration of single-pulse TMS, which depended on task-related desynchronization of beta-band oscillatory activity during a motor imagery task. State-triggered single-pulse TMS induced a stable LTP-like increase in corticospinal excitability, whereas the same number and pattern of stimuli applied in a brain-state independent fashion decreased corticospinal excitability. Brain-state independent single-pulse TMS was given at rest in the absence of motor imagery. Since the effects of motor imagery alone were not assessed, it is unclear whether the LTP-like increase in corticospinal excitability was caused by state-triggered single-pulse TMS, by motor imagery, or the combination of motor imagery and state-triggered single-pulse TMS. Based on a single-case study in a patient with motor stroke, it was suggested that a similar approach might be used to increase M1 excitability during neurorehabilitation [65]. Fluctuating neural states might not only be useful to define the time point of NTBS (i.e., state-triggered NTBS). State-informed NTBS may also be used to adjust other NTBS variables (e.g., intensity) according to a prespecified rule that is based on the expressed brain state.
Adaptive, closed-loop noninvasive transcranial brain stimulation
Standardizing the state of the brain at the time of NTBS can reduce the intra-individual and inter-individual variability and induce associative Hebbian plasticity. Another way to exploit online ‘read-outs’ of the brain state is to monitor the functional impact of NTBS online during stimulation. These acute changes may be predictive of NTBS after-effects and yield important information that can be used to dynamically adjust the stimulation settings in order to maximize its plasticity-inducing potential in each individual (Fig. 1).
Adaptive closed-loop neurostimulation that dynamically reacts to the occurrence of distinct neural activity patterns has already made significant progress in recent years in the field of invasive brain stimulation using implanted devices [66,67]. For instance, DBS of the STN that is dynamically controlled by STN-beta power resulted in approximately 30% greater clinical improvements than standard open-loop DBS in a small group of patients with Parkinson's disease [68▪▪]. A ‘on demand’ closed-loop stimulation device may deliver stimulation more efficiently by limiting stimulation only when brain function is impaired or aberrant neural activity is present [69].
Although this experience motivates the use of adaptive, closed-loop approaches in the field of NTBS, this is still in its infancy. Therefore, the label ‘closed-loop’ should be used very carefully. Especially, the use of state-triggered NTBS that lacks an adaptive property based on the NTBS-induced state changes should not be labeled as ‘closed-loop’ NTBS [64▪]. A real ‘closed-loop’ approach requires that several major challenges are solved. First, a state marker has to be identified which reliably signals the dynamic changes induced by NTBS. That is, rather than merely detecting the occurrence of a brain state to adjust the temporal application of the NTBS protocol to, we are interested in reading out the ongoing changes in the brain state because of NTBS. It should be noted that stable change detection requires integration over longer time windows, so that adaptive closed-loop will be slower than mere brain-state detection. Second, it has to be demonstrated that changes in the state marker are truly predictive of NTBS-induced after-effects of interest such as LTP-like or LTD-like plasticity or specific functional improvement. Finally, it has to be demonstrated that the iterative modification of selected stimulation settings does efficiently influence the identified state marker in a controlled way.
A first proof-of-principle example for closed-loop NTBS used TACS to target M1 at the individual tremor frequency in patients with Parkinson's disease to suppress tremor amplitude [70▪▪]. The individual tremor frequency was taken as a peripheral proxy for endogenous oscillatory activity and the goal was to weaken these spontaneous oscillations by phase-cancellation with exogenously applied TACS oscillations. To this end, the phase of TACS was constantly adjusted, informed by the ongoing tremor activity, to maintain the optimal phase-delay between TACS and the endogenous tremor rhythm as determined from simultaneous actigraphy measures. Using this method, the authors achieved tremor suppression of around 50%. Although this study is very encouraging, the demonstration that plasticity-inducing after-effects of NTBS can be improved by closed-loop adjustment of the stimulation settings is still missing.
CONCLUSION
The substantial intra-individual and inter-individual variability in the responsiveness to NTBS has given rise to a critical reflection on the conventional approach to apply NTBS in an ‘open-loop’ one-size-fits-all fashion. Many trait-related or state-related factors determine this variability and prompted novel strategies to reduce variability and maximize the plasticity-inducing effects of NTBS. Trait-related determinants can be identified before NTBS and used for selection of individuals sharing a similar responsiveness to NTBS. State-related determinants can inform priming interventions or PAS protocols to ‘standardize’ the brain state at the time of NTBS. The relevance of distinct brain states in determining the ability to induce a strong LTP-like or LTD-like effect motivates the use of state-informed open-loop NTBS where administration of NTBS is conditional upon the expression of predefined brain states. Adaptive closed-loop NTBS that dynamically adjusts stimulation settings to the occurrence of an NTBS-induced state change is another promising option to exploit state-dependent determinants and eventually boost plasticity-inducing effects of NTBS. Both, informed open-loop NTBS as well as adaptive closed-loop NTBS, are still in their infancy, and it remains to be shown whether they outperform the plasticity-inducing properties of standard rigid open-loop approaches.
Acknowledgements
None.
Financial support and sponsorship
This work was supported by the Lundbeck Foundation (Grant of Excellence ‘ContAct’ R59 A5399) and the Novo Nordisk Foundation (Interdisciplinary Synergy Programme Grant ‘BASICS’ NNF14OC0011413).
Conflicts of interest
H.R.S. has served on a scientific advisory board for Lundbeck A/S, Valby Denmark, has received honoraria as speaker from Biogen Idec, Denmark A/S, Genzyme, Denmark and Merck Serono, Denmark, has received honoraria as editor from Elsevier Publishers, Amsterdam, The Netherlands and Springer Publishing, Stuttgart, Germany, has received travel support from MagVenture, Denmark, and has received a research fund from Biogen Idec. The remaining authors have no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Bergmann TO, Karabanov A, Hartwigsen G, et al. Combining non-invasive transcranial brain stimulation with neuroimaging and electrophysiology: current approaches and future perspectives. Neuroimage 2016; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 2.Dayan E, Censor N, Buch ER, et al. Noninvasive brain stimulation: from physiology to network dynamics and back. Nat Neurosci 2013; 16:838–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziemann U, Siebner HR. Modifying motor learning through gating and homeostatic metaplasticity. Brain Stimul 2008; 1:60–66. [DOI] [PubMed] [Google Scholar]
- 4.Karabanov A, Ziemann U, Hamada M, et al. Consensus paper: Probing homeostatic plasticity of human cortex with non-invasive transcranial brain stimulation. Brain Stimul 2015; 8:442–454. [DOI] [PubMed] [Google Scholar]
- 5.Herz DM, Christensen MS, Bruggemann N, et al. Motivational tuning of fronto-subthalamic connectivity facilitates control of action impulses. J Neurosci 2014; 34:3210–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lefaucheur JP, Andre-Obadia N, Antal A, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol 2014; 125:2150–2206. [DOI] [PubMed] [Google Scholar]
- 7.George MS, Taylor JJ, Short EB. The expanding evidence base for rTMS treatment of depression. Curr Opin Psychiatry 2013; 26:13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ziemann U, Siebner HR. Inter-subject and inter-session variability of plasticity induction by non-invasive brain stimulation: boon or bane? Brain Stimul 2015; 8:662–663. [DOI] [PubMed] [Google Scholar]
- 9.Hamada M, Murase N, Hasan A, et al. The role of interneuron networks in driving human motor cortical plasticity. Cereb Cortex 2013; 23:1593–1605. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Alonso V, Cheeran B, Rio-Rodriguez D, Fernandez-Del-Olmo M. Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimul 2014; 7:372–380. [DOI] [PubMed] [Google Scholar]
- 11.McCambridge AB, Stinear JW, Byblow WD. ‘I-wave’ recruitment determines response to tDCS in the upper limb, but only so far. Brain Stimul 2015; 8:1124–1129. [DOI] [PubMed] [Google Scholar]
- 12.Wiethoff S, Hamada M, Rothwell JC. Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimul 2014; 7:468–475. [DOI] [PubMed] [Google Scholar]
- 13.Ridding MC, Ziemann U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J Physiol 2010; 588:2291–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li LM, Uehara K, Hanakawa T. The contribution of interindividual factors to variability of response in transcranial direct current stimulation studies. Front Cell Neurosci 2015; 9:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conde V, Vollmann H, Sehm B, et al. Cortical thickness in primary sensorimotor cortex influences the effectiveness of paired associative stimulation. Neuroimage 2012; 60:864–870. [DOI] [PubMed] [Google Scholar]
- 16.Opitz A, Paulus W, Will S, et al. Determinants of the electric field during transcranial direct current stimulation. Neuroimage 2015; 109:140–150. [DOI] [PubMed] [Google Scholar]
- 17.Raffin E, Pellegrino G, Di Lazzaro V, et al. Bringing transcranial mapping into shape: sulcus-aligned mapping captures motor somatotopy in human primary motor hand area. Neuroimage 2015; 120:164–175. [DOI] [PubMed] [Google Scholar]
- 18.List J, Kubke JC, Lindenberg R, et al. Relationship between excitability, plasticity and thickness of the motor cortex in older adults. Neuroimage 2013; 83:809–816. [DOI] [PubMed] [Google Scholar]
- 19.Thirugnanasambandam N, Grundey J, Paulus W, Nitsche MA. Dose-dependent nonlinear effect of L-DOPA on paired associative stimulation-induced neuroplasticity in humans. J Neurosci 2011; 31:5294–5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amadi U, Allman C, Johansen-Berg H, Stagg CJ. The homeostatic interaction between anodal transcranial direct current stimulation and motor learning in humans is related to GABAA activity. Brain Stimul 2015; 8:898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stagg CJ, Bachtiar V, Johansen-Berg H. The role of GABA in human motor learning. Curr Biol 2011; 21:480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sale MV, Ridding MC, Nordstrom MA. Cortisol inhibits neuroplasticity induction in human motor cortex. J Neurosci 2008; 28:8285–8293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamke MR, Ryan AE, Sale MV, et al. Visual spatial attention has opposite effects on bidirectional plasticity in the human motor cortex. J Neurosci 2014; 34:1475–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenkranz K, Seibel J, Kacar A, Rothwell J. Sensorimotor deprivation induces interdependent changes in excitability and plasticity of the human hand motor cortex. J Neurosci 2014; 34:7375–7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neuling T, Ruhnau P, Fusca M, et al. Friends, not foes: magnetoencephalography as a tool to uncover brain dynamics during transcranial alternating current stimulation. Neuroimage 2015; 118:406–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McAllister CJ, Ronnqvist KC, Stanford IM, et al. Oscillatory beta activity mediates neuroplastic effects of motor cortex stimulation in humans. J Neurosci 2013; 33:7919–7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raffin E, Siebner HR. Transcranial brain stimulation to promote functional recovery after stroke. Curr Opin Neurol 2014; 27:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freitas C, Farzan F, Pascual-Leone A. Assessing brain plasticity across the lifespan with transcranial magnetic stimulation: why, how, and what is the ultimate goal? Front Neurosci 2013; 7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheeran B, Talelli P, Mori F, et al. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol 2008; 586:5717–5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sale MV, Ridding MC, Nordstrom MA. Factors influencing the magnitude and reproducibility of corticomotor excitability changes induced by paired associative stimulation. Exp Brain Res 2007; 181:615–626. [DOI] [PubMed] [Google Scholar]
- 31.Downar J, Geraci J, Salomons TV, et al. Anhedonia and reward-circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biol Psychiatry 2014; 76:176–185. [DOI] [PubMed] [Google Scholar]
- 32.Liston C, Chen AC, Zebley BD, et al. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry 2014; 76:517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salomons TV, Dunlop K, Kennedy SH, et al. Resting-state cortico-thalamic-striatal connectivity predicts response to dorsomedial prefrontal rTMS in major depressive disorder. Neuropsychopharmacology 2014; 39:488–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berger B, Minarik T, Liuzzi G, et al. EEG oscillatory phase-dependent markers of corticospinal excitability in the resting brain. Biomed Res Int 2014; 2014:936096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sauseng P, Klimesch W, Gerloff C, Hummel FC. Spontaneous locally restricted EEG alpha activity determines cortical excitability in the motor cortex. Neuropsychologia 2009; 47:284–288. [DOI] [PubMed] [Google Scholar]
- 36.Zarkowski P, Shin CJ, Dang T, et al. EEG and the variance of motor evoked potential amplitude. Clin EEG Neurosci 2006; 37:247–251. [DOI] [PubMed] [Google Scholar]
- 37.Takemi M, Masakado Y, Liu M, Ushiba J. Event-related desynchronization reflects downregulation of intracortical inhibition in human primary motor cortex. J Neurophysiol 2013; 110:1158–1166. [DOI] [PubMed] [Google Scholar]
- 38.Schulz H, Ubelacker T, Keil J, et al. Now I am ready–now I am not: the influence of pre-TMS oscillations and corticomuscular coherence on motor-evoked potentials. Cereb Cortex 2014; 24:1708–1719. [DOI] [PubMed] [Google Scholar]
- 39.Maki H, Ilmoniemi RJ. EEG oscillations and magnetically evoked motor potentials reflect motor system excitability in overlapping neuronal populations. Clin Neurophysiol 2010; 121:492–501. [DOI] [PubMed] [Google Scholar]
- 40.Romei V, Brodbeck V, Michel C, et al. Spontaneous fluctuations in posterior alpha-band EEG activity reflect variability in excitability of human visual areas. Cereb Cortex 2008; 18:2010–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dugue L, Marque P, VanRullen R. The phase of ongoing oscillations mediates the causal relation between brain excitation and visual perception. J Neurosci 2011; 31:11889–11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sale MV, Ridding MC, Nordstrom MA. Factors influencing the magnitude and reproducibility of corticomotor excitability changes induced by paired associative stimulation. Exp Brain Res 2007; 181:615–626. [DOI] [PubMed] [Google Scholar]
- 43.Triesch J, Zrenner C, Ziemann U. Modeling TMS-induced I-waves in human motor cortex. Prog Brain Res 2015; 222:105–124. [DOI] [PubMed] [Google Scholar]
- 44.Sewerin S, Taubert M, Vollmann H, et al. Enhancing the effect of repetitive I-wave paired-pulse TMS (iTMS) by adjusting for the individual I-wave periodicity. BMC Neurosci 2011; 12:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klimesch W, Sauseng P, Gerloff C. Enhancing cognitive performance with repetitive transcranial magnetic stimulation at human individual alpha frequency. Eur J Neurosci 2003; 17:1129–1133. [DOI] [PubMed] [Google Scholar]
- 46▪.Cecere R, Rees G, Romei V. Individual differences in alpha frequency drive crossmodal illusory perception. Curr Biol 2015; 25:231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that occipital TACS stimulation adjusted around IAF had opposing behavioral effects: IAF+2Hz increased and IAF−2Hz decreased the temproal window in which an illusion was perserved. Stimulating at IAF did not cause a behavioral effect.
- 47.Thut G, Veniero D, Romei V, et al. Rhythmic TMS causes local entrainment of natural oscillatory signatures. Curr Biol 2011; 21:1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin Y, Potkin SG, Kemp AS, et al. Therapeutic effects of individualized alpha frequency transcranial magnetic stimulation (alphaTMS) on the negative symptoms of schizophrenia. Schizophr Bull 2006; 32:556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jin Y, Kemp AS, Huang Y, et al. Alpha EEG guided TMS in schizophrenia. Brain Stimul 2012; 5:560–568. [DOI] [PubMed] [Google Scholar]
- 50.Jin Y, Phillips B. A pilot study of the use of EEG-based synchronized transcranial magnetic stimulation (sTMS) for treatment of major depression. BMC Psychiatry 2014; 14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arns M, Spronk D, Fitzgerald PB. Potential differential effects of 9 Hz rTMS and 10 Hz rTMS in the treatment of depression. Brain Stimul 2010; 3:124–126. [DOI] [PubMed] [Google Scholar]
- 52.Saturnino GB, Antunes A, Thielscher A. On the importance of electrode parameters for shaping electric field patterns generated by tDCS. Neuroimage 2015; 120:25–35. [DOI] [PubMed] [Google Scholar]
- 53.Thielscher A, Opitz A, Windhoff M. Impact of the gyral geometry on the electric field induced by transcranial magnetic stimulation. Neuroimage 2011; 54:234–243. [DOI] [PubMed] [Google Scholar]
- 54.Cash RF, Murakami T, Chen R, et al. Augmenting plasticity induction in human motor cortex by disinhibition stimulation. Cereb Cortex 2016; 26:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nitsche MA, Muller-Dahlhaus F, Paulus W, Ziemann U. The pharmacology of neuroplasticity induced by non-invasive brain stimulation: building models for the clinical use of CNS active drugs. J Physiol 2012; 590:4641–4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stefan K, Kunesch E, Cohen LG, et al. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain 2000; 123:572–584. [DOI] [PubMed] [Google Scholar]
- 57▪.McNickle E, Carson RG. Paired associative transcranial alternating current stimulation increases the excitability of corticospinal projections in humans. J Physiol 2015; 593:1649–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that the concept of associative stimulation protocols can be transferred to TACS by pairing brief trains of peripheral afferent stimulation with short bursts of high frequency (≥80 Hz) TACS over contralateral M1.
- 58.Rizzo V, Siebner HS, Morgante F, et al. Paired associative stimulation of left and right human motor cortex shapes interhemispheric motor inhibition based on a Hebbian mechanism. Cereb Cortex 2009; 19:907–915. [DOI] [PubMed] [Google Scholar]
- 59.Chao CC, Karabanov AN, Paine R, et al. Induction of motor associative plasticity in the posterior parietal cortex-primary motor network. Cereb Cortex 2015; 25:365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davare M, Montague K, Olivier E, et al. Ventral premotor to primary motor cortical interactions during object-driven grasp in humans. Cortex 2009; 45:1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61▪.Polania R, Moisa M, Opitz A, et al. The precision of value-based choices depends causally on fronto-parietal phase coupling. Nat Commun 2015; 6:8090. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors studied behavioral effects of associative cortico-cortical pairing of TACS: antiphasic pairing of frontal and parietal TACS influenced behavior in a value-based choice task, while in-phase pairing was not different from sham-stimulation.
- 62▪▪.Udupa K, Bahl N, Ni Z, et al. Cortical plasticity induction by pairing subthalamic nucleus deep-brain stimulation and primary motor cortical transcranial magnetic stimulation in Parkinson's disease. J Neurosci 2016; 36:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]; An innovative extension of the PAS. By pairing deep brain stimulation of the subthalamic nucleus with single-pulse TMS of M1, the authors were able to induce plasticity effects in M1.
- 63.Bergmann TO, Molle M, Schmidt MA, et al. EEG-guided transcranial magnetic stimulation reveals rapid shifts in motor cortical excitability during the human sleep slow oscillation. J Neurosci 2012; 32:243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64▪.Kraus D, Naros G, Bauer R, et al. Brain state-dependent transcranial magnetic closed-loop stimulation controlled by sensorimotor desynchronization induces robust increase of corticospinal excitability. Brainstimulation 2016; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]; This study showed that state-triggered single-pulse TMS can induce LTP-like changes in corticospinal excitability. Task-related desynchronization of beta-band EEG during a motor imagery task triggered the administration of single-pulse TMS and induced a stable LTP-like increase in corticospinal excitability, whereas single-pulse TMS in the absence of motor imagery decreased corticospinal excitability.
- 65.Gharabaghi A, Kraus D, Leao MT, et al. Coupling brain-machine interfaces with cortical stimulation for brain-state dependent stimulation: enhancing motor cortex excitability for neurorehabilitation. Front Hum Neurosci 2014; 8:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosin B, Slovik M, Mitelman R, et al. Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron 2011; 72:370–384. [DOI] [PubMed] [Google Scholar]
- 67.Little S, Brown P. What brain signals are suitable for feedback control of deep brain stimulation in Parkinson's disease? Ann N Y Acad Sci 2012; 1265:9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68▪▪.Little S, Pogosyan A, Neal S, et al. Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol 2013; 74:449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]; An example of adaptive closed-loop invasive neurostimulation. DBS of the STN was dynamically adjusted by STN-beta power. Adaptive closed-loop stimulation improved clinical symptoms of Parkinson's disease 30% more than standard open-loop DBS.
- 69.Sun FT, Morrell MJ. Closed-loop neurostimulation: the clinical experience. Neurotherapeutics 2014; 11:553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70▪▪.Brittain JS, Probert-Smith P, Aziz TZ, Brown P. Tremor suppression by rhythmic transcranial current stimulation. Curr Biol 2013; 23:436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]; A first proof-of-principle example for closed-loop NTBS. In Parkinson's patients, TACS was applied to M1 as individual tremor frequency was able to suppress tremor amplitude. The phase of TACS was constantly adjusted, informed by the ongoing tremor activity, to maintain the optimal phase-delay between TACS and the endogenous tremor rhythm. Using this method the authors achieved tremor suppression of approximately 50%.


