Abstract
Protein restricted, high carbohydrate diets improve metabolic health in rodents, yet the precise dietary components that are responsible for these effects have not been identified. Further, the applicability of these studies to humans is unclear. Here, we demonstrate in a randomized controlled trial that a moderately protein restricted (PR) diet also improves markers of metabolic health in humans. Intriguingly, we find that feeding mice a diet specifically reduced in branched chain amino acids (BCAAs) is sufficient to improve glucose tolerance and body composition equivalently to a PR diet, via metabolically distinct pathways. Our results highlight a critical role for dietary quality at the level of amino acids in the maintenance of metabolic health, and suggest that diets specifically reduced in BCAAs, or pharmacological interventions in this pathway, may offer a translatable way to achieve many of the metabolic benefits of a PR diet.
Introduction
A calorie restricted (CR) diet, in which total caloric intake is reduced while maintaining adequate nutrition, promotes metabolic health and longevity in invertebrate model organisms and mammalian species ranging from mice to humans (Colman et al., 2014; Fontana et al., 2010; Lamming and Anderson, 2014; Mercken et al., 2012). Mammals, including humans, placed on a CR diet, significantly lose body weight and abdominal fat and display a robust improvement in glycemic control and insulin sensitivity (Barzilai et al., 1998; Foster et al., 2003; Weiss et al., 2006). A CR diet usually involves decreased consumption of all macronutrients, and the role of decreased protein in particular has received significant attention (Fontana and Partridge, 2015; Weindruch and Walford, 1988). Recently, protein restricted (PR) diets have been shown to significantly improve the metabolic health and longevity of rodents (Ables et al., 2014; Fontana and Partridge, 2015; Grandison et al., 2009; Solon-Biet et al., 2014; Solon-Biet et al., 2015).
While in humans, high protein, low carbohydrate diets have recently been popular for weight loss, epidemiological studies suggest that high protein intake correlates with increased mortality, while lower protein intake is associated with decreased mortality (Lagiou et al., 2007; Levine et al., 2014). Indeed, individuals consuming high protein diets are at increased risk of developing metabolic diseases including obesity and type 2 diabetes (Halkjaer et al., 2011; Sluijs et al., 2010; Vergnaud et al., 2013). Little is known about the molecular mechanisms through which PR regulates metabolic health. Moreover, many of the studies conducted thus far have utilized extremely restrictive protein diets, well below the estimated average requirement for human adults, and therefore likely unsustainable and unhealthy (Jennifer J. Otten et al., 2006). The impact of a moderate PR diet without CR on metabolic health in humans has not been considered.
Over the last decade, evidence has mounted that certain individual amino acids (AAs), the building blocks of protein, have distinct effects on metabolism (Brown-Borg et al., 2014; Edwards et al., 2015; Grandison et al., 2009; Miller et al., 2005). In particular, several studies have observed increased serum levels of the three branched-chain amino acids (BCAAs) – leucine, isoleucine, and valine – in insulin-resistant humans, and indeed BCAA levels are predictive of development of type 2 diabetes (Lynch and Adams, 2014). A possible causal role for BCAAs in the pathogenesis of type 2 diabetes has been suggested, and BCAA supplementation in the context of a high-fat diet promotes the development of insulin resistance in rats (Newgard et al., 2009). Studies using knockout diets – in which one AA is completely removed from the diet – have found that elimination of dietary leucine for one week improves glycemic control in mice (Xiao et al., 2011; Xiao et al., 2014).
In this study, we determined how a moderate PR diet impacts the metabolic health of both humans and mice, and tested the hypothesis that these effects are mediated by decreased consumption of BCAAs. We determined that moderate PR improves multiple indicators of metabolic health in both humans and mice, and that specific dietary restriction of all three BCAAs, but not of leucine alone, improves metabolic health, improving glucose tolerance and reducing fat accumulation. Unexpectedly, we observed negative effects of restricting dietary leucine alone on dermal and visceral adiposity. Our data suggest a critical role for dietary BCAAs in the regulation of metabolic health, and suggest that protein quality – the specific amino acid composition of the diet – plays an important role in the regulation of metabolic health.
Results
Moderate protein restriction improves metabolic health in mice and humans
We placed C57BL/6J wild-type mice on diets in which either 21% of calories or 7% of calories were derived from protein, which we have previously used to explore the impact of moderate PR on cancer (Fontana et al., 2013; Lamming et al., 2015). Mice fed the 7% PR diet demonstrated improved glucose tolerance (Figure 1A), as well as decreased fasting blood glucose and insulin levels, and reduced HOMA2-IR values (Figure 1B-1D). Mice fed the 7% PR diet also showed improved pyruvate tolerance and responded normally to insulin injection, indicating improved suppression of gluconeogenesis (Figure 1E, Figure S1A). Despite consuming more food, mice on the 7% PR diet gained less weight than mice on the 21% protein control diet over the course of 2 months (Figure 1F and 1G). Body composition analysis suggested that while consumption of a low protein diet slowed the gain of lean mass, fat mass accumulation was almost entirely blocked (Figure S1B and 1C). Mice eating the 7% PR diet had no change in spontaneous activity, but exhibited increased respiration throughout a 24 hour cycle, and had increased energy expenditure at night (Figure S2).
Figure 1. A naturally sourced low protein diet improves the metabolic health of mice.
Glucose (A) and pyruvate (B) tolerance tests on male C57BL/6J mice fed a naturally sourced 21% or 7% protein diet for 3 or 5 weeks, respectively (a, b: n=6-9/group; Tukey-Kramer test following ANOVA, * = p < 0.05). Mice were fasted overnight and (C) blood glucose and (D) insulin were measured, and (E) the HOMA2-IR was calculated after 6 weeks on the specified diets. (F) Food consumption was measured after 2 weeks on the 21% or 7% protein diets. (G) Weight was determined immediately prior to diet start and after 3 and 8 weeks (c-g: n=6-9/group; two-tailed t-test, * = p < 0.05). Error bars represent standard error.
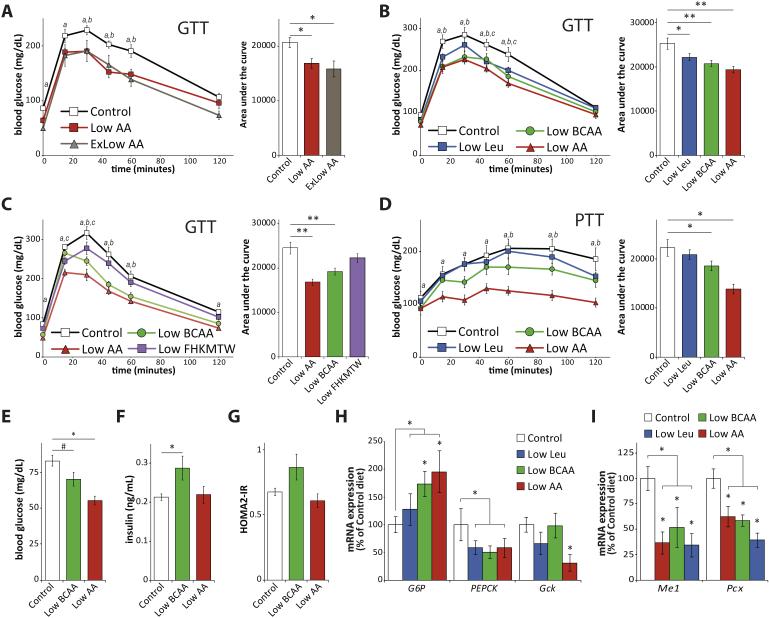
Figure 2. Reduced consumption of BCAAs improves glycemic control.
(A) Glucose tolerance test (GTT) on male C57BL/6J mice fed either an amino-acid defined Control diet, or one of two diets (Low AA and ExLow AA) with reduced amino acid content for three weeks (n=9 mice/group; for GTT, Dunnett’s test following ANOVA, a = p < 0.05 Control vs. Low AA, b = p < 0.05 Control vs. ExLow AA; for AUC, Dunnett’s test following ANOVA, * = p < 0.05). (B) GTT on male C57BL/6J mice fed a Control diet, a Low AA diet, or a diet in which either leucine (Low Leu) or all BCAAs (Low BCAA) is reduced by 2/3rds for three weeks (n=8-12 per group; for GTT, Dunnett’s test following ANOVA, a = p < 0.05 Control vs. Low AA, b = p < 0.05 Control vs. Low BCAA, c = p < 0.05 Control vs. Low Leu; for AUC, Dunnett’s test following ANOVA, * = p < 0.05, ** = p < 0.01). (C) GTT on male C57BL/6J mice fed a Control diet, a Low AA diet, a Low BCAA diet, or a Low FHKMTW diet in which six essential amino acids (F,H,K,M,T, and W) are reduced by 2/3rds, for three weeks (n=8 per group; for GTT, Dunnett’s test following ANOVA, a = p < 0.05 Control vs. Low AA, b = p < 0.05 Control vs. Low BCAA, c = p < 0.05 Control vs. Low FHKMTW; for AUC, Dunnett’s test following ANOVA, ** = p < 0.01). (D) Pyruvate tolerance test (PTT) on male C57BL/6J mice fed the indicated diets for 5 weeks (n=8-12 per group; for PTT, Dunnett’s test following ANOVA, a = p < 0.05 Control vs. Low AA (7%), b = p < 0.05 Control vs. Low BCAA, c = p < 0.05 Control vs. Low Leu; for AUC, Dunnett’s test following ANOVA, * = p < 0.05, ** = p < 0.01). Mice were fasted overnight and (E) blood glucose and (F) insulin were measured, and (G) the HOMA2-IR was calculated after 7 weeks on the specified diets (n=8-12 mice/group; Dunnett’s test following ANOVA, * = p < 0.05). (H-I) Gene expression in the liver of mice fasted overnight after 11 weeks of feeding the indicated diets was determined by quantitative PCR (n= 5-6/group, Dunnett’s test following ANOVA, * = p < 0.05 vs. control; for grouped analysis, n = 6-18/group, two-tailed t-test, * = p < 0.05). Error bars represent standard error.
To determine the relevance of these results to humans, we analyzed data from a randomized controlled trial (RCT) we conducted to determine the health effects of PR without calorie restriction in 38 middle-aged overweight and mildly obese (baseline BMI ~30 kg/m2) human males. The 19 volunteers randomized to PR were fed customized isocaloric 7-9% protein diets for an average of 43 days, while the 19 control subjects consumed their usual diets, consisting of ~50% more protein per day (Table S1). We measured physical parameters and collected blood for metabolic analysis in patients after an overnight fast, both at baseline and at a follow-up visit at the end of the trial. As this was a RCT, some baseline parameters were expected to vary between the control and PR groups; our analysis therefore focused on the changes within each diet group (within-group p) and the differences between the changes seen in each group (among-group p) (Table 1 and 2).
Table 1.
A human clinical trial of PR – physical parameters.
|
Protein
restricted |
within- group p |
Control | within- group p |
Among- group p |
|
|---|---|---|---|---|---|
| Age (yrs) | 52.46 ± 6.76 | 53.00 ± 7.71 | 0.82 | ||
| BMI (kg/m2) | |||||
| Baseline | 30.66 ± 5.43 | 30.15 ± 5.84 | |||
| Follow-up | 29.90 ± 5.32 | 30.18 ± 5.67 | |||
| Δ BMI | −0.76 ± 0.75 | < 0.0001 | 0.03 ± 0.51 | 0.81 | 0.001 |
| Weight (kg) | |||||
| Baseline | 101.46 ± 18.75 | 93.17 ± 17.25 | |||
| Follow-up | 98.84 ± 18.61 | 92.96 ± 16.87 | |||
| Δ Weight | −2.62 ± 2.18 | < 0.0001 | −0.21 ± 1.36 | 0.52 | 0.001 |
| Fat mass (kg) | |||||
| Baseline | 33.24 ± 11.91 | 31.07 ± 11.83 | |||
| Follow-up | 31.86 ± 11.09 | 30.72 ± 11.45 | |||
| Δ Fat mass | −1.37 ± 1.55 | 0.001 | −0.36 ± 1.02 | 0.15 | 0.02 |
Humans were randomly assigned to a 7-9% protein restricted or control diet group (19 male subjects per group), and physical parameters were assessed at baseline and at a follow-up visit 43 ± 11 days later. Change (Δ) represents the difference between the baseline and follow-up visit. Changes between and within the PR and control groups were tested with analysis of covariance and paired t-tests. Statistical tests were two-tailed, with significance accepted at p < 0.05. Errors represent standard deviation.
Table 2.
A human clinical trial of PR – blood parameters.
|
Protein
restricted |
within- group p |
Control | within- group p |
Among- group p |
|
|---|---|---|---|---|---|
| Glucose (mg/dL) | |||||
| Baseline | 112.95 ± 36.38 | 103.31 ± 16.00 | |||
| Follow-up | 105.95 ± 31.26 | 105.98 ± 20.46 | |||
| Δ Glucose | −7.00 ± 9.33 | 0.02 | 2.67 ± 8.50 | 0.23 | 0.01 |
| Insulin (μU/ml) | |||||
| Baseline | 7.33 ± 5.29 | 6.79 ± 4.20 | |||
| Follow-up | 7.38 ± 5.15 | 7.32 ± 4.55 | |||
| Δ Insulin | 0.05 ± 2.45 | 0.94 | 0.53 ± 2.68 | 0.4 | 0.56 |
| FGF21 (pg/ml) | |||||
| Baseline | 131.55 ± 101.62 | 231.08 ± 244.61 | |||
| Follow-up | 260.33 ± 172.67 | 223.43 ± 143.76 | |||
| Δ FGF21 | 128.78 ± 155.25 | 0.003 | −7.64 ± 176.06 | 0.86 | 0.02 |
| Leucine (μmol/L) | |||||
| Baseline | 132 .02 ± 13.62 | 132.42 ± 20.89 | |||
| Follow-up | 116.21 ± 21.87 | 136.41 ± 27.26 | |||
| Δ Leucine | −15.81 ± 21.03 | 0.004 | 3.99 ± 18.86 | 0.37 | 0.004 |
| Isoleucine (μmol/L) | |||||
| Baseline | 66.43 ± 10.01 | 64.97 ± 11.28 | |||
| Follow-up | 60.29 ± 13.57 | 70.06 ± 15.07 | |||
| Δ Isoleucine | −6.14 ± 13.22 | 0.06 | 2.08 ± 11.01 | 0.42 | 0.04 |
| Valine (μmol/L) | |||||
| Baseline | 237.82 ± 29.50 | 244.37 ± 42.29 | |||
| Follow-up | 193.18 ± 40.36 | 244.58 ± 47.90 | |||
| Δ Valine | −44.64 ± 42.72 | < 0.0001 | 0.21 ± 31.24 | 0.98 | 0.001 |
Humans were randomly assigned to a 7-9% protein restricted or control diet group (19 male subjects per group), and fasting blood levels of glucose, insulin, FGF21 and the indicated amino acids were assessed at baseline and at a follow-up visit 43 ± 11 days later. Change (Δ) represents the difference between the baseline and follow-up visit. Changes between and within the PR and control groups were tested with analysis of covariance and paired t-tests. Statistical tests were two-tailed, with significance accepted at p < 0.05. Errors represent standard deviation.
Humans eating isocaloric PR diets, but not those in the control group, showed very similar effects to mice placed on a PR diet, with a significant decrease in body weight (approximately 2.6 kg), fat mass, and BMI (Table 1). We also observed a significant decrease in fasting blood glucose levels, although there was no effect of PR on the level of insulin; however, we observed a doubling of levels of the insulin sensitizing hormone FGF21 in subjects fed a PR diet, with no change in FGF21 levels in the control group (Table 2); a similar increase in FGF21 was previously observed in humans fed a more severe PR diet (Laeger et al., 2014a). We also observed a significant decrease in plasma levels of the three branched-chain amino acids (BCAAs) – leucine, isoleucine, and valine – which are associated with insulin-resistance in humans and rodents (Table 2) (Lynch and Adams, 2014; Newgard et al., 2009). With the exception of lysine, we did not observe decreased plasma levels of any of the other essential amino acids in humans on a PR diet (Table S2).
Specific reduction of dietary branched chain amino acids improves glucose and pyruvate tolerance
In order to study the specific effects of reducing dietary levels of the three BCAAs on metabolic health, we designed and constructed an amino acid defined Control diet modelled on the 21% protein diet used in Figure 1 and in our recent study (Lamming et al., 2015). We also examined two diets with decreased levels of amino acids, in which either 7% or 5% of calories were derived from amino acids through a uniform reduction of every amino acid in the Control diet. The Low AA diet therefore is based on the 7% protein diet used in Figure 1, while the more restricted extremely Low AA (ExLow AA) diet reflects the 5% protein diets used in some recent studies (Laeger et al., 2014a; Solon-Biet et al., 2014; Solon-Biet et al., 2015). All three amino acid defined diets are isocaloric with identical levels of dietary fat; the exact formulations of these diets are provided in Table S3.
We first verified that PR (in naturally sourced diets) and amino acid restriction (in our newly constructed synthetic diets) were comparable interventions. Mice fed either the Low AA or ExLow AA diets had improved glucose tolerance relative to mice fed the Control diet (Figure 2A). Just as with naturally sourced PR diets, mice fed the Low AA and ExLow AA diets had improved pyruvate tolerance, and despite eating more gained significantly less weight and reduced gain of fat mass (Figure S2A-S2C). The improved glucose and pyruvate tolerance of mice fed the Low AA and ExLow AA diets persisted throughout the study (Figure S2D-S2E). Notably, while mice fed the Low AA diet were able to maintain their body weight over the course of 13 weeks, mice fed the ExLow AA diet lost both weight and lean mass (Figure S2C); we therefore proceeded to use the Low AA diet (7% of calories derived from amino acids) as a sustainable baseline for investigation of the specific contribution of reduced dietary BCAAs.
We proceeded to design two additional diets: a Low Leu diet in which the level of leucine was reduced by 2/3rds to match the level of the Low AA diet, while all other amino acids were kept at the level of the a Control diet; and a Low BCAA diet in which all three BCAAs were reduced by 2/3rds to match the levels of the Low AA diet, while all other amino acids were kept at the level of the a Control diet. As with the Low AA diet, all of these diets were isocaloric with the Control diet and had identical levels of dietary fat; the exact formulations of these diets are provided in Table S3. Mice fed either the Low Leu, Low BCAA or Low AA diets for 3 weeks had significantly improved glucose tolerance compared to mice fed the Control diet (Figure 2B).
We repeated the glucose tolerance test with a new group of mice, comparing the Control and Low AA diets to a Low BCAA diet and a new diet (Low FHKMTW) in which the other six essential amino acids (phenylalanine, threonine, tryptophan, methionine, lysine, and histidine) were reduced by 2/3rds to match the levels of the Low AA diet; the levels of the non-essential amino acids were increased in the Low BCAA and Low FHKMTW diets to match the caloric contribution from amino acids, carbohydrates and fats to the Control diet (the exact formulations of these diets are provided in Table S3). Mice fed the Low BCAA or Low AA diets for 3 weeks again showed improved glucose tolerance, but mice fed the Low FHKMTW diet showed no improvement in glucose tolerance (Figure 2C).
Mice fed either the Low BCAA or Low AA diet also had improved pyruvate tolerance but responded normally to insulin injection, indicating improved suppression of gluconeogenesis (Figure 2D, Figure S3A). As seen in mice fed a Low AA diet (Figure S2D and S2E), the improved glucose and pyruvate tolerance in mice fed a Low BCAA diet persisted for the duration of the study (Figure S3B and S3C). We collected blood from mice after an overnight fast for the determination of blood glucose and insulin levels. We observed decreased fasting blood glucose in mice fed the Low BCAA and Low AA diets; neither diet decreased fasting insulin levels or reduced HOMA2-IR values (Figure 2E-2G).
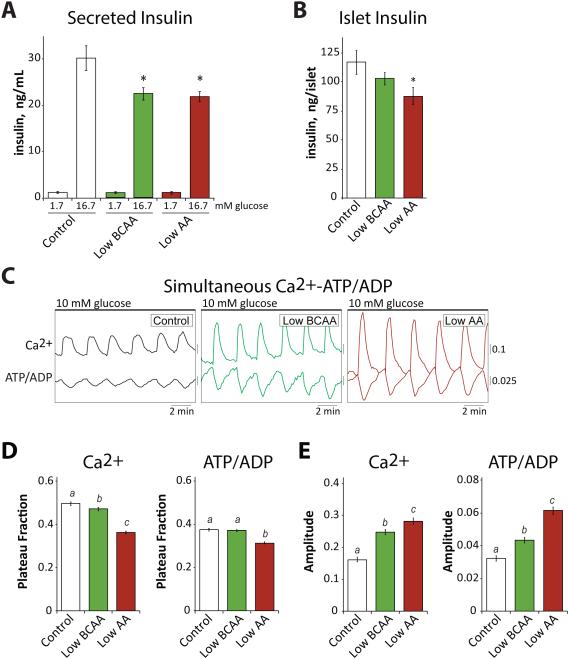
Figure 3. Ex vivo analysis of pancreatic islet and β cell function.
(A) An ex vivo insulin secretion assay was performed to assess (A) insulin secretion per islet and (B) islet insulin content in response to low (1.7 mM) and high (16.7 mM) glucose in mice fed either Control, Low AA, or Low BCAA diets for 11 weeks (n = 6 mice/group, Dunnett’s test following ANOVA, * = p < 0.05 vs. Control). (C) The impact of decreased BCAAs or total AAs on ATP/ADP and Ca2+ oscillations of pancreatic β cells was determined by simultaneous imaging after 17 weeks of feeding the indicated diets, and (D) plateau fraction and (E) amplitude was then calculated (n=149-176 islets; Dunnett’s test following ANOVA). Error bars represent standard error.
In combination, our results suggested that mice fed either a Low AA or Low BCAA diet might have altered hepatic gluconeogenesis. We examined the expression of the gluconeogenic genes glucose 6-phosphatase (G6p) and phosphoenolpyruvate carboxykinase (PEPCK), as well as the expression of glucokinase (Gck) in the livers of mice following an overnight fast. We observed a strong induction of G6p in mice fed either a Low AA or Low BCAA diet; however, PEPCK was not induced by any diet, but was indeed was downregulated by Low AA, Low BCAA and Low Leu diets, and Gck expression was decreased only in mice fed a Low AA diet (Figure 2H). We also examined the expression of pyruvate carboxylase (Pcx) and malic enzyme 1 (Me1), which are involved in the maintenance of pyruvate levels and function upstream of PEPCK during gluconeogenesis (Merritt et al., 2011); the expression of both genes was strongly decreased by all three diets (Figure 2I).
Reduction in dietary branched chain amino acids reduces β cell metabolic stress
We next examined the effect of Low BCAA and Low AA diets on pancreatic β cell function. We isolated pancreatic islets from mice on the Control, Low BCAA and Low AA diets, and examined ex vivo glucose stimulated insulin secretion as well as the metabolic and Ca2+ oscillations that drive secretion (Merrins et al., 2016; Merrins et al., 2013; Truchan et al., 2015). Islets isolated from the mice fed Low BCAA and Low AA diets secreted significantly less insulin per islet than islets from the mice fed Control diet (Figure 3A), while the islet insulin content was also decreased by both diets, significantly in the case of the Low AA diet (Figure 3B). By comparison with the Control diet, Low BCAA and Low AA diets reduced the plateau fraction (a measure of the time the islet spends in the active state (Merrins et al., 2016; Nunemaker et al., 2006)) of both ATP/ADP and Ca2+ oscillations (Figure 3C and 3D), reflecting decreased metabolic flux, a highly advantageous state as unchecked β cell metabolic workload can lead to β cell failure over time (Porat et al., 2011). Ca2+ oscillation amplitude was increased in these cases (Figure 3C and 3E), an efficiency which explains how the islets maintained adequate secretion on the Low BCAA and Low AA diets without the need for a higher metabolic rate. These data indicate that β cell glucose sensitivity is reduced in mice fed either the Low BCAA diet or the Low AA diet, an effect that is most likely due to reduced insulin demand on the pancreatic β cells of these mice rather than a primary β cell lesion as both diets improved glucose tolerance.
Reduced dietary leucine stimulates white adipose tissue mass
As with mice fed a Low AA or PR diet, mice fed a Low BCAA diet consume more food (Figure 4A). Despite consuming more calories, mice fed either a Low BCAA or Low AA diet gained less weight over the course of 10 weeks, with reduced gain of both fat mass and lean mass (Figure 4B). In contrast, mice fed a Low Leu diet did not eat more than Control diet mice (Figure 4A), and while not statistically different from Control mice with respect to weight, we observed that mice fed a Low Leu diet showed a trend towards increased adipose mass and decreased lean mass (Figure 4B); further, the mice appeared fatty upon necropsy. To quantify this, we collected skin (including dermal white adipose tissue, dWAT) (Alexander et al., 2015; Driskell et al., 2014) from mice fed each diet, as well as the epididymal and inguinal fat pads. All three adipocyte depots were assessed, since these depots can be independently regulated. We observed an approximately 70% increase in the thickness of the dWAT from mice fed a Low Leu diet (Figure 4C and 4D), with an 80% increase in the weight of the epididymal fat pads (Figure 4E).
Figure 4. Dietary branched chain amino acids regulate food intake, body composition and adipose mass.
(A) Food consumption after 3 weeks on diets (n=9 mice/group, means with the same letter are not significantly different from each other (Tukey–Kramer test following ANOVA, p < 0.05)). (B) Weight and body composition were measured immediately prior to diet start and after 3 and 10 weeks on the indicated diets (n= 7-12/group, means with the same letter are not significantly different from each other (Tukey–Kramer test following ANOVA, p < 0.05)). (C) Paraffin-embedded skin samples were collected after feeding mice the indicated diets for 11 weeks, sectioned, H&E stained and the thickness of dermal white adipose tissue (dWAT) was quantified (D) for non-anagen stage skin samples, measuring from muscle to dermis; scale bar = 100μM (n=5-11/group, means with the same letter are not significantly different from each other (Tukey–Kramer test following ANOVA, p < 0.05)). (E) The epididymal white adipose tissue (eWAT) and inguinal white adipose tissue (iWAT) was collected at necropsy and weighed (n=6-9/group, means with the same letter are not significantly different from each other (Tukey–Kramer test following ANOVA, p < 0.05)). Error bars represent standard error.
Reduction in dietary BCAAs is metabolically distinct from restriction of all dietary AAs.
Many of the metabolic effects of PR diet, including increased energy expenditure, are proposed to be mediated by the insulin sensitizing hormone FGF21. FGF21 is induced in rodents and humans fed an extremely restrictive protein diet (Laeger et al., 2014a), and we have found that FGF21 is also induced in humans eating a moderate PR diet (Table 1). We thus examined levels of FGF21 in mice fed either a Control, Low AA, Low BCAA or Low Leu diet. Surprisingly, we found that while a Low AA diet increases plasma FGF21 and induces FGF21 mRNA in both liver and skeletal muscle, we observed no increase in mice fed either a Low BCAA or Low Leu diet (Figure 5A and 5B). In concordance with this result, we observed increased nighttime energy expenditure only in mice fed a Low AA diet (Figure 5C). Further, FGF21 stimulates the production of adiponectin (Lin et al., 2013), and we observed increased plasma adiponectin only in mice fed a Low AA diet (Figure 5D). Transcription of Fgf21 is mediated by Pgc1α (Cornu et al., 2014), and indeed we observed increased hepatic Ppargc1a expression only in mice fed a Low AA diet (Figure 5E).
Figure 5. The effects of reduced branched chain amino acids are independent of FGF21.
(A) FGF21 was measured in the plasma of mice fed the indicated diets for 17 weeks and sacrificed following an overnight fast (n=8-9/group, Dunnett’s test following ANOVA, * = p < 0.05). (B) Fgf21 expression in the liver, skeletal muscle and adipose tissue of mice fasted overnight after 11 weeks of feeding the indicated diets was determined by quantitative PCR (n= 5-11/group, Dunnett’s test following ANOVA, * = p < 0.05). (C) Energy expenditure was measured after 4-6 weeks of feeding the indicated diets (n= 5-9/group, Dunnett’s test following ANOVA, * = p < 0.05). (D) Adiponectin was measured in the plasma of mice fed the indicated diets following an overnight fast after 17 weeks of diet feeding (n=5-9/group, Dunnett’s test following ANOVA, * = p < 0.05). (E) Ppargc1a expression in the liver of mice fasted overnight after 11 weeks of feeding the indicated diets was determined by quantitative PCR (n= 5-6/group, Dunnett’s test following ANOVA, * = p < 0.05 vs. control). Error bars represent standard error.
Discussion
Understanding how dietary choices impact metabolic health is an area of significant research interest, but until recently this has largely focused on one’s choice of foods – e.g., caloric intake of foods or choosing between vegetable, fish, and red meat as a protein source. Recently, diets with altered macronutrient ratios have received significant attention from the public as a potential means to combat obesity, while evidence suggesting that a lower protein intake is positively associated with increased health, survival, and insulin sensitivity has continued to mount (Levine et al., 2014; Solon-Biet et al., 2014; Solon-Biet et al., 2015). However, an understanding of the specific dietary components altered in a low protein diet that promote metabolic health has been lacking, and it has been unclear if humans will receive immediate benefits to metabolic health.
Here, we demonstrate that a moderate reduction in total dietary protein or selected amino acids can rapidly improve metabolic health in both humans and mice. Reduction of dietary protein or total amino acids decreases fasting blood glucose levels and improves glucose tolerance in both species in less than six weeks, while also decreasing BMI and fat mass in humans and decreasing weight and fat mass gain in young growing mice. A moderate reduction of total dietary protein/amino acids increases circulating FGF21 in both species just as efficiently as more severe forms of protein restriction (Laeger et al., 2014a).
Importantly, we have now found that altered dietary quality – the precise amino acid composition of the diet – regulates metabolic health. Specifically reducing the three branched chain amino acids (leucine, isoleucine, and valine) to the same level as found in a low protein diet is sufficient to improve many aspects of metabolic health, including glucose tolerance and body composition, as effectively as a 2/3rds reduction in total consumption of dietary amino acids. The three BCAAs contribute uniquely to the overall effect of dietary protein restriction on glucose tolerance, as a 2/3rds reduction in the other six essential amino acids is not sufficient to improve glucose tolerance (Figure 2C). This also clearly demonstrates that the effect of BCAAs on glucose homeostasis is independent from changes in body composition, as diets reduced in either the three BCAAs or the other six essential amino acids have similar impacts on body composition (Figure S4). However, not all of the effects of a low protein diet are attributable to reduced BCAAs; a specific reduction in dietary BCAAs does not induce hepatic Ppargc1a, increase circulating FGF21 and adiponectin, increase energy expenditure, or decrease hepatic Gck, effects we observe exclusively in mice fed a Low AA diet (Figures 2 and 5). It remains to be determined if these other effects are attributable to a reduction in total amino acids, the greater impact of a Low AA diet on body composition, or if other specific amino acids are responsible; for instance, recent research suggests that methionine restriction is sufficient to induce FGF21 (Lees et al., 2014).
Notably, while some recent studies have suggested that a low protein diet improves metabolic health due to a high carbohydrate to low protein ratio (Solon-Biet et al., 2014; Solon-Biet et al., 2015), we have determined that reduced dietary BCAAs improves metabolic health even in the absence of significant alterations in the dietary carbohydrate to protein ratio. An interesting question left unanswered by our work is if a Low BCAA diet is (like a low protein diet) most efficacious at promoting metabolic health when accompanied by elevated dietary carbohydrates. Other significant unanswered questions also remain, including the role of other essential amino acids in the response to a moderate PR diet, and understanding the full biochemical basis for the effect of reduced dietary BCAAs on hepatic gluconeogenesis. We also observed several other significant physiological effects that are ripe for future exploration, including alterations in pancreatic β cell metabolism and body composition. Notably, mice on the Low AA and Low BCAA diets ate significantly more than mice on the Control diet, yet gained less weight. In mice placed on a Low AA diet, this may be explained in part by an FGF21-mediated increase in energy expenditure (Laeger et al., 2014a), but mice eating a diet specifically reduced in the BCAAs do not have increased FGF21 or increased energy expenditure; the mechanism for this remains to be determined. Interestingly, a recent study in Sprague-Dawley rats determined that diets with reduced dietary BCAAs do not stimulate hyperphagia (Laeger et al., 2014b); whether this reflects differences between mice and rats, or experimental differences in the length of diet feeding and the degree of BCAA restriction remains to be determined.
While we determined that mice fed a diet specifically reduced in leucine did not eat more than control mice, we observed a dramatic effect of leucine reduction on white adipose tissue mass and distribution. In addition to increased visceral adipose tissue, which is associated with poor metabolic health (Bergman et al., 2006), we observed increased intradermal adipose tissue, which has been proposed to be key to thermal insulation (Cannon and Nedergaard, 2011; Kasza et al., 2014). Thus, thicker dWAT could potentially decrease the energy required for thermogenesis. As we did not observe increased dWAT or eWAT in mice restricted in all three BCAAs, it is plausible that the phenotypes we observed could result from an imbalance between the levels of leucine and either isoleucine or valine; both of these amino acids have been implicated in lipid and fatty acid metabolism (Du et al., 2012; Jang et al., 2016). However, the ultimate molecular mechanism which drives the effect of a leucine reduced diet on white adipose tissue is as yet unknown. If altered dietary levels of a single amino acid can also regulate adipose mass in humans, it suggests that the obesity epidemic sweeping the world could be impacted by relatively subtle changes in dietary quality at the level of amino acid composition.
Our findings highlight an important new avenue of investigation – specifically, how dietary quality at the level of individual amino acids, not simply the quantity of food consumed, regulates metabolic health. Our findings may be highly translatable to the clinic through the use of diet plans or through the prescription of already FDA-approved medical foods lacking specific branched chain amino acids. Our human clinical trial data suggests that even a quite modest PR regimen may have significant clinical benefits. In the long term, further investigation of the molecular mechanisms and biological pathways regulated by specific dietary amino acids may permit the development of pharmacological agents to target these pathways to promote metabolic health and to combat obesity and diabetes.
Experimental Procedures
For detailed procedure and reagents, see Supplemental Experimental Procedures.
Animals and Treatments
Male C57BL/6J mice were purchased from The Jackson Laboratory at 8 weeks of age, and diet changes occurred at 9 weeks of age. Glucose, insulin, and pyruvate tolerance tests were performed by fasting the mice overnight for 16 hours and then injecting either glucose (1g/kg), insulin (0.75U/kg), or pyruvate (2g/kg) intraperitoneally. Glucose measurements were taken using a Bayer Contour blood glucose meter and test strips. Animal diets were obtained from Envigo (formerly Harlan Laboratories), Madison, WI. Natural sourced diets used were 21% protein (TD.10193) and 7% protein (TD.10192). Amino acid defined diet compositions and item numbers are provided in Supplemental Table S3. Diets were adjusted such that the 7% amino acid diet had a proportional, 2/3rds reduction in all amino acids, the branched chain amino acid diet had a 2/3rds reduction in leucine, isoleucine, and valine, and the leucine diet had a 2/3rds reduction in only leucine. Mouse body composition was determined using an EchoMRI 3-in-1 Body Composition Analyzer (Houston, TX) according to the manufacturer’s procedures. For assay of multiple metabolic parameters (O2, CO2, food consumption) and activity tracking, mice were acclimated to housing in a Columbus Instruments Oxymax/CLAMS metabolic chamber system (Columbus, OH) for approximately 24 hours, and data from a continuous 24 hour period was then recorded and analyzed. All mice were sacrificed between 8am and 12pm after an approximately 16 hour fast. Plasma was prepared for analysis and stored at -80°C until needed; tissues for molecular analysis were flash-frozen in liquid nitrogen and prepared as described below. Samples of skin were isolated from the belly of mice on the indicated diets, and sections were paraformaldehyde-fixed (4%) overnight and then paraffin-embedded for evaluation.
Human clinical trial
Subjects-Experimental design and report were prepared following the CONSORT standards for randomized clinical trials (clinicaltrials.gov identifier NCT01692587). Male volunteers were recruited under a protocol approved by the IRB (No. 201011804) of the Washington University in St.Louis. Full details of this academic, single-center, parallel-group, Prospective, Randomized, Open-label, Blinded Endpoint (PROBE) trial and PR diet are provided in Supplemental Experimental Procedures.
Quantitative PCR
Total liver RNA was extracted with an RNeasy Mini Kit (Qiagen, Gaithersburg, MD, USA) according to the manufacturer’s instructions, or with Trireagent (Sigma). The concentration and purity of RNA were determined by absorbance at 260/280 nm, and 1μg ofRNA was used to generate cDNA (Superscript III; Invitrogen, Carlsbad, CA, USA). Oligo dT primers and primers for real-time PCR were obtained from Integrated DNA Technologies (Coralville, IA, USA). Reactions were run on an Applied Biosystems StepOne Plus machine (Applied Biosystems, Foster City, CA, USA) with Sybr Green PCR Master Mix (Invitrogen). Actin was used to normalize the results from gene-specific reactions. Primer sequences are given in Supplementary Table S4.
Statistics
Statistical analysis was conducted using Prism 6 (GraphPad Software). Glucose, insulin, and pyruvate tolerance tests were analyzed with two-way repeated-measures ANOVA, followed by a Tukey-Kramer or Dunnett’s post-hoc test as appropriate. All other comparisons of three or more means were analyzed by one-way ANOVA followed by a Dunnett’s or Tukey-Kramer post-hoc test as appropriate unless otherwise noted.
Islet isolation and ex vivo studies
Islets were isolated as previously described (Neuman et al., 2014). Full details of the ex vivo glucose stimulated insulin secretion (GSIS) assay and metabolic imaging are provided in Supplemental Experimental Procedures.
Study approval
Procedures involving animals were approved by the Institutional Animal Care and Use Committees of the University of Wisconsin-Madison and the William S. Middleton Memorial Veterans Hospital, Madison WI. Procedures involving human subjects were recruited under a protocol approved by the Institutional Review Board of the Washington University in St. Louis, and patients provided written informed consent prior to inclusion in the study.
Supplementary Material
Acknowledgements
We would like to thank all the members of the Lamming, Fontana, Kimple, Merrins and Alexander labs for their assistance and insight, and the Davis lab for their continual support. We would like to thank Dena Cohen for advice and consultation, Tina Herfel (Envigo) for assistance with the formulation of the amino acid defined diets, and the reviewers of this manuscript, which have greatly aided us by their suggestions. We thank Kathleen Obert, MS, RD, LD for the photograph of a sample dinner used in the slider image accompanying this manuscript, and Clint Thayer for assistance with preparation of the slider image. The Lamming lab is supported by a K99/R00 Pathway to Independence Award to D.W.L. from the National Institute of Health/National Institute on Aging (AG041765), a New Investigator Program Award from the Wisconsin Partnership Program, and a Glenn Foundation Award for Research in the Biological Mechanisms of Aging, as well as startup funds from the UW-Madison School of Medicine and Public Health and the UW-Madison Department of Medicine. This research was conducted while D.W.L. was an AFAR Research Grant recipient from the American Federation for Aging Research. The Fontana lab is supported by grants from the Bakewell Foundation, the Longer Life Foundation (an RGA/Washington University Partnership), the National Center for Research Resources (UL1 RR024992). This work was also supported by grants from the American Diabetes Association (1-14-BS-115) and the NIH/NIDDK (R01 DK102598) to M.E.K. The Merrins lab is supported in part by a Research Scientist Development Award from the NIH/NIDDK (K01 DK101683) and an Innovative Basic Science Award from the American Diabetes Association (1-16-IBS-212) to M.J.M. S.I.A.A. is supported by a fellowship from the American Diabetes Association (1-16-PMF-001). J.C.N. is supported in part by a training grant from the UW Institute on Aging (NIA T32 AG000213). I.K. is supported in part by McArdle Departmental Funds. S.E.C. was supported by the Rural and Urban Scholars in Community Health Program. This work was supported using facilities and resources from the William S. Middleton Memorial Veterans Hospital. This work does not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Author Contributions
LF, MJM, CMA, MEK, and DWL conceived the experiments and secured funding. NEC, SIAA, JCN, IK, BAS, EC, FS, VT, FAS, ELB, NV, SEC, RJF, BB, HKB, TP, ADB, RSF, GLD, and DWL performed the experiments. LF, NV, MJM, CMA, MEK and DWL analyzed the data. LF, NEC, MJM and DWL wrote the manuscript.
References
- Ables GP, Brown-Borg HM, Buffenstein R, Church CD, Elshorbagy AK, Gladyshev VN, Huang TH, Miller RA, Mitchell JR, Richie JP, et al. The first international mini-symposium on methionine restriction and lifespan. Front Genet. 2014;5:122. doi: 10.3389/fgene.2014.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander CM, Kasza I, Yen CE, Reeder SB, Hernando D, Gallo RL, Jahoda CA, Horsley V, MacDougald OA. Dermal White Adipose Tissue: A New Component of the Thermogenic Response. J Lipid Res. 2015 doi: 10.1194/jlr.R062893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, Banerjee S, Hawkins M, Chen W, Rossetti L. Caloric restriction reverses hepatic insulin resistance in aging rats by decreasing visceral fat. J Clin Invest. 1998;101:1353–1361. doi: 10.1172/JCI485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman RN, Kim SP, Catalano KJ, Hsu IR, Chiu JD, Kabir M, Hucking K, Ader M. Why visceral fat is bad: mechanisms of the metabolic syndrome. Obesity (Silver Spring) 2006;14(Suppl 1):16S–19S. doi: 10.1038/oby.2006.277. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy S, Wonderlich JA, Armstrong V, Rojanathammanee L. Altered dietary methionine differentially impacts glutathione and methionine metabolism in long-living growth hormone-deficient Ames dwarf and wild-type mice. Longev Healthspan. 2014;3:10. doi: 10.1186/2046-2395-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol. 2011;214:242–253. doi: 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nature communications. 2014;5:3557. doi: 10.1038/ncomms4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornu M, Oppliger W, Albert V, Robitaille AM, Trapani F, Quagliata L, Fuhrer T, Sauer U, Terracciano L, Hall MN. Hepatic mTORC1 controls locomotor activity, body temperature, and lipid metabolism through FGF21. Proc Natl Acad Sci U S A. 2014;111:11592–11599. doi: 10.1073/pnas.1412047111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Jahoda CA, Chuong CM, Watt FM, Horsley V. Defining dermal adipose tissue. Exp Dermatol. 2014;23:629–631. doi: 10.1111/exd.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Meng Q, Zhang Q, Guo F. Isoleucine or valine deprivation stimulates fat loss via increasing energy expenditure and regulating lipid metabolism in WAT. Amino Acids. 2012;43:725–734. doi: 10.1007/s00726-011-1123-8. [DOI] [PubMed] [Google Scholar]
- Edwards C, Canfield J, Copes N, Brito A, Rehan M, Lipps D, Brunquell J, Westerheide SD, Bradshaw PC. Mechanisms of amino acid-mediated lifespan extension in Caenorhabditis elegans. BMC Genet. 2015;16:8. doi: 10.1186/s12863-015-0167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Adelaiye RM, Rastelli AL, Miles KM, Ciamporcero E, Longo VD, Nguyen H, Vessella R, Pili R. Dietary protein restriction inhibits tumor growth in human xenograft models. Oncotarget. 2013;4:2451–2461. doi: 10.18632/oncotarget.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L. Promoting health and longevity through diet: from model organisms to humans. Cell. 2015;161:106–118. doi: 10.1016/j.cell.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, Klein S. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348:2082–2090. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- Grandison RC, Piper MD, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halkjaer J, Olsen A, Overvad K, Jakobsen MU, Boeing H, Buijsse B, Palli D, Tognon G, Du H, van der AD, et al. Intake of total, animal and plant protein and subsequent changes in weight or waist circumference in European men and women: the Diogenes project. Int J Obes (Lond) 2011;35:1104–1113. doi: 10.1038/ijo.2010.254. [DOI] [PubMed] [Google Scholar]
- Jang C, Oh SF, Wada S, Rowe GC, Liu L, Chan MC, Rhee J, Hoshino A, Kim B, Ibrahim A, et al. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat Med. 2016;22:421–426. doi: 10.1038/nm.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten Jennifer J., Hellwig Jennifer Pitzi, Meyers LD. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. The National Academies Press; Washington, D.C.: 2006. [Google Scholar]
- Kasza I, Suh Y, Wollny D, Clark RJ, Roopra A, Colman RJ, MacDougald OA, Shedd TA, Nelson DW, Yen MI, et al. Syndecan-1 is required to maintain intradermal fat and prevent cold stress. PLoS Genet. 2014;10:e1004514. doi: 10.1371/journal.pgen.1004514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, Munzberg H, Hutson SM, Gettys TW, Schwartz MW, et al. FGF21 is an endocrine signal of protein restriction. J Clin Invest. 2014a;124:3913–3922. doi: 10.1172/JCI74915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeger T, Reed SD, Henagan TM, Fernandez DH, Taghavi M, Addington A, Munzberg H, Martin RJ, Hutson SM, Morrison CD. Leucine acts in the brain to suppress food intake but does not function as a physiological signal of low dietary protein. Am J Physiol Regul Integr Comp Physiol. 2014b;307:R310–320. doi: 10.1152/ajpregu.00116.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagiou P, Sandin S, Weiderpass E, Lagiou A, Mucci L, Trichopoulos D, Adami HO. Low carbohydrate-high protein diet and mortality in a cohort of Swedish women. J Intern Med. 2007;261:366–374. doi: 10.1111/j.1365-2796.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- Lamming DW, Anderson RM. In eLS. John Wiley & Sons, Ltd; Chichester: 2014. Metabolic Effects of Caloric Restriction. [Google Scholar]
- Lamming DW, Cummings NE, Rastelli AL, Gao F, Cava E, Bertozzi B, Spelta F, Pili R, Fontana L. Restriction of dietary protein decreases mTORC1 in tumors and somatic tissues of a tumor-bearing mouse xenograft model. Oncotarget. 2015;6:31233–31240. doi: 10.18632/oncotarget.5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees EK, Krol E, Grant L, Shearer K, Wyse C, Moncur E, Bykowska AS, Mody N, Gettys TW, Delibegovic M. Methionine restriction restores a younger metabolic phenotype in adult mice with alterations in fibroblast growth factor 21. Aging Cell. 2014;13:817–827. doi: 10.1111/acel.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, Fontana L, Mirisola MG, Guevara-Aguirre J, Wan J, et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014;19:407–417. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Tian H, Lam KS, Lin S, Hoo RC, Konishi M, Itoh N, Wang Y, Bornstein SR, Xu A, et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013;17:779–789. doi: 10.1016/j.cmet.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nature reviews. Endocrinology. 2014;10:723–736. doi: 10.1038/nrendo.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R. Of mice and men: the benefits of caloric restriction, exercise, and mimetics. Ageing Res Rev. 2012;11:390–398. doi: 10.1016/j.arr.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrins MJ, Poudel C, McKenna JP, Ha J, Sherman A, Bertram R, Satin LS. Phase Analysis of Metabolic Oscillations and Membrane Potential in Pancreatic Islet beta-Cells. Biophysical journal. 2016;110:691–699. doi: 10.1016/j.bpj.2015.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrins MJ, Van Dyke AR, Mapp AK, Rizzo MA, Satin LS. Direct measurements of oscillatory glycolysis in pancreatic islet beta-cells using novel fluorescence resonance energy transfer (FRET) biosensors for pyruvate kinase M2 activity. J Biol Chem. 2013;288:33312–33322. doi: 10.1074/jbc.M113.508127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt ME, Harrison C, Sherry AD, Malloy CR, Burgess SC. Flux through hepatic pyruvate carboxylase and phosphoenolpyruvate carboxykinase detected by hyperpolarized 13C magnetic resonance. Proc Natl Acad Sci U S A. 2011;108:19084–19089. doi: 10.1073/pnas.1111247108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4:119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman JC, Truchan NA, Joseph JW, Kimple ME. A method for mouse pancreatic islet isolation and intracellular cAMP determination. Journal of visualized experiments : JoVE. 2014:e50374. doi: 10.3791/50374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunemaker CS, Bertram R, Sherman A, Tsaneva-Atanasova K, Daniel CR, Satin LS. Glucose modulates [Ca2+]i oscillations in pancreatic islets via ionic and glycolytic mechanisms. Biophysical journal. 2006;91:2082–2096. doi: 10.1529/biophysj.106.087296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porat S, Weinberg-Corem N, Tornovsky-Babaey S, Schyr-Ben-Haroush R, Hija A, Stolovich-Rain M, Dadon D, Granot Z, Ben-Hur V, White P, et al. Control of pancreatic beta cell regeneration by glucose metabolism. Cell Metab. 2011;13:440–449. doi: 10.1016/j.cmet.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluijs I, Beulens JW, van der AD, Spijkerman AM, Grobbee DE, van der Schouw YT. Dietary intake of total, animal, and vegetable protein and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-NL study. Diabetes Care. 2010;33:43–48. doi: 10.2337/dc09-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, Warren A, Huang X, Pichaud N, Melvin RG, et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014;19:418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon-Biet SM, Mitchell SJ, Coogan SC, Cogger VC, Gokarn R, McMahon AC, Raubenheimer D, de Cabo R, Simpson SJ, Le Couteur DG. Dietary Protein to Carbohydrate Ratio and Caloric Restriction: Comparing Metabolic Outcomes in Mice. Cell reports. 2015;11:1529–1534. doi: 10.1016/j.celrep.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truchan NA, Brar HK, Gallagher SJ, Neuman JC, Kimple ME. A single-islet microplate assay to measure mouse and human islet insulin secretion. Islets. 2015;7:e1076607. doi: 10.1080/19382014.2015.1076607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnaud AC, Norat T, Mouw T, Romaguera D, May AM, Bueno-de-Mesquita HB, van der AD, Agudo A, Wareham N, Khaw KT, et al. Macronutrient composition of the diet and prospective weight change in participants of the EPIC-PANACEA study. PLoS One. 2013;8:e57300. doi: 10.1371/journal.pone.0057300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, Walford RL. The retardation of aging and disease by dietary restriction. C.C. Thomas; Springfield, Ill., U.S.A.: 1988. [Google Scholar]
- Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Holloszy JO. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr. 2006;84:1033–1042. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F, Huang Z, Li H, Yu J, Wang C, Chen S, Meng Q, Cheng Y, Gao X, Li J, et al. Leucine deprivation increases hepatic insulin sensitivity via GCN2/mTOR/S6K1 and AMPK pathways. Diabetes. 2011;60:746–756. doi: 10.2337/db10-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F, Yu J, Guo Y, Deng J, Li K, Du Y, Chen S, Zhu J, Sheng H, Guo F. Effects of individual branched-chain amino acids deprivation on insulin sensitivity and glucose metabolism in mice. Metabolism. 2014;63:841–850. doi: 10.1016/j.metabol.2014.03.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.