Abstract
Given the increased utility and lack of consensus regarding carbon nanotube (CNT) environmental and human health hazards, there is a growing demand for guidelines that inform safer CNT design. In this study, the zebrafish (Danio rerio) model is utilized as a stable, sensitive biological system to evaluate the bioactivity of systematically modified and comprehensively characterized multi-walled carbon nanotubes (MWNTs). MWNTs were treated with strong acid to introduce oxygen functional groups, which were then systematically thermally reduced and removed using an inert temperature treatment. While 25 phenotypic endpoints were evaluated at 24 and 120 hours post fertilization (hpf), high mortality at 24 hpf prevented further resolution of the mode of toxicity leading to mortality. Advanced multivariate statistical methods are employed to establish a model that identifies those MWNT physicochemical properties that best estimate the probability of observing an adverse outcome. The physicochemical properties considered in this study include surface charge, percent surface oxygen, dispersed aggregate size and morphology, and electrochemical activity. Of the five physicochemical properties, surface charge, quantified as the point of zero charge (PZC), was determined as the best predictor of mortality at 24 hpf. From a design perspective, the identification of this property-hazard relationship establishes a foundation for the development of design guidelines for MWNTs with reduced hazard.
Keywords: Carbon Nanotube Toxicity, Zebrafish (Danio rerio), Predictive Toxicity, Safe Material Design, Property-Hazard Relationship
INTRODUCTION
There are numerous current and projected applications of carbon nanotubes (CNTs) spanning a range of sectors, including energy, electronics, and healthcare.(De Volder et al., 2013, Endo et al., 2008) These applications are inspired by the unique physical and chemical properties of CNTs, including tensile strength, electronic and thermal conductivity, aspect ratio, and antimicrobial activity, to name a few.(Endo et al., 2008, Popov, 2004) Of the two primary classes of CNTs, single- (SWNT) and multi-walled (MWNT), MWNTs are primarily utilized in high concentration, low order applications and accounted for 95% of the total 2010 CNT production value.(Patel, 2011) As such, there is increased likelihood of environmental and human exposure to MWNTs considering the various pathways of release throughout the life cycle.(U.S.E.P.A, 2014) Given the current lack of consensus regarding the MWNT hazard profile, this is potentially of concern for environmental and human health.
To better understand and resolve the specific material properties that can induce MWNT environmental and human health hazard, zebrafish (Danio rerio) were selected as the model organism. Zebrafish are considered a preferred in vivo vertebrate model organism for assessing the toxicity of nanomaterials for multiple reasons.(Fako and Furgeson, 2009, Lin et al., 2012, Usenko et al., 2007, Rizzo et al., 2013) Zebrafish possess genetic parallels with humans enabling projection and understanding of potential mechanisms of human toxicity.(Fako and Furgeson, 2009) Zebrafish also have a remarkable degree of molecular and physiological conservation with other vertebrates, particularly during embryonic development.(Amsterdam et al., 2004) Exposure to exogenous compounds during this sensitive life stage can disrupt key developmental processes such as cell differentiation, organogenesis, apoptosis, proliferation, ion regulation and neurogenesis, which manifest as craniofacial malformations, edema, disrupted vasculature and muscles, abnormal evasion response and even death.(Harper et al., 2011, Hill et al., 2005) Further, zebrafish are favorable for high throughput toxicity screening methodologies as they facilitate rapid testing of numerous chronic and acute endpoints, simultaneous testing of a range of concentrations, and the potential to probe detailed mechanisms of toxicity.(Harper et al., 2011)
While there is a significant body of research investigating the impacts of CNTs at the cellular level (Johnston et al., 2010, Kang et al., 2008a, Kang et al., 2008b, Kang et al., 2007, Vecitis et al., 2010, Pasquini et al., 2012, Pasquini et al., 2013, Liu et al., 2009) and using mammalian models (Kane and Hurt, 2008, Lam et al., 2004, Nel et al., 2006, Muller et al., 2005, Kolosnjaj-Tabi et al., 2010, Lam et al., 2006), those studies pertaining to higher order aquatic organisms are more disparate (Baun et al., 2008, Scown et al., 2010). In particular, only a handful of studies, to date, investigate the impact of CNTs on zebrafish (Adenuga et al., 2013, Asharani et al., 2008, Cheng et al., 2009, Cheng and Cheng, 2012, Cheng et al., 2007), and there are significant variations in the experimental methodologies. A seminal paper by Cheng, et al. found that the chorion serves as a protective barrier, preventing the interaction between the CNT aggregates and the embryo.(Cheng et al., 2007) While they observed delayed hatching, there were no observed developmental or mortality implications.(Cheng et al., 2007) To facilitate direct exposure between CNTs and the embryo, two alternative procedures were developed. The first is a microinjection technique, which is administered at the one-cell stage (Cheng et al., 2009, Cheng and Cheng, 2012) or 8-cell stage (Asharani et al., 2008). The second method involves enzymatic dechorionation of the embryos 4 hours post fertilization (hpf), prior to CNT exposure.(Mandrell et al., 2012, Truong et al., 2011) This latter technique is employed in the present study because it is automated, quick, cost effective, and more precise than the manual alternative.(Mandrell et al., 2012)
While the current CNT zebrafish literature thoroughly evaluates both lethal and sublethal endpoints, there is often a lack of comprehensive physicochemical characterization of the nanomaterials necessary to draw meaningful conclusions or to compare results between studies.(Asharani et al., 2008, Cheng et al., 2009, Cheng and Cheng, 2012, Cheng et al., 2007) The approach employed here is unique from those presented in previous CNT zebrafish studies in that the MWNTs: 1) originated from the same starting batch, which eliminates the potential of confounding variables associated with batch heterogeneity.(Jones et al., 2008); 2) were systematically modified, minimizing the number of properties being varied at one time; and 3) were comprehensively characterized for those intrinsic and extrinsic properties most pertinent to their toxicity including surface charge, oxygen content, dispersed aggregate radius and morphology, and electrochemical activity.(Fubini et al., 2010, Powers et al., 2007)
The ability to extrapolate relationships between physicochemical properties and observed trends in adverse outcomes will improve our understanding of the underlying toxicity mechanisms as well as promote the design of CNTs for enhanced performance and minimized hazard. To address the current knowledge gap, this study intends to inform the development of property-hazard relationships for a given set of MWNTs by developing a logistic model that relates MWNT physicochemical properties and zebrafish toxicity.
The study presented herein has three primary goals: 1) to determine whether the established correlating trend of MWNT electrochemical activity and bacterial cytotoxicity(Gilbertson et al., 2014, Pasquini et al., 2013) is maintained for higher trophic organisms (i.e., zebrafish), 2) to establish a model that relates the observed trend in the adverse effects in zebrafish with physicochemical properties of MWNTs, and 3) to utilize the model as a first step towards developing a foundational understanding of the underlying mechanism of the bioactivity of MWNTs. The combined results aim to elucidate the toxic potential of MWNTs and to inform their future inherently safer design.
METHODS
MWNT preparation
Pristine MWNTs were purchased from CheapTubes (Burlington, VT, CCVD, >95 wt %, 10–20 nm diameter, 10–20 μm length).(CheapTubes, 2014) These as-received MWNTs were acid treated in house via reflux in nitric acid (HNO3, 15.7 M) for 2 h. This acid treated (AT 2) MWNT sample was then annealed (heat treatment under inert conditions) at increased maximum temperature (400–900 °C, 100 °C increments) and served as the model training set. A separate set of MWNTs, purchased from NanoLab Inc. (Waltham, MA 15 ± 5 nm diameter, 5–20 μm length, >95% purity), was prepared and utilized to confirm the logistic model.(NanoLab, 2011) These as-received MWNTs (NL-AR) were treated by the manufacturer using a sulfuric-nitric acid mix and then annealed in house at a subset of the previously applied temperatures (400, 600, and 900 °C).
Annealing enabled systematic modifications of MWNT properties via reduction of surface oxygen groups that were added during the acid treatment. The various functional group moieties (e.g. carboxylic acid, hydroxyl, and carbonyl) possess different thermal properties.(Figueiredo et al., 1999) As the maximum annealing temperature increases, the more labile groups elude the MWNT surface while the more thermally stable groups remain (illustrated in the schematic in Table 2). The annealing conditions remained consistent for all samples, including heating under helium (He) at the indicated maximum temperature for 1 h (10° per min heating rate). Compiled treatment conditions for all of the MWNT samples utilized in this study can be found in Table 1.
Table 2.
Compiled characterization data including point of zero charge (PZC) by titration, percent oxygen (% O) by X-ray photoelectron spectroscopy (XPS), fractal dimension (Df) by static light scattering (SLS), aggregate radius by dynamic light scattering (DLS), electrochemical activity (E1/2) by oxygen reduction reaction (ORR) and the rotating disc electrode method (RDE), and a schematic representation of the distribution and type of functional group with increased annealing temperature.
| Model Development Sample Set *
| ||||||
|---|---|---|---|---|---|---|
| MWNT Sample | Point of Zero Charge (PZC, pH) | Surface Oxygen (%O) | Morphology (Df) | Aggregate Radius | Half-Wave Potential (E1/2, V) | Schematic Representation of Oxygen Functional Group Distribution |
|
| ||||||
| AT 2 | 3.08 | 7.6 | 1.85 | 37.5 | −0.219 |

|
| AT2-400 | 4.16 | 4.6 | 1.84 | 48.8 | −0.221 | |
| AT2-500 | 4.73 | 3.8 | 2.05 | 57.2 | −0.216 | |
| AT2-600 | 6.11 | 3.5 | 2.07 | 37.5 | −0.127 | |
| AT2-700 | 6.35 | 3 | 1.82 | 39.5 | −0.215 | |
| AT2-800 | 6.43 | 2.4 | 2.07 | 92.1 | −0.244 | |
| AT2-900 | 9.42 | 1.3 | 2.14 | 48.8 | −0.244 | |
|
| ||||||
| Model Validation Sample Set | ||||||
|
| ||||||
| NL-AR | 3.1 | 8.5* | 1.24 | 41.69 | −0.235* | |
| NL-400 | 4.9 | 4.4* | 1.92 | 51.52 | −0.215* | |
| NL-600 | 7.4 | 3* | 1.7 | 46.33 | −0.214* | |
| NL-900 | 8.6 | 1.2* | 2.42 | 67.67 | −0.238* | |
This data is previously published (Gilbertson et al., 2014)
Table 1.
MWNT samples and treatment conditions.
| Model Development Sample Set
| ||
|---|---|---|
| MWNT Sample | Acid Treatment | Annealing Treatment (Max Temp) |
|
| ||
| AT 2 | 2 hr reflux 70% HNO3 | |
| AT2-400 | 2 hr reflux 70% HNO3 | 400 °C |
| AT2-500 | 2 hr reflux 70% HNO3 | 500 °C |
| AT2-600 | 2 hr reflux 70% HNO3 | 600 °C |
| AT2-700 | 2 hr reflux 70% HNO3 | 700 °C |
| AT2-800 | 2 hr reflux 70% HNO3 | 800 °C |
| AT2-900 | 2 hr reflux 70% HNO3 | 900 °C |
|
| ||
| Model Validation Sample Set | ||
|
| ||
| NL-AR | H2SO4:HNO3 By Manufacturer | |
| NL-400 | H2SO4:HNO3 By Manufacturer | 400 °C |
| NL-600 | H2SO4:HNO3 By Manufacturer | 600 °C |
| NL-900 | H2SO4:HNO3 By Manufacturer | 900 °C |
MWNT characterization
The physicochemical properties of the MWNT samples used in this study were comprehensively characterized for sample purity by thermogravimetric analysis (TGA), surface charge by point of zero charge (PZC), elemental composition by x-ray photoelectron spectroscopy (XPS), dispersed aggregate size and size distribution by dynamic light scattering (DLS), dispersed aggregate morphology by static light scattering (SLS), and electrochemical activity by the oxygen reduction reaction (ORR) using the rotating disc electrode method (RDE). Dispersed aggregate morphology is quantified here by the fractal dimension (Df), which is evaluated using the static light scattering technique, and is representative of the aggregate compactness and shape (Df = 1: rod-like morphology, Df = 3: sphere-like morphology).(Schaefer et al., 2003, Chen et al., 2004) Relative MWNT surface charge was determined via PZC using a mass titration method.(Pasquini et al., 2013, Noh and Schwarz, 1988) The point of zero charge is the pH at which there is a net zero surface charge, and thus, the values reported in Table 2 are pH values obtained using the previously described titration method. Details on the characterization methods utilized in this study can be found in the Supplementary Information. Table 2 contains the compiled characterization data, including some previously published data associated with our bacterial cytotoxicity study.(Gilbertson et al., 2014)
Toxicity testing
At 4 hour post fertilization (hpf), the acellular chorion was enzymatically removed from tropical 5D zebrafish embryos using pronase following an established protocol described in Mandrell et al.(Mandrell et al., 2012) At 6 hpf, embryos were rinsed and transferred to a low ionic strength 62.5 μM CaCl2-2H2O (Sigma Aldrich) medium prepared in ultrapure water (Invitrogen) prior to loading (one embryo per well) into 96-well plates (Falcon) containing 0.05 mL of 62.5 μM CaCl2-2H2O. A low ionic strength 62.5 μM CaCl2-2H2O medium was employed in this assay to minimize MWNT aggregation while maintaining normal zebrafish development. On the day of exposure, MWNTs were suspended in a 10% dimethylsulfoxide (DMSO; J.T.Baker) solution prepared with ultrapure water to create a 1 mg/mL MWNT stock. The stock was sonicated indirectly for 30 minutes via Cup Horn with a Fisher Scientific 60 Sonic Dismembrator at 20 kHz. Immediately following sonication, MWNT stock was diluted in CaCl2-2H2O water to prepare a five-fold, 2X MWNT dilution series ranging from 100-0.16 mg/L with DMSO (1%) and CaCl2-2H2O (62.5 μM). At 8 hpf, 0.05 mL of the 2X MWNT suspension was added to 96-well plates for an n = 48 per exposure concentration. Zebrafish underwent a static nonrenewal exposure to 1X MWNT (50-0.08 mg/L), 0.5% DMSO and 62.5 μM CaCl2-2H2O for five days maintained at 28 ± 1°C in the dark. The concentration range utilized is the standard range for this established method.(Truong et al., 2011, Adenuga et al., 2013) At 24 hpf, embryos were visually evaluated by stereomicroscope for mortality, spontaneous tail flexion, notochord malformations and delayed developmental progression. At 120 hpf, zebrafish were evaluated for mortality, phenotypic malformations of the axis, yolk sac, eye, jaw, snout, brain, otic vesicle, somites, heart, circulation, caudal and pectoral fins, swim bladder and behavioral response to mechanical stimulus (Table S1). Results were recorded using a binary system of 1 for the presence of an endpoint and 0 for absence. For a more detailed description of the assay, refer to Truong et al.(Truong et al., 2011) All experiments were conducted in accordance with Institutional Animal Care and Use Committee protocols at Oregon State University
Statistical Analysis: Model Selection and Development
All statistical analyses were conducted using the R version 3.02 statistical software.(R_Core_Team., 2013)
The logistic model
A logistic model was used to fit the MWNT physicochemical property and zebrafish toxicity data to provide a means of deriving a relationship between the probability of zebrafish embryo mortality (p) and specific MWNT properties. The nature of the relationship between continuous property data and the binomial toxicity outcome suggests the appropriate use of binomial regression. Binomial models are commonly used for toxicity outcomes (Morgan, 1992) and have three major mathematical advantages when modeling binary data, including adequate consideration of inconsistent variance, no necessity for lack of normality assumptions, and no range restrictions imposed by binary response variables. The logistic binomial model was chosen for this study because it is easily interpretable and there was no available information on success asymmetry.(Collett, 1991)
In this study, logistic regression was used to predict the category of outcome (e.g. dead or alive) for individual embryos optimizing the model for minimal complexity. Logistic regression calculates the outcome as odds ratio of embryo death, or probability of death over the probability of survival according to Equation 1:
| ( Collett, 1991) | (1) |
where pi = p[xi=1] =1-p[xi=0] and xi is the independent variable corresponding to ith observation in the sample i = 1, 2, …, n. In this case pi or p[xi=1], is the probability of observing a toxic effect and 1-pi or p[xi=0] is the probability of embryo survival.
Five MWNT physicochemical properties were included as potential predictor variables in the univariate and multivariate analysis to elucidate the toxicity outcome. The affection of percent surface oxygen, point of zero charge, dispersed aggregate radius, dispersed aggregate morphology, and electrochemical activity, and their interactions, on zebrafish embryo toxicity is considered. In logistic regression, these predictor variables are related to the probability of mortality as shown in Equation 2:
| ( Collett, 1991) | (2) |
where β0 is the intercept coefficient, β1 through βk are parameter coefficients for independent variables 1 though k, and x1 through xk are measured parameters for the specific MWNT sample exposed to zebrafish embryo i.
Equation 3, a rearrangement of equations 1 and 2, represents the general form of logistic regression for the logit probability function.
| (3) |
The parameters of the logistic model β0 though βk are derived by the method of maximum likelihood. Under the assumption of the logistic regression, the chosen coefficients maximize the probability or likelihood of the observed data.(Collett, 1991)
Model training and selection
The model was trained on a set of seven systematically treated MWNT (see Table 1 for treatment details) with 240 embryonic zebrafish tested for each MWNT type (48 embryos per concentration, 5 exposure concentrations). An iterative model selection process was used to derive the most parsimonious model without over fitting the data. We used Akaike information criterion (AIC), Wald test, and likelihood-ratio test to compare and select model fits.(Fox, 1997) Parsimonious models with clear interpretations were preferred, when statistical parameters were similar. Z-score tests were used to assess individual parameter contribution to the overall model. Likelihood ratio, Wald, and the more conservative le Cessie-van Houswelingeen-Copas-Hosme tests (le Cessie and van Houwelingen, 1991) were also used to assess model differences
Model validation
The final multivariate model performance was tested on a distinct set of four MWNT samples (see Table 1 for treatment details) under the same methodological conditions as previously described for the toxicity evaluation.
RESULTS
Physicochemical properties of the MWNTs
Various techniques were utilized here to characterize the intrinsic and extrinsic properties of the MWNT sample set. The data is compiled in Table 2, including a schematic that illustrates the change in surface oxygen groups with increased annealing temperature.(Gilbertson et al., 2014) There are some identifiable trends in the compiled data. First, there is a systematic decrease in the percent surface oxygen with increased annealing temperature due to the varying thermal stability of oxygen functional groups on MWNT surfaces.(Kundu et al., 2008) The corresponding increase in the PZC with increased annealing temperature results from the lower thermal stability of the more acidic functional groups, including carboxylic acid (COOH) and hydroxyl (OH), relative to more basic oxygen moieties, such as carbonyl (C=O) containing anhydride, ester, and quinone.(Figueiredo et al., 1999, Montes-Moran et al., 2004, Gilbertson et al., 2014)
There are no discernable trends in the dispersed aggregate radius or morphology despite the significant change in surface oxygen and PZC. The addition of oxygen functional groups is commonly used to enhance SWNT dispersion yet, MWNTs are inherently more dispersible than SWNTs.(Hilding et al., 2003) As such, the changes in surface oxygen do not significantly influence the dispersity of the MWNT samples used in this study. Finally, there is a positive shift in the half-wave potential, E1/2, of the MWNT annealed at 600 °C, which is indicative of enhanced activity toward oxygen reduction.(Yeager, 1984) It was previously shown that this shift correlates with the observed trend in E.coli cytotoxicity.(Pasquini et al., 2013) Pasquini, et al. hypothesize that the combined reduction of carboxylic acid groups and presence of carbonyl groups, particularly quinone moieties, is optimized around 600 °C and results in a significant increase in the MWNT electrochemical and antimicrobial activity.(Gilbertson et al., 2014) The five characterized properties (percent oxygen, PZC, aggregate radius, aggregate morphology, and electrochemical activity) are used in the development of a predictive model for zebrafish toxicity.
MWNT toxicity profiles
Impact on Embryos
During early developmental stages, embryonic zebrafish interact with the MWNT samples in concentrations ranging from 0.8–50 mg/L. Figure 1 includes representative images under different concentrations and at various stages of development. The images indicate that the MWNTs under all exposure concentrations conditions aggregate and settle over time. When immobile, the interaction between embryo and the MWNTs depends on direct contact between the settled aggregates to the stationary embryo (Figure 1). Once mobile (> 36 hpf), the zebrafish movement within the plate well disrupts the MWNT aggregates thus, promoting interaction.
Figure 1.
Representative images of the zebrafish embryos under varying MWNT concentrations (a–c) and at different stages of development (d–e). a–c show an increasing presence of MWNT aggregates with increased concentration for embryos 8 hpf. The zebrafish shown in d–e are at 120 hpf, with e) indicating the accumulation of MWNT aggregates at the gills (red arrow).
Concentration-Response
Exposure to all MWNTs at concentrations below 50 mg/L induced minimal adverse response. However, several samples halted developmental progression at the highest exposure concentration (50 mg/L) due to significant mortality prior to 24 hpf. The high prevalence of mortality at 24 hpf prevented further evaluation and determination of whether MWNT exposure significantly influenced the manifestation of signature morphological malformations in the zebrafish indicative of more chronic endpoints. Thus, the statistical model development utilizes mortality as the most relevant endpoint for MWNT toxicity prediction. Figure 2 shows the mortality at 24 hpf induced by exposure to the seven MWNT samples treated as previously described.
Figure 2.
Compiled concentration-response curves for seven differently treated MWNTs and embryonic zebrafish mortality at 24 hpf. MWNT treatment details are outlined in Table 1. Briefly, AT 2 MWNTs were acid treated for 2 hours and then annealed at 100° increments from 400 – 900 °C (AT2-400, -500, -600, -700, -800, -900). Dispersed MWNT samples were pre loaded to wells at concentrations ranging 0 – 50 mg/L prior to adding the dechorionated embryos 6–8 hpf. Increased mortality at 24 hpf was observed only at 50 mg/L concentration.
There is a significant increase in mortality upon exposure at 50 mg/L compared to the other concentrations. This trend is most notable for the AT 2, AT2-400, and AT2-500 samples, and diminishes for those samples treated at higher annealing temperatures. Due to the highly pronounced differences in mortality at 50 mg/L exposure concentration, and little to no observed effects at and below 10 mg/L, the development of the logistic model relied exclusively on the 50 mg/L results.
Exploratory statistics used to inform the multivariate model
Parameter correlations
In order to create a model for adverse zebrafish effects, we first explored the linear relationships between five individual MWNT properties and zebrafish mortality at 24 hpf (Figure 3). As evident from Figure 3, PZC and percent surface oxygen result in the highest linear correlation with mortality at 24 hpf (R2 = 0.647 and 0.665, respectively). This suggests that of the previously described MWNT properties, percent surface oxygen and surface charge (PZC) are likely to have a significant influence on the observed trend in mortality. The remaining predictors show some linear correlation with observed zebrafish mortality in order of decreasing correlation: aggregate morphology, aggregate radius, and the electrochemical activity (E1/2).
Figure 3.
Linear correlations between the five measured MWNTs physicochemical properties and embryonic zebrafish mortality observed at 24 hpf. The MWNT properties are in the order of decreasing correlation with the 24 hpf mortality. Surface oxygen (%) and point of zero charge (PZC) are the best linear predictors of mortality. Dispersion measurements, including fractal dimension and aggregate radius are minimally correlated with the mortality endpoints. Finally, the correlation between MWNT reactivity (half-wave potential) and mortality is negligible. The 95% confidence interval for the linear regression between MWNT properties and mortality is shown in grey.
Parameter relationships
It is expected that certain MWNT properties would correlate highly with each other. Pearson correlation analysis was used to test property association and remove highly related properties prior to model development. The compiled correlation plots for the measured MWNT properties can be found in Figure S1. As expected, PZC and percent surface oxygen are highly correlated (R = 0.901). As explained above, this relationship is expected because the charge distribution on the MWNT surface is dependent on the nature of the surface functional group.(Figueiredo et al., 1999, Montes-Moran et al., 2004) When two highly co-linear predictors were available during the model development, only one parameter was selected. In the case of PZC and percent surface oxygen, PZC was chosen as the more ubiquitous property that would be more prudent for future model testing and implementation as it is applicable to CNTs functionalized or doped with elements other than oxygen including nitrogen and sulfur. In addition, conclusions drawn herein related to PZC are potentially relevant to other classes of nanomaterials. As such, PZC and the remaining three properties were considered in the model development as potential predictors for zebrafish toxicity.
Statistical model for predicting zebrafish mortality
A derived logistic regression was used to elucidate the relationship between intrinsic and extrinsic MWNT physicochemical properties and their influence on the mortality of zebrafish. The final four MWNT properties that were considered as potential predictors of mortality at 24 hpf include PZC, aggregate size, aggregate morphology, and electrochemical activity. The percent surface oxygen was eliminated due to co-linearity considerations as described above. The toxicity was assessed with seven different MWNTs and 48 embryos tested for a given concentration. The final model was selected based on highest explanatory power and biological relevance, while maintaining preference for minimal complexity. The logistic model uses PZC as the sole significant predictor (p = 0.000) of mortality to provide robust, interpretable results, without overfitting. The model and associated significance tests are provided in Table 3.
Table 3.
24-hour Zebrafish Mortality Model Summary
| Parameter | Estimate | Std. Error | z-score | p-value | Pr(>|z|) |
|---|---|---|---|---|---|
|
| |||||
| (Intercept) | 5.10 | 0.64 | 8.02 | 0.000 | *** |
| PZC | −1.10 | 0.12 | −8.85 | 0.000 | *** |
|
| |||||
| Null Deviance | 428.3 | DoF: 334 | AIC: 293.02 | ||
| Residual Deviance | 289.0 | DoF: 333 | |||
|
| |||||
| Likelihood Ratio Test | 139.3 | DoF: 1 | 0.000 | *** | |
| Le Cessie Test | 46.7 | 0.59 | −5.944 | 0.000 | *** |
Notes:
Significance codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘ ‘ 1
DoF - Degrees of Freedom
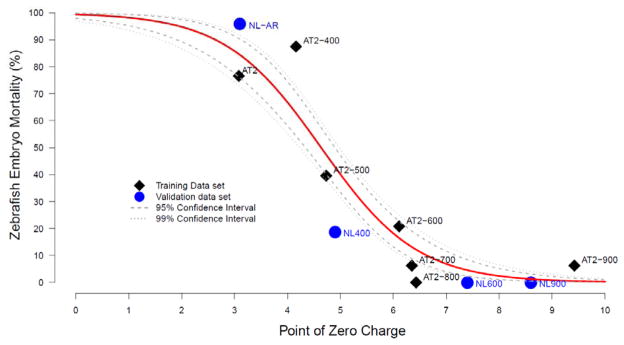
For each unit increase in PZC, the natural logarithm of toxicity odds ratio decreases by 1.1 (95% CI = 1.35:0.86). The relative decrease in odds ratio for each unit of PZC increase is 0.33 (95% CI = 0.26–0.42). The model significantly improves the understanding of the relationship between MWNT properties and embryonic zebrafish toxicity. Both the likelihood ratio and le Cessie-van Houwelingen-Copas-Hosmer unweighted sum of squares tests of significance confirm the explanatory power of the model (p = 0.000). Higher PZC is associated with lower probability of mortality at 24 hpf. Figure 4 illustrates the model relationship between probability of embryo mortality and MWNT PZC.
Figure 4.
A graphical representation of the established statistical model showing the effect of PZC on the 24 hpf mortality model when other covariates are held constant. Surface charge (quantified as the point of zero charge, PZC) is the best single estimator of toxicity and the correlation indicates that the greater the PZC, the lower the magnitude of toxicity.
Model validation
To validate the model and its ability to predict zebrafish mortality based on surface charge, four new MWNT samples were prepared and tested as described in the methods section. These MWNTs were purchased from another vendor and acid treated under different conditions to ensure robustness across MWNT batches and acid treatment conditions. The annealing temperatures for the test set include 400, 600, and 900 °C to obtain MWNT samples from each significant shift in PZC. All other conditions were held constant. Table 4 summarizes the predicted and measured results for the training set.
Table 4.
Model Validation Results
| Sample Name | NL-AR | NL-400 | NL-600 | NL-900 |
|---|---|---|---|---|
| Predicted Probability (CI) | 0.84 (0.76–0.90) | 0.42 (0.50–0.35) | 0.04 (0.08–0.02) | 0.01 (0.03–0.005) |
| Measured Probability | 0.98 | 0.19 | 0.00 | 0.00 |
| Numbr of Embryos Tested | 47 | 47 | 47 | 48 |
| Observed # of dead embryos | 46.0 | 9 | 0 | 0 |
| Expected # dead embryos (CI) | 39 (36–42) | 20 (16–23) | 2 (4–1) | 0 (1–0) |
The model was able to predict the mortality at 24 hpf for the MWNT validation sample set as illustrated by reasonable agreement between the predicted and measured probability in Table 4. The data for these four validation samples are also included in Figure 4 (blue circles) and follow the same trend as the training sample set.
DISCUSSION
As the field of nanotechnology matures, there is an increased demand to resolve the potential environmental and human health implications resulting from the utilization, handling, and disposal of nanomaterials. Lessons learned from toxic legacy chemicals have motivated a paradigm shift and the development of predictive toxicity models that can be used to estimate a molecule’s hazard potential without investing the time and resources necessary to complete the suite of conventional in vivo toxicity assessments (e.g. mammalian and aquatic studies).(Kostal et al., 2013, Voutchkova-Kostal et al., 2012, Connors et al.) While there are added complexities when applying this approach to nanomaterials, it is imperative to engage in the resolution of a set of design guidelines to inform the development of nanomaterials with reduced hazard, ultimately facilitating sustainable growth of the field by preventing unintended consequences.
The study presented herein, is the first to establish a statistical model to explain the observed adverse response of embryonic zebrafish upon exposure to carbon nanotubes. The model is informed by comprehensive physicochemical property characterization of systematically modified MWNTs from the same starting batch, which minimizes the number of intrinsic properties being altered at one time and avoids batch-to-batch heterogeneity, respectively. The importance of such an approach has recently been identified as a primary research objective and is intended for simultaneous resolution and optimization of the performance and safety of nanomaterials.(Harper et al., 2011, Liu et al., 2013, N.R.C., 2012, N.N.I., 2011)
The logistic model based on the analysis of seven MWNT samples and four independent physicochemical properties is summarized in Table 3 and indicates that MWNT surface charge is the best predictor of zebrafish mortality at 24 hpf. The model was developed on MWNT samples with PZC values between 3 and 9. Thus, there is high confidence in the relevance of the model for carbon nanotubes with PZC properties within this range.
The current one-parameter model explains the relationship between the MWNT surface charge and the associated zebrafish toxicity. The relationship explains a large portion of variability in the toxicity data (McFadden’s Pseudo R2 = 0.32). McFadden’s Pseudo R2 is a measure similar to R2 in linear regression and is used for maximum likelihood estimates, such as logistic regression. McFadden’s Pseudo R2 values between 0.2 and 0.4 are indicative of good fit.(McFadden, 1974) The established PZC-mortality relationship is promising from a material design perspective, providing a tunable parameter that can be impacted by rational design. However, with additional data (e.g. differently treated MWNTs and additional measured endpoints at MWNT concentrations between 10 and 50 mg/L), the remaining toxicity variability and the relationship between MWNT properties can be further explained leading to enhanced predictive power.
In previous studies, using the same sample set, MWNT electrochemical activity was found to correlate with the observed trend in bacterial cytotoxicity.(Gilbertson et al., 2014, Pasquini et al., 2013) Controlled modification of surface oxygen functional groups significantly influenced the potential for the MWNTs to facilitate redox reactions and thus, the potential to disrupt healthy cellular function.(Gilbertson et al., 2014) This finding highlights the important contribution of the chemical mechanism to cell viability.(Liu et al., 2013) A goal of the current study is to evaluate whether this correlation was maintained for a higher trophic level aquatic organism, which we found was not the case. This is an increasingly important consideration as various stake holder communities attempt to reconcile generalizable hazard profiles for all classes of nanomaterials.(N.R.C., 2012, Nel et al., 2013)
The results presented here indicate that MWNT surface charge, not electrochemical activity, has the greatest influence on zebrafish mortality. Surface charge is known to influence the aggregation behavior of nanomaterials.(Petosa et al., 2010) The available contact area will increase with enhanced dispersion, including decreased aggregate size and less compact morphology (Df ≤ 2). This will enhance contact between the developing embryos and MWNT samples as well as promote MWNT uptake, both of which can lead to increased lethality. In addition, enhanced dispersion will promote molecular level interactions necessary for the production of reactive oxygen species (ROS), which can induce oxidative stress related adverse developmental outcomes and embryonic mortality. Yet, characterization of the MWNTs used in this study reveal that dispersed aggregate properties do not play a critical role in the observed mortality at 24 hpf. Thus, the surface charge must be impacting the bioactivity of the MWNTs through a mechanism other than aggregation.
The charged nature of the MWNT surface may also influence the interaction with the organism.(Saxena et al., 2007) The pH of the media used in this study is between 6 and 7; the system was intentionally not buffered in an effort to reduce ionic strength induced aggregation of the MWNTs.(Truong et al., 2012) Therefore, the MWNTs associated with the greatest embryonic zebrafish mortality at 24 hpf possessed a negatively charged surface (PZC less than ~6). Several recent studies have explored the impact of nanomaterial surface charge, controlled by functionalizing with cationic, neutral, or anionic ligands, on observed adverse outcomes using either Daphnia magna or embryonic zebrafish as the in vivo model organism.(Harper et al., 2011, Lee et al., 2013, Bozich et al., 2014) Results from these studies elucidate the important role that surface charge plays in the observed toxicity trends, which is hypothesized to be governed primarily by the electrostatic interaction between the material and model organism. Yet, inconsistencies among the findings remain unresolved due to the complexities that result from differences in the nanomaterial studied (i.e. Au, Ag, C60, CNTs) and in vivo model organism utilized. While the importance of nanomaterial surface charge has been established, the underlying mechanism is not yet elucidated. As such, this is the subject of ongoing research.
The observed disparity in the driving force of viability between bacteria (E.coli ) and zebrafish for the same MWNT sample set is not entirely surprising considering variations in methodological exposure conditions and the differences in biological response between bacteria and zebrafish. That said, analysis and establishment of statistical models for alternative adverse developmental endpoints, particularly those sensitive to oxidative stress, would provide further resolution of the mechanism of zebrafish mortality and may reveal the important contribution of additional MWNT properties. Elucidation of the underlying mechanism of toxicity is the subject of ongoing research.
A primary limitation of the current study is the sample size. Due to the complexities of preparing and comprehensively characterizing a controlled sample set as well as the motivation to compare to previous studies completed at the cellular level, the sample set was limited here to seven MWNTs. As such, a confirmation sample set of four MWNTs, (also previously evaluated at the cellular level) was utilized to confirm the significance of the resulting model. With the model and statistical methods established, further robust analysis can be applied to sample sets of interest when they become available. Additional data would allow for more complex hypothesis testing with fewer concerns for overfitting during model development. Another limiting factor to consider in this study is the reduced number of observations of the other adverse endpoints. Since mortality at 24 hpf was significant for several of the MWNTs, the number of available observations for the remaining developmental endpoints was reduced. This prevented the ability to draw significant statistical conclusions for other developmental endpoints.
Future research will focus on resolving the underlying mechanism of toxicity probing additional endpoints and using more specific assays. In particular, measures of transcriptional, proteomic, and metabolomics profiles coupled with in situ detection methods can be used to identify adverse outcome pathways and the primary cellular targets of CNTs. These studies will provide additional resolution surrounding the contribution of the chemical versus physical mechanism to zebrafish toxicity. Since significant adverse effects were primarily observed at the 50 mg/L MWNT concentration, it will be important to include concentrations between 10 and 50 mg/L in future studies. The lower concentrations (< 50 mg/L) will also provide insight into the potential hazard of CNTs at more environmentally relevant concentrations. Finally, the current data suggests a threshold dose-response curve shape where mortality is observed upon reaching a given concentration. Analysis of additional concentrations (10–50 mg/L) will further resolve the shape of this curve as well as non-mortality effects of MWNTs.
CONCLUSIONS
Significant embryonic zebrafish mortality was observed at 24 hpf and the highest MWNT concentration studied here, 50 mg/L. The logistic model developed around this data set for seven systematically treated MWNTs and four independent physicochemical properties indicates that surface charge, quantified as the point of zero charge, is the most significant predictor of mortality at 24 hpf. This model was confirmed using a separate set of MWNTs purchased from a different vendor and systematically modified in the same manner. While MWNTs are the subject of this study, the approach is applicable to all classes of nanomaterials. A similar approach has been successfully applied to gold nanoparticles.(Harper et al., 2011) The study exemplifies the importance of comprehensively characterizing physicochemical properties of nanomaterials used in toxicological investigations as well as the utility of statistical methods for extrapolating generalizable relationships between nanomaterial properties and hazard endpoints. Since the observed toxicity is correlated with a universal property (i.e. PZC) rather than a specific batch of MWNTs, the findings are intended for comparison across studies, including those utilizing different cellular or organism models and different carbon nanomaterials. Furthermore, the approach and findings presented here are intended to inform the development of nanomaterial hazard profiles. The threshold observed in the concentration-response curve prevented resolution of the underlying mechanism of mortality due to the lack of observations at other developmental endpoints. Still, the establishment of the PZC-mortality relationship serves as a first step towards the development of predictive models to inform safe carbon nanotube design for minimization of unintended consequences.
Supplementary Material
Acknowledgments
The authors would like to thank Patrick Kelleher for assisting with the MWNT preparation, Eva Albalghiti for collecting PZC measurements, and David Goodwin for providing XPS analysis.
Footnotes
DECLARATION OF INTEREST
The authors declare no conflict of interest. The authors alone are responsible for the content and writing of the paper. This publication was developed under Assistance Agreement No. RD83558001-0 by the U.S. Environmental Protection Agency and the NIH grant No. T32 ES07060 and P30 ES000210. It has not been formally reviewed by EPA. The views expressed in this document are solely those of the authors and do not necessarily reflect those of the Agency. EPA does not endorse any products or commercial services mentioned in this publication.
References
- Adenuga AA, Truong L, Tanguay RL, Remcho VT. Preparation of water soluble carbon nanotubes and assessment of their biological activity in embryonic zebrafish. In J Biomed Nanosci Nanotech. 2013;3:39–51. doi: 10.1504/IJBNN.2013.054514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A, Nissen RM, Sun Z, Swindell EC, Farrington S, Hopkins N. Identification of 315 genes essential for early zebrafish development. Proc Natl Acad Sci. 2004;101:12792–12797. doi: 10.1073/pnas.0403929101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asharani PV, Serina NGB, Nurmawati MH, Wu YL, Gong Z, Valiyaveettil S. Impact of multi-walled carbon nanotubes on aquatic species. J Nanosci Nanotechnol. 2008;8:3603–3609. doi: 10.1166/jnn.2008.432. [DOI] [PubMed] [Google Scholar]
- Baun A, Hartmann NB, Grieger K, Kusk KO. Ecotoxicity of engineered nanoparticles to aquatic invertebrates: a brief review and recommendations for future toxicity testing. Ecotoxicology. 2008;17:387–395. doi: 10.1007/s10646-008-0208-y. [DOI] [PubMed] [Google Scholar]
- Bozich JS, Lohse SE, Torelli MD, Murphy CJ, Hamers RJ, Klaper RD. Surface chemistry, charge and ligand type impact the toxicity of gold nanoparticles to Daphnia magna. Environ Sci: Nano. 2014;1:260–270. [Google Scholar]
- Cheaptubes. Multi Walled Carbon Nanotubes. 2014 [Online] Available: http://www.cheaptubesinc.com/default.htm.
- Chen Q, Saltiel C, Manickavasagam S, Schadler LS, Siegel RW, Yang H. Aggregation behavior of single-walled carbon nanotubes in dilute aqueous suspension. J Colloid Interf Sci. 2004;280:91–97. doi: 10.1016/j.jcis.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Cheng J, Chan CM, Veca LM, Poon WL, KCP, Qu L, Sun YP, Cheng SH. Acute and long term effects after single loading of functionalized multi-walled carbon nanotubes into zebrafish (Danio rerio) Toxicol Appl Pharm. 2009;235:216–225. doi: 10.1016/j.taap.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Cheng J, Cheng SH. Influence of carbon nanotube length on toxicity to zebrafish embryos. Int J Nanomed. 2012;7:3731–3739. doi: 10.2147/IJN.S30459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Flahaut E, Cheng SH. Effect of carbon nanotubes on developing zebrafish (Danio rerio) embryos. Environ Toxicol Chem. 2007;26:708–716. doi: 10.1897/06-272r.1. [DOI] [PubMed] [Google Scholar]
- Collett D. Modelling Binary Data. London, UK: Chapman and Hall; 1991. [Google Scholar]
- Connors KA, Voutchkova-Kostal A, Kostal J, Anastas PT, Zimmerman JB, Brooks BW. Reducing aquatic hazard of industrial chemicals: Probabilistic assessment of sustainable molecular design principles. Environ Toxicol Chem. doi: 10.1002/etc.2614. In Press. [DOI] [PubMed] [Google Scholar]
- De Volder MFL, Tawfick SH, Baughman RH, Hart AJ. Carbon nanotubes: Present and future commercial applications. Science. 2013;339:535–539. doi: 10.1126/science.1222453. [DOI] [PubMed] [Google Scholar]
- Endo M, Strano MS, Ajayan PM. Potential applications of carbon nanotubes. Top Appl Phys. 2008;111:13–61. [Google Scholar]
- Fako VE, Furgeson DY. Zebrafish as a correlative and predictive model for assessing biomaterial nanotoxicity. Adv Drug Deliver Rev. 2009;61:478–486. doi: 10.1016/j.addr.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Figueiredo JL, Pereira MFR, Freitas MMA, Orfao JJM. Modification of the surface chemistry of activated carbons. Carbon. 1999;37:1379–1389. [Google Scholar]
- Fox J. Applied Regression Analysis, Linear Models, and Related Methods. Thousand Oaks, CA: Sage Publications; 1997. [Google Scholar]
- Fubini B, Ghiazza M, Fenoglio I. Physico-chemical features of engineered nanoparticles relevant to their toxicity. Nanotoxicology. 2010;4:347–363. doi: 10.3109/17435390.2010.509519. [DOI] [PubMed] [Google Scholar]
- Gilbertson LM, Goodwin DG, Taylor AD, Pfefferle LD, Zimmerman JB. Toward tailored functional design of multi-walled carbon nanotubes (MWNTs): Electrochemical and antimicrobial activity enhancement via oxidation and selective reduction. Environ Sci Technol. 2014;48:5938–5945. doi: 10.1021/es500468y. [DOI] [PubMed] [Google Scholar]
- Harper SL, Carriere JL, Miller JM, Hutchison JE, Maddux BLS, Tanguay RL. Systematic evaluation of nanomaterial toxicity: Utility of standardized materials and rapid assays. ACS Nano. 2011;5:4688–4697. doi: 10.1021/nn200546k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilding J, Grulke EA, Zhang ZG, Lockwood F. Dispersion of carbon nanotubes in liquids. J Disper Sci Technol. 2003;24:1–41. [Google Scholar]
- Hill AJ, Teraoka H, Heideman W, Peterson RE. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci. 2005;86:6–19. doi: 10.1093/toxsci/kfi110. [DOI] [PubMed] [Google Scholar]
- Johnston HJ, Hutchison GR, Christensen FM, Peters S, Hankin S, Aschberger K, Stone V. A critical review of the biological mechanisms underlying the in vivo and in vitro toxicity of carbon nanotubes: The contribution of physico-chemical characteristics. Nanotoxicology. 2010;4:207–246. doi: 10.3109/17435390903569639. [DOI] [PubMed] [Google Scholar]
- Jones CP, Jurkschat K, Crossley A, Banks CE. Multi-walled carbon nanotube modified basal plane pyrolytic graphite electrodes: Exploring heterogeneity, electro-catalysis and highlighting batch to batch variation. J Iran Che Soc. 2008;5:279–285. [Google Scholar]
- Kane AB, Hurt RH. The asbestos analogy revisted. Nature Nanotech. 2008;3:378–379. doi: 10.1038/nnano.2008.182. [DOI] [PubMed] [Google Scholar]
- Kang S, Herzberg M, Rodrigues DF, Elimelech M. Antibacterial effects of carbon nanotubes: Size does matter! Langmuir. 2008a;24:6409–6413. doi: 10.1021/la800951v. [DOI] [PubMed] [Google Scholar]
- Kang S, Mauter MS, Elimelech M. Physicochemical determinants of multiwalled carbon nanotube bacterial cytotoxicity. Environ Sci Technol. 2008b;42:7528–7534. doi: 10.1021/es8010173. [DOI] [PubMed] [Google Scholar]
- Kang S, Pinault M, Pfefferle LD, Elimelech M. Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir. 2007;23:8670–8673. doi: 10.1021/la701067r. [DOI] [PubMed] [Google Scholar]
- Kolosnjaj-Tabi J, Hartman KB, Boudjemaa S, Ananta JS, Morgant G, Szwarc H, Wilson LJ, Moussa F. In vivo behavior of large doses of ultrashort and full-length single-walled carbon nanotubes after oral and intraperitoneal administration to Swiss mice. ACS Nano. 2010;4:1481–1492. doi: 10.1021/nn901573w. [DOI] [PubMed] [Google Scholar]
- Kostal J, Voutchkova-Kostal A, Anastas PT, Zimmerman JB. Identifying and designing chemicals with minimal acute aquatic toxicity. Proc Natl Acad Sci. 2013 doi: 10.1073/pnas.1314991111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu S, Wang Y, Xia W, Muhler M. Thermal stability and reducibility of oxygen-containig functional groups on multiwalled carbon nanotube surfaces: A quantitative high-resolution XPS and TPD/TPR study. J Phys Chem C. 2008;112:16869–16878. [Google Scholar]
- Lam CW, James JT, Mccluskey R, Arepalli S, Hunter RL. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit Rev Toxicol. 2006;36:189–217. doi: 10.1080/10408440600570233. [DOI] [PubMed] [Google Scholar]
- Lam CW, TJJ, Mccluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Sci. 2004;77:126–134. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- Le Cessie S, Van Houwelingen JC. A goodness-of-fit test for binary regression models, based on smoothing methods. Biometrics. 1991;47:1267–1282. [Google Scholar]
- Lee KJ, Browning LM, Nallathamby PD, Xu XH. Study of charge-dependent transport and toxicity of peptide-functionalized silver nanoparticles using zebrafish embryos and single nanoparticle plasmonic spectroscopy. Chem Res Toxicol. 2013;26:904–917. doi: 10.1021/tx400087d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Zhao Y, Nel AE, Lin S. Zebrafish: An in vivo model for nano EHS studies. Small. 2012;9:1608–1618. doi: 10.1002/smll.201202115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Wei L, Hao L, Fang N, Chang MW, Xu R, Yang Y, Chen Y. Sharper and faster “nano darts” kill more bacteria: A study of antibacterial activity of individually dispersed pristine single-walled carbon nanotube. ACS Nano. 2009;3:3891–3902. doi: 10.1021/nn901252r. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhao Y, Sun B, Chen C. Understanding the toxicity of carbon nanotubes. Acc Chem Res. 2013;46:702–713. doi: 10.1021/ar300028m. [DOI] [PubMed] [Google Scholar]
- Mandrell D, Truong L, Jephson C, Sarker MR, Moore A, Lang C, Simonich MT, Tanguay RL. Automated zebrafish chorion removal and single embryo placement: Optimizing throughput of zebrafish developmental toxicity screens. J Lab Autom. 2012;17:66–74. doi: 10.1177/2211068211432197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcfadden D. Conditional logit analysis of qualitative choice behavior. Academic Press; 1974. [Google Scholar]
- Montes-Moran MA, Suarez D, Menendez JA, Fuente E. On the nature of basic sites on carbon surfaces: an overview. Carbon. 2004;42:1219–1225. [Google Scholar]
- Morgan BJT. Analysis of Quantal Response Data. London UK: Chapman and Hall; 1992. [Google Scholar]
- Muller J, Huaux F, Moreau N, Misson P, Heilier JF, Delow M, Arras M, Fonseca A, Nagy JB, Lison D. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol Appl Pharm. 2005;207:221–231. doi: 10.1016/j.taap.2005.01.008. [DOI] [PubMed] [Google Scholar]
- N.N.I. National Nanotechnology Initiative Environmental, Health, and Safety Reserach Strategy. National Science and Technology Council Committee on Technology and the Subcommittee on Nanoscale Science, Engineering, and Technology; 2011. [Google Scholar]
- N.R.C. A Research Strategy for Environmental, Health, and Safety Aspects of Engineered Nanomaterials. Washington, D.C: National Research Council of The National Academies; 2012. [PubMed] [Google Scholar]
- Nanolab. Products: Carbon Nanotubes and Nanomaterials. 2011 [Online]. Available: http://www.nano-lab.com/cooh-functionalized-nanotubes.html.
- Nel A, Xia T, Madler L, Li N. Toxic potential of matrials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- Nel A, Xia T, Meng H, Wang X, Lin S, Ji Z, Zhang H. Nanomaterial toxicity testing in the 21st century: Use of a predictive toxicological approach and high-throughput screening. Acc Chem Res. 2013;46:607–621. doi: 10.1021/ar300022h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh JS, Schwarz JA. Estimation of the point of zero charge of simple oxides by mass titration. J Colliod Interf Sci. 1988;130:157–164. [Google Scholar]
- Pasquini LM, Hashmi SM, Sommer TJ, Elimelech M, Zimmerman JB. Impact of surface functionalization on bacterial cytotoxicity of single-walled carbon nanotubes. Environ Sci Technol. 2012;46:6297–6305. doi: 10.1021/es300514s. [DOI] [PubMed] [Google Scholar]
- Pasquini LM, Sekol RC, Taylor AD, Pfefferle LD, Zimmerman JB. Realizing comparable oxidative and cytotoxic potential of single- and multiwalled carbon nanotubes through annealing. Environ Sci Technol. 2013;47:8775–8783. doi: 10.1021/es401786s. [DOI] [PubMed] [Google Scholar]
- Patel V. Global carbon nanotubes market-industry beckons. [Accessed February 10, 2014];Nanowerk Nanotechnology Spotlight. 2011 [Online] Available: nanowerk.com.
- Petosa AR, Jaisi DP, Quevedo IR, Elimelech M, Tufenkji N. Aggregation and deposition of engineered nanomaterials in aquatic environments: Role of physicochemial interactions. Environ Sci Technol. 2010;44:6532–6549. doi: 10.1021/es100598h. [DOI] [PubMed] [Google Scholar]
- Popov VN. Carbon nanotubes: properties and application. Mater Sci Engin R. 2004;43:61–102. [Google Scholar]
- Powers KW, Palazuelos M, Moudgil BM, Roberts SM. Characterization of the size, shape, and state of dispersion of nanoparticles for toxicological studies. Nanotoxicology. 2007;1:42–51. [Google Scholar]
- R_Core_Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Online] Available: http://www.R-project.org/ [Google Scholar]
- Rizzo LY, Golombek SK, Mertens ME, Pan Y, Laaf D, Broda J, Jayapaul J, Mockel D, Subr V, Kiessling F, Lammers T. In vivo nanotoxicity testing using the zebrafish embryo assay. J Mater Chem B. 2013;1:3918–3925. doi: 10.1039/C3TB20528B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena RK, William W, Mcgee JK, Daniels MJ, Boykin E, Gilmour MI. Enhanced in vitro and in vivo toxicity of poly-dispersed acid-functionalized single-wall carbon nanotubes. Nanotoxicology. 2007;1:291–300. [Google Scholar]
- Schaefer DW, Zhao J, Brown JM, Anderson DP, Tomlin DW. Morphology of dispersed carbon single-walled nanotubes. Chem Phys Lett. 2003;375:369–375. [Google Scholar]
- Scown TM, Van Aerle R, Tyler CR. Review: Do engineered nanoparticles pose a significant threat to the aquatic environment? Crit Rev Toxicol. 2010;40:653–670. doi: 10.3109/10408444.2010.494174. [DOI] [PubMed] [Google Scholar]
- Truong L, Harper SL, Tanguay RL. Evaluation of embryotoxicity using the zebrafish model. Methods Mol Biol. 2011;691:271–279. doi: 10.1007/978-1-60761-849-2_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong L, Zaikova T, Richman EK, Hutchison JE, Tanguay RL. Media ionic strength impacts embryonic responses to engineered nanoparticle exposure. Nanotoxicology. 2012;6:691–699. doi: 10.3109/17435390.2011.604440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S.E.P.A. Risk Management Sustainable Technology: Nanotechnology. 2014 [Online]. Available: http://www.epa.gov/nrmrl/std/nanotech.html.
- Usenko CY, Harper SL, Tanguay RL. In vivo evaluation of carbon fullerene toxicity using embryonic zebrafish. Carbon. 2007;45:1891–1898. doi: 10.1016/j.carbon.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecitis CD, Zodrow KR, Kang S, Elimelech M. Electronic-structure-dependent bacterial cytotoxicity of single-walled carbon nanotubes. ACS Nano. 2010;4:5471–5479. doi: 10.1021/nn101558x. [DOI] [PubMed] [Google Scholar]
- Voutchkova-Kostal A, Kostal J, Connors KA, Brooks BW, Anastas PT, Zimmerman JB. Toward rational molecular design for reduced chronic aquatic toxicity. Green Chem. 2012;14:1001–1008. [Google Scholar]
- Yeager E. Electrocatalysts for O2 reduction. Electrochim Acta. 1984;29:1527–1537. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.