Abstract
Migraine affects predominantly women. Furthermore, epidemiological studies suggest that obesity is a risk factor for migraine and this association is influenced by sex. However, the biological basis for this bias is unclear. To address this issue, we assessed light avoidant behavior, a surrogate of photophobia, in female C57BL/6J mice fed regular diet (RD) or high fat diet (HFD, 60% kcal from fat). We first assessed sex differences in basal photophobia in 20-25 week old mice and found that both obese and lean females spent significantly less time in light than their male counterparts. Next, we assessed photophobia evoked by trigeminal stimulation with intradermal capsaicin. Females at 20-25 weeks of age did not display capsaicin-evoked photophobic behavior unless they had diet-induced obesity. When we tested 8-11 week old females to determine if the diet alone could be responsible for this effect, we found that both HFD and RD 8-11 week old females exhibit capsaicin-evoked photophobic behavior. This is in contrast to what we have previously shown in males and indicates a sex difference in the photophobic behavior of mice. Comparison of 20-25 week old RD mice with 8-11 week old RD mice suggests that age or age-related weight gain may contribute to capsaicin-evoked photophobic behavior in males, but not in females. These findings suggest that obesity exacerbates photophobia in both sexes, but additional work is needed to understand the sex- and age-specific mechanisms that may contribute to photophobia and trigeminal pain.
Keywords: diet induced obesity, sex, photophobia, trigeminal, migraine, capsaicin
Migraine is a common and debilitating disorder that affects 36 million Americans. The prevalence of migraine is particularly high, between 20–28%, in women during reproductive years (Lipton et al., 2001; Buse et al., 2013). Furthermore, women report migraine symptoms, such as photophobia, more often than men (Lipton et al., 2001; Buse et al., 2013). Epidemiological studies have found that obesity increases the odds of having migraine, with the strongest association among women and individuals of reproductive age (18-50 years) (Peterlin et al., 2010; Peterlin et al., 2013). Despite these findings, we do not know how obesity interacts with female sex to influence the manifestation and progression of migraine.
Overall, the studies addressing sex differences in animal models of migraine have found that females are more sensitive to stimulation of the trigeminal system, which is critical to migraine pathophysiology. This has been demonstrated with respect to susceptibility to cortical spreading depression (Brennan et al., 2007), activation of dural afferents by inflammatory mediators (Scheff and Gold, 2011), and migraine-like behavior in response to chronic dural application of inflammatory mediators (Stucky et al., 2011). Sex differences also exist in the expression levels of signaling molecules thought to be involved in migraine pathophysiology, including calcitonin gene-related peptide and its receptor components (Stucky et al., 2011) and serotonin-synthesizing enzymes (Asghari et al., 2011). How obesity may influence any of these sex differences has not been investigated. Furthermore, how these differences contribute to aspects of migraine other than headache, like photophobia, also remains unknown.
Several studies have recently elucidated mechanisms contributing to migraine-related photophobia using rodent models (Recober et al., 2009; Noseda et al., 2010; Okamoto et al., 2010; Recober et al., 2010; Dolgonos et al., 2011; Kaiser et al., 2012), but only one study assessed both sexes (Recober et al., 2009). While no sex differences in photophobic behavior were found in the human Receptor Activity Modifying Protein 1 (RAMP1) transgenic mice (Recober et al., 2009), this should be verified in wildtype mice and in other experimental paradigms. This is especially important since photophobia occurs more frequently in women than men and seems to change with age among migraine patients (Wöber-Bingöl et al., 2004; Buse et al., 2013; Bolay et al., 2015).
In this study, we sought to characterize light avoidance, a surrogate measure of photophobia, in female mice, with and without diet-induced obesity, a broadly accepted obesity model. This builds on our previous work in male mice where we found that obesity enhances basal photophobic behavior and lowers the stimulus required to evoke photophobia (Rossi et al., 2016). While light aversion is worsened in both sexes by diet-induced obesity, our current findings suggest that sex may interact with age to modulate this behavior differently in males and females across the life span.
Experimental Procedures
Animals
Female C57BL/6J mice bred in our colony were randomly assigned to and maintained on either a regular chow diet (RD; Teklad) or a 60% high fat diet (HFD; Research Diets Inc. #D12491) from weaning (3-4 weeks of age). To evaluate photophobic behavior before and after the induction of obesity, a cohort of mice was tested at 8-11 weeks of age and a different cohort was tested at 20-25 weeks of age. Females are sexually mature at 8 weeks of age, and do not exhibit anestrus until 49 weeks of age (Felicio et al., 1984). The weights of the mice at weaning and during testing are presented in Table 1. We chose to test two cohorts rather than one cohort at different ages to limit the injections of capsaicin to one per whisker pad to avoid desensitization of nociceptors. All mice were maintained on a standard 12-hour light-dark cycle (lights on at 06:00), with food and water available ad libitum.
Table 1.
Weights of different age and diet groups of female mice at weaning (3–4 weeks old) and testing.
| Weaning | Testing | |||||
|---|---|---|---|---|---|---|
| Cohort Age | Diet | Mean | SEM | Mean | SEM | n |
| 8–11 weeks | HFD | 9.54 | 0.46 | 25.49*** | 0.64 | 17 |
| RD | 8.63 | 0.55 | 20.09 | 0.34 | 15 | |
|
| ||||||
| 20–25 weeks | HFD | 10.22 | 0.28 | 37.87*** | 0.85 | 49 |
| RD | 9.78 | 0.21 | 23.11 | 0.44 | 49 | |
p<0.0001 HFD vs RD
For comparison, we are showing data from male mice derived from the same colony that have been previously published (Rossi et al., 2016). The 8-11 week old males (RD n = 17, HFD n = 17) and females (RD n = 15, HFD n = 17) consisted of three smaller batches (n = 3–8 mice/diet and sex in each batch) tested together each baseline and post-treatment testing day. The 20-25 week old females (RD n = 49, HFD n = 49) consisted of five smaller batches used for baseline evaluation in Fig. 1 (RD: n = 6–11 mice/batch; HFD: n = 8–14 mice/batch). Two of these five batches (RD n = 21; HFD n = 24) also received post-treatment testing, but were not tested with males in the same day. All protocols were approved by the University of Iowa IACUC and were conducted in accordance with The Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80–23, revised 1996).
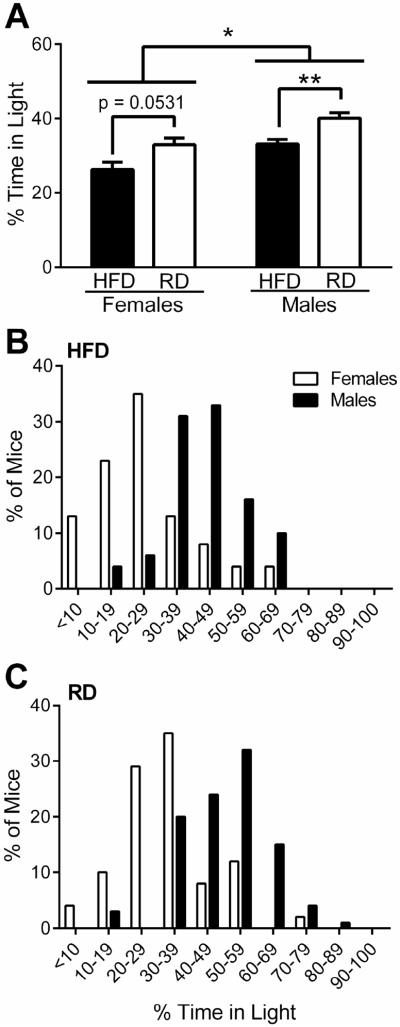
Fig. 1.
Effects of sex and diet on basal time spent in light in mice at 20-25 weeks of age. (A) Baseline percentage of time spent in light for HFD (black bars) and RD (white bars) females (n = 49/diet) and males (n = 74–80/diet). (B) Histogram stratifying the distribution of HFD female (n = 49, white bars) and male (n = 80, black bars) mice based on the percentage of time in light at baseline. (C) Histogram stratifying the distribution of RD female (n = 49, white bars) and male (n = 74, black bars) mice based on the percentage of time in light at baseline. *p < 0.05 and **p < 0.01 for indicated comparisons. (Male data has been previously published and is shown here for direct comparison with females (Rossi et al., 2016))
Light Avoidance Assay
Photophobia is a key clinical feature of migraine and light avoidance in mice can be a useful tool to evaluate mechanisms underlying migraine-related photophobia (Recober et al., 2009; Recober et al., 2010; Markovics et al., 2011; Kaiser et al., 2012; Chanda et al., 2013). Here we assessed light avoidance in mice as previously described (Rossi et al., 2016), using a modified commercially available apparatus (Med Associates) where mice are given a choice between light and darkness. To avoid the potential anxiety-inducing effects of the clear walls and open top on the lit side, we covered the walls of the chambers with black opaque foam panels and the top with clear Plexiglas to allow illumination of that side while being enclosed like the dark side. The light source in each box was a custom built array of LED lights affixed to the ceiling over light side of the testing chamber. All the arrays were controlled by a single dimmer with different settings and we always used the same setting for the light intensity. For these studies, the intensity was 775 ± 27 lux, while the light levels in the home cage was between 88–110 lux.
Mice were always placed first in the lit compartment and allowed to freely move between the lit and dark compartments for the duration of the test (20 minutes). Mice acclimated in the testing room for at least an hour prior to testing, with food available ad libitum in their cages. Six mice could be tested simultaneously in a 20 minute testing session, and a maximum of 26 mice (range 17–26) were tested in one day between 09:00 and 13:30, during the light cycle. All mice were tested twice to establish a baseline prior to any treatment. Baseline and post-treatment tests occurred on different days. Post-treatment tests were separated by five to eight days (see "Induction of Photophobic Behavior" below). The second baseline was used to compare with post-treatment behavior tests.
We measured the percentage of time that mice spend in the lit compartment (% time in light), as well as several locomotor parameters. These parameters included percentage of time spent resting (“% resting”), percentage of time spent moving (“% moving”), and the distance travelled divided by the time spent in each compartment (cm/time in zone). "Resting" is defined by the Med Associates software as a lack of adjacent beam breaks detected within 500 ms from the last detected beam break. The program is also capable of defining time spent in "stereotypic movement", representing smaller movements (e.g. grooming, sniffing, etc). However, to adequately identify the nature of these movements video recording would be required. This outcome is not included here because it lacks sensitivity and we have not found it to be significantly different between diets or after treatments. Because of the distinction between resting and stereotypic movement, the percentage of time resting and percentage of time moving are about 1–2% short of 100% when added together for the indicated zone.
Induction of Photophobic Behavior with Capsaicin
Five to eight days after baseline testing in the light avoidance assay, each mouse was treated with vehicle (5% each ethanol and Tween80 in phosphate buffered saline, PBS) by injection into one whisker pad using an injector attached to a Hamilton syringe. The injector is made from a 30 gauge needle removed from its hub and epoxied to PE 20 tubing, which fits tightly over the end of the cemented needle on a gastight Hamilton syringe (1701). Unanesthetized animals were manually restrained to receive whisker pad injections. Mice were tested in the light avoidance assay 18-20 hours after the treatment. Five to eight days after receiving vehicle, mice were treated with 0.01% capsaicin in the opposite whisker pad and tested 18-20 hours later. After another waiting period of five to eight days, this process was repeated with 0.1% capsaicin. Thus, each animal received capsaicin once on each side. All mice received these treatments in the order indicated (vehicle, 0.01% capsaicin, and 0.1% capsaicin) between 15:00 and 17:00 in a different location than the testing room. Mice acclimated in the room 40–60 minutes prior to being handled for injections and were returned to the housing space once all injections were completed.
We chose to test the mice 18-20 hours after capsaicin for the following reasons: (1) to allow mice time to recover from the stress of the injection and acute pain, (2) to ensure that animals would not be actively engaging in acute nocifensive grooming, and (3) to match the timing of testing and light exposure to morning measurements as done for baseline and previous studies.
Statistical Analysis
To evaluate the effect of sex and diet on basal light aversion, we used two-way ANOVAs and post-hoc Tukey's tests. To evaluate the effect of diet and treatment on light aversive behavior in females, we used two-way ANOVAs with repeated measures and post-hoc Sidak tests. All treatment post-hoc tests compared all other conditions (baseline, 0.01% and 0.1% capsaicin) to vehicle. Repeated measures two-way ANOVA was used to assess age and treatment effects in females but repeated measures could not be used for males. For all tests, p < 0.05 was considered significant and Prism (v.6, Graph Pad) was used to analyze the data.
Results
Female mice spend less time in light than males
First we sought to characterize light avoidance in female mice and the effects of high-fat diet induced obesity on this behavior. In this cohort, the average total body weight of the HFD group was 14.8 g higher than the RD group (Table 1). HFD and RD 20-week old female mice spent about 7% less time in light than their male counterparts at baseline (Diet: F1, 247 = 16.91, p < 0.0001; Sex: F1, 247 = 17.96, p < 0.0001; Interaction: F1, 247 = 0.009, p = 0.92). Furthermore, diet-induced obesity in females also produced a 7% decrease in basal time in light, which trended towards significance (p = 0.0531) (Fig 1A). In contrast with males, a strong preference for the dark was seen in females in both diet groups (Fig. 1B). Among obese mice on HFD, 70% of females spent less than a third of the time in the light compared with 10% of males (Fig. 1B). Among lean mice on RD, 43% of females versus 3% of males spent less than one third of the time in the light (Fig. 1C). This indicates that females are more photophobic than males at baseline and that diet-induced obesity in either sex enhances this behavior.
Obese 20-25 week old females spend less time in light in response to capsaicin
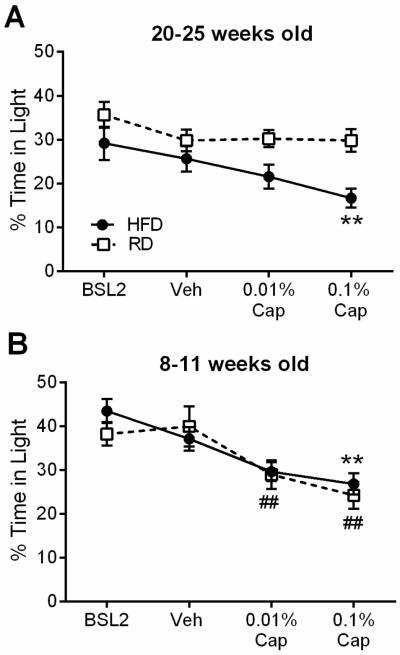
We previously found that HFD induced obesity lowered the threshold of capsaicin-evoked photophobia in male mice (Rossi et al. 2015). Obese HFD females at 20-25 weeks of age spent significantly less time in light after 0.1% capsaicin as compared to vehicle (Fig. 2A) (Treatment: F 3,117 = 7.82, p < 0.0001; Diet: F1,39 = 7.184, p = 0.0107; Interaction: F 3,117 = 1.919, p = 0.1303). Lean RD females at 20-25 weeks of age did not spend less time in light after capsaicin. This suggests that obesity increases susceptibility to capsaicin-evoked photophobic behavior in 20-25 week old female mice.
Fig. 2.
Effects of obesity and diet on capsaicin-induced photophobic behavior in female mice. (A) Female mice on HFD (n = 20) and RD (n = 21) tested at 20-25 weeks of age at baseline and 18-20 hours after vehicle, 0.01%, and 0.1% capsaicin in the whisker pad. (B) Female mice on HFD (n = 17) and RD (n = 15) tested at 8–11 weeks of age at baseline and 18-20 hours after vehicle, 0.01%, and 0.1% capsaicin in the whisker pad. **p < 0.01 as compared to vehicle for HFD females. ##p < 0.01 as compared to vehicle for RD females.
Lean 8-11 week old females spend less time in the light in response to capsaicin
Next we investigated the possible contribution of the high-fat diet itself independently from obesity. For this we used a different cohort of female mice that were fed HFD or RD and were tested at 8-11 weeks of age. At this age, the HFD group was only 5.4 g heavier than the RD group (Table 1). We found no effect of diet on photophobic behavior at any testing point. However, both HFD and RD females spent significantly less time in light following capsaicin injection as compared to vehicle (Fig. 2B) (Treatment: F 3, 90 = 17.24, p < 0.0001; Diet: F 1,30 = 0.2385, p = 0.6289; Interaction: F3,90 = 0.9264, p = 0.4314). Taken together, these findings suggest that young females (8-11 week old) are susceptible to capsaicin-evoked photophobia and that this is not affected by high-fat diet. This pattern is the opposite of what we observed in lean males, who did not develop capsaicin-evoked photophobia in young adulthood, but did at 20-25 weeks of age (Rossi et al., 2016).
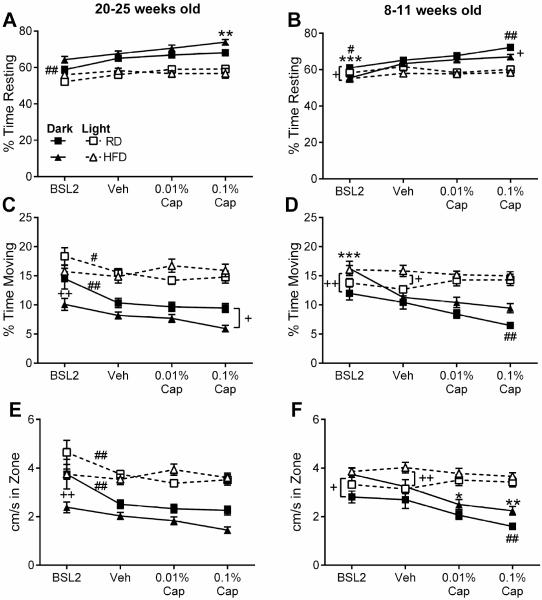
Capsaicin-evoked photophobia is associated with decreased locomotor activity in the dark
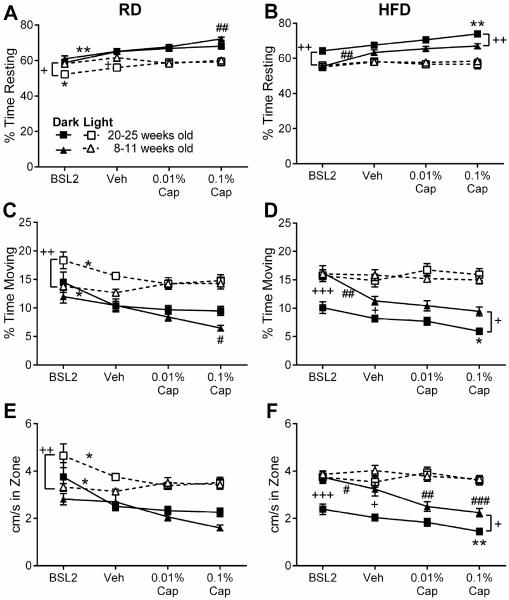
Our previous findings in males suggest that capsaicin-evoked photophobia is accompanied by reduced locomotor activity only in the dark compartment (Rossi et al., 2016). Among females, we also found that locomotor activity in the dark, but not the light, was significantly affected by capsaicin injection (Table 2, Fig. 3). While there were significant overall treatment effects in the light compartment on % time moving and cm/second in the 20-25 week old females (Table 2), post-hoc tests indicate that there was no difference between vehicle and either dose of capsaicin. Therefore, capsaicin had no effect on locomotor parameters in the light compartment. The reduced locomotor activity in the dark after capsaicin treatment was especially pronounced in the 8-11 week old females, who exhibited a greater degree of capsaicin-induced photophobia. Post-hoc tests revealed that 8-11 week old HFD mice moved significantly slower after both 0.01% and 0.1% capsaicin as compared to vehicle (Fig. 3F), and RD mice rested more, moved less, and slower after 0.1% capsaicin treatment as compared to vehicle (Fig. 3B,D,F). The only significant effects of treatment and treatment by diet interactions observed in the light compartment were seen in the 20-25 week old RD females, who spent more time moving and moved faster at baseline than after vehicle or either dose of capsaicin (Table 2, Fig. 3C,E).
Table 2.
Effect of diet, treatment and their interaction as determined by 2-way ANOVAs of locomotor parameters for 8–11 and 20–25 week old females analyzed separately (Figure 3). Only overall effects, as calculated by ANOVA, are reported here. See figure 3 for post-hoc comparisons and specific capsaicin effects compared to vehicle
| Diet effects | Treatment effects | Interaction | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Zone | Parameter | 20–25 week (F1,39) | 8–11 week (F1,30) | 20–25 week (F3, 117) | 8–11 week (F3,90) | 20–25 week (F3, 117) | 8–11 week (F3,90) |
| Dark | % resting | 7.064* | 6.298* | 18.91*** | 37.07*** | 0.6701 | 1.55 |
| % moving | 12.76** | 6.521* | 10.59*** | 28.92*** | 0.896 | 2.16 | |
| cm/s in zone | 12.71** | 6.272* | 8.350*** | 23.06*** | 1.282 | 0.6765 | |
|
| |||||||
| Light | % resting | 0.03297 | 3.301 | 4.492** | 3.080* | 3.513* | 0.7563 |
| % moving | 0.004705 | 6.051* | 2.502 | 0.3123 | 4.374** | 1.319 | |
| cm/s in zone | 0.1913 | 7.66** | 4.229** | 0.1461 | 4.641** | 1.939 | |
p <0.05,
p <0.01,
p <0.0001
Fig. 3.
Effects of capsaicin and diet on locomotor parameters for HFD and RD females mice. Locomotor parameters are % time resting (A, B), % time moving (C, D), and distance travelled relative to time spent in zone (cm/s spent in zone) (E, F). (A, C, E) Locomotor parameters in females tested at 20-25 weeks of age at baseline and 18-20 hours after vehicle, 0.01%, or 0.1% capsaicin in the whisker pad. (B, D, F) Locomotor parameters in females tested at 8-11 weeks of age at baseline and 18-20 hours after vehicle, 0.01%, or 0.1% capsaicin in the whisker pad. For each diet (HFD = triangles and RD = squares) the locomotor parameters in the dark are indicated by solid lines and in the light are indicated by dotted lines. HFD: *p < 0.05, **p < 0.01, ***p < 0.0001 as compared to vehicle. RD: #p < 0.05 and ##p < 0.01 as compared to vehicle. Between diet comparison: +p < 0.05 and ++p < 0.01 for indicated treatment.
A significant effect of diet on movement in the dark was also present in both age groups (Table 2). As one might expect, the obese 20-25 week old HFD females move less and slower than RD females, with post-hoc differences observed at baseline (percent time moving and distance travelled relative to time in that zone (cm/s), Fig. 3C,E) and after 0.1% capsaicin treatment (percent time moving, Fig. 3C). However, 8-11 week old HFD females actually moved slightly more than the RD females, with post-hoc differences observed at baseline for all parameters (Fig. 3B,D,F), and in percentage of time resting after 0.1% capsaicin treatment (Fig. 3B). Taken together, these data suggest that not only do female mice spend more time in the dark compartment after capsaicin treatment, they also move less while on that side. Furthermore, obesity causes this effect to persist in older female mice. Importantly, HFD and RD mice move similarly in the light compartment, suggesting that there are no diet- or obesity-related locomotor deficits driving the photophobic behavior.
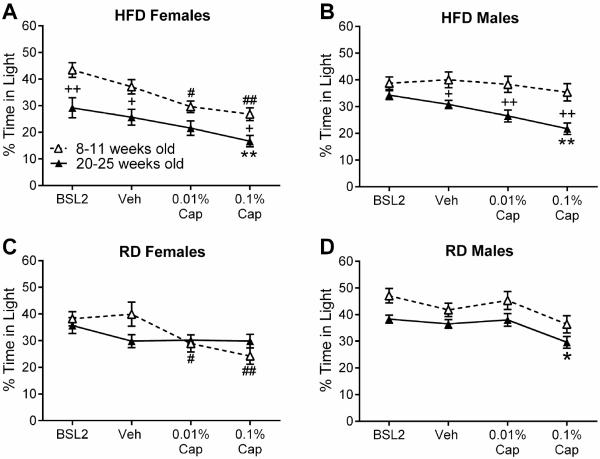
Obesity, not ageing, exacerbates photophobic behavior in both sexes
Since we had to allow several months for the development of diet-induced obesity, we felt that it was important to control for possible effects of ageing. To distinguish the relative contribution of age and obesity to our findings, we compared the two cohorts of mice (20-25 versus 8-11 weeks of age) on each of the diets. At 20-25 weeks of age, HFD females and HFD males weighed 12 g and 13 g more than sex-matched 8-11 week old HFD mice, respectively. In contrast, 20-25 week old RD females and RD males only weighed 3 g and 4.5 g more than sex-matched 8-11 week old RD mice, respectively. In both females and males, 20-25 week old mice on HFD spent less time in the light than 8-11 week old mice on HFD (Fig. 4A, Females: significant effect of age, F 1,35 = 12.22, p =0.0013; Fig. 4B, Males: significant effect of age, F 1,276 = 27.26 p < 0.0001). For HFD females, this was evident from baseline and after all injections; 20-25 week old HFD females spent 8–12% less time in light than 8-11 week old HFD females (Fig. 4A). For HFD males, this was evident only after injections; 20-25 week old HFD males spent 9–14% less time in light than 8-11 week old HFD males (Fig. 4B). This indicates that both female and male HFD mice at 20-25 weeks old are more photophobic than their 8-11 week old counterparts.
Fig. 4.
Effects of obesity, diet, and age on photophobic behavior in females and males. (A) HFD females tested at 8-11 weeks of age (n = 17) compared with HFD females tested at 20-25 weeks of age (n = 20) at baseline and 18-20 hours after vehicle, 0.01%, and 0.1% capsaicin. (B) HFD males tested at 8-11 weeks of age (n = 17) compared with HFD males (n = 28-44) tested at 20-25 weeks of age at baseline and 18-20 hours after vehicle, 0.01%, and 0.1% capsaicin (n = 44 in 20-25 week old cohort received baseline, vehicle, and 0.01% capsaicin; n = 28 received baseline, vehicle, and 0.1% capsaicin). (C) RD females tested at 8-11 weeks of age (n = 15) compared with RD females tested at 20-25 weeks of age (n = 21) at baseline and 18-20 hours after vehicle, 0.01%, and 0.1% capsaicin. (D) RD males tested at 8-11 weeks of age (n = 16) compared to RD males tested at 20-25 week old males (n = 29-38) after baseline and 18-20 hours after vehicle, 0.01%, or 0.1% capsaicin in one whisker pad (n = 38 in 20-25 week old cohort received baseline, vehicle, and 0.01% capsaicin, n = 29 received baseline vehicle and 0.1% capsaicin). For 20-25 weeks old: *p < 0.05 and **p < 0.01 for the indicated dose of capsaicin as compared to vehicle. For 8-11 weeks old: #p < 0.05 and ##p < 0.01 for the indicated dose of capsaicin as compared to vehicle. Between age comparison: +p < 0.05 and ++p < 0.01 for the indicated treatment. (Male data has been previously published and is shown here for direct comparison with females (Rossi et al., 2016)).
Among RD mice, age-related effects differed between the sexes. As noted above, RD females in the 8-11 week old cohort exhibited capsaicin-evoked photophobic behavior, while 20-25 week old RD females did not (Effect of age: F 1,34 = 0.2496, p = 0.6206; age by treatment interaction: F 3,102 = 3.784, p = 0.0128, Fig. 4C). At 20-25 weeks of age, RD males spent about 7% less time in light on average than 8-11 week old RD males (Effect of age: F 1,257 = 14.14 p = 0.0002), but there were no post-hoc differences between the two age groups at baseline or after injections (Age by treatment interaction: F 3,257 = 0.1613 p = 0.9223, Fig. 4D). As indicated above and previously (Rossi et al 2015), all groups exhibit significant effects of treatment (HFD females: F 3,105 = 21, p < 0.0001, HFD males: F 3,276 = 3.45 p = 0.0171, RD females: F 3,102 = 7.148, p = 0.0002, RD males: F 3,257 = 5.067 p = 0.002). Taken together, these findings indicate that among the RD cohort, males and females have the opposite pattern of age-related response to capsaicin. This may suggest that sex and age interact to influence photophobic behavior.
Age does not affect decreased locomotor activity associated with capsaicin-induced photophobia
We also compared locomotor data for the two age cohorts of each diet in females, and found that 20-25 week old HFD females moved less and slower in the dark compartment as compared to their younger counterparts (Table 3, Fig. 5, solid lines). There were no differences between the HFD age groups in the light compartment under any treatment condition (Table 3, Fig. 5, dotted lines). In contrast, the only difference in dark compartment locomotion of the RD age groups occurred at baseline for the distance travelled relative to the time spent in that zone. The 20-25 week old RD mice had higher locomotor activity in the light compartment than their younger counterparts at baseline and after vehicle treatment (Fig. 5, dotted lines). As noted previously, a significant effect of capsaicin on locomotion in the dark compartment was apparent only in the cohorts that exhibited reduced time in light after capsaicin (8-11 week old RD and both age cohorts of HFD mice). Specifically, after 0.1% capsaicin 8-11 week old RD females rested more and moved less. This was also true of HFD 20-25 week old females, who also travelled less distance relative to the time spent in the dark compartment after 0.1% capsaicin. While 8-11 week old HFD females exhibited a significant reduction in distance travelled relative to the time spent in the dark compartment, they did not exhibit significant changes in time spent resting or moving after capsaicin. Overall, this suggests that obesity, rather than diet or ageing, drive the decreased locomotor activity in the dark compartment associated with capsaicin-induced photophobia. Obesity does not affect locomotor behavior in the light compartment.
Table 3.
Effect of age, treatment and their interaction as determined by 2-way ANOVAs of locomotor parameters for RD and HFD females analyzed separately (Figure 5). Only overall effects, as calculated by ANOVA, are reported here. See figure 5 for post-hoc comparisons and specific capsaicin effects compared to vehicle
| Age effects | Treatment effects | Interaction | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Zone | Parameter | RD (F1,34) | HFD (F1,35) | RD (F3,102) | HFD (F3,105) | RD (F3,102) | HFD (F3,105) |
| Dark | % resting | 1.409 | 13.84** | 19.96*** | 30.73*** | 0.8416 | 1.554 |
| % moving | 3.422 | 17.48** | 10.28*** | 31.96*** | 0.9473 | 3.539* | |
| cm/s in zone | 2.839 | 19.6*** | 7.26** | 29.01*** | 1.25 | 2.685 | |
|
| |||||||
| Light | % resting | 3.775 | 0.07309 | 5.551** | 1.69 | 4.05** | 0.496 |
| % moving | 4.429* | 0.07209 | 2.323 | 0.4251 | 3.344* | 1.451 | |
| cm/s in zone | 3.729 | 0.2754 | 2.724* | 0.9875 | 4.026** | 1.864 | |
p <0.05,
p <0.01,
p <0.0001
Fig. 5.
Effects of obesity, diet, and age on locomotor parameters of HFD and RD females mice. Locomotor parameters are % time resting (A, B), % time moving (C, D), and distance moved relative to time spent in zone (cm/s spent in zone) (E,F). (A, C, E) Locomotor parameters in RD females tested at 8-11 weeks of age or 20-25 weeks of age at baseline and 18-20 hours after vehicle, 0.01%, or 0.1% capsaicin in one whisker pad. (B, D, F) Locomotor parameters in HFD females tested at 8-11 weeks of age or 20-25 weeks of age at baseline and 18-20 hours after vehicle, 0.01%, and 0.1% capsaicin in one whisker pad. In each age group (20-25 weeks old = squares and 8-11 weeks old = triangles), solid lines indicate locomotor parameter for the dark zone and dotted lines indicate the locomotor parameter for the light zone. For 20-25 week old: *p < 0.05 and **p < 0.01 as compared to vehicle. For 8-11 week old: #p < 0.05, ##p < 0.01, and ###p < 0.0001 as compared to vehicle. Between age comparison: +p < 0.05 and ++p < 0.01 for the indicated treatment.
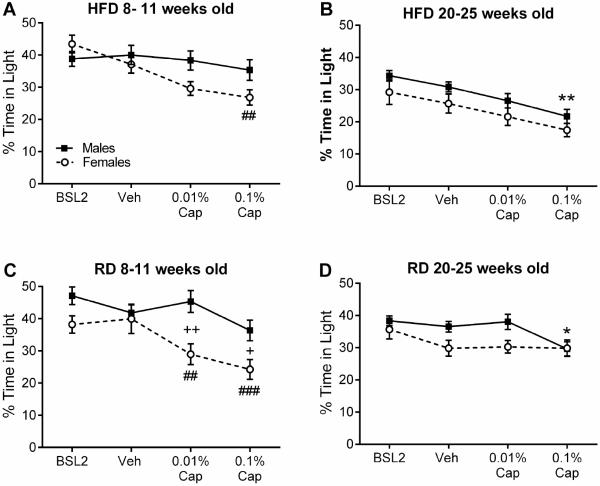
Capsaicin-evoked photophobia occurs in young females but not males
Our comparison of the age cohorts suggested that there may be some age-dependent differences between females and males in their response to capsaicin. Therefore, we directly compared the sexes within each diet and age group (Fig. 6). We found that there was a significant effect of sex in the RD group in both age cohorts (8-11 weeks: F1,29 = 7.822, p = 0.0091; 20-25 weeks: F 1, 277 = 6.351, p = 0.0123). However, in the HFD group, we only observed a sex effect in the 20-25 week old cohort (F 1, 288 = 7.847, p = 0.0054) but not in the 8-11 week cohort (F1,32 = 2.162, p = 0.1512). We found a significant sex by treatment interaction in both diet groups but only in the 8-11 weeks cohort (HFD: F3,32 = 3.857, p = 0.0118; RD: F 3, 87 = 3.417, p = 0.0208), not in the 20-25 weeks cohort (HFD: F3,32 = 0.01168, p = 0.9983; RD: F 3, 277 = 1.136, p = 0.3349). Overall, this indicates that 8-11 week old females respond to capsaicin, while males do not, which is true for both diets. In the 20-25 week old cohort, females spend less time in light than males in general, but do not exhibit an enhanced response to capsaicin. In contrast to males, young female mice in both diet groups display capsaicin-evoked photophobia.
Fig. 6.
Effects of sex and capsaicin treatment on percent time in light for HFD and RD mice in each age cohort. (A) Percentage of time in light for females (n = 17) compared with males (n = 17) in the 8-11 week old HFD cohort at baseline and 18-20 hours after vehicle, 0.01%, or 0.1% capsaicin in one whisker pad. (B) Percentage of time in light for females (n = 20) compared with males (n = 28-44) in the 20-25 week old HFD cohort at baseline and 18-20 hours after vehicle, 0.01%, or 0.1% capsaicin in one whisker pad. (C) Percentage of time in light for females (n = 15) compared with males (n = 16) in the 8-11 week old RD cohort at baseline and 18-20 hours after vehicle, 0.01%, or 0.1% capsaicin in one whisker pad. (D) Percentage of time in light for females (n = 21) compared with males (n = 29-38) in the 20-25 week old RD cohort at baseline and 18-20 hours after vehicle, 0.01%, or 0.1% capsaicin in one whisker pad. For males: *p < 0.05 and **p < 0.01 as compared to vehicle. For females: #p < 0.05, ##p < 0.01, and ###p < 0.0001 as compared to vehicle. Between sex comparison: +p < 0.05 and ++p < 0.01 between the sexes for the indicated treatment. (Male data has been previously published and is shown here for direct comparison with females (Rossi et al., 2016)).
Discussion
Sex-related differences are well established in migraine. Importantly, sex and age may also influence the known association between obesity and migraine (Peterlin et al., 2010; Peterlin et al., 2013). Here we characterized photophobic behavior in obese and lean female mice and compared them with our findings in males (Rossi et al., 2016). These experiments in females and males were conducted simultaneously. The main findings of this study are: (1) Overall, female mice were more photophobic than males, independent of obesity; (2) Young females were susceptible to capsaicin-evoked photophobic behavior, which did not occur in age-matched males (Rossi et al., 2016); (3) High fat diet without obesity did not affect photophobic behavior in either sex; and (4) Capsaicin-evoked photophobia is very consistently accompanied by reduced locomotor activity in the dark compartment, while movement in the light compartment remains unchanged. This indicates that obesity-related locomotor deficits cannot explain the photophobic behavior. Taken together, these findings suggest that sex plays an important role in photophobic behavior and that obesity is an exacerbating factor in both sexes.
Overall, these findings are consistent with our previous study examining the acute effect of capsaicin on the activation of second order neurons in the trigeminal nucleus caudalis (Rossi et al., 2013). The acute response to a single whisker pad injection with 0.01% capsaicin, was similar in males and females at 20 weeks of age for both diet groups (Rossi et al., 2013). We do not have any information regarding c-Fos expression in 8-11 week old males and females following acute injection of capsaicin to compare with the sex difference seen in photophobic behavior. Additionally, c-Fos immunoreactivity was measured after a single capsaicin injection and in the current study we used an alternating and escalating scheme of treatment. Thus, it is possible that some of the differences reported here could be mediated by sex-related differences in response to the repeated injections, although we tried to avoid this by alternating sides and allowing for recovery periods.
Independent of diet and obesity, sex-specific differences in pain processing could explain our observation that female mice are more photophobic than male mice. A greater degree of peripheral and central sensitization in females has been suggested in the literature. Human studies indicate that women have a greater pain response to intradermal capsaicin than men, indicating greater peripheral sensitization (Gazerani et al., 2005; Gazerani et al., 2007), and a larger area of secondary hyperalgesia, indicating central sensitization (Jensen and Petersen, 2006; Gazerani et al., 2007). Sex differences in nocifensive responses to intraplantar capsaicin have also been noted in rats (Lu et al., 2009). Multiple studies have shown differences in endogenous pain inhibition between males and females (Linnman et al., 2012) (Craft et al., 2004; Loyd and Murphy, 2014). Future work will determine to what extent sensitization versus lack of inhibition may be contributing to capsaicin-evoked photophobic behavior in mice.
Our findings mirror some clinical observations regarding migraine and photophobia across the life span of men and women. Adult women experience longer migraines and a greater incidence of photophobia than men (Wöber-Bingöl et al., 2004; Bolay et al., 2015). Moreover, there is a relationship between age and the occurrence of photophobia in women. Photophobia is most prevalent in women during reproductive years (Wöber-Bingöl et al., 2004), but seems to decline after the age of 50 (Bolay et al., 2015). We found age-dependent differences in the lean RD cohorts. Young females are more susceptible to capsaicin-evoked photophobia than old females. In contrast, young males are less susceptible than old males. There are several potential explanations for this. Photophobia is assessed 20 hours after the facial capsaicin injection in our experimental paradigm, which does not allow us to determine the exact onset and resolution of photophobia. It is possible that the duration of capsaicin-induced photophobia is shorter in older female mice. It is also possible that older females require a higher dose of capsaicin. The answer to these questions may provide insight into the mechanisms involved in initiating and suppressing photophobic behavior.
Clinical studies suggest that obesity specifically increases the odds of having migraine in adults within a reproductive age range (18-50 years), and that this effect is more pronounced in women (Peterlin et al., 2010, Peterlin et al., 2013). It is not clear how obesity specifically impacts the presentation of photophobia, but obese migraineurs report photophobia and phonophobia more frequently than non-obese migraineurs (Bigal et al., 2006; Winter et al., 2009). We found that obesity increases photophobic behavior in both sexes. Possible explanations for the effects of obesity on photophobia include sex hormone abnormalities, low-grade inflammation, or a combination of these. A limitation of our study is that we did not measure sex hormones and cannot speculate whether they were abnormal. The bidirectional influences of obesity and sex hormones in both sexes are well established (Tchernof and Després, 2000). Thus, obesity-related changes in hormones may have an impact on their effects on nociception or the neural substrates involved in photophobia. It will be important to assess sex hormones in future studies investigating the relationship between obesity and migraine. Obesity is also associated with low-grade inflammation (Ghigliotti et al., 2014; Vieira-Potter, 2014). It is well established that injury-related inflammation contributes to peripheral and central sensitization (Ren and Dubner, 2010), so it is possible that inflammation caused by obesity may alter nociceptive tone in a similar fashion. Ongoing research in our laboratory is addressing these questions.
Conclusion
In summary, we found sex differences in basal and capsaicin-evoked photophobic behavior that changed with age. Overall, female mice appear to be more sensitive to light. Furthermore, obesity enhanced this behavior in both sexes. This study provides a first step towards a better understanding of how sex, age, and obesity may interact to modulate trigeminal sensory processing across the life span. Given the current epidemic of obesity and the high prevalence of migraine and other headache disorders, further research in this area is crucial for the development of patient-tailored therapies for migraine.
Female mice are more photophobic than male mice.
Young female but not male mice are susceptible to capsaicin-evoked photophobia.
Obesity enhances capsaicin-evoked photophobia in female and male mice.
Acknowledgements
We thank Sunny Kothari and Kimberly Broadhurst for their assistance with data collection, and Drs. Andrew Russo and Randy Kardon for the use of equipment at the University of Iowa. This work was supported by NINDS/NIH grant NS066087 (Recober).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Dr. Recober has received licensing fees from Alder Biopharmaceutical, LLC (for anti-CGRP antibodies in the treatment of photophobia and migraine) unrelated to current manuscript.
References
- Asghari R, Lung MSY, Pilowsky PM, Connor M. Sex differences in the expression of serotonin-synthesizing enzymes in mouse trigeminal ganglia. Neuroscience. 2011;199:429–437. doi: 10.1016/j.neuroscience.2011.10.036. [DOI] [PubMed] [Google Scholar]
- Bigal ME, Liberman JN, Lipton RB. Obesity and migraine: A population study. Neurology. 2006;66:545–550. doi: 10.1212/01.wnl.0000197218.05284.82. [DOI] [PubMed] [Google Scholar]
- Bolay H, Ozge A, Saginc P, Orekici G, Uludüz D, Yalın O, Siva A, Bıçakçı Ş , Karakurum B, Öztürk M. Gender influences headache characteristics with increasing age in migraine patients. Cephalalgia. 2015;35:792–800. doi: 10.1177/0333102414559735. [DOI] [PubMed] [Google Scholar]
- Brennan KC, Romero-Reyes M, López Valdés HE, Arnold AP, Charles AC. Reduced threshold for cortical spreading depression in female mice. Ann Neurol. 2007;61:603–606. doi: 10.1002/ana.21138. [DOI] [PubMed] [Google Scholar]
- Buse DC, Loder EW, Gorman JA, Stewart WF, Reed ML, Fanning KM, Serrano D, Lipton RB. Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2013;53:1278–1299. doi: 10.1111/head.12150. [DOI] [PubMed] [Google Scholar]
- Chanda ML, Tuttle AH, Baran I, Atlin C, Guindi D, Hathaway G, Israelian N, Levenstadt J, Low D, Macrae L, O'Shea L, Silver A, Zendegui E, Lenselink AM, Spijker S, Ferrari MD, van den Maagdenberg AMJM, Mogil JS. Behavioral evidence for photophobia and stress-related ipsilateral head pain in transgenic Cacna1a mutant mice. Pain. 2013;154:1254–1262. doi: 10.1016/j.pain.2013.03.038. [DOI] [PubMed] [Google Scholar]
- Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain. 2004;8:397–411. doi: 10.1016/j.ejpain.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Dolgonos S, Ayyala H, Evinger C. Light-induced trigeminal sensitization without central visual pathways: another mechanism for photophobia. Invest Ophth Vis Sci. 2011;52:7852–7858. doi: 10.1167/iovs.11-7604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felicio L, Nelson J, Finch C. Longitudinal studies of estrous cyclicity in aging C57BL/6J mice: II. Cessation of cyclicity and the duration of persistent vaginal cornification. Biol Reprod. 1984;31:446–453. doi: 10.1095/biolreprod31.3.446. [DOI] [PubMed] [Google Scholar]
- Gazerani P, Andersen OK, Arendt-Nielsen L. A human experimental capsaicin model for trigeminal sensitization. Gender-specific differences. Pain. 2005;118:155–163. doi: 10.1016/j.pain.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Gazerani P, Andersen OK, Arendt-Nielsen L. Site-specific, dose-dependent, and sex-related responses to the experimental pain model induced by intradermal injection of capsaicin to the foreheads and forearms of healthy humans. J Orofac Pain. 2007;21:289–302. [PubMed] [Google Scholar]
- Ghigliotti G, Barisione C, Garibaldi S, Fabbi P, Brunelli C, Spallarossa P, Altieri P, Rosa G, Spinella G, Palombo D, Arsenescu R, Arsenescu V. Adipose tissue immune response: novel triggers and consequences for chronic inflammatory conditions. Inflammation. 2014;37:1337–1353. doi: 10.1007/s10753-014-9914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MT, Petersen KL. Gender differences in pain and secondary hyperalgesia after heat/capsaicin sensitization in healthy volunteers. J Pain. 2006;7:211–217. doi: 10.1016/j.jpain.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Kaiser EA, Kuburas A, Recober A, Russo AF. Modulation of CGRP-induced light aversion in wild-type mice by a 5-HT1B/D agonist. J Neurosci. 2012;32:15439–15449. doi: 10.1523/JNEUROSCI.3265-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C, Beucke J-C, Jensen KB, Gollub RL, Kong J. Sex similarities and differences in pain-related periaqueductal gray connectivity. Pain. 2012;153:444–454. doi: 10.1016/j.pain.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646–657. doi: 10.1046/j.1526-4610.2001.041007646.x. [DOI] [PubMed] [Google Scholar]
- Loyd DR, Murphy AZ. The neuroanatomy of sexual dimorphism in opioid analgesia. Exp Neurol. 2014;259:57–63. doi: 10.1016/j.expneurol.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y-C, Chen C-W, Wang S-Y, Wu F-S. 17β-Estradiol mediates the sex difference in capsaicin-induced nociception in rats. J Pharmacol Exp Thera. 2009;331:1104–1110. doi: 10.1124/jpet.109.158402. [DOI] [PubMed] [Google Scholar]
- Markovics A, Kormos V, Gaszner B, Lashgarara A, Szoke E, Sandor K, Szabadfi K, Tuka B, Tajti J, Szolcsanyi J, Pinter E, Hashimoto H, Kun J, Reglodi D, Helyes Z. Pituitary adenylate cyclase-activating polypeptide plays a key role in nitroglycerol-induced trigeminovascular activation in mice. Neurobiol Dis. 2011;45:633–644. doi: 10.1016/j.nbd.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Noseda R, Kainz V, Jakubowski M, Gooley JJ, Saper CB, Digre K, Burstein R. A neural mechanism for exacerbation of headache by light. Nat Neurosci. 2010;13:239–245. doi: 10.1038/nn.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Tashiro A, Chang Z, Bereiter DA. Bright light activates a trigeminal nociceptive pathway. Pain. 2010;149:235–242. doi: 10.1016/j.pain.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterlin BL, Rosso AL, Rapoport AM, Scher AI. Obesity and migraine: the effect of age, gender and adipose tissue distribution. Headache. 2010;50:52–62. doi: 10.1111/j.1526-4610.2009.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterlin BL, Rosso AL, Williams MA, Rosenberg JR, Haythornthwaite JA, Merikangas KR, Gottesman RF, Bond DS, He J-P, Zonderman AB. Episodic migraine and obesity and the influence of age, race, and sex. Neurology. 2013;81:1314–1321. doi: 10.1212/WNL.0b013e3182a824f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recober A, Kaiser EA, Kuburas A, Russo AF. Induction of multiple photophobic behaviors in a transgenic mouse sensitized to CGRP. Neuropharmacology. 2010;58:156–165. doi: 10.1016/j.neuropharm.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recober A, Kuburas A, Zhang Z, Wemmie JA, Anderson MG, Russo AF. Role of calcitonin gene-related peptide in light-aversive behavior: implications for migraine. J Neurosci. 2009;29:8798–8804. doi: 10.1523/JNEUROSCI.1727-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med. 2010;16:1267–1276. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi HL, Broadhurst KA, Luu AS, Lara O, Kothari SD, Mohapatra DP, Recober A. Abnormal trigeminal sensory processing in obese mice. Pain. 2016;157:235–246. doi: 10.1097/j.pain.0000000000000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi HL, Luu AKS, DeVilbiss JL, Recober A. Obesity increases nociceptive activation of the trigeminal system. Eur J Pain. 2013;17:649–653. doi: 10.1002/j.1532-2149.2012.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff NN, Gold MS. Sex differences in the inflammatory mediator-induced sensitization of dural afferents. J Neurophysiol. 2011;106:1662–1668. doi: 10.1152/jn.00196.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucky NL, Gregory E, Winter MK, He Y-Y, Hamilton ES, McCarson KE, Berman NEJ. Sex differences in behavior and expression of CGRP-related genes in a rodent model of chronic migraine. Headache. 2011;51:674–692. doi: 10.1111/j.1526-4610.2011.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernof A, Després JP. Sex steroid hormones, sex hormone-binding globulin, and obesity in men and women. Horm Metab Res. 2000;32:526–536. doi: 10.1055/s-2007-978681. [DOI] [PubMed] [Google Scholar]
- Vieira-Potter VJ. Inflammation and macrophage modulation in adipose tissues. Cell Microbiol. 2014;16:1484–1492. doi: 10.1111/cmi.12336. [DOI] [PubMed] [Google Scholar]
- Winter A, Berger K, Buring J, Kurth T. Body mass index, migraine, migraine frequency and migraine features in women. Cephalalgia. 2009;29:269–278. doi: 10.1111/j.1468-2982.2008.01716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöber-Bingöl Ç , Wöber C, Karwautz A, Auterith A, Serim M, Zebenholzer K, Aydinkoc K, Kienbacher C, Wanner C, Wessely P. Clinical features of migraine: a cross-sectional study in patients aged three to sixty-nine. Cephalalgia. 2004;24:12–17. doi: 10.1111/j.1468-2982.2004.00621.x. [DOI] [PubMed] [Google Scholar]