Abstract
Objective
To assess safety and feasibility of an intraoperative, minimally invasive NIR image-guided approach to lymphatic mapping in esophageal cancer patients. Although local lymph nodes (LNs) are removed with the esophageal specimen, no techniques are available to identify the regional LNs (separate from the esophagus) during esophagectomy. We hypothesize that NIR imaging can identify regional LNs with potential to improve staging and the extent of lymphadenectomy (LAD).
Methods
Of the 10 patients enrolled, nine had resectable esophageal adenocarcinoma and underwent NIR mapping following peritumoral, submucosal injection of Indocyanine Green (ICG) alone or pre-mixed in human serum albumin (ICG:HSA) prior to resection. NIR imaging was performed in situ and ex vivo.
Results
Intraoperative NIR imaging demonstrated NIR signal at all tumors and in 2–6 NIR+ regional LNs in six of the patients. NIR+ LNs were not identified in 4 cases: one occult stage IV patient, for which further imaging was not performed and was thus excluded from analysis, and 3 cases in which ICG was used without HSA. Identification of local LNs on the esophagus was obscured by peritumoral background. Importantly, pathologic status of NIR+ regional LNs reflected overall regional nodal status.
Conclusions
NIR lymphatic mapping is safe and feasible in esophageal cancer and can identify regional LNs when ICG:HSA is used. Although future work is needed to improve background signal and local LN identification, intraoperative detection of regional NIR+ LNs allows in-depth histologic analysis of LN basins not commonly scrutinized as part of the specimen and may improve detection of occult nodal disease.
Keywords: Esophageal cancer, sentinel lymph node, near infrared imaging
Introduction
Esophageal cancer is an aggressive malignancy with an overall 5-year survival rate of only 18%1. Even with limited N1 disease by the current TNM staging system, 5-year survival is less than 40%2. The incidence of esophageal cancer is increasing, with rates of adenocarcinoma in the U.S. up over 350% over the past 20 years3.
The AJCC 7th Edition pathologic nodal staging for esophageal cancer stratifies prognosis based on the total number of metastatic nodes present rather than by proximity to the tumor4. Given that a more extensive LAD is associated with better prognostication but it is unknown which lymph nodes drain a specific tumor and are at risk for metastatic disease, current surgical recommendations stipulate the removal of a minimum of 20 LNs for T2 (local) disease and ≥30 for more advanced T3/T4 disease in order to attempt to include as many lymph nodes as possible5–7. However, even an extensive 3-field LAD has a 5% risk of recurrent disease appearing in the locoregional nodes, suggesting that key nodes can be missed despite an aggressive LAD and can contain occult nodal metastases8. Although the more common two-field LAD has been shown to minimize operative morbidity compared to 3-field LAD, some studies suggest that recurrence rates may be higher than following an extended LAD9.
Innovative techniques to optimize intraoperative LAD have the potential to improve outcomes by sampling the specific tumor-associated LNs most likely to harbor metastatic disease and by increasing the pathologic scrutiny on these nodes. The goals of such an approach are to provide more accurate prognostic information, potentially reduce the likelihood of regional nodal recurrence by removing occult disease, and identify patient subsets that can benefit from additional adjuvant therapies or, in the case of early T1 disease, esophageal-sparing procedures.
Over the past ten years, efforts to improve intraoperative nodal staging in esophageal cancer have focused primarily on the application of radiocolloid tracers for sentinel lymph node (SLN) mapping. These efforts have not yet led to a reproducible technique for intraoperative in-situ SLN identification10–12. The frequent requirement for an open procedure with back table dissection of the esophagectomy specimen, along with exposure of patients and staff to radioactive tracers have hindered widespread clinical translation. Lastly, the inability to identify the SLN due to “shine-through” from the primary tumor makes the identification and removal of “second tier” nodes beyond SLNs of potential prognostic benefit.
Near-infrared (NIR) imaging has emerged as a safe and promising intraoperative technology for lymphatic mapping in melanoma, breast and lung cancers13–16. However, a single study using NIR imaging in esophageal cancer required preoperative CT lymphography for SLN localization rather than NIR imaging alone, and utilized open thoracotomy for identification and resection of nodes17. We hypothesized that NIR imaging could be developed for minimally invasive intraoperative lymphatic mapping of tumor-draining LNs, including “second tier” regional nodes beyond paraesophageal nodes, for the purposes of targeted analysis of regional nodes at risk for metastases. The clinical translation of such an approach has the potential to render oncologic LAD more attainable and more importantly, can personalize histologic scrutiny to the most critical regional LNs that identify the tumor-associated lymphatic pathway.
The aims of this pilot trial were to develop intraoperative in situ NIR image-guided lymphatic mapping in surgically resectable esophageal cancer and to evaluate safety and feasibility of this approach. This study focuses on NIR lymphatic mapping of the regional LNs as they are not removed with the esophageal specimen and may result in the identification of additional occult nodal disease.
2. Methods
Eligibility
Ten patients diagnosed with esophageal adenocarcinoma scheduled to undergo minimally invasive Ivor Lewis esophagectomy were enrolled between June 2013 and April 2014 after informed consent. This trial was approved by the Dana Farber Cancer Institute Institutional Review Board. Exclusion criteria included patients who choose not to proceed with surgery, age less than 18 years old, pregnancy or breastfeeding, history of iodide or seafood allergy, and discovery of occult metastatic disease at the time of surgery. All study patients underwent preoperative Chest CT, PET/CT and endoscopic ultrasound (EUS).
NIR Image-Guided Lymphatic Mapping and Surgical Resection
Indocyanine Green (ICG) is an FDA-approved NIR dye with a fluorescence emission wavelength ranging from 800–1000nm13. ICG signal is detectable in vivo with minimal NIR autofluorescence and background signal from normal human tissue. Based on a prior successful intrathoracic NIR dose escalation trial in lung cancer,13 a dose of 2.5mg ICG was used for lymphatic mapping in this trial. ICG (Novadaq Technologies, Bonita Springs, Fla) at an initial stock dose of 25mg was diluted to 2.5mg/ml with either sterile water (“ICG alone”) or 25% human serum albumin (ICG:HSA), as albumin has been shown to increase the effective hydrodynamic diameter of ICG almost 6-fold, resulting in increased lymphatic retention18.
Real-time NIR fluorescence images were obtained intraoperatively with the Novadaq PINPOINT minimally invasive system using a 10mm 30 degree NIR thoracoscopic camera. Three simultaneous images of the surgical field were collected: the standard visible light image, a black and white NIR image, and a merged image with NIR signal shown in green in the context of a visible light image. Detection was by qualitative color visualization as software for signal-to-background ratio calculation and semi-quantitative intensity evaluation were not available during this study.
Patients were randomized in block cohorts of two to receive ICG vs. ICG:HSA injected peritumorally via endoscopy prior to positioning the patient for esophagectomy (Fig 1). A total of 1cc ICG (n=5) or ICG:HSA (n=5) was administered peritumorally via 4-corner submucosal injection adjacent to each lesion. In some cases, an endoluminal mass was not evident on intraoperative endoscopic due to prior endoscopic lesion resection or neoadjuvant treatment response. The prior location of the mass as determined by pre-operative staging endoscopy as well as mucosal irregularities consistent with neoadjuvant treatment response guided the accuracy of dye injection in these cases. NIR imaging was performed throughout both the laparoscopic and thoracoscopic portions of each case. Patients were monitored throughout the case for signs of anaphylaxis or other adverse events, of which none occurred.
Figure 1.
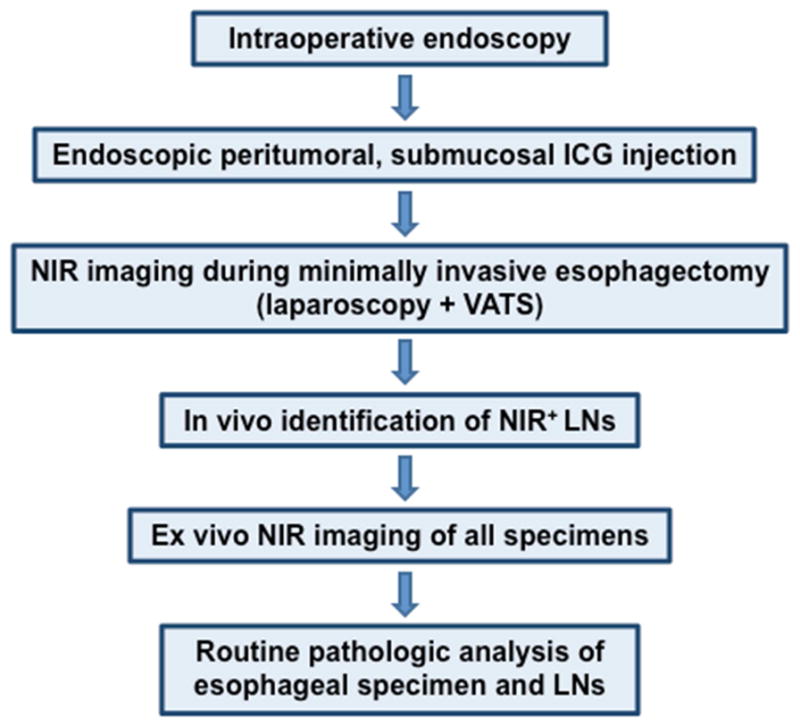
Intraoperative NIR imaging protocol for lymphatic mapping in esophageal cancer
All patients with localized disease (n=9) underwent a minimally invasive Ivor Lewis esophagectomy with two-field LAD. Four cases utilized robotic assistance for the thoracic portion of the procedure. The NIR videoscope was used during dissection of each nodal basin to assess for NIR+ nodes. All cases began with the laparoscopic abdominal portion except in the case of patient 1, who underwent left video-assisted thoracoscopy surgery (VATs) for LN staging and NIR imaging of a PET avid focus prior to the abdominal dissection and subsequent esophagectomy.
Pathologic Analysis
LNs removed with the esophageal specimen were dissected during gross pathologic analysis and were considered “local” LNs. In contrast, LNs identified intraoperatively at discrete stations away from the esophagus (such as pericardial, gastric and celiac stations) were defined as “regional” LNs. Location, including discrete stations, and NIR status were recorded for all LNs before being sent for independent pathologic analysis and interpretation.
Immunohistochemistry for micrometastatic disease in NIR+ LNs was performed on 4-μm thick formalin-fixed paraffin-embedded lymph node tissue tissue sections following proteinase antigen retrieval using a cocktail of anti-keratin mouse monoclonal antibodies (AE1/AE3; 1:200 dilution; 40 min incubation; Dako, Carpinteria, CA) and the EnVision+ System-HRP (Dako).
3. Results
Patient Demographics
A total of 10 patients (9 males and one female) were enrolled and underwent endoscopic peritumoral ICG injection. Two patients (5 and 9) had T1 disease and underwent surgery without neoadjuvant therapy (Table 1). Patient 5 had previous EMR of an intramucosal adenocarcinoma prior to enrollment and underwent esophagectomy for persistent multifocal high grade dysplasia. The other eight patients were assessed to have locally invasive disease and received neoadjuvant chemoradiation, although one was found intraoperatively to have occult stage IV disease and did not undergo resection and thus was excluded from NIR analysis.
Table 1.
Description of subjects by diagnosis, stage and esophageal tumor location
| Pt # | Location of adenocarcinomab | Clinical stage at diagnosisc | Neoadj chemorads | Post-Tx cN staged | Post-op pathologic stage |
|---|---|---|---|---|---|
| 1 | 42–45cm | T2N1 | Yes | N1 | T2N0 |
| 2 | 31–38cm | T3N1 | Yes | N0 | T3N0 |
| 3 | 39cm | T3N1 | Yes | N0 | T2N1 |
| 4 | 35–42cm | T3N1 | Yes | N0 | T3N0 |
| 5 | 30cm | T1N0 | No | - | T1N0 |
| 6a | 34–44cm | T2N1 | Yes | N0 | Stage IV due to M1 |
| 7 | 42cm | TxN0 | Yes | N0 | T3N1 |
| 8 | 34–40cm | T3N1 | Yes | N0 | T3N1 |
| 9 | 35cm | T1N0 | No | - | T1N1 |
| 10 | 39–44cm | TxN1 | Yes | N1 | T3N3 |
Patient excluded due to occult stage IV disease identified at time of laparoscopy.
Location of esophageal mass based on preoperative endoscopic utrasound (EUS)
Clinical stage at time of diagnosis based on EUS and PET/CT findings.
Post-neoadjuvant treatment clinical N stage is based on follow up restaging PET/CT prior to operative intervention.
The mean age was 67 +/− 7.62 years. Patient demographics including histology, clinical stage, tumor location and subsequent pathologic stage at resection are shown in Table 1. All lesions were considered lower third/GE junction tumors except for patient 5 with an intramucosal lesion at 30cm in the middle third of the esophagus.
Preoperative PET/CTs were node negative in 8 of 10 cases, five of which had undergone neoadjuvant chemoradiotherapy for suspected N1 disease by EUS or initial PET/CT. Two patients demonstrated new or persistent PET avidity at the time of surgery. Patient 1 was initially clinical stage T2N0, underwent neoadjuvant chemoradiation and subsequently developed avidity in the left mediastinum, correlating with a LN between the left mainstem bronchus and aorta. The initial PET/CT for patient 10 demonstrated avidity in the region of the gastrohepatic ligament with decreased but persistent avidity following neoadjuvant chemoradiation.
Feasibility of Submucosal Peritumoral NIR Lesion Marking and the Importance of Tracer Composition
In this pilot study, we evaluated the feasibility and safety of endoscopic submucosal injection of Indocyanine Green (ICG) dye (Fig 2), which, unlike the subadventitial approach, permits direct visualization of the lesion at the time of operative endoscopy and facilitates accurate peritumoral NIR marking. Furthermore, as lymphatic mapping requires that the tumor-associated lymphatic pathway remains intact, we sought to avoid potential lymphatic disruption that may occur with dissection and subadventitial injection.
Figure 2.

Submucosal esophageal ICG injection approach results in ICG dye migration through submucosal lymphatics (dark blue line) and to more distal regional LNs (black line).
Sclerotherapy needles were primed with ICG prior to injection to avoid inadequate dose delivery. ICG injection was performed via endoscopy under direct visualization in a 4-corner peritumoral manner in all patients without difficulty. No adverse events were noted.
To assess the importance of the ICG carrier for NIR lymphatic mapping, patients undergoing minimally invasive esophagectomy were randomized to endoscopic peritumoral injection of ICG mixed with saline (ICG alone, n=5) or ICG mixed with human serum albumin (ICG:HSA, n=5). Endoscopic peritumoral ICG injection was successfully performed in all cases (n=10) and in situ detection of NIR signal at the site of the esophageal tumor was achieved in all cases. The time from injection to initial videoscopic NIR imaging ranged from 20 minutes to one hour and 20 minutes. Patient 6 underwent peritumoral ICG:HSA injection of a clinically staged T2N0 tumor but was subsequently excluded from nodal analysis as occult stage IV disease was discovered at the time of laparoscopy. Given that esophagectomy and LAD were not performed and NIR imaging was not completed, the patient was excluded from further analysis. Of the nine patients that did undergo esophagectomy and LAD, ICG:HSA was the superior lymphatic imaging agent, with identification of regional lymph nodes in 100% (4 of 4 patients) vs 40% (2 of 5 patients) using ICG alone. There were no adverse events in any patients.
Results of Intraoperative NIR Lymphatic Mapping
The mean total number of LNs resected (including both local and regional nodes) for T1 and T2/T3 surgical specimens was 21.5 +/− 4.95 and 29.29 +/− 5.99 respectively. The total number of “local” LNs removed with the esophageal specimen and dissected at the time of formal pathologic analysis ranged from 15 to 30 per specimen (Table 2). The total number of regional LNs identified intraoperatively as separate from the esophageal specimen and removed ranged from 2 to 11.
Table 2.
Characteristics of lymphadenectomy specimens including NIR+ regional LNs
| Pt # | NIR tracer | Time to 1st imaging (mins) | Total # LNs removed | # NIR+ regional LNs | Nodal station of NIR+ LNsa (time to detection) | ||
|---|---|---|---|---|---|---|---|
| Lap | VATS | Local | Regional | ||||
| 1 | ICG:HSA | - | 39 | 30 | 10 | 6 | 8L (−), 16 (−) |
| 2 | ICG:HSA | 60 | - | 20 | 2 | 2 | 7 (−) |
| 3 | ICG alone | 60 | 222 | 15 | 11 | 0 | - |
| 4 | ICG alone | 26 | 192 | 25 | 3 | 0 | - |
| 5 | ICG alone | 80 | 378 | 16 | 2 | 0 | - |
| 7 | ICG alone | 34 | 275 | 28 | 5 | 1 | 16 (80 mins) |
| 8 | ICG:HSA | 25 | 240 | 17 | 8 | 2 | 16 (30 mins), 20 (60 mins) |
| 9 | ICG:HSA | 20 | 265 | 15 | 10 | 3 | 16 (90 mins), 17 (110 mins) |
| 10 | ICG alone | 45 | 310 | 25 | 6 | 4 | 16 (205 mins), 17 (210 mins) |
“Local” LNs are located directly on the esophageal specimen; “regional” LNs are separate from the specimen.
Regional LN stations: 7 subcarinal; 8L thoracic lower periesophageal; 16 pericardial; 17 left gastric; 20 celiac axis (see Figure 4). LN = lymph node; NIR+ = detection of near infrared fluorescence; ICG = Indocyanine green; ICG:HSA = Indocyanine green premixed with human serum albumin
Significant NIR background directly on the esophageal specimen, particularly around the injection site, was observed. No distinct NIR+ local LNs were identified directly on the specimen due to the high peritumoral ICG signal. However, two to six NIR+ regional LNs, separate from the esophagus, were readily identified in 6 of 9 patients. All 3 patients for whom regional NIR+ LNs were not identified had received an injection of ICG alone, confirming superiority of ICG:HSA as regional NIR+ nodes were identified in 4 of 4 patients undergoing esophagectomy. ICG nodal uptake was confirmed ex vivo on all identified NIR+ LNs (Fig 3). Bisection of NIR+ LNs demonstrated tracer uptake throughout the nodal parenchyma, indicative of lymphatic migration and not surface contamination.
Figure 3.
In vivo and ex vivo NIR image-guided identification of intra-abdominal esophageal LNs. A. In vivo NIR+ left periesophageal LN B. NIR+ lymphatic tract to LN C. Ex vivo confirmation of an NIR+ LN
Time to VATS from ICG injection ranged from 39 minutes (when VATS was performed prior to laparoscopy) to 6hrs and 18 minutes. On intrathoracic NIR imaging, there was evidence of dye migration on the esophagus and dye extravasation into the posterior mediastinum in some cases. These findings did create difficulty assessing lymph nodes directly on the esophagus, however, regional lymph nodes within the mediastinum could still be visualized without background NIR signal.
In vivo identification of NIR+ LNs was feasible as early as 30 minutes and as late as 3.5 hrs. NIR+ regional LNs were identified at LN stations in both abdominal and thoracic cavities, including the pericardial (n=5), left gastric (n=2), celiac axis (n=1), lower periesophageal (n=1) and subcarinal (n=1) stations (Fig 4). Cervical lymph nodes were sampled in one case and did not show evidence of NIR uptake.
Figure 4.

The distribution of NIR+ LNs in the patients studied is displayed by anatomic nodal station
Histopathologic Analysis
Routine histopathologic analysis of regional LNs distinct from the esophageal specimen demonstrated that all NIR+ regional LNs were negative for disease, which was consistent with the pathologic status of all other regional LNs removed (Table 3). Immunohistochemistry for keratins was also negative in regional NIR+ LNs indicating that micrometastatic disease was also not present in these regional NIR+ LNs. Pathologic “local” LNs were identified in 5 cases; all came as part of the esophageal specimen and were identified as malignant on routine pathologic analysis.
Table 3.
Location of histologically detected metastatic lymph nodes (LNs) in cases with identified NIR+ LNs.
| Pt # | # Metastatic/Total nodes identified | |
|---|---|---|
| Regional | Local | |
| 7 | 0/5 | 1/28 |
| 8 | 0/8 | 1/17 |
| 9 | 0/10 | 1/15 |
| 10 | 0/6 | 7/25 |
Includes NIR+ regional LNs
Patients 1 and 10, both of whom had neoadjuvant chemoradiation and preoperative PET/CT findings of FDG avidity, had negative regional LNs on histologic analysis, including a PET avid NIR+ left lower periesophageal LN identified at the time of surgery (patient 1) and a PET avid left gastrohepatic lymph node (patient 10). A total of three NIR+ left gastric LNs were identified and removed in patient 10 and all were negative on routine histopathologic and immunohistochemical analysis. However, it is of note that despite an otherwise negative preoperative PET/CT (aside from the avidity in the gastrohepatic region), this patient did have evidence of nodal disease in seven local LNs removed with the esophageal specimen.
4. Discussion
This pilot trial is a “first in human” study to develop the technique and demonstrate feasibility of NIR image-guided lymphatic mapping as the sole modality for the identification of regional LNs during minimally invasive esophagectomy. Although high background at the site of peritumoral injection prevented identification of local nodes removed with the esophageal specimen itself, discrete regional LNs distinct from the esophageal specimen were identified in 6 of 9 patients that underwent esophagectomy, and in all esophagectomy patients in which ICG:HSA was used as the lymphatic tracer. As seen with lung NIR imaging, peritumoral injection of ICG:HSA improved NIR+ LN identification with NIR+ nodes detected in all 4 esophagectomies, whereas injection of ICG alone led to NIR+ LN identification in only 2 of 5 cases.
Of the six cases in which NIR+ LNs were identified, five had abdominal NIR+ LNs, whereas only two had thoracic NIR+ LNs. The distribution of NIR+ LNs in our patients is relatively consistent with literature describing the metastatic patterns of LN metastases in lower esophageal and GE junction cancers, with an NIR+ pericardial node draining the tumor in over 80% of the patients studied19–21. In 181 cases of lower thoracic and GE junction adenocarcinomas treated with surgery alone or neoadjuvant chemoradiation and surgery, Castoro et al identified lymphatic metastasis predominantly in regional abdominal and thoracic periesophageal nodes, including pericardial (12.5–37.1%), perigastric (18.8–35.6%), celiac axis (14.4–18.8%) as well as thoracic periesophageal (21.9–29.6%) and subcarinal (5.3–9.4%) stations19. The similar distribution of regional nodes identified using NIR imaging suggests that this technology may reflect the relevant tumor-draining lymphatic pathways in this disease rather than the preferential identification of NIR+ nodes in the first cavity examined. The ability to identify and characterize these pathways has the potential to improve the likelihood of an oncologic LAD by identifying the regional LNs most likely to contain metastatic disease.
The current inability to accurately identify and sample the appropriate LNs in a systematic fashion for esophageal cancer has limited clinical decision-making. NIR identification of these regional nodes assures removal and, with further validation of their relationship to the primary tumor, may allow greater pathologic and molecular analysis of the lymphatic pathway(s) leading away from the tumor, and ultimately permit better staging and identification of patients that may benefit from neoadjuvant therapy before esophagectomy is performed. Although peritumoral NIR background likely obscured the ability to detect NIR+ nodes on the esophagus directly adjacent to the tumor, van de Ven reported that ~30% of GEJ tumors also have LN metastasis at other sites beyond the peritumoral region, and Hosch reported a 43% rate of “skip” metastasis (defined as nodes 3cm beyond the primary lesion)22. Failure to remove these metastatic regional nodes with the esophagectomy specimen results in a falsely low N stage in the current staging system as nodal staging is based on the number of metastatic LNs identified, rather than the nodal location or distance the metastases have traveled within the lymphatic system. Therefore, these studies suggest that NIR lymphatic mapping of regional LNs could be very useful in identifying regional metastatic nodes which are not removed with the specimen, and currently results in understaging and undertreating of metastatic nodal disease. Having now established initial safety and feasibility, NIR lymphatic mapping can permit targeted LN sampling of these LNs which might otherwise be missed. By accurate NIR localization of regional tumor-associated LNs and histologic classification of local and regional LN tiers, we envision that the LN station relative to the primary tumor will take on a greater role in prognosis as seen with many other solid organ tumors.
In the current study, NIR+ regional LNs were negative for metastatic disease, consistent with the status of all other regional LNs removed from these patients and is reflective of early stage in these surgically resectable patients. Findings suggest that histopathologic examination of NIR+ LN separate from the esophageal specimen may assist in determining adequate LN sampling for staging. It is of interest that several PET avid regional nodes identified as high risk for metastases on the pre-operative PET imaging, also resided within the lymphatic basins mapped by peritumoral ICG and identified as NIR+. This suggests that these reactive nodes are draining the tumor basin and thus indeed are at high risk for occult metastases, however, none of the NIR+ LNs, including those retrieved from PET avid nodal regions, stained positive for micrometastatic disease on immunohistochemical analysis. Given that PET avidity is not visible intraoperatively, the ability to use NIR imaging as an intraoperative guide to these high risk nodes may prove very helpful if this correlation persists in future studies. While the prognostic value of micrometastatic disease is not firmly established in esophageal cancer, there is growing evidence that nodal micrometastasis may confer poor prognosis and even provide additional prognostic information over node positive cases identified by H&E23,24. Interestingly, none of our patients found to have metastatic disease in local paraesophageal nodes demonstrated FDG activity adjacent to the esophagus on the preoperative PET/CT even where disease was ultimately found. This highlights the limitations of PET/CT to reliably identify metastatic LNs and suggests that additional imaging tools such as dose-adjusted NIR lymphatic mapping may improve the identification of LNs for increased scrutiny to rule out metastasis that cannot be detected by other current imaging modalities.
The current study is the first human trial to develop a technique for in situ minimally invasive NIR-guided lymphatic mapping in esophageal cancer and to directly compare ICG alone to ICG:HSA. Prior sentinel LN (SLN) mapping trials comparing ICG to ICG:HSA in breast and cervical cancer suggested that there was no significant difference in the NIR+ LN yield25,26. However, ICG mixed with HSA has been shown to increase the hydrodynamic diameter of the dye, improving fluorescence and nodal retention in a porcine model of SLN mapping18. Previous studies by our lab and others have shown that HSA is variably required for NIR imaging based on the tissue to be imaged13,14,27. The one published study on esophageal NIR lymphatic mapping using ICG required the addition of preoperative CT lymphography with iopamidol to guide nodal identification but the NIR+ nodal identification rate was 95% when assessed via open thoracotomy17. However, periesophageal background was not discussed. Thus, randomized comparison of ICG to ICG:HSA was included in the current trial design to identify the more promising tracer for esophageal lymphatic mapping.
NIR+ LNs were not identified in three of nine patients that underwent esophagectomy and surprisingly ICG suspended in sterile water without albumin was utilized in all three patients, suggesting that albumin enhances ICG retention in human LNs as well as at least in the thoracic cavity. In the three ICG:alone cases in which NIR+ nodes were not identified, abdominal imaging occurred between 30 minutes and 1 hour after dye injection and lasted for 2–2.5 hours, consistent with other cases in the trial that did lead to NIR nodal identification. It is possible that rapid lymphatic clearance and poor lymphatic retention with ICG alone led to missing the relevant LNs during the later thoracic component, which typically began about 4 hours after injection. We hypothesize that ICG:HSA may be preferable in esophageal cancer lymphatic mapping as this formulation may remain within the lymphatics and regional LNs for the longer periods of time required for esophagectomy and LAD.
NIR background on the esophageal specimen, as a result of transmucosal dye migration and dye spillage during dissection, may have precluded the intraoperative identification of local NIR+ LNs on the specimen. As the ICG dose used in this trial (2.5mg) was initially optimized for lung cancer lymphatic mapping where background levels are lower, we hypothesize that ICG dose reduction will significantly decrease the peritumoral background and improve the in vivo signal-to-noise ratio and thus identification of NIR+ “local” LNs. The ability to accurately sample tumor-associated nodes for histologic scrutiny prior to esophagectomy, thereby avoiding complete esophagectomy and LAD with back table nodal dissection as has been required in studies using radiocolloid approaches, would permit in situ staging and possible selection of truly N0 patients for esophageal-sparing endomucosal resection10,12. Given that a significant number of early lesions may have nodal metastasis which will be missed by PET imaging, an optimized NIR guided two-field LN mapping technique may be justified as a means to accurately assess the pathologic status of both local and regional NIR+ LNs in situ.
Given the theoretical risk of lymphatic ablation, particularly given reports of poor sentinel node identification following neoadjuvant treatment with other techniques, we were concerned that NIR imaging for lymphatic mapping in esophageal cancer would be limited by prior neoadjuvant radiation treatment18. Interestingly, this was not the case as NIR+ nodes were readily identified in the 3 neoadjuvant patients given ICG:HSA, adding further support for the use of ICG:HSA as the NIR fluorophore in esophageal patients. We recognize that this pilot trial includes only a small patient sample and that future trials are needed to truly determine the impact of neoadjuvant treatment on NIR+ LN yield and the ability to identify the correct tumor-associated nodes.
Limitations of the trial include a small sample size without identification of a large number of disease-positive regional lymph nodes to assess accuracy of NIR regional nodal mapping. Additionally, cervical and upper mediastinal lymph nodes were not assessed in most cases, as three-field LAD was not performed given the potential risks of further dissection in patients without clinical or radiographic evidence of disease. Although not sufficiently powered to definitively compare the performance of ICG:HSA to ICG alone, this pilot trial has aided in a preliminary determination of which tracer has the best likelihood of success such that a larger trial can focus on identifying the optimal dose of ICG:HSA to minimize peritumoral background on the esophageal specimen.
Future efforts in NIR lymphatic mapping in esophageal cancer will focus on improving the identification of both regional and adjacent “local” esophageal LNs such that in situ nodal staging may ultimately permit organ preservation for patients deemed node negative. The development of innovative dual modality approaches pairing NIR lymphatic mapping with other novel tracers may more directly identify suspicious nodes and enhance the detection of nodal metastases. Promising approaches such as preoperative MRI imaging with intravenous superparamagnetic iron oxide particles have demonstrated feasibility for LN staging in small pilot trials in other malignancies but merit further evaluation in intrathoracic malignancies such as esophageal cancer and the current manuscript establishes the groundwork for these upcoming studies28. Further optimization of minimally invasive NIR lymphatic mapping for esophageal cancer has the potential to provide a safe, easy to use, intraoperative approach to the identification of tumor-specific regional LNs for improved targeted staging.
Supplementary Material
Central picture.

NIR-guided in vivo regional esophageal LN detection
Video.

Intraoperative near infrared image-guided technique for regional node identification in esophageal cancer
central message
Near infrared lymphatic mapping is safe and feasible for in situ identification of regional lymph nodes in esophageal cancer.
perspective statement
This “first-in human” trial demonstrates preliminary safety and feasibility of intraoperative minimally invasive near-infrared (NIR) lymphatic mapping for identification of regional lymph nodes (LNs) in esophageal cancer. NIR imaging may have potential as a staging tool in the future to better select patients for neoadjuvant therapy and endomucosal resection.
Acknowledgments
Funding for this research study: Internal hospital funds
Glossary of Abbreviations
- NIR
near infrared
- SLN
sentinel lymph node
- ICG
indocyanine green
- HSA
human serum albumin
- LAD
lymphadenectomy
- PET
positron emission tomographic
- CT
computed tomography
Footnotes
Disclosures: Authors have no potential conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.http://seer.cancer.gov/statfacts/html/esoph.html
- 2.Talsma K, van Hagen P, Grotenhuis BA, Steyerberg EW, Tilanus HW, van Lanschot JJ, et al. Comparison of the 6th and 7th Editions of the UICC-AJCC TNM Classification for Esophageal Cancer. Ann Surg Oncol. 2012;19(7):2142–8. doi: 10.1245/s10434-012-2218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altorki NK, Zhou XK, Stiles B, Port JL, Paul S, Lee PC, et al. Total Number of Resected Lymph Nodes Predicts Survival in Esophageal Cancer. Ann Surg. 2008;248(2):221–26. doi: 10.1097/SLA.0b013e31817bbe59. [DOI] [PubMed] [Google Scholar]
- 4.Edge SB, et al., editors. AJCC cancer staging manual. 7. New York, NY: Springer; 2009. Esophagus and esophagogastric junction; pp. 103–16. [Google Scholar]
- 5.Herrera LJ. Extent of lymphadenectomy in esophageal cancer: how many lymph nodes is enough? Ann Surg Oncol. 2011;16:676–8. doi: 10.1245/s10434-009-0824-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenstein AJ, Litle VR, Swanson SJ, Divinio CM, Packer S, Wisnivesky JP. Effect of the number of lymph nodes sampled on postoperative survival of lymph node-negative esophageal cancer. Cancer. 2008;112:1239–46. doi: 10.1002/cncr.23309. [DOI] [PubMed] [Google Scholar]
- 7.Peyre CG, Hagen JA, DeMeester SR, Altorki NK, Ancano E, Griffin SM, et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg. 2008;248:549–56. doi: 10.1097/SLA.0b013e318188c474. [DOI] [PubMed] [Google Scholar]
- 8.Lerut T, Nafteux P, Moons J, Coosemans W, Decker G, De Leyn P, et al. Three-Field Lymphadenectomy for Carcinoma of the Esophagus and Gastroesophageal Junction in 174 R0 Resections: Impact on Staging, Disease-Free Survival, and Outcome: a Plea for Adaptation of TNM Classification in Upper-Half Esophageal Carcinoma. Ann Surg. 2004;240(6):962–74. doi: 10.1097/01.sla.0000145925.70409.d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishihara T, Hirayama K, Mori S. A prospective randomized trial of extended cervical and superior mediastinal lymphadenectomy for carcinoma of the thoracic esophagus. Am J Surg. 1998;175:47–51. doi: 10.1016/s0002-9610(97)00227-4. [DOI] [PubMed] [Google Scholar]
- 10.Takeuchi H, Kawakubo H, Takeda F, Omori T, Kitagawa Y, et al. Sentinel node navigation surgery in early-stage esophageal cancer. Ann Thorac Cardiovasc Surg. 2012;18(4):306–13. doi: 10.5761/atcs.ra.12.01951. [DOI] [PubMed] [Google Scholar]
- 11.Uenosono Y, Arigami T, Yanagita S, Kozono T, Arima H, Hirata M, et al. Sentinel node navigation surgery is acceptable for clinical T1 and N0 esophageal cancer. Ann Surg Oncol. 2011;18:2003–9. doi: 10.1245/s10434-011-1711-6. [DOI] [PubMed] [Google Scholar]
- 12.Thompson SK, Bartholomeusz D, Jamieson GG. Sentinel lymph node biopsy in esophageal cancer: should it be standard of care? J Gastrointest Surg. 2011;15(10):1762–8. doi: 10.1007/s11605-011-1634-3. [DOI] [PubMed] [Google Scholar]
- 13.Gilmore DM, Khullar OV, Jaklitsch MT, Chirieac LR, Frangioni JV, Colson YL. Identification of metastatic nodal disease in a phase I dose-escalation trial of intraoperative sentinel lymph node mapping in non-small cell lung cancer using near-infrared imaging. J Thorac Cardiovasc Surg. 2013;146(3):562–70. doi: 10.1016/j.jtcvs.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilmore DM, et al. Effective low-dose escalation of indocyanine green for near-infrared fluorescent sentinel lymph node mapping in melanoma. Ann Surg Oncol. 2013;20(7):2357–63. doi: 10.1245/s10434-013-2905-x. [DOI] [PubMed] [Google Scholar]
- 15.Mitsumori N, Nimura H, Takahashi N, Kawamura M, Aoki H, Shida A. Sentinel lymph node navigation surgery for early stage gastric cancer. World J Gastroenterol. 2013;20(19):5685–93. doi: 10.3748/wjg.v20.i19.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Troyan SL, Kianzad V, Gibbs-Strauss SL, Gioux S, Matsui A, Oketokoun R. The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in breast cancer sentinel lymph node mapping. Annals of Surg Onc. 2009;16:2943–52. doi: 10.1245/s10434-009-0594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuasa T, Seike J, Yoshida T, Takechi H, Yamai H, Yamamoto Y, et al. Sentinel lymph node biopsy using intraoperative indocyanine green fluorescence imaging navigated with preoperative CT lymphography for superficial esophageal cancer. Ann Surg Oncol. 2012;19(2):486–93. doi: 10.1245/s10434-011-1922-x. [DOI] [PubMed] [Google Scholar]
- 18.Ohnishi S, Lomnes SJ, Laurence RG, Gogbashian A, Mariani G, Frangioni JV. Organic alternatives to quantum dots for intraoperative near-infrared fluorescent sentinel lymph node mapping. Mol Imaging. 2005;4(3):172–181. doi: 10.1162/15353500200505127. [DOI] [PubMed] [Google Scholar]
- 19.Castoro C, Scarpa M, Cagol M, Ruol A, Cavallin F, Alfieri R, et al. Nodal Metastasis from locally advanced esophageal cancer: how neoadjuvant therapy modifies their frequency and distribution. Ann Surg Oncol. 2011;18(13):3743–54. doi: 10.1245/s10434-011-1753-9. [DOI] [PubMed] [Google Scholar]
- 20.van de Ven C, De Leyn P, Coosemans W, Van Raemdonck D, Lerut T. Three-field lymphadenectomy and pattern of lymph node spread in T3 adenocarcinoma of the distal esophagus and the gastro-esophageal junction. Eur J Cardiothorac Surg. 1999;15(6):769–73. doi: 10.1016/s1010-7940(99)00122-0. [DOI] [PubMed] [Google Scholar]
- 21.Stein HJ, et al. Early esophageal cancer : pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg. 2005;242(4):566–73. doi: 10.1097/01.sla.0000184211.75970.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosch SB, Stoecklein NH, Pichlmeier U, Rehders A, Scheunemann P, Niendorf A, et al. Esophageal cancer: the mode of lymphatic tumor cell spread and its prognostic significance. J Clin Oncol. 2001;19(7):1970–5. doi: 10.1200/JCO.2001.19.7.1970. [DOI] [PubMed] [Google Scholar]
- 23.Prenzel KL, Holscher AH, Drebber U, Agavonova M, Gutschow CA, Bollschweiler E. Prognostic impact of nodal micrometastasis in early esophageal cancer. Eur J Surg Oncol. 2012;38(4):314–8. doi: 10.1016/j.ejso.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Zingg U, Montani M, Busch M, Metzger U, Went P, Oertli D. Prognostic influence of immunohistochemically detected lymph node micrometastasis and histological subtype in pN0 oesophageal cancer. Eur J Surg Oncol. 2009;35(6):593–599. doi: 10.1016/j.ejso.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Polom K, Murawa D, Nowaczyk P, Rho YS, Murawa P. Breast cancer sentinel lymph node mapping using near infrared guided indocyanine green and indocyanine green—human serum albumin in comparison with gamma emitting radioactive colloid tracer. Eur J Surg Oncol. 2012;38(2):137–42. doi: 10.1016/j.ejso.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Schaafsma BE, van der Vorst JR, Gaarenstroom KN, Peters AA, Verbeek FP, de Kroon CD, et al. Randomized comparison of near-infrared fluorescence lymphatic tracers for sentinel lymph node mapping of cervical cancer. Gynecol Oncol. 2012;127(1):126–30. doi: 10.1016/j.ygyno.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamashita S, Tokuishi K, Miyawaki M, Anami K, Moroga T, Takeno S, et al. Sentinel node navigation surgery by thoracoscopic fluorescence imaging system and molecular examination in non-small cell lung cancer. Ann Surg Oncol. 2012;19(3):728–33. doi: 10.1245/s10434-011-2145-x. [DOI] [PubMed] [Google Scholar]
- 28.McDermott S, Thayer SP, Fernandez-Del Castillo C, Mino-Kenudson M, Weissleder R, Harisinghani MG. Accurate prediction of nodal status in preoperative patients with pancreatic ductal adenocarcinoma using next-gen nanoparticle. Transl Oncol. 2013;6(6):670–5. doi: 10.1593/tlo.13400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



