Abstract
Background
Previous studies in adults have suggested that donor dopamine treatment may improve recipient outcomes in organ transplantation; in this analysis, we aimed to determine if donor dopamine reduces the incidence of post-operative right heart failure in pediatric heart transplant recipients.
Methods
Data for recipients aged ≤ 18 transplanted at our institution between 1/1/2000–6/15/2011 and their respective donors were obtained. The presence of postoperative right heart failure was assessed for in all subjects. Donor dopamine dose was stratified into 3 groups: none, low-dose (≤ 5 mcg/kg/min), and high-dose (>5 mcg/kg/min). Logistic regression was used to assess the relationship between donor dopamine dose and recipient right heart failure.
Results
Of 192 recipients, 34 (18%) experienced postoperative right heart failure. There was no difference in baseline demographics between recipients with and without right heart failure. When controlling for pulmonary vascular resistance index, graft ischemic time, and cardiopulmonary bypass time, donor low-dose dopamine was independently associated with a decreased risk of right heart failure (OR=0.16 [0.04–0.70]; p=0.02); however high-dose DA was neither associated with, nor protective of, RHF (OR: 0.31 [0.06–1.6]; p=0.16).
Conclusions
Despite advances in perioperative care of the recipient, right heart failure persists as a complication of pediatric heart transplantation. In this study donor pre-treatment with low-dose dopamine is associated with a decreased risk of postoperative right heart failure in pediatric heart recipients. Further studies into this association may be useful in determining the utility of empiric donor pre-treatment with low-dose dopamine.
INTRODUCTION
Heart transplantation is an increasingly successful treatment strategy for end stage heart failure due to a wide variety of congenital and acquired cardiac diseases in children1. However, primary graft failure, and in particular postoperative acute right heart failure (RHF), remains a significant barrier to survival2–4.
Treatment of brain-dead donors with low-dose dopamine prior to graft procurement improves adult recipient outcomes in renal transplantation; reducing post-transplant dialysis requirements5. The use of inotropic support in potential cardiac donors, including dopamine, is usually considered a negative indicator of donor quality. However, low-dose dopamine pretreatment of brain-dead donors has recently been associated with improved long-term survival in adult cardiac transplant recipients6. Nevertheless, the possible peri-operative benefits of donor dopamine in pediatric cardiac transplant recipients have yet to be examined.
In this retrospective analysis, we examined the hypothesis that pretreatment of donors with dopamine is associated with improved postoperative outcomes, specifically the development of right heart failure, in pediatric heart transplant recipients.
METHODS
Study Population
After Institutional Review Board approval was obtained, the study population of all pediatric heart transplant recipients less than or equal to 18 years of age transplanted from Jan 1, 2000 through June 15, 2011 at our institution was identified. Clinical data were obtained through retrospective institutional recipient chart review and review of corresponding donor medical records. Donor data collected included age, gender, mechanism of death, requirement of cardiopulmonary resuscitation, use of thyroxine replacement, echocardiographic data and cardiac enzyme levels. Donor echocardiograms were considered abnormal if the report indicated an abnormal ejection fraction, wall motion abnormalities, or more than mild valvar regurgitation. Recipient demographics and pre-transplant factors along with operative variables such as graft ischemic time and cardiopulmonary bypass time were collected as well. For analysis, recipients were divided into 3 donor dopamine pre-treatment groups, determined at the time of organ procurement: no dopamine, low-dose dopamine (dose ≤5 mcg/kg/min), or high-dose dopamine (dose > 5 mcg/kg/min).
Outcome Measures
The primary outcome measure of this study was postoperative right heart failure of the recipient requiring treatment. This outcome was defined by either the need for mechanical circulatory support of the right ventricle or rising central venous pressure with right ventricular hypocontractility and progressive right ventricular dilatation on echocardiography requiring medical intervention (initiation or increase of inotropic support in conjunction with pulmonary vasodilator therapy, typically inhaled Nitric Oxide).
Secondary outcomes measures included complication frequency, time to extubation, intensive care unit (ICU) length of stay, total postoperative length of stay, and short- and long-term survival.
Statistical Analysis
Summary statistics are presented as median (interquartile range), mean ± standard deviation, or number (percent) as appropriate. Donor and recipient characteristics and short-term outcomes were analyzed using Chi-square, Fisher’s exact, Student’s t-test, Wilcoxon rank-sum test, ANOVA, or Kruskal-Wallis test, as appropriate to variable type and distribution. The primary outcomes of the relationship between donor dopamine dose and recipient postoperative RHF was assessed using logistic regression. Variables for building the multivariable logistic regression model were based on known clinical factors, as well as statistical interactions. Those variables with an unadjusted p-value of <0.1 were considered candidates for the multivariable model as were variable defined a priori to be assumed risk factors of RHF. In deference to parsimony, variables that were neither significant, nor had interactions (defined as a minimum of a 10% change in coefficient) with other variables were eliminated from the final model. Kaplan-Meier analysis with log-rank test was used to analyze actuarial survival. The conventional p-value < 0.05 was used to determine statistical significance, and all reported p-values are 2-sided. The data were analyzed with StataIC 11.0 (Stata Corp LP, College Station, TX).
RESULTS
Study Population
A total of 192 pediatric heart transplant recipients ≤18 years of age were transplanted from January 1, 2000 through June 15, 2011 at our institution. The total study population had a median age of 8.25 years (IQR: 1.7–13.8 years) and was 46.9% female. Indications for transplant were cardiomyopathy (59.4%), congenital heart disease (29.7%), retransplantation (6.8%), and other diagnoses (4.2%); including viral myocarditis and intracardiac tumor. At the time of transplant, 82% were UNOS status 1A with 13% on mechanical ventilation, 66% on high-dose or multiple inotropic support, and 19% on mechanical circulatory support. Of the 192 donors, 118 (61%) were on inotropic support (epinephrine, norepinephrine, milrinone, dobutamine or dopamine) at the time of organ procurement with 17 of those on 2 or more inotropic drips. Dopamine was the most commonly used inotrope in 77 donors (40%), followed by norepinephrine (n=30, 16%), epinephrine (n=19, 10%), dobutamine (n=9, 5%) and milrinone (n=2, 1%). With respect to donor dopamine administration, 115 (60%) recipients were included in the no dopamine group, 51 (26%) in the low-dose group, and 26 (14%) in the high-dose group. Recipient baseline characteristics did not differ significantly between donor dopamine groups (Table 1a). Furthermore, there were no significant differences in donor characteristics across dopamine dose groups (Table 1b).
Table 1.
| a. Recipient Demographics compared among donor dopamine groups. | ||||
|---|---|---|---|---|
| No Dopamine (n=115) |
Low-Dose Dopamine (n=51) |
High-Dose Dopamine (n=26) |
p-value | |
| Age (IQR) (years) | 8.9 (1.7–13.9) | 7.7 (1.7–15.8) | 7.2 (1.6–11.8) | 0.74 |
| BSA (IQR) (m2) | 0.9 (0.5–1.4) | 0.9 (0.4–1.6) | 0.8 (0.5–1.2) | 0.64 |
| Gender (% female) | 52 (45.2%) | 25 (49.0%) | 13 (50.0%) | 0.85 |
| Cardiac diagnosis | - | - | - | 0.38 |
| Cardiomyopathy | 65 (56.5%) | 36 (70.6%) | 13 (50.0%) | |
| Congenital | 36 (31.3%) | 11 (21.6%) | 10 (38.5%) | |
| Retransplant | 8 (7.0%) | 2 (3.9%) | 3 (11.5%) | |
| Ventilator support, pre-operative | 12 (10.4%) | 8 (15.7%) | 5 (19.2%) | 0.39 |
| Inotropic support, pre-operative | 82 (71.3%) | 32 (62.8%) | 13 (50%) | 0.11 |
| VAD, pre-operative | 13 (11.3%) | 9 (17.6%) | 2 (7.7%) | 0.42 |
| ECMO, pre-operative | 5 (4.4%) | 4 (7.8%) | 3 (11.5%) | 0.27 |
| PVRi, pre-operative (IQR) (WU × m2) | 2.7 (1.7–5.6) | 3.0 (2.1–5.1) | 2.4 (1.7–3.1) | 0.52 |
| Graft cold ischemic time (min) | 196 ± 55 | 214 ± 68 | 216 ± 80 | 0.13 |
| Cardiopulmonary bypass time (min) | 158 ± 60 | 150 ± 48 | 154 ± 61 | 0.73 |
| b. Donor demographics compared among donor dopamine groups | ||||
|---|---|---|---|---|
| No Dopamine (n=115) |
Low-Dose Dopamine (n=51) |
High-Dose Dopamine (n=26) |
p-value | |
| Donor Age (IQR) (years) | 11.0 (2.0–18.0) | 8.0 (1.6–16.6) | 6.6 (1.2–15.1) | 0.34 |
| Donor BSA (IQR) (m^2) | 1.2 (0.6–1.7) | 1.0 (0.5–1.6) | 0.9 (0.5–1.5) | 0.29 |
| Donor Gender (% female) | 50 (43.5%) | 25 (49.0%) | 14 (53.8%) | 0.58 |
| Cause of death | - | - | - | 0.29 |
| Anoxia | 37 (32.2%) | 17 (33.3%) | 5 (19.2%) | |
| Cerebrovascular | 16 (13.9%) | 10 (19.6%) | 1 (3.8%) | |
| Head trauma | 57 (49.6%) | 22 (43.1%) | 18 (69.2%) | |
| Downtime | 52 (45.2%) | 23 (45.1%) | 9 (34.6%) | 0.60 |
| Positive blood culture | 7 (6.1%) | 4 (7.8%) | 1 (3.8%) | 0.83 |
| Thyroxine replacement | 46 (40.0%) | 17 (33.3%) | 7 (23.1%) | 0.24 |
| Vasopressin administration | 46 (40.0%) | 19 (37.2%) | 15 (57.7%) | 0.20 |
| Other inotrope administration | 41 (36%) | 10 (20%) | 5 (20%) | 0.06 |
| Ejection fraction (%) | 61.1 ± 7.7 | 59.4 ± 5.4 | 62.4 ± 1.4 | 0.10 |
| Abnormal echocardiogram | 7 (6.1%) | 4 (7.8%) | 3 (11.5%) | 0.52 |
| Troponin, at procurement (IQR) (ng/mL) | 0.10 (0.04–0.66) | 0.10 (0.04–0.40) | 0.21 (0.04–0.80) | 0.85 |
Clinical outcomes based on donor dopamine dose
Of the 192 recipients, 34 (17.7%) experienced the primary outcome of postoperative RHF. With the exception of pulmonary vascular resistance index (PVRi), recipient baseline characteristics did not differ between those who had RHF and those who did not (Table 2). Importantly, perioperative characteristics of graft cold ischemic time and cardiopulmonary bypass time were not different between those who experienced postoperative RHF and those who did not.
Table 2.
Recipient characteristics by development of right heart failure (RHF)
| No RHF (n=158) |
RHF (n=34) |
p-value | |
|---|---|---|---|
| Age (IQR) (years) | 7.6 (1.5–13.9) | 10.6 (3.2–13.6) | 0.24 |
| BSA (IQR) (m^2) | 0.84 (.43–1.4) | 1.1 (.6–1.4) | 0.25 |
| Gender (% female) | 76 (48.1%) | 14 (41.2%) | 0.57 |
| Cardiac diagnosis, on admission | - | - | 0.07 |
| Cardiomyopathy | 100 (63.3%) | 14 (41.2%) | |
| Congenital | 44 (27.9%) | 13 (38.2%) | |
| Retransplant | 9 (5.7%) | 4 (11.8%) | |
| Ventilator support, pre-operative | 22 (13.9%) | 3 (8.8%) | 0.58 |
| High-dose or multiple inotropes | 101 (63.9%) | 26 (76.5%) | 0.23 |
| VAD, pre-operative | 21 (13.3%) | 3 (8.8%) | 0.58 |
| ECMO, pre-operative | 9 (5.7%) | 3 (8.8%) | 0.45 |
| PVRi, pre-operative (IQR) (WU × m2) | 2.7 (1.8–4.3) | 4.4 (1.8–8.6) | 0.01 |
| Graft cold ischemic time (mins) | 204.8 ± 64.2 | 197.7 ± 54.8 | 0.55 |
| Cardiopulmonary bypass time (mins) | 153.7 ± 59.4 | 163.2 ± 45.6 | 0.40 |
Donor administration of dopamine showed significant associations with recipient outcomes (Table 3). Specifically, there was a significant reduction in the development of RHF when the donor received dopamine, particularly low-dose dopamine (none: 23.5%, low-dose: 7.8%, high-dose: 11.5%; p=0.03). Those recipients whose donors received dopamine had a significantly shorter time to extubation and a shorter hospital length of stay compared to the no dopamine group. Of note, there was no significant difference in short-term recipient mortality (30-day and 1-year) by donor dopamine dose.
Table 3.
Clinical Outcomes by donor dopamine dose
| No Dopamine (n=115) |
Low-Dose Dopamine (n=51) |
High-Dose Dopamine (n=26) |
p-value | |
|---|---|---|---|---|
| Right Heart Failure | 27 (23.5%) | 4 (7.8%) | 3 (11.5%) | 0.03 |
| Mechanical Circulatory Support | 7 (6.1%) | 1 (2.0%) | 1 (3.8%) | 0.63 |
| Time to Extubation (IQR) (days) | 2 (1–5) | 1 (1–3) | 2 (1–4) | 0.01 |
| ICU Length of Stay (IQR) (days) | 10 (6–17) | 7 (5–11) | 9 (6–15) | 0.07 |
| Post-op Length of Stay (IQR) (days) | 21 (14–33) | 16 (11–21) | 18 (12–26) | 0.01 |
| 30-day Mortality | 4 (3.5%) | 1 (2.0%) | 1 (3.8%) | 0.84 |
| 1-year Mortality | 10 (8.7%) | 6 (11.7%) | 1 (3.8%) | 0.63 |
Clinical outcomes based on RHF status post-transplant
Recipient postoperative RHF was significantly associated with poorer clinical outcomes (Table 4). Median time to extubation (4 vs 2 days, p=0.0001) and ICU length of stay (12 vs 8 days, p=0.01) were significantly longer in recipients who developed RHF as compared to those who did not. Furthermore, the development of RHF was associated with worse 30-day mortality (11.8% vs. 1.3%, p=0.01). Despite the increase in 30-day mortality, RHF did not appear to be associated with poorer overall survival (p=0.41) (Figure 1).
Table 4.
Clinical outcomes by RHF status
| No RHF (n=158) |
RHF (n=34) |
p-value | |
|---|---|---|---|
| Mechanical Circulatory Support | 6 (3.8%) | 3 (8.8%) | 0.20 |
| Time to Extubation (IQR) (days) | 2 (1–4) | 4 (3–7) | 0.0001 |
| ICU Length of Stay (IQR) (days) | 8 (6–15) | 12 (9–18) | 0.01 |
| Post-op Length of Stay (IQR) (days) | 18 (12–28) | 24 (17–30) | 0.10 |
| 30-day Mortality | 2 (1.3%) | 4 (11.8%) | 0.01 |
| 1-year Mortality | 12 (8.9%) | 5 (19.3%) | 0.16 |
Figure 1.
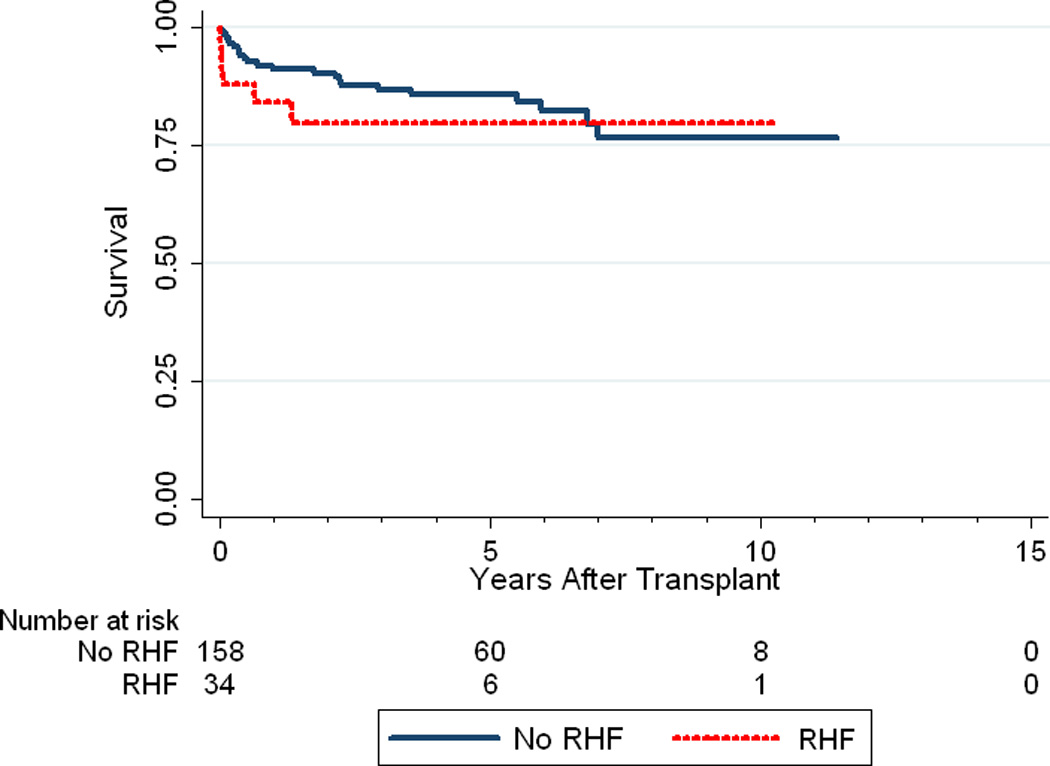
Kaplan-Meier survival curve depicting recipient survival between those who experienced RHF and those who did not (p=0.41).
Relationship between Donor Dopamine Administration and Recipient RHF
In univariable logistic regression analysis, only donor pretreatment with dopamine and recipient pretransplant PVRi were significantly associated with the development of postoperative RHF. A list of all variables considered for analysis is provided in Table 5a. Based on the univariable analysis, risk factors included in the multivariable logistic regression were donor dopamine group, recipient pre-operative PVRi (dichotomized at 6 WU × m2), and the a priori determined risk factors of graft cold ischemic time, and total cardiopulmonary bypass time. The results of the multivariable logistic regression are shown in Table 5b. As the nonsignficant risk factors did not show interaction with the primary variable of interest (donor dopamine), a reduced model was created by removing the nonsignificnat risk factors of graft cold ischemic time and total cardiopulmonary bypass time (Table 5c). Most importantly, when controlling for graft ischemic time and total cardiopulmonary bypass time; donor low-dose dopamine remained associated with a decreased risk of recipient postoperative RHF (OR: 0.16 [0.34–0.78]; p=0.02), independent of recipient pre-operative PVRi. However, high-dose dopamine was neither associated with, nor protective of recipient postoperative RHF (OR: 0.32 [0.06–1.6]; p=0.16). This relationship held true even when not controlling for ischemic and cardiopulmonary bypass times. Despite this association there was no significant difference in long-term survival when recipients were stratified by their donor’s dopamine dose (p=0.66) (Figure 2). To examine the lack of association with high-dose dopamine, the multivariable model was rerun using low-dose dopamine as the indicator variable. In this model, no dopamine was significantly associated with an increased risk of postoperative RHF (OR: 6.15 [1.28–29.4], p=0.02), and high-dose dopamine was again not significantly associated with the outcome (OR: 1.93 [0.235–15.84], p=0.54).
Table 5.
| a. Univariate analysis of proposed factors associated with postoperative RHF | |||
|---|---|---|---|
| Variable | Unadjusted Odds Ratio |
95% Confidence Interval | p-value |
| Donor: Weight (kg) | 1.4 | 0.8–2.6 | 0.24 |
| Donor: Cause of death | 1.1 | 0.7–1.6 | 0.68 |
| Donor: Low-dose dopamine pre-treatment | 0.3 | 0.1–0.8 | 0.02 |
| Donor: High-dose dopamine pre-treatment | 0.4 | 0.1–1.4 | 0.16 |
| Donor: Other inotrope pre-treatment | 1.8 | 0.6–4.9 | 0.27 |
| Donor: Thyroxine pre-treatment | 0.9 | 0.4–2.0 | 0.88 |
| Donor: Downtime (y/n) | 0.8 | 0.4–1.9 | 0.74 |
| Donor: Abnormal echocardiogram | 0.7 | 0.2–3.6 | 0.73 |
| Recipient: Admission diagnosis | 1.1 | 0.7–1.9 | 0.61 |
| Recipient: Ventilator support, pre-operative | 0.6 | 0.2–2.1 | 0.43 |
| Recipient: VAD placement, pre-operative | 0.6 | 0.2–2.3 | 0.48 |
| Recipient: ECMO, pre-operative | 1.6 | 0.4–6.3 | 0.50 |
| Recipient: High-dose/Multiple Inotropes | 1.8 | 0.8–4.3 | 0.17 |
| Recipient: Status 1A, prior to transplant | 1.8 | 0.6–5.4 | 0.32 |
| Recipient: PVRi, pre-operative | 1.2 | 1.1–1.4 | 0.006 |
| Graft cold ischemic time (mins) | 1.0 | 0.9–1.1 | 0.55 |
| Cardiopulmonary bypass time (mins) | 1.0 | 0.9–1.1 | 0.40 |
| b. Multivaraible model of risk factors associated with postoperative RHF | |||
|---|---|---|---|
| Variable | Adjusted Odds Ratio |
95% Confidence Interval | p-value |
| Donor: Low-dose dopamine | 0.16 | 0.04–0.78 | 0.02 |
| Donor: High-dose dopamine | 0.31 | 0.06–1.6 | 0.16 |
| Recipient: Elevated Pre-operative PVRi (>6 WU × m2) | 4.0 | 1.4–11.9 | 0.01 |
| Graft cold ischemic time (mins) | 0.99 | 0.98–1.0 | 0.40 |
| Cardiopulmonary bypass time (mins) | 1.0 | 0.99–1.01 | 0.61 |
| c: Reduced multivaraible model of risk factors associated with postoperative RHF | |||
|---|---|---|---|
| Variable | Adjusted Odds Ratio |
95% Confidence Interval | p-value |
| Donor: Low-dose dopamine | 0.14 | 0.03–0.69 | 0.02 |
| Donor: High-dose dopamine | 0.31 | 0.06–1.5 | 0.15 |
| Recipient: Elevated Pre-operative PVRi (>6 WU × m2) | 5.1 | 1.8–14.5 | 0.002 |
Figure 2.
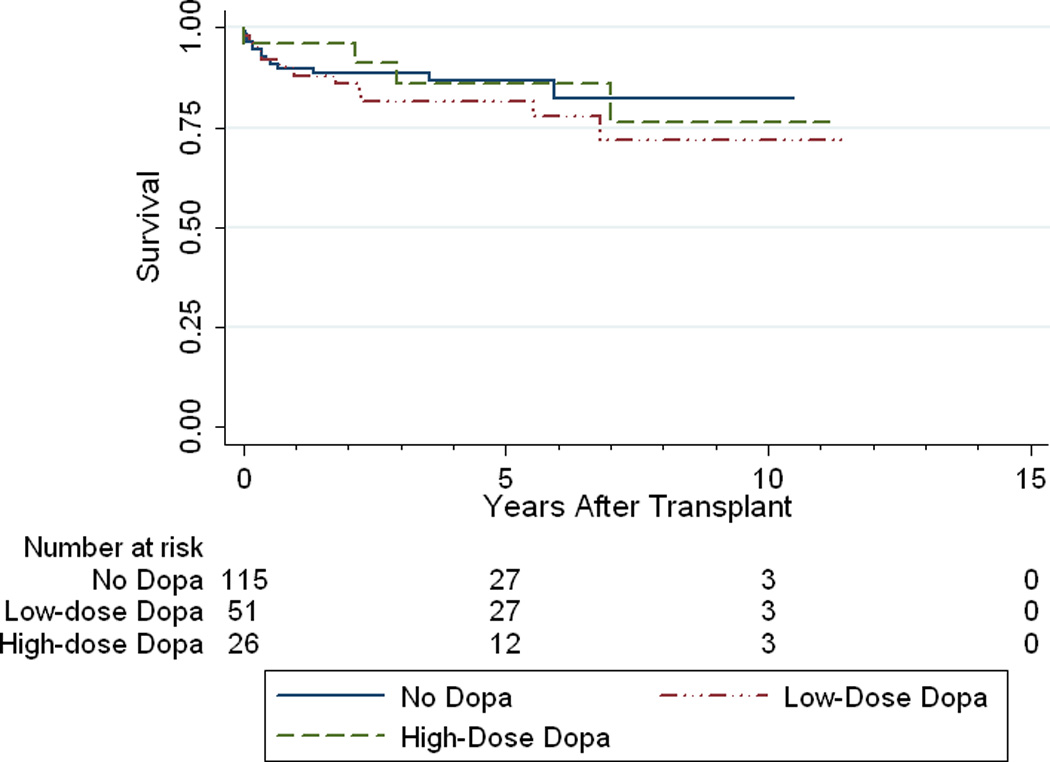
Kaplan-Meier recipient survival curve divided by donor dopamine dose
DISCUSSION
While heart transplantation is a largely successful therapy for children with end stage heart failure, early mortality remains a significant problem; despite the improved treatment of and decreasing mortality from early postoperative right heart failure (4). While the association of right heart failure and high recipient pulmonary vascular resistance is well described, the importance of donor factors prior to procurement is becoming increasingly clear2–4,7–9. The use of low-dose dopamine in donors prior to organ procurement has been shown to improve kidney graft outcomes, and may be linked to improved long-term outcomes in adult heart recipients5,6. The present study showed an association between the use of low-dose dopamine in donors and a decrease in the incidence of early postoperative right heart failure in pediatric heart recipients as well as benefits in duration of mechanical ventilation and hospital length of stay.
While elevated pulmonary vascular resistance is often considered the prime risk factor for right heart failure after orthotopic heart transplantation, it has been demonstrated that the cardiac graft is likely predisposed to right ventricular failure well before organ procurement. Animal studies have shown significantly decreased right and left ventricular preload recruitable stroke work in both the brain dead donor as well as the recipient of the graft8,10. Interestingly, the right ventricle appears to be more susceptible to this type of injury11. This decrease in right ventricular contractility pre-procurement is further worsened by cold ischemia; resulting in a graft that is significantly impaired in its ability to adapt to increases in right ventricular afterload8, a problem compounded by elevated recipient pulmonary vascular resistance. Besides stimulating dopamine specific receptors at low doses, recent studies have suggested that the benefit of low-dose dopamine may be secondary to antioxidant properties which can attenuate the damage myocytes experience during cold ischemia, thereby minimizing at least one of the factors contributing to postoperative right heart failure12. In this study, only low dose dopamine exhibited a protective effect on the development of right heart failure. It is possible that higher dose dopamine was an indicator of donor hemodynamic instability, cardiac dysfunction, or perhaps there may be negative effects upon the graft at higher doses due to increased myocardial oxygen consumption or other mechanisms. Additionally, as few donors were on high-dose dopamine, we may not be able to detect a true difference between high-dose dopamine and no dopamine due to inadequate statistical power. Certainly prospective studies would be needed to assess whether this protective benefit is limited to low-dose dopamine or if intermediate or higher doses could confer a similar benefit in a suitable donor.
The study population was derived from recipients at a single high-volume institution. This allowed for better ascertainment of the occurrence of right heart failure, data which is not routinely collected in current multicenter registries such as the UNOS/OPTN dataset or ISHLT registry. To that end, although the study period encompassed over a decade of pediatric heart transplants, there was uniformity of treatment throughout the study period in the ICU as the use of inhaled nitric oxide for posttransplant right heart failure began in 1995. The only difference in clinical care was the use of sildenafil instead of nifedipine to transition off of inhaled nitric oxide in recent years.4,13. Furthermore, donor management occurred at the local level, and the recipient medical team did not consider type or dose of donor medications into their treatment decisions for the recipient, making such bias unlikely. To this end, it is interesting to hypothesize why some donors received dopamine infusions while others did not. Of course, the lack of clarity as to the indication for dopamine administration in the donor is a major limitation. Due to the retrospective nature of this study not only are the decision making processes of the donor team impossible to know, but even the specifics of duration of treatment and any titration of the dopamine dose are unknown. It is entirely possible that these unknown variables are responsible for the findings of this study and donor dopamine administration is merely a mediator or causal partner in the effect model. This would not negate the association seen in this study, but rather offer a different understand of why it is. However, in preliminary analysis, no obvious donor factors seemed to play a role in the decision to administer dopamine, and as literature supporting the use of donor dopamine is quite recent, it is unlikely that this played a factor either5,6. Of note, we did not notice any time dependent effect in the proportion of donors receiving dopamine with similar rates of use occurring throughout the study period (unpublished data).
The usual limitations of retrospective data collection and analysis were minimized in this study as determination of right heart failure occurred independently and prior to donor data collection, eliminating this particular bias. While specific measurements of right heart function (central venous pressure, right ventricular end-diastolic dimension, etc.) were unable to be attained for all patients, the records were sufficiently complete to determine the initiation of treatment (mechanical circulatory support, inotropes, inhaled nitric oxide) for clinical right heart failure as described above. The association with poorer clinical outcomes (increased time of mechanical ventilation, hospital length of stay and 30-day mortality) appears to validate the determination of right heart failure as described; however, we acknowledge that the lack of an objective measure of right heart failure remains a major limitation of the study and tempers the findings. Furthermore, we were able to obtain all donor records for patients transplanted at our institution during the study period, confirming either the absence or use of donor dopamine and also the specific dose, thereby minimizing any selection bias that could have occurred due to incomplete records. Our institution has a high proportion (18%) of recipients with pretransplant elevated PVRi (>6 WU × m2), however even when controlling for recipient PVRi, low-dose donor dopamine remained protective of right heart failure, suggesting that the benefit of donor dopamine persists even in the face of elevated recipient PVRi. We similarly also controlled for factors previously defined as conferring risk of right heart failure (graft cold ischemic time, total cardiopulmonary bypass time)2,3, despite no significant associations in our study population. While this study did not show a survival benefit to the use of low-dose donor dopamine, this is likely due to improved treatment of right heart failure resulting in equivalent survival curves for patients with and without early right heart failure, as well as the low number of early deaths (6 of 192) in the study population, making this study underpowered to detect a difference for that time point.
Despite advances in perioperative care of the pediatric heart recipient, early right heart failure remains prevalent and can lead to further morbidities and mortality. Although early right heart failure may have a multifactorial etiology, donor pre-treatment with low-dose dopamine may abrogate the risk of the development of early right heart failure in pediatric heart recipients leading to decreases in duration of mechanical ventilation and overall hospital stay. Given the limitations of this study, further follow-up and larger, prospective studies are necessary to fully evaluate the effects of donor pre-treatment with low-dose dopamine on the pediatric heart recipient as well as the pediatric recipients of other solid organs.
Acknowledgments
FREEDOM OF INVESTIGATION
This work was supported in part by NIH Training Grant # 2T35HL007616-31A1 (R.E.)
Abbreviations
- RHF
Right Heart Failure
- PVRi
Pulmonary Vascular Resistance Index
- ICU
Intensive Care Unit
Footnotes
DISCLOSURES
Disclosure Statement: The authors declare no conflicts of interest
All authors have no financial or other conflicts of interest to disclose.
Author Contributions:
Marc Richmond (mr2306@columbia.edu) Participated in research design, performance of the research, data analysis and writing of the manuscript
Rachel Easterwood (rae7001@nyp.org) Participated in research design, performance of the research, data analysis and writing of the manuscript
Rakesh Singh (rsingh@rchsd.org) Participated in research design, performance of the research and editing of the manuscript
Lisa Gilmore (lagpnp@yahoo.com) Participated in performance of the research and editing of the manuscript
Kimberly Beddows (kdbeddows@gmail.com) Participated in performance of the research and editing of the manuscript
Warren Zuckerman (wz2116@columbia.edu) Participated in performance of the research and editing of the manuscript
Eric McFeely (eric.d.mcfeely@gmail.com) Participated in performance of the research and editing of the manuscript
Jonathan Chen (jonathan.chen@seattlechildrens.org) Participated in research design and editing of the manuscript
Linda Addonizio (lja1@columbia.edu) Participate in research design and editing of the manuscript
REFERENCES
- 1.Dipchand AI, Kirk R, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: Sixteenth Official Pediatric Heart Transplantation Report—2013; Focus Theme: Age. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2013;32(10):979–988. doi: 10.1016/j.healun.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Hoskote A, Carter C, Rees P, Elliott M, Burch M, Brown K. Acute right ventricular failure after pediatric cardiac transplant: predictors and long-term outcome in current era of transplantation medicine. The Journal of Thoracic and Cardiovascular Surgery. 2010;139(1):146–153. doi: 10.1016/j.jtcvs.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 3.Huang J, Trinkaus K, Huddleston CB, Mendeloff EN, Spray TL, Canter CE. Risk factors for primary graft failure after pediatric cardiac transplantation: importance of recipient and donor characteristics. The Journal of Heart and Lung Transplantation. 2004;23(6):716–722. doi: 10.1016/j.healun.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Ofori-Amanfo G, Hsu D, Lamour JM, et al. Heart transplantation in children with markedly elevated pulmonary vascular resistance: Impact of right ventricular failure on outcome. The Journal of Heart and Lung Transplantation. 2011;30(6):659–666. doi: 10.1016/j.healun.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Schnuelle P, Gottmann U, Hoeger S, et al. Effects of donor pretreatment with dopamine on graft function after kidney transplantation: A randomized controlled trial. Jama. 2009;302(10):1067–1075. doi: 10.1001/jama.2009.1310. [DOI] [PubMed] [Google Scholar]
- 6.Benck U, Hoeger S, Brinkkoetter PT, et al. Effects of Donor Pre-Treatment With Dopamine on Survival After Heart Transplantation: A Cohort Study of Heart Transplant Recipients Nested in a Randomized Controlled Multicenter Trial. Journal of the American College of Cardiology. 2011;58(17):1768–1777. doi: 10.1016/j.jacc.2011.05.060. [DOI] [PubMed] [Google Scholar]
- 7.Fukushima N, Gundry SR, Razzouk AJ, Bailey LL. Risk factors for graft failure associated with pulmonary hypertension after pediatric heart transplantation. J Thorac Cardiovasc Surg. 1994 Apr 1;107(4):985–989. 1994. [PubMed] [Google Scholar]
- 8.Bittner HB, Chen EP, Biswas SS, Van Trigt P, III, Davis RD. Right ventricular dysfunction after cardiac transplantation: primarily related to status of donor heart. Ann Thorac Surg. 1999 Nov 1;68(5):1605–1611. doi: 10.1016/s0003-4975(99)00987-x. 1999. [DOI] [PubMed] [Google Scholar]
- 9.Conway J, Chin C, Kemna M, et al. Donors' characteristics and impact on outcomes in pediatric heart transplant recipients. Pediatric Transplantation. 2013;17(8):774–781. doi: 10.1111/petr.12149. [DOI] [PubMed] [Google Scholar]
- 10.Van Trigt P, Bittner HB, Kendall SW, et al. Mechanisms of transplant right ventricular dysfunction. Ann Surg. 1995 Jun;221(6):666–675. doi: 10.1097/00000658-199506000-00006. discussion 675-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kendall SW, Bittner HB, Peterseim DS, et al. Right ventricular function in the donor heart. Eur J Cardiothorac Surg. 1997 Apr;11(4):609–615. doi: 10.1016/s1010-7940(97)01148-2. [DOI] [PubMed] [Google Scholar]
- 12.Vettel C, Hottenrott MC, Spindler R, et al. Dopamine and Lipophilic Derivates Protect Cardiomyocytes against Cold Preservation Injury. Journal of Pharmacology and Experimental Therapeutics. 2014 Jan 1;348(1):77–85. doi: 10.1124/jpet.113.207001. 2014. [DOI] [PubMed] [Google Scholar]
- 13.Singh RK, Richmond ME, Zuckerman WA, et al. The Use of Oral Sildenafil for Management of Right Ventricular Dysfunction After Pediatric Heart Transplantation. American Journal of Transplantation. 2013 doi: 10.1111/ajt.12552. n/a-n/a. [DOI] [PubMed] [Google Scholar]


