Abstract
Introduction
Familial hypercholesterolemia (FH) is a severely underdiagnosed and undertreated genetic disorder. Little is known about regional variation in the prevalence of FH, and information for Central and Eastern Europe (CEE) is scarce. This paper assesses the prevalence of FH and related cardiovascular disease (CVD) risk factors in Poland.
Material and methods
We performed a meta-analysis of six population-based studies in Poland. The FH was assessed using the Dutch Lipids Clinics Network (DLCN) criteria. The categories “definite” (> 8 points) and “probable” (6–8 points) were combined into “potential FH”. Combined estimates of proportions across studies were pooled by meta-analysis with a random effects model.
Results
A total of 37,889 persons aged 20–79 years were included in the analysis. The distribution of DLCN scores was skewed, and there were only 7 cases of definite FH. Prevalence of potential FH was 404/100,000 people (95% CI = 277–531/100,000). Familial hypercholesterolemia was more prevalent in women than in men, and the prevalence was the highest in the age group 45–54 years in men and 55–64 years in women. After adjustment for age and sex, compared to participants with normal cholesterol, persons with potential FH had twice the prevalence of hypertension (p < 0.01); smoking was more prevalent by about 80% (p < 0.01) and hypertriglyceridemia was nine times more frequent (p < 0.001). There was no difference in the prevalence of low high-density lipoprotein (HDL)-cholesterol or diabetes.
Conclusions
We believe that our study might facilitate the planning of a strategy to manage the disease at a population level, i.e. to develop a national strategy for the detection, diagnosis, and treatment of FH.
Keywords: familial hypercholesterolemia, Dutch Lipids Clinics Network criteria, risk factors, epidemiology, prevalence
Introduction
Familial hypercholesterolemia (FH) is an autosomal dominant condition characterized by the life-course elevated blood low-density lipoprotein (LDL)-cholesterol concentration. In most cases, mutations of the gene for the LDL receptor (LDLR) or for apoprotein B (ApoB) or for proprotein convertase subtilisin/kexin type 9 (PCSK9) are found, but other forms of genetic mutations can also exist as very rare mutations of the LDLR adaptor protein 1 pathway. A vast majority of people with FH are heterozygotes. Heterozygous mutations of LDLR are present in about 90% of FH cases, while ApoB and PCSK9 were found in 5% and 1%, respectively [1–3]. Familial hypercholesterolemia has been well recognized for over 50 years, but it has gained attention recently for several important reasons. First, it is related to early atherosclerosis and premature coronary heart disease (CHD) and people with heterozygous FH are automatically considered as high-risk patients [4, 5]. Second, in many countries, the condition remains severely underdiagnosed and undertreated. Out of 22 countries, in more than half the estimates of the proportion of individuals diagnosed with FH were not higher than 1% of all cases, and only in five countries did the proportion exceed 5%, reaching the absolutely exceptional maximum of 71% in the Netherlands [3]. Only about half of FH patients were receiving appropriate therapy [4, 6]. Furthermore, FH patients were more likely to smoke and to have a high concentration of blood triglycerides and lower chance of having blood pressure within the recommended limits [6]. Statins are considered to be the first-line therapy for patients with FH [7–9], but low-potency statins or moderate doses of higher potency statins are not effective enough. In consequence, higher doses of high-potency statins are often needed to reach the goal of lowering LDL-C levels by 50% at least. Combined treatment with ezetimibe, ER niacin, bile acid binding agents, or anti-PCSK9 antibodies is also postulated [10–12]. The problem of statin intolerance is of particular importance as it affects 10–15% of patients treated with statins in general, with some complications having been reported more with the use of synthetic, potent, and more lipophilic statins [13].
All this suggests that FH requires a specific detection and treatment strategy that is firmly based on epidemiological evidence. It used to be generally believed that homozygous FH occurs in about 1/100,000 people, and heterozygous FH in about 1/500. However, these numbers were questioned because they were derived mainly from old clinical data. More recent studies indicated that the prevalence may reach 1/200 [3]. Data on prevalence of FH are not available for most countries because there is a lack of national registers or screening strategies in action. Furthermore, uniform criteria for the diagnosis of FH are not agreed, and there are three sets of criteria at least in broader use depending on the region of the world [2]. Genetic testing would be particularly beneficial to confirm the diagnosis in every individual case, but genetic screening was not found to be a cost-effective tool [14, 15].
The spectrum of FH mutations in Europe varies by country [2]. It is likely that there is also regional variation in the prevalence of FH in general. Information for Eastern Europe is scarce. Even the flagship report on FH of the European Atherosclerosis Society does not provide an estimate for diagnosed FH for a single Central or East European (CEE) country [3]. The findings in patients after hospitalization due to CHD indicate that the prevalence of FH seems to be high in CEE countries including Poland, in which 11.4% of patients were found to have definite or probable FH [6]. In Poland, several large population-based studies have been conducted, but the problem of FH was not addressed in any of them, as each of these studies had too low statistical power to deliver a more precise estimate.
The goal of this study was to assess the prevalence of FH and related cardiovascular disease (CVD) risk factors by a meta-analysis of the results of six large, population-based, observational studies carried out in Poland, using available data on phenotype of FH.
Material and methods
We used data from six population studies, carried out in Poland in well-defined populations, in which at least 2,000 participants were examined. Detailed descriptions of the methods used in particular studies have been published elsewhere [16–23]. The list of populations studied and the information on recruitment and participation are presented in Table I.
Table I.
Description of the study populations, selection, and participation rates of the study samples
| Study name | Study population | Sampling | Years of observation | Total examined | Participation rate (%) | Included in meta-analysis | |||
|---|---|---|---|---|---|---|---|---|---|
| N | % | Age | % men | ||||||
| POL-MONICA Krakow | Residents of Tarnobrzeg Voivodship (province) | Random sampling after stratification by sex and 10-year age groups | 1983–1984 1987–1988 1992–1993 |
5362 | 81 73 74 |
5159 | 96.2 | 35–64 | 46.5 |
| POL-MONICA Warszawa | Residents of two districts in Warsaw, capital city | Random sampling after stratification by sex and 10-year age groups | 1984 1988 1993 |
5618 | 74 70 74 |
5385 | 95.9 | 35–64 | 49.2 |
| WOBASZ | Residents of Poland | Three-stage sampling | 2003–2004 | 14769 | 77 | 14011 | 94.9 | 20–74 | 47.0 |
| Pilot HAPIEE | Residents of Krakow town | Random sampling after stratification by sex and 5-year age groups | 2001–2002 | 2310 | 65 | 2043 | 88.4 | 45–64 | 49.8 |
| HAPIEE | Residents of Krakow town | Random sampling after stratification by sex and 5-year age groups | 2003–2005 | 9296 | 61 | 9128 | 98.2 | 45–70 | 48.3 |
| NATPOL 2011 | Residents of Poland | Three-stage sampling | 2011 | 2413 | 66 | 2163 | 89.6 | 20–74 | 48.2 |
For the present analysis, FH was assessed using the Dutch Lipids Clinics Network (DLCN) criteria for diagnosis of heterozygous FH in adults [3, 24]. Participants were classified as follows: “definite FH” if they scored > 8 points, “probable FH” if they scored 6–8 points, and “possible FH” if they scored 3–5 points. In addition, categories “definite FH” and “probable FH” were combined into “potential FH.” The category “normal blood cholesterol” was defined as having total cholesterol (TC) < 5 mmol/l and LDL-C < 3 mmol/l and not being on blood lipid-lowering treatment. In all studies, data on tendon xanthomas and arcus cornealis in participants or their families as well as data on first-degree relatives and children with LDL-C > 95th percentile by age and gender were not available. Also, no molecular genetic testing was available, so the final classification was based on phenotype characteristics, i.e. blood LDL-cholesterol and family or personal history of CHD and other acute atherosclerosis manifestations. The methods of blood processing and lipid determination are presented in Table II. In all studies, the information on family and personal history was collected by interview according to the questionnaire. All the available and relevant information on personal and family history of early CHD, stroke, and peripheral arterial disease (PAR) was used. Table III presents the information obtained to be used in the DLCN classification of FH.
Table II.
Methods of blood lipid determination
| Study name | Material | TC | HDL-C | TG | LDL-C |
|---|---|---|---|---|---|
| POL-MONICA Krakow | Plasma (frozen) | Manual, direct (Liebermann-Burchard procedure) | Manual, direct (Liebermann-Burchard procedure), after precipitation with MnCL2 and heparin | Manual, enzymatic | Calculated (Friedewald's formula) |
| POL-MONICA Warszawa | Plasma (frozen) | Manual, direct (Liebermann-Burchard procedure) | Manual, direct (Liebermann-Burchard procedure) after precipitation with MnCL2 and heparin | Manual, enzymatic | Calculated (Friedewald's formula) |
| WOBASZ | Serum (frozen) | Enzymatic-colorimetric test (CHOD/PAP) with cholesterol esterase, cholesterol oxidase, and 4-aminoantipyrine (Roche kit); INTEGA 400 automatic analyzer | Monochromatic colorimetric test with cholesterol esterase and cholesterol oxidase modified PEG after precipitation with MnCL2 and heparin (Roche kit); INTEGA 400 automatic analyzer | Enzymatic-colorimetric (GPO/PAD) with glycerol phosphate oxidase and 4-aminoantipyrine (Roche kit); INTEGA 400 automatic analyzer | Calculated (Friedewald's formula); if TG > 400 mg/dl monochromatic colorimetric test |
| Pilot HAPIEE | Plasma | Oxidation method with the MP3 Boehringer Mannheim chemical reagent kit in the Technicon RA-I000 automatic analyzer | Oxidation method with the MP3 Boehringer Mannheim chemical reagent kit in the Technicon RA-I000 automatic analyzer after precipitation with MnCL2 and heparin | Oxidation method with TG 30 Cormay chemical reagent kit | Calculated (Friedewald's formula) |
| HAPIEE | Plasma | Oxidation method with the MP3 Boehringer Mannheim chemical reagent kit in the Technicon RA-I000 automatic analyzer | Oxidation method with the MP3 Boehringer Mannheim chemical reagent kit in the Technicon RA-I000 automatic analyzer after precipitation with MnCL2 and heparin | Oxidation method with TG 30 Cormay chemical reagent kit | Calculated (Friedewald's formula) |
| NATPOL 2011 | Fasting serum (frozen) | Enzymatic/cholesterol esterase and cholesterol oxidases; Architect c8000 chemistry analyzer, Abbott Laboratories | Direct method – Accelerator Selective Detergent (ASD) with accelerated non-HDL-C oxidation and HDL-C dissolving; Architect c8000 chemistry analyzer, Abbott Laboratories | Enzymatic/glycerol kinase and glycerol phosphate oxidase; Architect c8000 chemistry analyzer, Abbott Laboratories | Calculated (Friedewald's formula) |
Table III.
Information on family and personal history of early coronary heart disease (CHD), cerebrovascular disease, and peripheral artery disease (PAR)
| Study name | Family history of early CHD | Personal history of early CHD, cerebrovascular disease, and PAR | |||||
|---|---|---|---|---|---|---|---|
| MI | CHD | Angina pectoris | CABG or PCI | PAR | Cerebrovascular disease | ||
| POL-MONICA Krakow | History of heart disease or death due to HA in parents‡ or death due to HA (MI or acute IHD) or CHD in parents, siblings, or children below age 60 years | History of chest pain followed by medical diagnosis of HA, i.e. MI, CHD, acute or chronic coronary insufficiency‡ | Positive rose angina questionnaire† | Not available | Positive history of IC (standard questionnaire)† | History of brain stroke, brain hemorrhage or brain ischemia‡ | |
| POL-MONICA Warszawa | |||||||
| WOBASZ | Death from HA or history of MI or brain stroke in parents‡ | History of hospitalization due to MI or acute CHD or medical diagnosis of past MI‡ | History of hospitalization due to chronic CHD or heart insufficiency† | Positive rose angina questionnaire† | History of hospitalization due to PCI or CABG† | History of medical diagnosis or PAR† | History of hospitalization due to brain stroke† |
| Pilot HAPIEE | History of MI, AP, or IHD in parents aged below 60 years | History of medical diagnosis of MI† | History of medical diagnosis of AP or IHD† | Not available | Not available | History of medical diagnosis of brain stroke† | |
| HAPIEE | History of MI, AP or IHD in parents aged below 60 years | History of medical diagnosis of MI† or incident MI‡ | Not available | Positive rose angina questionnaire† or history of medical diagnosis of AP‡ | 5 years incidence | History of medical diagnosis of brain stroke† or history of brain stroke‡ | |
| NATPOL 2011 | History of CHD, AP, MI, or brain stroke in parents or siblings aged below 65 years in women/55 years in men | History of medical diagnosis of MI‡ | History of medical diagnosis of CHD‡ | Positive Rose angina questionnaire† | History of PCI or CABG† | History of PAR‡ | History of medical diagnosis of brain stroke (brain hemorrhage, brain ischemia)‡ |
In men aged below 55 years and in women aged below 60 years at the date of examination
at age below 55 years in men and 60 years in women
AP – angina pectoris, HA – heart attack, MI – myocardial infarction, IHD – ischemic heart disease, PCI – percutaneous intervention, CABG – coronary artery bypass grafting, IC – intermittent claudication.
Statistical analysis
In the first stage, calculations in each of the studies were conducted separately. Proportions in persons with FH and in persons with normal blood cholesterol were standardized directly to the sex-specific age distribution of the Polish population at the end of 2013. Then, combined estimates of proportions across studies were pooled by meta-analysis technique. In the presence of heterogeneity within and between studies, random effects methodology was chosen to obtain pooled prevalence [25]. The prevalence of FH by sex and age strata was obtained by much the same procedure except for the two extreme age groups (20–34 years, 65 years and over), in which fixed effect meta-analysis was applied. A similar two-step approach was performed to determine the association between FH and CVD risk factors. First, a set of multiple regressions was carried out with risk factors as dependent variables and the FH vs. healthy group as the independent variable, adjusted for age and sex. The second step was to implement random effects model meta-analysis to calculate the combined measure of association across the studies. The statistical calculations were performed with IBM SPSS Statistics 22 for Windows except the meta-analysis procedure. The R Metafor package [26] was used to pool estimates across the studies p < 0.05 was considered statistically significant.
Results
Out of 39,768 participants examined in six studies, 37,889 individuals (47.8% men) were included in the present meta-analysis. About 1,885 (4.7%) participants were excluded, mainly because of missing LDL-C (1,343 persons) or the age being outside the range specific for each study at the date of examination (542 persons). The detailed recruitment and participation numbers by study are given in Table I.
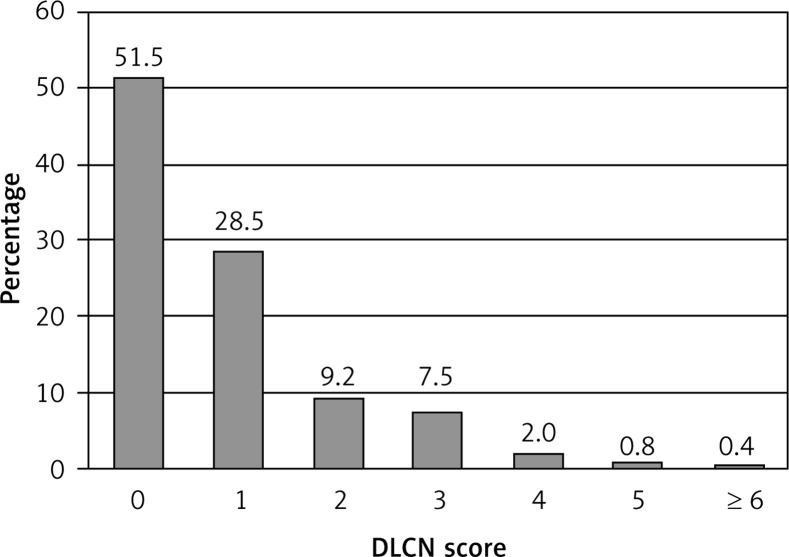
Severe hypercholesterolemia was not frequently found in the pooled sample included in the analysis. Very high LDL-cholesterol (≥ 6.5 mmol/l) was found only in 4.34‰ (95% CI: 3.19–5.48‰) and LDL-C ≥ 5 mmol/l was found in 5.79% (95% CI: 4.52–7.05%) of all participants. The distribution of the DLCN scores was skewed, and about half of all participants had a DLCN score of zero (Figure 1). In all 37,889 individuals studied, there were only 7 cases of DLCN score > 8 points. Potential FH, i.e. definite and probable FH combined (score ≥ 6 points), was more prevalent. The average prevalence was 404/100,000 people (95% CI: 277–531/100,000). However, the estimate of the average prevalence varied by study, with the minimum of 231/100,000 and maximum of 548/100,000 (Table IV).
Figure 1.
Distribution of Dutch Lipid Consensus Network (DLCN) score
Table IV.
Prevalence of potential (definite and probable combined) and possible familial hypercholesterolemia (FH) according to the Dutch Lipids Clinics Network (DLCN) criteria
| Study name | Potential FH | Possible FH | ||
|---|---|---|---|---|
| ‰ | 95% CI | % | 95% CI | |
| POL-MONICA Krakow | 4.46 | 2.64–6.28 | 12.1 | 11.2–13.0 |
| POL-MONICA Warszawa | 5.01 | 3.13–6.90 | 13.5 | 12.6–14.5 |
| WOBASZ | 2.46 | 1.63–3.28 | 8.1 | 7.6–8.5 |
| Pilot HAPIEE | 5.38 | 2.21–8.56 | 12.9 | 11.4–14.3 |
| HAPIEE | 5.48 | 3.96–6.99 | 12.2 | 11.5–12.9 |
| NATPOL 2011 | 2.31 | 0.29–4.34 | 5.9 | 4.9–6.9 |
| Total | 4.04 | 2.77–5.31 | 10.4 | 8.9–12.7 |
In Table V, the prevalence of potential and possible FH is presented by sex and by age group. Familial hypercholesterolemia was more prevalent in women than in men, and the prevalence was the highest in the age group 45–54 years in men and 55–64 years in women. At age 65 years and older in men, the prevalence of FH was almost three times lower than the peak, and at age 65 years and older in women it was twice as low as the peak.
Table V.
Percentage of potential (definite and probable combined) and possible familial hypercholesterolemia (FH) by sex and age group
| Age group | Men | Women | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potential FH | Possible FH | Potential FH | Possible FH | Potential FH | Possible FH | |||||||
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| 20–34 | 0 | – | 1.74 | 0.93–2.55 | 0 | – | 1.34 | 0.60–2.10 | 0 | – | 1.61 | 1.24–1.96 |
| 35–44 | 0.10 | 0.0001–0.21 | 6.24 | 5.33–7.15 | 0.24 | 0.0001–0.50 | 6.17 | 2.66–9.68 | 0.18 | 0.08–0.29 | 6.13 | 3.84–8.41 |
| 45–54 | 0.33 | 0.18–0.48 | 11.72 | 9.60–13.84 | 0.71 | 0.51–0.92 | 13.59 | 10.40–16.78 | 0.54 | 0.41–0.67 | 12.65 | 10.10–15.20 |
| 55–64 | 0.19 | 0.08–0.31 | 8.42 | 6.69–10.16 | 0.77 | 0.54–1.00 | 16.55 | 14.51–18.58 | 0.49 | 0.36–0.63 | 12.54 | 10.80–14.27 |
| ≥ 65 | 0.09 | 0.0001–0.24 | 7.25 | 1.35–13.11 | 0.37 | 0.10–0.64 | 11.76 | 8.75–14.78 | 0.25 | 0.09–0.41 | 10.10 | 7.11–13.10 |
In Table VI, the characteristics of participants with potential and possible FH are compared with persons with normal blood cholesterol, i.e. TC < 5 mmol/l and LDL-C < 3 mmol/l. Persons with FH were older and included a higher proportion of women. By definition, positive history of CHD and other acute manifestations of atherosclerosis were more frequent in participants with FH. Still, about 10% of participants with normal blood cholesterol had a history of stroke or acute or chronic CHD.
Table VI.
Descriptive statistics for participants with potential (definite and probable combined) and possible familial hypercholesterolemia (FH) according to the Dutch Lipids Clinics Network (DLCN) criteria and for participants with normal blood cholesterol (TC < 5 mmol/l and LDL-C < 3 mmol/l)
| Parameter | Potential FH | Possible FH | Normal blood lipids | |||
|---|---|---|---|---|---|---|
| Mean or% | 95% CI | Mean or% | 95% CI | Mean or% | 95% CI | |
| Mean age [years] | 53.8 | 52.8–54.8 | 53.4 | 51.7–55.1 | 46.2 | 39.7–52.7 |
| Men (%) | 27.3 | 20.3–34.4 | 39.6 | 36.3–43.0 | 49.3 | 46.1–52.5 |
| Mean BMI [kg/m2] | 27.8 | 27.0–28.5 | 28.1 | 28.0–28.3 | 26.1 | 25.2–27.1 |
| Current smokers (%) | 41.3 | 33.5–49.1 | 34.5 | 28.2–40.9 | 36.0 | 31.1–40.9 |
| Diabetes (%) | 1.8 | < 0.1–3.9 | 8.1 | 4.7–11.6 | 5.6 | 1.7–9.5 |
| Hypertension (%) | 68.6 | 60.9–76.3 | 62.9 | 61.3–64.4 | 41.1 | 30.4–51.9 |
| History of CVD (%) | 70.1 | 56.9–83.2 | 48.9 | 39.3–58.5 | 12.1 | 7.4–16.8 |
| History of MI (%) | 32.1 | 15.0–49.2 | 16.7 | 9.9–23.4 | 3.2 | 1.7–4.7 |
| History of CABG or PCI (%) | 6.5 | < 0.1–15.4 | 3.2 | 1.8–4.7 | 0.6 | 0.1–1.0 |
| History of AP (%) | 49.3 | 29.3–69.3 | 35.0 | 23.6–46.4 | 8.6 | 4.6–12.5 |
| History of brain stroke (%) | 3.2 | < 0.1–6.6 | 2.9 | 2.3–3.4 | 1.3 | 0.9–1.7 |
| History of PAR (%) | 2.5 | < 0.1–5.8 | 4.5 | 2.3 –6.7 | 1.2 | 0.7–1.6 |
| CVD and diabetes (%) | 1.8 | < 0.1–4.0 | 4.9 | 2.8–7.0 | 1.5 | 0.5–2.5 |
| Lipid-lowering treatment (%) | 14.1 | 3.6–24.6 | 16.9 | 8.6–25.2 | – | – |
| Mean TC [mmol/l] | 8.7 | 8.2–9.2 | 6.8 | 6.6–7.0 | 4.3 | 4.3–4.4 |
| Mean HDL-C [mmol/l] | 1.4 | 1.4–1.5 | 1.4 | 1.4–1.4 | 1.4 | 1.4–1.49 |
| Mean LDL-C [mmol/l] | 6.4 | 6.0–6.9 | 4.6 | 4.4–4.8 | 2.4 | 2.3–2.4 |
| TG (median, min–max) [mmol/l] | 1.9 | 0.4–4.4 | 1.6 | 0.3–4.7 | 1.0 | 0.2–4.5 |
BMI – body mass index, CVD – cardiovascular disease, AP – angina pectoris, HA – heart attack, MI – myocardial infarction, PCI – percutaneous intervention, CABG – coronary artery bypass grafting, PAR – peripheral artery disease, TC – total cholesterol, HDL-C – high-density lipoprotein cholesterol, LDL – low-density lipoprotein cholesterol, TG – triglycerides.
Participants with potential and with possible FH had higher exposure to some other risk factors. After adjustment for age and sex, compared to participants with normal cholesterol, persons with potential FH had twice as high prevalence of hypertension; smoking was more prevalent by about 80% and hypertriglyceridemia nine times more frequent. The prevalence of these risk factors was also higher in participants with possible FH; in particular, the prevalence of hypertriglyceridemia was four times higher compared to participants with normal blood cholesterol. Also, in participants with possible FH, higher prevalence of obesity was found. The prevalence of low HDL-cholesterol and diabetes was similar in participants with FH and in participants with normal blood cholesterol (Table VII).
Table VII.
Relation between familial hypercholesterolemia (FH) and prevalence of other cardiovascular disease (CVD) risk factors (reference group = participants with TC < 5 mmol/l and LDL < 3 mmol/l and not on blood lipid lowering treatment)
| Parameter | Potential FH | Possible FH | ||||
|---|---|---|---|---|---|---|
| OR* | 95% CI | P-value | OR* | 95% CI | P-value | |
| BMI ≥ 30 kg/m2 | 1.16 | 0.80–1.68 | 0.44 | 1.25 | 1.10–1.41 | 0.0006 |
| Smoking | 1.75 | 1.23–2.48 | 0.002 | 1.27 | 1.16–1.39 | < 0.0001 |
| Diabetes | 2.26 | 0.85–6.00 | 0.10 | 1.17 | 0.91–1.50 | 0.21 |
| Hypertension | 2.02 | 1.95–3.43 | 0.009 | 1.76 | 1.57–1.97 | < 0.0001 |
| Low HDL-C | 1.66 | 0.84–3.27 | 0.14 | 1.31 | 1.08–1.60 | 0.006 |
| TG > 1.7 mmol/l | 9.05 | 6.10–13.44 | < 0.0001 | 4.37 | 3.71–5.14 | < 0.0001 |
BMI – body mass index, TC – total cholesterol, HDL-C – high-density lipoprotein cholesterol, LDL – low-density lipoprotein cholesterol, TG – triglycerides
Adjusted for age and sex.
Discussion
We found that the prevalence of FH in Poland was between 277 and 531/100,000 people. The average estimate was 404/100,000, which equates to approximately 1/250 people.
To our best knowledge, this is the first estimate of the prevalence of FH in Poland, which is based on the results of larger studies carried out in well-defined populations. Furthermore, the studies included in the present meta-analysis used the standardized methods of observations, and in some cases, the methods were standardized across these studies to obtain comparable results. For example, the POL-MONICA Krakow and Warsaw studies used the same questionnaires and blood collection procedures, and laboratory procedures were subjected to the same external quality control programs carried out by the CDC in Atlanta (USA) and by the MONICA Project Lipid Reference Center [16–18]. The WOBASZ Study used questionnaires largely based on POL-MONICA experiences, and biochemical analyses were carried out in the same laboratory as in POL-MONICA Warsaw. The methods in HAPIEE Krakow and the Pilot HAPIEE study were the same, and biochemical analyses were done in a laboratory that participated in the POL-MONICA Krakow study. Also, the strength of the analysis is that we were able to include data from two studies in which samples studied were selected from the total Polish population (WOBASZ and NATPOL 2011).
There are, however, certain limitations in the interpretation of the results. The first is that none of the studies included in the meta-analysis was designed to assess FH according to the DLCN or any other standard set of diagnostic criteria. The information on phenotype and family history in particular varied between the studies. This could bias the final results, resulting in decreased numbers of people classified with definite or probable FH. The samples studied differed in age. Including the age group below 35 years could result in a decrease in the number of detected cases, as in heterozygous FH the onset of CHD frequently appears after the age of 35 years. On the other hand, in the older age group, the proportion of persons with FH was smaller because of lower life expectancy and higher frequency of the use of blood lipid-lowering agents. Indeed, in studies which involved samples with a broader age span (WOBASZ and NATPOL 2011), the rates of FH were lower. In participants aged 60–79 years (NATPOL 2011 study), the proportion of people taking statins was three times higher than the average for the total sample [22]. Blood lipid-lowering treatment could also lower the rates of FH in general. This would not be a problem of the early studies as treatment for hypercholesterolemia was infrequent, even marginal. The advantage of using data from the older studies might be that the bias due to the lipid-lowering treatment would be smaller than in more current observations. Indeed, in the later studies, the proportions of treated people were higher than in the old studies, but treatment of hypercholesterolemia was still not a standard practice. In the WOBASZ study (a representative sample for Poland), only 12% of people with hypercholesterolemia received treatment, and out of them, the treatment goals were reached only in every fifth person [19]. In the most recent study (NATPOL 2011), the proportion was similar [22]. It is likely that the effect of blood lipid-lowering treatment on our results was rather small. In the Finnish population study, mutation carriers who were treated with lipid-lowering medication had similar LDL-C to carriers who were not treated [27]. Also, lack of information on genetic mutations might be of smaller importance, as in the untreated, predominantly middle-age population, cases of FH with normal blood LDL-C or only slightly over the normal values should be very rare.
Like in the other studies, there were differences in FH prevalence according to age [4, 6, 28, 29], which could be explained by the impact of increasing age and weight on LDL-C when using DLCN criteria. Higher prevalence in women was also found in some studies [4, 6, 30] but not in all [28–30]. Besides the effects of age and weight, these observations can also be explained by the differences in life expectancy between men and women. Familial hypercholesterolemia was found to be related to higher exposure to other CVD risk factors. Our study design does not allow for conclusions on causality, but these results call for intensifying the intervention in clinical practice.
Direct comparisons between our findings and the results of other studies are difficult due to the differences in the design and methods used. Most evidence on the prevalence of FH is based on data from registers whose coverage is difficult to control [31]. In a few studies, the studied samples were representative of a larger population, but they were rarely large enough to provide reliable estimates of the prevalence. Our estimate is slightly lower compared to the results of the well-designed and frequently cited Danish study and close to the estimate of the European Atherosclerosis Society [3, 4]. Also, our results are close to the results obtained using similar methods from the US NHANES 2001–2012 datasets (nearly 60,000 persons) and similar to results of the studies from Australia and China (18,000 and 10,000 persons respectively) [28–30]. Our estimate of the prevalence is twice as high compared to the Finnish study (over 28,000 persons), which was based on finding genetic mutations which are present in about 70% of all FH cases in Finland [27].
Observations from the younger groups of the Polish population, i.e. below the age of 20 years, would add complementary information leading to better assessment of FH in the Polish population. However, it is unlikely that it will be accomplished in the near future, as cholesterol screening is not recommended for people below the age of 18 years. The postulated tool for the detection of FH is cascade screening. This is based on detailed examinations of the first- and second-degree relatives of the probands (index cases). The latter might emerge from either by-chance examination or from the population screening, which would provide information on blood cholesterol, premature CHD, and cardiac deaths in family members or tendon xanthomas in the proband or his/her family member [3, 5, 8, 32].
We believe that our study allows for a better understanding of how many cases of FH can emerge from the population cholesterol screening facilitating the planning of a strategy to manage the disease at a population level. We hope that our results will draw the attention of health managers and clinicians, particularly primary care physicians involved in population cholesterol screening in the group of people with potential FH. In Poland, a country with 38 million residents, the size of this group is about 150,000 ±50,000 adult people. These people require special diagnostics which would involve not only themselves but also all of their first- and second-degree relatives and which would include genetic testing and other more sophisticated biochemical diagnostics. Furthermore, it could be expected that many of them would require intensive treatment with high doses of highly potent statins alone or in combination with other lipid-lowering agents including new generations of efficacious drugs [24, 33]. All these points need to be addressed urgently, to develop a national strategy for the detection, diagnosis, and treatment of FH.
Acknowledgments
The authors express their gratitude to all participants and investigators of the POL-MONICA, HAPIEE, WOBASZ, and NATPOL studies.
POLMONICA and the HAPIEE pilot study were supported by the Polish Committee of Scientific Research [4 1474 9101, 1992-93, 4 P05D 036 08, 253//S4/92/02, 380/p05/95/08 and 4 PO5D 019 18]; WOBASZ by the Polish Ministry of Health [program POLKAD 2003-2005, 4.12.1]; HAPIEE by the Wellcome Trust [WT081081] and by the US National Institute of Aging [Grant Number R01 AG23522]; NATPOL 2011 was supported by unrestricted grants from Sanofi-Aventis, Abbott Laboratories Poland Ltd, Siemens Ltd and Polpharma. This work was supported in part by Amgen. Funding bodies and sponsors had no role in the design, data collection, data analysis, data interpretation, or writing of this report.
Conflict of interest
AP was paid an honorarium for consultancy from Amgen. PJ has received honoraria or grants from Amgen, KRKA, Polpharma, MSD, and Sanofi.
References
- 1.Austin M, Hutter C, Zimmerman R, et al. Genetic causes of monogenic heterozygous familiar hypercholesterolemia at HuGE prevalence review. Am J Epidemiol. 2004;160:407–420. doi: 10.1093/aje/kwh236. [DOI] [PubMed] [Google Scholar]
- 2.Nair DR, Sharifi M, Al-Rasadi K. Familiar hypercholesterolemia. Curr Opin Cardiol. 2014;29:381–8. doi: 10.1097/HCO.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 3.Nordestgaard B, Chapman M, Humphries S, et al. Familiar hypercholesterolemia is underdiagnosed and untreated in general population; guidance for clinicians to prevent coronary heart disease. Eur Heart J. 2013;34:3478–90. doi: 10.1093/eurheartj/eht273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benn M, Watts G, Tybjaerg-Hansen A, Nordestgaard B, Nordestgaard B. Familiar hypercholesterolemia in the Danish general population: prevalence, coronary artery disease and cholesterol-lowering medication. J Clin Endocrinol Metab. 2012;97:3956–64. doi: 10.1210/jc.2012-1563. [DOI] [PubMed] [Google Scholar]
- 5.The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012) Eur Heart J. 2012;33:1635–701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 6.De Backer G, Besseling J, Chapman J, et al. Prevalence and management of familiar hypercholesterolemia in coronary patients: an analysis of EUROASPIRE IV, a study of the European Society of Cardiology. Atherosclerosis. 2015;241:169–175. doi: 10.1016/j.atherosclerosis.2015.04.809. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg A, Hopkins P, Toth P, et al. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5:S1–8. doi: 10.1016/j.jacl.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 9.Minhas R, Humphries S, Qureshi N, Neil H. Controversies in familial hypercholesterolaemia: recommendations of the NICE Guideline Development Group for the identification and management of familial hypercholesterolaemia. Heart. 2009;95:584–7. doi: 10.1136/hrt.2008.162909. [DOI] [PubMed] [Google Scholar]
- 10.Robinson J. Management of familial hypercholesterolemia. J Manag Care Pharm. 2013;19:139–49. doi: 10.18553/jmcp.2013.19.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson J, Nedergaard B, Rogers W, et al. Effect of evolocumab or ezetimbide added to moderate of high-intensity statin therapy on LDL-C lowering in ptients with hypercholesterolemia. JAMA. 2014;311:1870–82. doi: 10.1001/jama.2014.4030. [DOI] [PubMed] [Google Scholar]
- 12.Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. JACC. 2014;63:2541–8. doi: 10.1016/j.jacc.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Banach M, Rizzo M, Tot P, et al. Statin intolerance – an attempt at a unified definition. Position paper from an International Lipid Expert Panel. Arch Med Sci. 2015;11:1–23. doi: 10.5114/aoms.2015.49807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marks D, Wonderling D, Thorogood M, Lambert H, Humphries S, Neil A. Cost effectiveness analysis of different approaches of screening for familial hypercholesterolaemia. BMJ. 2002;324:1303–6. doi: 10.1136/bmj.324.7349.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen C, Hay J. Cost-effectiveness analysis of alternative screening and treatment strategies for heterozygous familial hypercholesterolemia in the United States. Int J Cardiol. 2015;181:417–24. doi: 10.1016/j.ijcard.2014.12.070. [DOI] [PubMed] [Google Scholar]
- 16.Rywik S, Pająk A. Monitoring of cardiovascular incidence, fatality and mortality trends and their determinants – longitudinal study Pol MONICA. Part III: principles of standardization and quality control. Przegl Lek. 1985;42:280–4. [PubMed] [Google Scholar]
- 17.Rywik S, Sznajd J, Kulesza W, et al. Monitoring of cardiovascular incidence, fatality and mortality trends and their determinants – longitudinal study Pol MONICA. Part II: material and methods. Przegl Lek. 1985;42:256–60. [PubMed] [Google Scholar]
- 18.Döring A, Pająk A, Ferrario M, Grafnette RD, Kuulasmaa K. Methods of total cholesterol measurements in the baseline survey of the MONICA Project. Rev Epidem Sante Publ. 1990;38:455–61. [PubMed] [Google Scholar]
- 19.Pająk A, Wiercińska E, Polakowska M, Kozakiewicz KE. Rozpowszechnienie dyslipidemii u mężczyzn i kobiet w wieku 20–74 lat w Polsce. Wyniki programu WOBASZ. Kardiol Pol. 2005;63(Suppl. 4):S1–6. [PubMed] [Google Scholar]
- 20.Peasey A, Bobak M, Kubinova R, et al. Determinants of cardiovascular disease and other non-communicable diseases in Central and Eastern Europe: rationale and design of the HAPIEE study. BMC Public Health. 2006;6:255–60. doi: 10.1186/1471-2458-6-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pająk A. Psychosocial and nutritional cardiovascular disease risk factors – longitudinal study. Assumptions, goals and methods of the screening survey. Przegl Lek. 2002;59:993–8. [PubMed] [Google Scholar]
- 22.Zdrojewski T, Rutkowski M, Bandosz P, et al. Prevalence and control of cardiovascular risk factors in Poland. Assumptions and objectives of the NATPOL 2011 Survey. Kardiola Pol. 2013;71:381–92. doi: 10.5603/KP.2013.0066. [DOI] [PubMed] [Google Scholar]
- 23.Rywik S, Kupść W, Piotrowski W, et al. Wieloośrodkowe ogólnopolskie badanie stanu zdrowia ludności – projekt WOBASZ. Założenia metodyczne oraz logistyka. Kardiol Pol. 2005;63(Suppl. 4):S2–9. [PubMed] [Google Scholar]
- 24.Rynkiewicz A, Cybulska B, Banach M, et al. Management of familial heterozygous hypercholesterolemia: position paper of the Polish Lipid Expert Forum. J Clin Lipidol. 2013;7:217–21. doi: 10.1016/j.jacl.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Cochran W. The combination of estimates from different experiments. Biometrics. 1954;10:101–29. [Google Scholar]
- 26.Viechtbauer W. Conducting meta-analyses in R with the Metafor package. J Statist Software. 2010;36:1–48. [Google Scholar]
- 27.Lahtinen AM, Havulinna AS, Jula A, et al. Prevalence and clinical correlates of familial hypercholesterolemia founder mutations in the general population. Atherosclerosis. 2015;23:64–9. doi: 10.1016/j.atherosclerosis.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 28.De Ferranti SD, Rodday MA, Mendelson M, et al. What is the prevalence of familial hypercholesterolemia in the US? American Heart Association 2014. Circulation. 2014;130(Suppl II):A19656. [Google Scholar]
- 29.Shi Z, Youan B, Zhao D, et al. Familial hypercholesterolemia in China: prevalence and evidence of underdetection and undertreatment in a community population. Int J Cardiol. 2014;174:834–6. doi: 10.1016/j.ijcard.2014.04.165. [DOI] [PubMed] [Google Scholar]
- 30.Watts GF, Shaw JE, Pang J, et al. Prevalence and treatment of familial hypercholesterolemia in Australian communities. Int J Cardiol. 2015;185:69–71. doi: 10.1016/j.ijcard.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 31.Vallejo-Vaz AJ, Seshasai SRK, Cole D, et al. Familial hypercholesterolemia: a global call to arms. Atherosclerosis. 2015;243:257–9. doi: 10.1016/j.atherosclerosis.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 32.ESC/EAS Guidelines for the management of dyslipidaemias. Eur Heart J. 2011;32:1769–818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 33.Myśliwiec M, Walczak M, Małecka-Tendera E, et al. Management of familial hypercholesterolemia in children and adolescents. Position paper of the Polish Lipid Expert Forum. J Clin Lipidol. 2014;8:173–80. doi: 10.1016/j.jacl.2014.01.001. [DOI] [PubMed] [Google Scholar]