Abstract
Introduction
Glycosylated haemoglobin level (HbA1c) is an indicator of the average blood glucose concentrations over the preceding 2–3 months and is used as a convenient and well-known biomarker in clinical practice. Currently, epidemiological evidence suggests that HbA1c level is an independent risk factor for cardiovascular events such as myocardial infarction, stroke, coronary heart disease and heart failure. This protocol aim is to conduct a systematic review and meta-analysis to determine relationships of HbA1c levels with cardiovascular outcomes and cause of death, and to analyse the range of HbA1c levels that is a predictor of cardiovascular disease and/or mortality based on data from published observational studies.
Methods and analysis
The search will be conducted using Medline, EMBASE, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and Web of Science databases from their inception. Observational studies written in Portuguese, Spanish or English will be included. The Quality In Prognosis Studies tool will be used to assess the risk of bias for the studies included in the systematic review or meta-analysis. HRs for cardiovascular outcomes and causes of death with 95% CIs will be determined as primary outcomes. Subgroup analyses will be performed based on cardiovascular outcomes, cause of death studied, and type of population included in the studies.
Ethics and dissemination
This systematic review will synthesise evidence on the potential of using HbA1c level as a prognostic marker for cardiovascular disease outcomes and/or mortality. The results will be disseminated by publication in a peer-reviewed journal. Ethics approval will not be needed because the data used for this systematic review will be obtained from published studies and there will be no concerns about privacy.
Trial registration number
PROSPERO CRD42015032552.
Keywords: mortality, glycated haemoglobin, cardiovascular disease
Strengths and limitations of this study.
This review of evidence will be useful to improve future research on HbA1c level as a prognostic marker for cardiovascular disease outcomes and/or mortality.
Study selection, data extraction and quality assessment will be performed independently by two researchers.
Limitations and strengths will be discussed in our review, and the results will be put into context with other studies in the field.
Different population-based studies can be a source of variable quality and heterogeneity between studies and may limit the quality of the evidence of this meta-analysis and systematic review.
Introduction
Cardiovascular disease (CVD) is a chronic disorder that develops insidiously throughout an individual's life and usually has progressed to an advanced stage by the time symptoms occur.1 The percentage of all deaths due to CVD before the age of 75 years in Europe is 42% in women and 38% in men.2 CVD, especially coronary heart disease, is the leading cause of premature death worldwide.3
In 2007, The Reynolds Risk Score for predicting CVD risk was developed, which incorporates information on glycosylated haemoglobin (HbA1c), but this score was only used in people with known diabetes.4 In 2010, the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines considered HbA1c level to be an appropriate index for CVD risk assessment in asymptomatic adults without a diagnosis of diabetes.5 Finally, the Canadian Cardiovascular Society proposed that CVD risk could be stratified by measuring levels of fasting plasma glucose, HbA1c, or both.6
HbA1c level is an indicator of the average blood glucose concentrations over the preceding 2–3 months and is used as a convenient and well-known biomarker in clinical practice.7 8 Epidemiological evidence suggests that HbA1c level is an independent risk factor for cardiovascular events.9 There is also evidence that the association of HbA1c level with mortality from all causes and CVD can be found at lower levels than the diabetic threshold.10 A meta-analysis showed that HbA1c level is an independent predictor of mortality in patients with coronary artery disease without established diabetes but not in those with established diabetes.11
Currently, the association between chronic hyperglycaemia and cardiovascular complications is not well defined. Several observational studies have demonstrated that a higher HbA1c level is associated with increased risk of CVD and death.9 12 13 Thus, an elevated HbA1c level might contribute to the development of CVD, but the association between HbA1c level and the risk of CVD and mortality in the general population remains unclear. Therefore, this protocol aims to present a clear and transparent procedure for systematically reviewing, evaluating and summarising existing information on the relationship of HbA1c level with CVD and death, which could guide clinical decision making in further treatment strategies and also inform and facilitate future intervention research.
Objective
The aim of this protocol study is to establish a transparent and clear methodology for conducting a systematic review and meta-analysis aimed to (i) determine the relationship between HbA1c level and cause of death and cardiovascular outcomes based on data from observational studies, and (ii) analyse what level of HbA1c is a predictor of CVD and/or mortality.
Methods and analysis
Review design
This protocol was developed based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses Protocols (PRISMA-P)14 and was registered with PROSPERO (Registration number CRD42015032552). The MOOSE15 (Meta-analysis of observational studies in epidemiology: a proposal for reporting), PRISMA16 and Cochrane Collaboration Handbook17 will be used to guide the review methods.
Literature review
The literature search will be conducted using Medline (via PubMed), EMBASE, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and Web of Science databases from the date of their inception until August 2016. Study records will be managed with the Mendeley reference manager.
The following search terms will be combined using Boolean operators: glycosylated haemoglobin, HbA1c, haemoglobin levels, glycated haemoglobin, haemoglobin A1c, cardiovascular, cardiovascular disease, coronary heart disease, heart failure, stroke, peripheral arterial disease, cardiovascular events, coronary artery disease, myocardial infarction, cardiovascular outcomes, mortality, all-cause mortality, cardiovascular mortality, cause-specific mortality, death, cardiovascular death, observational study, cohort study and population-based (table 1).
Table 1.
Search strategy for Medline
| “glycosylated haemoglobin” OR “HbA1c” OR “haemoglobin levels” OR “glycated haemoglobin” OR “haemoglobin A1c” |
AND | Cardiovascular OR ‘cardiovascular disease’ OR ‘coronary heart disease’ OR ‘heart failure’ OR Stroke OR ‘peripheral arterial disease’ OR ‘cardiovascular events’ OR ‘coronary artery disease’ OR ‘myocardial infarction’ OR ‘cardiovascular outcomes’ OR mortality OR ‘all-cause mortality’ OR ‘cardiovascular mortality’ OR ‘cause-specific mortality’ OR death OR ‘cardiovascular death’ |
AND | ‘observational study’ OR ‘cohort study’ OR ‘population-based |
Previous systematic reviews and meta-analyses, and relevant references included in the selected studies, will be screened as supplemental sources.
Inclusion/exclusion criteria for study selection
Studies on HbA1c level and cardiovascular outcomes retrieved in the literature search that meet the following criteria will be included: (i) prospective or retrospective observational studies; (ii) studies that observed the following cardiovascular outcomes: myocardial infarction, stroke, major adverse cardiovascular events (MACE), coronary heart disease and heart failure; (iii) reports of all-cause mortality and/or cardiovascular mortality; (iv) outcomes measured using univariate and multivariate Cox proportional hazards models; (v) population of adults aged 18 or older with any restriction on the race, gender or diabetic status; and (vi) studies published in Portuguese, Spanish or English.
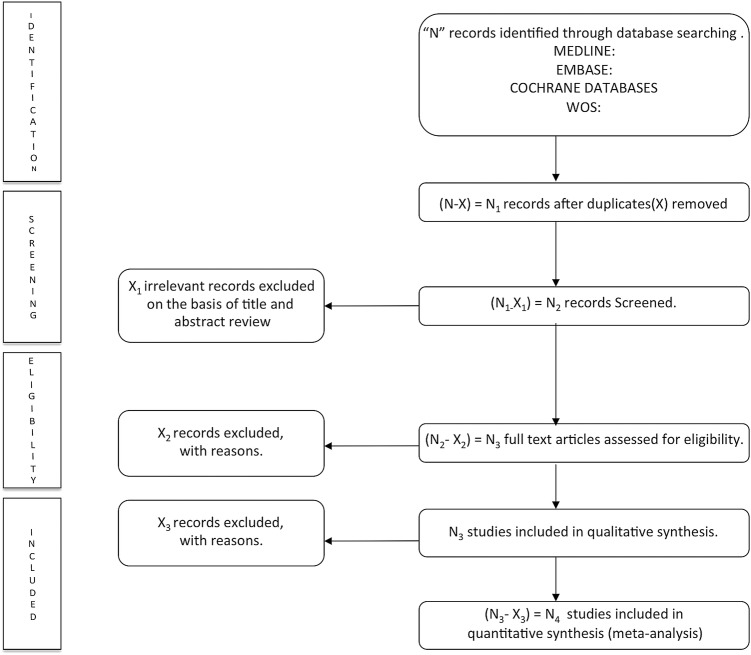
The process of identifying, screening of studies and inclusion or exclusion of those studies is shown in the PRISMA flow chart (figure 1).
Figure 1.
Literature search PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) consort diagram.
Study selection and data extraction
Two reviewers will independently check titles and abstracts to identify eligible studies according to the inclusion criteria. Then the full manuscripts of the identified studies will be examined. Finally, two reviewers will check the included and excluded studies and verify the reasons why they were included/excluded. Any discrepancies will be resolved by discussion; a third reviewer will be asked in cases of disagreement.
Two authors will independently extract the data on author information, year of publication, design of study, country, study project name and year of data collection, number and age of participants, population characteristics (diabetic or non-diabetic), methods used for HbA1c test certified by National Glycohemoglobin Standardization Program (NGSP), number of cardiovascular events, level of HbA1c used as the reference, and the HR for each HbA1c level (table 2).
Table 2.
Characteristics of studies included in the systematic review and/or meta-analysis
| Reference | Design | Country | Study/year of data collection | Age | n | n cardiovascular events | HbA1c method | HbA1c reference level | HR for HbA1c levels |
|---|---|---|---|---|---|---|---|---|---|
| Author information and year of publication | Design of the study | Country | Study project name and year of data collection | Age of participants | Number of participants | Number of cardiovascular events | Methods used for HbA1c test certified by NGSP | Level of HbA1c used as the reference | HR for each HbA1c level |
HbA1c, glycosylated haemoglobin; NGSP, National Glycohemoglobin Standardization Program.
Any disagreement will be resolved by discussion to reach a consensus. When necessary, authors of the potential included studies will be contacted to obtain any missing information.
Assessment of the risk of bias in the included studies
After blinding of two independent researchers to the author, title and year of publication of the included studies, the methodological quality will be assessed by the Quality in Prognosis Studies (QUIPS) tool.18 Any disagreement in the assessment of the risk of bias will be discussed to reach a consensus. A third reviewer will make the final decision if a consensus is not reached. The QUIPS tool involves the use of six domains for the risk of bias: study participation (sampling bias), study attrition (attrition bias), prognostic factor measurement, outcome measurement (ascertainment bias), confounding measurement and accounting, and analysis and reporting. Studies will be considered to have a low, moderate or high risk of bias according to scores of 5–6, 3–4 or 1–2, respectively, for the six bias domains.
Statistical analysis
The researchers will create tables to summarise the characteristics of the included studies and any important questions related to the aim of this systematic review. The reviewers will determine whether a meta-analysis is possible after the data have been extracted. At least five observations addressing HR for cardiovascular outcomes and mortality will be required to conduct a meta-analysis. If it is possible to carry out a meta-analysis, Stata 14 software will be used to combine the extracted HR with 95% CIs using an inverse variance model. We will compare adjusted and unadjusted estimates separately for each outcome. A fixed-effects model will be used if there is no evidence of heterogeneity; otherwise, a random-effects model will be used.19 For HbA1c levels, we will group studies by similar cut-off points to obtain meta-analysis results for each cut-off point whenever possible. We will use generalised least squares regression models to assess the pooled dose–response relation between HbA1c and CVD outcomes across prospective cohort studies that have heterogeneous categorisations of HbA1c.20 Each meta-analysis will be summarised by the pooled HR and 95% CIs. Studies providing insufficient data to perform the analyses will be omitted from the data synthesis. The heterogeneity of the studies will be assessed with an I2 statistic. Usually, I2 values of <25%, 25–50% and >50% are considered to represent small, medium and large amounts of heterogeneity, respectively.21 If a meta-analysis is not possible, we will undertake a narrative synthesis. Finally, publication bias will be visually evaluated using a funnel plot, as well as with the method proposed by Egger.22 The strength of the body of evidence will be evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool.23
Subgroup analyses and meta-regression
Subgroup analyses and meta-regression will be performed based on the cardiovascular outcomes (myocardial infarction, stroke, MACE, coronary heart disease, heart failure), cause of death studied (all causes of mortality or cardiovascular mortality), type of population included in the studies (diabetic, prediabetic or non-diabetic), or the age of the study participants (young adults aged 18–35 years, middle-aged adults aged 36–55 years, or older adults aged older than 55 years), because these may be major factors causing heterogeneity. Furthermore, design of the study and QUIPS score will be considered for additional subgroup analyses.
Sensitivity analysis
Sensitivity analyses will be performed by excluding the included studies from the analysis one by one and comparing the results.
Discussion
The utility of HbA1c level as a prognostic marker for CVD outcomes and/or mortality is currently a source of controversy in the medical literature. Therefore, we will conduct a systematic review to identify what HbA1c level might be able to predict CVD outcome and mortality.
There is currently no consensus on what percentages should be used to determine the level of heterogeneity in categorical terms. Therefore, in this study, we will use the definition suggested by Higgins and Thompson21 to indicate that there is heterogeneity when the I2 value is >50%.
Possible limitations of this research are publication bias, information bias, poor statistical analyses and inadequate reporting of methods and findings of the primary studies.24 However, it is important to summarise the information available on this issue. To overcome these limitations, we will follow the recommendations included in the MOOSE, PRISMA and Cochrane Collaboration Handbook. According to the Cochrane Prognosis Methods Group, we will use the QUIPS tool to assess the quality of the included studies.18
There have already been numerous studies on the use of HbA1c level as a prognostic marker for CVD outcome and mortality, but the individual studies have been controversial, so there is uncertainty regarding its use. It is therefore necessary to conduct a systematic review to provide a global overview of the current literature and to improve future research on this topic. This protocol provides a clear and structured procedure for maximising the extraction of relevant information and providing summarised information on the importance of HbA1c levels for controlling the risk of CVD outcomes and mortality.
Footnotes
Contributors: VM-V and IC-R designed the study. VM-V was the principal investigator and guarantor. IC-R and VM-V were the main coordinators of the study. BP, CA-B, FR-A and VM-V conducted the study. IC-R, BP and FR-A gave statistical and epidemiological support. IC-R wrote the article with the support of CA-B. All authors revised and approved the final version of the manuscript.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Dregan A, Charlton J, Chowienczyk P et al. . Chronic inflammatory disorders and risk of type 2 diabetes mellitus, coronary heart disease, and stroke: a population-based cohort study. Circulation 2014;130:837–44. 10.1161/CIRCULATIONAHA.114.009990 [DOI] [PubMed] [Google Scholar]
- 2.Perk J, De Backer G, Gohlke H et al. . European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). Eur Heart J 2012;33:1635–701. 10.1093/eurheartj/ehs092 [DOI] [PubMed] [Google Scholar]
- 3.Finegold JA, Asaria P, Francis DP. Mortality from ischaemic heart disease by country, region, and age: statistics from World Health Organisation and United Nations. Int J Cardiol 2013;168:934–45. 10.1016/j.ijcard.2012.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ridker PM, Buring JE, Rifai N et al. . Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA 2007;297:611–19. 10.1001/jama.297.6.611 [DOI] [PubMed] [Google Scholar]
- 5.Greenland P, Alpert JS, Beller GA et al. . American College of Cardiology Foundation; American Heart Association. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010;56:e50–e103. 10.1016/j.jacc.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 6.Anderson TJ, Grégoire J, Hegele RA et al. . 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol 2013;29:151–67. 10.1016/j.cjca.2012.11.032 [DOI] [PubMed] [Google Scholar]
- 7.Nathan DM, Turgeon H, Regan S. Relationship between glycated haemoglobin levels and mean glucose levels over time. Diabetologia 2007;50:2239–44. 10.1007/s00125-007-0803-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyons TJ, Basu A. Biomarkers in diabetes: hemoglobin A1c, vascular and tissue markers. Transl Res 2012;159:303–12. 10.1016/j.trsl.2012.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selvin E, Steffes MW, Zhu H et al. . Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010;362:800–11. 10.1056/NEJMoa0908359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khaw KT, Wareham N, Bingham S et al. . Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med 2004;141:413–20. 10.7326/0003-4819-141-6-200409210-00006 [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Yang YM, Zhu J et al. . Prognostic significance of hemoglobin A1c level in patients hospitalized with coronary artery disease. A systematic review and meta-analysis. Cardiovasc Diabetol 2011;10:98 10.1186/1475-2840-10-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eeg-Olofsson K, Cederholm J, Nilsson PM et al. . New aspects of HbA1c as a risk factor for cardiovascular diseases in type 2 diabetes: an observational study from the Swedish National Diabetes Register (NDR). J Intern Med 2010;268:471–82. 10.1111/j.1365-2796.2010.02265.x [DOI] [PubMed] [Google Scholar]
- 13.Oh HG, Rhee EJ, Kim TW et al. . Higher glycated hemoglobin level is associated with increased risk for ischemic stroke in non-diabetes Korean male adults. Diabetes Metab J 2011;35:551–7. 10.4093/dmj.2011.35.5.551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Shamseer L, Clarke M et al. . Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stroup DF, Berlin JA, Morton SC et al. . Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, Green S. Chapter 7: selecting studies and collecting data. Cochrane Handbook of Systematic Reviews of Interventions, Version 5.1.0 [updated March 2011]. Cochrane Collaboration, 2011. http://www.cochrane-handbook.org [Google Scholar]
- 18.Hayden JA, van der Windt DA, Cartwright JL et al. . Assessing bias in studies of prognostic factors. Ann Intern Med 2013;158:280–6. 10.7326/0003-4819-158-4-201302190-00009 [DOI] [PubMed] [Google Scholar]
- 19.Hedges LV, Vevea JL. Fixed-and random-effects models in meta-analysis. Psychological methods 1998;3:486 10.1037/1082-989X.3.4.486 [DOI] [Google Scholar]
- 20.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992;135:1301–9. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 22.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ 2001;323:101–5. 10.1136/bmj.323.7304.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owens DK, Lohr KN, Atkins D et al. . Grading the strength of a body of evidence when comparing medical interventions. 5 August 2009 In: Methods guide for effectiveness and comparative effectiveness reviews. Rockville, MD: Agency for Healthcare Research and Quality (US), 2008. [PubMed] [Google Scholar]
- 24.Egger M, Smith GD. Bias in location and selection of studies. BMJ 1998;316:61 10.1136/bmj.316.7124.61 [DOI] [PMC free article] [PubMed] [Google Scholar]