Crosspresentation is of vital significance to immune surveillance. External antigens, soluble or solid, enter cells via endophagocytic vesicles and are eventually delivered to MHC class I molecules on the cell surface. After antigen uptake, there are three proposed pathways leading to surface MHC class I/peptide complex presentation. Direct translocation into the cytosol to use the conventional proteasome/TAP-dependent ER peptide loading; phagosome autonomous presentation following fusion with selected ER components; and endocytic recycling whereby antigens are processed within the endosome and form a complex with internalized MHC class I, for redelivery back to cell surface.1 Endocytic recycling compartment (ERC), a major perinuclear tubular network considered critical for slow endosomal recycling, has been suggested to be one route for crosspresentation.2 This pathway is particularly relevant for soluble antigen presentation, as the former two routes are mostly used to describe particulate antigen processing, involving phagosomes. Here we report the unexpected finding that disruption of key regulatory factors of ERC, small GTPase proteins ARF6, Rab11a, and Rab22a, had no effect on soluble ovalbumin (OVA) crosspresentation in a model dendritic cell system.
Plasma membrane-bound MHC class I molecules are constitutively recycled into the endocytic pathway, mainly via a tyrosine-containing motif in its cytoplasmic tail3 or via ubiquitination. Along with other cargo, parts of class I molecules are routed to ERC where they are believed to meet antigenic peptides from endocytosed antigen that have been processed by endosomal Cathepsin S.4 Newly-synthesized MHC class I can also directly target the recycling pathway in an MHC class II invariant chain-dependent manner.5 The implication of this rendezvous on crosspresentation has been a major focus of attention. In its GTP-bound active state, ARF6 is translocated to the inner plasma membrane to assist clathrin-independent endocytosis. In its GDP-bound state, ARF6 is positioned on the tubular ERC. This shuffling contributes to the ERC-based recycling.6 Dominant-negative (DN) and constitutive-active (CA) ARF6 mutants are known to disrupt ERC functions.7 Rab22a has been implicated in early endosome-to-ERC transport and recycling of clathrin-independent cargo, including MHC class I.8 Rab22a activation is required for tubule formation from ERC and its subsequent inactivation facilitates fusion of recycling membranes with the surface. Likewise, Rab22a depletion or its CA form has been reported to reduce MHC class I recycling. Rab11a, a defining marker of ERC, interacts with many Rab11 family interaction proteins and regulates ERC traffic.9 These three GTPases are therefore regarded as key regulatory components of trafficking to and from ERC, and presumably participate in crosspresentation. However, most of the work on the regulatory functions of these proteins was performed in cells deficient in crosspresentation; a definitive association thus remains speculative.
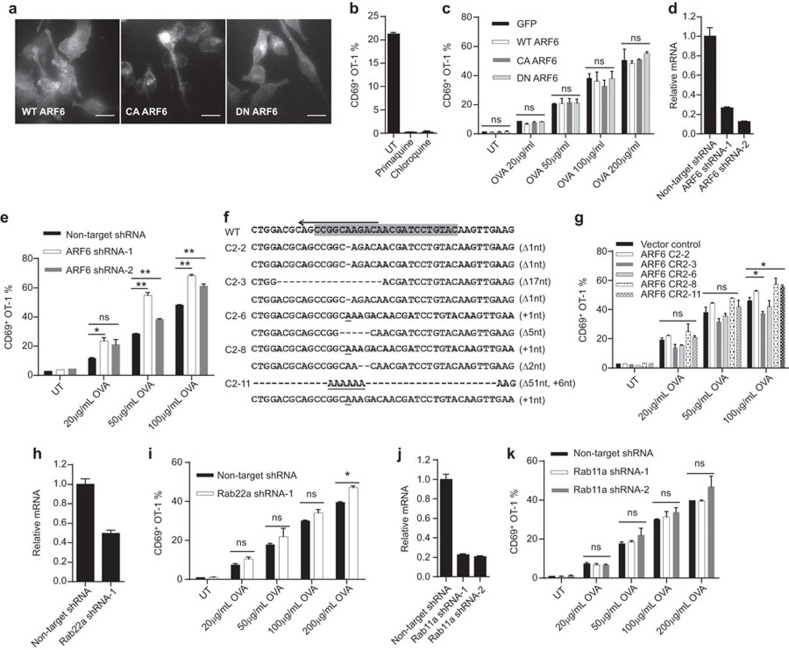
To show how this pathway was related to soluble antigen crosspresentation, we generated C-terminal mCherry-tagged DN (T27N) and CA (Q67L) ARF6 mutants, and transfected DC2.4 cells with retrovirus expression system. As expected, a fraction of CA mutant was associated with the plasma membrane while DN mutant was intracellular with little membrane association. Wild-type (WT) control showed a balance of two states (Figure 1a and Supplementary Movies 1, 2 and 3). These mutants did not significantly alter the H-2Kb distribution (Supplementary Figure 1a). While crosspresentation of soluble OVA was completely blocked by an endocytic recycling blocker primaquine and an endocytic protease inhibitor chloroquine (Figure 1b), surprisingly none of the mutants altered the crosspresentation of soluble OVA to OT-1 cells over a large dose range (Figure 1c).We produced shRNA to knock down ARF6 expression. Two shRNA variants greatly reduced ARF6 mRNA level (Figure 1d). However, the crosspresentation efficiency remained unchanged (Figure 1e) and in some instances showed a small enhancement (Supplementary Figure 1b). SIINFEKL peptide-pulsed ARF6-knockdown cells also showed statistically higher crosspresentation, which might indicate a lack of internalization of the surface MHC class I (Supplementary Figure 1c). Considering the previous two attempts might not have sufficiently outcompeted or reduced the endogenous expression, respectively, we resorted to Cas9-based genomic deletion. Five versions of mutant cells were produced with both loci carrying small out of frame shifts or a large truncation plus a random insertion (Figure 1f). In line with the earlier results, none of these mutants negatively impacted crosspresentation (Figure 1g). To rule out any peculiarity of DC2.4, we repeated shRNA knockdown on DC1940 cells, a recently generated C57BL/6 DC line with immature phenotype (Supplementary Figure 1d). This treatment did not consistently reduce the crosspresentation by this cell line (Supplementary Figure 1e). The data therefore suggested that while DN and CA mutants of ARF6 showed polarized distributions, these mutants as well as overexpression, downregulation and absence of ARF6 do not change soluble antigen crosspresentation.
Figure 1.
Disruption of key GTPase regulators of endocytic recycling compartment does not interfere with soluble antigen crosspresentation in dendritic cells. (a) DC2.4 cells stably transfected with C-terminal mCherry-tagged WT, CA or DN ARF6 by lentivirus were analyzed by live cell imaging. Scale bars are 15 µm. (b) Crosspresentation of soluble OVA by DC2.4 cells in the absence or presence of 80 µM primaquine or 25 µM chloroquine was evaluated by CD69 upregulation in OT-1 cells as described in the section on ‘Methods'. (c) GFP, WT or mutant ARF6-transfected DC2.4 cells were stimulated with soluble OVA at the indicated concentrations for 4 h and then cocultured with OT-1 cells for 24 h. CD69 on OT-1 cells was detected by flow cytometry. (d) qPCR analysis of ARF6 knockdown efficiency in DC2.4 cells transfected with lentivirus encoding ARF6 shRNA. GAPDH was used as internal reference. (e) The same as in c, crosspresentation of soluble OVA by DC2.4 cells transfected with non-target or ARF6 shRNA was evaluated by CD69 upregulation in OT-1 cells. (f) Indels introduced by CRISPR/Cas9 for ARF6 loci in DC2.4 cells. Letters in gray background were sgRNA target sequences. Nucleotide deletions and insertions in five clones are indicated by dashes and underlined letters, respectively. (g) As above, crosspresentation of soluble OVA by vector control DC2.4 cells and five ARF6 knockout clones was evaluated by CD69 upregulation in OT-1 cells. (h and j) Quantification of Rab22a and Rab11a shRNA knockdown in DC2.4 cells. (i and k) Rab22a or Rab11a shRNA-treated DC2.4 cells were analyzed for crosspresentation. Student's t-test results for all figures are indicated as follows: **P<0.01; *P<0.05; P at 0.05 or larger was considered non-significant: ns. DN, dominant-negative; CA, constitutive-active; OVA, ovalbumin; WT, wild-type.
To test the role of Rab22a, we produced CA mutant of Rab22a with N-terminal mCherry tag in DC2.4 cells. With total internal reflection microscopy, Rab22a was found to localize to small and large round-shaped vesicles, with interconnecting dynamic moving tubular structures (Supplementary Figure 1f and Supplementary Movie 4). WT Rab22a vesicles also continually fused with the surface (Supplementary Figure 1f). Cells expressing CA mutant (Q64L) exhibited prominent tubular structures, likely due to defects in cell plasma membrane fusion (Supplementary Figure 1f and Supplementary Movie 5). WT and CA mutant of Rab22a did not affect crosspresentation of soluble OVA as was the case of ARf6 mutants (Supplementary Figure 1g). To further determine whether endogenous Rab22a regulates DC crosspresentation, we used shRNA against Rab22a to deplete endogenous Rab22a. qPCR analysis confirmed the knockdown efficiency (Figure 1h), yet the treatment did not cause any change in the crosspresentation (Figure 1i). Similarly, Rab11a shRNA failed to downregulate the antigen presentation as well (Figure 1j and k).
Our results confirm previous findings that ARF6 DN and CA alter their cytoplasmic distribution. However, these changes did not alter soluble antigen crosspresentation in DC2.4 cells, arguably the most studied model dendritic cell line. shRNA knockdown and Cas9-mediated chromosomal deletion also failed to alter the presentation, which confirmed that in our system ERC was not a crucial compartment for soluble antigen crosspresentation. This observation was corroborated by the Rab11a and Rab22a shRNA knockdown data. Since the involvement of direct endocytic recycling is well established, our data infer that the rapid recycling pathway appears to be the main route of this type of antigen presentation.10
Acknowledgments
YS was supported by Peking-Tsinghua University Center for Life Sciences. This work was supported by Natural Sciences and Engineering Research Council of Canada (RGPIN-355350/396037), Canadian Institutes for Health Research (MOP-119295) and National Natural Science Foundation of China (31370878).
Footnotes
Supplementary information accompanies the paper on Cellular & Molecular Immunology's website(http://www.nature.com/cmi/).
Supplementary Information
References
- Kasturi SP, Pulendran B. Cross-presentation: avoiding trafficking chaos? Nat Immunol 2008; 9: 461–463. [DOI] [PubMed] [Google Scholar]
- Compeer EB, Flinsenberg TW, van der Grein SG, Boes M. Antigen processing and remodeling of the endosomal pathway: requirements for antigen cross-presentation. Front Immunol 2012; 3: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizee G, Basha G, Tiong J, Julien JP, Tian M, Biron KE et al. Control of dendritic cell cross-presentation by the major histocompatibility complex class I cytoplasmic domain. Nat Immunol 2003; 4: 1065–1073. [DOI] [PubMed] [Google Scholar]
- Shen L, Sigal LJ, Boes M, Rock KL. Important role of cathepsin S in generating peptides for TAP-independent MHC class I crosspresentation in vivo. Immunity 2004; 21: 155–165. [DOI] [PubMed] [Google Scholar]
- Basha G, Omilusik K, Chavez-Steenbock A, Reinicke AT, Lack N, Choi KB et al. A CD74-dependent MHC class I endolysosomal cross-presentation pathway. Nat Immunol 2012; 13: 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishna H, Donaldson JG. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J Cell Biol 1997; 139: 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol 2009; 10: 597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magadan JG, Barbieri MA, Mesa R, Stahl PD, Mayorga LS. Rab22a regulates the sorting of transferrin to recycling endosomes. Mol Cell Biol 2006; 26: 2595–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert R, Yeung AC, Li J, Donaldson JG. Rab22a regulates the recycling of membrane proteins internalized independently of clathrin. Mol Biol Cell 2004; 15: 3758–3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf S, Kautz A, Bohnert V, Knolle PA, Kurts C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science 2007; 316: 612–616. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.