Abstract
Epstein–Barr virus (EBV) is prevalent throughout the world and is associated with several malignant diseases in humans. Latent membrane protein 2 (LMP2) of EBV plays a crucial role in the pathogenesis of EBV-associated tumors; therefore, LMP2 has been considered to be a potential immunodiagnostic and immunotherapeutic target. A multi-epitope-based antigen is a promising option for therapeutic vaccines and diagnoses of such malignancies. In this study, we systematically screened cytotoxic T lymphocyte (CTL), helper T cell (Th) and B-cell epitopes within EBV-LMP2 using bioinformatics. Based on the screen, two peptides rich in overlapping epitopes of both T cells and B cells were selected to construct a plasmid containing the sequence for a chimeric multi-epitope protein referred to as EBV-LMP2m, which is composed of LMP2aa195∼232 and LMP2aa419∼436. The EBV-LMP2m protein was expressed in E. coli BL21 (DE3) after prokaryotic codon optimization. Inoculation of the purified chimeric antigen in BALB/c mice induced not only high levels of specific IgG in the serum and secretory IgA in the vaginal mucus but also a specific CTL response. By using purified EBV-LMP2m as an antigen, the presence of specific IgG in the serum specimens of 202 nasopharyngeal carcinoma (NPC) patients was effectively detected with 52.84% sensitivity and 95.40% specificity, which represents an improvement over the traditional detection method based on VCA-IgA (60.53% sensitivity and 76.86% specificity). The above results indicate that EBV-LMP2m may be used not only as a potential target antigen for EBV-associated tumors but also a diagnostic agent for NPC patients.
Keywords: Epstein–-Barr virus (EBV), epitope, latent membrane protein 2 (LMP2), vaccine
INTRODUCTION
Epstein-Barr virus (EBV), also known as human herpes virus 4, is prevalent worldwide, and approximately 90% of adults throughout the world have developed specific antibodies against this virus1 EBV was first discovered by Epstein and Barr upon culturing cells derived from patients suffering from endemic Burkitt lymphoma (BL). Similar to other members of the gamma herpes virus family, EBV typically establishes a lifelong persistent infection in human B lymphocytes and is also associated with several human tumors, including BL, Hodgkin lymphoma (HD), nasopharyngeal carcinoma (NPC), and a subset of cervical,2 gastric,3,4,5 lung,6 and breast cancers.7,8 Some researchers have focused on the relationship between EBV infection and NPC. Serological and molecular epidemiological studies have demonstrated the important etiological role of EBV in NPC carcinogenesis. EBV-specific serum antibodies can be detected in almost 100% of undifferentiated and poorly differentiated NPC patients, and the EBV genome and its products of gene expression can be detected in NPC tumor tissue.9,10,11
In a latent EBV infection, only a limited subset of the full repertoire of viral genes is transcribed. The latency-associated EBV genes express at least nine proteins: the nuclear proteins EBNA1, EBNA2, EBNA3A, EBNA3B, EBNA3C, and EBNA-LP, and the membrane proteins latent membrane protein (LMP)1, LMP2A, and LMP2B.12 LMP2A and LMP2B are two alternative forms of the hydrophobic membrane protein LMP2.13 Both of these variants are transcribed across the fused terminal repeats of the EBV episome using an alternative promoter. Through the regulation of lipid rafts and protein tyrosine kinases (e.g., Syk and Lyn of the Src family), LMP2 can inhibit tyrosine phosphorylation induced by the B-cell receptor (BCR) and prevent the proliferation of EBV to maintain the latent EBV infection.14,15 Moreover, LMP2A may contribute to the malignant transformation of the host genome by intervening with signaling pathways at multiple points, especially in the cell cycle and apoptotic pathway.16 LMP2B has been shown to co-localize with LMP2A.17 Both proteins can promote epithelial cell spreading and motility.1 Therefore, LMP2 is an ideal target protein for EBV-associated immunoprophylaxis and immunotherapy of malignant cancers.
Epitope-based vaccines, as a novel and unique type of vaccine, represent an alternative strategy in tumor immunotherapy. Compared with a whole protein vaccine, an epitope-based vaccine has the following advantages: it induces a conserved protective epitope-specific immune response without eliciting an autoimmune reaction or immune suppression; multi-epitope vaccines containing T- and B-cell epitopes can induce broader humoral and cellular immune responses in the host19; and the combination of different HLA-restricted CTL epitopes in one vaccine can be used to accelerate the effect of cellular immune responses against certain tumors due to the presence of HLA allelic variants that bind to distinct sets of peptides.20
Recently, several Th- and HLA-restricted CTL epitopes in LMP2 have been identified,21,22,23 and our group has reported B-cell epitopes of EBV-LMP2.24 In this study, we systematically screened CTL, Th, and B-cell epitopes within LMP2 using bioinformatics. Based on the results of the bioinformatics screening, two peptides composed of LMP2 amino acids 195–232 and 419–436 (referred to as LMP2aa195∼232 and LMP2aa419∼436, respectively) are rich in overlapping T and B-cell epitopes and were selected to construct the chimeric multi-epitope protein EBV-LMP2m. An immunogenicity analysis indicated that EBV-LMP2m induces high levels of specific IgG and IgA as well as a specific CTL immune response in BALB/c mice. Moreover, by using purified EBV-LMP2m as an antigen, the specific serum IgG of NPC patients can be effectively detected. The sensitivity of an ELISA assay using the EBV-LMP2m antigen to detect IgG in NPC patients reached 52.84% with a specificity of 95.40%, which is better than the values obtained by traditional detection using VCA-IgA (60.53% sensitivity and 76.86% specificity). Therefore, this EBV-LMP2 multi-epitope protein may be used not only as a potential target antigen for the immunotherapy of EBV-associated tumors but also as a serological diagnostic agent for NPC patients.
MATERIALS AND METHODS
Serum samples
Serum specimens from 202 patients with NPC, 12 patients with Hodgkin disease, 18 patients with malignant lymphadenoma, 6 patients with infectious mononucleosis, and 112 healthy individuals were submitted to our reference laboratory for EBV-LMP2-specific antibody testing by indirect ELISA. All of the cases were selected from the First Affiliated Hospital of Wenzhou Medical University (Zhejiang Province, China) and were confirmed by both clinical diagnosis and histopathological examination. Informed written consent was obtained from each patient, and the study was approved by the Human Research Ethics Committee from the First Affiliated Hospital of Wenzhou Medical University. The serum samples were stored at −20°C immediately after separation until further analysis.
Preparation of native LMP2 antigen
Native LMP2 protein was obtained from the B95-8 cell strain as previously described.24,25,26 The EBV-positive B95-8 cell line (ATCC CRL-1612) was obtained from the American Type Culture Collection and cultured in RPMI 1640 containing 10% fetal bovine serum (FBS). First, 1 × 107 cells in the exponential phase of growth were collected and washed several times with PBS (pH 7.0), followed by ultrasonication for 5 min on ice. The cell membrane proteins (containing native LMP2) were obtained using a membrane protein extraction kit (BestBio Biotechnology Co., Shanghai, China) and stored at −20°C until further analysis.
Prediction of the T epitopes
The gene and complete amino acid sequences of EBV-LMP2 were obtained from the GenBank database. Epitopes restricted by MHC classes I and II, termed CTL and Th epitopes, respectively, were predicted using SYFPEITHI software (http://www.syfpeithi.de/Scripts/MHCServer.dll/EpitopePrediction.htm). Mouse H2-Kd and the human HLA types prevalent in China (HLA-A*0201, HLA-A*2402, HLA-DRB1*0401, HLA-DRB1*0301, and HLA-DRB1*1501) were chosen for analysis. The score of each peptide was recorded based on whether the amino acid in the peptides was located in the anchor position, auxiliary residue, or advantage residue. Peptides with a high score were chosen as potential immunodominant CTL and Th epitopes.27
Prediction of the linear B-cell epitopes
Combined with their secondary structure and surface properties, the dominant B-cell epitopes of EBV-LMP2 were predicted.28,29,30 The secondary structure of EBV-LMP2 was predicted by the SOPMA,31 GOR,32 nnPredict (http://www.cmpharm.ucsf.edu/˜nomi/nnpredict.html) and HNN (http://npsa-pbil.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_hnn.html) software packages, and the transmembrane domains were analyzed by SOSUI.33 The software was provided by the EXPASY Internet Server (http://www.expasy.ch/tools). The surface properties of EBV-LMP2, such as its hydrophilicity, flexibility, accessibility, and antigenicity, were analyzed using the following three software packages: Hopp and Woods,34 Zimmerman,35 and Jameson and Wolf.36 Based on the results, peptides with good hydrophilicity, high accessibility, sufficient flexibility, and strong antigenicity were chosen. The antigenic determinants of LMP2 were further obtained using the approach of Kolaskar and Tongaonkar37 and the immunodominant linear B-cell epitopes of LMP2. Peptides located in the α-spiral and β-sheet regions, which do not readily form epitope regions, were excluded.
Design of the fused multi-epitope antigen from EBV-LMP2
For the multi-epitope antigen, amino acid sequences rich in CTL, Th epitopes, and B-cell epitopes were selected. These sequences were then connected in series to construct a novel multi-epitope antigen and re-predicted to validate whether any of the T- and B-cell epitopes in the fused sequence were changed. The fused sequence with unchanged epitopes was selected for further analysis and termed the EBV-LMP2 multi-epitope antigen (EBV-LMP2m).
Expression, verification, and purification of EBV-LMP2m recombinant protein
To express the fused multi-epitope antigen, the amino acid sequence of EBV-LMP2m was translated into a DNA sequence with codon usage of a prokaryotic system (http://www.jcat.de/Start.jsp). The EBV-LMP2m gene was synthesized by Beijing Sunbiotech Co. Ltd. (Beijing, China) and cloned into the BamH I and Hind III sites of the prokaryotic expression vector pET32a(+) to construct the recombinant plasmid pET32a(+)/EBV-LMP2m, which was confirmed by both restriction endonuclease digestion and sequencing. Following confirmation of the inserted sequences, E. coli BL21 (DE3) was transformed with positive plasmids and induced with 1 mM isopropyl-D-thiogalactopyranoside (IPTG, Sigma). As the recombinant protein contained the His-tag protein, the expression and characterization of the EBV-LMP2m recombinant protein were verified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting analysis with an HRP-anti-His monoclonal antibody (KPL, Gaithersburg, MD, USA). The recombinant protein was purified using Ni-NTA agarose following the procedure recommended by Qiagen (Hilden, Germany). The protein was renatured by dialysis against phosphate-buffered saline (PBS, pH 7.4) and fractionated by SDS-PAGE, after which the concentration was determined by the bicinchoninic acid (BCA) protein quantitation method. The samples were stored at −20°C until further use.
Animals and vaccination schedule
Six- to eight-week-old female BALB/c mice (H-2d) were purchased from Shanghai SLAC Laboratory Animal Co. Ltd and kept at the animal facility of Wenzhou Medical University (Wenzhou, China). All of the animal procedures were performed according to approved protocols and in accordance with recommendations for the proper use and care of laboratory animals. The mice were randomly divided into three equal groups (nine mice per group) and immunized with purified EBV-LMP2m protein, the His tag or PBS buffer. Each animal was subcutaneously immunized with 50 μg of proteins delivered in 0.1 ml PBS formulated with an equal volume of incomplete Freund's adjuvant (IFA) except for the first dose, which contained Freund's complete adjuvant. The control groups received the same volumes of adjuvants only. Three vaccinations were given at two-week intervals with the same amount of antigen. To boost the immunization of the Freund's adjuvant groups, IFA was used. One week after each immunization, 100 μl of PBS was used to wash the vagina of each mouse 6–8 times, and all of the vaginal washing fluid was collected. After centrifugation at 12 000 rpm for 5 min at 4°C, the supernatants were collected and stored in −20°C until testing. Concurrently, serum samples taken from the tail vein of the mice were separated and stored in −20°C. Three weeks after the final immunization, the mice were sacrificed. The spleen cells were separated under sterile condition for use in the CTL assay.
Detection of specific sera IgG and vaginal IgA of the immunized mice
The specific antibody response of mice immunized to either artificial EBV-LMP2m or native EBV-LMP2 was assayed by indirect ELISA. Microplates were coated with purified EBV-LMP2m (10 μg/ml) and the EBV membrane protein (1 × 107/ml) (BestBio Biotechnology Co., Shanghai, China) in carbonate coating buffer (100 μl/well). After an overnight incubation at 4°C, the plates were rinsed with PBS solution containing 0.05% Tween 20 (PBST). The coated wells were blocked with 100 μl blocking buffer (PBST containing 5% nonfat dry milk w/v) at 37°C for 1 h. The plates were washed 5 times with PBST after blocking. Then, either 100 μl of 1:1000 diluted immune sera or 100 μl of vaginal washing fluid was added to the wells in triplicate, and the plates were incubated at 37°C for 2 h. After washing 5 times, 100 μl of either a 1:1000 dilution of the HRP-conjugated goat anti-mouse IgG (H+L) (ABR, USA) or a 1:2000 diluted HRP-conjugated goat anti-mouse IgA (ABR, USA) was added to the corresponding wells and incubated for 1 h at 37°C. The wells were washed again, followed by the addition of 100 μl/well of 3,3′,5,5′-tetramethylbenzidine (TMB)–H2O2 solution. The reaction was carried out at room temperature for 20 min and halted with 50 μl of 2 mol/l of H2SO4 per well, and the absorbance (OD) at 490 nm was measured using a Bio-tek ELISA microplate reader. All of the experiments were performed in triplicate.
CTL activity detection in spleen lymphocytes from immunized mice (lactate dehydrogenase)
Three weeks after the final immunization, cells from the spleens of the BALB/c mice (H-2d) were collected, and erythrocytes in single-cell suspensions were abolished with an NH4Cl solution (pH 7.2). The cells were washed and resuspended in RPMI 1640 complete medium (Gibco) supplemented with 2 mM L-glutamine, 100 U of penicillin per ml, 0.1 mg of streptomycin per ml, 30 mM HEPES, 3.7% sodium bicarbonate, and 15% heat-inactivated newborn calf serum. The cells were counted and adjusted to 5 × 106/ml as the effector cells. For preparation of the target cells, 1 × 105/ml of P815 cells (H-2d, ATCC TIB-64, Shanghai Institute of Cell Resource Center, Chinese Academy of Sciences) were fully mixed with 10 μg/ml of either the synthetic CTL epitope peptide (TTYGPVFMCL, H-2kd) or control peptide (KYAVTVETRL, H-2kd) from Chlamydia trachomatis MOMP, aliquoted into 96-well U-bottom plates (Cosca) at 1 × 104 cells per well and incubated at 37°C for 3 h. Then, the effector cells were plated on the target cells in quadruplicate at ratios of 5:1, 10:1, and 25:1 and incubated in a 37°C 5% CO2 incubator for 4–6 h. The supernatants were collected, and the CTL activity was measured using the CytoTox 96 Non-Radioactive Cytotoxicity Assay Kit (Promega) according to the manufacturer's instructions. The following formula was used to determine the percentage of the specific cytotoxicity: Cytotoxicity (%) = (A490 value of the experimental group – A490 value of the target cell spontaneous release group – A490 value of the effector cell spontaneous release group)/(A490 value of the target cell maximum release group – A490 value of the target cell spontaneous release group) × 100%.
Western blot analysis
To evaluate the antigenicity of the expressed epitope-fused proteins, the purified samples were run on a 12% gel using SDS-PAGE and analyzed via western blot. 6 × His protein purified from E. coli BL21 (DE3) transformed with the pET32a (+) blank vector was used as a control. Rabbit anti-EBV membrane protein serum and sera from NPC patients were obtained as the primary antibody, and either HRP-conjugated goat anti-rabbit IgG (H+L) (ABR Inc, USA) or HRP-conjugated goat anti-human IgG (H+L) (eBioscience) were used as the secondary antibody. The protein bands were visualized using 0.005% (w/v) 4-chloro-1-naphthol and a 0.015% (v/v) hydrogen peroxidase color development substrate.
Detection of specific IgG in the sera of EBV-associated malignant patients
Using EBV-LMP2m as the antigen-specific antibodies within the sera of the EBV-associated malignant patients were detected by indirect ELISA. Microplates were coated with 1 μg/ml purified EBV-LMP2m, and the sera were diluted in blocking buffer. HRP-goat anti-human IgG (H+L) diluted to 1:5000 was used as the secondary antibody. Each sample was prepared in triplicate, and and the His-tag was used as a negative control. Other procedures were performed as described above. For the sera of the NPC cases, the serum antibody titers of EBV VCA/IgA were additionally detected by using an enzyme-linked immunoadsorbent assay (Zeus Scientific, Inc.).
Statistical analysis
One-way analysis of variance (ANOVA) and independent t-tests were used to evaluate the differences in the antibody levels between different groups. The data were considered to be statistically significant when P < 0.05. All calculations were performed with the SPSS software (version 16.0).
RESULTS
Screening of EBV-LMP2m
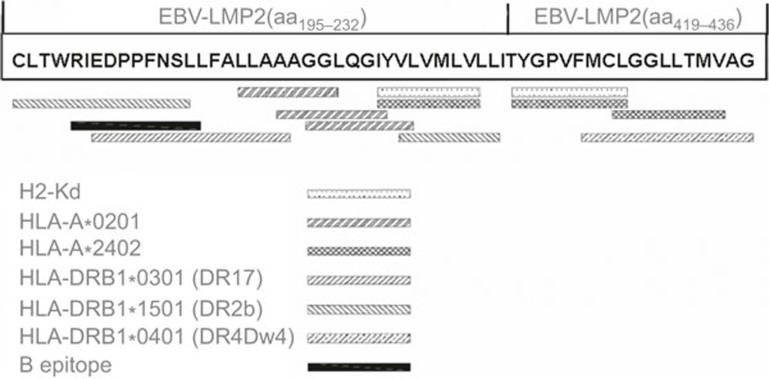
Two sequences that were rich in T- and B-cell epitopes, EBV-LMP2 (aa195∼232) and EBV-LMP2 (aa419∼436), were selected to construct a multi-epitope gene for EBV-LMP2 (EBV-LMP2m) (Figure 1). EBV-LMP2 (aa195∼232) contains three putative HLA-A2 restricted CTL epitopes (LMP2aa211∼219, 215∼223, 217∼225), one putative H-2Kd-restricted CTL epitope (LMP2aa222-230), one confirmed HLA-A2-restricted CTL epitope (LMP2aa222∼230),38 three putative Th epitopes (LMP2aa195∼208,201∼216,224∼232), and one confirmed B epitope (LMP2aa199∼209).24 EBV-LMP2 (aa419∼436) contains one predictive H-2Kd restricted CTL epitope (LMP2aa419-427), one putative Th cell epitope (LMP2aa424∼436), and two confirmed HLA-A24 restricted CTL epitopes (LMP2aa419∼427, 426∼434).39,40
Figure 1.
Map of the different epitopes in the EBV-LMP2 multi-epitope sequence. Two sequences rich in T- and B-cell epitopes, EBV-LMP2 (aa195∼232) and EBV-LMP2 (aa419∼436), were selected to construct the multi-epitope gene of EBV-LMP2 (EBV-LMP2m). EBV-LMP2 (aa195∼232) contains three putative HLA-A2-restricted CTL epitopes (LMP2aa211∼219, 215∼223, 217∼225), one putative H-2Kd-restricted CTL epitope (LMP2aa222-230), one confirmed HLA-A2-restricted CTL epitope (LMP2aa 222∼230), three putative Th epitopes (LMP2aa195∼208, 201∼216, 224∼232) and one confirmed B epitope (LMP2aa199∼209), whereas EBV-LMP2 (aa419∼436) contains one predictive H-2Kd-restricted CTL epitope (LMP2aa419-427), one putative Th cell epitope (LMP2aa424∼436) and two confirmed HLA-A24-restricted CTL epitopes (LMP2aa419∼427, 426∼434).
Production of the EBV-LMP2m protein
The synthesized EBV-LMP2m gene was cloned into the prokaryotic expression vector pET32a(+) (Figure 2). The identified pET32a(+)/EBV-LMP2m plasmid was then transformed into E.coli. BL21 (DE3) and induced by IPTG. As shown in Figure 3, the monoclonal antibody against the His tag readily detected the recombinant proteins. The expected molecular mass of the recombinant proteins was approximately 27 kDa. These His-tag fusion proteins were further purified by Ni-NTA agarose affinity chromatography.
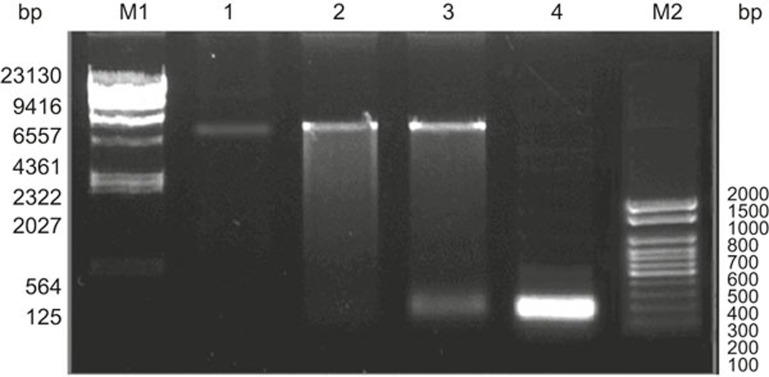
Figure 2.
Restriction endonuclease digestion of pET32a(+)/EBV-LMP2m. The EBV-LMP2m gene was synthesized and cloned into the BamH I and Hind III sites of the prokaryotic expression vector pET32a(+) to construct the recombinant plasmid pET32a(+)/EBV-LMP2m. The recombinant plasmid was digested by restriction enzymes (BamH I and Hind III). Lane M1: λDNA/Hind III DNA marker; Lane M2: DL 2000 DNA marker; Lane 1: pET32a(+); Lane 2: pET32a(+)/EBV-LMP2m; Lane 3: pET32a(+)/EBV-LMP2m/Hind III +BamH I; Lane 4: PCR product of EBV-LMP2m.
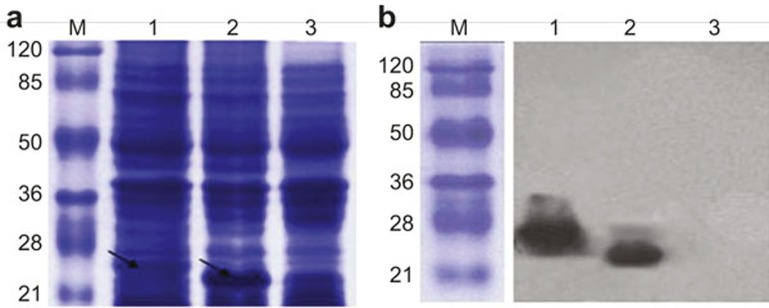
Figure 3.
Expression and identification of EBV-LMP2m recombinant protein. EBV-LMP2m recombinant protein and pET32a(+) basal plasmid protein (His-tag) expression (indicated by the arrow) detection by SDS-PAGE analysis. (A) Protein expression was under the control of the T7 promoter and was induced by IPTG. (B) EBV-LMP2m recombinant protein expression detection by western blotting using a monoclonal antibody against the His-tag protein. The protein lysates of pET32a(+)/EBV-LMP2m and the pET32a(+) blank control were transferred onto nitrocellulose filters. Lane M: pre-stained protein marker; Lane 1: pET32a(+)/EBV-LMP2m at 27 kDa; Lane 2: pET32a basal plasmid expression protein (His-tag) at 19 kDa; Lane 3: Lysate of E. coli BL21.
Immunogenicity analysis of EBV-LMP2m
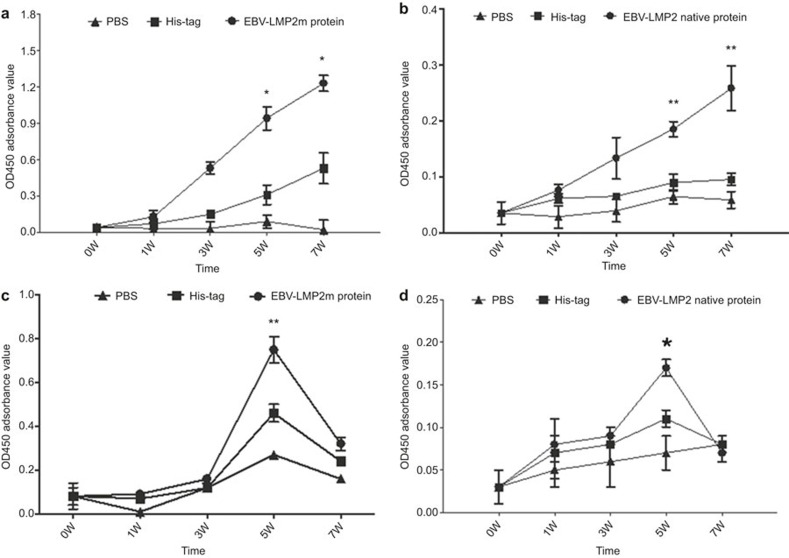
After immunization with EBV-LMP2m, the levels of specific IgG in immune sera and specific IgA in vaginal washing fluid were analyzed by ELISA (Figure 4). The results showed that specific reactions of IgG and IgA with the EBV-LMP2m and native LMP2 antigen were induced in the EBV-LMP2m group. The levels of specific IgG in the EBV-LMP2m group were significantly higher than those in the His-tag group and PBS group after the fifth week (P < 0.05), whereas the specific IgA levels in the vaginal washing fluid peaked during the fifth week (P < 0.05) but showed no significant difference at week 7.
Figure 4.
ELISA analysis of EBV-LMP2 with specific IgG in immune sera and specific IgA in vaginal washing fluid. Mice (nine per group) were immunized with purified EBV-LMP2m recombinant protein at two-week intervals using the His-tag protein and PBS buffer as negative controls. Serum and vaginal secretions were collected for ELISA analysis at 0, 1, 3, 5, and 7 w. (a) The specific IgG response to EBV-LMP2m protein in the immune sera compared to the controls, F = 88.450, *P < .01 (5 W); F = 174.150, *P < 0.01 (7 W). (b) The specific IgG to EBV-LMP2 native protein in the immune sera compared to the controls, F = 12.495, **P < 0.05; F = 21.720, **P < 0.05. (c) The specific IgA to EBV-LMP2m protein in the vaginal mucosa compared to the controls, F = 60.300, **P < 0.05. (d) The specific IgA to EBV-LMP2 native protein in the vaginal mucosa compared to the controls, F = 37.226, *P < 0.01. The results are presented as the mean OD450 absorbance value and standard deviation (error bars) for three experiments. The data were analyzed by one-way ANOVA.
CTL activity analysis of spleen lymphocytes
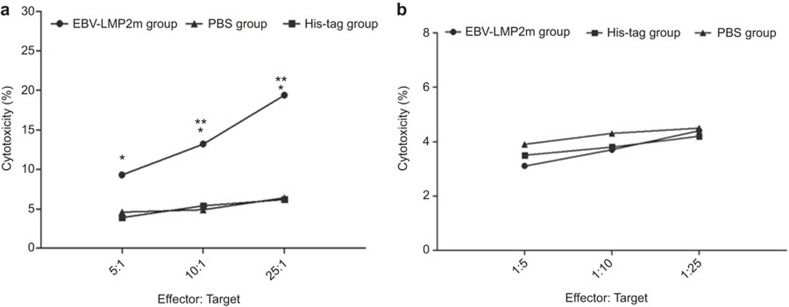
The specific CTL activity of spleen lymphocytes from the immunized mice was further analyzed (Figure 5). With the P815 cells as target cells, the specific CTL activity of spleen lymphocytes in the EBV-LMP2m group at 5:1, 10:1, and 25:1 effector (E):target (T) ratios was statistically higher than in the His-tag and PBS groups (P < 0.05). The CTL activities were 19.45% ± 2.12%, 13.21% ± 1.53%, and 9.30% ± 1.39% for the EBV-LMP2m groups; 6.20% ± 1.14%, 5.42% ± 1.07%, and 3.92% ± 0.85% for the His-tag groups; and 6.43% ± 0.92, 4.90% ± 0.63, and 4.60% ± 0.22% for the PBS groups, respectively. When target cells were mixed with the control peptide, KYAVTVETRL, there were no significant differences between the EBV-LMP2m group and the His-tag and PBS groups.
Figure 5.
The specific CTL activity of spleen lymphocytes. Splenocytes were collected from the BALB/c mice (H-2d) group immunized with purified EBV-LMP2m protein and from the control groups immunized with the His-tag protein and PBS buffer and used as effector cells. (a) The P815(H-2d) cells were used as target cells and coated with the corresponding CTL peptide (TTYGPVFMCL, H-2kd) for 3 h. (b) The P815 (H-2d) cells were used as target cells and were coated with the control peptide (KYAVTVETRL, H-2kd, from Chlamydia trachomatis MOMP) for 3 h. The cytotoxic activity of the CTL lines was tested using the CytoTox 96 Non-Radioactive Cytotoxicity Assay Kit (Promega). The results are presented as the mean cytotoxicity (%) and standard deviation (error bars) for three experiments. The data were analyzed by independent t-tests. When the P815 (H-2d) cells were coated with the corresponding CTL peptide (TTYGPVFMCL, H-2kd), and the ratio of effector cells to target cells was 5:1, 10:1, and 25:1, the cytotoxicity (%) of the EBV-LMP2m group was significantly higher than that of the His-tag (t = 3.873, **P < 0.05; t = 3.9121, **P < 0.05; t = 10.122, **P < 0.05, respectively) and PBS groups (t = 3.935, **P < 0.05; t = 5.001, *P < 0.01; t = 11.539, *P < 0.01, respectively). When target cells were coated with the control peptide, there were no significant differences between the EBV-LMP2m group and either the His-tag or PBS groups.
Antigenicity analysis of EBV-LMP2m
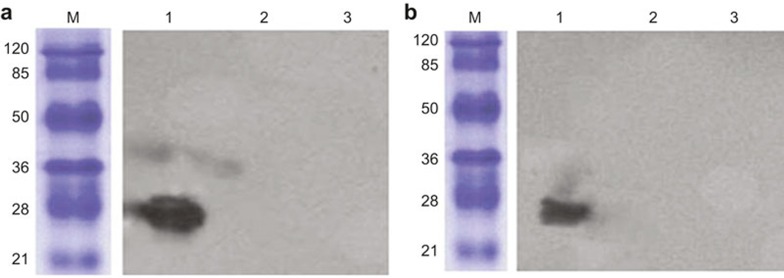
Rabbit anti-EBV membrane protein sera and the sera of NPC patients, which contain the specific antibody for EBV-LMP2, were used to evaluate the antigenicity of EBV-LMP2m. As shown in Figure 6, the EBV-LMP2m protein was specifically recognized by the NPC sera mixture and the rabbit anti-EBV membrane protein antibodies, whereas the His-tag control failed to be specifically recognized.
Figure 6.
Western blot analysis of the EBV-LMP2m protein. The EBV-LMP2m protein was detected using a western blot assay with rabbit immune sera anti-EBV membrane protein (1:100) and the sera of NPC patients (1:800) as the primary antibody. (a) The immune sera of the anti-EBV membrane protein recognized the EBV-LMP2m protein. (b) The sera of NPC patients recognized the EBV-LMP2m protein. Lane M: pre-stained protein marker; Lane 1: pET32a(+)/EBV-LMP2m at 27 kDa; Lane 2: pET32a basal plasmid expression protein (His-tag); Lane 3: lysate of E. coli BL21.
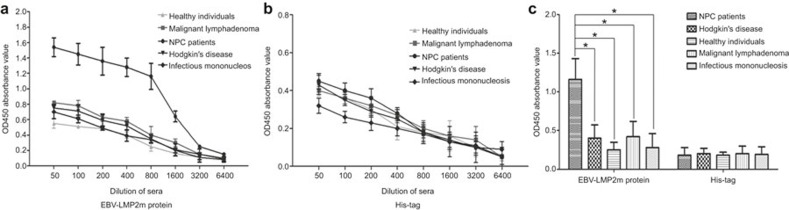
To confirm the prevalence of EBV-LMP2m-specific immune responses in NPC individuals, the EBV-LMP2m reactivity of sera from 202 NPC patients; 36 EBV-infected, non-NPC patients (12 HD, 18 malignant lymphoma and 6 infectious mononucleosis); and 112 healthy control subjects were evaluated by indirect ELISA (Figure 7). The mean OD450 value for EBV-LMP2m in 1:800 diluted sera of NPC patients showed a significant difference (F = 110.306, P < 0.01) between the NPC group and the other two groups (the EBV-infected, non-NPC group and the healthy control group). However, the differences among the mean OD450 values for the His-tag control were statistically insignificant (t1 = 0.537, t2 = 0.283, P > 0.05).
Figure 7.
The reactivity of sera from NPC patients, EBV-infected non-NPC patients, and healthy individuals to EBV-LMP2m protein. The reactivity sera from NPC patients, Hodgkin disease patients, malignant lymphoma patients, infectious mononucleosis patients, and healthy individuals were analyzed by an indirect ELISA assay. (A) The specificity of the sera from different groups to the EBV-LMP2m protein was analyzed using a serial dilution. (B) The specificity of the serum from the different groups to the His-tag protein was analyzed by a serial dilution. (C) Analysis of the specificity of patients' sera to the EBV-LMP2m protein and the His-tag, as determined by the indirect ELISA assay. Serum samples (1:800) from 112 healthy adults, 202 NPC patients, 12 Hodgkin disease patients, 18 malignant lymphoma patients, and 6 infectious mononucleosis patients were analyzed. Each column represents the mean OD450 absorbance value and standard deviation (error bars) for three experiments. The data were analyzed by one-way ANOVA. The data indicate significant differences between the NPC patients and other patients and healthy individuals when the EBV-LMP2m protein was used as an antigen (*P < 0.01), whereas there were no significant differences between the NPC patients and the other EBV patients and healthy individuals when the His-tag protein was used as an antigen (P > 0.05).
Sensitivity and specificity analysis of EBV-LMP2m-based ELISA
The serum antibody positive rates for EBV-LMP2m in the NPC and healthy groups were further evaluated in 202 NPC patients and 112 healthy subjects. Samples with OD450 values greater than the cutoff value (i.e., the mean OD450 value of sera ± 2 SD for healthy individuals)41,42,43 were determined to be positive. As shown in Table 1, the serum antibody positive rates in the NPC group and healthy control group were 52.97% (107/202) and 4.46% (5/112), respectively. When the same serum samples were analyzed using an EBV VCA-IgA ELISA kit (Zeus Scientific, Inc.), which is the gold standard for serologic diagnosing EBV infection, the positive percentages of the NPC group and healthy control group were 60.39% (122/202) and 24.10% (27/112) respectively. This result indicates that the sensitivity and specificity of the EBV-LMP2m-based ELISA were 52.97% (107/202) and 95.54% (107/112), respectively, for the NPC group.
Table 1. Sensitivity and specificity analysis of the EBV-LMP2m-based ELISA.
| Group | Number | EBV-LMP2m | Ratio (%) | VCA-IgA | Ratio (%) |
|---|---|---|---|---|---|
| NPC | 202 | 0.450 ± 0.367 | 52.97(107/202) | 0.325 ± 0.304 | 60.39% (122/202) |
| Control | 112 | 0.184 ± 0.041 | 4.46% (5/112) | 0.033 ± 0.024 | 24.10% (27/112) |
DISCUSSION
Since the discovery of EBV in the 1970s, the relationship between EBV infection and malignancies has been studied in detail. EBV is the cause of BL, and its persistent infection is also associated with B-cell lymphomas, some T-cell lymphomas, gastric carcinomas, Hodgkin disease, NPC, and oral hairy leukoplakia.44 As shown in previous reports, cell-mediated immunity has been thought to play an important role in inducing an immune response to EBV. Cytotoxic T cells target all of the EBV latent gene protein products except for EBNA1; thus, synthetic peptides based on LMP may be used as a possible vaccine for EBV-associated tumors.45 Previous studies have suggested that the HLA-A*0201-restricted CTL epitopes LLWTLVVLL (LMP2aa329∼337)46 and LLLIVAGIL (LMP2aa396∼404)21 from EBV-LMP2 may be potentially useful as a vaccine. The HLA-A2-restricted CTL epitopes YLLEMLWRL (LMP1aa125∼133) and YLQQNWWTL (LMP1aa159∼167)47 from LMP1 and the HLA-A2-restricted CTL epitopes TYGPVFMCL (LMP2aa419∼427),48 FLYALALLL (LMP2aa356-364),49 IYVLVMLVL (LMP2aa222∼230),38 and CLGGLLTMV (LMP2aa426∼434)40 from LMP2 can be identified by the CTL cells of PBMCs in EBV-infected patients, which can induce specific CTL effects in the body. Therefore, these CTL epitopes have been considered to be important target antigens with respect to EBV-associated malignancies and are susceptible to the LMP2-targeting immunotherapy.
In this study, using SYFPEITHI prediction analysis, nine CTL epitopes were predicted in the LMP2aa195∼232 sequence. In this sequence, seven CTL epitopes were predicted (LMP2aa208∼216, aa211∼219, aa215∼223, aa217∼225, aa218∼227, aa222∼230, and aa223∼232), two HLA-DRB1-restricted Th cell epitopes were predicted (LMP2aa208∼216 and 224∼232) and one B-cell epitope was predicted (LMP2aa199∼209). Additionally, HLA-A*2402- and H2-Kd-restricted CTL epitopes were predicted in LMP2aa222∼230 and LMP2aa419∼427. Furthermore, the LMP2aa419∼436 region also included HLA-DRB1-restricted Th cell epitopes, LMP2aa420∼435 and aa424∼436. Therefore, the two sequences of EBV-LMP2aa195∼232 and LMP2aa419∼436, which are rich in T- and B-cell epitopes, were selected for further study as a possible EBV-LMP2 multi-epitope gene. Thus, the designed EBV-LMP2m contained not only a variety of HLA-specific CTL and Th epitopes but also the H2-Kd for mouse CTL epitopes, which could be used in animal experiments to verify the immunogenicity of EBV-LMP2m. We also codon-optimized the EBV-LMP2m gene for prokaryotic systems and obtained a high yield of the recombinant proteins.
An effective EBV vaccine will likely induce both broad and potent CTL responses.50 In our study, LMP2m was found to contain several possible T-cell epitopes, which may induce broad and strong cellular immune responses. Moreover, these possible T-cell epitopes can be easily identified using web prediction services.51 The use of LMP2m in combination with a variety of MHC-restricted epitopes can resolve the issues encountered with a single MHC-restricted epitope peptide such that a wider scope of vaccine applications and more adapted populations will be available. Our results showed that the specific CTL activity was significantly different from that of the control groups, indicating that LMP2m may be able to mediate anti-tumor effects in vivo.
A multi-epitope vaccine that includes T- and B-cell epitopes can induce a strong immune response.52 Recombinant multi-epitopes of the CTL from various HIV subtypes have been used to target multiple HIV-1 regions to overcome HIV's evasion of the host immune system.19 Tian et al. selected seven epitopes of the S1 and S2 spike proteins and nucleocapsid (N) protein from infectious bronchitis virus and constructed a multi-epitope DNA vaccine, which led to a dramatic augmentation of humoral and cellular responses and provided up to an 80.0% rate of immune protection.53 In another study, a multi-epitope vaccine was constructed with five epitopes: three Th epitopes from UreB and two B-cell epitopes from UreB and HpaA of Helicobacter pylori (H. pylori). The vaccine could induce a therapeutic effect against H. pylori infection. The effects of the vaccine were correlated with antigen-specific CD4+ T cells as well as IgG and mucosal IgA antibody responses.54 A different group constructed a multi-epitope DNA vaccine based on six antigens from toxoplasma. These antigens induced a strong humoral immune response and Th1 cell-mediated immune effects.55 These studies demonstrate that a multi-epitope vaccination treatment is a potential strategy for the control of viruses, bacteria, parasitic infections, and related tumors. In this study, an EBV-LMP2 multi-epitope located in epitope-enriched or overlapping regions was predicted and selected. A specific serum IgG and a genital mucosal IgA reaction to the EBV membrane protein could be induced in immunized BALB/c mice. Furthermore, EBV-LMP2-specific CTL cytotoxicity was achieved, and EBV-LMP2m can be recognized by the specific serum antibodies in NPC patients.
Because LMP2 is located on the cell membrane surface of EBV-related tumors in cases of NPC, Hodgkin disease and other tumors, this protein is a promising target antigen in immune therapy and immune detection.56 Currently, issues exist regarding the insufficient accuracy and high false positive rates in EBV VCA-IgA detection and EBV diagnosis. In the present study, using the EBV-LMP2 multi-epitope protein as a diagnostic antigen in ELISA, the serum titers from NPC patients showed significantly higher positive rates than the serum titers from infectious mononucleosis patients and patients with malignant lymphomas such as Hodgkin disease. Thus, high specificity was obtained by ELISA based on EBV-LMP2 multi-epitope protein, and we plan to further use these ELISA diagnostic kits to study EBV infection and its associated tumors.
In conclusion, we report that a multi-epitope EBV-LMP2m-containing epitopes from EBV-LMP2 antigens can induce a specific cellular immune response and a specific humoral immune effect in the whole body and in the genital mucosa, respectively. With a variety of HLA-specific CTL and Th epitopes, this multi-epitope protein can exert immunogenic effects for the majority of the population and may also be used to generate ex vivo EBV-specific T cells that could be administered to patients. Additionally, the specific murine immune serum IgG can bind to the EBV membrane protein, and a higher specificity was obtained with ELISAs based on the EBV-LMP2 multi-epitope in NPC patients. The EBV-LMP2 multi-epitope has a strong antigenicity and immunogenicity and may be used as a potential agent for the immunotherapy and immunodiagnosis of EBV-associated malignancies.
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China (No: 81372447).
References
- Rickinson AB, Lane PJ.Epstein-Barr virus: co-opting B-cell memory and migration. Curr Biol 10 February 2000; 10: R120–R123. [DOI] [PubMed] [Google Scholar]
- Sasagawa T, Shimakage M, Nakamura M, Sakaike J, Ishikawa H, Inoue M. Epstein-Barr virus (EBV) genes expression in cervical intraepithelial neoplasia and invasive cervical cancer: a comparative study with human papillomavirus (HPV) infection. Hum Pathol 2000; 31: 318–326. [DOI] [PubMed] [Google Scholar]
- Oh ST, Seo JS, Moon UY, Kang KH, Shin DJ, Yoon SK et al. A naturally derived gastric cancer cell line shows latency I Epstein-Barr virus infection closely resembling EBV-associated gastric cancer. Virology 2004; 320: 330–336. [DOI] [PubMed] [Google Scholar]
- Sairenji T, Tajima M, Kanamori M, Takasaka N, Gao X, Murakami M et al. Characterization of EBV-infected epithelial cell lines from gastric cancer-bearing tissues. Curr Top Microbiol Immunol 2001; 258: 185–198. [DOI] [PubMed] [Google Scholar]
- Xiao YP, Han CB, Mao XY, Li JY, Xu L, Ren CS et al. Relationship between abnormality of FHIT gene and EBV infection in gastric cancer. World J Gastroenterol 2005; 11: 3212–3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillinger S, Yang SC, Zhu L, Huang M, Duckett R, Atianzar K et al. EBV-induced molecule 1 ligand chemokine (ELC/CCL19) promotes IFN-gamma-dependent antitumor responses in a lung cancer model. J Immunol 2003; 171: 6457–6465. [DOI] [PubMed] [Google Scholar]
- Arbach H, Viglasky V, Lefeu F, Guinebretière JM, Ramirez V, Bride N et al. Epstein-Barr virus (EBV) genome and expression in breast cancer tissue: effect of EBV infection of breast cancer cells on resistance to paclitaxel (Taxol). J Virol 2006; 80: 845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande CG, Badve S, Kidwai N, Longnecker R. Lack of expression of the Epstein-Barr Virus (EBV) gene products, EBERs, EBNA1, LMP1, and LMP2A, in breast cancer cells. Lab Invest 2002; 82: 1193–1199. [DOI] [PubMed] [Google Scholar]
- Hu B, Hong G, Li Z, Xu J, Zhu Z, Li L. Expression of VCA (viral capsid antigen) and EBNA1 (Epstein-Barr-virus-encoded nuclear antigen 1) genes of Epstein-Barr virus in Pichia pastoris and application of the products in a screening test for patients with nasopharyngeal carcinoma. Biotechnol Appl Biochem 2007; 47(Pt 1): 59–69. [DOI] [PubMed] [Google Scholar]
- Li J, Zhang XS, Xie D, Deng HX, Gao YF, Chen QY et al. Expression of immune-related molecules in primary EBV-positive Chinese nasopharyngeal carcinoma: associated with latent membrane protein 1 (LMP1) expression. Cancer Biol Ther 2007; 6: 1997–2004. [DOI] [PubMed] [Google Scholar]
- Li JH, Chia M, Shi W, Ngo D, Strathdee CA, Huang D et al. Tumor-targeted gene therapy for nasopharyngeal carcinoma. Cancer Res 2002; 62: 171–178. [PubMed] [Google Scholar]
- Farwell DG, McDougall JK, Coltrera MD. Expression of Epstein-Barr virus latent membrane proteins leads to changes in keratinocyte cell adhesion. Ann Otol Rhinol Laryngol 1999; 108: 851–859. [DOI] [PubMed] [Google Scholar]
- Longnecker R. Epstein-Barr virus latency: LMP2, a regulator or means for Epstein-Barr virus persistence? Adv Cancer Res 2000; 79: 175–200. [DOI] [PubMed] [Google Scholar]
- Sutkowski N, Chen G, Calderon G, Huber BT. Epstein-Barr virus latent membrane protein LMP-2A is sufficient for transactivation of the human endogenous retrovirus HERV-K18 superantigen. J Virol 2004; 78: 7852–7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Pagano JS. Interferon regulatory factor 7: a key cellular mediator of LMP-1 in EBV latency and transformation. Semin Cancer Biol 2001; 11: 445–453. [DOI] [PubMed] [Google Scholar]
- Pang MF, Lin KW, Peh SC. The signaling pathways of Epstein-Barr virus-encoded latent membrane protein 2A (LMP2A) in latency and cancer. Cell Mol Biol Lett 2009; 14: 222–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch DT, Zimmerman JS, Rowe DT. Epstein-Barr virus latent membrane protein 2B (LMP2B) co-localizes with LMP2A in perinuclear regions in transiently transfected cells. J Gen Virol 2002; 83(Pt 5): 1025–1035. [DOI] [PubMed] [Google Scholar]
- Allen MD, Young LS, Dawson CW. The Epstein-Barr virus-encoded LMP2A and LMP2B proteins promote epithelial cell spreading and motility. J Virol 2005; 79: 1789–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Piontkivska H. Discovery of novel targets for multi-epitope vaccines: screening of HIV-1 genomes using association rule mining. Retrovirol 2009; 6: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depil S, Moralès O, Castelli FA, Delhem N, François V, Georges B et al. Determination of a HLA II promiscuous peptide cocktail as potential vaccine against EBV latency II malignancies. J Immunother 2007; 30: 215–226. [DOI] [PubMed] [Google Scholar]
- Lalonde A, Avila-Cariño J, Caruso M, de Campos-Lima PO. Rescue of the immunotherapeutic potential of a novel T cell epitope in the Epstein-Barr virus latent membrane protein 2. Virology 2007; 361: 253–262. [DOI] [PubMed] [Google Scholar]
- Straathof KC, Leen AM, Buza EL, Taylor G, Huls MH, Heslop HE et al. Characterization of latent membrane protein 2 specificity in CTL lines from patients with EBV-positive nasopharyngeal carcinoma and lymphoma. J Immunol 2005; 175: 4137–4147. [DOI] [PubMed] [Google Scholar]
- Wang B, Yao K, Liu G, Xie F, Zhou F, Chen Y. Computational prediction and identification of Epstein-Barr virus latent membrane protein 2A antigen-specific CD8+ T-cell epitopes. Cell Mol Immunol 2009; 6: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X1, Zhu S, Li W, Chen J, Ou Q, Zheng M et al. Identification and characterization of novel B-cell epitopes within EBV latentmembrane protein 2 (LMP2). Viral Immunol 2011; 24: 227–236. [DOI] [PubMed] [Google Scholar]
- Li W1, Chen Q, Chen H, Rao P, Xue X, Chen S et al. Immune response of mice to a latency membrane protein 2 multiepitope antigen of Epstein-Barr virus applied as DNA vaccine and/or peptide vaccine. Acta Virol 2013; 57: 51–58. [DOI] [PubMed] [Google Scholar]
- Lennette ET, Ward E, Henle G, Henle W et al. Detection of antibodies to Epstein-Barr virus capsid antigen by immune adherence hemagglutination. J Clin Microbiol 1982; 15: 69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao DH, Feng XG. Prediction for helper T cell epitopes and its application in vaccine development against parasite infection. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2008; 26: 228–233. [PubMed] [Google Scholar]
- Freund NT, Enshell-Seijffers D, Gershoni JM. Phage display selection, analysis, and prediction of B cell epitopes. Curr Protoc Immunol 2009; Chapter 9: Unit 9.8. [DOI] [PubMed] [Google Scholar]
- Reimer U. Prediction of linear B-cell epitopes. Methods Mol Biol 2009; 524: 335–344. [DOI] [PubMed] [Google Scholar]
- Saha S, Raghava GP. Prediction methods for B-cell epitopes. Methods Mol Biol 2007; 409: 387–394. [DOI] [PubMed] [Google Scholar]
- Geourjon C, Deléage G. SOPMA: significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Comput Appl Biosci 1995; 11: 681–684. [DOI] [PubMed] [Google Scholar]
- Garnier J, Gibrat JF, Robson B. GOR method for predicting protein secondary structure from amino acid sequence. Methods Enzymol 1996; 266: 540–553. [DOI] [PubMed] [Google Scholar]
- Hirokawa T, Boon-Chieng S, Mitaku S. SOSUI: classification and secondary structure prediction system for membrane proteins. Bioinformatics 1998; 14: 378–379. [DOI] [PubMed] [Google Scholar]
- Hopp TP, Woods KR. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A 1981; 78: 3824–3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JM, Eliezer N, Simha R. The characterization of amino acid sequences in proteins by statistical methods. J Theor Biol 1968; 21: 170–201. [DOI] [PubMed] [Google Scholar]
- Jameson BA, Wolf H. The antigenic index: a novel algorithm for predicting antigenic determinants. Comput Appl Biosci 1988; 4:181–186. [DOI] [PubMed] [Google Scholar]
- Kolaskar AS, Tongaonkar PC. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett 1990; 276: 172–174. [DOI] [PubMed] [Google Scholar]
- Ito Y, Kondo E, Demachi-Okamura A, Akatsuka Y, Tsujimura K, Tanimoto M et al. Three immunoproteasome-associated subunits cooperatively generate a cytotoxic T-lymphocyte epitope of Epstein-Barr virus LMP2A by overcoming specific structures resistant to epitope liberation. J Virol 2006; 80: 883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SP, Tierney RJ, Thomas WA, Brooks JM, Rickinson AB. Conserved CTL epitopes within EBV latent membrane protein 2: a potential target for CTL-based tumor therapy. J Immunol 1997; 158: 3325–3334. [PubMed] [Google Scholar]
- Marastoni M, Bazzaro M, Gavioli R, Micheletti F, Traniello S, Tomatis R. Design of dimeric peptides obtained from a subdominant Epstein-Barr virus LMP2-derived epitope. Eur J Med Chem 2000; 35: 593–598. [DOI] [PubMed] [Google Scholar]
- Carter JJ, Wipf GC, Hagensee ME, McKnight B, Habel LA, Lee SK et al. Use of human papillomavirus type 6 capsids to detect antibodies in people with genital warts. J Infect Dis 1995; 172: 11–18. [DOI] [PubMed] [Google Scholar]
- Heim K, Christensen ND, Hoepfl R, Wartusch B, Pinzger G, Zeimet A et al. Serum IgG, IgM, and IgA reactivity to human papillomavirus types 11 and 6 virus-like particles in different gynecologic patient groups. J Infect Dis 1995; 172: 395–402. [DOI] [PubMed] [Google Scholar]
- Marais DJ, Rose RC, Lane C, Kay P, Nevin J, Denny L, et al. Seroresponses to human papillomavirus types 16, 18, 31, 33, and 45 virus-like particles in South African women with cervical cancer and cervical intraepithelial neoplasia. J Med Virol 2000; 60: 403–410. [DOI] [PubMed] [Google Scholar]
- De Paschale M, Clerici P. Serological diagnosis of Epstein-Barr virus infection: problems and solutions. World J Virol 12 February 2012; 1: 31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong B, Peng G, Berry L, Gottschalk S, Jung JU, Chen SY et al. Generating CTLs against the subdominant EBV LMP antigens by transient expression of an A20 inhibitor with EBV LMP proteins in human DCs. Gene Ther 2012; 19: 818–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SP, Thomas WA, Blake NW, Rickinson AB. Transporter (TAP)-independent processing of a multiple membrane-spanning protein, the Epstein-Barr virus latent membrane protein 2. Eur J Immunol 1996; 26: 1875–1883. [DOI] [PubMed] [Google Scholar]
- Khanna R, Burrows SR, Nicholls J, Poulsen LM. Identification of cytotoxic T cell epitopes within Epstein-Barr virus (EBV) oncogene latent membrane protein 1 (LMP1): evidence for HLA A2 supertype-restricted immune recognition of EBV-infected cells by LMP1-specific cytotoxic T lymphocytes. Eur J Immunol 1998; 28: 451–458. [DOI] [PubMed] [Google Scholar]
- Okugawa K, Itoh T, Kawashima I, Takesako K, Mazda O, Nukaya I et al. Recognition of Epstein-Barr virus-associated gastric carcinoma cells by cytotoxic T lymphocytes induced in vitro with autologous lymphoblastoid cell line and LMP2-derived, HLA-A24-restricted 9-mer peptide. Oncol Rep 2004; 12: 725–731. [PubMed] [Google Scholar]
- Lautscham G, Haigh T, Mayrhofer S, Taylor G, Croom-Carter D, Leese A et al. Identification of a TAP-independent, immunoproteasome-dependent CD8+ T-cell epitope in Epstein-Barr virus latent membrane protein 2. J Virol 2003; 77: 2757–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münz C, Moormann A. Immune escape by Epstein Barr virus associated malignanci. Semin Cancer Biol 2008; 18: 381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depil S, Moralès O, Castelli FA, Delhem N, François V, Georges B et al. Determination of a HLA II promiscuous peptide cocktail as potential vaccine against EBV latency II malignancies. J Immunother 2007; 30: 215–226. [DOI] [PubMed] [Google Scholar]
- Toure-Balde A, Perlaza BL, Sauzet JP, Ndiaye M, Aribot G, Tall A et al. Evidence for multiple B- and T-cell epitopes in Plasmodium falciparum liver-stage antigen 3. Infect Immun 2009; 77: 1189–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Wang HN, Lu D, Zhang YF, Wang T, Kang RM. The immunoreactivity of a chimeric multi-epitope DNA vaccine against IBV in chickens. Biochem Biophys Res Commun 2008; 377: 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou WY, Shi Y, Wu C, Zhang WJ, Mao XH, Guo G. Therapeutic efficacy of a multi-epitope vaccine against Helicobacter pylori infection in BALB/c mice model. Vaccine 2009; 27: 5013–5019. [DOI] [PubMed] [Google Scholar]
- Liu S, Shi L, Cheng YB, Fan GX, Ren HX, Yuan YK. Evaluation of protective effect of multi-epitope DNA vaccine encoding six antigen segments of Toxoplasma gondii in mice. Parasitol Res 2009; 105: 267–274. [DOI] [PubMed] [Google Scholar]
- Lin CL, Lo WF, Lee TH, Ren Y, Hwang SL, Cheng YF et al. Immunization with Epstein-Barr Virus (EBV) peptide-pulsed dendritic cells induces functional CD8+ T-cell immunity and may lead to tumor regression in patients with EBV-positive nasopharyngeal carcinoma. Cancer Res 2002; 63: 6952–6958. [PubMed] [Google Scholar]