Abstract
Bacterial flagellin is a unique pathogen-associated molecular pattern (PAMP), which can be recognized by surface localized Toll-like receptor 5 (TLR5) and the cytosolic NOD-like receptor (NLR) protein 4 (NLRC4) receptors. Activation of the TLR5 and/or NLRC4 signaling pathways by flagellin and the resulting immune responses play important roles in anti-bacterial immunity. However, it remains unclear how the dual activities of flagellin that activate the TLR5 and/or NLRC4 signaling pathways orchestrate the immune responses. In this study, we assessed the effects of flagellin and its mutants lacking the ability to activate TLR5 and NLRC4 alone or in combination on the adaptive immune responses against flagellin. Flagellin that was unable to activate NLRC4 induced a significantly higher antibody response than did wild-type flagellin. The increased antibody response could be eliminated when macrophages were depleted in vivo. The activation of NLRC4 by flagellin downregulated the flagellin-induced and TLR5-mediated immune responses against flagellin.
Keywords: flagellin, NLRC4, TLR5, macrophage, adaptive immunity
INTRODUCTION
Bacterial flagellin is one of a small number of protein pathogen-associated molecular patterns (PAMPs), which can be recognized by cell surface Toll-like receptor 5 (TLR5)1 and the cytosolic NOD-like receptor protein 4 (NLRC4) inflammasome receptor NAIP5.2,3 Flagellin consists of two highly conserved domains (D0 and D1) and one central hypervariable domain (D2/D3).4,5,6 The conserved D0 and D1 domains are required for the immune activity of flagellin as a PAMP. The N-terminal amino acids 90–97 (QRVRELAV) of D1 form a highly conserved motif that is essential for both high-affinity binding and signaling to TLR5.7,8 The C-terminal 35 amino acids of flagellin in the D0 domain are responsible for the interaction between flagellin and NLRC4. The substitution of three conserved leucine residues L502, L504, and L505 for alanine in the 35 C-terminal amino acids could abolish the ability of flagellin to activate NLRC4.9 Flagellin-mediated activation of TLR5 activates inflammatory genes via the MyD88 pathway, whereas flagellin-activated NLRC4 triggers the assembly of the inflammasome, which culminates in caspase-1 activation, IL-1β/IL-18 secretion, and cellular pyroptosis.10,11
As an important virulence factor in pathogenic bacteria, flagellin might regulate their virulence and pathogenesis.12,13 During bacterial infections, shedding flagellin plays multiple roles as an immune target in the interactions between the pathogen and the host immune system. First, flagellin has been shown to be the dominant pro-inflammatory determinant and regulates pro-inflammatory gene expression when pathogenic bacteria such as Salmonella invade and infect gut epithelial cells.14 Second, flagellin activates TLR5 and/or NLRC4, which might elicit different innate and adaptive immune responses qualitatively and quantitatively.15 Finally, flagellin is a potent immunogen that can induce strong adaptive immune responses against itself.16,17,18
Innate immunity is responsible for detecting bacterial invasion and infection by using the dual sensors TLR5 and NLRC4 to recognize flagellin, and then initiating immune responses to protect the host from infection. The activation of these sensors might have significantly different outputs depending on the structural properties, amount released, and distribution of flagellin. However, it is unclear whether the activation of TLR5 and/or NLRC4 is beneficial to the host in its defense against bacterial infection. For example, TLR5-deficient mice are less prone to infection with Salmonella than are wild-type (WT) mice,19 and NLRC4 activation might protect mice from the mucosal and systemic dissemination of salmonella.20 But, Letran et al. observed no significant difference in the susceptibility of mice to bacterial infection in WT and TLR5-deficient mice.21 On the other hand, Salmonella could downregulate NLRC4 expression to prevent the inflammasome response and thereby promote bacterial persistence and dissemination.22 NLRC4-dependent IL-1β production by intestinal phagocytes could discriminate pathogenic from commensal bacteria, thereby contributing to the immune defense against enteric bacterial infection.10 Therefore, the activation of both of TLR5 and NLRC4 by flagellin should be taken into consideration regarding the interaction between pathogenic bacteria and immune responses. Further studies on the interaction and cross-talk between TLR5 and NLRC4 will be valuable to increase understanding of the complexities of the innate immune recognition of flagellated pathogens and also critical for the reasonable design of flagellin-based vaccines.
In the present study, we assessed the ability of flagellin and its mutants lacking ability to activate either TLR5 or NLRC4, or both TLR5 and NLRC4 to stimulate the immune responses against flagellin. Abolishing NLRC4 activation by flagellin increased the antibody responses against flagellin significantly compared with WT flagellin. This increased antibody response could be eliminated when macrophages were depleted in mice. These data revealed an interaction between NLRC4 and TLR5-mediated activity that affects the immune responses against flagellin. It is possible that NLRC4-mediated activation negatively regulates the TLR5-mediated immune response against flagellin due to the decreased amount of macrophages and corresponding cytokines secretion.
MATERIALS AND METHODS
Animals
Female C57BL/6 mice aged 6–8 weeks were purchased from Beijing Laboratory Animal Research Center and housed under specific pathogen-free (SPF) conditions in the Animal Center of Wuhan Institute of Virology (WIV), Chinese Academy of Sciences (CAS). All animals were divided randomly into different groups before the immunization experiments. Animal studies were performed according to Regulations for the Administration of Affairs Concerning Experimental Animals in China (1988), and the Guidelines for Animal Care and Use, WIV, CAS (permit number WIVA09201203). All animal studies and methods conformed to ARRIVE guidelines.
Construction, expression, and purification of recombinant FliC, FliCΔ90-97, FliC-L3A, and FliCΔ90-97:L3A
WT flagellin protein and its three modified proteins FliC (WT), FliCΔ90-97 (unable to activate TLR5), FliC-L3A (unable to activate NLRC4 due to mutation of C-terminal L502, L504, and L505 to A), and FliCΔ90-97:L3A (unable to activate both TLR5 and NLRC4) were constructed as described previously.23 Appropriate oligonucleotide primers containing restriction enzyme sites were designed based upon the full-length fiagellin gene fliC from Salmonella enterica subsp. (GenBank Accession No. 1070204) to construct truncated, deleted, and/or chimeric fliC.24 The DNA fragments were PCR amplified and cloned into pET-28a(+) plasmid (Invitrogen/Life Technologies, Carlsbad, CA, USA). All recombinant plasmids were transformed into competent E. coli BL21 (DE3), selected, and their sequences were confirmed using DNA sequencing (Invitrogen/Life Technologies).
Transformed E. coli BL21 (DE3) containing recombinant flagellin expression constructs were grown and induced as described previously.24 The resulting recombinant proteins were prepared and purified using affinity chromatography on a Ni-NTA column (Qiagen, Venlo, Limburg, the Netherlands). The concentrations of the purified proteins were determined using a Bradford protein assay. The purified proteins were verified by western blotting using an anti-His-tag monoclonal antibody (Qiagen) followed by a secondary alkaline phosphatase (AP)-conjugated goat anti-mouse IgG antibody (Southern Biotech). The antibody–antigen complexes were visualized using SuperSignal West Pico Chemiluminescent Substrate (Pierce/Thermo Fisher Scientific, Waltham, MA, USA) and imaged on a Versadoc 3000 imager (Bio-Rad, Hercules, CA, USA).
Lipopolysaccharide (LPS) was removed from the purified recombinant proteins using the Affinity Pak Detoxi Gel Endotoxin Removing gel (Pierce), and the residual LPS content of the protein was determined using a Limulus assay (Associates of Cape Cod, East Falmouth, MA, USA). The endotoxin value of the purified recombinant flagellin preparation was <0.03 EU/mg. Any contamination with other PAMPs was assessed using the purified proteins to stimulate the flagellin-nonresponsive RAW264.7 cells25,26 overnight at the concentration of 4000 nM.
MCP-1 and IL-8 release by Caco-2 cells
Caco-2 cells were maintained in the Dulbecco's modified Eagle medium (Invitrogen/Life Technologies) supplemented with 10% fetal bovine serum (Gibco/Life Technologies, Carlsbad, CA, USA). The cells were seeded at a density of 1 × 105/well in 48-well plates (Costar) and incubated at 37 °C in an atmosphere containing 5% CO2. For IL-8 and MCP-1 release assays, Caco-2 cells were cultured for 5 days. After an overnight cultivation in media without FBS, cells were stimulated with different concentrations of recombinant flagellin proteins for 6 h. The supernatants were collected and IL-8 and MCP-1 levels were quantified using enzyme-linked immunosorbent assay (ELISA) kits (BD Biosciences, Franklin Lakes, NJ, USA). The experiment was repeated at least three times.
Preparation of splenocytes, peritoneal cells, and peritoneal macrophages
Splenocyte suspensions were prepared by homogenization and cultured as described previously.27 Briefly, spleens were obtained from 6- to 8-week-old female C57BL/6 mice, smashed using syringe pistons in phosphate-buffered saline (PBS), filtered through 70-μm cell strainers (Becton Dickinson, Franklin Lakes, NJ, USA), and then resuspended in RPMI1640 containing 10% FBS. Peritoneal cells were isolated from naive animals by collecting peritoneal washes, and then resuspended in RPMI 1640 containing 10% FBS.28,29 Peritoneal cells were seeded into 96-well plates at a density of 3 × 105 cells per well and cultured overnight. Macrophages were prepared by gentle washing peritoneal cells with RPMI1640 to remove the non-adhered cells.
Stimulating macrophages with flagellin
It had been reported that LPS pretreatment of macrophages allowed a robust IL-1β released. So, macrophages were pretreated with 50 ng/ml LPS for 3 h, and then stimulated with flagellin using or no using DOTAP transfection (Roche Diagnostics, BASEL, Switzerland).27,29 Briefly, 15 μl of DOTAP liposomal transfection reagent was incubated with 5 μg of purified flagellin for 20 min in serum-free media, and then 1 ml of RPMI 1640 medium was added. For no DOTAP transfection, 100 nM flagellin was added to macrophages directly in a 96-well plate, and supernatants were collected after 6 h to measure IL-1β and IL-18 levels. For LDH release assays, macrophages were stimulated directly with 1000 nM flagellin without DOTAP transfection for 20 h, and then analyzed using the Cytotoxicity Detection Kit PLUS (LDH) (Roche Diagnostics). The experiments were repeated at least three times.
Cytokine detection
The concentrations of IL-8 and MCP-1 secreted by Caco-2 cells, IL-1β, and IL-18 secreted by macrophages, and MCP-1, KC, IL-6, IL-1β, and IL-18 in mouse serum was measured using ELISA according to the manufacturer's instructions. Human IL-8 and MCP-1 ELISA kits were purchased from BD Biosciences, and mouse MCP-1, KC, IL-6, IL-1β, and IL-18 ELISA kits were purchased from eBioscience (San Diego, CA, USA).
Clodronate- and PBS-liposome preparation and macrophage depletion
Clodronate-liposomes (Clo-LP) were prepared as described previously.30 Briefly, 25 mg cholesterol (Sigma, St Louis, MO, USA, C8667) was dissolved in 10 ml chloroform, and 100 mg l-α-phosphatidylcholine (Sigma, P3556) was dissolved in 1 ml chloroform. Next, 500 μl phosphatidylcholine and 5 ml cholesterol were mixed, and evaporated slowly (58 mbar, 150 rpm, 40 °C) until a thin phospholipid film formed. Then, 6 ml PBS was added to prepare PBS-liposomes, or clodronate solution (Sigma, D4434; 200 mg/ml in PBS) was added to prepare Clo-LP. The mixture was exposed to N2 gas and incubated for 2 h at room temperature with gentle shaking (100 rpm), and sonicated in 55 kHz for 5 min, then incubated for another 2 h to allow liposome swelling. Finally, the solution was centrifuged at 10,000g for 15 min. The liposome-encapsulated clodronate or PBS-liposomes were collected and washed twice with sterile PBS, and then stored at 4 °C until use. For macrophage depletion, 100 μl Clo-LP was injected intraperitoneally (i.p.) into mice, and macrophage-depleted mice were used in experiments 1 day later.
Mice immunization, sample preparation, and antibody detection
Female C57BL/6 mice were injected i.p. with recombinant flagellin (FliC or mutants, 10 μg in 100 μl PBS) and/or HIV p24 protein (5 μg) twice at 4-week intervals. Serum was collected from each mouse on day 21 after immunization and stored at −20 °C until antibody analysis using ELISA. Briefly, 96-well ELISA plates were coated with 100 μl FliC or p24 (3 μg/ml) in coating buffer overnight at 4 °C. Sera from the individual mice were serially diluted in PBS containing 1% newborn calf serum (NCS), and then analyzed using AP-labeled goat anti-mouse IgG, (Southern Biotechnology), IgG1, IgG2a, or IgG2b (BETHYL) polyclonal antibodies followed by substrate p-nitrophenyl phosphate (PNPP) (Sigma). The absorbance at 405 nm was read using a microplate reader (Thermo Labsystems). The reciprocal values of the last dilution giving an OD reading that was twice the background of empty wells were determined as the titer.
Flow cytometry for cell surface staining
Cell surface staining was performed using BD Biosciences or eBioscience reagents. Dendritic cells (DCs) or macrophages in splenocytes and peritoneal cell cultures were characterized using anti-CD11c (clone N418) or anti-F4/80 (clone BM8) antibodies, respectively. For cell surface staining, cells were blocked using anti-CD16/32 (BD Biosciences) first, then stained with anti-CD86 (clone GL1), anti-CD80 (clone 16-10A1), and anti-MHCII (M5/114.15.2) antibodies followed by 7-amino-actinomycin D (7-AAD) (BD Biosciences). Flow cytometry data were acquired on a FACS AriaIII flow cytometer (BD Biosciences), and 100 000–150 000 lymphocyte-gated events were collected per sample. Analyses were performed using the FlowJo (Tree Star, Ashland, OR, USA) software.
Statistical analysis
All data were analyzed using one-way analysis of variance for more than three groups, or unpaired Student's t-tests for comparing two groups using Graphpad Prism software (version 5.0). Statistical significance is indicated by *p < 0.05, **p < 0.01, ***p < 0.001, and “n.s.” (p ≥ 0.05). A p-value < 0.05 was considered to be statistically significant.
RESULTS
Preparation of flagellins and verification of their TLR5- and/or NLRC4-mediated activities
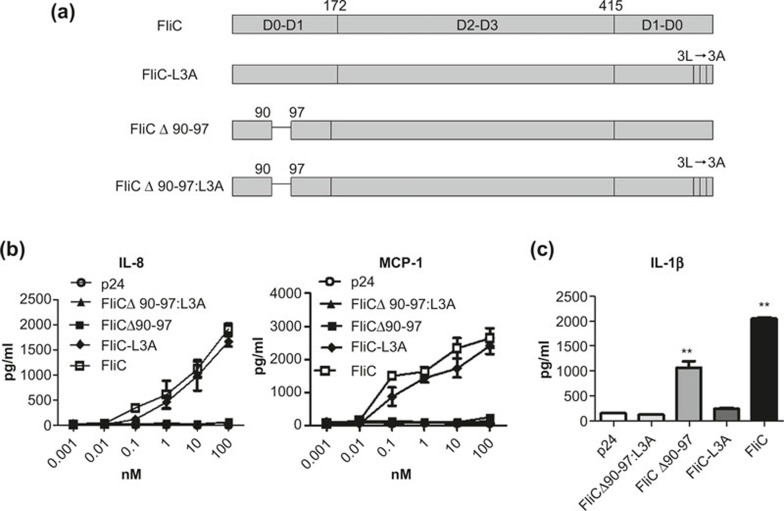
Flagellin can activate innate immunity by stimulating the TLR5 or/and NLRC4 pathways. To evaluate the cross-talk and interaction between TLR5 and NLRC4 signaling during specific immune responses we constructed recombinant WT and three variant flagellin proteins: FliC, FliCΔ90-97, FliC-L3A, and FliCΔ90-97:L3A (Figure 1a).23 To assess the ability of recombinant flagellin proteins to activate the TLR5 signal pathway, the epithelial cell line Caco-2 (which expresses TLR5 constitutively) was used to test the efficacy of TLR5 agonists by measuring IL-8 and MCP-1 secretion. Recombinant HIV-1 p24 protein, prepared using the same method as flagellin, was used as a negative control. FliC-L3A induced IL-8 and MCP-1 secretion in a dose-dependent manner, similar to WT FliC. Meanwhile, FliCΔ90-97, FliCΔ90-97:L3A, and P24 did not induce detectable levels of either IL-8 or MCP-1 (Figure 1b). This confirmed that the flagellin variants FliCΔ90-97 and FliCΔ90-97:L3A lost their TLR5 agonist activity, as expected. To determine whether the recombinant flagellin proteins could activate the NLRC4 signaling pathway, peritoneal macrophages were isolated from naive mice and analyzed ex vivo, as described previously.29 As shown in Figure 1c, FliCΔ90-97 induced significant IL-1β secretion, although with lower efficacy than did FliC. FliC-L3A and FliCΔ90-97:L3A did not induce detectable IL-1β secretion, even at a high concentration of 100 nM, which confirmed that both FliC-L3A and FliCΔ90-97:L3A had lost their NLRC4 agonist activity. Therefore, FliCΔ90-97 lost TLR5 but retained NLRC4 agonist activity, FliC-L3A lost NLRC4 but retained TLR5 agonist activity, and FliCΔ90-97:L3A lost both TLR5 and NLRC4 agonist activity, as expected.
Figure 1.
Generation and characterization of recombinant flagellin proteins. (a) Schematic representation of the recombinant WT and variant proteins used in this study. (b) IL-8 and MCP-1 were detected to confirm the bioactivity of the recombinant proteins on TLR5 activation. (c) Peritoneal macrophages were stimulated and analyzed using an IL-1β assay to confirm the effects of the recombinant proteins on NLRC4 activation. Data are presented as means ± SEM from triplicates of one experiment that was repeated at least three times.
FliC-L3A, which lacks the ability to activate NLRC4, induces stronger antibody responses than does FliC
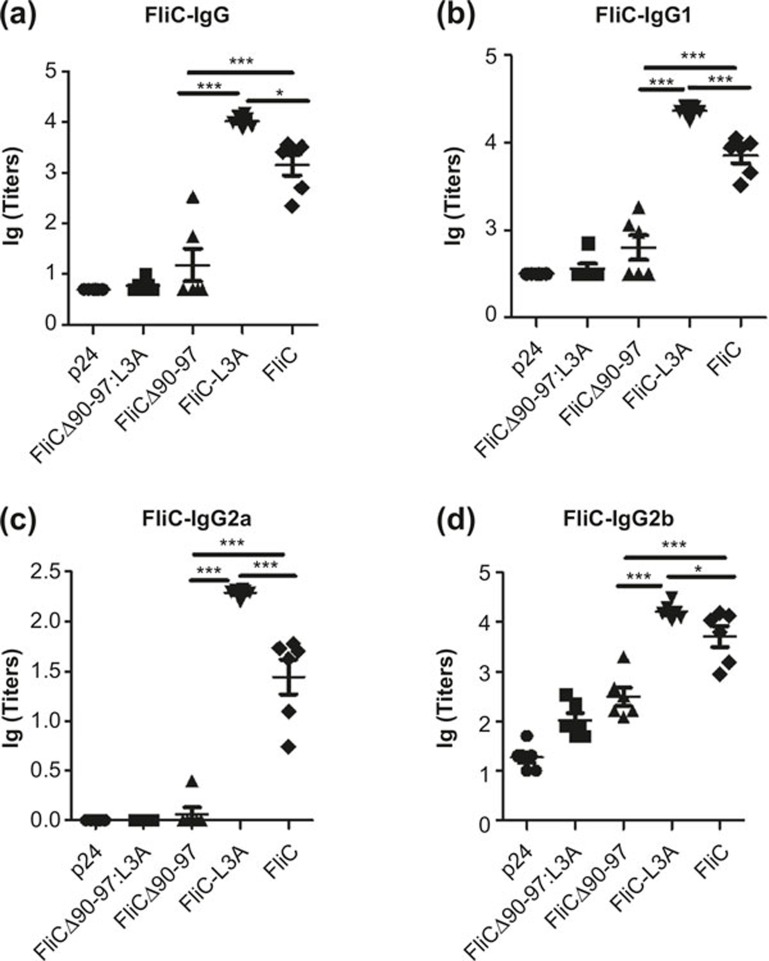
Because innate immune signaling instructs the development of antigen-specific adaptive immune responses,31 we speculated that cross-talk and interaction between the TLR5 and NLRC4 pathways might further affect adaptive immune responses. Therefore, mice were immunized i.p. with FliCΔ90-97:L3A, FliCΔ90-97, FliC-L3A, FliC, or p24, and the antibody responses against flagellin were then tested. Data revealed that FliCΔ90-97:L3A and FliCΔ90-97 did not induce significant IgG responses against flagellin in serum compared with FliC-L3A and FliC (Figure 2a). This suggests that TLR5-stimulating activity was important for activating the antibody response against flagellin, as observed previously,32 whereas the NLRC4-stimulating activity contributed much less to the antibody response against flagellin. Interestingly and surprisingly, FliC-L3A induced a significantly higher IgG response than did FliC (Figure 2a). Specifically, FliC-L3A (which activated TLR5 but not NLRC4) induced higher IgG1, IgG2a, and IgG2b responses against flagellin than did FliC (Figure 2b–d). This suggests that NLRC4 activation might downregulate the TLR5-mediated adaptive immune responses against flagellin. This difference in antibody responses is maintained at later time points and is independent of immunization routes (data not shown). This suggests that the NLRC4 activity of flagellin had a negative effect on the TLR5-mediated antibody responses. Further investigation of the interaction and cross-talk between NLRC4- and TLR5-mediated activity might help us to understand the complexities of the immune recognition and responses initiated by this multi-signaling PAMP protein.
Figure 2.
Flagellin-specific antibody response activated via the NLRC4 or TLR5 pathway (a–d). C57BL/6 WT mice were immunized with 10 μg FliCΔ90-97:L3A, FliCΔ90-97, FliC-L3A, or FliC (six mice per group) on days 0 and 28. Sera were taken from each mouse on day 21, and FliCspecific IgG (a), IgG1 (b), IgG2a (c), and IgG2b (d) production was analyzed using ELISA. The results are presented as means ±6 SEM, and the data are representative of three independent experiments.
FliC induces IL-1β and IL-18 production and macrophage death via NLRC4 signaling in vivo
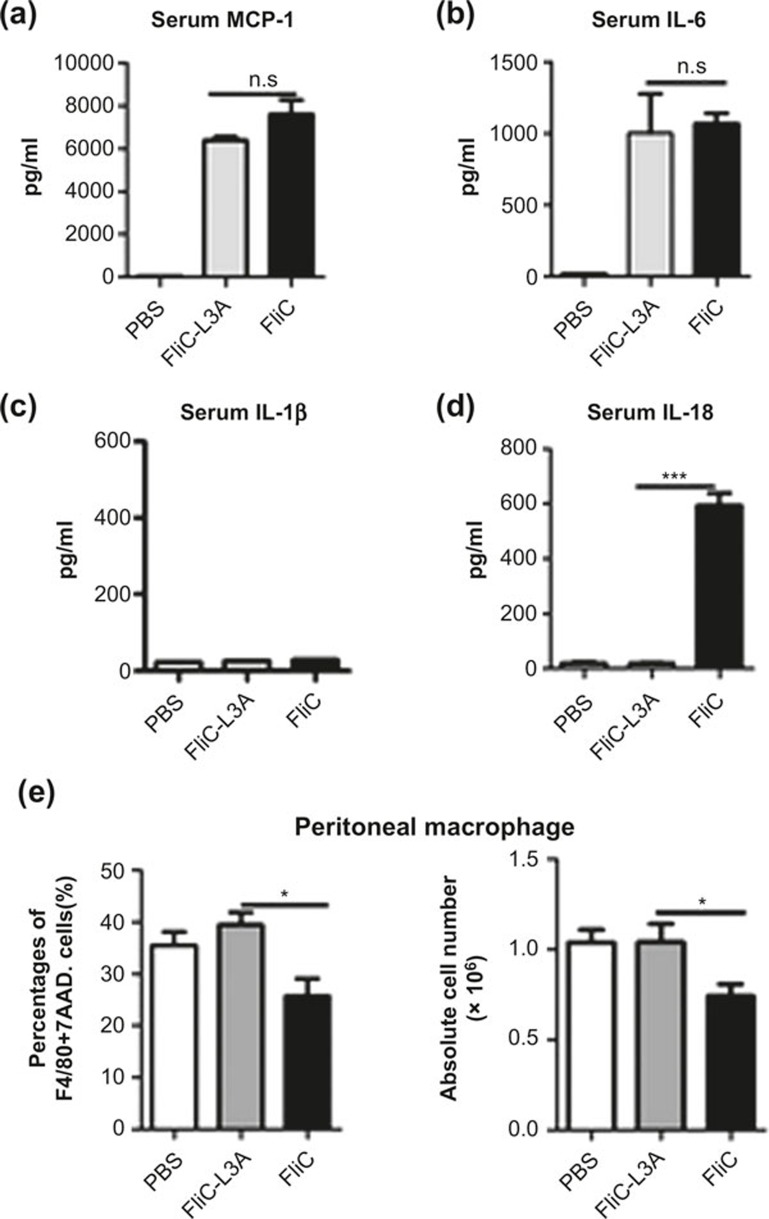
Since the NLRC4-mediated activity of flagellin might impair the TLR5-mediated immune responses, we next assessed whether NLRC4 activation modulated the TLR5-mediated specific immune responses against flagellin. First, we speculated that there might be differences in the cytokine production induced by FliC and FliC-L3A in vivo. To determine the serum cytokine responses, each mouse was injected i.p. with 10 μg protein for 3 h. As shown in Figure 3a–c, FliC-L3A induced a similar production of MCP-1, IL-6, and IL-1β as did FliC. In contrast, significant serum IL-18 production was detected in mice injected with FliC but not FliC-L3A (Figure 3d). These results suggest that the main difference between FliC and FliC-L3A on cytokine production in vivo was IL-18, which might be an important factor that downregulates the TLR5-mediated adaptive immune responses against flagellin.
Figure 3.
The effect of FliC and FliC-L3A on cytokine production and macrophage death (a–d). The ability of recombinant flagellin proteins to stimulate cytokine production was tested in vivo. Mouse sera were collected to analyze the cytokine response 3 h after the i.p. injection of 10 μg of FliC-L3A or FliC or PBS, and the concentrations of MCP-1 (a), IL-6 (b), IL-1β (c), and IL-18 (d) in serum were assayed using ELISA. The results are presented as means ± SEM, and the data are representative of three independent experiments. (e) C57BL/6 WT mice were injected i.p. with 10 μg FliC-L3A or FliC (four mice per group), and the percentage and absolute numbers of live macrophages in the peritoneal lavage fluids were detected 12 h later. The results are presented as means ± SEM, and the data are representative of three independent experiments.
Macrophages and DCs are two important antigen-presenting cells (APCs) that mediate the flagellin-specific adaptive immune response. Therefore, we speculated that there might be difference in the macrophage and DC activation stimulated by FliC and FliC-L3A. To test this hypothesis, we analyzed the effects of flagellin on the quantity and function of macrophages and DCs. It was reported that the NLRC4 activated by flagellin in macrophages might stimulate IL-1β secretion and cell death.29,33,34 Therefore, we isolated macrophages from naive C57BL/6 mice and stimulated them with FliC or FliC-L3A in vitro without LPS pretreatment or transfection for 20 h. The results showed that FliC induced significant IL-1β secretion and macrophage death compared with FliC-L3A or p24 (Figure S1a and b). These results are consistent with Figure 3d, where FliC could activate NLRC4 in the absence of LPS pretreatment or transfection in vivo.
To investigate whether FliC-L3A and FliC exert the same effect on macrophages in vivo, mice were treated with FliC-L3A or FliC by i.p. injection for 12 h, peritoneal cell and splenocytes were isolated, and the frequency of F4/80high macrophages in peritoneum or spleen were analyzed using flow cytometry. Data revealed that the frequency of macrophages was decreased with FliC, but not FliC-L3A, treatment in the peritoneal cavity. Moreover, the absolute number of peritoneal macrophages was decreased with FliC treatment, but not with FliC-L3A or PBS control (Figure 3e). The frequency and the absolute number of peritoneal macrophages in FliC-L3A-treated animals was ∼1.4-fold (38% vs. 26% and 1.04 × 106 vs. 0.74 × 106) (Figure 3e) higher than in the FliC-treated group. These results suggest that FliC treatment in vivo activated NLRC4 on macrophages, resulting in substantial damage to systemic and local macrophages. In contrast, FliC-L3A did not exhibit the same activity to decrease the number of macrophages. The same analysis was performed using DC cells, which revealed that both FliC-L3A and FliC exerted the same effects on increasing the frequency of splenic DCs compared with PBS control (Figure S2a).
Next, the effect of flagellin on the function of macrophages and DCs was evaluated using flow cytometry to detect the expression of cell surface markers. CD80 and MHCII expression on macrophages and DCs, which indicates T cell co-stimulating and antigen-presenting activity, respectively, was analyzed 12 h after the i.p. injection of FliC-L3A or FliC in mice. As shown in Figure S2b, FliC-L3A and FliC upregulated CD80 and MHCII on F4/80high peritoneal macrophages to a similar extent compared with PBS control. The same results were observed for F4/80+ splenic macrophages (Figure S2c) and splenic DCs (Figure S2d) in vivo. These results suggest that FliC-L3A and FliC could upregulate CD80 and MHCII expression in macrophages and DCs to a similar extent via TLR5. However, there were no IL-18 production and the decreased number of macrophage, which were due to the loss of NLRC4-mediated activity of FliC-L3A. This further suggests that the activation of NLRC4 might downregulate the TLR5-mediated adaptive immune response against flagellin, which might be mainly macrophage-associated.
Macrophage depletion in vivo abolishes the different immune responses induced by FliC-L3A and FliC
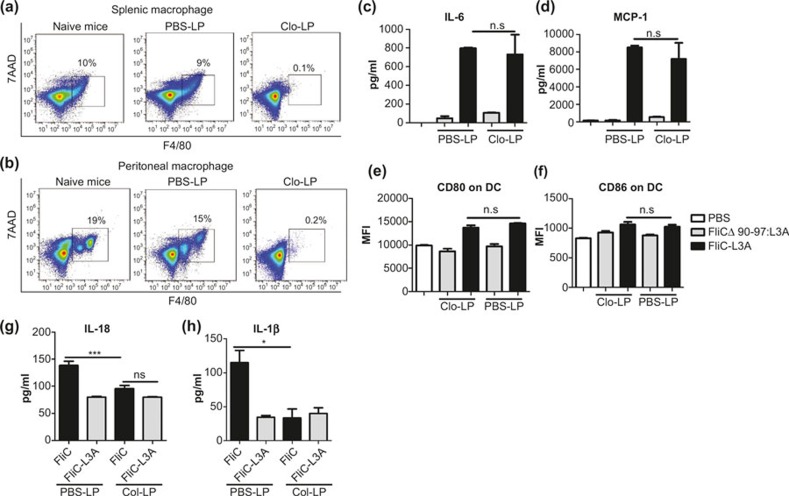
There are two possible explanations for the decreased number of macrophages. (1) The activation of NLRC4 resulted in the death of macrophages. (2) Macrophages departed from the peritoneal cavity. We hypothesized that the decreased number of peritoneal macrophages and macrophage-secreted cytokines might be associated with the NLRC4-stimulated downregulation of the TLR5-mediated adaptive immune response. To confirm this hypothesis, we tested the immune response of mice in which macrophages had been depleted using Clo-LP. Clo-LP is a highly efficient reagent for depleting macrophages. Specifically, it is swallowed by macrophages and induces their apoptosis when the intracellular clodronate concentration reaches a certain level; it has no effect on DCs or neutrophils.35,36 Macrophages were decreased rapidly both in the spleen (Figure 4a) and peritoneal cavity (Figure 4b) after the i.p. injection of Clo-LP, and their number remained very low for at least 3 days. TLR5-dependent cytokine secretion (Figure 4c and d) and DC activation (Figure 4e and f) were unchanged by macrophage depletion, whereas the levels of IL-18 (Figure 4g) and IL-1β (Figure 4h) in the peritoneal washes were decreased markedly. Macrophage-depleted mice were used to clarify that the different effects of FliC and FliC-L3A on the specific immune response against flagellin were macrophage-dependent.
Figure 4.
Depleting macrophages using Clo-LP abolishes the NLRC4- but not TLR5-mediated innate immune response. (a, b) Clo-LP depleted macrophages effectively. Splenocytes or peritoneal cells was isolated from C57BL/6 mice 1 day after treatment with Clo-LP, respectively, stained with F4/80-APC and 7AAD and analyzed using Accuri C6 (BD Biosciences). Naive and PBS-LP-treated mice were used as controls. (c–f) The depletion of macrophages did not abolish the TLR5-mediated innate immune response. Macrophage-depleted WT C57BL/6 mice were immunized i.p. with 10 μg FliCΔ90-97:L3A or FliC-L3A, and serum IL-6 (c) and MCP-1 (d) levels were assayed after 4 h using ELISA. Twenty-four hours later, splenocytes were isolated and CD80 (e) and CD86 (f) expression on CD11+ DCs were detected using Accuri C6 (BD Biosciences; n = 4). The results are presented as means ± SEM, and the data are representative of three independent experiments. (g, h) The effect of macrophage depletion on cytokine levels in the peritoneal lavage fluid via activation of the NLRC4 pathway. PBS-LP- or Clo-LP-pretreated C57BL/6 mice were injected i.p. with 10 μg FliC or FliC-L3A. Four hours later 600 μl PBS was injected into the abdominal cavity, and peritoneal lavage fluid was collected from each mouse and assayed for IL-18 (g) and IL-1β (h) levels (n = 4). The results are presented as means ± SEM, and the data are representative of three independent experiments.
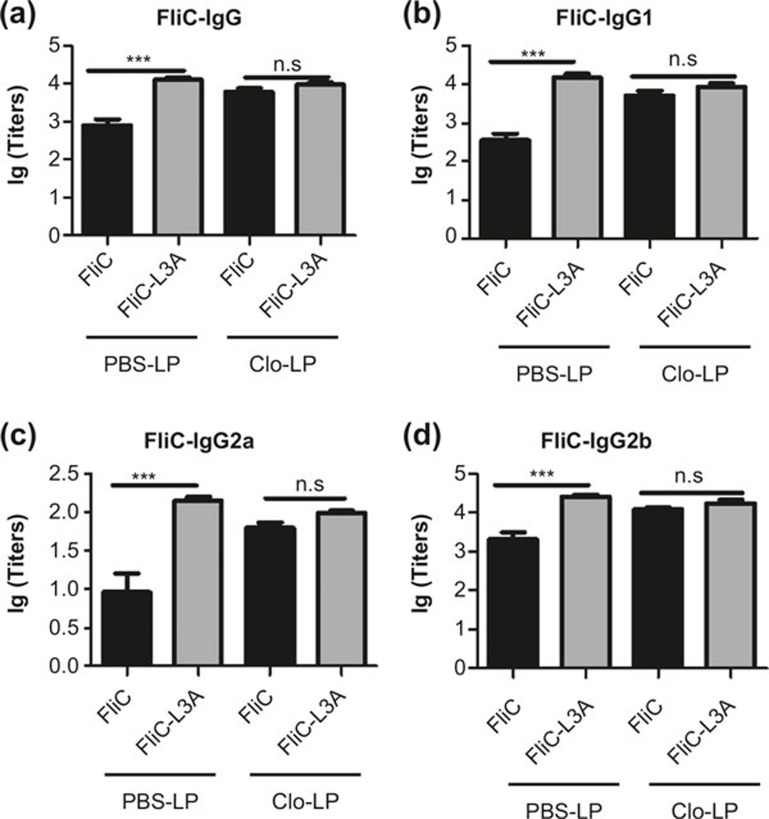
PBS-LP and Clo-LP treated mice were immunized with FliC or FliC-L3A, and the flagellin-specific humoral immune response was then analyzed. Data revealed that FliC-L3A and FliC induced similar immune response patterns against flagellin in mice treated with PBS-LP (Figure 5/PBS-LP) as without PBS-LP (Figure 2), indicating that PBS-LP had no significant effect on the FliC- or FliC-L3A-induced immune response. The difference in the flagellin-specific antibody response induced by FliC and FliC-L3A was eliminated in mice lacking macrophages (Figure 5/Clo-LP). Interestingly, the amount of FliC-induced flagellin-specific IgG subtypes, including total IgG, IgG1, IgG2a, and IgG2b, increased and reached a similar level as those induced by FliC-L3A (Figure 5a–d). These results suggested that depleting macrophages in mice abolished the differences in the immune responses induced by the flagellin variants FliC-L3A and FliC, which further confirmed that macrophages were the main effector cell that suppressed the TLR5-mediated adaptive immune response against flagellin by activating NLRC4.
Figure 5.
The different flagellin-specific antibody responses induced by FliC-L3A and FliC were abolished after macrophage depletion. (a–d) Macrophage-depleted C57BL/6 mice and PBS-LP pretreated mice were immunized with 10 μg FliC-L3A or FliC (five mice per group). Sera were collected from each mouse on day 21 after immunization, and flagellin-specific IgG (a), IgG1 (b), IgG2a (c), and IgG2b (d) levels in serum were analyzed using ELISA. The results are presented as means ± SEM, and the data are representative of three independent experiments (n = 5).
DISCUSSION
Flagellin is a unique bacterial structural protein. It is a PAMP that can be recognized by two sensors: surface localized TLR5 and the cytosolic NLRC4 receptor. Flagellin functions as an immunogen to induce strong immune response against itself and co-administered antigens.16,17,18,37,38,39,40,41,42 Based on its biological properties, flagellin or flagellin-based fusion proteins are being developed as therapeutic agents or vaccines to treat infectious diseases, toxic exposures, and even cancer.43,44,45,46,47 In recent years, studies have shown that the immune activities of flagellin, such as adjuvancy, are dependent on TLR5 recognition and the NLRC4 inflammasome.48,49 Vijay-Kumar et al. demonstrated that TLR5 and the NLRC4 inflammasome played redundant roles in flagellin-induced antibody responses,18 but that MyD88 was not required for the flagellin-induced IgG antibody responses. Most recently, López-Yglesias et al. defined roles for known flagellin receptors and MyD88 in the antibody responses generated toward flagellin using purified flagellin from Salmonella and a series of mice that were deficient genetically in the flagellin recognition pathways. They demonstrated that flagellin could induce antibody responses via a TLR5- and inflammasome-independent pathway, and speculated that a third MyD88-independent pathway might exist for flagellin recognition and antibody responses. Together, these studies revealed intriguing features of flagellin and warranted further investigation to enhance our understanding of flagellin and promote the rational design of flagellin-based prophylactic and therapeutic agents.32
Most previous studies used TLR5 and/or NLRC4 knockout (KO) mice to analyze the flagellin-induced adaptive immune responses.18,32 It is hardly to study interaction and cross-talk between NLRC4- and TLR5-mediated activity using KO mice because of the total loss of the receptor and its downstream signaling pathway. The current study compared the effects of flagellin and its variant proteins that lacked TLR5 and/or NLRC4 activity on the flagellin-induced immune responses, which made it possible to explore the specific role of NLRC4 activation and its cross-talk with TLR5 activation in the specific immune responses against flagellin. Vijay-Kumar et al. demonstrated that TLR5 or NLRC4 was necessary and sufficient to promote the humoral immunity against flagellin in TLR5 and/or NLRC4 KO mice.18 López-Yglesias et al. found that the deletion of Naip5 in mice did not change the number of IgG1 anti-FliC antibodies using Naip5 KO mice.32 However, flagellin-immunized A/J MyD88−/− mice (natural partially defective in Naip5) induced higher IgG1 and IgG2a responses against flagellin than did C57BL/6 mice.32 Nordlander et al. found that NLRC4 KO mice mounted a stronger specific humoral response compared with WT mice. The current results showed that flagellin that lacked NLRC4-mediated activity could induce higher flagellin-specific antibody responses than could WT FliC. It is possible that using flagellin that lacks activity (FliCΔ90-97 or FliC-L3A) as an agonist might provide different information and output regarding the flagellin-induced immune responses compared with KO mice lacking the host receptor (TLR5 and/or NLRC4). The current observations whereby flagellin lacking NLRC4-mediated activity could induce higher antibody responses against flagellin than could WT FliC was consistent with previous observations using A/J MyD88−/−32 and NLRC4 KO mice.50 We also observed that FliC-L3A (deficient in NLRC4-mediated activity) induced significantly higher antibody titers than did FliC after intranasal immunization (data not shown). In summary, we could conclude that abrogating the NLRC4-mediated activity of flagellin upregulated the TLR5-mediated adaptive immune responses against flagellin.
An interesting question is how the NLRC4-mediated activity of flagellin downregulates the TLR5-mediated adaptive immune responses against flagellin. To further assess what type of cells was involved, we first excluded DCs. Flagellin mainly activates the cytosolic NLRC4 signaling pathway in macrophages and induces pyroptosis and cytokine secretion in macrophages.33 Therefore, we next assessed the effects of NLRC4-mediated flagellin activity on the number of macrophages and cytokine secretion. Data revealed that the i.p. injection of 10 μg flagellin decreased the number of peritoneal macrophages significantly, whereas the flagellin variant FliC-L3A did not. This suggests that the activation of NLRC4 affected the TLR5-mediated adaptive immune responses against flagellin mainly via macrophages. There are two possibilities that could explain the underlying mechanism for these effects: the decrease in the number of macrophages directly impaired flagellin antigen presentation and the resulting immune response, or inhibited a panel of macrophage-secreted factors to further modulate the TLR5-mediated adaptive immune responses against flagellin. The current results obtained in mice with depleted macrophages support the latter hypothesis, whereby macrophage depletion did not decrease the IgG response completely (Figure 5). The levels of IL-18 and IL-1β, which are mainly NLRC4-dependent, were decreased markedly in the peritoneal washes after macrophage depletion (Figure 4g and h). This suggests that the cytokines produced by macrophages via NLRC4 might play a role in attenuating the humoral immunity than did the total number of macrophages. It was reported that IL-18 is essential for inducing antigen-specific regulatory T cells and a functional downregulator for immunogenic DCs.51 However, it was also reported that L. monocytogenes-L.p.FlaA induced defective T-cell responses compare to L. monocytogenes, which is dependent of NLRC4 but independent of IL-1β/IL-18.52 It seems NLRC4 activation modulate the adaptive immunity through multiple factors, which should be investigated further.
Many studies demonstrate that flagellin-specific T cell and antibody responses play very important roles in flagellated bacterial infection and anti-bacterial immunity.53,54 Enteropathogenic Escherichia coli usually adhere to the intestinal mucosa via a flagellin-dependent mechanism.55 Some pathogenic bacteria evade immune surveillance by switching off the expression of flagellin after disseminating into the deeper mucosal layer.56 Some bacteria, such as Helicobacter pylori and Campylobacter jejuni, escape the flagellin-specific host immune responses by mutating their flagellin molecules to a form that cannot be detected by TLR5.57 Many additional bacteria such as Salmonella typhimurium elicit beneficial effects by either inhibiting or avoiding inflammasome activation or IL-1β production.58 How the cross-talk and interaction between NLRC4 and TLR5 signaling on specific immune responses affects bacterial infections remains unclear and should be investigated further. Elucidating the underlying mechanisms will facilitate the development of novel approaches toward controlling infectious diseases and other inflammasome-dependent inflammatory processes.
It is well known that flagellin is a potent adjuvant.37,38,40,41,42 The current data support the conclusion that the adjuvancy of flagellin is more dependent on TLR5 than the NLRC4 inflammasome.32 However, they also suggested that deficiency in the NLRC4-mediated activity of flagellin might make it a better adjuvant for enhancing the antibody response. So we dissected the adaptive immune responses against co-administered antigens by comparing the adjuvant activity of FliC and its variant proteins that lack TLR5 and/or NLRC4 activity. We used p24 as model antigen, and the results demonstrated that FliC-L3A also induced higher p24-specific antibody responses than did FliC (Figure S3a–c). Furthermore, FliC-L3A exhibited a higher adjuvant activity toward co-administered immunogens when fused with the immunogens (data not shown). Collectively, FliC-L3A, which was deficient in NLRC4-mediated flagellin activity, was a better adjuvant candidate.
In summary, the current study found that abolishing the NLRC4-mediated activity of flagellin significantly increased the antibody responses against flagellin compared with WT FliC. The results revealed that there was an interaction between NLRC4 activation and TLR5 activation by flagellin, which affects the output of the macrophage-mediated specific immune responses.
CONFLICT OF INTERESTS
The authors have no conflicts of interest of disclose.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant numbers 81202381 and 81202312), the National Basic Research Program of China (973 Program) (Grant number 2012CB518904), the National S&T Major Project on Major Infectious Diseases (Grant numbers 2012ZX10001-008 and 2008ZX10001-010) from the Ministry of Science and Technology of the People's Republic of China. We sincerely thank the Core Facility and Technical Support, Wuhan Institute of Virology and Xuefang An for valuable assistance in the animal studies and Ying Sun, Rong Bao, and Benxia He for their help with the sample collection.
Footnotes
Supplementary Information accompanies the paper on Cellular & Molecular Immunology's website (http://www.nature.com/cmi)
Supplementary Information
References
- Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 2001; 410: 1099–1103. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Yang J, Shi J, Gong Y-N, Lu Q, Xu H et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 2011; 477: 596–600. [DOI] [PubMed] [Google Scholar]
- Kofoed EM, Vance RE. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 2011; 477: 592–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly MA, Steiner TS. Two nonadjacent regions in enteroaggregative Escherichia coli flagellin are required for activation of toll-like receptor 5. J Biol Chem 2002; 277: 40456–40461. [DOI] [PubMed] [Google Scholar]
- Malapaka RR, Adebayo LO, Tripp BC. A deletion variant study of the functional role of the Salmonella flagellin hypervariable domain region in motility. J Mol Biol 2007; 365: 1102–1116. [DOI] [PubMed] [Google Scholar]
- Mortimer CK, Gharbia SE, Logan JM, Peters TM, Arnold C. Flagellin gene sequence evolution in Salmonella. Infect Genet Evol 2007; 7: 411–415. [DOI] [PubMed] [Google Scholar]
- Smith KD, Andersen-Nissen E, Hayashi F, Strobe K, Bergman MA, Barrett SL et al. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat Immunol 2003; 4: 1247–1253. [DOI] [PubMed] [Google Scholar]
- Yoon SI, Kurnasov O, Natarajan V, Hong M, Gudkov AV, Osterman AL et al. Structural basis of TLR5-flagellin recognition and signaling. Science 2012; 335: 859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightfield KL, Persson J, Brubaker SW, Witte CE, von Moltke J, Dunipace EA et al. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat Immunol 2008; 9: 1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Kamada N, Nakamura Y, Burberry A, Kuffa P, Suzuki S et al. NLRC4-driven production of IL-1beta discriminates between pathogenic and commensal bacteria and promotes host intestinal defense. Nat Immunol 2012; 13: 449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 2011; 477: 596–600. [DOI] [PubMed] [Google Scholar]
- Carsiotis M, Weinstein DL, Karch H, Holder IA, O'Brien AD. Flagella of Salmonella typhimurium are a virulence factor in infected C57BL/6J mice. Infect Immun 1984; 46: 814–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecher B, Hapfelmeier S, Muller C, Kremer M, Stallmach T, Hardt WD. Flagella and chemotaxis are required for efficient induction of Salmonella enterica serovar Typhimurium colitis in streptomycin-pretreated mice. Infect Immun 2004; 72: 4138–4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Carlson AQ, Guo YW, Yu YM, Collier-Hyams LS, Madara JL et al. Flagellin is the major proinflammatory determinant of enteropathogenic Salmonella. J Immunol 2003; 171: 3668–3674. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol 2004; 4: 499–511. [DOI] [PubMed] [Google Scholar]
- Sanders CJ, Yu Y, Moore DA, 3rd, Williams IR, Gewirtz AT. Humoral immune response to flagellin requires T cells and activation of innate immunity. J Immunol 2006; 177: 2810–2818. [DOI] [PubMed] [Google Scholar]
- Sanders CJ, Franchi L, Yarovinsky F, Uematsu S, Akira S, Nunez G et al. Induction of adaptive immunity by flagellin does not require robust activation of innate immunity. Eur J Immunol 2009; 39: 359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay-Kumar M, Carvalho FA, Aitken JD, Fifadara NH, Gewirtz AT. TLR5 or NLRC4 is necessary and sufficient for promotion of humoral immunity by flagellin. Eur J Immunol 2010; 40: 3528–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu S, Jang MH, Chevrier N, Guo Z, Kumagai Y, Yamamoto M et al. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol 2006; 7: 868–874. [DOI] [PubMed] [Google Scholar]
- Carvalho FA, Nalbantoglu I, Aitken JD, Uchiyama R, Su Y, Doho GH et al. Cytosolic flagellin receptor NLRC4 protects mice against mucosal and systemic challenges. Mucosal Immunol 2012; 5: 288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letran SE, Lee SJ, Atif SM, Flores-Langarica A, Uematsu S, Akira S et al. TLR5-deficient mice lack basal inflammatory and metabolic defects but exhibit impaired CD4 T cell responses to a flagellated pathogen. J Immunol 2011; 186: 5406–5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Lopez A, Rosales-Reyes R, Alpuche-Aranda CM, Ortiz-Navarrete V. Salmonella downregulates Nod-like receptor family CARD domain containing protein 4 expression to promote its survival in B cells by preventing inflammasome activation and cell death. J Immunol 2013; 190: 1201–1209. [DOI] [PubMed] [Google Scholar]
- Kozlova D, Sokolova V, Zhong M, Zhang E, Yang J, Li W et al. Calcium phosphate nanoparticles show an effective activation of the innate immune response in vitro and in vivo after functionalization with flagellin. Virol Sin 2014; 29: 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Yang J, Zhang Y, Zhou D, Chen Y, Gai W et al. Recombinant flagellins with partial deletions of the hypervariable domain lose antigenicity but not mucosal adjuvancy. Biochem Biophys Res Commun 2010; 392: 582–587. [DOI] [PubMed] [Google Scholar]
- Mizel SB, Bates JT. Flagellin as an adjuvant: cellular mechanisms and potential. J Immunol 2010; 185: 5677–5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy SL, Kurtz SE, Hausman FA, Trune DR, Bennett RM, Hefeneider SH. Activation of RAW264.7 macrophages by bacterial DNA and lipopolysaccharide increases cell surface DNA binding and internalization. J Biol Chem 2004; 279: 17217–17223. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Liu F, Yang J, Zhong M, Zhang E, Li Y et al. Over-activation of TLR5 signaling by high-dose flagellin induces liver injury in mice. Cell Mol Immunol 2014; 12, 729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol 2008; Chapter 14:Unit 14 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zhang E, Liu F, Zhang Y, Zhong M, Li Y et al. Flagellins of Salmonella Typhi and nonpathogenic Escherichia coli are differentially recognized through the NLRC4 pathway in macrophages. J Innate Immun 2014; 6: 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooijen N, Hendrikx E. Liposomes for specific depletion of macrophages from organs and tissues. Methods Mol Biol 2010; 605: 189–203. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science 2010; 327: 291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Yglesias AH, Zhao X, Quarles EK, Lai MA, Vandenbos T, Strong RK et al. Flagellin induces antibody responses through a TLR5- and inflammasome-independent pathway. J Immunol 2014; 192: 1587–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol 2010; 11: 1136–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol 2006; 7: 569–575. [DOI] [PubMed] [Google Scholar]
- van Rooijen N, Hendrikx E. Liposomes for specific depletion of macrophages from organs and tissues. Methods Mol Biol 2010; 605: 189–203. [DOI] [PubMed] [Google Scholar]
- McKee AS, Munks MW, MacLeod MK, Fleenor CJ, Van Rooijen N, Kappler JW et al. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J Immunol 2009; 183: 4403–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honko AN, Sriranganathan N, Lees CJ, Mizel SB. Flagellin is an effective adjuvant for immunization against lethal respiratory challenge with Yersinia pestis. Infect Immun 2006; 74: 1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizel SB, Graff AH, Sriranganathan N, Ervin S, Lees CJ, Lively MO et al. Flagellin-F1-V fusion protein is an effective plague vaccine in mice and two species of nonhuman primates. Clin Vaccine Immunol 2009; 16: 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupz A, Guarda G, Gebhardt T, Sander LE, Short KR, Diavatopoulos DA et al. NLRC4 inflammasomes in dendritic cells regulate noncognate effector function by memory CD8(+) T cells. Nat Immunol 2012; 13: 162–169. [DOI] [PubMed] [Google Scholar]
- McSorley SJ, Ehst BD, Yu Y, Gewirtz AT. Bacterial flagellin is an effective adjuvant for CD4+ T cells in vivo. J Immunol 2002; 169: 3914–3919. [DOI] [PubMed] [Google Scholar]
- Lee SE, Kim SY, Jeong BC, Kim YR, Bae SJ, Ahn OS et al. A bacterial flagellin, Vibrio vulnificus FlaB, has a strong mucosal adjuvant activity to induce protective immunity. Infect Immun 2006; 74: 694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zhong M, Zhang Y, Zhang E, Sun Y, Cao Y et al. Antigen replacement of domains D2 and D3 in flagellin promotes mucosal IgA production and attenuates flagellin-induced inflammatory response after intranasal immunization. Hum Vaccin Immunother 2013; 9: 1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Sanchez A, Shi Z, Zhang T, Liu M, Zhang D. Activation of Toll-like receptor 5 on breast cancer cells by flagellin suppresses cell proliferation and tumor growth. Cancer Res 2011; 71: 2466–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfondrini L, Rossini A, Besusso D, Merlo A, Tagliabue E, Menard S et al. Antitumor activity of the TLR-5 ligand flagellin in mouse models of cancer. J Immunol 2006; 176: 6624–6630. [DOI] [PubMed] [Google Scholar]
- Garaude J, Kent A, van Rooijen N, Blander JM. Simultaneous targeting of toll- and nod-like receptors induces effective tumor-specific immune responses. Sci Transl Med 2012; 4: 120ra16. [DOI] [PubMed] [Google Scholar]
- Cummings LA, Barrett SL, Wilkerson WD, Fellnerova I, Cookson BT. FliC-specific CD4+ T cell responses are restricted by bacterial regulation of antigen expression. J Immunol 2005; 174: 7929–7938. [DOI] [PubMed] [Google Scholar]
- Simon R, Tennant SM, Wang JY, Schmidlein PJ, Lees A, Ernst RK et al. Salmonella enterica serovar enteritidis core O polysaccharide conjugated to H:g,m flagellin as a candidate vaccine for protection against invasive infection with S. enteritidis. Infect Immun 2011; 79: 4240–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates JT, Uematsu S, Akira S, Mizel SB. Direct stimulation of tlr5+/+ CD11c+ cells is necessary for the adjuvant activity of flagellin. J Immunol 2009; 182: 7539–7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao EA, Andersen-Nissen E, Warren SE, Aderem A. TLR5 and Ipaf: dual sensors of bacterial flagellin in the innate immune system. Semin Immunopathol 2007; 29:275–288. [DOI] [PubMed] [Google Scholar]
- Nordlander S, Pott J, Maloy KJ. NLRC4 expression in intestinal epithelial cells mediates protection against an enteric pathogen. Mucosal Immunol 2014; 7: 775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji NM, Nowak B. IL-18 and antigen-specific CD4(+) regulatory T cells in Peyer's patches. Ann N Y Acad Sci 2004; 1029: 413–415. [DOI] [PubMed] [Google Scholar]
- Sauer JD, Pereyre S, Archer KA, Burke TP, Hanson B, Lauer P et al. Listeria monocytogenes engineered to activate the Nlrc4 inflammasome are severely attenuated and are poor inducers of protective immunity. Proc Natl Acad Sci U S A 2011; 108: 12419–12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaun B, Romero P, Lebecque S. Toll-like receptors' two-edged sword: when immunity meets apoptosis. Eur J Immunol 2007; 37: 3311–3318. [DOI] [PubMed] [Google Scholar]
- Lavelle EC, Murphy C, O'Neill LA, Creagh EM. The role of TLRs, NLRs, and RLRs in mucosal innate immunity and homeostasis. Mucosal Immunol 2010; 3: 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giron JA, Torres AG, Freer E, Kaper JB. The flagella of enteropathogenic Escherichia coli mediate adherence to epithelial cells. Mol Microbiol 2002; 44: 361–379. [DOI] [PubMed] [Google Scholar]
- Hughes EA, Galan JE. Immune response to Salmonella: location, location, location? Immunity 2002; 16: 325–328. [DOI] [PubMed] [Google Scholar]
- Andersen-Nissen E, Smith KD, Strobe KL, Barrett SL, Cookson BT, Logan SM et al. Evasion of Toll-like receptor 5 by flagellated bacteria. Proc Natl Acad Sci U S A 2005; 102: 9247–9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taxman DJ, Huang MT, Ting JP. Inflammasome inhibition as a pathogenic stealth mechanism. Cell Host Microbe 2010; 8: 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.