Abstract
Programmed cell death 4 (Pdcd4) is a newly defined inhibitor of transcription and translation and a tumor suppressor. Recent studies have suggested that Pdcd4 may also be involved in some inflammatory diseases. However, its role in atherosclerosis, a chronic inflammation of the arterial wall, remains to be investigated. Here, we found that Pdcd4 deficiency in mice increased the expression of IL-10 in macrophages and decreased the expression of IL-17 in T cells in the presence of an atherosclerosis-associated stimulator in vitro and in high fat-induced atherosclerotic plaques. Importantly, knocking out Pdcd4 led to a decrease in atherosclerotic lesions in Apoe−/− mice fed a high fat diet. This effect could be partly reversed by blocking IL-10 with a neutralizing antibody but not by the application of exogenous IL-17. Further mechanistic studies revealed that Pdcd4 negatively regulated the expression of IL-10 in an ERK1/2- and p38-dependent manner. These results demonstrate that Pdcd4 deficiency attenuates atherosclerosis in hyperlipidemic mice in part through the upregulation of the anti-inflammatory cytokine IL-10. This indicates that endogenous Pdcd4 promotes atherosclerosis and therefore represents a potential therapeutic target for patients with atherosclerosis.
Keywords: atherosclerosis, IL-10, macrophage, Pdcd4
Introduction
Programmed cell death 4 (Pdcd4) was initially discovered during screening for genes activated during apoptosis and was subsequently found to be a tumor suppressor and an inhibitor of gene transcription and translation1,2. It inhibits gene translation by binding to eukaryotic translation initiation factor (eIF)-4A or by binding directly to the target gene3 and suppresses AP-1-dependent transcription by downregulating MAP4K14,5. Its expression is lost or reduced in several forms of human cancer, including lung cancer6, colorectal cancer7, and glioma8. Furthermore, Pdcd4 can suppress the malignant phenotype of tumors9 and enhance their chemosensitivity10.
Recent studies have suggested that Pdcd4 may also be involved in some inflammatory diseases. However, the role of Pdcd4 in inflammatory diseases demonstrates opposing effects (promotion or inhibition) in various disease models. Some researchers have shown that Pdcd4-deficient mice are resistant to autoimmune encephalomyelitis, LPS-induced shock, and type 1 diabetes11,12,13 and that Pdcd4 deficiency prevents diet-induced obesity, adipose tissue inflammation, and insulin resistance14. Knockdown of Pdcd4 by specific siRNA suppressed allergic pulmonary inflammation, as demonstrated by attenuated airway eosinophil infiltration, bronchial collagen deposition, and mucus production15. These results imply that endogenous Pdcd4 promotes inflammation. However, our more recent research has shown that Pdcd4-deficient mice were more sensitive to LPS/D-galactosamine-induced acute liver injury. In this model, the Pdcd4-deficient mice had more necrotic and apoptotic hepatocytes, inflammatory cell infiltration, and liver internal hemorrhage, as well as increased aspartate transaminase (AST) and alanine transaminase (ALT) compared with wild-type (WT) mice, indicating that endogenous Pdcd4 may inhibit inflammation16. Therefore, the role of Pdcd4 in inflammatory diseases remains unclear.
Atherosclerosis is a chronic inflammatory process involving the arterial wall that is responsible for the morbidity and mortality of most cardiovascular diseases17. Atherosclerotic plaques are characterized by an accumulation of lipids within the intima of the artery, together with infiltration of immunocytes. Macrophages and T cells are the major components of this infiltrate, and the cytokines secreted by these cells play a critical role in the development of atherosclerosis18. Beneath the vascular intima, the macrophages phagocytize modified low-density lipoprotein (LDL) particles and become foam cells that produce many pro-inflammatory cytokines, including IL-6 and TNF-α that promote the formation of atherosclerotic plaques, as well as anti-inflammatory cytokines, including IL-10 and TGF-β, that inhibit the formation of plaques19,20 and initiate an adaptive immune response. Accumulating evidence indicates that CD4+ T cells are the major type of T cells involved in atherosclerosis21. Reconstitution of immune-deficient scid/scid apolipoprotein E knockout (Apoe KO, Apoe−/−) mice with CD4+ T cells accelerated atherosclerosis, whereas CD4-deficient C57BL/6 mice were protected against fatty streak formation22,23, indicating that CD4+ T cells play a pathological role in atherosclerosis. Further studies demonstrated that Th1 (IFN-γ+CD4+) and Th17 (IL-17+CD4+) cells promote the development of atherosclerosis primarily by releasing the cytokines IFN-γ and IL-1724,25,26.
Although multiple cytokines are involved in atherosclerosis, IL-10 is one of the most important cytokines in this context27. Several studies have found that IL-10 has anti-inflammatory and antithrombotic potency28,29. In the past few years, a series of studies have confirmed the role of endogenous IL-10 in animal models of atherosclerosis30,31,32. In Apoe and IL-10 double-KO mice, feeding a high fat diet was shown to enhance the formation of early atherosclerotic plaques compared with the Apoe single knockout mice33. Consistent with these observations, intramuscular gene transfer of IL-10 cDNA has been proven to reduce atherosclerotic plaques in Apoe−/− mice34. These results provided evidence that IL-10 is instrumental in preventing the development of atherosclerotic lesions. Therefore, molecules that can regulate the expression of IL-10 will affect atherosclerosis.
Previous studies have reported that Pdcd4 inhibits the expression of IL-10. Pdcd4 deficiency or knockdown upregulates expression of IL-10 in activated splenocytes and macrophages, and overexpression of Pdcd4 suppresses the expression of IL-10 in macrophages11,12,35,36. More recent research has shown that Pdcd4 inhibits IL-10 expression by directly binding to Twist2 and then inhibiting Twist2-initiated c-Maf transcription36. These data suggest that Pdcd4 may affect the development of atherosclerosis by regulating the expression of IL-10.
In this study, we found that Pdcd4 deficiency in mice increased the expression of IL-10 in macrophages stimulated by oxidized LDL (oxLDL) in vitro and in high fat-induced atherosclerotic plaques. Importantly, knocking out Pdcd4 led to decreased atherosclerotic lesions in Apoe−/− mice fed with high fat diet, which could be partly reversed by blocking IL-10 with a neutralizing antibody. Furthermore, Pdcd4 negatively regulates the expression of IL-10 in an ERK1/2- and p38-dependent manner, suggesting that endogenous Pdcd4 promotes atherosclerosis partly by inhibiting the expression of IL-10.
Materials and methods
Mice
Apoe−/− mice on a C57BL/6 background and WT C57BL/6 mice were purchased from Beijing University (Beijing, China). The Pdcd4−/− mice on C57BL/6 background were generated as described previously11 and were mated with the Apoe−/− mice to obtain the Pdcd4−/−Apoe−/− mice. All animal study protocols were approved by the Animal Care and Utilization Committee of Shandong University.
Induction of atherosclerosis
Sex-matched Pdcd4+/+Apoe−/− and Pdcd4−/−Apoe−/− mice were fed a high fat diet (0.25% cholesterol and 15% cocoa butter) from 8 weeks until 16 weeks of age to induce atherosclerotic lesions.
Histopathology, immunohistochemistry (IHC)
After the mice were sacrificed, the hearts with the attached aortic roots were removed and fixed in 4% paraformaldehyde overnight and then embedded in OCT compound at −20 °C. Serial frozen sections (6 μm thick) were cut along the aortic root for up to 200 μm until the valve leaflets were no longer detectable. Sections of the aortic root with aortic valve were stained with hematoxylin and eosin (H&E) to evaluate the amount of plaque. To detect the IL-17 expression in the plaques, corresponding sections on separate slides were incubated in 3% H2O2 solution for 10 min at room temperature (RT) to block the endogenous peroxidase activity, incubated with rabbit anti-mouse IL-17 Ab (sc-7927, Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 1 h at RT, and then with horseradish peroxidase (HRP) conjugated goat-anti-rabbit IgG (sc-2004, Santa Cruz Biotechnology) for 15 min at RT. After staining with DAB solution, the sections were treated with hematoxylin to stain the nuclei. The total areas of lesions and the positively stained regions were measured using Image Proplus 6.0 software, and the ratio of positively stained region to the total area of the lesion was calculated.
Immunofluorescence (IF)
To examine the expression of IL-17 in the CD4+ T cells in the plaques, corresponding frozen sections on separate slides were analyzed by double IF staining with rabbit anti-mouse IL-17 Ab and FITC-conjugated goat-anti-rabbit IgG (sc-2012, Santa Cruz Biotechnology), and rat anti-mouse CD4 Ab (H129.19; BD Pharmingen, San Diego, CA, USA) and TRITC-conjugated goat-anti-rat IgG (3030-03, Southern Biotech, Birmingham, AL, USA). The expression of IL-10 in macrophages from plaques was detected by double IF staining with rat anti-mouse IL-10 Ab (ab33471, Abcam, Cambridge, UK) and TRITC-conjugated goat-anti-rat IgG, and rabbit anti-mouse CD68 Ab (sc-9139, Santa Cruz Biotechnology) and FITC-conjugated goat-anti-rabbit IgG, respectively. The cell nuclei were stained with Hoechst (94403, Sigma-Aldrich, Saint Louis, MO, USA). The different histological stains were examined using an Olympus microscope (IX71; Olympus Corporation, Tokyo, Japan).
Macrophages culture and treatment
Sex-matched Pdcd4−/− and C57BL/6 mice at 7–8 weeks old were intraperitoneally injected with 6% starch. After 3 days, the peritoneal macrophages were washed out and cultivated in 6-well flat bottom plates with Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and incubated in humidified 5% CO2 at 37 °C for 2 h to allow macrophage adherence. Non-adherent cells were removed by three washes with DMEM. The adherent cells were harvested and the purity of macrophages was determined using flow cytometry following staining with PECy5-conjugated anti-F4/80 Ab (BM8, eBioscience, San Diego, CA, USA). The purified macrophages (purity ≥95%) were cultured for 24 h. The next day, the cultured macrophages were stimulated with 50 μg/mL of oxLDL (Beijing Union-Biology Co., Ltd., Beijing, China) in the absence or presence of the ERK-specific inhibitor PD98059 (S1177, Selleck Chemicals, Houston, TX, USA) or the p38-specific inhibitor SB203580 (S1076, Selleck Chemicals, Houston, TX, USA) for the indicated times.
PCR and real-time PCR
To identify the genotype of the Pdcd4−/− mice, template genomic DNA was extracted from a small piece of tail from mice at age 8 weeks. PCR were performed using the following primers: Pdcd4-WT: sense: 5′-AGCCATTTCAGCCTTGGTGC-3′, anti-sense: 5′-AATCTGTGTCTATGGTGAGGGTGG-3′ (398-bp product length); Pdcd4-KO: sense: 5′-GTTTGGAGGGAGGAAATGGAAG-3′, anti-sense: 5′-AATCTGTGTCTATGGTGAGGGTGG-3′ (485-bp product length).
To measure cytokine expression, total RNA was isolated from the aortic arch with atherosclerotic plaques or cultured macrophages and then reverse transcribed into cDNA. Real-time PCR was performed using UltraSYBR Mixture (CW0956; CWBIO, Beijing, China) and specific primer pairs. The sequences of the sense and antisense primers were as follows: IL-10, sense: 5′-GCCAGAGCCACATGCTCCTA-3′, antisense: 5′-GATAAGGCTTGGCAACCCAAGTAA-3′; IL-6, sense: 5′-CTGCAAGAGACTTCCATCCAG-3′, antisense: 5′-AGTGGTATAGACAGGTCTGTTGG-3′; β-actin, sense: 5′-CATCCGTAAAGACCTCTATGCCAAC-3′, antisense: 5′-ATGGAGCCACCGATCCACA-3′. The relative expression data for the real-time quantitative PCR data were presented using the ΔΔCt model. Using the 2̂ΔΔCt method, our data are reported as the fold change in experimental group normalized to an endogenous reference gene (β-actin) and relative to the control group.
To assess the IL-10 RNA stability, WT and Pdcd4−/− peritoneal macrophages were treated with actinomycin D (10 μg/mL) for various times (0 min, 20 min, 40 min) and the IL-10 mRNA levels were analyzed by real-time PCR and normalized to 18S. To determine the effect of mitogen-activated protein kinase inhibitors on mRNA decay, the macrophages were treated with an inhibitor of ERK (SB203580) or of p38 (PD98059), each at 20 μM, and mRNA expression was assayed as described above.
Western blot
The proteins (50 μg) were separated by SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA), which were blocked with 2% bovine serum albumin (BSA) in Tris-buffered saline + 0.1% Tween-20 (TBST) for 1 h, then incubated overnight at 4 °C with rabbit anti-mouse antibodies against IL-10, p-JNK, JNK, p-ERK, ERK, p-p38, p38, STAT3, p-STAT3, SCOS3 (all at 1:1000; Cell Signaling Technology, Danvers, MA, USA) or rat anti-mouse β-actin (sc-1616-R, 1:2000; Santa Cruz Biotechnology), then incubated with HRP-conjugated goat anti-rabbit or HRP-conjugated anti-rat IgG for 1 h at RT. After washing three times, the signals were visualized using SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology, Rockford, IL, USA) and semi-quantified with Quantity One v4.62 software.
ELISA
The supernatant was collected from cultured macrophages after stimulation with oxLDL for 6 h, 12 h, 24 h, and 48 h. The cytokines were quantified using mouse IL-10, IL-6, TNF-α, TGF-β, and IL-17 ELISA kits (eBioscience) according to the manufacturer's instructions.
Flow cytometry
Splenocytes isolated from Pdcd4+/+Apoe−/− and Pdcd4−/−Apoe−/− mice fed with a high fat diet for 8 weeks were stimulated with functional grade purified anti-mouse CD3e Ab (145-2C11; eBioscience) for 3 days. The supernatant was collected and used for the measurement of IL-2, IL-4, IL-6, IFN-γ, TNF-α, IL-17, and IL-10 by BD Cytometric Bead Array (CBA) Mouse Th1/Th2/Th17 Cytokine Kit (560485; BD Biosciences) according to the manufacturer's instructions. At least 2100 gated beads were acquired and analyzed with a Cytomics FC500 (Beckman Coulter, Brea, CA, USA).
In vivo application of exogenous IL-17 and neutralizing anti-IL-10 antibody
Male Pdcd4+/+Apoe−/− mice (n = 8) and male Pdcd4−/−Apoe−/− mice (n = 16) were fed a high fat diet from 8 weeks of age. Four weeks later, the Pdcd4−/−Apoe−/− mice were divided into four groups (n = 4 for each group) and subjected to the following treatments: intraperitoneal injection of exogenous 0.02% recombinant IL-17A in normal saline (2 μg/mouse/time, PMC0175, Invitrogen, Carlsbad, CA, USA) or normal saline containing 0.02% albumin as control twice a week for 5 weeks; intraperitoneal injection with neutralizing anti-IL-10 antibody (250 μg/mouse/time, MAB417, rat IgG2A; R&D Systems, Minneapolis, MN, USA) or the isotype Ab (rat IgG2A, MAB006; R&D Systems) as a control once a week for 5 weeks. The control Pdcd4+/+Apoe−/− mice were divided into two groups (n = 4 for each group) and given normal saline or isotype Ab, respectively. The levels of IL-17 and IL-10 in the sera of the mice after treatment with recombinant IL-17A or the neutralizing anti-IL-10 antibody were measured using an ELISA kit (eBioscience) according to the manufacturer's instructions.
RNA-binding protein immunoprecipitation (RIP)
Peritoneal macrophages from the C57BL/6 mice were collected, cultured, and stimulated with oxLDL for 24 h. The cells (1 × 107) were lysed by RIP lysis buffer from the RIP kit (17-701; Millipore Corporation, Billerica, MA, USA). Approximately 10% of the supernatant (cytoplasmic fraction) was saved as the input sample, and the remainder was used for IP overnight at 4 °C, using rabbit anti-mouse Pdcd4 Ab or rabbit anti-mouse IgG (negative control) or rabbit anti-mouse SNRNP70 Ab (positive control) magnetic bead complexes to obtain a RNA-protein-magnetic bead complex. The protein-magnetic beads were removed digesting by proteinase K buffer. Then, the RNAs bound to the Pdcd4, IgG, or SNRNP70 were purified and reverse transcribed into cDNA. IL-10 expression in RNA bound to the Pdcd4 or the negative control IgG was measured using real-time PCR with IL-10-specific primers as described above, while U1 snRNA expression in the RNA bound to the positive control SNRNP70 was measured using the U1 snRNA-specific primers, sense: 5′-GGGAGATACCATGATCACGAAG GT-3′, anti-sense: 5′-CCACAAATTATGCAGTCGAGTTTCCC-3′.
Statistical analysis
All analyses were performed using the SPSS 11.0 program (SPSS, Chicago, IL, USA). One-way analysis of variance (ANOVA) and unpaired Student's t-tests were used to compare the data for multi-group and between-group differences, respectively. The data are shown as the means ± SEM. A probability value <0.05 was considered statistically significant.
Results
Pdcd4 deficiency increased the expression of IL-10 in macrophages and decreased the expression of IL-17 in T cells in vitro
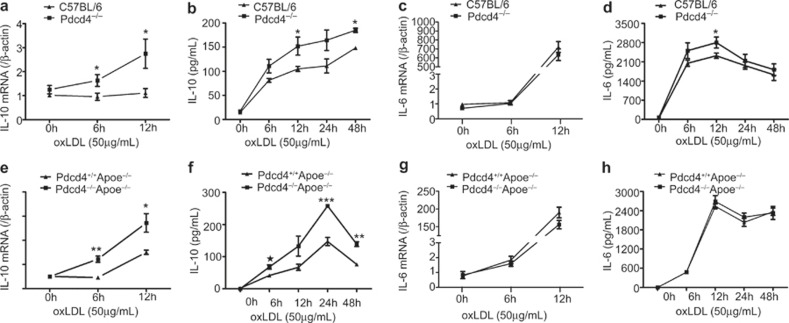
Macrophages and T cells are major components of atherosclerotic plaques, and the cytokines secreted by these cells play a critical role in the development of atherosclerosis. To explore the possible role of Pdcd4 in atherosclerosis, we first investigated the impact of Pdcd4 on the secretion of cytokines by macrophages in vitro. Primary peritoneal macrophages from Pdcd4−/− mice and their littermate WT C57BL/6 animals were stimulated by the atherosclerosis-associated stimulant, ox-LDL, for various times, and the cytokine expression was determined using real-time PCR and ELISA. As shown in Figure 1, the expression of IL-10 was persistently elevated in the oxLDL-stimulated Pdcd4−/− macrophages at both the mRNA level (Figure 1a) and the protein level (Figure 1b), and this stimulation occurred for a longer time than for the WT macrophages. However, the expression of IL-6 at the mRNA level did not differ between the Pdcd4-deficient macrophages and their WT controls (Figure 1c). Meanwhile, the expression of IL-6 at protein level was also not different between the two groups following long-term stimulation with oxLDL. However, the expression of IL-6 in the Pdcd4−/− macrophages increased at early stage (12 h) (Figure 1d). In addition, the Pdcd4 deficiency had no obvious effect on the levels of TNF-α (Supplementary Figure 1a) and TGF-β (Supplementary Figure 1b). To further clarify the effect of Pdcd4 deficiency on IL-10 and IL-6 secreted by the macrophages in hyperlipidemic environment, Pdcd4−/−Apoe−/− mice were generated by crossing Pdcd4−/− with Apoe−/− mice and the genotype with respect to Pdcd4 was identified (Supplementary Figure 2). We measured the expression of IL-10 and IL-6 in oxLDL-treated macrophages from the Pdcd4−/−Apoe−/− and Pdcd4+/+Apoe−/− mice. The results showed that the IL-10 expression at both RNA and protein levels significantly increased in the Pdcd4−/−Apoe−/− mice compared with Pdcd4+/+Apoe−/− mice (Figure 1e and f), which is consistent with the results from the Pdcd4−/− mice. In contrast, the IL-6 expression did not differ between the Pdcd4+/+Apoe−/− and Pdcd4−/−Apoe−/− macrophages at any time point at either the RNA or protein level (Figure 1g and 1h). This result indicates that macrophages lacking Pdcd4 produce more IL-10 under chronic stimulation by oxLDL in vitro.
Figure 1.
Pdcd4 deficiency increased the expression of IL-10 in oxLDL-stimulated macrophages in vitro. Peritoneal macrophages from C57BL/6 and Pdcd4−/− mice were collected, cultured in vitro and stimulated with oxLDL (50 μg/mL) for the indicated time periods. (a) The expression of IL-10 mRNA was measured by real-time PCR. (b) The concentrations of IL-10 in the culture supernatants were detected by ELISA. (c–d) The mRNA levels and concentrations of IL-6 in the culture supernatants were measured by real-time PCR (c) and ELISA (d), respectively. (e–h) Peritoneal macrophages from Pdcd4+/+Apoe−/− and Pdcd4−/−Apoe−/− mice were stimulated with oxLDL (50 μg/mL) for the indicated time periods. The mRNA levels and concentrations of IL-10 (e, f) and IL-6 (g, h) in the supernatants were assessed by real-time PCR and ELISA, respectively. The data are shown as the means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. The data are derived from four mice per group.
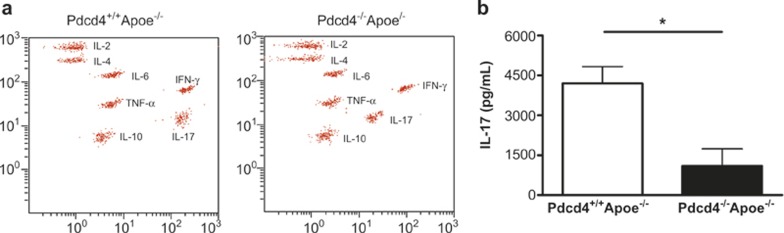
To explore the effect of Pdcd4 deficiency on the secretion of cytokines by T cells in a hyperlipidemic environment, after feeding the mice a high fat diet for 8 weeks, the splenocytes from Pdcd4+/+Apoe−/− and Pdcd4−/−Apoe−/− mice were stimulated with anti-CD3 monoclonal antibody for 3 days. The cytokines in the culture supernatants were detected using BD CBA (Figure 2a). As shown in Figure 2b, the T cells lacking Pdcd4 showed a 75% reduction in the level of IL-17. However, the other cytokines (IL-2, IL-4, IL-6, IFN-γ, TNF-α, and IL-10) were not significantly different between the Pdcd4+/+Apoe−/− and Pdcd4−/−Apoe−/− mice (Supplementary Figure 3). These data demonstrate that Pdcd4 deficiency increased the expression of IL-10 in macrophages and decreased the expression of IL-17 in T cells in presence of an atherosclerosis-associated stimulator in vitro.
Figure 2.
Pdcd4 deficiency decreased the expression of IL-17 in CD3-stimulated T cells in vitro. Pdcd4+/+Apoe−/− and Pdcd4−/−Apoe−/− mice were fed a high fat diet between age 8 weeks to 16 weeks. The splenocytes were stimulated with anti-CD3 monoclonal antibody (5 μg/mL) in vitro for 3 days. (a) The concentrations of IL-2, IL-4, IL-6, IFN-γ, TNF-α, IL-17, and IL-10 in the culture supernatants were detected using the BD CBA Mouse Th1/Th2/Th17 Cytokine Kit. (b) The differences in the concentrations of IL-17 between two genotypes of mice were analyzed using an unpaired t-test. The data are shown as the means ± SEM. *P < 0.05. The data are derived from six Pdcd4+/+Apoe−/− mice and four Pdcd4−/−Apoe−/− mice.
High levels of IL-10 and low levels of IL-17 were detected in the atherosclerotic lesions of Pdcd4−/−Apoe−/− mice
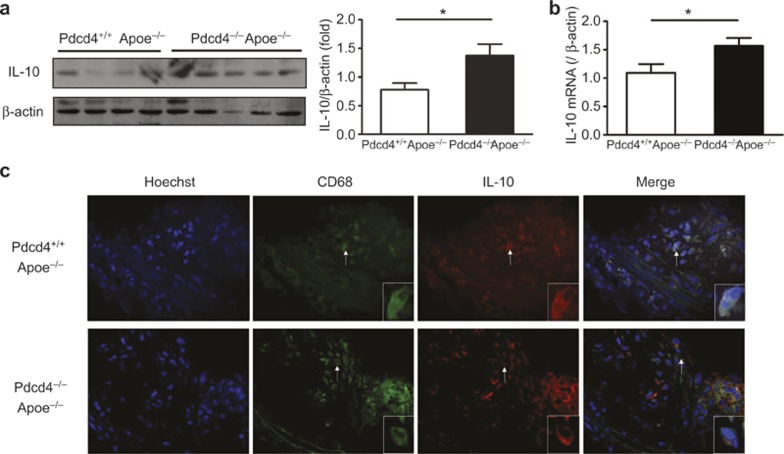
To determine whether Pdcd4 deficiency also upregulates the expression of IL-10 in plaques in hyperlipidemic mice, Pdcd4+/+Apoe−/− and Pdcd4−/−Apoe−/− mice were fed a high fat diet for 8 weeks to induce atherosclerotic lesions. The aortic arch with atherosclerotic plaques was isolated, and the expression of IL-10 was measured using real-time PCR and Western blot. As shown in Figure 3, the expression of IL-10 was markedly increased in Pdcd4−/−Apoe−/− mice compared with Pdcd4+/+Apoe−/− mice at both the protein level (Figure 3a) and the mRNA level (Figure 3b). Further, frozen sections were prepared from the aortic root with its atherosclerotic plaques, and the IL-10 and CD68 proteins were co-localized on the same sections using dual IF staining. The results showed that IL-10 was co-expressed with CD68 in the same cells (Figure 3c). These results indicate that the expression of IL-10 in macrophages from atherosclerotic plaques increased in Pdcd4-deficient mice.
Figure 3.
A high level of IL-10 expression was detected in the atherosclerotic lesions of the Pdcd4−/−Apoe−/− mice. Pdcd4+/+Apoe−/− and Pdcd4−/−Apoe−/− mice were fed a high fat diet from age 8 weeks to 16 weeks. The aortic arches with their atherosclerotic plaques were isolated. The expression of IL-10 was measured at the protein level by Western blot (a) and at the mRNA level by real-time PCR (b). The data are shown as the means ± SEM. *P < 0.05. (c) The aortic roots with their atherosclerotic plaques were isolated to prepare serial frozen sections. The cell nuclei were stained blue with Hoechst. IL-10 and CD68 were co-localized in the same sections by dual IF staining. CD68 (in green) was stained with rabbit anti-mouse antibody and FITC-conjugated anti-rabbit second antibody, and IL-10 (in red) was stained with rat anti-mouse antibody and TRITC-conjugated anti-rat second antibody. The merged image showed co-expression of IL-10 and CD68 (yellow) in the same cells. The cell indicated by the white arrows is shown magnified in the lower right corner. The data are derived from four Pdcd4+/+Apoe−/− mice and five Pdcd4−/−Apoe−/− mice.
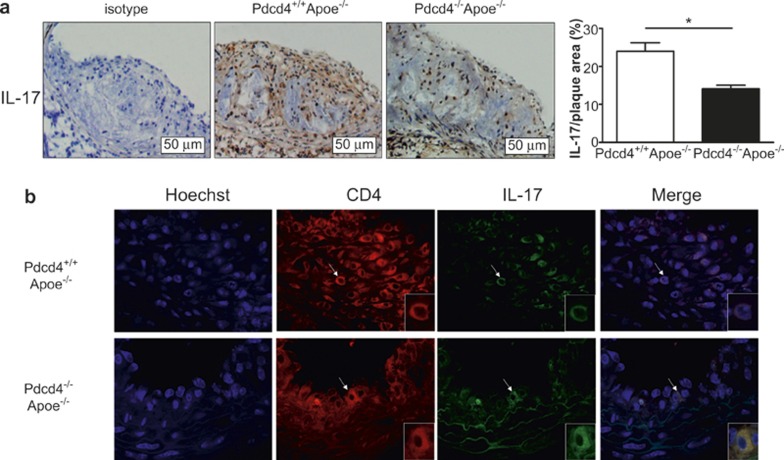
To determine whether the changes in IL-17 associated with Pdcd4 deficiency also occur in the aortic lesions, additional sections of the aortic root on separate slides were used to evaluate the expression of IL-17 by IHC and IF. The results showed that the expression of IL-17 in the aortic lesions was significantly decreased in the Pdcd4−/−Apoe−/− mice compared with the Pdcd4+/+Apoe−/− mice (Figure 4a). Moreover, dual IF staining for IL-17 and CD4 in the same section showed that IL-17 and CD4 were co-expressed in the same cells, which demonstrated that IL-17 was expressed in the CD4+ T cells (Figure 4b). These results indicate that the expression of IL-17 in CD4+ T cells decreased in the atherosclerotic lesions of Pdcd4-deficient mice.
Figure 4.
A low level of IL-17 was expressed in the atherosclerotic lesions of the Pdcd4−/−Apoe−/− mice. The Pdcd4+/+Apoe−/− and Pdcd4−/−Apoe−/− mice were fed a high fat diet from the age of 8 weeks to 16 weeks. The aortic roots with their atherosclerotic plaques were isolated to prepare serial frozen sections. (a) IHC staining of serial frozen sections using an anti-IL17 Ab revealed IL-17 expression primarily in the atherosclerotic plaques (original magnification ×200). The data are shown as the means ± SEM. *P < 0.05. (b) The cell nuclei were stained blue with Hoechst, and IL-17 and CD4 proteins were co-localized in the same section by dual IF staining. CD4 (in red) was stained with rat anti-mouse antibody and TRITC-conjugated anti-rat second antibody, and IL-17 (in green) was stained with rabbit anti-mouse antibody and FITC-conjugated anti-rabbit second antibody. The merged image shows co-expression of IL-17 and CD4 in the same cells (yellow). The cell indicated by the white arrows is shown magnified in the lower right corner. The data are derived from four Pdcd4+/+Apoe−/− mice and five Pdcd4−/−Apoe−/− mice.
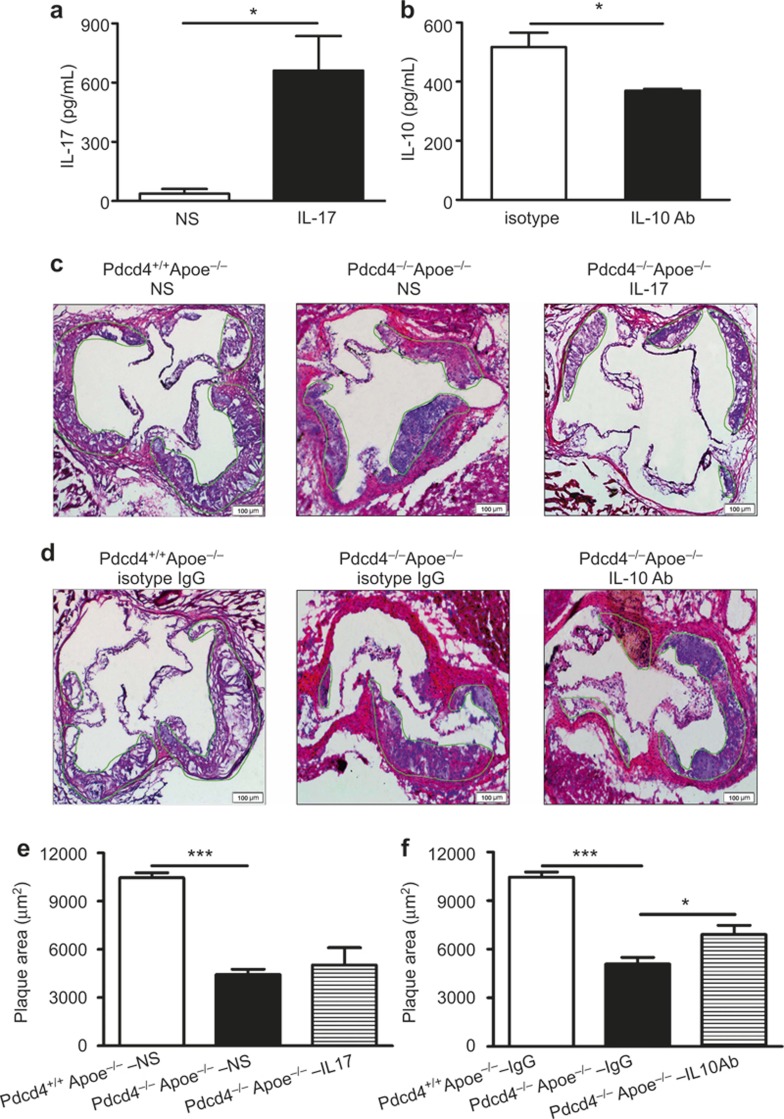
Pdcd4 deficiency decreased the atherosclerotic plaques in Apoe−/− mice: this could be partly reversed by blockage of IL-10 with a neutralizing antibody but not by supplementation with exogenous IL-17
To investigate the role of Pdcd4 in the formation of atherosclerotic plaques and the possible contributions of IL-17 and IL-10 expression to this process, we compared the size of the plaques in the Pdcd4+/+Apoe−/− mice with those in the Pdcd4−/−Apoe−/− mice fed with a high fat diet. In addition, we attempted to block the effects of IL-10 by injecting neutralizing anti-IL-10 antibodies and to enhance the effects of IL-17 by injecting recombinant IL-17 as described in the Methods section. The results showed that aortic walls without plaques (8 weeks) expressed a very low level of Pdcd4, whereas that with established plaques (16 weeks) expressed increased Pdcd4 in Pdcd4+/+Apoe−/− mice (Supplementary Figure 4). Pdcd4 deficiency led to a decrease in atherosclerotic lesions in the Pdcd4−/−Apoe−/− mice compared with the Pdcd4+/+Apoe−/− mice (Figure 5). Supplementation with exogenous recombinant IL-17 did not significantly change the size of the aortic lesions, although the level of IL-17 in the serum markedly increased (Figure 5a, c, and e). However, injection of the neutralizing anti-IL10 antibody significantly decreased the level of IL-10 in the serum and reversed the protective effect of Pdcd4 deficiency on the aortic lesions (Figure 5b, d, and f). These results indicate that upregulation of IL-10 is at least partially responsible for the reduced aortic lesions observed in the Pdcd4-deficient mice.
Figure 5.
Blockage of IL-10, but not additional recombinant IL-17, partially reversed the reduced aortic lesion in Pdcd4−/−Aope−/− mice. After being fed a high fat diet for 4 weeks, the Pdcd4−/−Apoe−/− mice were divided into two groups. One group received an injection of exogenous recombinant IL-17 or neutralizing IL-10 Ab, while the other group received injection of normal saline or isotype IgG as controls. The control Pdcd4+/+Apoe−/− mice received only the injection of normal saline or isotype IgG. The degree of IL-17 supplementation (a) and IL-10 sequestration (b) was determined in the serum of the Pdcd4−/−Apoe−/− mice using ELISA. The serial frozen sections of the aortic roots after treatment with exogenous recombinant IL-17 (c) or neutralizing IL-10 Ab (d) stained with H&E are shown. (e–f) The aortic lesion areas were measured using Image Proplus 6.0 software. The data are shown as the means ± SEM. P values of ANOVA for three groups were both less than 0.001. *P < 0.05; ***P < 0.001. The data are derived from four mice per group.
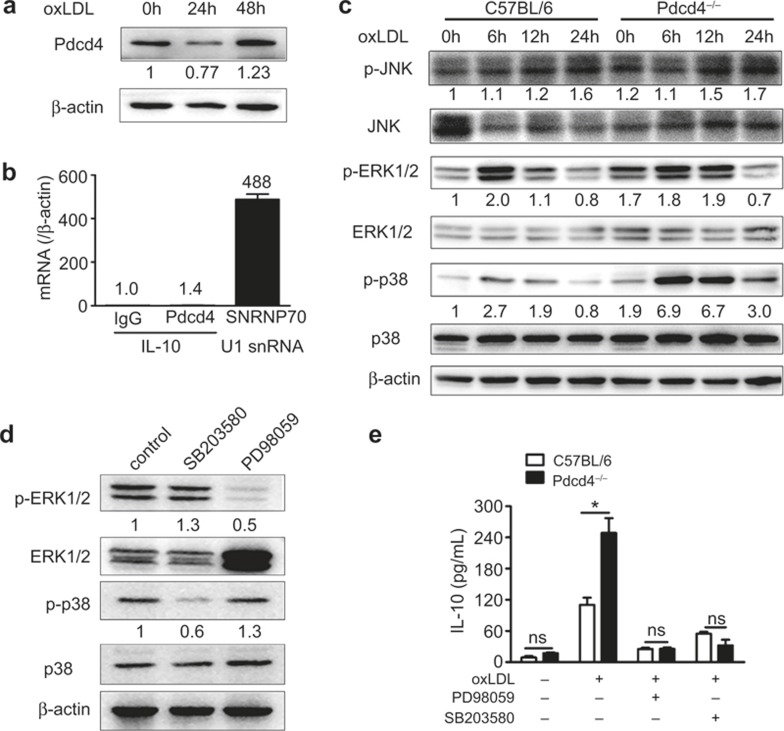
Pdcd4 regulated IL-10 expression in ERK1/2 and p38 MAPK pathways
Previous studies have reported that LPS stimulation upregulates IL-10 expression in macrophages by downregulating Pdcd4 expression12. We therefore measured Pdcd4 expression in C57BL/6 macrophages stimulated with oxLDL. The results showed that the expression of Pdcd4 was reduced at 24 h after stimulation, which is consistent with the results for macrophages treated with LPS as described in a previous report12, while the levels were elevated at 48 h after oxLDL stimulation (Figure 6a). These data suggest that long-term stimulation with this toxic lipid increased the expression of Pdcd4, which is consistent with the data from the plaques. Pdcd4 has been recognized as a suppressor of both gene transcription and translation via binding to eIF4A, which interacts with mRNAs containing 5′-untranslated regions (UTRs)5, and direct binding to the target genes, respectively3. Because the IL-10 mRNA contains 5′-UTRs, it has been speculated that IL-10 may be inhibited by the Pdcd4-eIF4A complex. We therefore explored the mechanism by which Pdcd4 regulates IL-10 expression using the RIP assay. As shown in Figure 6b, the positive control (U1 snRNA from anti-SNRNP70) was detected, but the IL-10 amplification product was not detected by the anti-Pdcd4 Ab, indicating that Pdcd4 did not bind the IL-10 mRNA to inhibit its translation. Because the MAPK, STAT3 and SOCS3 pathways are responsible for the regulation of IL-10 expression in macrophages37, we examined these pathways by Western blotting. The results showed that Pdcd4 deficiency markedly increased the phosphorylation of ERK1/2 and P38 but did not affect the phosphorylation of JNK (Figure 6c), STAT3 and SOCS3 (Supplementary Figure 5). Furthermore, inhibition of ERK1/2 and P38 activation using their specific inhibitors (PD98059 and SB203580, Figure 6d) reversed the difference in the IL-10 levels induced by Pdcd4 deficiency, respectively (Figure 6e). To exclude an effect of Pdcd4 on the stability of IL-10 mRNA, we also measured the changes in the levels of IL-10 mRNA after treatment of actinomycin D, an inhibitor of de novo transcription. As shown in Supplementary Figure 6, the level of IL-10 mRNA in all groups (the presence or absence of Pdcd4 or the presence or absence of ERK and P38 inhibitors, PD98095 and SB203580) decreased to less 50% within 40 min of treatment with actinomycin D, suggesting that Pdcd4 had no effect on the stability of the IL-10 mRNA (Supplementary Figure 6). Taken together, these results indicate that Pdcd4 inhibits the transcription of IL-10 through the ERK1/2 and P38 MAPK pathways in macrophages.
Figure 6.
Pdcd4 regulated IL-10 expression through the ERK1/2 and p38 MAPK pathways. (a) Peritoneal macrophages of C57BL/6 mice were collected, cultured and stimulated with oxLDL (50 μg/mL) for 24 h and 48 h. The expression of Pdcd4 was detected by Western blotting and semi-quantified. The number of replicates at 24 h and 48 h compared with 0 h are indicated. (b) Lysates of the activated macrophages were used for RIP with rabbit-anti-mouse Pdcd4 antibody, normal rabbit IgG as negative control or anti-SNRNP70 antibody as positive control. The purified RNA was then analyzed by real-time PCR using specific primers for IL-10 or the positive control U1 snRNA. (c) Peritoneal macrophages of C57BL/6 and Pdcd4−/− mice were collected, cultured in vitro and stimulated with oxLDL (50 μg/mL) for 6 h, 12 h, and 24 h. The phosphorylated (activated) forms of JNK, ERK1/2 and p38 in macrophages were detected by Western blotting and semi-quantified. The numbers of replicates in experimental groups compared with 0 h of C57/BL6 mice are indicated. (d) The peritoneal macrophages from C57BL/6 and Pdcd4−/− mice were collected, cultured in vitro, stimulated with oxLDL (50 μg/mL) and blocked with ERK1/2 inhibitor (PD98059) or p38 inhibitor (SB203580) for 12 h. The expression of p-ERK1/2 and p-P38 after blocking with the inhibitors was detected by Western blotting and semi-quantified. The number of replicates in experimental groups compared with control was indicated. (e) The concentrations of IL-10 in the culture supernatants were measured using ELISA. The pooled data ± SEM are shown. *P < 0.05. The data are derived from three separate experiments.
Discussion
In the present study, we demonstrated that a deficiency in Pdcd4 attenuates the development of atherosclerotic lesions in hyperlipidemic mice by upregulating the anti-inflammatory cytokine IL-10. Our finding suggests that Pdcd4 is a potential novel target for an anti-atherosclerosic therapeutic agent.
The accumulating evidence from both murine models and human primary tumors indicates that Pdcd4 is an oncogene suppressor that inhibits the proliferation of multiple types of tumor cells and suppresses their malignant phenotype6,7,8,9,10,11,38. In the present study, we provide the first evidence that Pdcd4 deficiency suppresses the formation of atherosclerotic lesions. These data are consistent with previous studies of autoimmune encephalomyelitis, LPS-induced shock, type 1 diabetes, and diet-induced obesity11,12,13,14. In addition, downregulation of Pdcd4 by miR-21 was shown to protect cardiac cells against ischemia/reperfusion or reactive oxygen species (ROS)-induced injury39,40. Taken together, these results indicate that Pdcd4 plays an essential role in promoting inflammation.
Although data from several disease models indicate that a deficiency in Pdcd4 attenuates inflammatory diseases, the underlying mechanisms for these effects are unclear. Previous studies have reported that Pdcd4 inhibits the expression of the anti-inflammatory cytokine IL-10 and that KO or knockdown of Pdcd4 significantly upregulates IL-10 production11,12,35. Consistent with these previous reports, in the present study, we observed that Pdcd4-deficient macrophages stimulated with oxLDL produce a higher level of IL-10 than WT cells, and high levels of IL-10 also existed in the local lesions of Pdcd4−/−Apoe−/− mice. More importantly, the administration of a neutralizing anti-IL10 antibody significantly prevented the anti-atherosclerotic effects of Pdcd4 deficiency. These results establish a link between the increased IL-10 levels and the reduced atherosclerotic lesions associated with the Pdcd4 deficiency. However, although the level of IL-10 in the serum was significantly decreased by treatment with an anti-IL-10 antibody in the Pdcd4−/−Apoe−/− mice, this treatment only partially restored the plaque size in the Pdcd4+/+Apoe−/− mice (Figure 5d and 5f). These results suggest that other factors may also be involved in the Pdcd4-mediated regulation of atherosclerosis. In our previous studies, we have found that a deficiency in Pdcd4 attenuates adipocyte foam cell formation14 and more recent data show that the deficiency in Pdcd4 also attenuated macrophage foam cell formation (data not shown). These data suggest that the Pdcd4 deficiency-associated attenuated formation of macrophage foam cells may contribute to the decrease in lesions. However, more experiments are needed to address this issue in the future.
Although a previous report indicated that Pdcd4 regulates IL-10 expression at the translational level12, by using the RIP assay, we found that Pdcd4 cannot bind directly or indirectly to the IL-10 mRNA, suggesting that Pdcd4 does not inhibit IL-10 translation. Consistent with two recent reports35,36, we observed that the Pdcd4-deficient macrophages from both Pdcd4−/− mice and Pdcd4−/−Apoe−/− mice expressed high levels of IL-10 mRNA, suggesting that Pdcd4 regulates the expression of IL-10 at the transcriptional level. Our further results indicated that the increased IL-10 mRNA transcription observed in macrophages lacking Pdcd4 was mediated by activation of ERK1/2 and p38 rather than through the JNK MAPK, STAT3 or SOCS3 pathways. Furthermore, we have excluded an effect of Pdcd4 on the stability of IL-10 mRNA using inhibitors of de novo transcription (actinomycin D) in the presence or absence of Pdcd4 and in the presence or absence of ERK1/2 and p38 inhibitors. More recent research has shown that Pdcd4 can inhibit the transcription of IL-10 through the Twist2/c-Maf pathway. Under normal condition, Pdcd4 forms a complex with the transcription factor Twist2 and then blocks the binding of Twist2 to the c-Maf promoter. The degradation of Pdcd4 induced by LPS stimulation leads to the disruption of this complex, allowing Twist2 to bind to the c-Maf promoter, which induces transcription of IL-10. It has been reported that the phosphorylation of c-Maf is essential for its transcriptional and biological properties and that p38/ERK MAP kinase can phosphorylate c-Maf, which regulates the activity of c-Maf41,42. These results suggest that Pdcd4 may inhibit the expression of IL-10 by inhibiting the p38/ERK-c-Maf pathway. However, more experiments are needed to address this issue.
Recently, studies have shown that IL-17, a major effector molecule of Th17 cells, is involved in atherosclerosis. However, its role is unclear, given conflicting results that have shown both protective and pathogenic effects43,44. In the present study, we found that Pdcd4 deficiency led to decreased production of IL-17 by T cells in vitro and a low level of IL-17 expression in CD4+ T cell of atherosclerotic lesion in Pdcd4−/−Apoe−/− mice. However, supplementation with exogenous IL-17 did not reverse the attenuated atherosclerosis. A similar phenomenon was also observed in diabetic models in which the change in IL-17 in the splenocytes from Pdcd4−/− NOD mice was not consistent with the attenuated diabetes after Pdcd4 deficiency13. These results suggest that, although Pdcd4 deficiency can change IL-17 expression, this change had no causal relationship with the attenuation of atherosclerotic plaques observed in Pdcd4 deficiency. Blocking IL-10 with a neutralizing antibody significantly reversed the Pdcd4 deficiency-induced decrease in the atherosclerotic lesions but supplementation with exogenous IL-17 did not, despite the fact that the level of IL-17 was markedly increased following the IL-17 treatment (Figure 5). These results suggest that effect of Pdcd4 on IL-17 may be indirect. The reduction of IL-17 in the plaques of the Pdcd4−/−Apoe−/− mice may be due to the improved inflammatory environment induced by the high level of IL-10.
Taken together, these results indicate that Pdcd4 negatively regulates the expression of IL-10 in an ERK1/2- and p38-dependent manner and thereby promotes atherosclerosis.
Acknowledgments
We are grateful to the Key Laboratory of Cardiovascular Remodeling and Function Research, Qilu Hospital, Shandong University for expert technical assistance. This work was supported by the National 973 Basic Research Program of China (Nos. 2011CB503906, 2012CB518603), grants from the National Natural Science Foundation of China (81172863, 31470856 and 91439124) and the Natural Science Foundation of Shandong (Z2008C02), and a grant from the China Postdoctoral Science Foundation (2014 M551912).
The authors declare that there is no conflict of interest in this article.
Footnotes
Supplementary information accompanies the article can be found on the Cellular & Molecular Immunology's website (http://www.nature.com/cmi).
Supplementary Information
References
- Yang HS, Jansen AP, Komar AA, Zheng X, Merrick WC, Costes S et al. The transformation suppressor Pdcd4 is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation. Mol Cell Biol 2003; 23: 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibahara K, Asano M, Ishida Y, Aoki T, Koike T, Honjo T. Isolation of a novel mouse gene MA-3 that is induced upon programmed cell death. Gene 1995; 166: 297–301. [DOI] [PubMed] [Google Scholar]
- Singh P, Wedeken L, Waters LC, Carr MD, Klempnauer KH. Pdcd4 directly binds the coding region of c-myb mRNA and suppresses its translation. Oncogene 2011; 30: 4864–4873. [DOI] [PubMed] [Google Scholar]
- Wang Q, Sun Z, Yang HS. Downregulation of tumor suppressor Pdcd4 promotes invasion and activates both beta-catenin/Tcf and AP-1-dependent transcription in colon carcinoma cells. Oncogene 2008; 27: 1527–1535. [DOI] [PubMed] [Google Scholar]
- Waters LC, Veverka V, Bohm M, Schmedt T, Choong PT, Muskett FW et al. Structure of the C-terminal MA-3 domain of the tumour suppressor protein Pdcd4 and characterization of its interaction with eIF4A. Oncogene 2007; 26: 4941–4950. [DOI] [PubMed] [Google Scholar]
- Chen Y, Knosel T, Kristiansen G, Pietas A, Garber ME, Matsuhashi S et al. Loss of PDCD4 expression in human lung cancer correlates with tumour progression and prognosis. J Pathol 2003; 200: 640–646. [DOI] [PubMed] [Google Scholar]
- Mudduluru G, Medved F, Grobholz R, Jost C, Gruber A, Leupold JH et al. Loss of programmed cell death 4 expression marks adenoma-carcinoma transition, correlates inversely with phosphorylated protein kinase B, and is an independent prognostic factor in resected colorectal cancer. Cancer 2007; 110: 1697–1707. [DOI] [PubMed] [Google Scholar]
- Gao F, Zhang P, Zhou C, Li J, Wang Q, Zhu F et al. Frequent loss of PDCD4 expression in human glioma: possible role in the tumorigenesis of glioma. Oncol Rep 2007; 17: 123–128. [PubMed] [Google Scholar]
- Wei ZT, Zhang X, Wang XY, Gao F, Zhou CJ, Zhu FL et al. PDCD4 inhibits the malignant phenotype of ovarian cancer cells. Cancer Sci 2009; 100: 1408–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang X, Song X, Liu C, Shi Y, Wang Y et al. Programmed cell death 4 enhances chemosensitivity of ovarian cancer cells by activating death receptor pathway in vitro and in vivo. Cancer Sci 2010; 101: 2163–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard A, Hilliard B, Zheng SJ, Sun H, Miwa T, Song W et al. Translational regulation of autoimmune inflammation and lymphoma genesis by programmed cell death 4. J Immunol 2006; 177: 8095–8102. [DOI] [PubMed] [Google Scholar]
- Sheedy FJ, Palsson-McDermott E, Hennessy EJ, Martin C, O'Leary JJ, Ruan Q et al. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol 2010; 11: 141–147. [DOI] [PubMed] [Google Scholar]
- Ruan Q, Wang T, Kameswaran V, Wei Q, Johnson DS, Matschinsky F et al. The microRNA-21-PDCD4 axis prevents type 1 diabetes by blocking pancreatic beta cell death. Proc Natl Acad Sci U S A 2011; 108: 12030–12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Dong Z, Liu X, Song X, Song Q, Shang Q et al. Programmed cell death-4 deficiency prevents diet-induced obesity, adipose tissue inflammation, and insulin resistance. Diabetes 2013; 62: 4132–4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong B, Yang X, Sun Q, Liu L, Lan X, Tian J et al. Pdcd4 modulates markers of macrophage alternative activation and airway remodeling in antigen-induced pulmonary inflammation. J Leukoc Biol 2014; 96: 1065–1075. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang L, Wei Z, Zhang X, Gao Q, Ma Y et al. The inhibitory action of PDCD4 in lipopolysaccharide/D-galactosamine-induced acute liver injury. Lab Invest 2013; 93: 291–302. [DOI] [PubMed] [Google Scholar]
- Di Tullio MR, Russo C, Jin Z, Sacco RL, Mohr JP, Homma S et al. Aortic arch plaques and risk of recurrent stroke and death. Circulation 2009; 119: 2376–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352: 1685–1695. [DOI] [PubMed] [Google Scholar]
- Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell 2011; 145: 341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedgui A, Mallat Z. Anti-inflammatory mechanisms in the vascular wall. Circ Res 2001; 88: 877–887. [DOI] [PubMed] [Google Scholar]
- Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol 2011; 12: 204–212. [DOI] [PubMed] [Google Scholar]
- Huber SA, Sakkinen P, David C, Newell MK, Tracy RP. T helper-cell phenotype regulates atherosclerosis in mice under conditions of mild hypercholesterolemia. Circulation 2001; 103: 2610–2616. [DOI] [PubMed] [Google Scholar]
- Zhou X, Nicoletti A, Elhage R, Hansson GK. Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation 2000; 102: 2919–2922. [DOI] [PubMed] [Google Scholar]
- Whitman SC, Ravisankar P, Elam H, Daugherty A. Exogenous interferon-gamma enhances atherosclerosis in apolipoprotein E−/− mice. Am J Pathol 2000; 157: 1819–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Jiang Y, Ma T, Zhu F, Gao F, Zhang P et al. A critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J Immunol 2010; 185: 5820–5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, Ley K et al. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation 2010; 121: 1746–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux H. Type 1 T-regulatory cells: their role in the control of immune responses. Transplantation 2003; 75: 8S-12S. [DOI] [PubMed] [Google Scholar]
- Halvorsen B, Waehre T, Scholz H, Clausen OP, von der Thusen JH, Muller F et al. Interleukin-10 enhances the oxidized LDL-induced foam cell formation of macrophages by antiapoptotic mechanisms. J Lipid Res 2005; 46: 211–219. [DOI] [PubMed] [Google Scholar]
- Arjuman A, Chandra NC. Effect of IL-10 on LOX-1 expression, signalling and functional activity: an atheroprotective response. Diab Vasc Dis Res 2013; 10: 442–451. [DOI] [PubMed] [Google Scholar]
- Mallat Z, Besnard S, Duriez M, Deleuze V, Emmanuel F, Bureau MF et al. Protective role of interleukin-10 in atherosclerosis. Circ Res 1999; 85: e17–e24. [DOI] [PubMed] [Google Scholar]
- Pinderski Oslund LJ, Hedrick CC, Olvera T, Hagenbaugh A, Territo M, Berliner JA et al. Interleukin-10 blocks atherosclerotic events in vitro and in vivo. Arterioscler Thromb Vasc Biol 1999; 19: 2847–2853. [DOI] [PubMed] [Google Scholar]
- Almer G, Frascione D, Pali-Scholl I, Vonach C, Lukschal A, Stremnitzer C et al. Interleukin-10: an anti-inflammatory marker to target atherosclerotic lesions via PEGylated liposomes. Mol Pharm 2013; 10: 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiuri G, Rudling M, Ollivier V, Jacob MP, Michel JB, Hansson GK et al. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol Med 2003; 9: 10–17. [PMC free article] [PubMed] [Google Scholar]
- Namiki M, Kawashima S, Yamashita T, Ozaki M, Sakoda T, Inoue N et al. Intramuscular gene transfer of interleukin-10 cDNA reduces atherosclerosis in apolipoprotein E-knockout mice. Atherosclerosis 2004; 172: 21–29. [DOI] [PubMed] [Google Scholar]
- Das A, Ganesh K, Khanna S, Sen CK, Roy S. Engulfment of apoptotic cells by macrophages: a role of microRNA-21 in the resolution of wound inflammation. J Immunol 2014; 192: 1120–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bosch MW, Palsson-Mcdermott E, Johnson DS, O'Neill LA. LPS induces the degradation of programmed cell death protein 4 (PDCD4) to release Twist2, activating c-Maf transcription to promote interleukin-10 production. J Biol Chem 2014; 289: 22980–22990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM et al. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci U S A 2006; 103: 2274–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen AP, Camalier CE, Colburn NH. Epidermal expression of the translation inhibitor programmed cell death 4 suppresses tumorigenesis. Cancer Res 2005; 65: 6034–6041. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Liu X, Zhang S, Lin Y, Yang J, Zhang C. MicroRNA-21 protects against the H(2)O(2)-induced injury on cardiac myocytes via its target gene PDCD4. J Mol Cell Cardiol 2009; 47: 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Zhu P, Yang J, Liu X, Dong S, Wang X et al. Ischaemic preconditioning-regulated miR-21 protects heart against ischaemia/reperfusion injury via anti-apoptosis through its target PDCD4. Cardiovasc Res 2010; 87: 431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sii-Felice K, Pouponnot C, Gillet S, Lecoin L, Girault JA, Eychene A et al. MafA transcription factor is phosphorylated by p38 MAP kinase. FEBS Lett 2005; 579: 3547–3554. [DOI] [PubMed] [Google Scholar]
- Benkhelifa S, Provot S, Nabais E, Eychene A, Calothy G, Felder-Schmittbuhl MP. Phosphorylation of MafA is essential for its transcriptional and biological properties. Mol Cell Biol 2001; 21: 4441–4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taleb S, Tedgui A, Mallat Z. Interleukin-17: friend or foe in atherosclerosis? Curr Opin Lipidol 2010; 21: 404–408. [DOI] [PubMed] [Google Scholar]
- Yu XH, Jiang N, Zheng XL, Cayabyab FS, Tang ZB, Tang CK. Interleukin-17A in lipid metabolism and atherosclerosis. Clin Chim Acta 2014; 431: 33–39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.