Abstract
The skin of patients with atopic dermatitis (AD) has a unique predisposition for colonization by Staphylococcus aureus (S. aureus), which contributes to the inflammation and grim prognosis of AD. Although the mechanism underlying the S. aureus-induced exacerbation of AD remains unclear, recent studies have found a pivotal role for pattern recognition receptors in regulating the inflammatory responses in S. aureus infection. In the present study, we used a typical mouse model of AD-like skin inflammation and found that S. aureus-associated nucleotide-binding oligomerization domain-containing protein 2 (NOD2) and toll-like receptor 2 (TLR2) ligands exacerbated AD-like symptoms, which were further deteriorated by the in vivo expansion of basophils and eosinophils. Subsequent histological analyses revealed that dermal fibroblasts were pervasive in the AD-like skin lesions. Co-culture of human dermal fibroblasts with basophils and eosinophils resulted in a vigorous cytokine/chemokine response to the NOD2/TLR2 ligands and the enhanced expression of intercellular adhesion molecule-1 on the dermal fibroblasts. Basophils and eosinophils were primarily responsible for the AD-related cytokine/chemokine expression in the co-cultures. Direct intercellular contact was necessary for the crosstalk between basophils and dermal fibroblasts, while soluble mediators were sufficient to mediate the eosinophil–fibroblast interactions. Moreover, the intracellular p38 mitogen-activated protein kinase, extracellular signal-regulated kinase, and nuclear factor-kappa B signaling pathways were essential for NOD2/TLR2 ligand-mediated activation of basophils, eosinophils, and dermal fibroblasts in AD-related inflammation. This study provides the evidence of NOD2/TLR2-mediated exacerbation of AD through activation of innate immune cells and therefore sheds light on a novel mechanistic pathway by which S. aureus contributes to the pathophysiology of AD.
Keywords: atopic dermatitis; basophils, eosinophils; NOD2; TLR2
Introduction
Atopic dermatitis (AD) is a chronic, relapsing type of inflammatory skin disorder characterized by eczematous skin lesions, elevated serum immunoglobulin E (IgE) levels, and increased the expression of T helper type 2 (Th2) cytokines and chemokines.1 The pathogenesis of AD involves an abnormal immune response to environmental allergens that is putatively related to compromised epidermal barrier functions.2 The skin of AD patients is prone to colonization by microbial organisms, notably the gram-positive bacteria Staphylococcus aureus (S. aureus).3 Therapeutic interventions to eliminate S. aureus have been shown to alleviate AD symptoms, suggesting a deteriorating role for S. aureus in the disease.4,5 Percutaneous application of peptidoglycan (PGN), which is a cell wall component of S. aureus, induces eosinophil infiltration in the skin.6 Lipoteichoic acid (LTA), another cell wall component of S. aureus, is widely present in AD skin lesions, and its amount detected in the skin correlate with the severity of AD.7 Although it has long been postulated that superficial infection with S. aureus contributes to AD pathophysiology, the underlying detailed relationship between S. aureus infection and allergic inflammation in AD remains to be addressed.
Unlike adaptive immune responses that mainly rely upon the clonal expansion of antigen-specific cells, innate immunity can efficiently detect a board spectrum of pathogens through pattern recognition receptors (PRRs), including toll-like receptors (TLRs), nucleotide-binding oligomerization domain-like receptors, and retinoid acid-inducible gene I-like receptors.8 The PRRs recognize pathogen-associated molecular patterns, which are evolutionarily conserved microbial structural components that are essential for the survival of microorganisms.8 Among the different PRRs, nucleotide-binding oligomerization domain-containing protein 2 (NOD2) and toll-like receptor 2 (TLR2) have been assigned crucial roles in mediating immune recognition of S. aureus. NOD2, but not NOD1, was shown to be crucial for the recognition of S. aureus and the induction of antibacterial immunity in a mouse model of skin infection.9 TLR2 expressed in the skin recognizes S. aureus-derived PGN and LTA and induces innate immune responses via intracellular signal transduction.10,11 TLR2-deficient mice are highly susceptible to S. aureus invasion, and macrophages from these mice release less interleukin (IL)-6 and IL-10 in response to heat-killed S. aureus.12 Importantly, TLR2 is involved in AD-associated immune dysregulation13 because the TLR2 ligand PGN (but not the TLR4 ligand lipopolysaccharide) activates basophils to secrete IL-4 and IL-13 in both an IgE-dependent and IgE-independent manner.14
Basophils and eosinophils have been reported to be associated with a variety of skin diseases, including AD.15,16 As the rarest circulating leukocytes with unique functions, basophils are renowned as important regulators of allergic inflammation.17 Large quantities of basophils are found in the skin biopsies of patients with AD, especially following provocative allergen challenges.15 Eosinophils are the principal effector cells of allergic inflammation and have long been accepted as the hallmark of Th2-type immune responses.18 Activated eosinophils release cytotoxic granular proteins and inflammatory mediators, causing epidermal damage, tissue swelling, and recruitment of inflammatory cells.19 Interestingly, both basophils and eosinophils have been shown to express NOD2 and TLR2, which facilitate the detection of S. aureus by these innate immune cells.20,21,22,23 Although NOD2 and TLR2 have been reported to be crucial for the inflammatory response in S. aureus infection, whether these PRRs are involved in the pathophysiology of AD remains unclear. Therefore, in the present study, we aimed to reveal the underlying relationship between concurrent S. aureus infection and AD by demonstrating that the NOD2 and TLR2 ligands trigger the activation of basophils and eosinophils by interacting with dermal fibroblasts.
Materials and methods
Mice
Inbred female BALB/c mice (8-weeks-old and 20 g weight) were purchased from The Laboratory Animal Services Centre, The Chinese University of Hong Kong (Hong Kong, China). All animal experiments were approved by the Animal Experimentation Ethics Committee of the Chinese University of Hong Kong.
AD mouse model
The AD mouse model was established according to previous publications.24,25,26 A total of 2 nmol of MC903 (Sigma-Aldrich Corp, St. Louis, MO, USA), which is a Th2-related AD-like skin inflammation-inducing compound, was topically applied in 5 μL of ethanol to the ears of the mice every other day for 14 days (days 0–14, eight times in total).24,25,26 Ear thickness was measured using a dial thickness gauge (Model G, Peacock, OZAKI MFG. CO., LTD, Tokyo, Japan) and recorded every other day for 16 days. Photographs of the mouse ears were taken on day 16, followed by the killing of the mice and histological analysis of the AD skin lesions. In certain experiments (Figures 1, 2, 3), 10 μg of the S. aureus-associated NOD2 ligand muramyl dipeptide (MDP) and the TLR2 ligands PGN and LTA (Invivogen, San Diego, CA, USA) were also topically administered to the mouse ears either separately or together with MC903 (2 nmol).
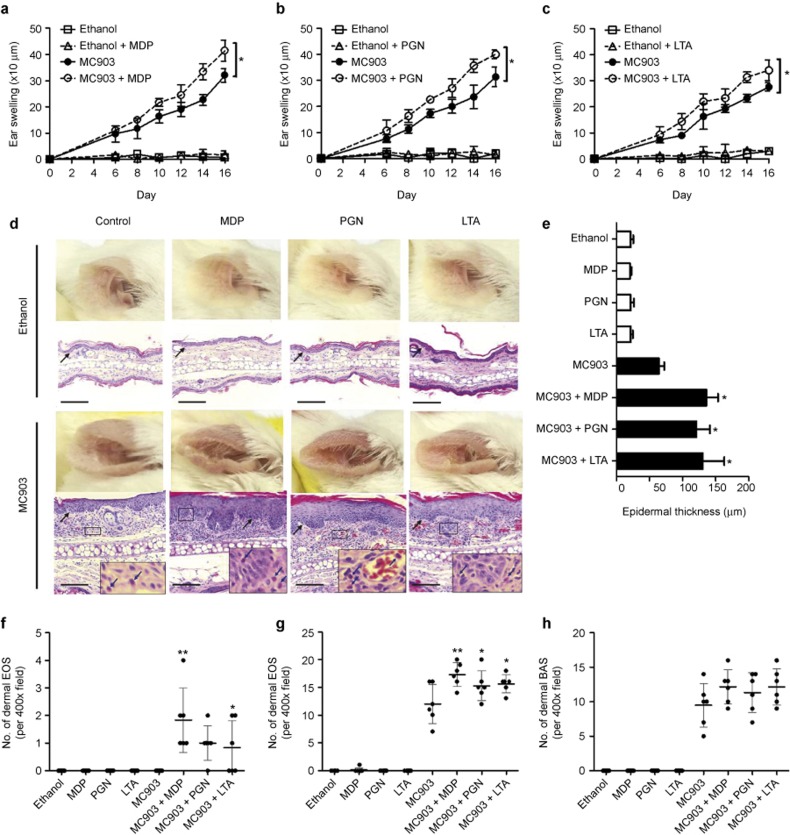
Figure 1.
Topical NOD2/TLR2 ligands exacerbate AD-like symptoms. An AD-like mouse model was established by administering MC903 on mouse ears. (a–c) Ear thickness was measured prior to the topical application of ethanol, MC903, or MC903 plus MDP (a), PGN (b), or LTA (c). (d and e) Photographs were taken and H&E staining was performed for the ears on day 16 (d), and epidermal thickness was measured (e). Blue arrows point to eosinophils. Black arrows point to the dermal/epidermal junction. Scale bars = 150 μm. (f–h) The number of eosinophils present in the epidermal (f) or dermal layer (g) and the number of basophils present in the dermal layer (h) per 400× field were counted with a microscope. BAS, basophils; EOS, eosinophils. The results are shown as the arithmetic mean ± SD. *P < 0.05, **P < 0.01.
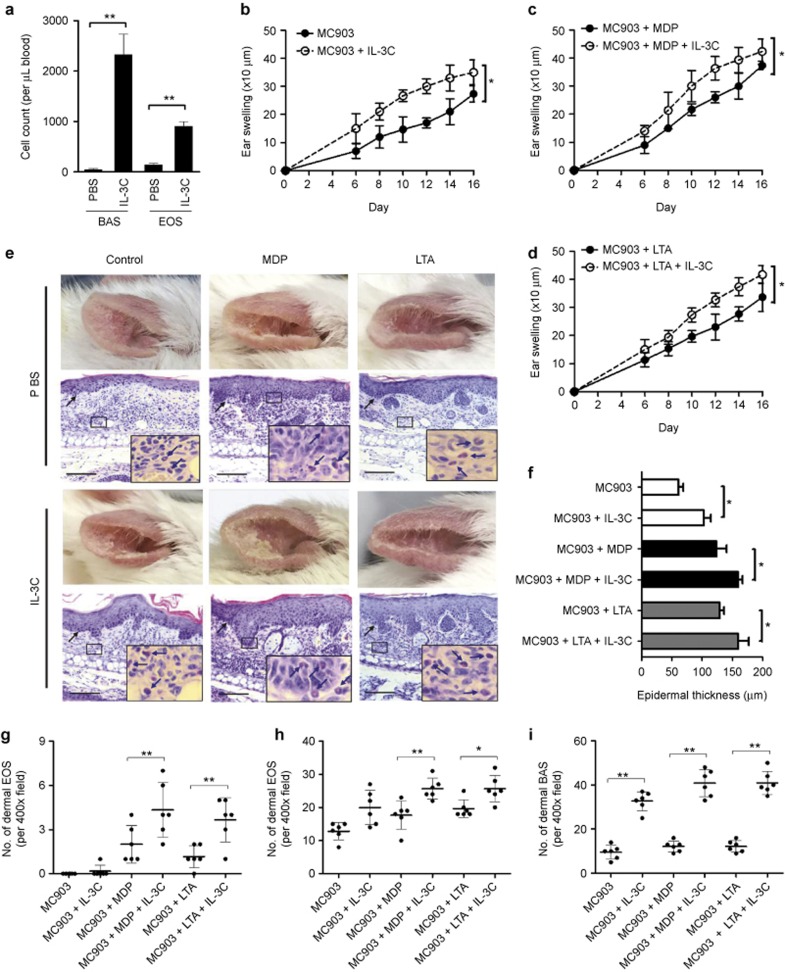
Figure 2.
Expansion of basophils and eosinophils further deteriorates AD-like skin inflammation in the presence of NOD2/TLR2 ligands. (a) Blood cell counts of basophils and eosinophils in PBS/IL-3C-treated mice. (b–d) Ear thickness was measured prior to topical administration of MC903 or MC903 plus ethanol (b), MDP (c), or LTA (d) in PBS/IL-3C-treated mice. (e and f) Photographs were taken, H&E staining was performed and epidermal thickness was measured as previously described. Blue arrows point to eosinophils. Black arrows point to the dermal/epidermal junction. Scale bars = 150 μm. (g–i) The numbers of eosinophils infiltrated into the epidermal (g) or dermal layer (h) and the number of basophils present in the dermal layer (i) were counted as previously described. The results are shown as the arithmetic mean ± SD. *P < 0.05, **P < 0.01.
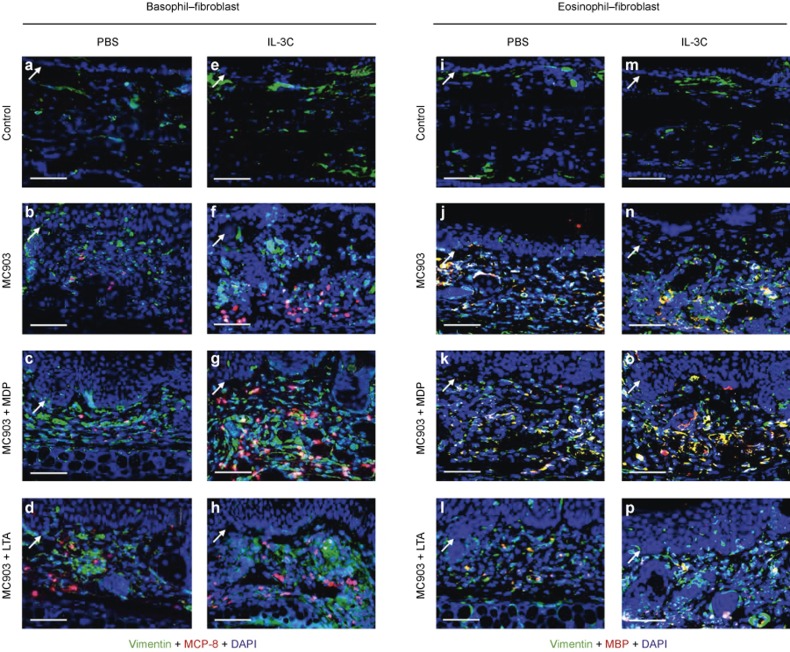
Figure 3.
Spatial analysis of basophils, eosinophils, and dermal fibroblasts in AD-like skin lesions. (a–h) Immunohistofluorescence analysis of basophils and dermal fibroblasts in AD-like skin lesions. The co-staining of dermal fibroblasts and basophils was identified using the anti-Vimentin (green) and anti-MCP-8 (red) antibodies, respectively. (i–p) Immunohistofluorescence analysis of eosinophils and dermal fibroblasts in AD-like skin lesions. The co-staining of dermal fibroblasts and eosinophils was identified using the anti-Vimentin (green) and anti-MBP (red) antibodies, respectively. Nuclei were visualized by DAPI (blue) staining. White arrows point to the dermal/epidermal junction. Scale bars = 100 μm.
Expansion of basophils and eosinophils in vivo
IL-3 complex (IL-3C) treatment was performed as previously described with some modifications.27 Briefly, 200 ng/mouse of recombinant mouse IL-3 (Peprotech Inc., Rocky Hill, NJ, USA) was incubated with 1 μg/mouse of rat anti-mouse IL-3 antibody (Biolegend Inc., San Diego, CA, USA) for 15 min at room temperature to form IL-3C, which was subsequently injected intraperitoneally (i.p.) into the mice every other day for 14 days (day 0–14, eight times in total). The mice were killed on day 16, and the blood, spleen, bone marrow, ear, and peritoneal exudate were collected for flow cytometric analysis or histological examination.
Depletion of basophils and eosinophils in vivo
Basophils and eosinophils were depleted by i.p. injection of 5 μg/mouse MAR-1 antibody or 10 μg/mouse TRFK5 antibody (Biolegend), respectively, in 200 μL of phosphate-buffered saline (PBS) each day for 14 consecutive days (days 0–13, 14 times in total). The mice were killed, and blood basophils and eosinophils were analyzed on day 16.
Histological examination and immunohistofluorescence
Hematoxylin and eosin (H&E) staining was performed as previously reported.28 Briefly, excised ears were fixed for 72 h at 4 °C with fixative (8% formalin). The fixed mouse ears were rinsed with PBS, dehydrated and embedded in paraffin. Sections (4 μm) were stained with H&E to assess the general morphology and eosinophil infiltration. For the detection of mast cells in the mouse ears, paraffin sections (4 μm) were stained with 0.1% toluidine blue (Sigma-Aldrich) for 1 min at room temperature.
To identify basophils, eosinophils, and dermal fibroblasts by immunohistofluorescence, paraffin sections of mouse ears were immunostained with lineage-specific antibodies. Briefly, 4 μm paraffin sections were subjected to antigen retrieval with citric acid buffer (10 mM, pH 6.0) and blocked with 5% fetal bovine serum (FBS) (Gibco Invitrogen Corp, Carlsbad, CA, USA), followed by incubation with a rat anti-mouse mast cell protease (MCP)-8 antibody (specific for basophils, Biolegend), rat anti-mouse major basic protein (MBP) antibody (specific for eosinophils, provided by James J. Lee, PhD, Mayo Clinic, Scottsdale, AZ, USA), or rabbit anti-Vimentin antibody (specific for dermal fibroblasts, Cell Signaling Technology, Beverly, MA, USA). A Cy3-conjugated goat anti-rat immunoglobulin G (IgG) antibody (Beyotime Co., Shanghai, China) and Alexa Fluor 488-conjugated goat anti-rabbit IgG antibody (Beyotime Co.) were used as the secondary antibodies. All images were acquired with a Leica DM6000B microscope (Leica Microsystems GmbH, Wetzlar, Germany) and processed using the Leica Application Suite software (Leica Microsystems GmbH). The epidermal thickness of five random areas was evaluated by measuring the distance between the basal membrane and stratum corneum using the Leica Application Suite software.
Flow cytometry
To detect specific cell populations in mice, cells from the blood, spleen, bone marrow, and peritoneal exudate were incubated with fluorescently labeled antibodies (all purchased from Biolegend, except for the Alexa Fluor 647-conjugated rat anti-mouse Siglec-F antibody from BD Biosciences, San Jose, CA, USA) for 30 min at 4 °C, followed by the lysis of red blood cells (RBCs) using a RBC lysis buffer (Biolegend). After washing with PBS, the cells were analyzed with a flow cytometer (FACSCalibur, BD Biosciences). Specific murine cell types were characterized using phenotypic markers: FcεRIα+CD49b+ for basophils, CCR3+Siglec-F+ for eosinophils, CD11b+Ly6G+ for neutrophils, CD11b+F4/80+ for monocytes, CD3+CD4+ for CD4+ T cells, CD3+CD8+ for CD8+ T cells, CD19+ for B cells, and FcεRIα+c-kit+ for mast cells.
To determine the surface expression of TLR2 and intercellular adhesion molecule (ICAM)-1 on human basophils, eosinophils, and dermal fibroblasts, the cells were resuspended in ice-cold PBS and blocked with 2% human pooled serum for 30 min at 4 °C, followed by incubation with a fluorescein isothiocyanate (FITC)-conjugated mouse anti-human TLR2 antibody (Imgenex, Novus Biologicals, Ltd., Cambridge, UK), a FITC-conjugated mouse anti-human ICAM-1 antibody (Biolegend) or FITC-conjugated mouse IgG1 isotypic control antibody (BD Biosciences) for an additional 30 min at 4 °C in the dark. After washing with PBS, the cells were subjected to flow cytometric analysis.
To determine the intracellular expression of NOD2 in human basophils, eosinophils, and dermal fibroblasts, the cells were fixed with 4% formaldehyde for 30 min at room temperature and permeabilized in ice-cold methanol for 30 min at 4 °C, followed by staining with a mouse anti-human NOD2 antibody (Biolegend) or mouse IgG1 isotypic control antibody (BD Biosciences). A FITC-conjugated goat anti-mouse IgG antibody (Invitrogen) was used as the secondary antibody. Then, the cells were washed and subjected to flow cytometric analysis. Data analyses were performed using FCS Express software version 4.0 (De Novo Software, Los Angeles, CA, USA).
Isolation of human basophils and eosinophils from the buffy coat
Fresh human buffy coats obtained from healthy volunteers at the Hong Kong Red Cross Blood Transfusion Service were diluted 1:2 with PBS at 4 °C and centrifuged using an isotonic Percoll solution (density 1.082 g mL–1; Amersham and Pharmacia Biotech, Uppsala, Sweden) for 30 min at 1000g.
For basophil isolation, the peripheral blood mononuclear cell (PBMC) fraction was collected and washed twice with cold PBS containing 2% FBS. Basophils were purified from the PBMC fraction using a Basophil Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) via the magnetic depletion of non-basophils by passing through an LS+ column (Miltenyi Biotec) within a magnetic field. For the isolation of eosinophils, the eosinophil-rich granulocyte fraction was collected and washed twice with cold PBS containing 2% FBS. Then, the cells were incubated with anti-CD16 magnetic beads (Miltenyi Biotec) at 4 °C for 45 min, and CD16-positive cells were depleted by passing through an LS+ column within a magnetic field.
The drop-through fractions of the above preparations contained basophils or eosinophils with a purity of at least 99% as assessed by the Hemacolor rapid blood smear stain (E Merck Diagnostica, Darmstadt, Germany). The isolated basophils or eosinophils were cultured in Roswell Park Memorial Institute 1640 medium (Gibco Invitrogen Corp) supplemented with 10% FBS and 20 mM Hepes (Gibco Invitrogen Corp). The above protocol using human basophils and eosinophils purified from human buffy coats was approved by the Clinical Research Ethics Committee of The Chinese University of Hong Kong-New Territories East Cluster Hospitals according to the Declaration of Helsinki.
Co-culture of human basophils/eosinophils and dermal fibroblasts
Primary human dermal fibroblasts isolated from adult skin were purchased from Life Technologies (Carlsbad, CA, USA) and grown in Medium 106 supplemented with the low serum growth supplement (Gibco Invitrogen Corp) until the monolayer reached confluence. For co-culture, the medium was replaced by Roswell Park Memorial Institute 1640 containing 10% FBS, and basophils or eosinophils (3 × 105 cells) and confluent fibroblasts (1 × 105 cells) were co-cultured with or without the NOD2/TLR2 ligands for the indicated time prior to the determination of the expression of cytokines/chemokines and ICAM-1. Except where indicated, the concentration of the NOD2/TLR2 ligands used in the experiments was 10 μg mL–1.
Co-culture of fixed baosphils/eosinophils and dermal fibroblasts
Confluent dermal fibroblasts or basophils or eosinophils were treated with paraformaldehyde (1% in PBS) for 15 min to prevent the release of cytokines/chemokines from the cells while preserving cell membrane integrity to maintain intercellular interactions. Then, the cells were washed three times with large volumes of 2% FBS in PBS, and fixed or unfixed cells were co-cultured in Roswell Park Memorial Institute 1640 containing 10% FBS.
Co-culture of baosphils/eosinophils and dermal fibroblasts in the presence of Transwell inserts
To prevent direct interaction between basophils or eosinophils and dermal fibroblasts in the co-culture, Transwell inserts (pore size 0.4 μm, BD Biosciences) were used to separate the cells into two compartments. Confluent dermal fibroblasts and basophils/eosinophils were cultured together in the presence of Transwell inserts in which the basophils/eosinophils and fibroblasts were placed in the upper and lower compartments, respectively.
Measurement of cytokines and chemokines
Concentrations of human CXCL8, CCL2, and CCL5 were quantitated with a human chemokine cytometric bead array (CBA) kit (BD Pharmingen Corp., San Diego, CA, USA). Concentrations of human IL-6, CXCL8, and CCL4 were measured by ELISA kits purchased from Biolegend.
Selective inhibition of cell signaling pathways
For the selective inhibition of cell signaling pathways, small molecule inhibitors specific to the p38 mitogen-activated protein kinase (p38 MAPK), nuclear factor kappa light-chain enhancer of activated B cells (NF-κB), and extracellular signal-regulated protein kinase (ERK) pathways were used as described in our previous publications.21,22,23 Briefly, human basophils or eosinophils (3 × 105 cells) co-cultured with confluent dermal fibroblasts (1 × 105 cells) were pretreated with 7.5 μM SB203580, 1 μM BAY11-7082, 10 μM U0126, 5 μM LY294002, or 3 μM SP600125 (Calbiochem, Merck Millipore, Darmstadt, Germany) for 1 h, followed by incubation with or without the NOD2/TLR2 ligands in the presence of these inhibitors for an additional 20 h. Then, cytokine/chemokine release and ICAM-1 expression were determined.
Detection of phosphorylated and total signaling molecules
KU812, which is a human basophilic cell line obtained from ATCC (Manassas, VA, USA), was used as a substitute for primary basophils for cell signaling analysis due to the scarcity of primary human basophils isolated from the buffy coats. After appropriate stimulation in co-culture, human dermal fibroblasts were separated from the KU812 cells and eosinophils by thorough rinsing (five times with large volumes of ice-cold PBS), followed by the determination of intracellular phosphorylated and total p38 MAPK, nuclear factor kappa light polypeptide gene enhancer in B-cell inhibitor alpha (IκBα) and ERK in the KU812 cells, eosinophils, and dermal fibroblasts using a Luminex Multiplex Kit (MAPmate Cell Signaling Buffer and Detection kit together with MAPmate Magnetic Beads, Merck Millipore, Darmstadt, Germany). The purity of the cells after separation was >99% for basophils/eosinophils and >85% for dermal fibroblasts (Supplementary Figure S1). The plates were read with the Bio-Plex 200 System (Bio-Rad, Hercules, CA, USA), and the results were expressed as the mean fluorescence intensity (MFI).
Statistical analysis
All data were analyzed with the SPSS 13.0 software (IBM Corporation, Armonk, NY, USA). One-way analysis of variance (ANOVA) was used to determine the statistical significance of differences between groups. Changes in ear thickness between different groups were assessed using repeated-measures ANOVA. Differences with P values < 0.05 were considered significant.
Results
Topical application of NOD2/TLR2 ligands induced exacerbation of AD-like skin inflammation
To test the in vivo effects of the S. aureus-associated NOD2 ligand MDP and the TLR2 ligands PGN and LTA in AD, we established a mouse model of AD-like skin inflammation using MC903, which was reported to mediate AD-like symptoms by inducing the expression of thymic stromal lymphopoietin (TSLP) in keratinocytes.24,25,26 As shown in Figure 1a and e–h and the left-most panel of Figure 1d, topical administration of MC903 on the ears for 14 days induced tissue swelling, epidermal thickening, and the infiltration of eosinophils and basophils into the dermal layer. Strikingly, topical application of the NOD2/TLR2 ligands together with the AD-inducing agent MC903 further deteriorated the AD-like symptoms, including enhanced ear swelling (Figure 1a–c), more extensive epidermal thickening (Figure 1d and e), and the presence of a larger number of eosinophils in the dermal layer (Figure 1g). NOD2/TLR2 ligand treatment alone did not induce effects in the absence of MC903 (Figure 1a–e and g and h), suggesting that the deteriorating effects of the NOD2/TLR2 ligands might only occur in pre-established skin lesions wherein the epidermal barrier function is disturbed. Previous publications have shown that eosinophil infiltration might only be observed in the dermal layer of AD skin lesions;24,29 however, here we found that topical S. aureus-associated NOD2/TLR2 ligands led to further epidermal infiltration of eosinophils in our mouse model of AD-like skin inflammation induced by MC903 (Figure 1f).
In vivo expansion of basophils and eosinophils amplified the deteriorating effects of the NOD2/TLR2 ligands on AD-like skin inflammation
In addition to their predisposition for cutaneous S. aureus infections, patients with AD are prone to basophilia and eosinophilia, which are often accompanied by the activation of circulating basophils and eosinophils.30,31,32 Therefore, we evaluated whether basophilia and eosinophilia (both of which are commonly observed in AD patients) might affect NOD2/TLR2 ligand-exacerbated AD-like skin inflammation. Herein, we examined the effects of the previously reported basophil-expansion agent IL-3C27 in our mouse model. As shown in Supplementary Figure S2 (left panel) and Figure 2a, repeated IL-3C i.p. treatments for 14 days dramatically increased the proportions of FcεRIα+CD49b+ basophils in the peripheral blood, spleen, and bone marrow, as well as the blood cell counts of basophils. IL-3C treatments also enhanced the eosinophil pool sizes because increased ratios of eosinophils were observed in all examined organs and systems (Supplementary Figure S2, right panel). Additionally, significantly increased eosinophil counts were found in the peripheral blood (Figure 2a). Nevertheless, IL-3C did not significantly affect the cellular pool sizes of neutrophils, monocytes, CD4+ T cells, CD8+ T cells, B cells, or skin/peritoneal mast cells (Supplementary Figure S3a–i), which confirmed the high specificity of IL-3C-mediated basophilia and eosinophilia.
Next, we investigated whether the IL-3C-expanded basophils and eosinophils affected the AD-like skin inflammation in the presence of the NOD2/TLR2 ligands. First, IL-3C treatments were found to deteriorate MC903-induced AD-like symptoms, as validated by enhanced ear swelling (Figure 2b), more extensive epidermal thickening (Figure 2e, left most panel, and Figure 2f), and a significantly increased number of eosinophils and basophils present in the dermal layer (Figure 2h and i). Synergistic effects were observed when i.p. IL-3C was administered together with topical NOD2/TLR2 ligands in the MC903-treated mice that further exacerbated the AD-like inflammation (Figure 2c–i). In mice administered IL-3C plus the NOD2/TLR2 ligands, severe skin lesions culminated in dry skin and eczematous ears (Figure 2e) that featured an intensely thickened epidermis (Figure 2e and f) and large quantities of eosinophils and basophils infiltrated into the epidermal and dermal layers (Figure 2g–i). To validate the role of the IL-3C-expanded basophils and eosinophils in the exacerbation of skin inflammation, anti-mouse FcεRIα antibody (MAR-1) and anti-mouse IL-5 antibody (TRFK5) were used for the in vivo depletion of basophils and eosinophils. As shown in Supplementary Figure S4a, i.p. administration of the MAR-1 antibody drastically reduced the quantity of basophils in the blood. Consequently, the IL-3C-deteriorated ear swelling was significantly alleviated in the MAR-1-administered mice that were deficient in basophils (Supplementary Figure S4b). Likewise, the reduction of blood eosinophils and alleviation of ear swelling were also observed in mice subjected to TRFK5 administration (Supplementary Figure S4c and d). The insignificant attenuation of ear swelling induced by the TRFK5 antibody might be partially attributed to the incomplete depletion of eosinophils (∼90% of total blood eosinophils depleted) as well as the infiltration of residual eosinophils into the inflamed skin (Supplementary Figure S4e). Together, these results indicate that IL-3C-mediated basophilia and eosinophila further deteriorated NOD2/TLR2 ligand-exacerbated AD-like skin inflammation.
Spatial analysis of basophils, eosinophils, and dermal fibroblasts in AD-like skin lesions
Because dermal fibroblasts have long been thought to be an important regulator of skin disorders due to their expression of adhesion molecules and the release of pro-inflammatory cytokines,33 here we hypothesized that these cells might participate in the pathophysiology of AD by interacting with basophils and eosinophils. To test this hypothesis, first, we investigated whether basophils and eosinophils were accessible to dermal fibroblasts in the lesional skin. Using immunohistofluorescence, the spatial distributions of basophils, eosinophils, and dermal fibroblasts were examined in the skin of mouse ears. The results showed that although they were pervasive in normal ear skin, dermal fibroblasts were enriched in the AD skin lesions (Figure 3a and b). A large number of infiltrated basophils and eosinophils were observed in the dermal layer and embedded in a backdrop of large numbers of dermal fibroblasts (Figure 3b and j). Consistent with the above results, similar cell distributions were observed in skin lesions of mice treated with MC903 that were co-administered IL-3C, the NOD2/TLR2 ligands, or IL-3C plus the NOD2/TLR2 ligands (Figure 3c and d, f–h, k and l, and n and p). These results collectively suggest that dermal fibroblasts, which are pervasive in the AD-like skin lesions, are accessible for the infiltrated basophils and eosinophils and that this accessibility is necessary for the interaction between these innate immune cells and adjacent dermal fibroblasts.
Co-culture of basophils/eosinophils and dermal fibroblasts significantly induced the expression of pro-inflammatory cytokines/chemokines and ICAM-1 in the presence of NOD2/TLR2 ligands
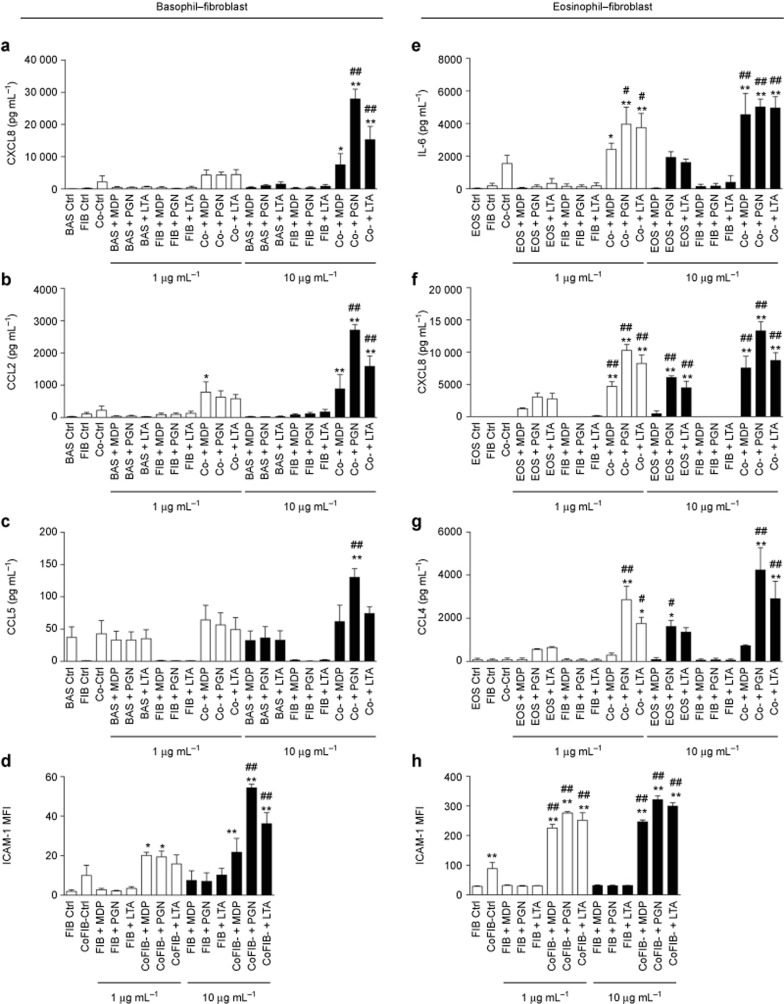
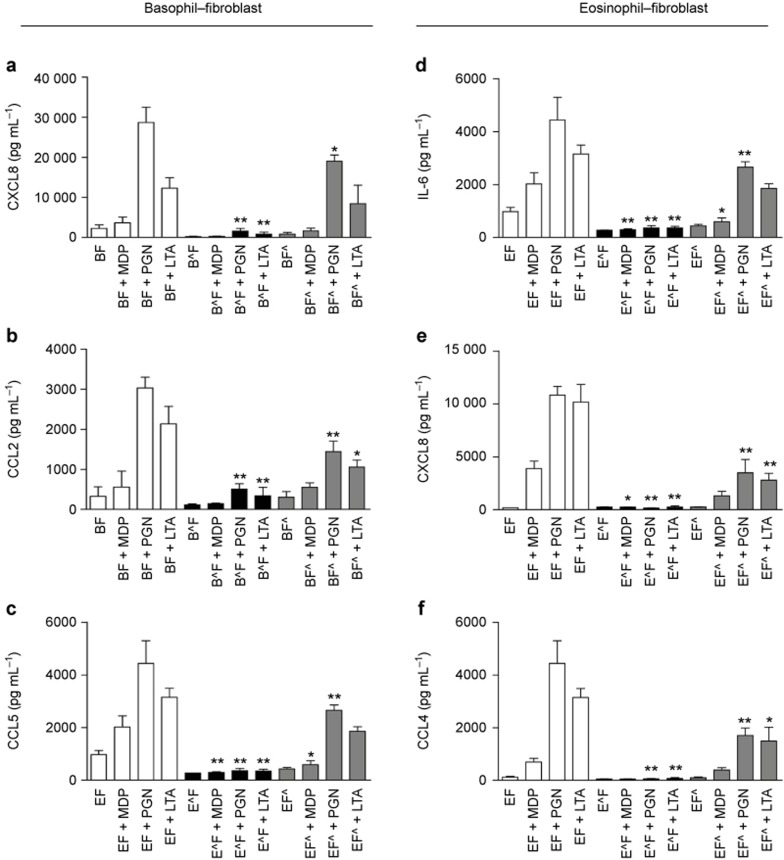
Next, we explored whether the interactions between basophils/eosinophils and dermal fibroblasts might enhance the activation of these cells upon exposure to the NOD2/TLR2 ligands using in vitro co-culture systems. Considering the scarcity of mouse basophils and eosinophils, and the difficulty of obtaining large amounts of these cells from murine sources, we chose to use their human counterparts to mimic human AD. As shown in Supplementary Figure S5a–f, human basophils, eosinophils, and dermal fibroblasts constitutively expressed intracellular NOD2 and cell surface TLR2, thereby facilitating the efficient detection of S. aureus by these innate immune cells. Upon co-culture of human basophils with dermal fibroblasts, large amounts of the AD-related chemokines CXCL8, CCL2, and CCL5 were released under the stimulation of the S. aureus-associated NOD2/TLR2 ligands (Figure 4a–c). Although no significant variation in ICAM-1 expression was detected on basophils (data not shown), a significant increase in ICAM-1 expression on dermal fibroblasts was observed under stimulation with the NOD2/TLR2 ligands in the basophil–fibroblast co-culture (Figure 4d). Similarly, human eosinophils co-cultured with dermal fibroblasts released larger amounts of IL-6, CXCL8, and CCL4 (Figure 4e–g) in the presence of the NOD2/TLR2 ligands, while the dermal fibroblasts expressed higher levels of ICAM-1 (Figure 4h).
Figure 4.
NOD2/TLR2 ligands activate human basophils and eosinophils in co-culture with dermal fibroblasts. (a–d) Basophils and dermal fibroblasts were cultured either together or separately with or without the NOD2/TLR2 ligands for 20 h. The release of CXCL8 (a), CCL2 (b), and CCL5 (c) was determined. (d) ICAM-1 expression on dermal fibroblasts was analyzed by flow cytometry and shown as MFI. (e–h) Likewise, eosinophils and dermal fibroblasts were cultured and stimulated. The release of IL-6 (e), CXCL8 (f), and CCL4 (g) were determined. (h) ICAM-1 expression on dermal fibroblasts was analyzed as previously described. BAS, basophils; EOS, eosinophils; FIB, dermal fibroblasts; Co, co-culture of dermal fibroblasts with basophils or eosinophils; CoFIB, dermal fibroblasts in co-culture. The results are shown as the arithmetic mean plus SD. *P < 0.05, **P < 0.01 compared between the denoted groups and the basophil (a–c), eosinophil (e–g), or fibroblast (d and h) control groups. #P < 0.05, ##P < 0.01 when compared between the denoted groups and the co-culture control groups.
Source of the cytokine/chemokine release in the co-culture system upon stimulation with NOD2/TLR2 ligands
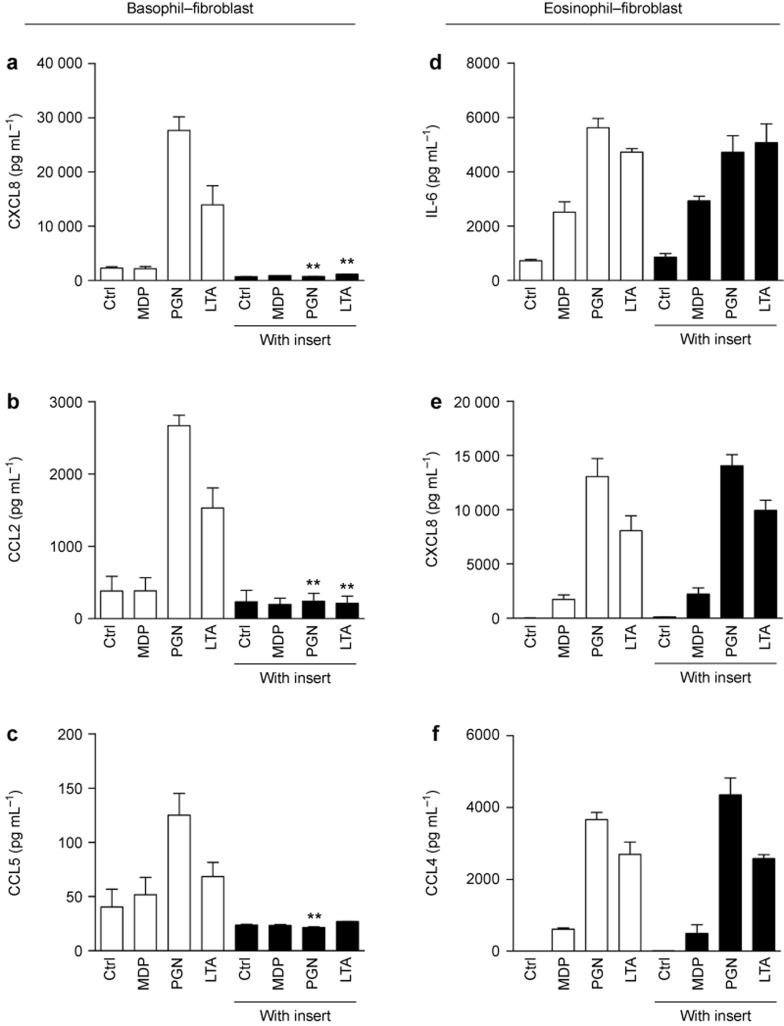
To investigate the source of the cytokines/chemokines released in the co-culture system, we compared the release of cytokines/chemokines from the co-culture of normal intact cells with paraformaldehyde-fixed cells. In the basophil-dermal fibroblast co-culture, the release of CXCL8, CCL2, and CCL5 were completely abrogated upon fixation of basophils alone but not the dermal fibroblasts (Figure 5a–c). Similarly, in the eosinophil–dermal fibroblast co-culture, the fixation of eosinophils but not dermal fibroblasts greatly suppressed the secretion of IL-6, CXCL8, and CCL4 (Figure 5d–f). These results collectively indicate that basophils and eosinophils co-cultured with dermal fibroblasts are the main source of cytokine/chemokine expression upon stimulation with the NOD2/TLR2 ligands.
Figure 5.
Source of cytokine/chemokine expression in co-cultures of basophils/eosinophils and dermal fibroblasts under stimulation with the NOD2/TLR2 ligands. (a–c) Basophils and dermal fibroblasts were treated with or without paraformaldehyde prior to being cultured together with or without the NOD2/TLR2 ligands for 20 h. The release of CXCL8 (a), CCL2 (b), and CCL5 (c) were determined. (d–f) Likewise, eosinophils and dermal fibroblasts were fixed, stimulated, and cultured. The release of IL-6 (d), CXCL8 (e), and CCL4 (f) were determined. B, unfixed basophils; B^, fixed basophils; E, unfixed eosinophils; E^, fixed eosinophils; F, unfixed dermal fibroblasts; F^, fixed dermal fibroblasts. The results are shown as the arithmetic mean plus SD. *P < 0.05, **P < 0.01 when compared between the denoted groups and the corresponding control groups of unfixed cells with MDP, PGN, or LTA treatment.
Effects of the Transwell insert on cytokine/chemokine release in the co-culture system upon stimulation with NOD2/TLR2 ligands
To explore whether direct intercellular contact was essential for the induction of cytokines/chemokines in the co-culture of basophils/eosinophils and dermal fibroblasts, Transwell inserts with a pore size of 0.4 μm were used to separate the co-cultured cells into two compartments. Intercellular communication by soluble mediators rather than direct intercellular interaction was allowed in this Transwell co-culture system. In the basophil–dermal fibroblast co-culture, the release of CXCL8, CCL2, and CCL5 was completely abrogated in the presence of the Transwell inserts (Figure 6a–c). However, in the eosinophil–dermal fibroblast co-culture, the separation of the cells barely suppressed IL-6, CXCL8, and CCL4 secretion (Figure 6d–f). Therefore, direct intercellular contact is necessary for the crosstalk between basophils and dermal fibroblasts to induce the expression of cytokines and chemokines, while soluble mediators are sufficient for the eosinophil–fibroblast interactions.
Figure 6.
Effect of Transwell inserts on cytokine/chemokine expression in co-cultures of basophils/eosinophils and dermal fibroblasts upon stimulation with the NOD2/TLR2 ligands. (a–c) Basophils and dermal fibroblasts were cultured together with or without MDP, PGN, or LTA in the presence or absence of Transwell inserts for 20 h. The release of CXCL8 (a), CCL2 (b), and CCL5 (c) were determined. (d–f) Likewise, eosinophils and dermal fibroblasts were stimulated and cultured. The release of IL-6 (d), CXCL8 (e), and CCL4 (f) were determined. The results are shown as the arithmetic mean plus SD. **P < 0.01 when compared between the denoted groups and the corresponding control groups with MDP, PGN, LTA, or Pam treatment in the absence of Transwell inserts.
Signal transduction via the p38 MAPK, NF-κB, and ERK pathways is responsible for the activation of basophils, eosinophils, and dermal fibroblasts in response to the NOD2/TLR2 ligands
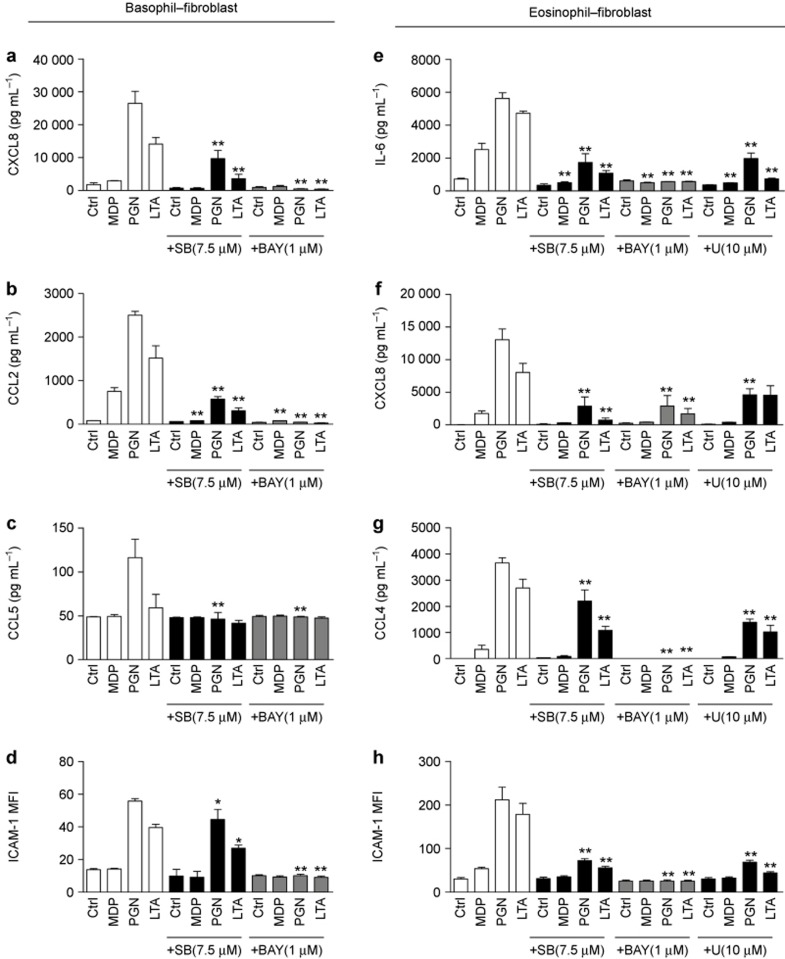
To address the intracellular signal transduction pathways responsible for NOD2/TLR2 ligand-induced activation of human basophils, eosinophils, and dermal fibroblasts, we adopted a previously reported approach to screen for the potential signaling pathways involved in the activation of these cells using small molecule inhibitors.33 As shown in Figure 7a–d, the p38 MAPK inhibitor SB203580 and NF-κB inhibitor BAY11-7082 significantly suppressed the NOD2/TLR2 ligand induced the release of CXCL8, CCL2, and CCL5 in basophils co-cultured with dermal fibroblasts and the increased expression of ICAM-1 on the dermal fibroblasts. However, neither the release of cytokines/chemokines nor the up-regulation of ICAM-1 expression was suppressed by the PI3K-AKT inhibitor LY294002, ERK inhibitor U0126, or JNK inhibitor SP600125 in basophils co-cultured with dermal fibroblasts under NOD2/TLR2 ligand stimulation (data not shown). These results suggested that the p38 MAPK and NF-κB pathways were essential for NOD2/TLR2-mediated activation of basophils and fibroblasts. In contrast, in the eosinophil–fibroblast co-culture, SB203580, BAY11-7082, and U0126 extensively suppressed the release of cytokines/chemokines and the increased ICAM-1 expression on dermal fibroblasts (Figure 7e–h). This indicated that in addition to p38 MAPK and NF-κB, ERK played an important role in the NOD2/TLR2 ligand-induced activation of eosinophils co-cultured with dermal fibroblasts.
Figure 7.
Inhibitors for the p38 MAPK, NF-κB, and ERK-MAPK pathways abolish NOD2/TLR2 ligand-induced activation of human basophils/eosinophils co-cultured with dermal fibroblasts. (a–d) Basophils co-cultured with dermal fibroblasts were pretreated with SB203580/BAY11-7082, followed by incubation with the NOD2/TLR2 ligands for an additional 20 h. The release of CXCL8 (a), CCL2 (b), and CCL5 (c) were determined. (d) ICAM-1 expression on dermal fibroblasts was analyzed and expressed as previously described. (e–h) Likewise, eosinophils co-cultured with dermal fibroblasts were pretreated with SB203580/BAY11-7082/U0126 and stimulated. The release of IL-6 (e), CXCL8 (f), CCL4 (g) were determined. (h) ICAM-1 expression on dermal fibroblasts was analyzed and expressed as described. SB, SB203580; BAY, BAY11-7082; U, U0126. The results are shown as the arithmetic mean plus SD. *P < 0.05, **P < 0.01 when compared between the denoted groups and the corresponding control groups with MDP, PGN, or LTA treatment.
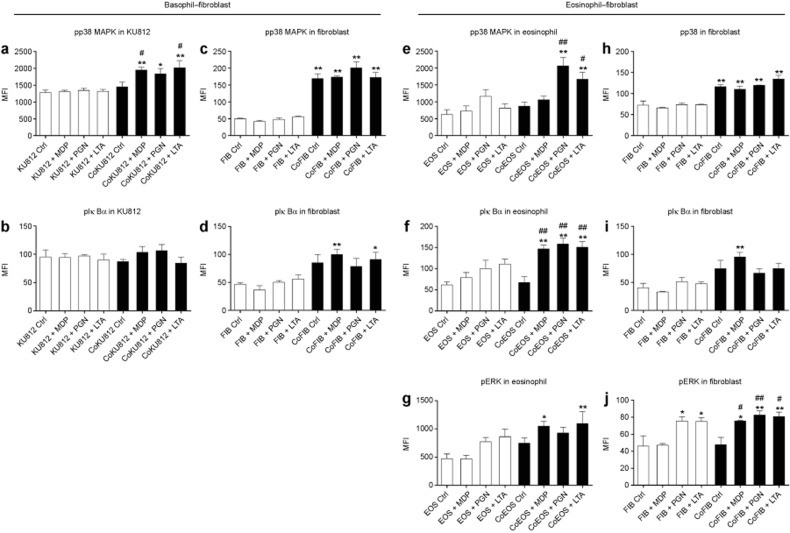
Moreover, using bead-based multiplex assays, we observed differentially phosphorylated p38 MAPK (pp38 MAPK) and phosphorylated IκBα (pIκBα) in KU812 cell lysates (a human basophilic cell line) and dermal fibroblasts in co-culture 10 min after stimulation with the NOD2/TLR2 ligands (Figure 8a–d and Supplementary Figure S6a–d). An elevated level of pp38 MAPK was detected in KU812 cells in co-culture with dermal fibroblasts upon MDP and LTA stimulation (Figure 8a). Co-culture by itself significantly activated p38 MAPK and IκBα in the dermal fibroblasts; this activation was further enhanced by NOD2/TLR2 ligand stimulation (Figure 8c and d). Likewise, in the eosinophil–fibroblast co-cultures MDP, PGN, and LTA stimulated the phosphorylation of p38 MAPK, IκBα, and ERK in eosinophils and ERK in dermal fibroblasts (Figure 8e–g and j and Supplementary Figure S6e–g and j). Additionally, elevated pp38 MAPK and pIκBα were also detected in fibroblasts co-cultured with eosinophils (Figure 8h and i and Supplementary Figure S6h and i).
Figure 8.
Activation of the p38 MAPK, IκBα, and ERK-MAPK pathways in human basophils/eosinophils co-cultured with dermal fibroblasts under NOD2/TLR2 ligand stimulation. (a–d) KU812 cells and dermal fibroblasts were cultured either together or separately with or without NOD2/TLR2 ligands for 10 min. Intracellular phosphorylated p38 MAPK and IκBα in KU812 cells and fibroblasts were determined using a Multiplex assay. (e–j) Likewise, eosinophils and dermal fibroblasts were cultured and stimulated. Intracellular phosphorylated p38 MAPK, IκBα, and ERK in eosinophils and fibroblasts were determined. CoKU812, KU812 cells in co-culture; Eos, eosinophils; CoEOS, eosinophils in co-culture; FIB, dermal fibroblasts; CoFIB, dermal fibroblasts in co-culture. The results are expressed as the MFI and shown as the arithmetic mean plus SD. *P < 0.05, **P < 0.01 when compared between the denoted groups and the KU812 cell (a and b), eosinophil (e–g), or fibroblast (c and d, h–j) control groups. #P < 0.05, ##P < 0.01 when compared between the denoted groups and the co-culture control groups.
Discussion
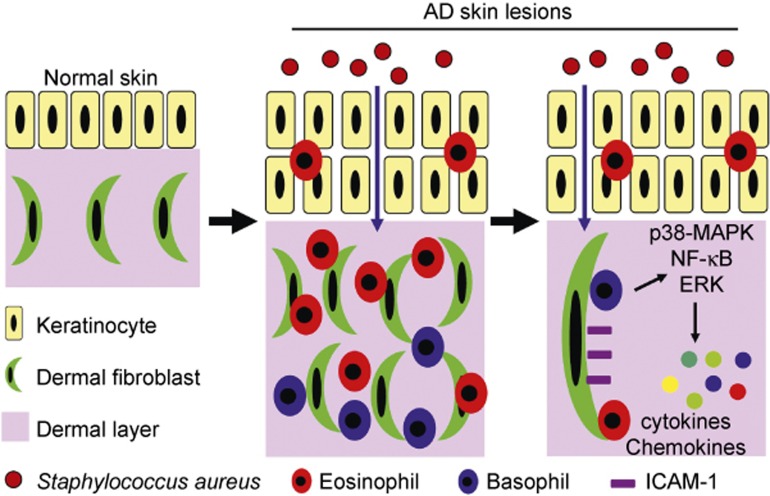
Previous studies have shown that patients with AD have a unique predisposition for colonization by S. aureus, which contributes to the persistent skin inflammation through the activation of bacterial-related PRRs such as NOD2 and TLR2.3,9,10,11 It has also been noted that the elimination of the bacteria can relieve the symptoms in AD patients.4,5 However, the pathological mechanism underlying S. aureus-exacerbated AD remains to be addressed, and no publications have elucidated the cellular and molecular pathways by which epicutaneous S. aureus infection leads to the deterioration of AD. Therefore, the current study focused on the in vivo and in vitro effects of the S. aureus-associated NOD2/TLR2 ligands and revealed that NOD2 and TLR2 exacerbated AD-like skin inflammation and induced the activation of basophils and eosinophils by interacting with dermal fibroblasts. A summary of these results are illustrated in Figure 9.
Figure 9.
S. aureus-derived NOD2/TLR2 ligands induce the activation of basophils and eosinophils by interacting with dermal fibroblasts in AD-like skin lesions. Patients with AD are prone to the epicutaneous colonization of S. aureus, which penetrates into the dermal layer through the disrupted epidermal barriers. Consequently, large amounts of NOD2/TLR2 ligands derived from S. aureus trigger the activation of basophils and eosinophils by interacting with dermal fibroblasts through the p38 MAPK, NF-κB, and ERK pathways and induce the release of pro-inflammatory cytokines/chemokines and up-regulated ICAM-1 expression on dermal fibroblasts.
One novel aspect of this study was that S. aureus-derived NOD2/TLR2 ligands were topically applied onto mouse ears together with the AD-inducing agent MC903, thereby mimicking the colonization of S. aureus in AD skin lesions and its penetration through the disrupted epidermal barrier. We also found that the infiltration of the NOD2/TLR2 ligands through the disrupted skin barrier further exacerbated tape stripping-induced lesions (data not shown). Enhanced AD severity was observed in mice that received the topical application of a combination of the MC903 and the NOD2/TLR2 ligands (Figure 1). It is worth noting that epidermal eosinophil infiltration could not be detected in the mice treated with MC903 alone unless it was applied together with the NOD2/TLR2 ligands (Figure 1f). Therefore, these results imply that topical S. aureus component diffuse through the disrupted epidermal barrier and may trigger an inflammatory response by recruiting inflammatory cells such as eosinophils to the local pathological sites (e.g., the epidermis). Although bacterial-derived NOD2/TLR2 ligands are not limited to S. aureus and PGN/LTA from Bacillus subtilis (a common commensal bacterium) was observed to aggravate skin inflammation (Supplementary Figure S7), AD-associated skin lesions are predominantly colonized by S. aureus, which comprises more than 80% of the skin flora at the culmination of AD symptoms.34 Therefore, our finding regarding the common and S. aureus-specific NOD2 and TLR2 ligands represent the pro-inflammatory effects of concurrent S. aureus colonization in AD patients afflicted with a disrupted skin barrier.
Basophils and eosinophils play crucial roles in both the sensitization and effector stages of AD. Basophils can function as the antigen-presenting cells in an allergen-induced Th2 response35 as well as serve as the initiator and major source of IL-4 in the memory immune response.36 In contrast, eosinophils are considered to be the hallmark of allergic diseases and are mainly involved in the effector stage of AD by expressing inflammatory cytokines/chemokines and releasing toxic granule proteins.19 Importantly, atopic diathesis is commonly observed in AD patients and is accompanied by an elevated level of circulating basophils and eosinophils with an activated cell status.30,31,32 In this study, we tried to extend our finding of NOD2/TLR2 ligand-mediated exacerbation of AD-like skin inflammation by exploring whether experimental basophilia/eosinophilia might aggravate AD in the presence of NOD2/TLR2 ligands. Our results subsequently depicted an efficient way to expand basophils and eosinophils in vivo, thereby mimicking the elevated levels of circulating basophils and eosinophils frequently observed in AD. Cytokines combined with specific antibodies form immune complexes that are resistant to degradation, thereby improving their stability and biological activity in vivo.37 IL-3, a cytokine that induces the differentiation of basophils and mast cells,38 has previously been shown to expand basophils in vivo without affecting the number of tissue mast cells.27 Although previous studies have emphasized IL-3C-mediated basophil expansion,27 our experiments demonstrated a dramatic increase in both basophils and eosinophils in the peripheral blood as well as in the AD-like skin lesions in the IL-3C-treated mice (Figure 2a, Supplementary Figures S2 and S3). These results confirmed the specificity of IL-3C-induced basophil and eosinophil expansion by showing that mice that received IL-3C treatment were comparable to normal mice in terms of the cell counts of mast cells in the peritoneum and ear tissue and the blood cell counts of neutrophils, monocytes, T cells, and B cells (Supplementary Figure S3).
The TLR2 ligand Pam2Cys was recently reported to induce chronic AD by sustaining adaptive immunity through IL-4-mediated suppression of IL-10.13 However, our investigations favor the view that innate immune cells actively take part in S. aureus-accentuated AD symptoms by focusing on the NOD2/TLR2-mediated activation of basophils, eosinophils, and dermal fibroblasts. NOD2 and TLR2 are the pivotal PRRs responsible for the immune recognition of S. aureus and are prevalently expressed on innate immune cells, including basophils and eosinophils.20,21,22,23 Moreover, our results provide the first evidence showing that IL-3C-expanded basophils and eosinophils further deteriorate AD-like inflammation in mice treated with epicutaneous S. aureus components and that the targeted experimental depletion of these innate immune cells protects mice from IL-3C-exacerbated skin inflammation (Figure 2 and Supplementary Figure S4).
Basophils were reported to cooperate with fibroblasts to induce the recruitment of eosinophils through up-regulated expression of CCL5 and eotaxin/CCL11 in a mouse model of irritant contact dermatitis.39 Co-cultivation with fibroblasts increased basophil CCL5 expression, while basophils induced elevated eotaxin/CCL11 expression in the fibroblasts.39 In our results, a large number of dermal fibroblasts and a dramatic infiltration of basophils and eosinophils were observed in the AD-like skin lesions (Figure 3). Because they are accessible to each other in vivo, we hypothesized that basophils and eosinophils might be activated in synergy with dermal fibroblasts in response to NOD2/TLR2 ligands. We validated this hypothesis in vitro by separately co-culturing basophils and eosinophils with dermal fibroblasts in the presence of the NOD2/TLR2 ligands. The results showed an increased release of pro-inflammatory cytokines/chemokines and enhanced expression of ICAM-1 on dermal fibroblasts in both the basophil–fibroblast and eosinophil–fibroblast co-cultures (Figure 4). All of the cytokines/chemokines evaluated in this study were primarily released by basophils and eosinophils following NOD2/TLR2 stimulation, although dermal fibroblasts were found to produce marginal amounts of CXCL8, CCL2, and IL-6 (Figures 4 and 5). Direct intercellular contact was necessary for the cooperation of basophils with dermal fibroblasts, while soluble mediators were sufficient for the interaction between eosinophils and dermal fibroblasts (Figure 6). Moreover, although synergistic effects were likewise observed in a co-culture between eosinophils and keratinocytes (the main cell type in the epidermal layer) (Supplementary Figure S8), the dermal layer was found to be the main pathological site of AD and the site of the vast majority of the basophil and eosinophil infiltration (Figures 1d and 3).
Regarding the intracellular signaling mechanisms, previous reports characterized the signal transduction pathways involved in the NOD2/TLR2-mediated activation of basophils and eosinophils co-cultured with bronchial epithelial cells and highlighted an important role for the MAPK and NF-κB pathways in the pathogenesis of allergic asthma.21,22,23,40 Using optimal concentrations of the small molecule inhibitors SB203580 (7.5 μM), BAY11-7082 (1 μM), U0126 (10 μM), LY294002 (5 μM), and SP600125 (3 μM) that had maximum inhibitory effects but no observable toxicity in the cells,33 we demonstrated the involvement of the NF-κB, p38 MAPK, and ERK pathways in the NOD2/TLR2-mediated activation of basophils, eosinophils, and dermal fibroblasts (Figure 7). Moreover, the phosphorylation of pivotal cell signaling molecules necessary for the activation of these pathways was confirmed using high-throughput bead-based multiplex assays (Figure 8).
Different signaling pathways have been reported to be accountable for the MDP-induced activation of basophils and eosinophils via interactions with bronchial epithelial cells.22,23 In the present study, both the p38 MAPK and NF-κB pathways were found to be essential for the NOD2/TLR2 ligand-mediated activation of basophils and eosinophils by interacting with dermal fibroblasts. However, the ERK pathway was indispensable for the activation of eosinophils (but not basophils) co-cultured with dermal fibroblasts (Figure 7). The incomplete coincidence of the cell signaling pathways responsible for NOD2/TLR2-mediated activation between basophils and eosinophils interacting with dermal fibroblasts may be partly due to variations in their preferences for signaling pathways and the distinct cellular responses (i.e., different cytokine/chemokine expression) to the same stimulus by different cells.
In conclusion, this study used an AD mouse model and ex vivo co-culture systems to illustrate a novel NOD2/TLR2-stimulated pathway that induced the activation of basophils and eosinophils via interaction with dermal fibroblasts. Taken together with previous publications demonstrating a key role for S. aureus in AD, the results of the current study depict a mechanism by which S. aureus induces the accentuation of AD through PRRs and innate immunity. Furthermore, our results concerning the vicious effects of S. aureus on AD shed light on the hypothesis that implementing therapeutic interventions to eliminate S. aureus may help to alleviate AD symptoms.
Acknowledgments
We thank James J. Lee from the Mayo Clinic (Scottsdale, AZ, USA) for providing us the rat anti-mouse MBP antibody. This work was supported by the Research Grant Committee General Research Fund, Hong Kong (project reference no.: CUHK 476813, principal investigator: CKW).
The authors declare no competing commercial interests in relation to this work.
Footnotes
Supplementary information of this article can be found on the Cellular & Molecular Immunology's website (http://www.nature.com/cmi).
Supplementary Information
References
- Bieber T. Atopic dermatitis. N Engl J Med 2008; 358: 1483–1494. [DOI] [PubMed] [Google Scholar]
- Sicherer SH, Leung DY. Advances in allergic skin disease, anaphylaxis, and hypersensitivity reactions to foods, drugs, and insects in 2013. J Allergy Clin Immunol 2014; 133: 324–334. [DOI] [PubMed] [Google Scholar]
- Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev 2011; 242: 233–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JT, Abrams M, Tlougan B, Rademaker A, Paller AS. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics 2009; 123: e808–e814. [DOI] [PubMed] [Google Scholar]
- Gong JQ, Lin L, Lin T, Hao F, Zeng FQ, Bi ZG et al. Skin colonization by Staphylococcus aureus in patients with eczema and atopic dermatitis and relevant combined topical therapy: a double-blind multicentre randomized controlled trial. Br J Dermatol 2006; 155: 680–687. [DOI] [PubMed] [Google Scholar]
- Matsui K, Wirotesangthong M, Nishikawa A. Percutaneous application of peptidoglycan from Staphylococcus aureus induces eosinophil infiltration in mouse skin. Clin Exp Allergy 2007; 37: 615–622. [DOI] [PubMed] [Google Scholar]
- Travers JB, Kozman A, Mousdicas N, Saha C, Landis M, Al-Hassani M et al. Infected atopic dermatitis lesions contain pharmacologic amounts of lipoteichoic acid. J Allergy Clin Immunol 2010; 125: 146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010; 140: 805–820. [DOI] [PubMed] [Google Scholar]
- Hruz P, Zinkernagel AS, Jenikova G, Botwin GJ, Hugot JP, Karin M et al. NOD2 contributes to cutaneous defense against Staphylococcus aureus through alpha-toxin-dependent innate immune activation. Proc Natl Acad Sci USA 2009; 106: 12873–12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo IH, Carpenter-Mendini A, Yoshida T, McGirt LY, Ivanov AI, Barnes KC et al. Activation of epidermal toll-like receptor 2 enhances tight junction function: implications for atopic dermatitis and skin barrier repair. J Invest Dermatol 2013; 133: 988–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebuhr M, Baumert K, Werfel T. TLR-2-mediated cytokine and chemokine secretion in human keratinocytes. Exp Dermatol 2010; 19: 873–877. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol 2000; 165: 5392–5396. [DOI] [PubMed] [Google Scholar]
- Kaesler S, Volz T, Skabytska Y, Koberle M, Hein U, Chen KM et al. Toll-like receptor 2 ligands promote chronic atopic dermatitis through IL-4-mediated suppression of IL-10. J Allergy Clin Immunol 2014; 134: 92–99. [DOI] [PubMed] [Google Scholar]
- Bieneman AP, Chichester KL, Chen YH, Schroeder JT. Toll-like receptor 2 ligands activate human basophils for both IgE-dependent and IgE-independent secretion. J Allergy Clin Immunol 2005; 115: 295–301. [DOI] [PubMed] [Google Scholar]
- Irani AM, Huang C, Xia HZ, Kepley C, Nafie A, Fouda ED et al. Immunohistochemical detection of human basophils in late-phase skin reactions. J Allergy Clin Immunol 1998; 101: 354–362. [DOI] [PubMed] [Google Scholar]
- Simon D, Braathen LR, Simon HU. Eosinophils and atopic dermatitis. Allergy 2004; 59: 561–570. [DOI] [PubMed] [Google Scholar]
- Karasuyama H, Mukai K, Obata K, Tsujimura Y, Wada T. Nonredundant roles of basophils in immunity. Annu Rev Immunol 2011; 29: 45–69. [DOI] [PubMed] [Google Scholar]
- Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol 2010; 125: S73–S80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P et al. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy 2008; 38: 709–750. [DOI] [PubMed] [Google Scholar]
- Komiya A, Nagase H, Okugawa S, Ota Y, Suzukawa M, Kawakami A et al. Expression and function of toll-like receptors in human basophils. Int Arch Allergy Immunol 2006; 140: 23–27. [DOI] [PubMed] [Google Scholar]
- Wong CK, Cheung PF, Ip WK, Lam CW. Intracellular signaling mechanisms regulating toll-like receptor-mediated activation of eosinophils. Am J Respir Cell Mol Biol 2007; 37: 85–96. [DOI] [PubMed] [Google Scholar]
- Qiu HN, Wong CK, Chu IM, Hu S, Lam CW. Muramyl dipeptide mediated activation of human bronchial epithelial cells interacting with basophils: a novel mechanism of airway inflammation. Clin Exp Immunol 2013; 172: 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CK, Hu S, Leung KM, Dong J, He L, Chu YJ et al. NOD-like receptors mediated activation of eosinophils interacting with bronchial epithelial cells: a link between innate immunity and allergic asthma. Cell Mol Immunol 2013; 10: 317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci USA 2006; 103: 11736–11741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Kim JR, Kim H, Kim YA, Lee HJ, Kim J et al. The atopic dermatitis-like symptoms induced by MC903 were alleviated in JNK1 knockout mice. Toxicol Sci 2013; 136: 443–449. [DOI] [PubMed] [Google Scholar]
- Rebane A, Runnel T, Aab A, Maslovskaja J, Ruckert B, Zimmermann M et al. MicroRNA-146a alleviates chronic skin inflammation in atopic dermatitis through suppression of innate immune responses in keratinocytes. J Allergy Clin Immunol 2014; 134: 836–847. [DOI] [PubMed] [Google Scholar]
- Ohmori K, Luo Y, Jia Y, Nishida J, Wang Z, Bunting KD et al. IL-3 induces basophil expansion in vivo by directing granulocyte-monocyte progenitors to differentiate into basophil lineage-restricted progenitors in the bone marrow and by increasing the number of basophil/mast cell progenitors in the spleen. J Immunol 2009; 182: 2835–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc 2008; 2008: t4986. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Midoro K, Kamei T, Gyoten M, Kawano Y, Ashida Y et al. Inhibition of allergic dermal inflammation by the novel imidazopyridazine derivative TAK-427 in a guinea pig experimental model of eczema. J Pharmacol Exp Ther 2002; 303: 1283–1290. [DOI] [PubMed] [Google Scholar]
- James JM, Kagey-Sobotka A, Sampson HA. Patients with severe atopic dermatitis have activated circulating basophils. J Allergy Clin Immunol 1993; 91: 1155–1162. [DOI] [PubMed] [Google Scholar]
- Jenerowicz D, Czarnecka-Operacz M, Silny W. Peripheral blood eosinophilia in atopic dermatitis. Acta Dermatovenerol Alp Pannonica Adriat 2007; 16: 47–52. [PubMed] [Google Scholar]
- Lewis SA, Pavord ID, Stringer JR, Knox AJ, Weiss ST, Britton JR. The relation between peripheral blood leukocyte counts and respiratory symptoms, atopy, lung function, and airway responsiveness in adults. Chest 2001; 119: 105–114. [DOI] [PubMed] [Google Scholar]
- Wong CK, Leung KM, Qiu HN, Chow JY, Choi AO, Lam CW. Activation of eosinophils interacting with dermal fibroblasts by pruritogenic cytokine IL-31 and alarmin IL-33: implications in atopic dermatitis. PLoS One 2012; 7: e29815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Glatz M, Horiuchi K, Kawasaki H, Akiyama H, Kaplan DH et al. Dysbiosis and Staphylococcus aureus colonization drives inflammation in atopic dermatitis. Immunity 2015; 42: 756–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol 2009; 10: 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodoun MV, Orekhova T, Potter C, Morris S, Finkelman FD. Basophils initiate IL-4 production during a memory T-dependent response. J Exp Med 2004; 200: 857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman FD, Madden KB, Morris SC, Holmes JM, Boiani N, Katona IM et al. Anti-cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J Immunol 1993; 151: 1235–1244. [PubMed] [Google Scholar]
- Lantz CS, Boesiger J, Song CH, Mach N, Kobayashi T, Mulligan RC et al. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature 1998; 392: 90–93. [DOI] [PubMed] [Google Scholar]
- Nakashima C, Otsuka A, Kitoh A, Honda T, Egawa G, Nakajima S et al. Basophils regulate the recruitment of eosinophils in a murine model of irritant contact dermatitis. J Allergy Clin Immunol 2014; 134: 100–107. [DOI] [PubMed] [Google Scholar]
- Jeon JH, Kim SK, Baik JE, Kang SS, Yun CH, Chung DK et al. Lipoteichoic acid of Staphylococcus aureus enhances IL-6 expression in activated human basophils. Comp Immunol Microbiol Infect Dis 2012; 35: 363–374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.