Abstract
Nutrition is a key component of life-history theory, yet we know little about how diet quality shapes life-history evolution across species. Here, we test whether quantitative measures of nutrition are linked to life-history evolution across 96 species of butterflies representing over 50 independent diet shifts. We find that butterflies feeding on high nitrogen host plants as larvae are more fecund, but their eggs are smaller relative to their body size. Nitrogen and sodium content of host plants are also both positively related to eye size. Some of these relationships show pronounced lineage-specific effects. Testis size is not related to nutrition. Additionally, the evolutionary timing of diet shifts is not important, suggesting that nutrition affects life histories regardless of the length of time a species has been adapting to its diet. Our results suggest that, at least for some lineages, species with higher nutrient diets can invest in a range of fitness-related traits like fecundity and eye size while allocating less to each egg as offspring have access to a richer diet. These results have important implications for the evolution of life histories in the face of anthropogenic changes in nutrient availability.
Keywords: life history, fecundity, nutrition, nitrogen, ecological stoichiometry
1. Significance statement
Why do organisms vary drastically in life-history traits? Some have many offspring, while others have relatively few; some have large brains and long lives, while others do not. In this research, we show that variation across species in such fitness-related traits may sometimes be due to differences in nutrition. Species of butterflies that feed on more nutrient-dense plant families as caterpillars produce more eggs (that are relatively smaller) and have larger eyes. The length of time a species has been adapting to their present diet does not matter, suggesting that nutrition may be a fundamental constraint on life-history evolution in some lineages. This is particularly relevant today because humans have drastically increased the availability of many nutrients.
2. Introduction
Biologists have long sought to understand why fitness-related traits vary across species [1,2]. It is well established that nutrition affects the expression of life-history traits and trade-offs within species [3–5], and it has been hypothesized to shape life-history evolution across species [6,7]. Indeed, variation in diet quality across species has been suggested to influence the evolution of development and body size in mammals and insects [8,9], brain size, fecundity and lifespan in humans [10–12], and lifespan in butterflies [13]. However, no systematic studies exist to test how quantitative variation in nutrition affects life-history traits across species. On the one hand, extending the theory developed for life-history variation within species, we might surmise that species with access to higher quality diets can afford to allocate more nutrients to a range of life-history traits, masking underlying trade-offs between traits [3,14]. Thus, one might predict that species on more nutrient rich diets would be more fecund and have larger traits tied to fitness, such as brains and testes. On the other hand, nutrition may play a minor role in life-history evolution if organisms can cope with low nutrient conditions through adaptive nutritional plasticity, shifts in gut morphology, metabolic adaptations or symbiotic associations [15–19]. Determining the extent to which nutrient availability affects life-history evolution is particularly important today given anthropogenic change in nutrients once limited in availability such as nitrogen and phosphorus [20]: changes in nutrition have major consequences for the expression and evolution of life-history traits and hence demographic and evolutionary processes [21].
Investigating the evolutionary importance of nutrition is hampered by the difficulty in quantifying nutrient availability across species over their evolutionary histories. A number of studies have approached this question using various proxies for nutrition [22] or comparing broad classes of foraging modes [23,24]. Specialist herbivores such as phytophagous insects are a useful system because nutrient content has been shown to vary systematically across plant families [25,26]. Butterflies in particular are a powerful system because most of an individual's essential nutrients (e.g. nitrogen, phosphorus) come from larval feeding and larval host plants are known for most species. Furthermore, by mapping these host records onto lepidopteran phylogenies, we can estimate the relative timing of diet shifts [27].
Here, we investigate how nutrition affects life-history evolution across 96 species of butterflies that represent over 50 independent diet shifts across 38 plant families (figure 1). We focus on nitrogen and phosphorus, both important components of proteins and nucleic acids, and generally the major limiting macronutrients in the growth and development of terrestrial herbivores [9,28]. We secondarily focus on sodium as a major limiting micronutrient for herbivores as sodium is important in brain and muscle growth and function in both invertebrates and vertebrates [29,30]. Such a stoichiometric approach [31] allows direct quantitative comparison of specific nutrients across plant families and integration with the ecosystem literature on changing nutrient availability. Although other elements are clearly important in organismal development (e.g. carbon, oxygen, hydrogen), our focal elements are particularly important for herbivores because they are much less concentrated in plant relative to animal tissue [30,32]. We take a broad view of life histories [33], focusing on whole organism investment in maintenance, reproduction and survival; we consider not only traits directly tied to fecundity (egg number and size, testis size), but also body size, which is correlated with fecundity in insects [34]. We additionally measure eye size because vision is important for males to secure a mate [35,36] and host finding in females [37,38]. We test the prediction that species on higher nutrient diets will be larger, more fecund, invest more in individual offspring and have larger eyes. Additionally, by looking at the effect of timing of a diet shift, we test the alternate prediction that adaptation to diet over time relaxes the extent to which diet constrains life-history traits.
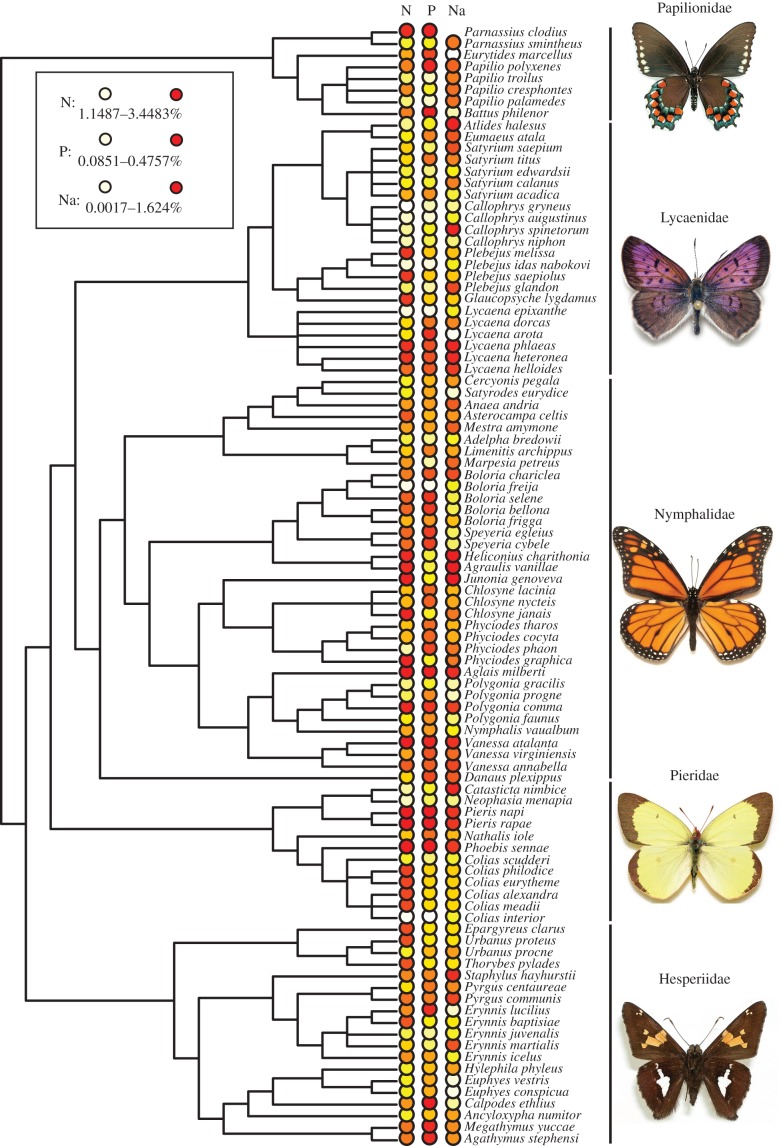
Figure 1.
Phylogeny of all species included in the analysis. A representative of each family is pictured (photos courtesy of Andrew Warren). Nutrient content of plant families consumed as host plants is indicated in heat-map colours (white representing low values and red representing high values) for nitrogen, phosphorus and sodium (per cent dry weight). See the electronic supplementary material, file 1, for a complete list of all butterfly specimens used in the analysis. See the electronic supplementary material, figures S1–S3 for summaries of nitrogen, phosphorus and sodium content of plant families consumed by these butterflies. (Online version in colour.)
3. Material and methods
(a). Overview of butterfly specimens
We focused our sampling efforts on 96 species of butterflies that were fairly specialized (feeding on one to three host plant families) and represented as many independent host shifts as possible. We used both fresh-caught specimens (which we could dissect for fecundity measures) and museum specimens (for which only external measures could be taken). Field-caught specimens (65 species, n > 2 individuals per species, n = 644 individuals) were obtained from 11 states from across the USA (see the electronic supplementary material, file 1). Live individuals were transported back to the laboratory where they were stored in sealed containers at −20°C prior to abdomen dissections and wing measurements. As detailed below, field-caught specimens were used for measures of body size, egg number, egg size and testis size. We additionally measured species obtained from the University of Minnesota Insect Collection, Department of Entomology (n = 68 species, 518 individuals; see the electronic supplementary material, file 1). We focused our measurements on museum specimens from 25 US states; 6% of specimens originated in Canada, Central or South America. Only species with at least three individuals available were targeted for measurements. As detailed below, museum specimens were used for measures of body size and eye size. Sex was determined based on wing pattern and either gonad dissection (field specimens) or genitalia inspection (museum specimens).
(b). Quantification of host plant nutrition
We obtained plant nutrient data from three sources. Data on nitrogen and phosphorus were obtained from Borer [39]. Plant sodium content was obtained from Watanabe et al. [25]. We additionally collected 117 samples of host plants commonly used by butterflies that were poorly represented for the nutrients in which we were interested, in addition to a subset of re-sampled species to ensure our methods were in alignment with previous studies (samples from eight states across the USA; see the electronic supplementary material, file 1). We measured nitrogen using the Dumas method [40] and used inductively coupled plasma mass spectrometry to measure phosphorus and sodium. Together, we drew from 8381 plant samples for our measurements of plant nutrient levels. We focused on percentage nutrient content for dried leaf samples. Angiosperm phylogeny group III (APG III) [41] was used to classify all plant genera into currently accepted families. As found in other studies [25,42], there were significant differences in nutrient availability across plant families (electronic supplementary material, figure S1). For the subset of plant families fed on by butterflies in this study, family-level variation was more pronounced for nitrogen (n = 4843; R2 = 0.32, F238,4604 = 14.6, p < 0.0001) and sodium (n = 2148; R2 = 0.52, F141,2006 = 15.2, p < 0.0001) than for phosphorus (n = 4947; R2 = 0.32, F226,46 720 = 9.64, p < 0.0001).
Plant family-level nutrient values were calculated as the average nutrient level for all samples from a given family. Median sample sizes per family were 24, 25 and 8, for nitrogen, phosphorus and sodium, respectively. Plant families were, on average, represented by nutrient values from five genera. In our analyses, we treat each nutrient separately because tight correlations between nitrogen and phosphorus content [42] make it statistically challenging to test for interactions between nutrients.
Host plant records for butterflies were obtained from Scott [43]. We recorded the number of host plant species per plant genus for each butterfly species, ignoring records listed as ‘dubious’ or ‘oviposition only’. APG III was used to classify all plant genera into currently accepted families. We calculated a host family-level metric of butterfly nutrition with a weighted average for each nutrient. For instance, if a species had five host records on Fabaceae and five host records on Brassicaceae, the average nutrient value was calculated as the sum of (1/2) × (family-level nitrogen of Fabaceae) and (1/2) × (family-level nitrogen of Brassicaceae).
(c). Measurement of life-history traits
On all specimens, we measured wing length as the length from the articulation of the forewing with the thorax to the wing apex. For field-caught specimens, forewings were completely removed from the butterfly and placed flat for imaging (mean = 9.9 individuals per species; 644 total measurements). For museum specimens, individuals were oriented such that the focal forewing was in the same plane for imaging (mean = 7.2 individuals per species; 511 total measurements). Wing length was measured in Image J (NIH). We analysed wing length as a response variable in addition to using it as a measure of body size in models testing for effects of nutrients on the relative size of traits.
Three reproductive measures were taken for field-caught individuals. For females, paired ovaries were dissected out in 1× PBS buffer under approximately 20× magnification using a Leica M165C microscope. All mature, chorionated eggs were counted and egg size was measured for one to five eggs per individual (mean = 2.8 eggs per individual, 499 total eggs) as the area of each mature egg. For egg measurements, we eliminated specimens that were desiccated or had zero mature eggs (e.g. due to reproductive diapause), averaging egg measurements for 3.9 individuals per species (195 total). For most male butterflies, the ancestrally paired testes are fused into one testis. The single testis was dissected out in 1× PBS and imaged. Testis size was measured as the area of the testis (mean = 4 individuals per species; 192 total). Within butterflies, at least two reversions to paired testes have occurred. For these species, testis investment was measured as the summed area of each testis. For field-caught specimens, we made efforts to sample both males and females (mean sex ratio = 0.81 females : males; see the electronic supplementary material, file 1).
For museum specimens, we measured eye width (in addition to wing length). Individuals were oriented face-on under the microscope (as in [44]). Eye width was measured on each eye as the distance from where the eye meets the head, to the furthest distance from the midline. By measuring both eyes, we could use asymmetry of the eye width measure to ensure that specimens were oriented properly (for analysis, we excluded eye width measurements that had an eye width difference of more than 25% of the average eye size). Eye width averaged across both eyes was used in analyses. For museum specimens, we sought to measure at least three females and three males per species (mean females per species = 3.46; mean males per species = 3.95; average sex ratio across species = 0.87 females : males; see the electronic supplementary material, file 1). Collection dates of specimens ranged from 1899 to 2010. There were significant differences across species in collection date (F69,430 = 7.11, p < 0.0001); however, a species' collection date was not related to eye size (F1,67 = 0.67, p = 0.42) or wing length (F1,68 = 0.91, p = 0.34), suggesting changes in nutrient availability over time were not affecting the development of our traits.
(d). Phylogeny
Because there is no comprehensive phylogeny for butterflies, we constructed a phylogeny by combining available family- and tribe-level phylogenies with a phylogeny giving relationships among families [45]. We used a number of criteria when choosing phylogenetic references. We preferred molecular phylogenies over morphological and also preferred phylogenies containing more species from our analysis. We used references that resolved polytomies wherever possible. Relationships within Papilionidae were taken from Simonsen et al. [46]. Relationships among Colias and their close relatives were based on Pollock et al. [47]. Relationships within Pierinae and Hesperiidae were taken from Braby & Trueman [27] and Warren et al. [48,49], respectively. Relationships within Nymphalidae were taken from a number of sources, including [50–56]. Relationships within the Lycaenidae are not currently well resolved. Relationships within the proposed sub-tribe Polyommatina and Lycaena were taken from Talavera et al. [57] and van Dorp [58], respectively, with clarifications from Eliot [59]. A few relationships within the Lycaenidae for which there were no references were built based on current taxonomic classification.
(e). Timing of host shifts
Age, or timing, of a larval host shift, is an estimate of how long the larvae of a given butterfly species have been feeding primarily on the plant family in question. Specifically, we focus here on whether the common ancestor of the entire genus most probably fed on the plant family in question. We designate a ‘recent shift’ when a focal species primarily feeds on a different plant family than the common ancestor of the genus. If the focal species primarily feeds on the same plant family as the common ancestor of the genus, we assume the host shift is relatively more ancient. Determinations were made primarily using the HOSTS caterpillar host-plant database [60] and [43]. Additional references for individual host records include [27,43,46,61–71]. While the ancestral determination was clear for most genera, a few required significant interpretation (see the electronic supplementary material, file 2).
(f). Data analysis
To assess the relationship between nutrition and life history, we used mean life-history trait values for each species. Because analyses among species must consider the degree to which species are related to one another, we performed phylogenetic generalized least-squares regressions with the ‘caper’ package [72] in R v. 3.1.3 [73]. For each model, we allowed λ, a measure of the degree of phylogenetic autocorrelation, to take its maximum-likelihood estimate (MLE). Each life-history variable was analysed both in its body size-corrected form by including wing length in the model (e.g. ‘relative eye width’) and its uncorrected form (e.g. ‘absolute eye width’). Analysing data calculated from males versus females did not qualitatively alter our results, so the sexes were pooled for analyses of eye width and wing length.
We were interested in whether links between nutrition and life history varied across butterfly families and between ancient and recent host shifts. To each model, we first added an interaction effect between the nutrient of interest and either butterfly family or host shift timing. We inspected the change in the sample-size corrected Akaike's information criterion (ΔAICc) due to the additional term. If the ΔAICc was at least two, we then took this to mean the model was improved enough to account for the increase in the number of parameters in the model. Family- and timing-specific analyses are presented only if they improved the model fit.
4. Results
We made use of both museum specimens (n = 68 species, 518 individuals) and wild-caught specimens (n = 65 species, 644 individuals). Across all specimens, wing length was weakly positively correlated with host plant sodium content (p = 0.07), but not nitrogen or phosphorus (figure 2; electronic supplementary material, table S2). In subsequent analyses, we consider both absolute values of life-history traits and trait values relative to body size.
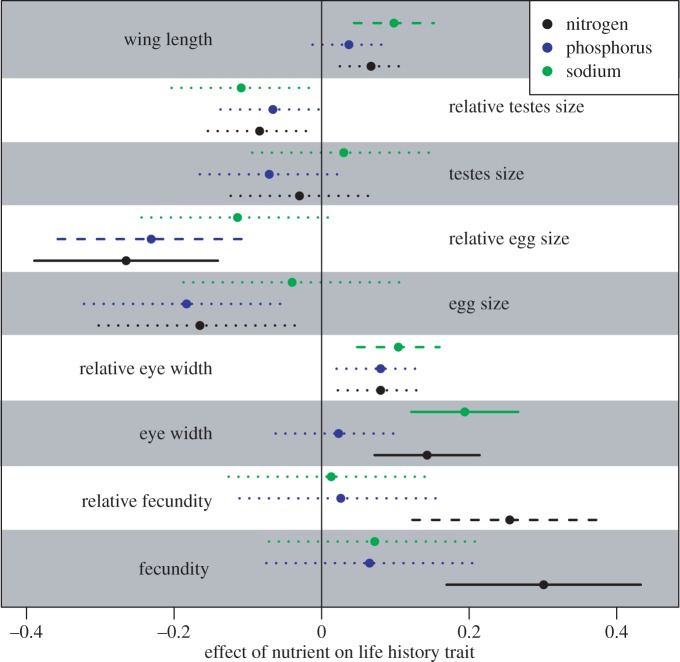
Figure 2.
Effects of host plant nutrient content on life-history traits. Shown are mean centred and standardized effect sizes for each variable combination. Within each shaded or un-shaded block (each representing a different life-history trait), the top line represents sodium (in green), the middle line represents phosphorus (in blue) and the lower line represents nitrogen (in black). Solid lines are significant at an alpha of 0.05, dashed lines are significant at an alpha of 0.10 and dotted lines are not significant. Full statistical details are presented in electronic supplementary material, tables S1–S3. (Online version in colour.)
For field-caught specimens, we dissected out reproductive tissue and measured total mature eggs, egg size and testis size. Butterflies that feed on plant families higher in nitrogen had higher fecundity, whether corrected (p = 0.06) or uncorrected (p = 0.03) for body size, although the relationship is weaker for relative fecundity (figures 2 and 3; electronic supplementary material, table S1). Female butterflies that feed on plant families with more nitrogen and phosphorus had smaller eggs, but only when egg size was corrected for body size (p = 0.04 and 0.08, respectively; figure 2; electronic supplementary material, tables S1 and S3). Neither absolute nor relative testis size was influenced by any nutrient (figure 2; electronic supplementary material, tables S1–S3). For museum specimens, nitrogen (p < 0.05) and sodium (p = 0.009) were both significantly positively related to absolute eye width, but not relative eye width (figures 2 and 3; electronic supplementary material, tables S1 and S2).
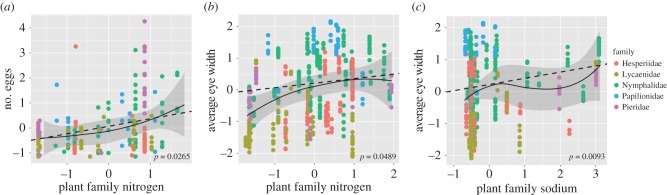
Figure 3.
Effects of nitrogen and sodium on fecundity and eye width. Shown are plots of data for three of the significant univariate analyses (figure 2): (a) nitrogen and fecundity, (b) nitrogen and eye width and (c) sodium and eye width. Data shown are individual-level data, although the regressions performed were at the species-level. All data were mean centered and standardized. Dotted lines and p-values given are from PGLS regressions, while solid lines and shaded area are the predicted values and standard errors from a LOESS regression uncorrected for phylogeny, both at the species-level. Full statistical details are presented in electronic supplementary material, tables S1 and S3. (Online version in colour.)
The relative effects of different nutrients varied across the different butterfly families sampled. The effects of nitrogen on fecundity were particularly pronounced within Pieridae and Nymphalidae, while the effect of sodium on eye width was pronounced within Lycaenidae (electronic supplementary material, figure S4 and table S4). We only investigated relationships within specific families when corrected AICc suggested that including family improved the model (electronic supplementary material, tables S1–S3). Although the measure of phylogenetic signal (λ) we estimated varied among traits (electronic supplementary material, table S5), the traits exhibiting important family-level variation did not show a consistent pattern of phylogenetic autocorrelation. Rather, the morphological traits exhibited moderate to high phylogenetic signal, while the fecundity-related traits exhibited low phylogenetic signal.
We were additionally interested in whether the age of a diet shift influenced the extent to which nutrition shaped life-history traits. For our age variable, we classified diet shifts as ‘ancient’ or ‘recent’ with regards to whether they occurred before or after the diversification of the genus. Using corrected AICc for model selection, we found that the role of nutrition in shaping life-history traits did not differ between ancient and recent host shifts (electronic supplementary material, tables S1–S3).
5. Discussion
Our results support the idea that diet quality can shape life-history evolution across species. There was a positive relationship between host plant nitrogen content and fecundity (figures 2 and 3), despite the fact that many of these species have been adapting to a low nutrient diet for millions of years. We also found that species feeding on more nitrogen-rich, and to some extent phosphorus-rich, host plants had eggs that were smaller relative to their body size (figures 2 and 3). Smaller relative egg size in response to a better quality diet, in conjunction with higher fecundity suggests that these species may be adopting a different reproductive strategy. These species may invest relatively less in each individual offspring because the offspring will be feeding on higher nutrient plants, thus allowing the parents to have higher fecundity. Indeed, rearing experiments of several satyrine butterflies suggest that egg size confers a larval survival advantage on nitrogen-poor plants [74]. Unexpectedly, there were no significant links between nutrition and testis size. This could reflect strong selection on testis size such that males prioritize allocation to this trait even on low nutrient diets (as for eyes [75]); alternatively, noise from variation in sexual selection intensity (e.g. re-mating rate [76]), may also be obscuring a relationship.
We additionally found that species that feed on plant families with higher nitrogen and sodium content had larger eyes. In butterflies, vision is important for males to secure a mate [35,36] and females to locate host plants [37,38], both important components of fitness. These results speak to more general ideas relating nutrition to brain evolution [10–12] given that species with larger eyes probably also have greater total neural investment. This is in part because 75% of the butterfly brain is dedicated to visual processing [77,78], and in part due to correlations between eye size and the size of brain regions dedicated to processing visual information in both vertebrates and invertebrates [79,80].
Insects can often compensate for low nutrient diets through changes in foraging behaviour, such as changes in feeding rate or development time [81,82]. However, the fact that we see a signature of nutrient content across some butterfly families suggests that changes in feeding cannot fully compensate for low nutrient diets (although it could explain some of the added variation seen in figure 3). It is possible that high larval mortality imposes significant costs to increases in development time [83]. Indeed, the skipper family, which includes one of the most successful butterfly radiations on a low nutrient plant family (grasses) employs larval shelter building, presumably an adaptation to avoid predation and parasitism [84]. There may be additional costs to compensatory feeding, such as increased exposure to toxins [85], which results in species incompletely compensating for low nutrient diets.
Interestingly, some of the relationships between nutrition and life-history traits varied across butterfly families. The link between sodium and eye size was particularly strong within Lycaenidae and the correlation between fecundity and nitrogen was especially pronounced in Pieridae, and to some extent, Nymphalidae (electronic supplementary materials, figure S4 and table S4). It is possible that some adaptation to diet, such as shelter building in skippers (see above), may result in a family being less constrained by nutrition. It is also possible that the ancestral diet of a family may pre-adapt them to certain diet shifts. For instance, the ancestral hosts of Pieridae were high-nitrogen legumes [27], which could explain their poor performance on low nitrogen diets. It is also possible that the subsample of hosts used by a particular family could constrain statistical analyses across families. For instance, the hosts of Lycaenidae span a much broader range of sodium availability than the hosts of Papilionidae (figure 3).
Significant relationships between diet and life-history evolution emerged in our analyses despite the challenges of quantifying ‘nutrition’. We focused on elemental measures of nutrient availability as a way to standardize fundamental nutrient requirements across species, and because extensive data are available on plant nutrient content. However, such an approach glosses over the extent to which nutrients are bio-available to a species, especially when nutrients are tied up in plant defences. This is an important limitation to our results, but still represents an increase in resolution over measures of diet quality such as ‘herbivorous’ or ‘carnivorous’ (e.g. [86]). Accounting for the true nutritional value of a resource will remain challenging because what is bio-available depends on the organism—for instance, nitrogen may be incorporated into plant defences, which a specialist, but not a generalist, may be able to metabolize [87]. Patterns linking plant nutrient levels and herbivore life-history traits also emerged despite considerable variability within plants in nutrient content, whether due to geographical [39] or seasonal variation [8].
We were interested in whether the timing of a diet shift influenced the relationship between nutrition and life-history traits, reasoning that nutrition may be more likely to constrain life-history evolution following a recent diet shift, prior to the longer-term evolution of adaptations to that diet through changes to gut morphology, metabolic adaptations or symbiotic associations [15–19]. We did not find any effect of timing on the link between diet and life history (electronic supplementary material, tables S1–S3). Of course, it is possible that this negative result could be a consequence of our methods for quantifying ‘timing’. We focused on host use at the level of butterfly genus because we were able to trace most diet shifts to this level, and it seemed a likely level for such timing effects to play out. ‘Recent’ shifts were classified as those that occurred within a genus with respect to the ancestral host of that genus. Given the age of some genera, the ‘recent shift’ classification may still correspond to a very long timescale—20–40 million years (e.g. [70]), no doubt an adequate period of time for species to adapt to low nutrient diets. As phylogenetic information becomes more resolved across butterflies, it may be possible to gain more precise estimates of timing of host shifts, allowing for tests of a timing signature at a finer scale.
Our research suggests that variation across species in nutrient availability may sometimes explain variation in life histories across species. In some cases, species with higher quality diets can afford to allocate more to traits related to fitness, such as fecundity and eye size, paralleling theory linking resource availability and life-history traits within species [3,14]. It is possible that nutrient availability across species is obscuring underlying life-history trade-offs [3,14,22,88], although further analyses are necessary to test this idea. As a result of this life-history variation, species that differ in diet may vary systematically in major ecological and evolutionary processes, such as survival in novel environments [89] or rates of evolution and diversification [90]. Indeed, butterfly species specialized on more nitrogen rich larval diets have shown more pronounced northward range expansions over the last 40 years [91]. Our results are particularly important given that humans are drastically altering the availability of many once-limited nutrients, especially nitrogen, phosphorus and sodium [21]. Anthropogenic increases in nutrient availability may have evolutionary impacts on the reproductive strategies of a range of organisms. For instance, an evolutionary increase in the fecundity of pest species could be one of many unintended consequences of increases in fertilizer use and atmospheric nitrogen deposition over the last 100 years.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
Many thanks to Christopher Boser, Natalie Eichten, Naomi Wick, Nathan Fremling, Supra Khare, Jeffrey Rock and Rhea Smykalski for additional help with specimen collection and processing. We are indebted to Art Shapiro, Dan Papaj, Mike Reese, Jaret Daniels, Sandy Koi, Katy Prudic and Jeff Oliver for helping to collect specimens. Dean Hansen, Steve Kohler, Virginia Scott, Jeff Marcus, Kyle Johnson, Ann and Scott Swengel, Ron Huber, Robert Dana, Joel Kingsolver and Dave Biesboer provided valuable assistance locating field sites and butterflies. Thanks to Robin Hopkins, Ralph Holzenthal, Paul Tinerella and the University of Minnesota Insect Collection, Department of Entomology for access to museum specimens. Thanks to Cedar Creek LTER, Cloquet Forestry Center, O'Leno State Park, Fairchild Botanic Garden and Custer National Forest for permission to collect samples used in this research and Andrew Warren for allowing the use of his photos (http://butterfliesofamerica.com). Elizabeth Borer provided access to plant nutrient data prior to publication. Comments from the Snell-Rood lab, Eric Lind and two anonymous reviewers improved earlier versions of this manuscript.
Data accessibility
All data are available in Dryad (doi:10.5061/dryad.447sq).
Authors' contributions
E.C.S.-R. devised the approach and supervised collection of the data with input from other authors. All authors contributed substantially to specimen and data collection. E.M.S. analysed the data, with contribution from E.C.S.-R. E.M.S. and E.C.S.-R. interpreted the results and wrote the majority of the text, and other authors contributed to both interpretation and text.
Competing interests
We have no competing interests.
Funding
E.M.S. was supported by an NSF postdoctoral fellowship (#1306627). This research was supported by a Grant-in-Aid of Research from the University of Minnesota OVPR; the Snell-Rood lab was supported in part through NSF IOS-1354737.
References
- 1.Stearns S. 1992. The evolution of life histories. New York, NY: Oxford University Press. [Google Scholar]
- 2.Roff D. 2001. Life history evolution. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 3.Van Noordwijk A, De Jong G. 1986. Acquisition and allocation of resources: their influence on variation in life history tactics. Am. Nat. 128, 137–142. ( 10.1086/284547) [DOI] [Google Scholar]
- 4.Nylin S, Gotthard K. 1998. Plasticity in life-history traits. Annu. Rev. Entomol. 43, 63–83. ( 10.1146/annurev.ento.43.1.63) [DOI] [PubMed] [Google Scholar]
- 5.Flatt T, Heyland A. 2011. Mechanisms of life history evolution: the genetics and physiology of life history traits and trade-offs. New York, NY: Oxford University Press. [Google Scholar]
- 6.Arnold SJ. 1992. Constraints on phenotypic evolution. Am. Nat. 140, S85–S107. ( 10.1086/285398) [DOI] [PubMed] [Google Scholar]
- 7.Antonovics J, van Tienderen PH. 1991. Ontoecogenophyloconstraints? The chaos of constraint terminology. Trends Ecol. Evol. 6, 166–168. ( 10.1016/0169-5347(91)90059-7) [DOI] [PubMed] [Google Scholar]
- 8.Scriber JM, Slansky F. 1981. The nutritional ecology of immature insects. Annu. Rev. Entomol. 26, 183–211. ( 10.1146/annurev.en.26.010181.001151) [DOI] [Google Scholar]
- 9.Mattson WJ. 1980. Herbivory in relation to plant nitrogen content. Annu. Rev. Ecol. Syst. 11, 119–161. ( 10.1146/annurev.es.11.110180.001003) [DOI] [Google Scholar]
- 10.Leonard WR, Robertson ML. 1994. Evolutionary perspectives on human nutrition: the influence of brain and body size on diet and metabolism. Am. J. Hum. Biol. 6, 77–88. ( 10.1002/ajhb.1310060111) [DOI] [PubMed] [Google Scholar]
- 11.Kaplan H, Hill K, Lancaster J, Hurtado AM. 2000. A theory of human life history evolution: diet, intelligence, and longevity. Evol. Anthropol. 9, 156–185. ( 10.1002/1520-6505(2000)9:4%3C156::AID-EVAN5%3E3.0.CO;2-7) [DOI] [Google Scholar]
- 12.Boback SM, Cox CL, Ott BD, Carmody R, Wrangham RW, Secor SM. 2007. Cooking and grinding reduces the cost of meat digestion. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 148, 651–656. ( 10.1016/j.cbpa.2007.08.014) [DOI] [PubMed] [Google Scholar]
- 13.Beck J, Fiedler K. 2009. Adult life spans of butterflies (Lepidoptera: Papilionoidea plus Hesperioidea): broadscale contingencies with adult and larval traits in multi-species comparisons. Biol. J. Linn. Soc. 96, 166–184. ( 10.1111/j.1095-8312.2008.01102.x) [DOI] [Google Scholar]
- 14.Reznick D, Nunney L, Tessier A. 2000. Big houses, big cars, superfleas and the costs of reproduction. Trends Ecol. Evol. 15, 421–425. ( 10.1016/s0169-5347(00)01941-8) [DOI] [PubMed] [Google Scholar]
- 15.Lynch JP, Brown KM. 2001. Topsoil foraging—an architectural adaptation of plants to low phosphorus availability. Plant Soil 237, 225–237. ( 10.1023/a:1013324727040) [DOI] [Google Scholar]
- 16.Breznak JA, Brune A. 1994. Role of microorganisms in the digestion of lignocellulose by termites. Annu. Rev. Entomol. 39, 453–487. ( 10.1146/annurev.ento.39.1.453) [DOI] [Google Scholar]
- 17.Cork SJ. 1996. Optimal digestive strategies for arboreal herbivorous mammals in contrasting forest types. Why koalas and colobines are different. Aust. J. Ecol. 21, 10–20. ( 10.1111/j.1442-9993.1996.tb00581.x) [DOI] [Google Scholar]
- 18.Douglas AE. 2009. The microbial dimension in insect nutritional ecology. Funct. Ecol. 23, 38–47. ( 10.1111/j.1365-2435.2008.01442.x) [DOI] [Google Scholar]
- 19.Axelsson E, et al. 2013. The genomic signature of dog domestication reveals adaptation to a starch-rich diet. Nature 495, 360–364. ( 10.1038/nature11837) [DOI] [PubMed] [Google Scholar]
- 20.Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman D. 1997. Human alteration of the global nitrogen cycle: sources and consequences. Ecol. Appl. 7, 737–750. ( 10.2307/2269431) [DOI] [Google Scholar]
- 21.Snell-Rood E, Cothran R, Espeset A, Jeyasingh P, Hobbie S, Morehouse N. 2015. Life history evolution in the anthropocene: effects of increasing nutrients on traits and tradeoffs. Evol. Appl. 8, 635–649. ( 10.1111/eva.12272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glazier DS. 1999. Trade-offs between reproductive and somatic (storage) investments in animals: a comparative test of the Van Noordwijk and De Jong model. Evol. Ecol. 13, 539–555. ( 10.1023/a:1006793600600) [DOI] [Google Scholar]
- 23.Jones KE, MacLarnon AM. 2004. Affording larger brains: testing hypotheses of mammalian brain evolution on bats. Am. Nat. 164, E20–E31. ( 10.1086/421334) [DOI] [PubMed] [Google Scholar]
- 24.Conway CJ, Martin TE. 2000. Evolution of passerine incubation behavior: influence of food, temperature, and nest predation. Evolution 54, 670–685. ( 10.1111/j.0014-3820.2000.tb00068.x) [DOI] [PubMed] [Google Scholar]
- 25.Watanabe T, Broadley MR, Jansen S, White PJ, Takada J, Satake K, Takamatsu T, Tuah SJ, Osaki M. 2007. Evolutionary control of leaf element composition in plants. New Phytol. 174, 516–523. ( 10.1111/j.1469-8137.2007.02078.x) [DOI] [PubMed] [Google Scholar]
- 26.Wright IJ, et al. 2004. The worldwide leaf economics spectrum. Nature 428, 821–827. ( 10.1038/nature02403) [DOI] [PubMed] [Google Scholar]
- 27.Braby MF, Trueman JWH. 2006. Evolution of larval host plant associations and adaptive radiation in pierid butterflies. J. Evol. Biol. 19, 1677–1690. ( 10.1111/j.1420-9101.2006.01109.x) [DOI] [PubMed] [Google Scholar]
- 28.Perkins MC, Woods HA, Harrison JF, Elser JJ. 2004. Dietary phosphorus affects the growth of larval Manduca sexta. Arch. Insect. Biochem. Physiol. 55, 153–168. ( 10.1002/arch.10133) [DOI] [PubMed] [Google Scholar]
- 29.Bursey RG, Watson ML. 1983. The effect of sodium restriction during gestation on offspring brain development in rats. Am. J. Clin. Nutr. 37, 43–51. [DOI] [PubMed] [Google Scholar]
- 30.Snell-Rood EC, Espeset A, Boser CJ, White WA, Smykalski R. 2014. Anthropogenic changes in sodium affect neural and muscle development in butterflies. Proc. Natl Acad. Sci. USA 111, 10 221–10 226. ( 10.1073/pnas.1323607111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elser JJ, et al. 2000. Biological stoichiometry from genes to ecosystems. Ecol. Lett. 3, 540–550. ( 10.1046/j.1461-0248.2000.00185.x) [DOI] [Google Scholar]
- 32.Sterner R, Elser J. 2002. Ecological stoichiometry: the biology of elements from molecules to the biosphere, pp. 209–211 Princeton, NJ: Princeton University Press. [Google Scholar]
- 33.Badyaev AV, Qvarnstrom A. 2002. Putting sexual traits into the context of an organism: a life-history perspective in studies of sexual selection. Auk 119, 301–310. ( 10.1642/0004-8038(2002)119%5B0301:pstitc%5D2.0.co;2) [DOI] [Google Scholar]
- 34.Garcia-Barros E. 2000. Body size, egg size, and their interspecific relationships with ecological and life history traits in butterflies (Lepidoptera: Papilionoidea, Hesperioidea). Biol. J. Linn. Soc. 70, 251–284. ( 10.1111/j.1095-8312.2000.tb00210.x) [DOI] [Google Scholar]
- 35.Rutowski RL, McCoy L, Demlong MJ. 2001. Visual mate detection in a territorial male butterfly (Asterocampa leilia): effects of distance and perch location. Behaviour 138, 31–43. ( 10.1163/156853901750077772) [DOI] [Google Scholar]
- 36.Silberglied RE, Taylor OR. 1978. Ultraviolet reflection and its behavioral role in courtship of sulfur butterflies Colias eurytheme and Colias philodice (Lepidoptera, Pieridae). Behav. Ecol. Sociobiol. 3, 203–243. ( 10.1007/bf00296311) [DOI] [Google Scholar]
- 37.Papaj DR, Rausher MD. 1987. Components of conspecific host descrimination behavior in the butterfly Battus philenor. Ecology 68, 245–253. ( 10.2307/1939254) [DOI] [Google Scholar]
- 38.Hern A, Edwards-Jones G, McKinlay RG. 1996. A review of the pre-oviposition behaviour of the small cabbage white butterfly, Pieris rapae (Lepidoptera: Pieridae). Ann. Appl. Biol. 128, 349–371. ( 10.1111/j.1744-7348.1996.tb07328.x) [DOI] [Google Scholar]
- 39.Borer ET, et al. 2013. Global biogeography of autotroph chemistry: is insolation a driving force? Oikos 122, 1121–1130. ( 10.1111/j.1600-0706.2013.00465.x) [DOI] [Google Scholar]
- 40.Matejovic I. 1995. Total nitrogen in plant material determined by means of dry combustion: a possible alterntive to determination by Kjedahl digestion. Commun. Soil Sci. Plant Anal. 26, 2217–2229. ( 10.1080/00103629509369441) [DOI] [Google Scholar]
- 41.Bremer B, et al. 2009. An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG III. Bot. J. Linn. Soc. 161, 105–121. ( 10.1111/j.1095-8339.2009.00996.x) [DOI] [Google Scholar]
- 42.Kerkhoff AJ, Fagan WF, Elser JJ, Enquist BJ. 2006. Phylogenetic and growth form variation in the scaling of nitrogen and phosphorus in the seed plants. Am. Nat. 168, E103–E122. ( 10.1086/507879) [DOI] [PubMed] [Google Scholar]
- 43.Scott J. 1992. The butterflies of North America: a natural history and field guide. Red wood City, CA: Stanford University Press. [Google Scholar]
- 44.Rutowski RL. 2000. Variation of eye size in butterflies: inter- and intraspecific patterns. J. Zool. 252, 187–195. ( 10.1111/j.1469-7998.2000.tb00614.x) [DOI] [Google Scholar]
- 45.Kawahara AY, Breinholt JW. 2014. Phylogenomics provides strong evidence for relationships of butterflies and moths. Proc. R. Soc. B 281, 20140970 ( 10.1098/rspb.2014.0970) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simonsen TJ, Zakharov EV, Djernaes M, Cotton AM, Vane-Wright RI, Sperling FAH. 2011. Phylogenetics and divergence times of Papilioninae (Lepidoptera) with special reference to the enigmatic genera Teinopalpus and Meandrusa. Cladistics 27, 113–137. ( 10.1111/j.1096-0031.2010.00326.x) [DOI] [PubMed] [Google Scholar]
- 47.Pollock DD, Watt WB, Rashbrook VK, Iyengar EV. 1998. Molecular phylogeny for Colias butterflies and their relatives (Lepidoptera: Pieridae). Ann. Entomol. Soc. Am. 91, 524–531. ( 10.1093/aesa/91.5.524) [DOI] [Google Scholar]
- 48.Warren AD, Ogawa JR, Brower AVZ. 2008. Phylogenetic relationships of subfamilies and circumscription of tribes in the family Hesperiidae (Lepidoptera: Hesperioidea). Cladistics 24, 642–676. ( 10.1111/j.1096-0031.2008.00218.x) [DOI] [Google Scholar]
- 49.Warren AD, Ogawa JR, Brower AVZ. 2009. Revised classification of the family Hesperiidae (Lepidoptera: Hesperioidea) based on combined molecular and morphological data. Syst. Entomol. 34, 467–523. ( 10.1111/j.1365-3113.2008.00463.x) [DOI] [Google Scholar]
- 50.Pena C, Wahlberg N, Weingartner E, Kodandaramaiah U, Nylin S, Freitas AVL, Brower AVZ. 2006. Higher level phylogeny of Satyrinae butterflies (Lepidoptera: Nymphalidae) based on DNA sequence data. Mol. Phylogenet. Evol. 40, 29–49. ( 10.1016/j.ympev.2006.02.007) [DOI] [PubMed] [Google Scholar]
- 51.Brower AVZ. 2000. Phylogenetic relationships among the Nymphalidae (Lepidoptera) inferred from partial sequences of the wingless gene. Proc. R. Soc. Lond. B 267, 1201–1211. ( 10.1098/rspb.2000.1129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freitas AVL, Brown KS. 2004. Phylogeny of the Nymphalidae (Lepidoptera). Syst. Biol. 53, 363–383. ( 10.1080/10635150490445670) [DOI] [PubMed] [Google Scholar]
- 53.Wahlberg N, Weingartner E, Nylin S. 2003. Towards a better understanding of the higher systematics of Nymphalidae (Lepidoptera: Papilionoidea). Mol. Phylogenet. Evol. 28, 473–484. ( 10.1016/s1055-7903(03)00052-6) [DOI] [PubMed] [Google Scholar]
- 54.Wahlberg N, Brower AVZ, Nylin S. 2005. Phylogenetic relationships and historical biogeography of tribes and genera in the subfamily Nymphalinae (Lepidoptera: Nymphalidae). Biol. J. Linn. Soc. 86, 227–251. ( 10.1111/j.1095-8312.2005.00531.x) [DOI] [Google Scholar]
- 55.Wahlberg N, Wheat CW. 2008. Genomic outposts serve the phylogenomic pioneers: designing novel nuclear markers for genomic DNA extractions of Lepidoptera. Syst. Biol. 57, 231–242. ( 10.1080/10635150802033006) [DOI] [PubMed] [Google Scholar]
- 56.Wahlberg N, Weingartner E, Warren AD, Nylin S. 2009. Timing major conflict between mitochondrial and nuclear genes in species relationships of Polygonia butterflies (Nymphalidae: Nymphalini). BMC Evol. Biol. 9, 92 ( 10.1186/1471-2148-9-92) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Talavera G, Lukhtanov VA, Pierce NE, Vila R. 2013. Establishing criteria for higher-level classification using molecular data: the systematics of Polyommatus blue butterflies (Lepidoptera, Lycaenidae). Cladistics 29, 166–192. ( 10.1111/j.1096-0031.2012.00421.x) [DOI] [PubMed] [Google Scholar]
- 58.van Dorp K. 2004. Molecular systematics of Lycaena F, 1807 (Lepidoptera: Lycaenidae)—some preliminary results. Proc. Neth. Entomol. Soc. 15, 65–70. [Google Scholar]
- 59.Eliot JN. 1973. The higher classification of the Lycaenidae (Lepidoptera): a tentative arrangement. Bull. Br. Mus. Nat. Hist. 28. [Google Scholar]
- 60.Robinson GG, Ackery PR, Kitching IJ, Beccaloni GW, Hernandez LM. 2010. HOSTS—a database of the World's lepidopteran hostplants. London, UK: Natural History Museum. [Google Scholar]
- 61.Aubert J, Legal L, Descimon H, Michel F. 1999. Molecular phylogeny of swallowtail butterflies of the tribe Papilionini (Papilionidae, Lepidoptera). Mol. Phylogenet. Evol. 12, 156–167. ( 10.1006/mpev.1998.0605) [DOI] [PubMed] [Google Scholar]
- 62.Mullen SP, Savage WK, Wahlberg N, Willmott KR. 2011. Rapid diversification and not clade age explains high diversity in neotropical Adelpha butterflies. Proc. R. Soc. B 278, 1777–1785. ( 10.1098/rspb.2010.2140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nylin S, Slove J, Janz N. 2014. Host plant utilization, host range oscillations and diversification in Nymphalid butterflies: a phylogenetic investigation. Evolution 68, 105–124. ( 10.1111/evo.12227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ehrlich PR, Hanski I. 2004. On the wings of checkerspots. New York, NY: Oxford University Press. [Google Scholar]
- 65.Janz N, Nyblom K, Nylin S. 2001. Evolutionary dynamics of host-plant specialization: a case study of the tribe Nymphalini. Evolution 55, 783–796. ( 10.1554/0014-3820(2001)055%5B0783:edohps%5D2.0.co;2) [DOI] [PubMed] [Google Scholar]
- 66.Silva-Brandao KL, Solferini VN. 2007. Use of host plants by Troidini butterflies (Papilionidae, Papilioninae): constraints on host shift. Biol. J. Linn. Soc. 90, 247–261. ( 10.1111/j.1095-8312.2007.00727.x) [DOI] [Google Scholar]
- 67.Simonsen TJ. 2006. Fritillary phylogeny, classification, and larval host plants: reconstructed mainly on the basis of male and female genitalic morphology (Lepidoptera: Nymphalidae: Argynnini). Biol. J. Linn. Soc. 89, 627–673. ( 10.1111/j.1095-8312.2006.00697.x) [DOI] [Google Scholar]
- 68.Weingartner E, Wahlberg N, Nylin S. 2006. Dynamics of host plant use and species diversity in Polygonia butterflies (Nymphalidae). J. Evol. Biol. 19, 483–491. ( 10.1111/j.1420-9101.2005.01009.x) [DOI] [PubMed] [Google Scholar]
- 69.Scriber JM. 1994. The swallowtail butterflies: their ecology and evolutionary biology. Gainesville, FL: Scientific Publisher, Inc. [Google Scholar]
- 70.Wheat CW, Vogel H, Wittstock U, Braby MF, Underwood D, Mitchell-Olds T. 2007. The genetic basis of a plant–insect coevolutionary key innovation. Proc. Natl Acad. Sci. USA 104, 20 427–20 431. ( 10.1073/pnas.0706229104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.New TR. 1993. Conservation biology of Lycaenidae (butterflies). Gland, Switzerland: IUCN. [Google Scholar]
- 72.Orme D, et al. 2012. CAPER: comparative analyses of phylogenetics and evolution in R. R package version 0.5. See http://CRAN.R-project.org/package=caper.
- 73.R Development Core Team. 2013. R: A language and environment for statistical computing, 3.0.1 edn Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 74.Braby MF. 1994. The significance of egg size variation in butterflies in relation to host plant quality. Oikos 71, 119–129. ( 10.2307/3546179) [DOI] [Google Scholar]
- 75.Merry JW, Kemp DJ, Rutowski RL. 2011. Variation in compound eye structure: effects of diet and family. Evolution 65, 2098–2110. ( 10.1111/j.1558-5646.2011.01285.x) [DOI] [PubMed] [Google Scholar]
- 76.Gage MJG. 1994. Associations between body size, mating pattern, testis size and sperm lengths across butterflies. Proc. R. Soc. Lond. B 258, 247–254. ( 10.1098/rspb.1994.0169) [DOI] [Google Scholar]
- 77.Sivinski J. 1989. Mushroom body development in Nymphalid butterflies: a correlate of learning. J. Insect Behav. 2, 277–283. ( 10.1007/BF01053299) [DOI] [Google Scholar]
- 78.Ali F. 1974. Structure and metamorphosis of the brain and suboesophageal ganglion of Pieris brassicae (L.) (Lepidoptera: Pieridae). Trans. R. Entomol. Soc. Lond. 125, 363–412. ( 10.1111/j.1365-2311.1974.tb02306.x) [DOI] [Google Scholar]
- 79.Gronenberg W, Holldobler B. 1999. Morphologic representation of visual and antennal information in the ant brain. J. Comp. Neurol. 412, 229–240. ( 10.1002/(sici)1096-9861(19990920)412:2%3C229::aid-cne4%3E3.0.co;2-e) [DOI] [PubMed] [Google Scholar]
- 80.Pearce E, Dunbar R. 2012. Latitudinal variation in light levels drives human visual system size. Biol. Lett. 8, 90–93. ( 10.1098/rsbl.2011.0570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee KP, Raubenheimer D, Simpson SJ. 2004. The effects of nutritional imbalance on compensatory feeding for cellulose-mediated dietary dilution in a generalist caterpillar. Physiol. Entomol. 29, 108–117. ( 10.1111/j.0307-6962.2004.00371.x) [DOI] [Google Scholar]
- 82.Berner D, Blanckenhorn WU, Korner C. 2005. Grasshoppers cope with low host plant quality by compensatory feeding and food selection: N limitation challenged. Oikos 111, 525–533. ( 10.1111/j.1600-0706.2005.14144.x) [DOI] [Google Scholar]
- 83.Martin TE. 1995. Avian life history evolution in relation to nest sites, nest predation, and food. Ecol. Monogr. 65, 101–127. ( 10.2307/2937160) [DOI] [Google Scholar]
- 84.Jones MT, Castellanos I, Weiss MR. 2002. Do leaf shelters always protect caterpillars from invertebrate predators? Ecol. Entomol. 27, 753–757. ( 10.1046/j.1365-2311.2002.00465.x) [DOI] [Google Scholar]
- 85.Slansky F, Wheeler GS. 1992. Caterpillars compensatory feeding response to diluted nutrients leads to toxic allelochemical dose. Entomol. Exp. Appl. 65, 171–186. ( 10.1111/j.1570-7458.1992.tb01641.x) [DOI] [Google Scholar]
- 86.Swanson EM, Holekamp KE, Lundrigan BL, Arsznov BM, Sakai ST. 2012. Multiple determinants of whole and regional brain volume among terrestrial carnivorans. PLoS ONE 7, e38447 ( 10.1371/journal.pone.0038447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Karban R, Agrawal AA. 2002. Herbivore offense. Annu. Rev. Ecol. Syst. 33, 641–664. ( 10.1146/annurev.ecolsys.33.010802.150443) [DOI] [Google Scholar]
- 88.King EG, Roff DA, Fairbairn DJ. 2011. Trade-off acquisition and allocation in Gryllus firmus: a test of the Y model. J. Evol. Biol. 24, 256–264. ( 10.1111/j.1420-9101.2010.02160.x) [DOI] [PubMed] [Google Scholar]
- 89.Sol D, Duncan RP, Blackburn TM, Cassey P, Lefebvre L. 2005. Big brains, enhanced cognition, and response of birds to novel environments. Proc. Natl Acad. Sci. USA 102, 5460–5465. ( 10.1073/pnas.0408145102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reznick DN, Ghalambor CK. 2001. The population ecology of contemporary adaptations: what empirical studies reveal about the conditions that promote adaptive evolution. Genetica 112, 183–198. ( 10.1023/A:1013352109042) [DOI] [PubMed] [Google Scholar]
- 91.Betzholtz P-E, Pettersson LB, Ryrholm N, Franzen M. 2013. With that diet, you will go far: trait-based analysis reveals a link between rapid range expansion and a nitrogen-favoured diet. Proc. R. Soc. B 280, 20122305 ( 10.1098/rspb.2012.2305) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in Dryad (doi:10.5061/dryad.447sq).