Abstract
Evidence from grassland experiments suggests that a plant community's phylogenetic diversity (PD) is a strong predictor of ecosystem processes, even stronger than species richness per se. This has, however, never been extended to species-rich forests and host–parasitoid interactions. We used cavity-nesting Hymenoptera and their parasitoids collected in a subtropical forest as a model system to test whether hosts, parasitoids, and their interactions are influenced by tree PD and a comprehensive set of environmental variables, including tree species richness. Parasitism rate and parasitoid abundance were positively correlated with tree PD. All variables describing parasitoids decreased with elevation, and were, except parasitism rate, dependent on host abundance. Quantitative descriptors of host–parasitoid networks were independent of the environment. Our study indicates that host–parasitoid interactions in species-rich forests are related to the PD of the tree community, which influences parasitism rates through parasitoid abundance. We show that effects of tree community PD are much stronger than effects of tree species richness, can cascade to high trophic levels, and promote trophic interactions. As during habitat modification phylogenetic information is usually lost non-randomly, even species-rich habitats may not be able to continuously provide the ecosystem process parasitism if the evolutionarily most distinct plant lineages vanish.
Keywords: ecological networks, environmental gradients, Gutianshan National Nature Reserve, parasitism, species interactions, trap-nesting Hymenoptera
1. Introduction
Consumptive and antagonistic interactions between species are central processes in ecosystems. The pressures exerted by higher trophic levels, often termed top-down forces, structure species communities [1] and are probably central to maintaining local and global diversity [2], for example by increasing niche diversity and availability [3].
Insect parasitoids and their hosts are prime examples of antagonistic trophic interactions: larval parasitoids develop on individual arthropod hosts, which are ultimately killed [4]. Because of their high trophic position, mostly narrow host ranges, and specialized habitat requirements, parasitoids are predicted to rapidly respond to changing habitat conditions [5]. Parasitism, the ecosystem process delivered by parasitoids, can be sensitive to environmental change [6], making it crucial to understand how the environment relates to host–parasitoid interactions. A range of studies mostly from anthropogenically influenced ecosystems showed that parasitism and trophic interactions are influenced by habitat age (e.g. [7]), habitat fragmentation (e.g. [8]), or land-use type (e.g. [9]), mostly via lower parasitoid diversity and abundance in more severely modified habitats.
However, host–parasitoid interactions have been less studied in natural ecosystems and findings from modified, agricultural, and silvicultural ecosystems may not simply be transferable to structurally more complex and temporally more stable ecosystems, such as natural forests [10]. Globally, forests account for a third of all terrestrial ecosystems and, due to the astonishing diversity of low-latitude forests, are estimated to maintain over 80% of all terrestrial species [11]. Thus, it is important to understand how the abiotic and biotic environment changes host–parasitoid interactions and the associated ecosystem process parasitism in forests. The biodiversity-ecosystem-functioning (BEF) theory [12] predicts that forests with locally more tree species should support more parasitoids translating to more trophic interactions and higher parasitism. So far, several studies on the effect of tree species richness on parasitoids found a positive relationship between tree diversity and parasitoids [13,14], albeit without considering trophic interactions (but see [15]). The properties of such interactions can mathematically be quantified by various indices describing the structure of species interaction networks [16,17]. Thus, relating network properties to the environment can provide insight on how trophic interactions are influenced by changing habitat conditions [9,18,19].
There is evidence that plant (tree) species richness might not be the most informative predictor for BEF relationships [20,21]. Plants interact with their surroundings not merely by the number of species in a community but by their functional morphological and physiological properties, commonly termed traits. Thus, it would be desirable to use species traits rather than species numbers when exploring functional relationships in ecology [22]. However, measuring all biologically meaningful traits on the community level is logistically challenging if not impossible, especially for species-rich communities [23]. Also, if closely related species share similar traits, phylogenetic diversity (PD) is a more inclusive measure of overall trait similarity [21,24]. Estimates of the evolutionary divergence among different lineages within a community may be good surrogates for niche differences, assuming that changes in PD are proportional to changes in niche space or ecological function, i.e. that functionally relevant plant traits are phylogenetically conserved ([20,21]; but see [25]). Consequently, the PD of a plant community is expected to be a comprehensive predictor for ecosystem processes, as demonstrated in experimental grasslands: more productive and stable plant communities had more evolutionary diverse lineages; PD was superior in predictive power compared with species richness or traits [20]. Dinnage et al. [26] showed in the same BEF experiment that plant PD is positively related to the species richness and community composition of arthropods across trophic levels including parasitoids. If and how plant PD influences host–parasitoid interactions, however, remains to be studied.
We exposed standardized trap nests for solitary cavity-nesting Hymenoptera and their parasitoids in a subtropical forest to test if tree PD influences hosts, parasitoids, and their interactions. By mimicking easily available natural nesting sites for the host species (illustrated in [27]), trap nests sample species via natural reproduction, excluding potential ‘tourists’. Such trap nests have readily been used as a model system to study host–parasitoid interactions along environmental gradients (e.g. [7,9]). The host species (bees and wasps) often have short foraging distances following optimal foraging (e.g. [28]), making them and their parasitoids sensitive and responsive to local habitat properties.
Our study was conducted on the 27 well-studied plots of the BEF-China Project (www.bef-china.de, [29]) that allowed the inclusion of a broad set of abiotic (e.g. elevation) and biotic (e.g. cover of vegetation layers) environmental variables. This is important, because potential relationships between PD and host–parasitoid interactions are not independent of, and might be mediated by, the environment. Using a range of complementary analyses, we specifically test the hypotheses that hosts and parasitoids (abundance, richness) and their interactions (parasitism rate, descriptors of quantitative interaction networks) are positively related to tree PD and that parasitism is more strongly related to tree PD compared with tree species richness.
2. Material and methods
(a). Study site
This study was conducted in the Gutianshan National Nature Reserve (GNNR; 29°08′–29°17′ N, 118°02′–118°11′ E), subtropical southeast China, climatically characterized by warm and wet summers and relatively mild winters (mean annual temperature ca 15°C, mean annual precipitation ca 2 000 mm). On sloped land between 250–1 260 metres above sea level (m.a.s.l.), the GNNR comprises around 8 000 ha of species-rich broad-leaved forest. In 2008, 27 study plots with a size of 30 × 30 m were established. Plot selection was based on successional age (less than 20 to more than 80 years since the last land use) and tree species richness (25–69 species), which were not correlated. For more details and botanical information, refer to Bruelheide et al. [29] and for a map of the study site to Staab et al. [30].
(b). Sampling
Standardized trap nests made from reed internodes to collect solitary cavity-nesting Hymenoptera were exposed from September 2011 to October 2012 as described in [27]. Per plot, two wooden posts with four trap nests each were used. Monthly, internodes containing Hymenoptera nests were removed and replaced with similar empty internodes. For every nest, the number of brood cells (from here on, host brood cells) and the number of parasitized brood cells, i.e. brood cells killed by parasitoids, were counted. We did not distinguish between the two fundamental life-history strategies of parasitoids (true parasitoids, kleptoparasitoids) as the ecological result is the same: the death of host brood cells. Similarly, analyses were not separated by host species ecology (bees: pollinators; wasps: predators) as we were interested in host–parasitoid interactions at a community level. Nests were reared at ambient conditions until specimens hatched. More information on sampling can be found in the electronic supplementary material.
(c). Environmental variables
In heterogeneous ecosystems such as the GNNR, single environmental variables are rarely fully independent from each other, making a comprehensive characterization of the environment desirable. Hence, a broad range of plot-specific environmental variables were included in the analyses. Tree species richness was the number of all woody plant species of more than 1 m height and tree abundance was the number of those individuals. Canopy layer cover (%) was the proportion covered by the highest tree layer and shrub layer cover (%), the proportion covered by low woody vegetation. Successional age (years) was determined from stem core drillings (details in [29]). Herb layer cover (%) was the proportion covered by vegetation less than 1 m in the central 100 m2 of each plot. Herb species richness was not analysed as most plants smaller than 1 m were recruits of woody species growing on the same plot [29].
To obtain a variable referring to some functional aspects of the tree community, leaf functional diversity was calculated as Rao's Q from the 26 leaf traits analysed in [31]. The trait matrix included a comprehensive set of morphological (e.g. specific leaf area) and chemical (e.g. leaf carbon content) traits. Calculations were based on trait distances weighted by abundance. As a measure of PD, mean phylogenetic distance (MPD) was calculated as the mean abundance weighted phylogenetic distance among all angiosperm tree species in a plot based on a phylogeny [32,33] of all 147 tree species recorded on the 27 plots (electronic supplementary material). Four non-angiosperm species were excluded [34]. These species were rare (3.6% of individuals) but would, due to their disproportionally long branch lengths, bias MPD [35], as even few non-angiosperm individuals strongly increase plot-scale MPD because of their high distance to all other individuals (Spearman's rs = 0.88 for the correlation between the proportion of gymnosperm individuals and total MPD).
In addition to these biotic environmental variables, the abiotic variables plot elevation (m.a.s.l.) and aspect, i.e. the orientation of a plot on a slope as aspect eastness and northness, were extracted from a digital elevation model. More detailed descriptions of environmental variables can be found in [29,30] and electronic supplementary material, tables S1 and S2.
(d). Statistical analyses
All statistical analyses were conducted with R v. 3.2.4 (https://www.r-project.org). Prior to analyses, samples from the four trap nests attached to one wooden post were pooled, resulting in two data points per plot. To assess sampling efficiency for hosts and parasitoids, first-order jackknife species richness estimators and species accumulation curves (10 000 permutations) in the R-package vegan were used (https://www.cran.r-project.org/package=vegan).
To test for relationships between environmental variables and the response variables host abundance (host brood cell number), host species richness, parasitoid abundance (number of parasitized host brood cells), parasitoid species richness, and parasitism rate, generalized linear mixed-effect models (GLMMs) in the R-package lme4 were calculated (https://www.cran.r-project.org/package=lme4). Poisson error distribution was used for the count data on abundance and species richness, and binomial error distribution for the incidence data parasitism rate.
Tree species richness, tree abundance, canopy layer cover, shrub layer cover, and herb layer cover were log-transformed to improve normality and homoscedasticity. Before fitting models, all environmental variables were standardized (mean = 0, s.d. = 1) and tested for collinearity to prevent biased model estimates. If two environmental variables were correlated with Spearman's rs > 0.7, only one variable was retained following Dormann et al. [36]. This was the case for successional age, which was strongly correlated with canopy layer cover (rs = 0.76) and tree abundance (rs = −0.74), and retained as the more comprehensive variable describing stand-age related habitat properties (electronic supplementary material, table S2). Hence, the full GLMMs were fitted with the fixed effects aspect eastness, aspect northness, herb layer cover, elevation, leaf functional diversity, MPD, shrub layer cover, successional age, and tree species richness. The interaction term between tree species richness and MPD was included to account for the possible interdependence between the two measures of diversity [26], which were weakly positively correlated (rs = 0.47; electronic supplementary material, table S2 and figure S1). Host brood cell number (log-transformed) was included as a fixed effect in all models for parasitoid abundance, parasitoid richness, and parasitism rate to account for possible dependencies between host population density and parasitoids [37]. Plot identity was treated as a random factor to account for plot-specific environmental variation and for the hierarchical structure of the data. All Poisson GLMMs showed signs of possible overdispersion and a single-level observation random factor was included to improve model fits [38].
To examine the relative importance of the environmental variables for explaining the response variables we used model averaging [39] in the R-package MuMIn (https://www.cran.r-project.org/package=MuMIn). We calculated all possible models based on the fixed effects of the full models and ranked them by lowest Akaike information criterion (AICc). For each response variable, all equally likely candidate models within two AICc units of the model with the lowest AICc were averaged using the ‘model.avg’ command in MuMIn. All full models were tested for spatial autocorrelation with Moran's I coefficients of model residuals using the R-package ape (https://www.cran.r-project.org/package=ape). P-values were obtained by comparing observed and expected (10 000 permutations) Moran's I coefficients.
To test for and to illustrate direct and indirect pathways among the environmental variables most strongly influencing host–parasitoid interactions, path analysis was applied in the R-package piecewiseSEM (https://www.cran.r-project.org/package=piecewiseSEM). We used the same standardized data as for the GLMMs and treated plot identity as a random factor [40]. An a priori path model with the direct paths of MPD and elevation on parasitism rate as well as the indirect paths of the two variables via parasitoid abundance was constructed. Additionally, a path of host abundance on parasitoid abundance was included. This model structure was chosen with the a priori knowledge obtained by the averaged GLMMs on which variables are most meaningful in our study system and allows assessing if environmental effects on parasitism rates are indirectly mediated via parasitoid abundance.
Non-metric multidimensional scaling (NMDS) on data pooled per plot was used to analyse variation in host and parasitoid communities among plots. NMDS ordinations were based on Morisita–Horn dissimilarities of square-root transformed, Wisconsin-double standardized abundance data. Ordinations were calculated for two dimensions, which gave satisfying stress levels, centred and rotated until the first NMDS-axis explained maximum variance [41]. In a post hoc procedure, the environmental variables were correlated with the ordination results (command ‘envfit’ in Vegan) to explore associations with community variation and the environment (10 000 permutations). Procrustes rotation (10 000 permutations) was used to test for the non-randomness between host and parasitoid ordinations. All multivariate analyses were performed in vegan.
To obtain a quantitative measure of host–parasitoid interactions, Shannon interaction diversity was calculated with the R-package Bipartite (https://www.cran.r-project.org/package=bipartite) that was also used to describe and illustrate species-level host–parasitoid interactions. Many potential indices are available to quantify properties of species interactions in networks (reviewed and mathematically described in [17]), of which ‘linkage density’ (LD) and ‘H2’, the latter being a measure of interaction specialization at the network level, were chosen. Those indices and the associated null models were calculated for the total network and for a subset of plot-level networks. The influence of environmental variables on plot-level Shannon interaction diversity, LD, and H2 was tested with linear models using the same variables, model averaging, and spatial autocorrelation approach as for the GLMMs (see the electronic supplementary material for more details on network analyses).
3. Results
(a). General community patterns
Diverse hosts and parasitoids were reared from the trap nests. In total, 2 933 brood cells of 25 cavity-nesting solitary Hymenoptera species were found, of which 11% (335 brood cells) were parasitized by 27 parasitoid species belonging to Coleoptera (five brood cells/one species), Diptera (122/8), and Hymenoptera (202/18). More detailed descriptions of host and parasitoid communities are given in the electronic supplementary material, table S3 and figure S2. Species richness estimation and accumulation curves indicated that host and parasitoid (electronic supplementary material, figure S3) communities were sampled equally well.
(b). Influence of environmental variables
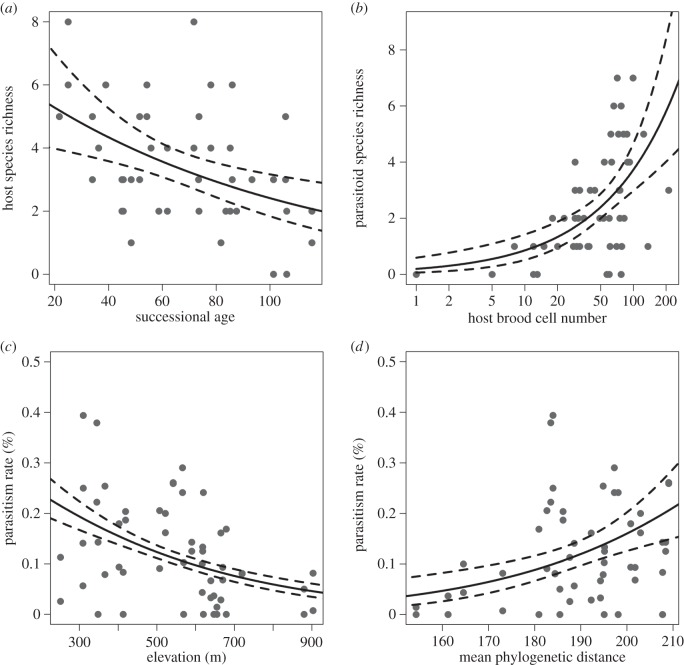
Host abundance could not be explained by the environment. Host species richness declined with successional age (figure 1a; p = 0.014; significant averaged model parameters are given in table 1; full parameters of averaged models can be found in electronic supplementary material, table S4) while increasing on more east-facing slopes (p = 0.028).
Figure 1.
Relationships between environmental variables and host species richness (a), parasitoid species richness (b), and parasitism rates (c,d). Shown are model predictions (solid lines) and 95% CI (dashed lines). Please note that the x-axis of (b) is log-scaled. See table 1 and electronic supplementary material, table S4 for detailed model parameters and significances.
Table 1.
Summary results of the averaged mixed-effect models (within two AICc units of the model with the lowest AICc) for parasitism rate (binomial models) and abundance and species richness (Poisson models) of parasitoids and hosts. Shown are standardized model estimates ± standard error (s.e.) of significant variables allowing a direct comparison of effect sizes, z-values, p-values of the z-statistics, and the relative importance of variables in the averaged models. Detailed output of averaged models can be found in the electronic supplementary material, table S4.
| environmental variable | estimate ± s.e. | z-values | p-values | relative importance |
|---|---|---|---|---|
| parasitism rate | ||||
| elevation | −0.439 ± 0.139 | 3.073 | 0.002 | 1.00 |
| mean phylogenetic distance | 0.427 ± 0.149 | 2.801 | 0.005 | 1.00 |
| parasitoid abundance | ||||
| host brood cell numbera | 1.050 ± 0.182 | 5.611 | <0.001 | 1.00 |
| elevation | −0.361 ± 0.126 | 2.868 | 0.004 | 1.00 |
| mean phylogenetic distance | 0.388 ± 0.134 | 2.825 | 0.005 | 1.00 |
| parasitoid species richness | ||||
| host brood cell numbera | 0.808 ± 0.165 | 4.775 | <0.001 | 1.00 |
| elevation | −0.229 ± 0.111 | 2.023 | 0.043 | 1.00 |
| host abundance | ||||
| none | — | — | — | — |
| host species richness | ||||
| aspect eastness | 0.180 ± 0.080 | 2.193 | 0.028 | 0.91 |
| successional age | −0.223 ± 0.089 | 2.458 | 0.014 | 0.86 |
aHost brood cell number is our definition of host abundance and consequently not included in models for host abundance and species richness.
Parasitoid abundance and species richness were influenced by more variables. Unsurprisingly, both strongly increased with host abundance (p < 0.001 each; figure 1b). Plots at higher elevations had consistently lower parasitoid abundance and species richness (abundance: p = 0.004; richness: p = 0.043). While tree species richness was never significantly related to hosts and parasitoids, there was a positive correlation between tree MPD and parasitoid abundance (p = 0.005).
Similarly, MPD was the only variable with a significantly positive relationship with parasitism rates (p = 0.005): in plots with a tree community consisting of phylogenetically more distantly related lineages, a higher proportion of host brood cells was attacked by parasitoids (figure 1c). However, parasitism was also related to elevation (p = 0.002) and strongly decreasing towards higher plots (table 1 and figure 1d). Examination of Moran's I coefficients showed no spatial autocorrelation.
(c). Direct and indirect pathways among environmental variables
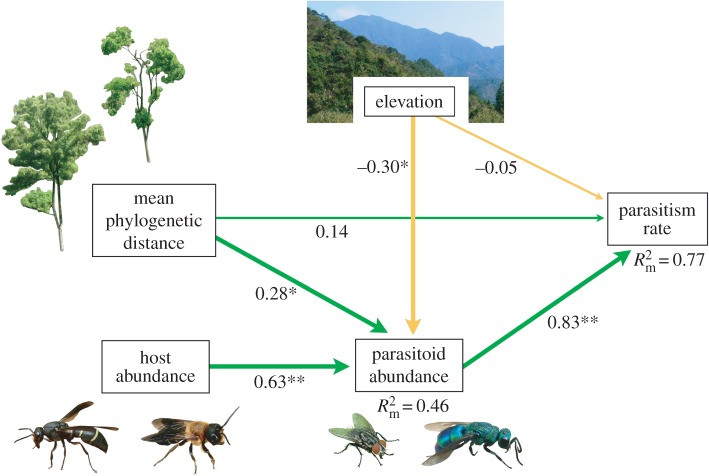
The path analysis (figure 2) indicated that the positive influence of MPD and the negative influence of elevation on parasitism rates are indirectly (MPD, p = 0.034; elevation, p = 0.18) mediated via parasitoid abundance and not direct, albeit the sign of the direct path of MPD was positive (p = 0.101). Host abundance was also strongly positively associated with parasitoid abundance (p < 0.001), which was positively associated with parasitism rates (p < 0.001). In addition to indicating the indirect causal links, the path analysis supported and strengthened the results previously obtained by modelling.
Figure 2.
Path model (C = 4.75, p = 0.14) showing that parasitism rate is influenced by MPD and elevation via parasitoid abundance. Numbers next to the arrows give the standardized path coefficients. Significant causal paths are indicated with bold arrows. Green (darker) arrows indicate positive relationships, golden (lighter) arrows negative relationships. Significances are **p < 0.01 and *p < 0.05. R² values are marginal R². All photographs by Michael Staab. (Online version in colour.)
(d). Community variation
Variation of host and parasitoid species among the study plots was indicated by two-dimensional NMDS ordinations (figure 3). Elevation (p = 0.004) and MPD (p = 0.006) were the environmental variables most strongly correlated with host species community variation and MPD was the only environmental variable significantly correlated with parasitoid species community variation (MPD: p = 0.009; electronic supplementary material, table S6). For hosts, shrub layer cover (p = 0.015) and leaf functional diversity (p = 0.017) were also significantly correlated with the NMDS ordination, but with influences opposing MPD. Host and parasitoid ordinations were not significantly related (Procrustes sum of squares = 0.912, p = 0.172).
Figure 3.
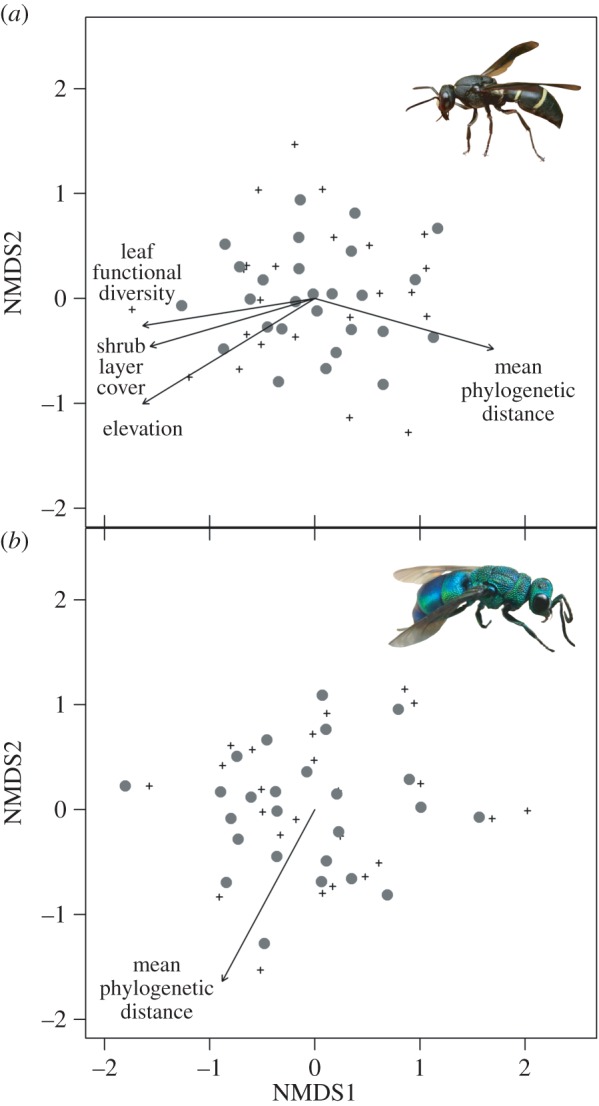
NMDS ordinations of (a) hosts (stress = 0.21) and (b) their associated parasitoids (stress = 0.17). Grey dots refer to the 27 study plots, black crosses to the species in each community. Arrows indicate significant correlations of environmental variables with plot-based axis scores. The length of the arrows is proportional to the strength of a given correlation. Details on correlations are given in electronic supplementary material, table S6. (Online version in colour.)
(e). Host–parasitoid interactions
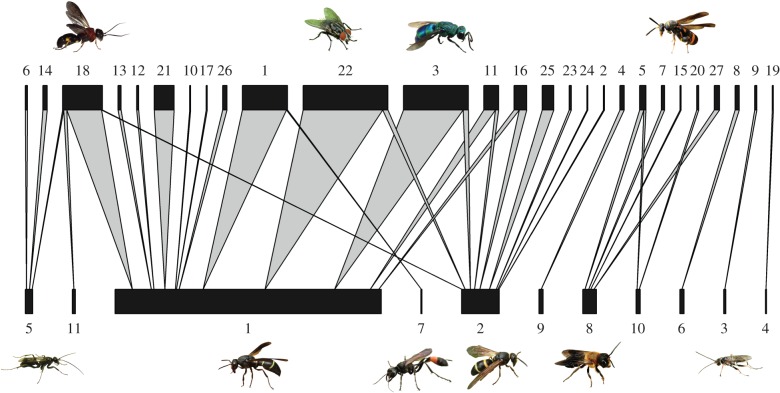
The Shannon interaction diversity was strongly and positively influenced by host abundance (p < 0.001; parameters of the averaged model can be found in the electronic supplementary material, table S5 and figure S4). There was no sign of spatial autocorrelation. The full host–parasitoid interaction network (figure 4) revealed relatively specialized interactions (H2 = 0.72). Linkage density was intermediate (LD = 3.64), indicating that species interacted on average with three to four species in the other trophic level. Plot-level network indices were unrelated to the environment. Null models showed that the observed networks were more specialized and less linked than expected by chance (electronic supplementary material).
Figure 4.
Total quantitative bipartite host–parasitoid network. Width of bars corresponds to the number of parasitized host brood cells per species; width of arrows corresponds to the number of interactions between hosts (below) and parasitoids (above). Single interactions are indicated by the most narrow bars and arrows. Numerical codes refer to electronic supplementary material, table S3. Pictures of specimens correspond to the numerical codes next to them. All photographs by Michael Staab. (Online version in colour.)
4. Discussion
We provide here the first evidence that parasitoid abundance and parasitism rates in a highly diverse forest are related to the PD of the local tree community. The influence of tree PD on parasitism rates was mediated by parasitoid abundance and much stronger than the influence of tree species richness.
(a). Influence of tree mean phylogenetic distance on host–parasitoid interactions
Positive correlations between the species richness of trees and other taxa are common [42]. However, for parasitoids and host–parasitoid systems evidence for cross-taxon congruence with tree species richness or other coarse measures of habitat heterogeneity is sparse and inconclusive. Depending on the study system, parasitoid abundance and species richness increased [13–15], were unrelated [43] or declined [44] with habitat heterogeneity, but no study considered descriptors of PD such as tree MPD. As introduced above, a plant community's PD is a suitable surrogate for overall niche availability and habitat heterogeneity [21,23], and more diverse evolutionary lineages will result in more diverse microhabitats. This might be especially pronounced in forests: trees are long-lived organisms with a high biomass, making them key-stone structures [45], potentially amplifying the positive influence of plant MPD on niche availability.
The specific mechanisms behind the correlation of PD with parasitoids probably depend on the specific host–parasitoid system and cannot be revealed by observational studies such as ours. For the system studied and for other host–parasitoid systems (e.g. leaf miner-parasitoid [8,46] or aphid-parasitoid [47]), the biologically most relevant niches are probably food (i.e. food for hosts, hosts of parasitoids; see below) and shelter [4]. While for systems with phytophagous hosts, plant MPD directly relates to food availability and diversity [21,26], it is less intuitive how MPD might influence systems with hosts from higher trophic levels. Host–parasitoid interactions in tropical forests are sensitive to microclimate [48], but the cause is unknown. Forest stands with higher MPD might support more diverse shelter and thus microclimates, on which the often small-bodied parasitoids could be particularly dependent [4]. Remarkably, increasing MPD not only increased parasitoid abundance but, via parasitoid abundance, also parasitoid performance measured as parasitism rates. Noteworthy, there was never a significant correlation of the response variables with tree species richness or the interaction between tree species richness and MPD. This indicates independent statistical effects of species richness and phylogeny, and that the diversity of evolutionary lineages is more important than species richness alone, despite the weakly positive relationship between both measures of diversity. Our results support the accumulating evidence that a plant community's PD is a strong predictor for the strength of ecosystem processes in that community [20,49]. For example, Parker et al. [50] demonstrated that the susceptibility of a plant species to diseases and invasion potential (via escape from diseases) is predicted well by the PD of the surrounding plant community. Also, the dietary specialization of herbivorous insects correlates positively with the PD of the plant community they live in [51]. Unfortunately, we lack data on herbivores (e.g. caterpillars, which are prey of many host species) and thus miss the direct link between tree MPD and host species. Nevertheless, we show that bottom-up effects of MPD can prevail to, and influence interactions in, high trophic levels, which has until now only been shown for structurally simple experimental ecosystems (e.g. [26,47]) but not for heterogeneous and species-rich natural forests.
(b). Direct and indirect environmental influences on hosts
Parasitoids depend directly on their hosts. Following the more individuals hypothesis [52], resource availability in a lower trophic level (hosts) translates to the performance of a higher trophic level (parasitoids); an increase of parasitoid abundance and species richness with host abundance has often been demonstrated (e.g. [7]). In this study, tree MPD was surprisingly, for as yet unknown reasons, unrelated to host abundance and richness, although hosts, similar to parasitoids, should theoretically benefit from higher habitat heterogeneity. As opposed to most parasitoids, many host species are strong flyers. Albeit having a preference for short foraging distances, those species might cover large areas [53] considerably exceeding the size of the plots on which the environmental variables were measured. Thus, it might be that in our study system hosts are less related to local tree MPD than parasitoids but more dependent on habitat properties affecting their nesting. Host species richness was consistently lower in older forest stands, which probably reflects less favourable conditions for thermophilic nest-provisioning Hymenoptera in old-growth forests. Canopy cover influences a forests’ climate [54] and is in the GNNR highly correlated with successional age. Also, older plots had fewer deciduous trees [29] and a higher leaf area index, resulting in less insolation of the understory and wetter and cooler conditions [55]. Solitary nest-provisioning Hymenoptera, however, need light and warmth for effective foraging [28] and brood development [56]. This is also reflected in the increase of host species richness with aspect eastness, because east-facing slopes get direct sunlight early in the day and thus warm up quickly.
The local plant species composition can predict arthropod community composition across trophic levels [57]. Unsurprisingly, tree MPD influenced host and parasitoid community composition, albeit not congruently, as NMDS ordinations were unrelated. Plots that differ in tree MPD are likely to support different species communities, confirming the found bottom-up effect. This is also supported by the (albeit weak compared with MPD) association between leaf functional diversity and host communities, which might be mediated by the food objects of the hosts (e.g. caterpillars) that directly interact with trees.
(c). Influence of elevation on host–parasitoid interactions
Elevation was associated with host community variation; parasitoid abundance and species richness declined with elevation, too. Changes of species diversity and communities along elevation gradients are long known [58]. By contrast, very little is known about the relationship between elevation and host–parasitoid interactions. A decline of parasitism towards higher elevations is likely, as the frequency of trophic interactions decreases polewards [59] and local elevation gradients reflect large-scale climatic gradients. To our knowledge, only two recent studies from subtropical Australia have addressed this topic and found a decrease of parasitism with elevation [18,46], which we support. Higher parasitism at lower and warmer lower elevation might be caused by higher attack rates of parasitoids, as demonstrated in controlled warming experiments [60]. Thus, increasing temperatures with climatic change will probably increase parasitism rates but influence host–parasitoid interactions in unpredictable ways, as it is unclear if hosts and parasitoids will react similarly [5].
(d). Environmental influences on host–parasitoid interaction networks
The selected quantitative network indices showed that host–parasitoid interactions were considerably more specialized and with less linkage density than expected by random species interactions; plot-level indices were not related to MPD or other environmental variables. Albeit limited by the relatively small size of our networks, this may indicate that network structure can be independent of the environment (but see [9]). In this regard, our results conform to a global meta-analysis [61], which demonstrated that host–parasitoid networks are generally specialized, independent of geography, scale, taxa or, host–parasitoid system.
That a phylogenetically diverse tree community supports parasitoids and parasitism agrees with earlier evidence that ‘healthier’ ecosystems [8] are characterized by high parasitism [62]. However, extinction risk due to anthropogenic disturbance is not random. Evolutionarily distinct plant lineages vanish disproportionally faster during anthropogenic habitat modification and global change [63]. This non-random loss of diversity from plant communities can negatively influence ecosystem processes, such as parasitism, because habitats with high plant MPD might contribute to the conservation of parasitoids and species interactions [64].
(e). Conclusion
We have demonstrated for the first time that the PD of a tree community is a predictor of parasitoid abundance and parasitism rates. This extends the literature on the relationship between plant PD and ecosystem processes from primary production and experimental grasslands [20,49] to host–parasitoid interactions and species-rich forests. Also, MPD was superior to tree species richness in predictive power, supporting theoretical considerations [21,23] and illustrating that a phylogenetic perspective in BEF research is vital to understand the role of diversity for ecosystem processes. Future work should not only extend our results to additional host–parasitoid systems but also incorporate species-specific life-history traits that can strongly influence parasitism [27]. Ideally, those studies would include experimental manipulations of plant MPD, hosts, and parasitoids (sensu [65]) to disentangle the mechanisms behind the bottom-up effect of MPD on host–parasitoid interactions in natural ecosystems.
Supplementary Material
Acknowledgements
We thank the administration of the GNNR for granting research permissions and the BEF-China coordination team, particularly Xiaojuan Liu, for continuous support. Working in the GNNR's challenging terrain would not have been possible without Jan Peters, Wenzel Kröber, several local workers, and our driver ‘A-de-we’ Yu. We are indebted to the taxonomists Michael Ohl (Sphecidae), Oliver Niehuis (Chrysididae), Tingjing Li (Vespidae), and Niu Ze-Qing (Megachilidae). The manuscript benefited from comments of five anonymous reviewers.
Data accessibility
Data are deposited in Dryad: http://dx.doi.org/10.5061/dryad.46n26
Authors' contributions
A.-M.K. and M.S. conceived the study. M.S. carried out fieldwork, analysed the data, and drafted the manuscript. W.D., S.M., and O.P. contributed to the phylogenetic data. All authors critically revised the manuscript and gave final approval for publication.
Competing interests
We have no competing interests.
Funding
This study was funded by the German Science Foundation (DFG; FOR 891/2, KL 1849/6-1).
References
- 1.Hunter MD, Price PW. 1992. Playing chutes and ladders—heterogeneity and the relative roles of bottom-up and top-down forces in natural communities. Ecology 73, 724–732. [Google Scholar]
- 2.Janzen DH. 1970. Herbivores and the number of tree species in tropical forests. Am. Nat. 104, 501–528. ( 10.1086/282687) [DOI] [Google Scholar]
- 3.Condon MA, Scheffer SJ, Lewis ML, Wharton R, Adams DC, Forbes AA. 2014. Lethal interactions between parasites and prey increase niche diversity in a tropical community. Science 343, 1240–1244. ( 10.1126/science.1245007) [DOI] [PubMed] [Google Scholar]
- 4.Godfray HCJ. 1994. Parasitoids: behavioral and evolutionary ecology. Princeton, NJ: Princeton University Press. [Google Scholar]
- 5.Jeffs CT, Lewis OT. 2013. Effects of climate warming on host–parasitoid interactions. Ecol. Entomol. 38, 209–218. ( 10.1111/een.12026) [DOI] [Google Scholar]
- 6.Tylianakis JM, Didham RK, Bascompte J, Wardle DA. 2008. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 11, 1351–1363. ( 10.1111/j.1461-0248.2008.01250.x) [DOI] [PubMed] [Google Scholar]
- 7.Tscharntke T, Gathmann A, Steffan-Dewenter I. 1998. Bioindication using trap-nesting bees and wasps and their natural enemies: community structure and interactions. J. Appl. Ecol. 35, 708–719. ( 10.1046/j.1365-2664.1998.355343.x) [DOI] [Google Scholar]
- 8.Fenoglio MS, Srivastava D, Valladares G, Cagnolo L, Salvo A. 2012. Forest fragmentation reduces parasitism via species loss at multiple trophic levels. Ecology 93, 2407–2420. ( 10.1890/11-2043.1) [DOI] [PubMed] [Google Scholar]
- 9.Tylianakis JM, Tscharntke T, Lewis OT. 2007. Habitat modification alters the structure of tropical host–parasitoid food webs. Nature 445, 202–205. ( 10.1038/nature05429) [DOI] [PubMed] [Google Scholar]
- 10.Scherber C, Vockenhuber EA, Stark A, Meyer H, Tscharntke T. 2014. Effects of tree and herb biodiversity on Diptera, a hyperdiverse insect order. Oecologia 174, 1387–1400. ( 10.1007/s00442-013-2865-7) [DOI] [PubMed] [Google Scholar]
- 11.FAO. 2010. Global forest resources assessment 2010. FAO Forestry Paper 163, 1–340. [Google Scholar]
- 12.Loreau M, et al. 2001. Biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294, 804–808. ( 10.1126/science.1064088) [DOI] [PubMed] [Google Scholar]
- 13.Sperber CF, Nakayama K, Valverde MJ, Neves FD. 2004. Tree species richness and density affect parasitoid diversity in cacao agroforestry. Basic Appl. Ecol. 5, 241–251. ( 10.1016/j.baae.2004.04.001) [DOI] [Google Scholar]
- 14.Fraser SEM, Dytham C, Mayhew PJ. 2007. Determinants of parasitoid abundance and diversity in woodland habitats. J. Appl. Ecol. 44, 352–361. ( 10.1111/j.1365-2664.2006.01266.x) [DOI] [Google Scholar]
- 15.Sobek S, Tscharntke T, Scherber C, Schiele S, Steffan-Dewenter I. 2009. Canopy vs. understory: does tree diversity affect bee and wasp communities and their natural enemies across forest strata? Forest Ecol. Manage. 258, 609–615. ( 10.1016/j.foreco.2009.04.026) [DOI] [Google Scholar]
- 16.Bersier LF, Banasek-Richter C, Cattin MF. 2002. Quantitative descriptors of food-web matrices. Ecology 83, 2394–2407. ( 10.1890/0012-9658(2002)083%5B2394:QDOFWM%5D2.0.CO;2) [DOI] [Google Scholar]
- 17.Dormann C, Fründ J, Blüthgen N, Gruber B. 2009. Indices, graphs and null models: analyzing bipartite ecological networks. Open Ecol. J. 2, 7–24. ( 10.2174/1874213000902010007) [DOI] [Google Scholar]
- 18.Morris RJ, Sinclair FH, Burwell CJ. 2015. Food web structure changes with elevation but not rainforest stratum. Ecography 38, 792–802. ( 10.1111/ecog.01078) [DOI] [Google Scholar]
- 19.Staab M, Blüthgen N, Klein AM. 2015. Tree diversity alters the structure of a tri-trophic network in a biodiversity experiment. Oikos 124, 827–834. ( 10.1111/oik.01723) [DOI] [Google Scholar]
- 20.Cadotte MW, Cavender-Bares J, Tilman D, Oakley TH. 2009. Using phylogenetic, functional and trait diversity to understand patterns of plant community productivity. PLoS ONE 4, e5695 ( 10.1371/journal.pone.0005695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srivastava DS, Cadotte MW, MacDonald AAM, Marushia RG, Mirotchnick N. 2012. Phylogenetic diversity and the functioning of ecosystems. Ecol. Lett. 15, 637–648. ( 10.1111/j.1461-0248.2012.01795.x) [DOI] [PubMed] [Google Scholar]
- 22.Diaz S, Cabido M. 2001. Vive la difference: plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 16, 646–655. ( 10.1016/s0169-5347(01)02283-2) [DOI] [Google Scholar]
- 23.Webb CO, Ackerly DD, McPeek MA, Donoghue MJ. 2002. Phylogenies and community ecology. Ann. Rev. Ecol. Syst. 33, 475–505. ( 10.1146/annurev.ecolsys.33.010802.150448) [DOI] [Google Scholar]
- 24.Tucker CM, et al. 2016. A guide to phylogenetic metrics for conservation, community ecology and macroecology. Biol. Rev. ( 10.1111/brv.12252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Purschke O, et al. 2013. Contrasting changes in taxonomic, phylogenetic and functional diversity during a long-term succession: insights into assembly processes. J. Ecol. 101, 857–866. ( 10.1111/1365-2745.12098) [DOI] [Google Scholar]
- 26.Dinnage R, Cadotte MW, Haddad NM, Crutsinger GM, Tilman D. 2012. Diversity of plant evolutionary lineages promotes arthropod diversity. Ecol. Lett. 15, 1308–1317. ( 10.1111/j.1461-0248.2012.01854.x) [DOI] [PubMed] [Google Scholar]
- 27.Staab M, Ohl M, Zhu CD, Klein AM. 2014. A unique nest-protection strategy in a new species of spider wasp. PLoS ONE 9, e101592 ( 10.1371/journal.pone.0101592) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein AM, Steffan-Dewenter I, Tscharntke T. 2004. Foraging trip duration and density of megachilid bees, eumenid wasps and pompilid wasps in tropical agroforestry systems. J. Anim. Ecol. 73, 517–525. ( 10.1111/j.0021-8790.2004.00826.x) [DOI] [Google Scholar]
- 29.Bruelheide H, et al. 2011. Community assembly during secondary forest succession in a Chinese subtropical forest. Ecol. Monogr. 81, 25–41. ( 10.1890/09-2172.1) [DOI] [Google Scholar]
- 30.Staab M, Schuldt A, Assmann T, Bruelheide H, Klein AM. 2014. Ant community structure during forest succession in a subtropical forest in South-East China. Acta Oecol. 61, 32–40. ( 10.1016/j.actao.2014.10.003) [DOI] [Google Scholar]
- 31.Kröber W, Böhnke M, Welk E, Wirth C, Bruelheide H. 2012. Leaf trait-environment relationships in a subtropical broadleaved forest in South-East China. PLoS ONE 7, e35742 ( 10.1371/journal.pone.0035742) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuldt A, Bruelheide H, Durka W, Michalski SG, Purschke O, Assmann T. 2014. Tree diversity promotes functional dissimilarity and maintains functional richness despite species loss in predator assemblages. Oecologia 174, 533–543. ( 10.1007/s00442-013-2790-9) [DOI] [PubMed] [Google Scholar]
- 33.Baruffol M, Schmid B, Bruelheide H, Chi X, Hector A, Ma K, Michalski SG, Tang Z, Niklaus PA. 2013. Biodiversity promotes tree growth during succession in subtropical forest. PLoS ONE 8, e81246 ( 10.1371/journal.pone.0081246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Letcher SG. 2010. Phylogenetic structure of angiosperm communities during tropical forest succession. Proc. R. Soc. B 277, 97–104. ( 10.1098/rspb.2009.0865) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lososova Z, Smarda P, Chytry M, Purschke O, Pysek P, Sadlo J, Tichy L, Winter M. 2015. Phylogenetic structure of plant species pools reflects habitat age on the geological time scale. J. Veg. Sci. 26, 1080–1089. ( 10.1111/jvs.12308) [DOI] [Google Scholar]
- 36.Dormann CF, et al. 2013. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36, 27–46. ( 10.1111/j.1600-0587.2012.07348.x) [DOI] [Google Scholar]
- 37.Hassell MP, Waage JK. 1984. Host–parasitoid population interactions. Annu. Rev. Entomol. 29, 89–114. ( 10.1146/annurev.en.29.010184.000513) [DOI] [Google Scholar]
- 38.Harrison XA. 2014. Using observation-level random effects to model overdispersion in count data in ecology and evolution. PeerJ 2, e616 ( 10.7717/peerj.616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grueber CE, Nakagawa S, Laws RJ, Jamieson IG. 2011. Multimodel inference in ecology and evolution: challenges and solutions. J. Evol. Biol. 24, 699–711. ( 10.1111/j.1420-9101.2010.02210.x) [DOI] [PubMed] [Google Scholar]
- 40.Lefcheck JS. 2015. piecewiseSEM: piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 7, 573–579. ( 10.1111/2041-210x.12512) [DOI] [Google Scholar]
- 41.Quinn GP, Keough MJ. 2002. Experimental design and data analysis for biologists. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 42.Basset Y, et al. 2012. Arthropod diversity in a tropical forest. Science 338, 1481–1484. ( 10.1126/science.1226727) [DOI] [PubMed] [Google Scholar]
- 43.Veddeler D, Tylianakis J, Tscharntke T, Klein AM. 2010. Natural enemy diversity reduces temporal variability in wasp but not bee parasitism. Oecologia 162, 755–762. ( 10.1007/s00442-009-1491-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maleque MA, Maeto K, Makino S, Goto H, Tanaka H, Hasegawa M, Miyamoto A. 2010. A chronosequence of understorey parasitic wasp assemblages in secondary broad-leaved forests in a Japanese 'satoyama' landscape. Insect Conserv. Divers 3, 143–151. ( 10.1111/j.1752-4598.2010.00087.x) [DOI] [Google Scholar]
- 45.Jones CG, Lawton JH, Shachak M. 1994. Organisms as ecosystem engineers. Oikos 69, 373–386. ( 10.2307/3545850) [DOI] [Google Scholar]
- 46.Maunsell SC, Kitching RL, Burwell CJ, Morris RJ. 2015. Changes in host–parasitoid food web structure with elevation. J. Anim. Ecol. 84, 353–363. ( 10.1111/1365-2656.12285) [DOI] [PubMed] [Google Scholar]
- 47.Petermann JS, Müller CB, Weigelt A, Weisser WW, Schmid B. 2010. Effect of plant species loss on aphid–parasitoid communities. J. Anim. Ecol. 79, 709–720. ( 10.1111/j.1365-2656.2010.01674.x) [DOI] [PubMed] [Google Scholar]
- 48.Stangler ES, Hanson PE, Steffan-Dewenter I. 2015. Interactive effects of habitat fragmentation and microclimate on trap-nesting Hymenoptera and their trophic interactions in small secondary rainforest remnants. Biodivers. Conserv. 24, 563–577. ( 10.1007/s10531-014-0836-x) [DOI] [Google Scholar]
- 49.Cadotte MW, Dinnage R, Tilman D. 2012. Phylogenetic diversity promotes ecosystem stability. Ecology 93, S223–S233. ( 10.1890/11-0426.1) [DOI] [Google Scholar]
- 50.Parker IM, Saunders M, Bontrager M, Weitz AP, Hendricks R, Magarey R, Suiter K, Gilbert GS. 2015. Phylogenetic structure and host abundance drive disease pressure in communities. Nature 520, 542–544. ( 10.1038/nature14372) [DOI] [PubMed] [Google Scholar]
- 51.Forister ML, et al. 2015. The global distribution of diet breadth in insect herbivores. Proc. Natl Acad. Sci. USA 112, 442–447. ( 10.1073/pnas.1423042112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Srivastava DS, Lawton JH. 1998. Why more productive sites have more species: an experimental test of theory using tree-hole communities. Am. Nat. 152, 510–529. ( 10.1086/286187) [DOI] [PubMed] [Google Scholar]
- 53.Zurbuchen A, Landert L, Klaiber J, Müller A, Hein S, Dorn S. 2010. Maximum foraging ranges in solitary bees: only few individuals have the capability to cover long foraging distances. Biol. Conserv. 143, 669–676. ( 10.1016/j.biocon.2009.12.003) [DOI] [Google Scholar]
- 54.Aussenac G. 2000. Interactions between forest stands and microclimate: ecophysiological aspects and consequences for silviculture. Annu. Forest Sci. 57, 287–301. ( 10.1051/forest:2000119) [DOI] [Google Scholar]
- 55.Hardwick SR, Toumi R, Pfeifer M, Turner EC, Nilus R, Ewers RM. 2015. The relationship between leaf area index and microclimate in tropical forest and oil palm plantation: forest disturbance drives changes in microclimate. Agric. Forest Meteorol. 201, 187–195. ( 10.1016/j.agrformet.2014.11.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Radmacher S, Strohm E. 2011. Effects of constant and fluctuating temperatures on the development of the solitary bee Osmia bicornis (Hymenoptera: Megachilidae). Apidologie 42, 711–720. ( 10.1007/s13592-011-0078-9) [DOI] [Google Scholar]
- 57.Schaffers AP, Raemakers IP, Sykora KV, Ter Braak CJF. 2008. Arthropod assemblages are best predicted by plant species composition. Ecology 89, 782–794. ( 10.1890/07-0361.1) [DOI] [PubMed] [Google Scholar]
- 58.Rahbek C. 1995. The elevational gradient of species richness—a uniform pattern. Ecography 18, 200–205. ( 10.1111/j.1600-0587.1995.tb00341.x) [DOI] [Google Scholar]
- 59.Schemske DW, Mittelbach GG, Cornell HV, Sobel JM, Roy K. 2009. Is there a latitudinal gradient in the importance of biotic interactions? Annu. Rev. Ecol. Evol. Syst. 40, 245–269. ( 10.1146/annurev.ecolsys.39.110707.173430) [DOI] [Google Scholar]
- 60.de Sassi C, Staniczenko PPA, Tylianakis JM. 2012. Warming and nitrogen affect size structuring and density dependence in a host–parasitoid food web. Phil. Trans. R. Soc. B 367, 3033–3041. ( 10.1098/rstb.2012.0233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morris RJ, Gripenberg S, Lewis OT, Roslin T. 2014. Antagonistic interaction networks are structured independently of latitude and host guild. Ecol. Lett. 17, 340–349. ( 10.1111/ele.12235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hudson PJ, Dobson AP, Lafferty KD. 2006. Is a healthy ecosystem one that is rich in parasites? Trends Ecol. Evol. 21, 381–385. ( 10.1016/j.tree.2006.04.007) [DOI] [PubMed] [Google Scholar]
- 63.Vamosi JC, Wilson JRU. 2008. Nonrandom extinction leads to elevated loss of angiosperm evolutionary history. Ecol. Lett. 11, 1047–1053. ( 10.1111/j.1461-0248.2008.01215.x) [DOI] [PubMed] [Google Scholar]
- 64.Tylianakis JM, Laliberte E, Nielsen A, Bascompte J. 2010. Conservation of species interaction networks. Biol. Conserv. 143, 2270–2279. ( 10.1016/j.biocon.2009.12.004) [DOI] [Google Scholar]
- 65.Fründ J, Dormann CF, Holzschuh A, Tscharntke T. 2013. Bee diversity effects on pollination depend on functional complementarity and niche shifts. Ecology 94, 2042–2054. ( 10.1890/12-1620.1) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are deposited in Dryad: http://dx.doi.org/10.5061/dryad.46n26