Abstract
Bees are thought to be strict users of carbohydrates as metabolic fuel for flight. Many insects, however, have the ability to oxidize the amino acid proline at a high rate, which is a unique feature of this group of animals. The presence of proline in the haemolymph of bees and in the nectar of plants led to the hypothesis that plants may produce proline as a metabolic reward for pollinators. We investigated flight muscle metabolism of hymenopteran species using high-resolution respirometry performed on permeabilized muscle fibres. The muscle fibres of the honeybee, Apis mellifera, do not have a detectable capacity to oxidize proline, as those from the migratory locust, Locusta migratoria, used here as an outgroup representative. The closely related bumblebee, Bombus impatiens, can oxidize proline alone and more than doubles its respiratory capacity when proline is combined with carbohydrate-derived substrates. A distant wasp species, Vespula vulgaris, exhibits the same metabolic phenotype as the bumblebee, suggesting that proline oxidation is common in hymenopterans. Using a combination of mitochondrial substrates and inhibitors, we further show that in B. impatiens, proline oxidation provides reducing equivalents and electrons directly to the electron transport system. Together, these findings demonstrate that some bee and wasp species can greatly enhance the oxidation of carbohydrates using proline as fuel for flight.
Keywords: proline, mitochondria, metabolism, evolution, bees, pollinators
1. Introduction
Animals can power cellular metabolism with carbohydrates, lipids or proteins. The relative importance of these various fuels does not only depend on the intensity of energy metabolism (reviewed by Weber [1]), but also on ecological requirements and dietary opportunities shaped by evolutionary mechanisms. In insects, such diversity in metabolic fuel use and muscle cell phenotypes has been well characterized during flight [2], a strictly aerobic activity [3]. Species that perform long-term flights have been exemplified by the migratory locust (Locusta migratoria), whose muscles use carbohydrates during the initial phase of flight and lipids to sustain activity [4]. By contrast, some blood-feeding insects use the amino acid proline as their main fuel even though most animals avoid relying on proteins. This unique strategy has been well documented in the tsetse fly [5,6], whereas other species such as the honeybee (Apis mellifera) are thought to have become exclusive carbohydrate users, probably because of the high intensity of hovering flight and dietary specialization [7]. The honeybee has become the poster child for all bees, a group estimated at over 20 000 species [8]. Multiple lines of evidence support the idea that A. mellifera only fuels flight with carbohydrates as indicated by its respiratory quotient of 1 (ratio of CO2 produced to O2 consumed) measured during flight [9,10]. Respirometry studies conducted on other bee species also support the notion that carbohydrates are used as the primary fuel [7,11]. Studies conducted at the muscle tissue level clearly show that the metabolic phenotype of A. mellifera is geared towards the oxidation of carbohydrates and cannot use lipids [3,12]. Such metabolic organization has become the blueprint for studies investigating energy metabolism in this group of animals [13–16].
The ability of the honeybee to use proline has been investigated, given the substantial concentration of this amino acid in haemolymph and in honey [17–21]. Several studies measuring changes in thoracic or haemolymph proline content of A. mellifera have concluded that the contribution of this amino acid is minimal [22,23]. Similarly, in A. mellifera drones, some proline may be oxidized, but the amount is negligible compared with carbohydrates [17]. The activity of enzymes involved in proline oxidation is too low in A. mellifera to use it at a high rate [12]. In contrast, a recent study indicates that proline may be an important mitochondrial substrate in A. mellifera [24]. Some plant studies indicate that flower nectar contains a substantial amount of proline, and that it is actively produced by the plant rather than contamination from pollen [25]. Proline can have multiple functional roles in plants against abiotic stress such as drought or high salinity [26,27], but its use as a metabolic fuel by pollinators has been proposed [28].
The capacity of insect flight muscles to use proline has been initially described in the blood-feeding tsetse fly [5,6,29], and more recently in mosquitoes [30–32], but it is not phylogenetically constrained to dipterans. Other groups of insects such as beetles display the ability to oxidize proline in combination with carbohydrates [33–38]. Therefore, capacity for proline metabolism appears labile within insects and could have played a significant role in the metabolic evolution of this group of animals. Proline oxidation involves a few steps catalysed by enzymes of the mitochondrial matrix before integration in the tricarboxylic acid cycle (TCA; figure 1). Initial accounts in insects showed that proline acts as a carbon source to replenish TCA cycle intermediates (defined as an anaplerotic role), thereby allowing maximal flux through the TCA cycle during the oxidation of carbohydrates [39–41], especially important during the transition from rest to flight [42]. A wide range of strategies is found across beetle species, with some using proline as a co-substrate with carbohydrates and others using proline nearly exclusively as a metabolic fuel [33].
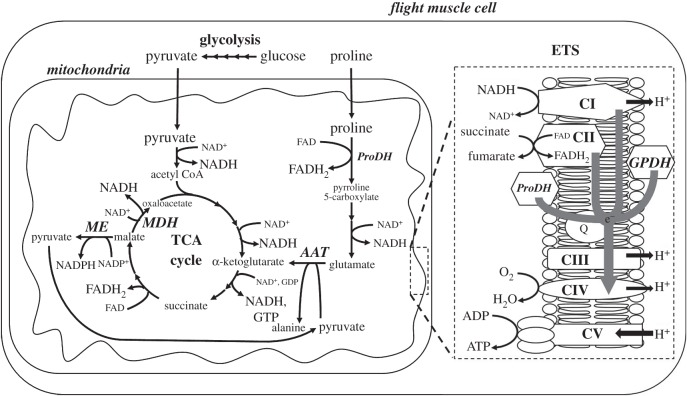
Figure 1.
Energy metabolism pathways of the flight muscle of hymenopteran species such as the bumblebee Bombus impatiens and the wasp Vespula vulgaris. AAT, alanine aminotransferase; ETS, electron transfer system; GPDH, glycerol-3-phosphate dehydrogenase; MDH, malate dehydrogenase; ME, malic enzyme; ProDH, proline dehydrogenase; Q, ubiquinone; TCA, tricarboxylic acid.
The proposed hypothesis that proline may be used as a metabolic reward for pollinating insects, combined with the observation that proline is important to elicit maximal respiration of bumblebee flight muscle [15], led us to investigate proline metabolism in hymenopterans. The goals of this study were (i) to determine if hymenopterans can oxidize proline in flight muscles and if this ability varies across species and (ii) to assess if proline acts in a strictly anaplerotic role to assist carbohydrate oxidation or if it can be oxidized alone. To achieve these goals, we have conducted high-resolution respirometry measurements on isolated flight muscle from four different species: the honeybee A. mellifera, the closely related bumblebee Bombus impatiens, the distantly related yellow-jacket wasp Vespula vulgaris and the migratory locust L. migratoria as an outgroup species.
2. Methods
(a). Insects
Mature adult bumblebees (B. impatiens), honeybees (A. mellifera) and yellow-jacket wasps (V. vulgaris) were captured on the University of Ottawa campus on the day of each experiment. Migratory locusts (L. migratoria) were used as outgroup species and obtained from a captive colony maintained by Dr Jeff Dawson (Carleton University, Ottawa, ON).
(b) Flight muscle fibres isolation and permeabilization
Individuals were placed at 4°C for 15 min, and then weighed and dissected to isolate the thorax. All further manipulations were performed on ice. Flight muscle fibres were isolated in an ice-cold preservation buffer (BIOPS [43], containing 10 mM Ca-EGTA buffer, 0.1 µM free calcium, 20 mM imidazole, 20 mM taurine, 50 mM K-2-(N-morpholino)ethanesulfonic acid (MES), 0.5 mM Dithiothreitol (DTT), 6.56 mM MgCl2, 5.77 mM ATP, 15 mM phosphocreatine, pH 7.1). Between 1 and 2 mg of muscle fibre were weighed after removing excess buffer by dabbing fibres on a dry Petri dish and immediately placed in 100 µl of respiration buffer (mitochondrial respiration medium, MIR05 [43], containing 0.5 mM EGTA, 3 mM MgCl2, 60 mM K-lactobionate, 20 mM taurine, 10 mM KH2PO4, 20 mM HEPES, 110 mM sucrose and 1 g l−1 of fatty acid-free bovine serum albumin, pH 7.1 at 30°C) before transfer to the respirometer chamber. During preliminary experiments, we tested other conditions for chemical permeabilization that did not improve respiration rates and, therefore, conducted all experiments using mechanical permeabilization only.
(c). High-resolution respiration rate
Oxygen consumption and the respiration rate of thorax muscle fibres were measured using a high-resolution respirometer (Oxygraph-2 k, Oroboros® Instruments, Innsbruck, Austria). The muscle fibres were placed in 2 ml of MIR05 in respirometer chambers, and O2 was added to reach a concentration of approximately 350 nmol O2 ml−1. Additional experiments showed that respiration rates remained unaffected by O2 concentration in the range of 500 and 150 nmol ml−1. Therefore, chambers were reoxygenated when reaching 150 nmol O2 ml−1. All assays were conducted at 37°C, selected as a representative of thoracic temperature of the studied hymenopteran species in flight (A. mellifera [44], B. impatiens [16], V. vulgaris [45]). Electrodes were calibrated daily, and background rates were assessed every two weeks [46]. Substrates and inhibitors were added successively with Hamilton syringes following different protocols (see next paragraph), and concentrations used were based on [47] or adjusted to saturating levels during preliminary experiments. Respiration rate of permeabilized fibres was measured following addition of substrates for complex I of the electron transport system (ETS), pyruvate (5 mM) and malate (2 mM), plus ADP (2.5 mM). Complex II was stimulated by addition of succinate (10 mM). Proline was added at a concentration of 10 mM, and the concentrations of the other substrates tested were 16 mM for glycerol-3-phosphate (G3P) and 0.1 mM for palmitoylcarnitine (PC). Mitochondrial membrane integrity was verified by the addition of cytochrome c (10 µM). Statistical analyses showed that respiration rates did not differ between samples that responded or not to cytochrome c, and, therefore, all samples were combined for further analysis. The concentrations of inhibitors were 0.5 µM for rotenone (Rot), 15 mM for malonic acid (Mna) and 2.5 µM for antimycin A (Ama).
(d). Respiration rate measurements: substrates and inhibitors protocols
To test differences in proline oxidation capacities among species (B. impatiens, n = 13; A. mellifera, n = 11; V. vulgaris, n = 11, L. migratoria, n = 6), we stimulated respiration associated with complex I by adding pyruvate, malate and ADP. We then assessed the subsequent oxidation of proline, followed by succinate, the substrate for complex II. We then sequentially inhibited complex I, II and III by adding the specific inhibitors rotenone (Rot—complex I), malonic acid (Mna—complex II) and antimycin A (Ama—complex III). Muscle fibre respiration rates are within the range reported for other insect species [48,49], reflecting a similar yield of functional mitochondria as commonly found with such preparation [50].
To identify the relative contribution of substrates and the points of entry of reducing equivalents in the ETS, we further characterized mitochondrial metabolism in B. impatiens (n = 8 for each protocol) by contrasting respiration rates obtained when (i) proline alone was present, (ii) after stimulating complex I (pyruvate + malate + ADP) and II (succinate), (iii) after stimulating complex I and adding proline, and (iv) by stimulating complex I and II first, before adding proline. These protocols were followed by sequential addition of inhibitors of complex I, II and III (Rot, Mna, Ama, respectively), to determine respiration rates independent of each complex. We also assessed the capacity of B. impatiens muscle fibres to oxidize PC, and tested if G3P can enhance respiration in the presence of substrates eliciting maximal respiration (pyruvate, malate, ADP and proline).
(e). Calculations and statistical analyses
Respiration rates (pmol O2 s−1 mg−1) obtained for each substrate or inhibitor added were calculated using the Oroboros DatLab software (see electronic supplementary material, figure S1 for traces). To take into account variation in fibre preparation, quadruplicate measurements were performed for each individual and averaged to represent individual data. Mean values and standard errors for each substrate or inhibitor are reported. Also, all data were analysed and normalized to maximal respiration with all substrates present. This allowed comparing relative changes in respiration rate associated with each substrate while controlling for variation in maximal respiration rate between preparations and species. Results were essentially the same, and therefore, only absolute values are reported, except figure 3 that reports relative respiration rate associated with proline.
Figure 3.
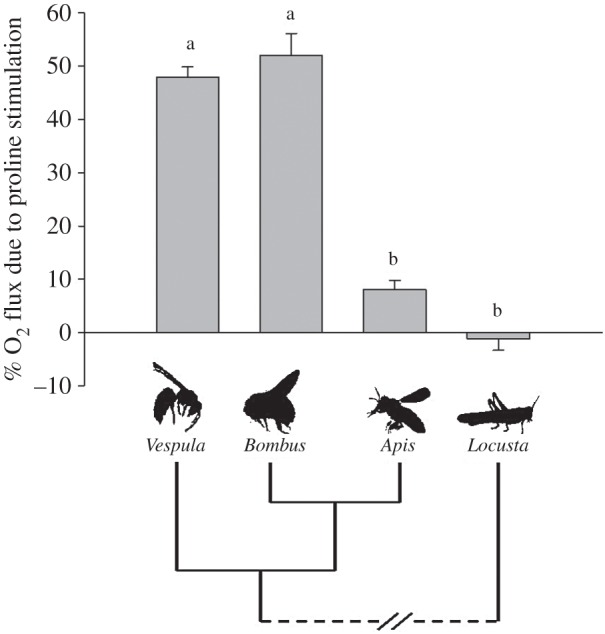
Diversity in proline oxidation capacities by hymenopterans flight muscle. Proline was added subsequently to complex I substrates. Oxidation rates are expressed as a percentage of the maximal respiration rate of the muscle fibres when all substrates were present. Two distinct patterns are shown: yellow-jacket wasps (Vespula vulgaris, n = 11) and bumblebees (Bombus impatiens, n = 13) show a strong increase in respiration with addition of proline, whereas honeybees (Apis mellifera, n = 11) and locusts used as outgroup species (Locusta migratoria, n = 6) do not exhibit a significant increase following addition of proline. Bars with different letters are significantly different (p < 0.05).
Interspecific differences and the effects of sequential addition of substrates and inhibitors on respiration rates were tested using two-way ANOVA for repeated measures, using SigmaPlot v. 12 software. Pairwise comparisons with adjustments for multiple comparisons (Holm–Sidak method) were conducted to detect further differences between species and substrates or inhibitors. For the relative respiration rate associated with proline presented in figure 3, statistical results reported were obtained from a two-way ANOVA for repeated measures conducted on all data normalized for maximal respiration. Further analysis on the bumblebee B. impatiens data used one-way ANOVA for repeated measures and Holm–Sidak post hoc test adjusted for multiple comparisons. All data were tested for normality and homoscedasticity, and the level of significance was set at p < 0.05.
3. Results
(a). Interspecific differences in proline oxidation
The sequential addition of substrates and inhibitors showed marked differences in the rates of oxygen consumption among species (figure 2). A two-way ANOVA with repeated measures showed an interaction between substrate and species (substrate: F5,185 = 225.0, p < 0.001; species: F3,185 = 9.5, p < 0.001; substrate × species: F15,185 = 3.8, p < 0.001). Pairwise comparisons first showed that the rates measured after stimulation of complex I using pyruvate + malate did not differ among the four species (range: 75.0 ± 15.2–124.6 ± 20.7 pmol O2 s−1 mg−1, 0.312 < p < 0.991); note that the rates obtained before the addition of ADP were minimal (5.86 ± 18.7 pmol O2 s−1 mg−1). The addition of proline increased the respiration rate in bumblebees and yellow-jacket wasps (p < 0.001), and the respiration rate did not differ between them (291.7 ± 41.7 and 256.5 ± 37.3; p = 0.979). Proline did not increase the respiration rate in the honeybee or locust (p = 0.275 and p = 0.931, respectively), and both species did not differ in respiration rate in the presence of this substrate (p = 0.09). The relative contribution of proline to flight muscle fibre maximal respiration capacity, calculated as the increase in rate owing to proline addition divided by the maximal respiration rate with all substrates present, corresponds to 52 ± 4% and 48 ± 2% in the bumblebee B. impatiens and the wasp V. vulgaris, respectively, but proline shows no significant contribution in the honeybee A. mellifera and locust L. migratoria (figure 3). The addition of succinate, the specific substrate for complex II, did not further increase the respiration rate in bumblebees and wasps (p = 0.51 and p = 0.87, respectively). By contrast, succinate increased the respiration rate in honeybees by 40% (p = 0.038), but the increase in locusts was not detected as significant (p = 0.139). In the presence of all substrates, bumblebees and wasps had higher respiration rates than locusts (p = 0.006 and p = 0.010, respectively), but honeybees could not be distinguished from other species.
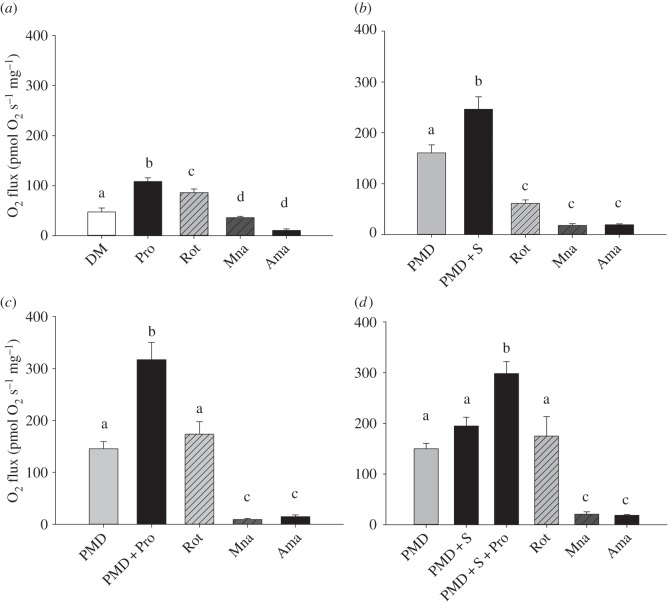
Figure 2.
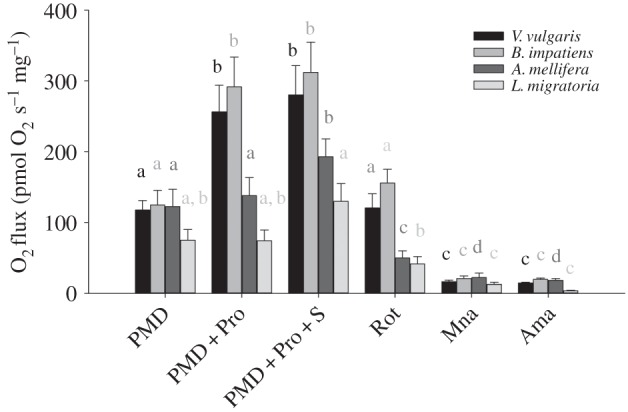
Respiration rates measured in flight muscle fibres of four insect species (Vespula vulgaris, V, n = 11; Bombus impatiens, B, n = 13; Apis mellifera, A, n = 11; Locusta migratoria, L, n = 6). Oxygen fluxes (pmol O2 s−1 mg−1) were determined after successive addition of pyruvate + malate + ADP (PMD), proline (PMD + Pro), succinate (PMD + Pro + S), and the inhibitors rotenone (Rot), malonate (Mna) and antimycin A (Ama). Bars with different letters denote significant differences between substrates or inhibitors within each species (p < 0.05). Differences among species are as follow: PMD: no difference; PMD + Pro: V = B > A = L; PMD + Pro + S: V = B > L; Rot: V = B > A = L; Mna: no difference; Ama: B > L.
The addition of inhibitors further shows the differences in mitochondrial metabolism among species. All species had a decreased respiration rate when complex I inhibitor, rotenone, was added (p < 0.01; figure 2). Moreover, the pair of species B. impatiens/V. vulgaris differed from A. mellifera/L. migratoria in the presence of rotenone (p < 0.005), indicating a large difference in complex I-independent respiration. The respiration rate obtained in the presence of rotenone was 50% and 43% of maximal respiration in B. impatiens and V. vulgaris, respectively, whereas only 26% and 32% of maximal respiration in A. mellifera and L. migratoria. The subsequent addition of complex II inhibitor, malonate, decreased respiration in all species (p < 0.05) that all showed the same low oxygen flux (0.176 < p < 0.774), ranging from 6% to 12% of maximal respiration rates. Finally, the addition of complex III inhibitor, antimycin A, did not further decrease respiration in all species (0.080 < p < 0.904), where all species had similar values except L. migratoria that had lower respiration than bumblebees (p = 0.021).
(b). Mitochondrial metabolism of bumblebee flight muscle
The importance of proline as a substrate for bumblebee flight muscle is further demonstrated by contrasting oxidation of other substrates, such as pyruvate + malate (complex I) or succinate (complex II) with proline (figure 4). Respiration rates obtained using pyruvate + malate did not differ between the different protocols tested (p > 0.05) and ranged from 145.3 ± 13.9 to 160.5 ± 15.8 pmol O2 s−1 mg−1. The addition of proline enhanced bumblebee muscle respiration rate by more than twofold to reach values over 300 pmol O2 s−1 mg−1 (figure 4c), with no further increase after addition of succinate (figure 2). By comparison, the addition of succinate, a specific substrate for complex II, led to a smaller increase in respiration rate, corresponding to about 1.5-fold increase from complex I (figure 4b,d). Moreover, once complex I and complex II were stimulated using pyruvate + malate and succinate, the addition of proline increased respiration values to more than 300 pmol O2 s−1 mg−1 (figure 4d), the same rate observed when complex I and proline were tested alone (figure 4c).
Figure 4.
Effects of pyruvate and malate, succinate and/or proline on respiration rates (pmol O2 s−1 mg−1) measured in flight muscle fibres of bumblebees (Bombus impatiens, n = 8 for each protocol). Substrates and inhibitors were successively added to determine each effect separately. DM, ADP + malate; PMD, pyruvate + malate + ADP; S, succinate; Pro, proline; Rot, rotenone; Mna, malonate; Ama, antimycin A. Values are means + s.e.m. Bars with different letters are significantly different (p < 0.05).
The effect of inhibitors differed in the presence of proline, except with the inhibitor of complex III, antimycin A that lowered respiration rates to 20 pmol O2 s−1 mg−1 or less in all cases (figure 4). In the absence of proline (figure 4b), the complex I inhibitor rotenone reduced respiration by 75% (p < 0.001), and addition of malonate (complex II inhibitor) further reduced respiration by 18% of maximal respiration (p < 0.001). The addition of antimycin A did not further decrease respiration (p = 0.636). In the presence of proline (figure 4a), rotenone reduced respiration by 21% (p < 0.005), and addition of malonate further reduced respiration by 46% of the maximal respiration (p < 0.001). The addition of antimycin A reduced the respiration rate by another 24%, but the rate obtained could not be distinguished from the rate with malonate (p = 0.093). When proline was added in the presence of succinate (figure 4d), rotenone inhibited respiration by 41% (p < 0.001), malonate by an additional 52% (p < 0.001) and antimycin A did not further decrease respiration (p = 0.638).
Finally, we assessed the capacity of bumblebee muscle to oxidize fatty acids using PC as a substrate and found no increase in respiration from routine respiration (electronic supplementary material, figure S2). The contribution of G3P to fibre respiration was also tested to assess if maximal respiration capacity of the fibre was achieved. The addition of G3P did not increase respiration in the presence of pyruvate + malate and proline (electronic supplementary material, figure S2; p = 0.856).
4. Discussion
(a). Proline metabolism in hymenopterans
Bees are thought to be strict users of carbohydrates as metabolic fuel for flight. This study shows that the isolated flight muscle of the honeybee A. mellifera appears to use carbohydrates exclusively to power mitochondrial metabolism, but that a species of its sister group, the bumblebee B. impatiens, exhibits a large potential for ATP production via proline oxidation. Moreover, the capacity to oxidize this amino acid may be an important metabolic feature of hymenopterans as a group, because the more distant species of wasp, V. vulgaris, exhibits the same mitochondrial phenotype as the bumblebee. These findings support the hypothesis that some hymenopteran pollinators can use proline from nectar as a metabolic reward [28], although capacity for proline metabolism is a diverse phenotype among bee species. This study also shows that using the honeybee, A. mellifera, to exemplify the metabolic physiology of hymenopteran muscle in general can be misleading. The second part of this work characterizes the mitochondrial physiology of B. impatiens and shows that proline more than doubles mitochondrial oxygen consumption, compared with carbohydrate oxidation alone. Proline is also oxidized alone and its metabolism supplies electrons directly to the ETS.
The current results on nectar-feeding hymenopteran species emphasize that proline metabolism is not strictly associated with a protein-rich diet. The use of proline as a fuel to power flight was initially associated with the protein-rich meal of blood-sucking insects such as the tsetse fly [5,6] and more recently mosquitoes [30–32]. However, the capacity to use proline is also clearly associated with another important physiological role: enhancing the oxidation of carbohydrates. This observation is not only supported by our experiments on hymenopterans, but also by previous work on dipterans [39–41] as well as coleopterans [33–38]. Proline is used as a single or combined fuel in many groups of insects and, therefore, should be considered an important metabolic feature for this class of animals.
Why some species of hymenopterans use proline as a metabolic fuel to power flight may be explained by the unique properties of this amino acid. First, compared with other animals, insects can store fuels at high concentrations in their haemolymph [51]. Carbohydrates are found in high concentration largely owing to the presence of the non-reducing disaccharide trehalose. Insects are also distinct in the high level of free circulating amino acids, where proline is predominant in many species [17,52–57]. The high solubility of proline makes it a readily available fuel in the flight muscle and haemolymph, and does not necessitate any specific carrier protein [1,33]. Proline can also serve as a carbon shuttling molecule between lipid reserves in the fat body and flight muscle [33,58,59]. Its partial oxidation producing alanine yields 0.52 mol of ATP per gram, making it much more similar to lipids (0.65 mol ATP g−1) than carbohydrates (0.18 mol ATP g−1) in terms of packing energy [33]. Additionally, proline partial oxidation is osmotically neutral and free of nitrogen waste products. Clearly, the potential of proline as metabolic fuel in insects has many advantages beyond simple dietary opportunities.
Proline oxidation is common in insects, but how this property evolved is not understood. Our results on hymenopterans show that two species belonging to sister groups have opposite phenotypes, where the honeybee has no appreciable capacity to oxidize proline on its own or in combination with carbohydrates, whereas the bumblebee and the more distant wasp exhibit an impressive and equal ability to metabolize proline and carbohydrates. Other accounts on bee species also suggest little or no contribution of proline to mitochondrial metabolism [11,22,23], including another bumblebee species [60]. The ultimate evolutionary causes for these interspecific differences and the proximate mechanisms underlying them remain to be elucidated.
A recent study suggests that proline may increase honeybee mitochondrial respiration by up to threefold [24], which is in direct contrast with our findings and the multiple lines of evidence supporting that proline may not be an important metabolic fuel in A. mellifera [9,10,12,17,22,23]. However, the interpretation of the results obtained by Campbell et al. [24] presents some difficulties. First, it is possible that isolated mitochondria and permeabilized muscle fibre preparations show different functional properties as reported in vertebrates [61]. A recent study on mosquitoes does not support this possibility as the two preparations responded similarly to various substrates, including proline [48]. Second, pyruvate and proline concentrations in the Campbell et al. study were approximately 10-fold lower than normally used for isolated mitochondria in insects [48,60]. The impact of working with such subsaturating conditions is that pyruvate-stimulated respiration could be well underestimated and that fold changes in respiration could vary widely given a typical Michaelis–Menten kinetic function. Lastly, the results of Campbell et al., cannot be used to distinguish if proline simply plays an anaplerotic role by increasing TCA cycle intermediates to oxidize pyruvate at high rates, as suggested for other bees [7,60], or if proline is a substantial oxidative substrate. As shown in another bee species, the oxidation of pyruvate at its highest rate is achieved by combining it with anaplerotic substrates that increase TCA cycle intermediates, regardless of whether it was malate, glutamate or proline [60]. To truly address if proline is a major oxidative fuel or mainly an anaplerotic substrate, as well as to determine the points of entry in the ETS, a combination of substrates and inhibitors will have to be used as in [48] and our study. Further work is clearly needed, but the current balance of evidence suggests that proline contributes little to energy production capacity in A. mellifera, as supported by our results on permeabilized muscle fibres.
(b). Flight muscle metabolic properties
Permeabilized muscle fibres enabled the investigation of the fundamental metabolic properties of the flight muscle of the bumblebee B. impatiens. Bumblebee permeabilized fibres confirm their inability to oxidize fatty acids. This feature relates to the diet of bees and likely to their high metabolic rate. This has been previously documented in the honeybee and a bumblebee species based on the low activity of enzymes involved in lipid catabolism [12], as well as orchid bee species where muscle homogenate cannot oxidize PC [7]. This lack of capacity for fatty acid oxidation is probably a generalized feature of bees and possibly hymenopterans as a group.
Insect mitochondria have been shown to be impermeable to many TCA cycle intermediates [62], which led to the use of proline to increase TCA cycle intermediates, also referred to as a ‘sparker’ metabolite in several studies conducted on insects and viewed mostly as an NAD-linked substrate [7,49,63,64]. Proline is indeed an important metabolite used to replenish TCA cycle intermediates and maintain the potential for pyruvate oxidation ([7,39–41,65], and possibly [24]). In such a scenario, proline metabolism maintains metabolites of the TCA cycle and the oxygen consumed by the mitochondria would be largely NAD-linked. Our results show that addition of proline alone induces cellular respiration, which is subsequently only minimally inhibited by the complex I inhibitor rotenone (figure 4a). Moreover, adding proline following stimulation of respiration by pyruvate (+ malate) in the presence of ADP more than doubles the respiration rate, but inhibition of complex I only reduces respiration by less than 50% (figure 4c). The addition of complex II inhibitor malonate shuts down oxygen consumption in all protocols tested through its direct effect on the electron transfer system, but possibly combined to the backing up of metabolites of the TCA cycle and affecting proline dehydrogenase by mass action. In bumblebees, the role of proline is more than simply an NAD-linked substrate contributing to anaplerotic reaction to maintain pathway intermediates. It is also a metabolic fuel as demonstrated by the occurrence of partial or complete oxidation of proline at a high rate.
The addition of proline to mitochondria oxidizing pyruvate showed a greater increase in respiration than the addition of complex II substrate succinate (figure 4b,c). In fact, the addition of proline following stimulation of both complex I and II shows a further increase in respiration rate (figure 4d). This result is likely explained by electron convergence at the Q-junction (ubiquinone; figure 1). The enhanced respiration following complex I and II maximal respiration is typical of the contribution of other enzymes reducing ubiquinone (Q oxidoreductase), a property that has been described for proline dehydrogenase in a variety of organisms [66–69] and recently described in the dipterans Drosophila melanogaster [70] and Aedes aegypti [48]. Another Q oxidoreductase that is typically active in insects is the G3P dehydrogenase, an enzyme located on the outer face of the inner mitochondrial membrane. The addition of proline is reminiscent of the response of insect mitochondria to G3P that elicits maximal mitochondrial respiration [49,60]. Moreover, we show that the ETS reaches its maximal flux capacity when using proline as G3P cannot further increase respiration (electronic supplementary material, figure S2). Finally, mitochondrial respiration when pyruvate (+ malate) and proline are combined is more than the sum of respiration rates obtained with substrates used individually. Together, these findings indicate that proline enhances pyruvate oxidation and provides reducing equivalents to the ETS of B. impatiens mitochondria (figure 1).
In conclusion, the use of proline as a metabolic fuel by flight muscles can greatly enhance carbohydrate oxidation in bee and wasp species. The metabolic properties found in B. impatiens show the potential to use proline as single fuel, at a lower rate or as co-substrate with carbohydrates at much higher rates. These findings suggest the possible role of proline as alternate fuel to support muscle metabolism from resting conditions to the high rate of energy production required during flight. This phenotype, however, appears diverse even among bee species. The diverse ability to oxidize proline in hymenopterans could be explained by the predictability of carbohydrates sources, where species with honey stores may have lost this phenotype and favoured strict carbohydrate oxidation. Alternatively, proline oxidation may be associated with the mobilization of fuel stored in the fat bodies, which cannot be oxidized directly as fatty acids but could be shuttled in the form of proline [33,58,59]. This phenotype may be especially important during periods when dietary carbohydrates are unavailable, such as early spring or overwintering. The importance of proline as fuel for flight in vivo will need to be clarified in this group of insects, as well as how and why this trait evolved in hymenopterans.
Supplementary Material
Acknowledgements
We thank Enrique Rodriguez for his help during laboratory work and Dr Jeff Dawson for providing the L. migratoria. The comments of two anonymous referees helped clarify the manuscript.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
C.A.D. conceived and designed the study, coordinated the study, helped with laboratory work, participated in data analysis and drafted the manuscript; L.T. carried out the laboratory work, conducted data analysis, participated in the design of the study and drafted the manuscript; J.C. carried out the laboratory work and participated in data analysis; J.M.W. helped draft the manuscript.
Competing interests
We have no competing interests.
Funding
L.T. was the recipient of an international mobility fellowship from la Région Rhône-Alpes (France) and a travel fellowship from The Company of Biologists. This work was supported by an NSERC Discovery Grant to C.A.D.
References
- 1.Weber J-M. 2011. Metabolic fuels: regulating fluxes to select mix. J. Exp. Biol. 214, 286–294. ( 10.1242/jeb.047050) [DOI] [PubMed] [Google Scholar]
- 2.Storey KB. 1985. Metabolic biochemistry of insect flight. In Circulation, respiration, and metabolism (ed. Gilles R.), pp. 193–207. Berlin, Germany: Springer. [Google Scholar]
- 3.Beenakkers AMT, Vanderhorst DJ, Vanmarrewijk WJA. 1984. Insect flight-muscle metabolism. Insect Biochem. 14, 243–260. ( 10.1016/0020-1790(84)90057-X) [DOI] [Google Scholar]
- 4.Worm RAA, Beenakkers AMT. 1980. Regulation of substrate utilization in the flight muscle of the locust, Locusta migratoria, during flight. Insect Biochem. 10, 53–59. ( 10.1016/0020-1790(80)90038-4) [DOI] [Google Scholar]
- 5.Bursell E. 1975. Substrates of oxidative metabolism in dipteran flight muscle. Comp. Biochem. Phys. B 52, 235–238. ( 10.1016/0305-0491(75)90057-7) [DOI] [PubMed] [Google Scholar]
- 6.Bursell E. 1963. Aspects of the metabolism of amino acids in the tsetse fly, Glossina (Diptera). J. Insect Physiol. 9, 439–452. ( 10.1016/0022-1910(63)90054-4) [DOI] [Google Scholar]
- 7.Suarez RK, Darveau CA, Welch KC Jr, O'Brien DM, Roubik DW, Hochachka PW. 2005. Energy metabolism in orchid bee flight muscles: carbohydrate fuels all. J. Exp. Biol. 208, 3573–3579. ( 10.1242/jeb.01775) [DOI] [PubMed] [Google Scholar]
- 8.Michener CD. 2000. The bees of the world. Baltimore, MD: The John Hopkins University Press. [Google Scholar]
- 9.Nachtigall W, Rothe U, Feller P, Jungmann R. 1989. Flight of the honey bee. III. Flight metabolic power calculated from gas analysis, thermoregulation and fuel consumption. J. Comp. Physiol. B 158, 729–737. ( 10.1007/BF00693011) [DOI] [Google Scholar]
- 10.Rothe U, Nachtigall W. 1989. Flight of the honey bee. J. Comp. Physiol. B 158, 739–749. ( 10.1007/BF00693012) [DOI] [Google Scholar]
- 11.Gäde G, Auerswald L. 1998. Flight metabolism in carpenter bees and primary structure of their hypertrehalosaemic peptide. In EBO experimental biology online annual 1998 (eds Bridges C, Sanders D, Curtis A), pp. 75–88. Berlin, Germany: Springer. [Google Scholar]
- 12.Crabtree B, Newsholme E. 1970. The activities of proline dehydrogenase, glutamate dehydrogenase, aspartate-oxoglutarate aminotransferase and alanine-oxoglutarate aminotransferase in some insect flight muscles. Biochem. J. 117, 1019 ( 10.1042/bj1171019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darveau CA, Billardon F, Belanger K. 2014. Intraspecific variation in flight metabolic rate in the bumblebee Bombus impatiens: repeatability and functional determinants in workers and drones. J. Exp. Biol. 217, 536–544. ( 10.1242/jeb.091892) [DOI] [PubMed] [Google Scholar]
- 14.Darveau CA, Hochachka PW, Welch KC Jr, Roubik DW, Suarez RK. 2005. Allometric scaling of flight energetics in Panamanian orchid bees: a comparative phylogenetic approach. J. Exp. Biol. 208, 3581–3591. ( 10.1242/jeb.01776) [DOI] [PubMed] [Google Scholar]
- 15.Skandalis DA, Roy C, Darveau CA. 2011. Behavioural, morphological, and metabolic maturation of newly emerged adult workers of the bumblebee, Bombus impatiens. J. Insect Physiol. 57, 704–711. ( 10.1016/j.jinsphys.2011.02.001) [DOI] [PubMed] [Google Scholar]
- 16.Skandalis DA, Darveau CA. 2012. Morphological and physiological idiosyncrasies lead to interindividual variation in flight metabolic rate in worker bumblebees (Bombus impatiens). Physiol. Biochem. Zool. 85, 657–670. ( 10.1086/665568) [DOI] [PubMed] [Google Scholar]
- 17.Berger B, Crailsheim K, Leonhard B. 1997. Proline, leucine and phenylalanine metabolism in adult honeybee drones (Apis mellifica carnica Pollm). Insect Biochem. Mol. 27, 587–593. ( 10.1016/S0965-1748(97)00034-9) [DOI] [Google Scholar]
- 18.Cotte JF, Casabianca H, Giroud B, Albert M, Lheritier J, Grenier-Loustalot MF. 2004. Characterization of honey amino acid profiles using high-pressure liquid chromatography to control authenticity. Anal. Bioanal. Chem. 378, 1342–1350. ( 10.1007/s00216-003-2430-z) [DOI] [PubMed] [Google Scholar]
- 19.Crailsheim K, Leonhard B. 1997. Amino acids in honeybee worker haemolymph. Amino Acids 13, 141–153. ( 10.1007/Bf01373212) [DOI] [Google Scholar]
- 20.Hrassnigg N, Leonhard B, Crailsheim K. 2003. Free amino acids in the haemolymph of honey bee queens (Apis mellifera L.). Amino Acids 24, 205–212. ( 10.1007/s00726-002-0311-y) [DOI] [PubMed] [Google Scholar]
- 21.Leonhard B, Crailsheim K. 1999. Amino acids and osmolarity in honeybee drone haemolymph. Amino Acids 17, 195–205. ( 10.1007/BF01361882) [DOI] [PubMed] [Google Scholar]
- 22.Barker RJ, Lehner Y. 1972. Free amino acids in thoraces of flown honey bees, Apis mellifera L. (Hymenoptera: Apidae). Comp. Biochem. Phys. B 43, 163–167. ( 10.1016/0305-0491(72)90213-1) [DOI] [Google Scholar]
- 23.Micheu S, Crailsheim K, Leonhard B. 2000. Importance of proline and other amino acids during honeybee flight. Amino Acids 18, 157–175. ( 10.1007/s007260050014) [DOI] [PubMed] [Google Scholar]
- 24.Campbell JB, Nath R, Gadau J, Fox T, DeGrandi-Hoffman G, Harrisson JF. 2016. The fungicide Pristine® inhibits mitochondrial function in vitro but not flight metabolic rates in honey bees. J. Insect Physiol. 86, 11–16. ( 10.1016/j.jinsphys.2015.12.003) [DOI] [PubMed] [Google Scholar]
- 25.Delauney AJ, Verma DPS. 1993. Proline biosynthesis and osmoregulation in plants. Plant J. 4, 215–223. ( 10.1046/j.1365-313X.1993.04020215.x) [DOI] [Google Scholar]
- 26.Szabados L, Savoure A. 2010. Proline: a multifunctional amino acid. Trends Plant Sci. 15, 89–97. ( 10.1016/j.tplants.2009.11.009) [DOI] [PubMed] [Google Scholar]
- 27.Verslues PE, Sharma S. 2010. Proline metabolism and its implications for plant–environment interaction. The Arabidopsis Book 8, e0140 ( 10.1199/tab.0140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carter C, Shafir S, Yehonatan L, Palmer RG, Thornburg R. 2006. A novel role for proline in plant floral nectars. Naturwissenschaften 93, 72–79. ( 10.1007/s00114-005-0062-1) [DOI] [PubMed] [Google Scholar]
- 29.Olembo NK, Pearson DJ. 1982. Changes in the contents of intermediates of proline and carbohydrate metabolism in flight muscle of the tsetse fly Glossina morsitans and the fleshfly Sarcophaga tibialis. Insect Biochem. 12, 657–662. ( 10.1016/0020-1790(82)90053-1) [DOI] [Google Scholar]
- 30.Giulivi C, Ross-Inta C, Horton A, Luckhart S. 2008. Metabolic pathways in Anopheles stephensi mitochondria. Biochem. J 415, 309–316. ( 10.1042/BJ20080973) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pennington JE, Goldstrohm DA, Wells MA. 2003. The role of hemolymph proline as a nitrogen sink during blood meal digestion by the mosquito Aedes aegypti. J. Insect Physiol. 49, 115–121. ( 10.1016/s0022-1910(02)00267-6) [DOI] [PubMed] [Google Scholar]
- 32.Scaraffia P, Wells M. 2003. Proline can be utilized as an energy substrate during flight of Aedes aegypti females. J. Insect Physiol. 49, 591–601. ( 10.1016/S0022-1910(03)00031-3) [DOI] [PubMed] [Google Scholar]
- 33.Gäde G, Auerswald L. 2002. Beetles’ choice: proline for energy output: control by AKHs. Comp. Biochem. Physiol. B 132, 117–129. ( 10.1016/S1096-4959(01)00541-3) [DOI] [PubMed] [Google Scholar]
- 34.Auerswald L, Gade G. 1995. Energy substrates for flight in the blister beetle Decapotoma lunata (Meloidae). J. Exp. Biol. 198, 1423–1431. [DOI] [PubMed] [Google Scholar]
- 35.Auerswald L, Gade G. 1999. The fate of proline in the African fruit beetle Pachnoda sinuata. Insect Biochem. Mol. 29, 687–700. ( 10.1016/S0965-1748(99)00045-4) [DOI] [Google Scholar]
- 36.Weeda E, De Kort CAD, Beenakkers AMT. 1979. Fuels for energy metabolism in the Colorado potato beetle, Leptinotarsa decemlineata Say. J. Insect Physiol. 25, 951–955. ( 10.1016/0022-1910(79)90108-2) [DOI] [Google Scholar]
- 37.Weeda E. 1981. Hormonal regulation of proline synthesis and glucose release in the fat body of the Colorado potato beetle, Leptinotarsa decemlineata. J. Insect Physiol. 27, 411–417. ( 10.1016/0022-1910(81)90020-2) [DOI] [Google Scholar]
- 38.Weeda E, De Kort C, Beenakkers A. 1980. Oxidation of proline and pyruvate by flight muscle mitochondria of the Colorado beetle, Leptinotarsa decemlineata Say. Insect Biochem. 10, 305–311. ( 10.1016/0022-1910(79)90108-2) [DOI] [Google Scholar]
- 39.van den Bergh SG. 1964. Pyruvate oxidation and the permeability of housefly sarcosomes. Biochem. J. 93, 128–136. ( 10.1042/bj0930128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brosemer RW, Veerabhadrappa P. 1965. Pathway of proline oxidation in insect flight muscle. Biochim. Biophys. Acta 110, 102 ( 10.1016/S0926-6593(65)80099-6) [DOI] [PubMed] [Google Scholar]
- 41.Sacktor B, Childress CC. 1967. Metabolism of proline in insect flight muscle and its significance in stimulating the oxidation of pyruvate. Arch. Biochem. Biophys. 120, 583–588. ( 10.1016/0003-9861(67)90522-x) [DOI] [Google Scholar]
- 42.Sacktor B. 1975. Biochemistry of insect flight. In Insect biochemistry and function (eds Candy DJ, Kilby BA), pp. 1–88. New York, NY: Springer. [Google Scholar]
- 43.Kuznetsov A, Lassnig B, Stadlmann S, Rieger G, Gnaiger E. 1998. Selected media and chemicals for respirometry with mitochondria and permeabilized cells. Mitochondr. Physiol. Network 3, 1–10. [Google Scholar]
- 44.Stabentheiner A, Kovac H, Hetz SK, Käfer H, Stabentheiner G. 2012. Assessing honeybee and wasp thermoregulation and energetics — new insights by combination of flow-through respirometry with infrared thermography. Thermochim Acta 534, 77–86. ( 10.1016/j.tca.2012.02.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kovac H, Stabentheiner A, Schmaranzer S. 2009. Thermoregulation of water foraging wasps (Vespula vulgaris and Polistes dominulus). J. Insect Physiol. 55, 959–966. ( 10.1016/j.jinsphys.2009.06.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gnaiger E. 2008. Polarographic oxygen sensors, the oxygraph and high-resolution respirometry to assess mitochondrial function. Mitochondrial Dysfunct. Drug-Induced Toxicity 327, 352. [Google Scholar]
- 47.Pesta D, Gnaiger E. 2012. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. In Mitochondrial bioenergetics, pp. 25–58. Berlin, Germany: Springer. [DOI] [PubMed] [Google Scholar]
- 48.Soares JB, Gaviraghi A, Oliveira MF. 2015. Mitochondrial physiology in the major arbovirus vector Aedes aegypti: substrate preferences and sexual differences define respiratory capacity and superoxide production. PLoS ONE 10, e0120600 ( 10.1371/journal.pone.0120600) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pichaud N, Ballard JWO, Tanguay RM, Blier PU. 2011. Thermal sensitivity of mitochondrial functions in permeabilized muscle fibers from two populations of Drosophila simulans with divergent mitotypes. Am. J. Physiol. Reg. I. 301, R48–R59. ( 10.1152/ajpregu.00542.2010) [DOI] [PubMed] [Google Scholar]
- 50.Gnaiger E. 2009. Capacity of oxidative phosphorylation in human skeletal muscle: new perspectives of mitochondrial physiology. Int. J. Biochem. Cell Biol. 41, 1837–1845. ( 10.1016/j.biocel.2009.03.013) [DOI] [PubMed] [Google Scholar]
- 51.Wyatt GR. 1961. The biochemistry of insect hemolymph. Annu. Rev. Entomol. 6, 75–102. ( 10.1146/annurev.en.06.010161.000451) [DOI] [Google Scholar]
- 52.Benassi CA, Colombo G, Allegri G. 1961. Free amino acids of the haemolymph of Schistocerca gregaria Forsk. Biochem. J. 80, 332–336. ( 10.1042/bj0800332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chamberlin ME, Phillips JE. 1982. Regulation of hemolymph amino acid levels and active secretion of proline by Malpighian tubules of locusts. Can. J. Zool. 60, 2745–2752. ( 10.1139/z82-351) [DOI] [Google Scholar]
- 54.Consoli FL, Vinson SB. 2002. Hemolymph of reproductives of Solenopsis invicta (Hymenoptera: Formicidae)—amino acids, proteins and sugars. Comp. Biochem. Phys. B 132, 711–719. ( 10.1016/S1096-4959(02)00087-8) [DOI] [PubMed] [Google Scholar]
- 55.Lefevere KS, Koopmanschap AB, De Kort CAD. 1989. Changes in the concentrations of metabolites in haemolymph during and after diapause in female Colorado potato beetle, Leptinotarsa decemlineata. J. Insect Physiol. 35, 121–128. ( 10.1016/0022-1910(89)90045-0) [DOI] [Google Scholar]
- 56.Morgan TD, Chippendale GM. 1983. Free amino acids of the haemolymph of the southwestern corn borer and the European corn borer in relation to their diapause. J. Insect Physiol. 29, 735–740. ( 10.1016/0022-1910(83)90001-x) [DOI] [Google Scholar]
- 57.Sowa SM, Keeley LL. 1996. Free amino acids in the hemolymph of the cockroach, Blaberus discoidalis. Comp. Biochem. Physiol. A 113, 131–134. ( 10.1016/0300-9629(95)02043-8) [DOI] [PubMed] [Google Scholar]
- 58.Bursell E. 1977. Synthesis of proline by fat body of the tsetse fly (Glossina morsitans): metabolic pathways. Insect Biochem. 7, 427–434. ( 10.1016/s0020-1790(77)90068-3) [DOI] [Google Scholar]
- 59.Arrese EL, Soulages JL. 2010. Insect fat body: energy, metabolism, and regulation. Annu. Rev. Entomol. 55, 207 ( 10.1146/annurev-ento-112408-085356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Syromyatnikov MY, Lopatin AV, Starkov AA, Popov VN. 2013. Isolation and properties of flight muscle mitochondria of the bumblebee Bombus terrestris (L.). Biochemistry-Moscow 78, 909–914. ( 10.1134/S0006297913080075). [DOI] [PubMed] [Google Scholar]
- 61.Picard M, Taivassalo T, Ritchie D, Wright KJ, Thomas MM, Romestaing C, Hepple RT. 2011. Mitochondrial structure and function are disrupted by standard isolation methods. PLoS ONE 6, e18317 ( 10.1371/journal.pone.0018317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van den Bergh SG, Slater EC. 1962. The respiratory activity and permeability of housefly sarcosomes. Biochem. J. 82, 362–371. ( 10.1042/bj0820362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferguson M, Mockett RJ, Shen Y, Orr WC, Sohal RS. 2005. Age-associated decline in mitochondrial respiration and electron transport in Drosophila melanogaster. Biochem. J. 390, 501–511. ( 10.1042/BJ20042130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goncalves RL, Oliveira JH, Oliveira GA, Andersen JF, Oliveira MF, Oliveira PL, Barillas-Mury C. 2012. Mitochondrial reactive oxygen species modulate mosquito susceptibility to Plasmodium infection. PLoS ONE 7, e41083 ( 10.1371/journal.pone.0041083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moyes CD, Suarez RK, Hochachka PW, Ballantyne JS. 1990. A Comparison of fuel preferences of mitochondria from vertebrates and invertebrates. Can. J. Zool. 68, 1337–1349. ( 10.1139/Z90-201) [DOI] [Google Scholar]
- 66.Johnson AB, Strecker HJ. 1962. The interconversion of glutamic acid and proline. IV. The oxidation of proline by rat liver mitochondria. J. Biol. Chem. 237, 1876–1882. [PubMed] [Google Scholar]
- 67.Rasmusson AG, Geisler DA, Moller IM. 2008. The multiplicity of dehydrogenases in the electron transport chain of plant mitochondria. Mitochondrion 8, 47–60. ( 10.1016/j.mito.2007.10.004) [DOI] [PubMed] [Google Scholar]
- 68.Servet C, Ghelis T, Richard L, Zilberstein A, Savoure A. 2012. Proline dehydrogenase: a key enzyme in controlling cellular homeostasis. Front. Biosci. (Landmark Ed.) 17, 607–620. ( 10.2741/3947) [DOI] [PubMed] [Google Scholar]
- 69.Quinlan CL, Perevoshchikova IV, Hey-Mogensen M, Orr AL, Brand MD. 2013. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol. 1, 304–312. ( 10.1016/j.redox.2013.04.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goncalves RL, Rothschild DE, Quinlan CL, Scott GK, Benz CC, Brand MD. 2014. Sources of superoxide/H2O2 during mitochondrial proline oxidation. Redox Biol. 2, 901–909. ( 10.1016/j.redox.2014.07.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.