Abstract
Marine protected areas (MPAs) are commonly employed to protect ecosystems from threats like overfishing. Ideally, MPA design should incorporate movement data from multiple target species to ensure sufficient habitat is protected. We used long-term acoustic telemetry and network analysis to determine the fine-scale space use of five shark and one turtle species at a remote atoll in the Seychelles, Indian Ocean, and evaluate the efficacy of a proposed MPA. Results revealed strong, species-specific habitat use in both sharks and turtles, with corresponding variation in MPA use. Defining the MPA's boundary from the edge of the reef flat at low tide instead of the beach at high tide (the current best in Seychelles) significantly increased the MPA's coverage of predator movements by an average of 34%. Informed by these results, the larger MPA was adopted by the Seychelles government, demonstrating how telemetry data can improve shark spatial conservation by affecting policy directly.
Keywords: animal telemetry, conservation, management, ecology
1. Introduction
Marine ecosystems provide highly valuable services, including food production, climate regulation and nutrient cycling [1,2]. However, the sustainability of these services is threatened globally by factors such as overfishing, pollution and habitat degradation [3,4]. Predators help promote ecosystem diversity and stability by exerting strong, top-down forces that shape communities over large spatio-temporal scales [5–7]. Sharks, for instance, occupy high trophic levels in most marine food webs, are typically well connected trophically and can elicit strong avoidance behaviours in prey [8–10]. Yet most fisheries target large predators, potentially exacerbating the impacts of overfishing on ecosystem stability by selectively removing influential predators like sharks and tuna [2].
Fishing pressure on sharks has increased to the point where an estimated 63–273 million sharks are caught each year [11], with some populations appearing to have undergone significant declines [12,13]. A common tool to combat overfishing, especially in tropical ecosystems, is the designation of marine protected areas (MPAs), which can be very effective, depending on their size, level of restriction and associated enforcement [14,15]. The initial design of an MPA should be informed by the movements and habitat use of the target species, to ensure that it covers sufficient critical habitat to be effective [16,17]. Yet such information is rarely available at the point of inception, and MPA boundaries can be established with limited information, making them less likely to succeed [18,19]. To conserve ecosystem services, MPA design should also consider multiple species [20,21], as efficacy will probably vary between species with different behaviours, life-history traits and vulnerability to fishing pressure [15].
Most declines in shark populations have been inferred from Atlantic and Pacific fisheries, which have historically kept the most comprehensive catch records [7,11,22]. For instance, catch rates for some shark species in the Atlantic Ocean are estimated to have declined by over 90% [12,23], with similar declines (more than 70%) also indicated for the Pacific Ocean [22,24]. Data on Indian Ocean shark populations are severely deficient by comparison, but available reports suggest declines in this region (e.g. in the Seychelles) may be similarly severe [25,26]. Shark fishing in the Seychelles has long been of strong socio-economic importance, but has intensified in recent years, following a temporary European Union (EU) ban on import of local swordfish Xiphias gladius, and persecution of sharks after two fatal shark attacks in 2011 [25,27]. Yet the relative importance of shark to Seychelles fisheries has decreased by an order of magnitude in the past 70 years [25]. Thus, even now with stocks seemingly depleted, there is intense, unregulated fishing pressure on sharks in the Seychelles [25], and associated impacts on their ecosystem services could be severe. Consequently, shark populations in Seychelles require some level of precautionary management to promote their sustainability.
In the Seychelles, most MPAs have been established to protect seabird colonies, coral reefs or turtle species [28]—the beaches of Seychelles host one of the world's largest nesting populations of the critically endangered hawksbill turtle Eretmochelys imbricata [29]. However, the largest MPA in the Seychelles currently extends only 1 km from mean high water (MHW) and others to only 400 m, and may be ineffective for protecting vulnerable, wide-ranging groups such as sharks and turtles, which may be exposed to exploitation over larger areas [30,31]. Therefore, while these MPAs may be effective in protecting some target species, they may not achieve the wider goal of sustaining ecosystem functionality in the long term [30].
Presently, there are insufficient data concerning the behavioural ecology of sharks in the Seychelles [32] to predict whether an MPA designed for turtles or reefs would also be effective for predators such as sharks. A combined appreciation of shark behaviour, habitat use and population structure can help frame the scale at which management efforts may be required [15]. Consequently, this study analysed detailed, long-term movements of hawksbill turtles and five shark species at a remote atoll in the Seychelles, specifically investigating whether an MPA designed for reefs and turtles would also be sufficient for the local sharks, and if not how could it be adjusted to accommodate them.
2. Material and methods
(a). Study site
The study focused on the islands of D'Arros and St Joseph in the Amirantes, Seychelles (electronic supplementary material, figure S1), where existing data suggest that these islands may provide rare, critical habitat in the Seychelles for a variety of species, including important nesting and foraging habitat for the regions' recovering turtle populations [32–34]. D'Arros Island (05°24′ S, 53°17′ E) is a small sand cay (approx. 1.6 km2) situated on a patch reef (approx. 3.6 km2), while St Joseph (approx. 22 km2; 05°25′ S, 53°20′ E) is 1 km east, separated by a channel of 60–70 m depth. St Joseph Atoll has 16 small islands atop an uninterrupted reef flat that encloses a shallow (3–9 m), access-restricted lagoon of approximately 5 km2. The flats surrounding St Joseph lagoon are largely exposed at low tide, causing temporary isolation of the lagoon from the outer reef. Up to 2 m of water covers the flats at high tide.
(b). Animal telemetry
Between August 2012 and March 2015, a total of 116 sharks of five different species (blacktip reef Carcharhinus melanopterus, sicklefin lemon Negaprion acutidens, grey reef Carcharhinus amblyrhynchos, tawny nurse Nebrius ferrugineus and silvertip shark Carcharhinus albimarginatus) and 25 hawksbill turtles were tagged with acoustic transmitters (either V13 180 s nominal delay or V16 120 s nominal delay, Vemco Ltd, Bedford, Canada; see the electronic supplementary material for details). Sharks and turtles were tracked using an array of 88 acoustic receivers (VR2 W, Vemco Ltd; electronic supplementary material, figure S1) with tags detected within 165 m ± 33 (s.d.) of the receiver, as determined by range testing. However, to accommodate the staggered deployment of acoustic receivers, the study was restricted to 67 receivers active November 2013–November 2015, providing an effective sample of 110 tagged individuals (see the electronic supplementary material for details).
(c). Network analysis
Network analysis was used to determine animal space use with receivers being treated as nodes and pairs of subsequent pings between nodes treated as a connection between those nodes [35]. Several network metrics were used to describe each network (see the electronic supplementary material for details). In brief, ‘occupancy’ provides a measure of how much time individuals spent at each receiver location. ‘Connectivity’ is the proportion of other nodes to which there is a connection. ‘Transit’ represents the extent to which a node is part of a corridor of movement as opposed to an area of occupancy. ‘Node density’ measures the extent of the array occupied, and ‘edge density’ provides a measure of mobility within the network, both ranging 0–1.
To test whether the observed movements were different from random, random networks were generated (see the electronic supplementary material for details) and their node metrics were tested against those of the real tracks using Wilcoxon matched-pairs signed-rank tests.
Each receiver location was designated a habitat type: lagoon (habitat within St Joseph Atoll, including the flats), coastal reef (sloped reefs bordering islands), plateau (flat-bottomed areas of patchy reef rubble and seagrass beds) or drop-off (the edge of the Amirantes plateau, before it drops to hundreds of metres). To reveal differences in space use between habitats for each species, node metrics were grouped according to habitat type and had their values compared to those of the same habitat type in the random networks. This was achieved by calculating a randomization index
where Om is the observed and Rm the random metric. Mean values were then plotted for each node metric in each habitat type, according to species. For each individual, a residency index was calculated representing the percentage of days during its track that it was detected within the array:
where Dd is days detected and Dal is days at liberty.
(d). Grid occupancy analysis
The data were further used to evaluate the potential efficacy of two MPA designs. Each design had its boundary radius restricted to 1 km, as this matches the current best in Seychelles for the UNESCO World Heritage Site of Aldabra Atoll. The first MPA model, the null MPA, matches the Aldabra designation with the boundary being formed by 1 km from the beach at MHW (figure 1). The second, proposed MPA keeps the same boundary radius of 1 km, but instead measures it from the edge of the reef flat at the lowest astronomical tide (figure 1). Owing to the extensive reef flats at D'Arros and St Joseph, which are exposed at low tide and can exceed 1 km width, this forces the boundary to include all of the lagoon and coastal reefs, some of which remain exposed in the null MPA (figure 1). The smaller null MPA encompasses an area of approximately 42.3 km2, whereas the larger proposed MPA covers approximately 64.9 km2 (approx. 50% increase in area).
Figure 1.
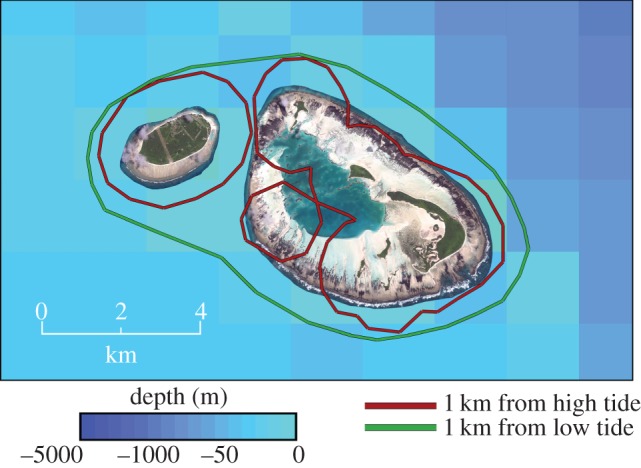
A map showing boundaries of the two MPAs: 1 km from the high-tide mark (smaller null MPA, red) and 1 km from the low-tide mark (larger proposed MPA, green). Map created in ArcGIS, using satellite imagery from LAND INFO Worldwide Mapping and ETOPO2v2 bathymetry data.
Grid occupancy analysis was used to evaluate the efficacy of both MPAs (see the electronic supplementary material for details). In brief, the array was divided into 0.5 km grid squares, and the number of days each individual occurred within each grid square was summed. Using the boundaries of both MPAs, it was then possible to calculate the percentage of their track each individual would have spent within the boundaries of each MPA.
3. Results
During the study period, 110 tagged individuals of six different species were tracked: blacktip reef (n = 25), grey reef (n = 22), sicklefin lemon (n = 20), tawny nurse (n = 6), silvertip sharks (n = 13) and hawksbill turtle (n = 24), providing over 50 477 tracking days (table 1). A range of juveniles and adults was tagged for each species, apart from silvertip sharks and hawksbill turtles, all of which were juvenile. Mean track duration across all sharks (n = 86) was 484 days ± 265 (s.d.), with 64.0% of tracks lasting more than a year. Mean turtle track (n = 24) duration was 368 days ± 210 (s.d.), with 62.5% of tracks lasting more than a year. All shark species showed a bias towards females among tagged individuals (table 1), while sex determination was not undertaken for the juvenile turtles, as it can only be achieved through costly and potentially invasive procedures (laparoscopy and blood sampling). Full details of all results are available in the electronic supplementary material with pertinent details reported here.
Table 1.
Summary data for the 110 tags (86 sharks and 24 turtles) used for data analysis. The curved carapace length was used as the corresponding total length (TL) for turtles (RI, residency index).
| species | n | TL range (cm) | mean TL (cm) | sex ratio (m : f) | liberty range (days) | mean liberty (days) | mean RI |
|---|---|---|---|---|---|---|---|
| blacktip | 25 | 77–130 | 107.6 | 1.0 : 2.6 | 34–753 | 563.8 | 54.2 |
| grey | 22 | 84–158 | 127.5 | 1.0 : 6.3 | 49–746 | 473.2 | 20.1 |
| lemon | 20 | 109–213 | 168.1 | 1.0 : 2.3 | 3–755 | 589.6 | 64.0 |
| nurse | 6 | 155–274 | 210.3 | 1.0 : 2.0 | 79–749 | 559.3 | 50.1 |
| silvertip | 13 | 79–120 | 95.7 | 1.0 : 3.3 | 11–349 | 154.1 | 22.1 |
| hawksbill | 24 | 36–71 | 46.7 | n.a. : n.a. | 6–756 | 367.6 | 28.6 |
(a). Species-specific habitat use
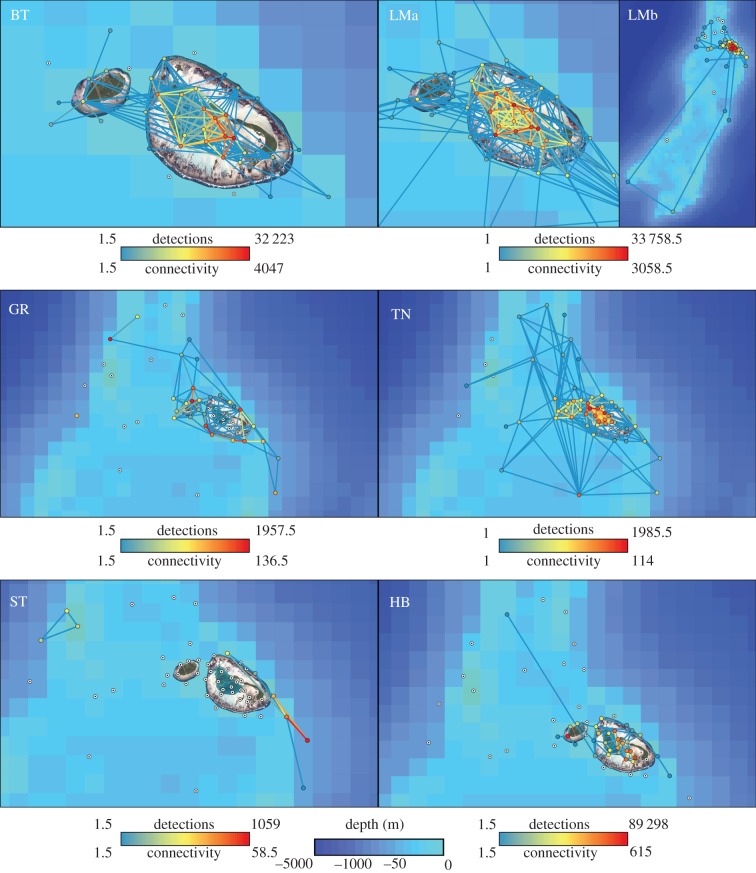
All metrics of the real networks of all species were statistically different from those generated by the random networks (electronic supplementary material, tables S1 and S2). Blacktip reef sharks displayed very restricted movements (figure 2), with 99.8% of all detections occurring within the confines of St Joseph Atoll, residency that is reflected by their very high occupancy of lagoon habitats compared with random networks (figure 3). Movements were highly focused on the eastern end of the lagoon (figure 2), consistent with their very low edge density of 0.09, versus 0.72 for the random sharks.
Figure 2.
Networks displaying species-specific detection frequency at each receiver (node colour) and how often each receiver was connected by subsequent detections (edge colour). Receivers with no detections marked with ⊙. BT, blacktip reef; LMa, lemon fine-scale; LMb, lemon broad-scale; GR, grey reef; TN, tawny nurse; ST, silvertip; HB, hawksbill. Maps created in ArcGIS, using satellite imagery from LAND INFO Worldwide Mapping and ETOPO2v2 bathymetry data.
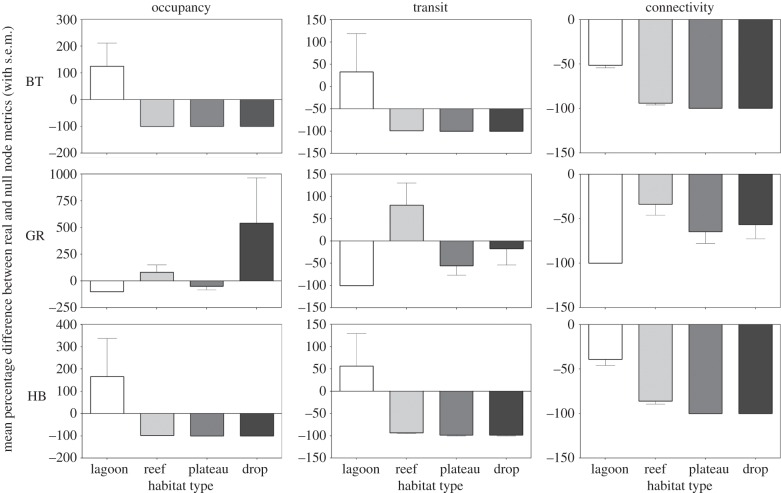
Figure 3.
Charts showing, for three species that exemplify the different patterns observed, the mean percentage difference between the actual node metrics and those from the randomly generated networks (n = 100 per species), with nodes grouped by habitat type. BT, blacktip reef; GR, grey reef; HB, hawksbill. Positive deviations denote where actual metric values were higher for that habitat than random, and vice versa. Note the different scales on the y-axes. Error bars represent the standard error of the mean.
For the sicklefin lemon sharks 98.8% of all detections occurred within the atoll (figure 2), with elevated occupancy of lagoon habitats in real versus random networks (electronic supplementary material, figure S2). However, lemon sharks were also recorded making wider movements across the Amirantes plateau, including to Desnoeufs Island 94 km south of D'Arros (figure 2). This is reflected in their high node and edge densities of 0.84 and 0.15, respectively, revealing much greater use of the array than blacktip reef sharks. One tagged lemon shark was also caught by fishermen at Marie-Louise 80 km south of D'Arros, while another was caught at Bird Island, 300 km away across deep water (more than 1000 m). All lemon sharks recorded moving across the plateau (n = 9) were at least 177 cm, whereas smaller individuals remained exclusively within the confines of the atoll and its coastal reefs.
By contrast, grey reef sharks were largely recorded along the coastal reefs and drop-offs (62.1% and 30.4% of detections, respectively), and not at all in the atoll (figure 2), with elevated occupancy of drop-off and coastal reef habitats in real versus random sharks (figure 3). One grey reef shark tag was returned from the reefs of D'Arros by fishermen.
The tawny nurse sharks displayed a range of movements similar to the lemon sharks (figure 2), reflected by similar node and edge densities (0.76 and 0.12, respectively). The majority of nurse shark detections (70.0%) occurred within the atoll with regular movement throughout. Almost all (98.1%) of nurse shark detections within the lagoon were from individuals less than 200 cm (n = 3), whereas 84.0% of all nurse shark detections outside the lagoon were from individuals more than 200 cm (n = 3). These larger nurse sharks frequently travelled more widely across the plateau (figure 2).
Silvertip sharks showed the most restricted movements (node density 0.13, edge density 0.01), producing fragmented networks that almost exclusively associated with the drop-off (96.5% of all silvertip detections in drop-off habitats; figure 2). Real silvertip sharks occupied drop-off habitats much more than random sharks, even transiting along the drop-offs more than random sharks did (electronic supplementary material, figure S2). Four of the 19 tagged silvertip sharks are known to have been caught by fishermen, contributing to their low mean time at liberty (table 1).
Hawksbill turtles displayed movements largely restricted to the atoll (figure 2), with 99.0% of all detections occurring in lagoon habitats. Hawksbill movements were highly focused with comparatively few connections made (edge density was only 0.03, node density 0.46). Hawksbill turtles also displayed very high occupancy of lagoon habitats compared with random networks (figure 3).
Apart from silvertip sharks along the drop-offs, all real networks displayed lower connectivity in all habitats than random networks for all species, revealing that all tracked individuals displayed more directed movement between nodes than their random counterparts (figure 3; electronic supplementary material, figure S2). This is also consistent with the universally low edge densities for all species, which are significantly lower than their random counterparts (electronic supplementary material, table S2).
(b). Occupancy of marine protected area
Grid occupancy analysis revealed that overall the proposed (larger) MPA increased coverage of predator movements by 33.8 ± 150.3% (s.d.) compared with the null (smaller) MPA, with all species apart from silvertip sharks displaying a significant increase in coverage from the larger MPA (see the electronic supplementary material, table S3). Although a high percentage (89.9%) of blacktip reef shark tracks occurred within the boundaries of the smaller MPA, 98.7% occurred within the larger MPA (figure 4). Similarly for lemon sharks, 83.5% of recorded tracks occurred within the smaller MPA versus 96.5% for the larger MPA (figure 4).
Figure 4.
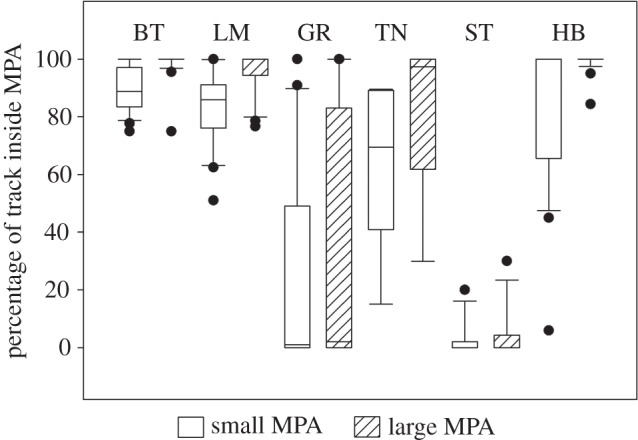
Box plots of the proportion of their recorded track each species spent inside the small MPA (white, 1 km from high tide) and the larger MPA (hatched, 1 km from low tide). BT, blacktip reef; LM, lemon; GR, grey reef; TN, tawny nurse; ST, silvertip; HB, hawksbill.
Grey reef sharks overall received very poor coverage from both MPAs, but still received a significant increase in coverage from the larger MPA (26.6% of time in the smaller versus 32.8% inside the larger; figure 4). This increase is largely driven by greater coverage of smaller individuals patrolling coastal reefs: two of the smallest grey reef sharks (79 cm and 99 cm) both had their coverage more than double to over 97% in the larger MPA.
Nurse sharks also receive a significant increase in coverage from the smaller to larger MPA (from 63.7 to 82.9%). Silvertip sharks spend very little time in either MPA (2.7 and 4.0%), with no significant difference between the two, as movements are largely focused along the offshore drop-offs (figure 2). Hawksbill turtles received similar coverage from the smaller MPA (84.9%) to blacktip reef sharks, and had significantly higher coverage from the larger MPA (99.1%; figure 4).
(c). Management of marine protected area
An early form of the results presented here was communicated to the Ministry of Environment, Energy and Climate Change, Seychelles, to demonstrate the value of habitat provided by D'Arros and St Joseph, and to indicate the increased efficacy of the larger MPA for protecting sharks. This contributed in part to the Seychelles government formally adopting the larger MPA, and declaring D'Arros and St Joseph a Special Reserve (International Union for the Conservation of Nature, IUCN, Category 1a) with a no-take zone extending 1 km from the low-tide mark [36]. An implementation plan was also agreed where the Save Our Seas Foundation would provide facilities (e.g. a patrol boat) to promote enforcement.
4. Discussion
While efforts have been made to assess the efficacy of existing MPAs (e.g. [14,37,38]), this study is novel in using the dynamic habitat use of sharks and turtles to inform the design of an MPA at a remote atoll in the Indian Ocean. In particular, the telemetry-based network and grid occupancy analyses allowed complex animal movements to be collapsed into a few axes that could be more easily interpreted within and between species in relation to spatial areas. An early form of the data on habitat use presented here was used not only to emphasize the importance of D'Arros and St Joseph as important habitat worthy of protection, but also to justify having a boundary beyond the 1 km from MHW used elsewhere in the Seychelles, informing the subsequent adoption of the Special Reserve [36]. Moreover, there has since been a proposal to extend the MPA around Aride Island in the Seychelles from 400 m offshore to 1 km [39].
In the light of global threats to marine ecosystems, conservation efforts are increasingly turning to spatial management options, with over 9000 MPAs having been declared to date [19]. A recent review of MPAs that have successfully increased biomass found that the chances of MPA success increased with the designation of a no-take zone, effective enforcement, age, size and isolation [14]. Yet over 90% of MPAs still permit some level of fishing, and the median size is only 4.5 km2, leaving significant gaps in coverage [19,31]. In comparison, the D'Arros and St Joseph Special Reserve is isolated, will not permit any fishing, will be over 65 km2 and will have effective enforcement, all of which suggest it has the potential to be effective.
Although an MPA of 1 km from MHW at D'Arros and St Joseph may have still been effective in protecting juvenile hawksbill turtles and some shark species, a change in definition to delineate the boundary according to the low tide mark predicts a significant increase in protection for all tracked species bar the silvertip shark. This increase can be explained by an understanding of movements and local topography—extending the boundary from the low-tide means it starts at the edge of the wide reef flats that surround the islands, forcing the boundary out beyond the coastal reefs and covering the lagoon, the two habitats used most frequently by the majority of tracked species. The smaller MPA would not have covered all of the lagoon or outer reefs (figure 1), leaving many sharks frequently exposed to fishing pressure. Indeed, shark finning has previously been recorded in the lagoon [40].
From the recorded tracks, it appears as though D'Arros and St Joseph may provide important nursery habitats for sharks within the Amirantes and across the Seychelles. Juveniles of blacktip reef, sicklefin lemon, grey reef and tawny nurse sharks were all found to display long-term, perennial use of the lagoon and coastal reef habitats, fulfilling previously established nursery criteria [41]. The confined, access-restricted habitat provided by the lagoon presumably provides refuge from predation alongside foraging opportunities, as suggested for similar shark nurseries in the Bahamas [42]. Consequently, its protection through the designation of a more effective MPA is particularly important, and may help promote survival and recruitment into regional populations, especially if larger individuals of certain species disperse broadly upon reaching maturity.
The differences in habitat use between the hawksbill turtles and different shark species correspond to the varied efficacy of the MPA between species, highlighting the importance of understanding movements of multiple species in order for MPA design to be effective. Given the historic focus on turtle conservation in the Seychelles, following intense exploitation for their shells and meat [29], the hawksbill turtles were the basis from which the null MPA was assessed, with the sharks being used as the justification for its extension. Although protected nationwide in Seychelles since 1994, hawksbill turtles are critically endangered in every ocean basin [43], and there is still some level of poaching in Seychelles [34].
Effective management of sicklefin lemon shark populations is particularly important as they are considered Vulnerable on the IUCN Red List and have been exploited to extirpation in several areas, including India and Thailand [44]. Consistent with previous work in Seychelles [32,45], smaller lemon sharks displayed high fidelity to lagoon habitats within MPA boundaries, but larger individuals of both lemon and nurse sharks adopted broader movements across the Amirantes plateau. Similarly, most grey reef and silvertip sharks favoured particular drop-off habitats, receiving little coverage from either MPA.
The more extensive distribution of larger lemon, grey reef and nurse sharks means that certain individuals remain exposed to fishing exploitation, and reveals the need for alternative management strategies. Potential nurseries such as St Joseph Atoll may be maintained by relatively few mature females; in Atol das Rocas off Brazil, it is estimated that a population of approximately 100 juvenile Atlantic lemon sharks Negaprion brevirostris could be maintained by as few as five to seven mature females [46]. Consequently, even infrequent shark finning events, as have been reported within St Joseph Atoll [40], pose significant risk to shark population stability. Although the MPA should prevent finning events in the lagoon, the risk is further realized by the capture of tagged lemon sharks at Marie-Louise and Bird Island. These captures emphasize that for wider-ranging species management tools like the MPA need to be coupled with broader fisheries management strategies in order to reduce mortality of wider-ranging adults and be effective at promoting recruitment [15,47], such as catch quotas, size limits, time/area closures or even a larger shark sanctuary that covers at least the Amirantes.
Furthermore, MPAs need to be linked with reduced fishing capacity to ensure that effort is not simply displaced [47]. Indeed, the mean increase in coverage of 33.8 ± 150.3% (s.d.) across all individuals comes at the expense of a 50% increase in area, which may incur a greater cost to local fishing capacity. However, this masks the fact that while some species (e.g. silvertip) receive little to no increase in coverage, the absolute coverage of the larger MPA for other species (e.g. blacktip reef, lemon) starts to approach 100% for most individuals, suggesting the change in boundary may be particularly valuable for the species using the atoll as a refuge or nursery, with recruitment benefits potentially outweighing the raw ratio of increase between coverage and MPA size.
In summary, this study reveals how a detailed understanding of habitat use, determined with acoustic telemetry and network analysis, was used to inform the design of a no-take MPA at the point of inception, defining its boundaries to enhance its efficacy significantly. This highlights the importance of an evidence-driven approach to MPA design, and the value of incorporating multiple species over the long term. Our study emphasizes how an MPA designed for one species (e.g. turtles) may not be as effective for others (e.g. sharks), and could therefore fall short of protecting the ecosystem as a whole. Even when the larger MPA in this study is in place, however, broader management efforts will need to be framed at regional scales, as movements of certain species and size classes continue to traverse MPA boundaries and the high seas.
Supplementary Material
Acknowledgements
The authors wish to thank the Seychelles Ministry of Environment, Energy and Climate Change for their collaboration with the present work and subsequent adoption of the D'Arros and St Joseph Special Reserve. All of the divers, ship crews, engineers and volunteers at the D'Arros Research Centre provided invaluable assistance during fieldwork and preparation. J.S.E.L. thanks D. M. P. Jacoby for initial advice on network analysis techniques. Additionally, the authors thank the fishermen who returned tags found in caught sharks.
Ethics
All fieldwork was approved by, and conducted with the knowledge of, the Ministry of Environment, Energy, and Climate Change, Seychelles. The animal handling and tagging methods were performed in accordance with the approved guidelines of the University of Plymouth, UK.
Data accessibility
Given ongoing exploitation of sharks in the Seychelles, including of species considered Vulnerable on the IUCN Red List [44], the detailed location data are considered sensitive information. The raw data have been deposited into a database at the Marine Biological Association of the UK, from where they may be available on request.
Authors' contributions
J.S.E.L. and C.R.C. conceived the study and performed the shark tracking. R.G.v.B. originated and performed the turtle tracking. D.W.S. conceived the network analysis approach and oversaw the manuscript structure and analysis techniques. N.E.H. designed the database, wrote software for network analysis and automation of track processing, and assisted with track analysis techniques. J.S.E.L. performed the analyses and created all figures. J.S.E.L., N.E.H. and D.W.S. wrote the manuscript.
Competing interests
We have no competing interests.
Funding
The authors thank the Founder of the Save Our Seas Foundation for funding and providing all facilities for this work. Funding for data analysis was provided by the UK Natural Environment Research Council (NERC) ‘Oceans 2025’ Strategic Research Programme in which D.W.S. was a principal investigator. J.S.E.L. was supported by the Founder of the Save Our Seas Foundation and the Marine Biological Association of the UK (MBA), and D.W.S. by an MBA Senior Research Fellowship. R.G.v.B. was supported by the Save Our Seas Foundation D'Arros Research Centre.
References
- 1.Holmlund CM, Hammer M. 1999. Ecosystem services generated by fish populations. Ecol. Econ. 29, 253–268. ( 10.1016/S0921-8009(99)00015-4) [DOI] [Google Scholar]
- 2.McCauley DJ, Pinsky ML, Palumbi SR, Estes JA, Joyce FH, Warner RR. 2015. Marine defaunation: animal loss in the global ocean. Science 347, 1255641 ( 10.1126/science.1255641) [DOI] [PubMed] [Google Scholar]
- 3.Worm B, Branch TA. 2012. The future of fish. Trends Ecol. Evol. 27, 594–599. ( 10.1016/j.tree.2012.07.005) [DOI] [PubMed] [Google Scholar]
- 4.Juan-Jordá MJ, Mosqueira I, Cooper AB, Freire J, Dulvy NK. 2011. Global population trajectories of tunas and their relatives. Proc. Natl Acad. Sci. USA 108, 20 650–20 655. ( 10.1073/pnas.1107743108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heithaus MR, Frid A, Wirsing AJ, Worm B. 2008. Predicting ecological consequences of marine top predator declines. Trends Ecol. Evol. 23, 202–210. ( 10.1016/j.tree.2008.01.003) [DOI] [PubMed] [Google Scholar]
- 6.Beschta RL, Ripple WJ. 2009. Large predators and trophic cascades in terrestrial ecosystems of the western United States. Biol. Conserv. 142, 2401–2414. ( 10.1016/j.biocon.2009.06.015) [DOI] [Google Scholar]
- 7.Ferretti F, Worm B, Britten GL, Heithaus MR, Lotze HK. 2010. Patterns and ecosystem consequences of shark declines in the ocean. Ecol. Lett. 13, 1055–1071. ( 10.1111/j.1461-0248.2010.01489.x) [DOI] [PubMed] [Google Scholar]
- 8.Cortés E. 1999. Standardized diet compositions and trophic levels of sharks. ICES J. Mar. Sci. J. du Conserv. 56, 707–717. ( 10.1006/jmsc.1999.0489) [DOI] [Google Scholar]
- 9.Bascompte J, Melián CJ, Sala E. 2005. Interaction strength combinations and the overfishing of a marine food web. Proc. Natl Acad. Sci. USA 102, 5443–5447. ( 10.1073/pnas.0501562102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heithaus MR, et al. 2013. Apparent resource partitioning and trophic structure of large-bodied marine predators in a relatively pristine seagrass ecosystem. Mar. Ecol. Prog. Ser. 481, 225–237. ( 10.3354/meps10235) [DOI] [Google Scholar]
- 11.Worm B, Davis B, Kettemer L, Ward-Paige CA, Chapman D, Heithaus MR, Kessel ST, Gruber SH. 2013. Global catches, exploitation rates, and rebuilding options for sharks. Mar. Policy 40, 194–204. ( 10.1016/j.marpol.2012.12.034) [DOI] [Google Scholar]
- 12.Ferretti F, Myers RA, Serena F, Lotze HK. 2008. Loss of large predatory sharks from the Mediterranean Sea. Conserv. Biol. 22, 952–964. ( 10.1111/j.1523-1739.2008.00938.x) [DOI] [PubMed] [Google Scholar]
- 13.Dulvy NK, et al. 2014. Extinction risk and conservation of the world's sharks and rays. eLife 3, e00590 ( 10.7554/eLife.00590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edgar GJ, et al. 2014. Global conservation outcomes depend on marine protected areas with five key features. Nature 506, 216–220. ( 10.1038/nature13022) [DOI] [PubMed] [Google Scholar]
- 15.Osgood GJ, Baum JK. 2015. Reef sharks: recent advances in ecological understanding to inform conservation. J. Fish Biol. 87, 1489–1523. ( 10.1111/jfb.12839) [DOI] [PubMed] [Google Scholar]
- 16.Speed CW, Meekan MG, Field IC, McMahon CR, Harcourt RG, Stevens JD, Babcock RC, Pillans RD, Bradshaw CJA. 2015. Reef shark movements relative to a coastal marine protected area. Reg. Stud. Mar. Sci. 3, 58–66. ( 10.1016/j.rsma.2015.05.002) [DOI] [Google Scholar]
- 17.Allen AM, Singh N. 2015. Linking movement ecology with wildlife management and conservation. Front. Ecol. Evol. 3, 155 ( 10.3389/fevo.2015.00155) [DOI] [Google Scholar]
- 18.Brown CJ, et al. 2015. Fisheries and biodiversity benefits of using static versus dynamic models for designing marine reserve networks. Ecosphere 6, 1–14. ( 10.1890/ES14-00429.1) [DOI] [Google Scholar]
- 19.Costello MJ, Ballantine B. 2015. Biodiversity conservation should focus on no-take Marine Reserves: 94% of Marine Protected Areas allow fishing. Trends Ecol. Evol. 30, 507–509. ( 10.1016/j.tree.2015.06.011) [DOI] [PubMed] [Google Scholar]
- 20.Mouillot D, et al. 2016. Global marine protected areas do not secure the evolutionary history of tropical corals and fishes. Nat. Commun. 7, 10359 ( 10.1038/ncomms10359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pendoley KL, Schofield G, Whittock PA, Ierodiaconou D, Hays GC. 2014. Protected species use of a coastal marine migratory corridor connecting marine protected areas. Mar. Biol. 161, 1455–1466. ( 10.1007/s00227-014-2433-7) [DOI] [Google Scholar]
- 22.Clarke SC, Harley SJ, Hoyle SD, Rice JS. 2013. Population trends in Pacific Oceanic sharks and the utility of regulations on shark finning. Conserv. Biol. 27, 197–209. ( 10.1111/j.1523-1739.2012.01943.x) [DOI] [PubMed] [Google Scholar]
- 23.Baum JK, Myers RA. 2004. Shifting baselines and the decline of pelagic sharks in the Gulf of Mexico. Ecol. Lett. 7, 135–145. ( 10.1111/j.1461-0248.2003.00564.x) [DOI] [Google Scholar]
- 24.Ward P, Myers RA. 2005. Shifts in open-ocean fish communities coinciding with the commencement of commercial fishing. Ecology 86, 835–847. ( 10.1890/03-0746) [DOI] [Google Scholar]
- 25.Nevill J, Robinson J, Giroux F, Isidore M. 2007. Seychelles National Plan of Action for the Conservation and Management of Sharks. Victoria, Seychelles: Seychelles Fishing Authority. [Google Scholar]
- 26.Graham NA, Spalding MD, Sheppard CR. 2010. Reef shark declines in remote atolls highlight the need for multi-faceted conservation action. Aquat. Conserv. Mar. Freshwater Ecosyst. 20, 543–548. ( 10.1002/aqc.v20:5) [DOI] [Google Scholar]
- 27.Seychelles Nation. 2015. Bull shark killed British honeymooner in Seychelles. See http://sharkyear.com/2011/bull-shark-killed-british-honeymooner-in-seychelles.html (accessed 15 December 2015). [Google Scholar]
- 28.Ministry of Environment, Energy and Climate Change 2012. National Parks and Nature Conservancy Act. Victoria, Seychelles: Ministry of Environment, Energy and Climate Change. [Google Scholar]
- 29.Mortimer JA, Collie J. 1998. Status and conservation of sea turtles in the Republic of Seychelles. In Proc. 17th Annu. Sea Turtle Symp. NOAA Tech Memo NMFS-SEFSC-415 (eds SE Epperly, J Braun), pp. 74–75. Miami, FL: National Marine Fisheries Service.
- 30.Jennings S, Marshall SS, Polunin NV. 1996. Seychelles’ marine protected areas: comparative structure and status of reef fish communities. Biol. Conserv. 75, 201–209. ( 10.1016/0006-3207(95)00081-X) [DOI] [Google Scholar]
- 31.Mazaris AD, Almpanidou V, Wallace BP, Pantis JD, Schofield G. 2014. A global gap analysis of sea turtle protection coverage. Biol. Conserv. 173, 17–23. ( 10.1016/j.biocon.2014.03.005) [DOI] [Google Scholar]
- 32.Filmalter JD, Dagorn L, Cowley PD. 2013. Spatial behaviour and site fidelity of the sicklefin lemon shark Negaprion acutidens in a remote Indian Ocean atoll. Mar. Biol. 160, 2425–2436. ( 10.1007/s00227-013-2237-1) [DOI] [Google Scholar]
- 33.Von Brandis RG, Mortimer JA, Reilly BK. 2010. In-water observations of the diving behaviour of immature hawksbill turtles, Eretmochelys imbricata, on a coral reef at D'Arros Island, Republic of Seychelles. Chelonian Conserv. Biol. 9, 26–32. ( 10.2744/CCB-0815.1) [DOI] [Google Scholar]
- 34.Mortimer JA, Camille J-C, Boniface N. 2011. Seasonality and status of nesting hawksbill (Eretmochelys imbricata) and green turtles (Chelonia mydas) at D'Arros Island, Amirantes Group, Seychelles. Chelonian Conserv. Biol. 10, 26–33. ( 10.2744/CCB-0830.1) [DOI] [Google Scholar]
- 35.Jacoby DMP, Brooks EJ, Croft DP, Sims DW. 2012. Developing a deeper understanding of animal movements and spatial dynamics through novel application of network analyses. Methods Ecol. Evol. 3, 574–583. ( 10.1111/j.2041-210X.2012.00187.x) [DOI] [Google Scholar]
- 36.Payet R. 2014. National Parks (D'Arros and St Joseph Special Reserve) Designation Order. Victoria, Seychelles: Ministry of Environment, Energy and Climate Change. [Google Scholar]
- 37.Bond ME, Babcock EA, Pikitch EK, Abercrombie DL, Lamb NF, Chapman DD. 2012. Reef sharks exhibit site-fidelity and higher relative abundance in marine reserves on the Mesoamerican Barrier Reef. PLoS ONE 7, e0032983 ( 10.1371/journal.pone.0032983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Espinoza M, Lédée EJ, Simpfendorfer CA, Tobin AJ, Heupel MR. 2015. Contrasting movements and connectivity of reef-associated sharks using acoustic telemetry: implications for management. Ecol. Appl. 25, 2101–2118. ( 10.1890/14-2293.1) [DOI] [PubMed] [Google Scholar]
- 39.Seychelles News Agency. 2015. Proposed extension of Aride's boundaries aims for more protection of the Seychelles special reserve. See http://www.seychellesnewsagency.com/articles/4160/Proposed+extension+of+Arides+boundaries+aims+for+more+protection+of+the+Seychelles+special+reserve (accessed 15 December 2015). [Google Scholar]
- 40.Vejarano CC, Engelhardt U. 2008. Report on shark-finning incidents at St Joseph Atoll, Amirantes from 24–26 January 2008. D'Arros, Seychelles: D'Arros Research Centre. [Google Scholar]
- 41.Heupel MR, Carlson JK, Simpfendorfer CA. 2007. Shark nursery areas: concepts, definition, characterization and assumptions. Mar. Ecol. Prog. Ser. 337, 287–297. ( 10.3354/meps337287) [DOI] [Google Scholar]
- 42.Guttridge TL, Gruber SH, Franks BR, Kessel ST, Gledhill KS, Uphill J, Krause J, Sims DW. 2011. Deep danger: intra-specific predation risk influences habitat use and aggregation formation of juvenile lemon sharks Negaprion brevirostris. Mar. Ecol. Prog. Ser. 445, 279–291. ( 10.3354/meps09423) [DOI] [Google Scholar]
- 43.Mortimer JA, Donnelly M. 2008. Marine turtle specialist group 2007 IUCN Red List status assessment hawksbill turtle (Eretmochelys imbricata). Gland, Switzerland: IUCN Marine Turtle Specialist Group. [Google Scholar]
- 44.Pillans R. 2003. Negaprion acutidens. The IUCN Red List of Threatened Species 2003. (SSG Aust, Ocean. Reg. Work. March 2003). e.T41836A10576957.
- 45.Stevens JD. 1984. Life-history and ecology of sharks at Aldabra Atoll, Indian Ocean. Proc. R. Soc. Lond. B 222, 79–106. ( 10.1098/rspb.1984.0050) [DOI] [Google Scholar]
- 46.Freitas RHA, Rosa RS, Wetherbee BM, Gruber SH. 2009. Population size and survivorship for juvenile lemon sharks (Negaprion brevirostris) on their nursery grounds at a marine protected area in Brazil. Neotrop. Ichthyol. 7, 205–212. ( 10.1590/S1679-62252009000200011) [DOI] [Google Scholar]
- 47.Kinney MJ, Simpfendorfer CA. 2009. Reassessing the value of nursery areas to shark conservation and management. Conserv. Lett. 2, 53–60. ( 10.1111/j.1755-263X.2008.00046.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Given ongoing exploitation of sharks in the Seychelles, including of species considered Vulnerable on the IUCN Red List [44], the detailed location data are considered sensitive information. The raw data have been deposited into a database at the Marine Biological Association of the UK, from where they may be available on request.