Abstract
A comprehensive description of the spectral characteristics of retinal photoreceptors in palaeognaths is lacking. Moreover, controversy exists with respect to the spectral sensitivity of the short-wavelength-sensitive-1 (SWS1) opsin-based visual pigment expressed in one type of single cone: previous microspectrophotometric (MSP) measurements in the ostrich (Struthio camelus) suggested a violet-sensitive (VS) SWS1 pigment, but all palaeognath SWS1 opsin sequences obtained to date (including the ostrich) imply that the visual pigment is ultraviolet-sensitive (UVS). In this study, MSP was used to measure the spectral properties of visual pigments and oil droplets in the retinal photoreceptors of the emu (Dromaius novaehollandiae). Results show that the emu resembles most other bird species in possessing four spectrally distinct single cones, as well as double cones and rods. Four cone and a single rod opsin are expressed, each an orthologue of a previously identified pigment. The SWS1 pigment is clearly UVS (wavelength of maximum absorbance [λmax] = 376 nm), with key tuning sites (Phe86 and Cys90) consistent with other vertebrate UVS SWS1 pigments. Palaeognaths would appear, therefore, to have UVS SWS1 pigments. As they are considered to be basal in avian evolution, this suggests that UVS is the most likely ancestral state for birds. The functional significance of a dedicated UVS cone type in the emu is discussed.
Keywords: spectral tuning, opsin evolution, microspectrophotometry, oil droplets, Casuariiformes
1. Introduction
The avian retina typically possesses four spectrally distinct single cone classes, a single double cone class, and a single rod class, together with their associated visual pigments [1]. A significant omission from the literature, however, is a comprehensive study of the photoreceptor complement and spectral characteristics in a member of the Palaeognathae (Order Struthioniformes), the large flightless birds that occur at the base of the avian radiation [2].
An early microspectrophotometric (MSP) study of the emu (Dromaius novaehollandiae) found a single type of rod, containing a visual pigment with a wavelength of maximum absorbance (λmax) at 502 nm, and three different cone types, all with a λmax at 567 nm but either lacking an oil droplet or possessing an oil droplet with differing wavelengths of half maximum transmission (λT0.5) [3]. As no other cone types were found in the emu retina, it was suggested that palaeognath colour discrimination might be limited when compared with neognaths such as the chicken (Gallus gallus) and pigeon (Columba livia), which are known to possess four spectrally distinct cone pigments with λmax values at 413 nm, 467 nm, 507 nm, and 562 nm, in addition to a rod pigment [4,5]. A subsequent study of the ostrich (Struthio camelus) and rhea (Rhea americana), however, found four single cone classes containing visual pigments with λmax values at 405 nm (ostrich only), 445 nm, 505 nm, and 570 nm, one double cone with a λmax at 570 nm in both members, and one rod (λmax at 505 nm) [6]. Collectively, this suggested that palaeognaths are similar to neognaths in terms of their retinal photoreceptor complement.
The λmax value (405 nm; n = 1) obtained for the most short-wavelength-sensitive (SWS) cone in the ostrich [6] would classify it as violet-sensitive (VS). VS SWS1 pigments are generally found in species with relatively large eyes (e.g. primates, dogs, cats, elephants, and horses), so a VS pigment might be expected to be present in palaeognaths. However, all palaeognaths studied to date, including the ostrich, have been shown to possess a SWS1 opsin with Cys90 [7,8], which in birds indicates a UV-sensitive (UVS) SWS1 pigment [9–11]. Although the available palaeognath SWS1 opsin sequence data are restricted to a few residues flanking site 90, this is clearly in contradiction to the reported presence of a VS SWS1 pigment in the ostrich [6].
In this study, MSP was used to measure the spectral absorption characteristics of emu retinal photoreceptors, and the coding sequences of the expressed visual pigments were determined by molecular analysis. These results provide, for the first time, a detailed assessment of palaeognath photoreceptor spectral sensitivities and address directly the issue of UVS/VS pigment evolution in birds.
2. Material and methods
(a). Animals
Two adult emus, reared in outdoor pens with exposure to full daylight and natural light : dark cycles, were dark adapted for 1 h prior to euthanization with an overdose of pentobarbitone (Lethabarb®, Virbac; 160 mg kg−1 delivered intravenously). Eyes were removed immediately under dim light and placed on ice until used.
(b). Microspectrophotometric measurements
Eyes were dissected under infrared illumination with the aid of an image converter and retinal tissue was prepared for MSP as described elsewhere [12]. Transverse absorbance spectra (330–800 nm) of individual photoreceptor outer segments and oil droplets were measured using a single-beam wavelength-scanning microspectrophotometer [13] and analysed using established methods [14].
(c). Spectral transmittance of the ocular media
Following removal of the posterior segment of the eye, the spectral transmittance (300–800 nm) of the combined ocular media (cornea, aqueous humour, and lens) in the anterior segment was measured along the optical axis. Light from a 175 W xenon broadband light source (Spectral Products) was delivered to the cornea via a 2 m, 200 µm diameter, quartz fibre-optic cable fitted with a quartz collimating lens. Light transmitted through the tissue fell on a Teflon diffuser placed in front of a second collimated quartz lens and was delivered to a charge-coupled device spectroradiometer (S2000; Ocean Optics) via a 2 m, 600 µm diameter, quartz fibre-optic cable. Each transmittance measurement comprised an average of 100 spectral scans. Three separate transmittance measurements were made from two eyes (taken from different emus) and averaged. The mean transmittance spectrum of each of the eyes was qualitatively similar, so both spectra were averaged together and normalized.
(d). Opsin sequences
Oligo-dT-primed retinal mRNA (2 µg) was converted to complementary DNA (cDNA) using the miScript polymerase chain reaction (PCR) Starter Kit (Qiagen) after extraction using the mirVana™ RNA Isolation Kit (Life Technologies), according to the manufacturers' protocols. Opsin coding sequences were amplified using nested PCRs and degenerate primers targeting LWS (long-wavelength-sensitive), SWS1, SWS2 (short-wavelength-sensitive-2), RH2 (rhodopsin-like-2), and RH1 (rod-opsin or rhodopsin-like-1) pigment genes (electronic supplementary material, table S1), as previously described [15]. All excised PCR products were purified using the FavorPrep Gel Purification Mini Kit (Fisher Biotech) and sequenced directly.
(e). Phylogenetic analysis
The MEGA 6 package [16] was used to generate maximum-likelihood trees from opsin amino acid sequences aligned in Clustal X [17]. Parameters used include 1 000 bootstraps, and the application of the Poisson model with uniform rates and partial deletion.
3. Results
(a). Microspectrophotometric measurements of retinal photoreceptors
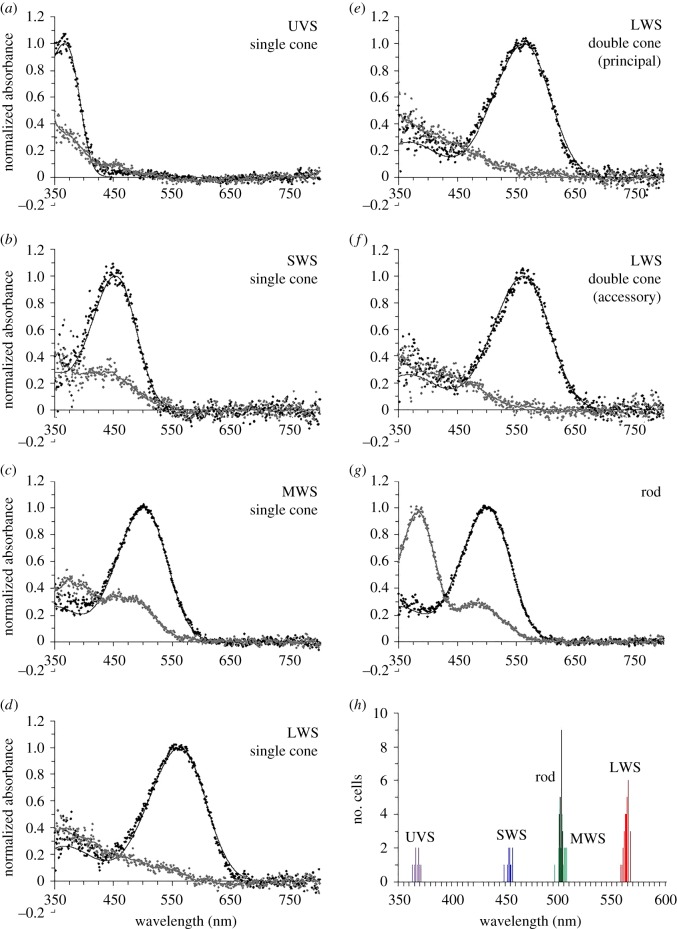
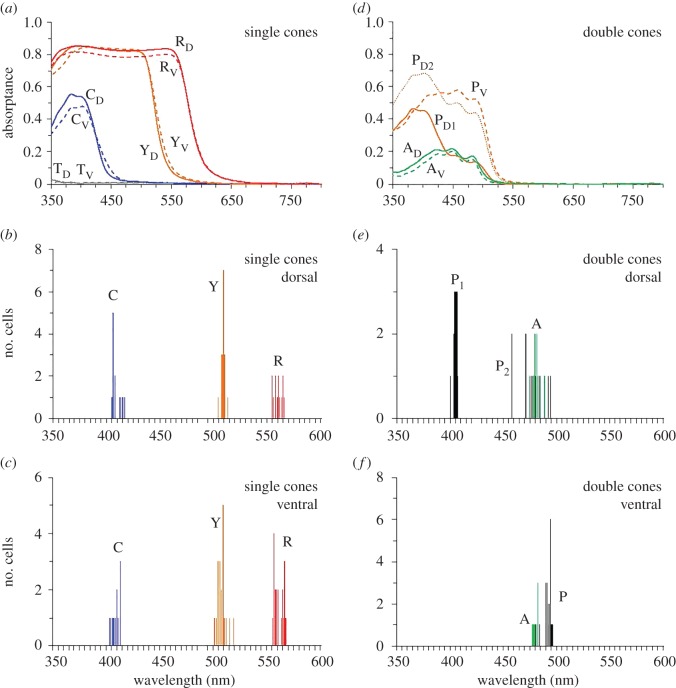
MSP data for the pigments (figure 1; electronic supplementary material, figure S1) and oil droplets (figure 2) present in emu retinal photoreceptors are summarized in the electronic supplementary material, table S2. Based on the absorbance spectra fit to published templates [18], all emu pigments contain a retinal chromophore based on vitamin A1. The retina contains a single medium-wavelength-sensitive (MWS) rod (λmax = 501 nm) that is devoid of oil droplets. There are four spectrally distinct single cones: (i) a UVS cone (λmax = 367 nm) with a transparent T-type oil droplet that has negligible absorptance between 330 and 800 nm, (ii) an SWS cone (λmax = 453 nm) with a colourless/pale-green C-type oil droplet that has a cut-off wavelength λcut (the wavelength of the intercept at the value of maximum absorptance by a line tangential to the absorptance curve at half-maximum absorptance) at 408 nm, (iii) an MWS cone (λmax = 502 nm) with a yellow Y-type oil droplet that has a λcut at 508 nm, and (iv) an LWS cone (λmax = 562 nm) with a red R-type oil droplet that has a λcut at 559 nm. An asymmetric double cone is also present, with both principal and accessory cone members containing the same pigment found in LWS single cones (λmax = 562–563 nm). Unlike the pigmented oil droplets in single cones, there is considerable variation in the λcut of the pale-green/yellow P-type oil droplet located in the double cone principal member. Two kinds of P-type oil droplet are present in the dorsal retina, with λcut values at 404 nm and 476 nm. By contrast, only a single P-type oil droplet with a λcut at 492 nm occurs in the ventral retina. The double cone accessory member is fairly uniform across the retina and does not contain an oil droplet; nonetheless, diffuse greenish-yellow pigmentation is present in the distal region of the inner segment with a λcut at 480 nm.
Figure 1.
(a–g) Normalized mean pre- (black circles) and post-bleach (grey circles) spectra of pigments measured using MSP from emu retinal photoreceptor outer segments. Pre-bleach spectra are overlayed with best-fit rhodopsin (vitamin A1) templates (black line). Post-bleach spectra are fitted with variable-point running average (grey line). (h) Histogram shows the spectral distribution of the wavelength of maximum absorbance (λmax) values for individual photoreceptor outer segments that were used to generate the mean spectra. The data for the distribution of LWS pigment λmax values includes measurements from LWS single cones, as well as both the principal and accessory members of double cones. λmax values are UVS 367 nm, SWS 453 nm, MWS 502 nm, and LWS 562 nm. UVS, ultraviolet-sensitive; SWS, short-wavelength-sensitive; MWS, medium-wavelength-sensitive; LWS long-wavelength-sensitive.
Figure 2.
Mean spectral absorptance (a,d) and spectral distribution of cut-off wavelength (λcut) values (b,c,e,f) of oil droplets or diffuse spectral filters located in the inner segments of emu single (a–c) and double (d–f) cones. T, C, Y, R, and P, refer to T-, C-, Y-, R-, and P-type oil droplets located in UVS, SWS, MWS, LWS single cones and the principal members of LWS double cones, respectively, and A refers to the diffuse A-type oil droplet-like pigment located in the distal region of the inner segment of LWS double cone accessory members. D, dorsal retina (solid/dotted lines); V, ventral retina (dashed lines). Two distinct types of P-type oil droplet were present in the dorsal retina, which are identified by (arbitrary) subscripts 1 and 2.
(b). Spectral transmittance of the ocular media
The transmittance of the combined ocular media is relatively high and uniform for wavelengths between 400 and 800 nm, but transmittance decreases sharply below 400 nm (electronic supplementary material, figure S2). The wavelength of 0.5 normalized transmittance (λT0.5) is 355 nm and the short wavelength cut-off, below which negligible light is transmitted to the retina, is approximately 335 nm.
(c). Opsin coding sequences
The coding sequences for five visual opsins were determined (GenBank Accession nos. LWS: KU568452, SWS1: KU568453, SWS2: KU568454, RH2: KU568455, RH1: KU568456) and subjected to maximum-likelihood phylogenetic analysis, which confirmed them as orthologues of the four cone (LWS, SWS1, SWS2, and RH2) and single rod (RH1) opsin genes found in vertebrates (electronic supplementary material, figures S3–S5).
(i). Short-wavelength-sensitive-1
The MSP data clearly show that SWS1-expressing single cones are UVS (λmax = 367 nm). This is consistent with the presence of Phe86 and Cys90, as each is capable of generating a UVS pigment [9,10]. The presence of these residues in the emu SWS1 opsin has been reported previously [7]; however, since the sequenced region was restricted to amino acids 80–92, the potential impact of other tuning sites could not be ruled out. The emu SWS1 sequenced region reported here now extends to 90% of the coding region (with only the first 13 residues at the N-terminus and 23 residues at the C-terminus missing), thereby enabling a more complete study of amino acid changes across avian UVS and VS SWS1 pigments (electronic supplementary material, table S3). Unique changes in the emu pigment are present at three sites 49, 93, and 118, but for sites 49 and 118, the amino acid present in the emu sequence is also found in the UVS pigment of the green anole so it is unlikely that either is uniquely involved in spectral tuning of the pigment. Met93 would, however, appear to be unique to palaeognaths [7], so a functional role in spectral tuning cannot be ruled out.
(ii). Short-wavelength-sensitive-2
A number of residues are important for the spectral tuning of SWS2 pigments [19,20]. In particular, a Thr269Ala substitution results in a 10 nm short-wavelength shift [21]. The λmax of the emu SWS2 pigment at 453 nm is similar to the majority of avian SWS pigments studied so far, including the chicken (λmax = 453 nm) and pigeon (λmax = 452 nm) [1]. In a few species, however, the λmax of the SWS2 pigment is short-wavelength shifted to approximately 440 nm (e.g. rosella [22], canary [23], and zebra finch [24]). Significantly, in the emu (electronic supplementary material, figure S4), chicken [25], and pigeon pigments [26], the residue at site 269 is polar Ser (emu and pigeon) or polar Thr (chicken), whereas it is non-polar in the rosella (Ala [22]), canary (Cys), and zebra finch (Cys) pigments [23,24]. This polar to non-polar substitution appears sufficient, therefore, to account for the spectral shifts between avian species.
(iii). Rhodopsin-like-2
Five tuning sites (49, 52, 83, 86, and 97) were identified when spectrally distinct RH2 pigments of the Tokay gecko (Gecko gecko) and the green anole (Anolis carolinensis) were compared [27]. The sequenced region of the emu RH2 opsin (λmax = 502 nm) does not extend to sites 49 and 52, but like other avian RH2 sequences, tuning sites 83, 86, and 97 are identical to those of the spectrally similar green anole pigment (λmax = 497 nm).
(iv). Long-wavelength-sensitive pigments
Spectral tuning of LWS pigments depends largely on substitutions at five sites: 164, 181, 261, 269, and 292, which when occupied by Ser, His, Tyr, Thr, and Ala, respectively, result in a pigment with a λmax at approximately 560–570 nm [28,29]. The residues at these sites in the emu LWS pigment are identical to those in other birds [22,23], with the exception of a Ser164Ala substitution. This change is predicted to cause a 7 nm short-wavelength shift in the λmax of the pigment [30], which is consistent with the spectral peak of the emu LWS pigment at 562 nm compared with that of other birds at 566–571 nm.
(v). Rhodopsin-like-1
The key tuning residues for RH1 pigments at sites 83, 122, 207, 211, 261, 265, 269, 292, and 295 have been largely identified from studies of fish RH1 gene sequences [31,32]. The only substitution across these sites in avian RH1 opsins is seen in the emu pigment, where Asn rather than Asp is found at site 83. This substitution is predicted to cause a 6 nm short-wavelength shift [28]; however, as the emu RH1 pigment (λmax = 501 nm) is spectrally almost identical to other birds (λmax = 502–503 nm) [25,26], it would appear that site 83 is not important for avian spectral tuning.
4. Discussion
The emu retina contains a similar photoreceptor complement to almost every other bird species studied to date (i.e. single rod, single double cone, and four spectrally distinct single cones) [1]. The four single cones generate a tetrachromatic visual system capable of excellent colour discrimination across the visible spectrum [33], and this is further refined by the presence of pigmented oil droplets that filter incoming light [34,35]. The only exceptions to this general format appear in some species of penguin, which have lost their MWS cone visual pigment through pseudogenization of the RH2 opsin gene [36] and appear to possess only three spectral types of single cone [37].
Molecular analysis of the emu retina confirmed the expression of a single rod and four cone opsin genes, which accounts for the observed spectral characteristics of the different cone and rod photoreceptors. MSP has demonstrated unequivocally that the emu SWS cone is UVS: this establishes the SWS1 pigment in the emu as UVS and enables a re-assessment of the evolution of UVS/VS in birds. Key tuning sites for SWS1 opsins have been identified at positions 86, 90, and 93 [9,10,38]. Opsin gene sequencing in lampreys [39], a basal vertebrate, indicates that the ancestral SWS1 pigment in vertebrates was UVS with the presence of Phe86, Ser90, and Thr93. Spectral shifts from UVS to VS have occurred several times in vertebrate SWS1 pigment evolution as a result of substitutions at only two sites: 86 and 90. For most vertebrates, site 86 is critical for UVS tuning, with a shift to VS being mediated by Phe86Met in amphibians [40], Phe86Ser in birds [41], and Phe86Tyr, Phe86Ser, or Phe86Val in mammals [38,42,43]. In birds, however, an additional key change is at site 90, with Cys in UVS and Ser in VS pigments [9,10].
A previous study proposed that the ancestral avian SWS1 pigment was VS [41], which was largely based on the reported presence of Ser86 and Ser90 in the ostrich SWS1 opsin [44]. However, a recent report [7] showed that the SWS1 opsins in a number of palaeognath birds (including the ostrich) possess Phe86 and Cys90, a result that was unexpected, as both are capable of generating UVS pigments as single substitutions [9,10,38,45]. The SWS1 pigments in these basal birds all resemble, therefore, the emu opsin, and we conclude from this that all palaeognaths possess a UVS SWS1 pigment. Since palaeognaths occupy a basal position [2], this implies that UVS pigments were present at the start of the avian radiation.
For the palaeognath SWS1 sequences determined by Aidala et al. [7], the sequenced region of the SWS1 opsin was limited to amino acids 80–92 or 93; our extended emu SWS1 sequence not only confirms the presence of Phe86 and Cys90, but demonstrates that the only unique change at a potential tuning site [46] in palaeognath SWS1 pigments is Met93 (electronic supplementary material, table S3). Substitution at this site has been implicated in the evolution of VS SWS1 pigments in primates [47], but in this case, it is Pro that is present at site 93.
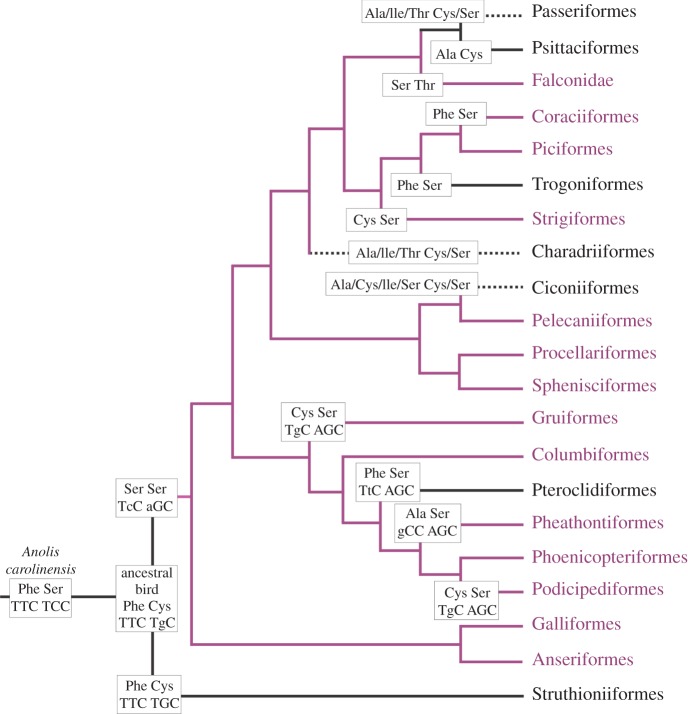
In extant diapsids (i.e. crocodiles, lizards, and snakes) that possess a common ancestor with birds, the amino acids present at sites 86 and 90 are Phe and Ala/Ser, respectively [15,48,49]. The phylogeny shown in figure 3 is based on a recent study of the relationships among 169 species of birds [2]. By superimposing residues 86 and 90 onto this phylogeny, it is evident that Phe86 is retained in the palaeognath lineage, with Ser90 replaced by Cys. By following the likely nucleotide changes for codons 86 and 90 that generated each amino acid substitution, it is apparent that just a single change in codon 90 of the SWS1 sequence is required to generate a Cys90 substitution as found in the palaeognaths, whereas in the neognaths, VS pigments arise from single changes in both codons to generate Phe86Ser and Cys90Ser substitutions. Subsequent changes in codon 86 give rise to additional changes (e.g. Ala, Cys, and Ser) in several avian orders in both the lower and upper parts of the phylogeny, including reverse mutations in the Pteroclidiformes and Trogoniformes to give Phe86 and presumably UVS pigments. It is only in the Ciconiiformes, Charadriiformes, Psittaciformes, and Passeriformes that Ser90 is frequently replaced by Cys to yield UVS pigments.
Figure 3.
Evolution of UVS/VS SWS1 pigments in birds. The ancestral states of amino acids at sites 86 and 90 are shown [2]. For the branches in the lower part of the tree, codons 86 and 90 are also shown. Nucleotide changes in these codons are designated with lower case letters. The ancestral avian pigment is derived from that of the green anole (Anolis carolinensis). Where there is inter-Order variation at these sites, all alternatives are shown. Black lines indicate UVS pigments, violet lines represent VS pigments, and dotted lines designate the presence of both UVS and VS pigments within the Order. Adapted from [8,9].
Sequencing of the emu SWS2 opsin has enabled further comparisons with the same pigment in other avian species. In all cases where the coding sequence is known, the longer wavelength shifted SWS2 pigments (including that of the emu) differ from the shorter wavelength shifted pigments at site 269, with polar Thr or Ser replaced by non-polar Cys or Ala. This site is located within helix VI in close proximity to the β-ionone ring of the chromophore and, therefore, in an ideal position to affect spectral tuning.
The peak sensitivities of avian RH2 pigments show relatively little variation, ranging from 497 nm in the rook (Corvus frugileus) to 507 nm in the chicken [1]. The emu is no exception with a λmax at 502 nm, and consistent with this, does not differ at the three known tuning sites [27] included in the sequenced emu opsin.
Avian rod (RH1) pigments likewise show relatively little variation, with peaks ranging from 501 to 509 nm [50–52]. Not surprisingly, the RH1 amino acid sequences are also highly conserved across potential tuning sites, with the emu RH1 opsin differing at just one site where Asp83 is replaced by Asn. This substitution has been implicated in relatively small spectral shifts in fish RH1 pigments [31], but does not appear to alter significantly the spectral sensitivity of emu rods. Finally, the small short-wavelength shift to 562 nm in the emu LWS pigment compared with orthologous pigments in other avian species (with λmax values between 566 and 571 nm) can be accounted for adequately by the presence of Ala164, which is one of the five LWS tuning sites identified in vertebrates [28].
Optically, the presence of a UVS rather than a VS SWS1 pigment in the emu (and most likely other palaeognaths) is perhaps surprising given the large size of their eyes. In birds, eye axial length is inversely correlated with the amount of UV light transmitted by the ocular media, which can be defined by the λT0.5 value [53]. Based on the two-term exponential relationship between λT0.5 and log10 axial length (mm) [53], the predicted λT0.5 for the emu eyes measured in this study (eye axial lengths 32–34 mm) would be approximately 360 nm, which is consistent with the measured value of 355 nm. Both emu and ostrich have λT0.5 values that are characteristic of bird species that possess VS (mean λT0.5 = 358 ± 20 nm) rather than UVS SWS1 pigments (mean λT0.5 = 323 ± 10 nm) [53]. Thus, neither eye size nor ocular media λT0.5 are totally reliable predictors of the SWS1 λmax value, although there remains a general relationship that warrants further investigation. Nonetheless, modelling in other bird species [53] suggests that, despite a λT0.5 at longer wavelengths than would be expected, based on the λmax value of the SWS1 pigment (and the consequent reduction in light transmission at wavelengths close to the peak sensitivity of the SWS1 pigment), the emu should still possess good detection and discrimination of UV wavelengths by virtue of a UVS rather than a VS SWS1 pigment.
Secondly, some of the most important optical aberrations that affect image quality in the vertebrate eye, by introducing blur (defocus) and reducing contrast (e.g. entoptic scatter [54], longitudinal [55] and transverse chromatic aberration [56]), are wavelength-dependent and increase markedly as the wavelength of light decreases. Although the actual magnitude of longitudinal chromatic aberration does not necessarily scale with eye size across species [55], its effects on image quality are theoretically worse in larger eyes (especially where the pupil is large relative to the focal length, i.e. a low f-number) as they have a shorter depth of focus (by virtue of their longer focal length) compared with small eyes [57].
In general, therefore, it might be expected that animals with larger eyes would have a more restricted range of pigment spectral sensitivities than animals with smaller eyes [58,59] and, in the case of the emu, to possess a VS rather than a UVS SWS1 pigment. Although the very largest vertebrate eyes (e.g. dichromatic elephants [43], monochromatic whales, seals [60], and sharks [13]) do contain relatively few spectrally distinct cones, this may be the result of phylogenetic inertia and/or adaptations to environmental light rather than optical factors. Indeed, many animals, including several birds, have multifocal optical systems that compensate somewhat for longitudinal chromatic aberration through concentric zones of varying refractive power [61,62]. It is interesting to note, however, that while the ostrich appears to possess a multifocal optical system, the emu has a monofocal optical system, despite an apparently similar complement of photoreceptors [62].
The functional significance of a UVS rather than a VS SWS1 pigment in palaeognaths is unclear. Generally, their plumage has a low spectral reflectance in the UV region of the spectrum, with no notable peaks compared to other spectral regions [63], and at least some of the structural and optical properties of the mostly greyish-brown plumage in the emu may be adapted for thermoregulation [64] rather than intraspecific communication. Adult emus have patches of blue skin on their head and neck that are (subjectively to humans) ‘darker’ in females than males and absent from juveniles [65], which may be important in sexual selection. The blue colouration is a non-iridescent structural colour; similar integumentary colours in other birds tend to reflect UV wavelengths [66], although this cannot be confirmed for the emu as skin reflectance spectra are unavailable. Emus also have a varied diet that includes fruits, seeds, flowers, insects, and foliage [67], and it is well established that many of these food items have high UV reflectance or characteristic UV reflectance patterns that may be used by emus (as with other birds) to detect or discriminate them against other objects [68,69].
It is also possible that the emu and other palaeognaths retain an ancestral and perhaps ‘generalist’ form of colour vision that evolved to facilitate a wide variety of visual tasks under a range of light environments. These flightless birds might not have been subjected to selection pressures of sufficient magnitude to drive a shift in the spectral range of their visual system, as appears to have occurred in birds that have subsequently evolved a VS SWS1 pigment. Future work should concentrate on identifying such selection pressures, given that a large eye size in itself does not appear to necessitate the shift in spectral sensitivity from UVS to VS.
In conclusion, therefore, we have established that members of the Palaeognathae have not only retained the ancestral UVS SWS1 pigment but also possess ocular media that will allow transmission of UV light, thereby conferring UV sensitivity in these large flightless birds.
Supplementary Material
Acknowledgements
The authors are grateful to Dr Irek Maleki for providing the emus used in this study and the staff of the UWA Biomedical Research Facility for their assistance with animal husbandry.
Ethics
All procedures were approved by the Animal Ethics Committee of the University of Western Australia.
Data accessibility
The electronic supplemental material contains the GenBank accession numbers for the new emu visual pigment sequences and their alignments with visual pigment sequences from other species, a phylogenetic tree identifying the emu sequences as orthologues, bleaching difference spectra for the emu pigments, spectral transmittance data for the emu's ocular media, the spectral absorption characteristics of retinal photoreceptors in the emu, and the amino acid differences between UVS and VS pigments at known tuning sites.
Authors' contributions
N.S.H. conducted the MSP and ocular media transmittance measurements; J.K.M. and W.I.L.D. carried out the molecular work; W.I.L.D. and D.M.H. analysed the sequencing data and generated phylogenetic trees; N.S.H., D.M.H., and W.I.L.D. generated the manuscript and S.P.C. assisted with editing; D.M.H., N.S.H., and W.I.L.D. conceived the study. All authors confirm final approval for publication.
Competing interests
There are no competing interests.
Funding
Financial support was provided by a WA Premier's Fellowship to S.P.C., the Australian Research Council (ARC) in the form of a Future Fellowship to W.I.L.D. (FT110100176), an ARC Discovery Project grant to W.I.L.D. and D.M.H. (DP140102117), and the Western Australian (WA) State Government in the form of three applied research grants to N.S.H. and S.P.C.
References
- 1.Hart NS, Hunt DM. 2007. Avian visual pigments: characteristics, spectral tuning, and evolution. Am. Nat. 169, S7–S26. ( 10.1086/510141) [DOI] [PubMed] [Google Scholar]
- 2.Hackett SJ, et al. 2008. A phylogenomic study of birds reveals their evolutionary history. Science 320, 1763–1768. ( 10.1126/science.1157704) [DOI] [PubMed] [Google Scholar]
- 3.Sillman AJ, Bolnick DA, Haynes LW, Walter AE, Loew ER. 1981. Microspectrophotometry of the photoreceptors of palaeognathous birds—the emu and tinamou. J. Comp. Physiol. 144, 271–276. ( 10.1007/BF00612558) [DOI] [Google Scholar]
- 4.Bowmaker JK. 1977. The visual pigments, oil droplets and spectral sensitivity of the pigeon. Vision Res. 17, 1129–1138. ( 10.1016/0042-6989(77)90147-X) [DOI] [PubMed] [Google Scholar]
- 5.Bowmaker JK, Knowles A. 1977. The visual pigments and oil droplets of the chicken retina. Vision Res. 17, 755–764. ( 10.1016/0042-6989(77)90117-1) [DOI] [PubMed] [Google Scholar]
- 6.Wright MW, Bowmaker JK. 2001. Retinal photoreceptors of paleognathous birds: the ostrich (Struthio camelus) and rhea (Rhea americana). Vision Res. 41, 1–12. ( 10.1016/S0042-6989(00)00227-3) [DOI] [PubMed] [Google Scholar]
- 7.Aidala Z, et al. 2012. Ultraviolet visual sensitivity in three avian lineages: paleognaths, parrots, and passerines. J. Comp. Physiol. A. 198, 495–510. ( 10.1007/s00359-012-0724-3) [DOI] [PubMed] [Google Scholar]
- 8.Odeen A, Hastad O. 2013. The phylogenetic distribution of ultraviolet sensitivity in birds. BMC Evol. Biol. 13, 36 ( 10.1186/1471-2148-13-36) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilkie SE, Robinson PR, Cronin TW, Poopalasundaram S, Bowmaker JK, Hunt DM. 2000. Spectral tuning of avian violet- and ultraviolet-sensitive visual pigments. Biochemistry 39, 7895–7901. ( 10.1021/bi992776m) [DOI] [PubMed] [Google Scholar]
- 10.Yokoyama S, Radlwimmer FB, Blow NS. 2000. Ultraviolet pigments in birds evolved from violet pigments by a single amino acid change. Proc. Natl Acad. Sci. USA 97, 7366–7371. ( 10.1073/pnas.97.13.7366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carvalho LS, Knott B, Berg ML, Bennett ATD, Hunt DM. 2011. Ultraviolet-sensitive vision in long-lived birds. Proc. R. Soc. B 278, 107–114. ( 10.1098/rspb.2010.1100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hart NS, Partridge JC, Cuthill IC. 1998. Visual pigments, oil droplets and cone photoreceptor distribution in the European starling (Sturnus vulgaris). J. Exp. Biol. 201, 1433–1446. [DOI] [PubMed] [Google Scholar]
- 13.Hart NS, Theiss SM, Harahush BK, Collin SP. 2011. Microspectrophotometric evidence for cone monochromacy in sharks. Naturwissenschaften 98, 193–201. ( 10.1007/s00114-010-0758-8) [DOI] [PubMed] [Google Scholar]
- 14.Hart NS. 2004. Microspectrophotometry of visual pigments and oil droplets in a marine bird, the wedge-tailed shearwater Puffinus pacificus: topographic variations in photoreceptor spectral characteristics. J. Exp. Biol. 207, 1229–1240. ( 10.1242/jeb.00857) [DOI] [PubMed] [Google Scholar]
- 15.Davies WL, Cowing JA, Bowmaker JK, Carvalho LS, Gower DJ, Hunt DM. 2009. Shedding light on serpent sight: the visual pigments of henophidian snakes. J. Neurosci. 29, 7519–7525. ( 10.1523/JNEUROSCI.0517-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729. ( 10.1093/molbev/mst197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larkin MA, et al. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948. ( 10.1093/bioinformatics/btm404) [DOI] [PubMed] [Google Scholar]
- 18.Govardovskii VI, Fyhrquist N, Reuter T, Kuzmin DG, Donner K. 2000. In search of the visual pigment template. Vis. Neurosci. 17, 509–528. ( 10.1017/S0952523800174036) [DOI] [PubMed] [Google Scholar]
- 19.Chinen A, Matsumoto Y, Kawamura S. 2005. Spectral differentiation of blue opsins between phylogenetically close but ecologically distant goldfish and zebrafish. J. Biol. Chem. 280, 9460–9466. ( 10.1074/jbc.M413001200) [DOI] [PubMed] [Google Scholar]
- 20.Yokoyama S, Tada T. 2003. The spectral tuning in the short wavelength-sensitive type 2 pigments. Gene 306, 91–98. ( 10.1016/S0378-1119(03)00424-4) [DOI] [PubMed] [Google Scholar]
- 21.Cowing JA, Poopalasundaram S, Wilkie SE, Bowmaker JK, Hunt DM. 2002. Spectral tuning and evolution of short wave-sensitive cone pigments in cottoid fish from Lake Baikal. Biochemistry 41, 6019–6025. ( 10.1021/bi025656e) [DOI] [PubMed] [Google Scholar]
- 22.Knott B, et al. 2013. How parrots see their colours: novelty in the visual pigments of Platycercus elegans. J. Exp. Biol. 216, 4454–4461. ( 10.1242/jeb.094136) [DOI] [PubMed] [Google Scholar]
- 23.Das D, Wilkie SE, Hunt DM, Bowmaker JK. 1999. Visual pigments and oil droplets in the retina of a passerine bird, the canary Serinus canaria: microspectrophotometry and opsin sequences. Vision Res. 39, 2801–2815. ( 10.1016/S0042-6989(99)00023-1) [DOI] [PubMed] [Google Scholar]
- 24.Yokoyama S, Blow NS, Radlwimmer FB. 2000. Molecular evolution of color vision of zebra finch. Gene 259, 17–24. ( 10.1016/S0378-1119(00)00435-2) [DOI] [PubMed] [Google Scholar]
- 25.Okano T, Kojima D, Fukada Y, Shichida Y, Yoshizawa T. 1992. Primary structures of chicken cone visual pigments: vertebrate rhodopsins have evolved out of cone visual pigments. Proc. Natl Acad. Sci. USA 89, 5932–5936. ( 10.1073/pnas.89.13.5932) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawamura S, Blow NS, Yokoyama S. 1999. Genetic analyses of visual pigments of the pigeon (Columba livia). Genetics 153, 1839–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takenaka N, Yokoyama S. 2007. Mechanisms of spectral tuning in the RH2 pigments of Tokay gecko and American chameleon. Gene 399, 26–32. ( 10.1016/j.gene.2007.04.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yokoyama S. 2000. Molecular evolution of vertebrate visual pigments. Prog. Retin. Eye Res. 19, 385–419. ( 10.1016/S1350-9462(00)00002-1) [DOI] [PubMed] [Google Scholar]
- 29.Davies WI, Collin SP, Hunt DM. 2012. Molecular ecology and adaptation of visual photopigments in craniates. Mol. Ecol. 21, 3121–3158. ( 10.1111/j.1365-294X.2012.05617.x) [DOI] [PubMed] [Google Scholar]
- 30.Merbs SL, Nathans J. 1992. Absorption spectra of human cone pigments. Nature 356, 433–435. ( 10.1038/356433a0) [DOI] [PubMed] [Google Scholar]
- 31.Hunt DM, et al. 2001. The molecular basis for spectral tuning of rod visual pigments in deep-sea fish. J. Exp. Biol. 204, 3333–3344. [DOI] [PubMed] [Google Scholar]
- 32.Hunt DM, Fitzgibbon J, Slobodyanyuk SJ, Bowmakers JK. 1996. Spectral tuning and molecular evolution of rod visual pigments in the species flock of cottoid fish in Lake Baikal. Vision Res. 36, 1217–1224. ( 10.1016/0042-6989(95)00228-6) [DOI] [PubMed] [Google Scholar]
- 33.Goldsmith TH, Butler BK. 2005. Color vision of the budgerigar (Melopsittacus undulatus): hue matches, tetrachromacy, and intensity discrimination. J. Comp. Physiol. A 191, 933–951. ( 10.1007/s00359-005-0024-2) [DOI] [PubMed] [Google Scholar]
- 34.Govardovskii VI. 1983. On the role of oil drops in colour vision. Vision Res. 23, 1739–1740. ( 10.1016/0042-6989(83)90192-X) [DOI] [PubMed] [Google Scholar]
- 35.Vorobyev M, Osorio D, Bennett ATD, Marshall NJ, Cuthill IC. 1998. Tetrachromacy, oil droplets and bird plumage colours. J. Comp. Physiol. A 183, 621–633. ( 10.1007/s003590050286) [DOI] [PubMed] [Google Scholar]
- 36.Zhang G, et al. 2014. Comparative genomics reveals insights into avian genome evolution and adaptation. Science 346, 1311–1320. ( 10.1126/science.1251385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bowmaker JK, Martin GR. 1985. Visual pigments and oil droplets in the penguin, Spheniscus humboldti. J. Comp. Physiol. A 156, 71–77. ( 10.1007/BF00610668) [DOI] [Google Scholar]
- 38.Cowing JA, Poopalasundaram S, Wilkie SE, Robinson PR, Bowmaker JK, Hunt DM. 2002. The molecular mechanism for the spectral shifts between vertebrate ultraviolet- and violet-sensitive cone visual pigments. Biochem. J. 367, 129–135. ( 10.1042/bj20020483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collin SP, Knight MA, Davies WL, Potter IC, Hunt DM, Trezise AEO. 2003. Ancient colour vision: multiple opsin genes in the ancestral vertebrates. Curr. Biol. 13, R864–R865. ( 10.1016/j.cub.2003.10.044) [DOI] [PubMed] [Google Scholar]
- 40.Starace DM, Knox BE. 1998. Cloning and expression of a Xenopus short wavelength cone pigment. Exp. Eye Res. 67, 209–220. ( 10.1006/exer.1998.0507) [DOI] [PubMed] [Google Scholar]
- 41.Carvalho LS, Cowing JA, Wilkie SE, Bowmaker JK, Hunt DM. 2007. The molecular evolution of avian ultraviolet- and violet-sensitive visual pigments. Mol. Biol. Evol. 24, 1843–1852. ( 10.1093/molbev/msm109) [DOI] [PubMed] [Google Scholar]
- 42.Parry JWL, Poopalasundaram S, Bowmaker JK, Hunt DM. 2004. A novel amino acid substitution is responsible for spectral tuning in a rodent violet-sensitive visual pigment. Biochemistry 43, 8014–8020. ( 10.1021/bi049478w) [DOI] [PubMed] [Google Scholar]
- 43.Yokoyama S, et al. 2005. Elephants and human color-blind deuteranopes have identical sets of visual pigments. Genetics 170, 335–344. ( 10.1534/genetics.104.039511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ödeen A, Håstad O. 2003. Complex distribution of avian color vision systems revealed by sequencing the SWS1 opsin from total DNA. Mol. Biol. Evol. 20, 855–861. ( 10.1093/molbev/msg108) [DOI] [PubMed] [Google Scholar]
- 45.Fasick JI, Applebury ML, Oprian DD. 2002. Spectral tuning in the mammalian short-wavelength sensitive cone pigments. Biochemistry 41, 6860–6865. ( 10.1021/bi0200413) [DOI] [PubMed] [Google Scholar]
- 46.Yokoyama S, Shi Y. 2000. Genetics and evolution of ultraviolet vision in vertebrates. FEBS Lett. 486, 167–172. ( 10.1016/S0014-5793(00)02269-9) [DOI] [PubMed] [Google Scholar]
- 47.Carvalho LS, Davies WL, Robinson PR, Hunt DM. 2012. Spectral tuning and evolution of primate short-wavelength-sensitive visual pigments. Proc. R. Soc. B 279, 387–393. ( 10.1098/rspb.2011.0782) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawamura S, Yokoyama S. 1996. Phylogenetic relationships among short wavelength-sensitive opsins of American chameleon (Anolis carolinensis) and other vertebrates. Vision Res. 36, 2797–2804. ( 10.1016/0042-6989(96)00034-X) [DOI] [PubMed] [Google Scholar]
- 49.Yokoyama S, Blow NS. 2001. Molecular evolution of the cone visual pigments in the pure rod-retina of the nocturnal gecko, Gekko gekko. Gene 276, 117–125. ( 10.1016/S0378-1119(01)00643-6) [DOI] [PubMed] [Google Scholar]
- 50.Bowmaker JK, Heath LA, Wilkie SE, Hunt DM. 1997. Visual pigments and oil droplets from six classes of photoreceptor in the retinas of birds. Vision Res. 37, 2183–2194. ( 10.1016/S0042-6989(97)00026-6) [DOI] [PubMed] [Google Scholar]
- 51.Hart NS, Partridge JC, Bennett ATD, Cuthill IC. 2000. Visual pigments, cone oil droplets and ocular media in four species of estrildid finch. J. Comp. Physiol. A 186, 681–694. ( 10.1007/s003590000121) [DOI] [PubMed] [Google Scholar]
- 52.Maier EJ, Bowmaker JK. 1993. Colour vision in the passeriform bird, Leiothrix lutea: correlation of visual pigment absorbance and oil droplet transmission with spectral sensitivity. J. Comp. Physiol. A 172, 295–301. ( 10.1007/BF00216611) [DOI] [Google Scholar]
- 53.Lind O, Mitkus M, Olsson P, Kelber A. 2014. Ultraviolet vision in birds: the importance of transparent eye media. Proc. R. Soc. B 281, 20132209 ( 10.1098/rspb.2013.2209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coppens JE, Franssen L, van den Berg TJ. 2006. Wavelength dependence of intraocular straylight. Exp. Eye Res. 82, 688–692. ( 10.1016/j.exer.2005.09.007) [DOI] [PubMed] [Google Scholar]
- 55.Mandelman T, Sivak JG. 1983. Longitudinal chromatic aberration of the vertebrate eye. Vision Res. 23, 1555–1559. ( 10.1016/0042-6989(83)90169-4) [DOI] [PubMed] [Google Scholar]
- 56.Thibos LN, Bradley A, Still DL, Zhang X, Howarth PA. 1990. Theory and measurement of ocular chromatic aberration. Vision Res. 30, 33–49. ( 10.1016/0042-6989(90)90126-6) [DOI] [PubMed] [Google Scholar]
- 57.Green DG, Powers MK, Banks MS. 1980. Depth of focus, eye size and visual acuity. Vision Res. 20, 827–835. ( 10.1016/0042-6989(80)90063-2) [DOI] [PubMed] [Google Scholar]
- 58.Kröger RHH. 2000. Optical and developmental constraints on colour vision with lens eyes. J. Opt. A 2, R39 ( 10.1088/1464-4258/2/6/203) [DOI] [Google Scholar]
- 59.Rucker FJ, Osorio D. 2008. The effects of longitudinal chromatic aberration and a shift in the peak of the middle-wavelength sensitive cone fundamental on cone contrast. Vision Res. 48, 1929–1939. ( 10.1016/j.visres.2008.06.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Newman LA, Robinson PR. 2005. Cone visual pigments of aquatic mammals. Vis. Neurosci. 22, 873–879. ( 10.1017/S0952523805226159) [DOI] [PubMed] [Google Scholar]
- 61.Kröger RHH, Fernald RD, Wagner H-J. 1999. Multifocal lenses compensate for chromatic defocus in vertebrate eyes. J. Comp. Physiol. A 184, 361–369. ( 10.1007/s003590050335) [DOI] [PubMed] [Google Scholar]
- 62.Lind OE, Kelber A, Kröger RHH. 2008. Multifocal optical systems and pupil dynamics in birds. J. Exp. Biol. 211, 2752–2758. ( 10.1242/jeb.018630) [DOI] [PubMed] [Google Scholar]
- 63.Mullen P, Pohland G. 2008. Studies on UV reflection in feathers of some 1 000 bird species: are UV peaks in feathers correlated with violet-sensitive and ultraviolet-sensitive cones? Ibis 150, 59–68. ( 10.1111/j.1474-919X.2007.00736.x) [DOI] [Google Scholar]
- 64.Maloney SK, Dawson TJ. 1995. The heat load from solar radiation on a large, diurnally active bird, the emu (Dromaius novaehollandiae). J. Therm. Biol. 20, 381–387. ( 10.1016/0306-4565(94)00073-R) [DOI] [Google Scholar]
- 65.Schodde R, Tidemann SC. 1997. Reader's digest complete book of Australian birds. Sydney, Australia: Reader's Digest (Australia) Pty Ltd. [Google Scholar]
- 66.Prum RO, Torres R. 2003. Structural colouration of avian skin: convergent evolution of coherently scattering dermal collagen arrays. J. Exp. Biol. 206, 2409–2429. ( 10.1242/jeb.00431) [DOI] [PubMed] [Google Scholar]
- 67.Davies SJJF. 1978. The food of emus. Aust. J. Ecol. 3, 411–422. ( 10.1111/j.1442-9993.1978.tb01189.x) [DOI] [Google Scholar]
- 68.Burkhardt D. 1982. Birds, berries and UV. Naturwissenschaften 69, 153–157. ( 10.1007/BF00364887) [DOI] [PubMed] [Google Scholar]
- 69.Stobbe N, Dimitrova M, Merilaita S, Schaefer HM. 2009. Chromaticity in the UV/blue range facilitates the search for achromatically background-matching prey in birds. Phil. Trans. R. Soc. B 364, 511–517. ( 10.1098/rstb.2008.0248) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The electronic supplemental material contains the GenBank accession numbers for the new emu visual pigment sequences and their alignments with visual pigment sequences from other species, a phylogenetic tree identifying the emu sequences as orthologues, bleaching difference spectra for the emu pigments, spectral transmittance data for the emu's ocular media, the spectral absorption characteristics of retinal photoreceptors in the emu, and the amino acid differences between UVS and VS pigments at known tuning sites.