Abstract
Congenital deafness causes large changes in the auditory cortex structure and function, such that without early childhood cochlear-implant, profoundly deaf children do not develop intact, high-level, auditory functions. But how is auditory cortex organization affected by congenital, prelingual, and long standing deafness? Does the large-scale topographical organization of the auditory cortex develop in people deaf from birth? And is it retained despite cross-modal plasticity? We identified, using fMRI, topographic tonotopy-based functional connectivity (FC) structure in humans in the core auditory cortex, its extending tonotopic gradients in the belt and even beyond that. These regions show similar FC structure in the congenitally deaf throughout the auditory cortex, including in the language areas. The topographic FC pattern can be identified reliably in the vast majority of the deaf, at the single subject level, despite the absence of hearing-aid use and poor oral language skills. These findings suggest that large-scale tonotopic-based FC does not require sensory experience to develop, and is retained despite life-long auditory deprivation and cross-modal plasticity. Furthermore, as the topographic FC is retained to varying degrees among the deaf subjects, it may serve to predict the potential for auditory rehabilitation using cochlear implants in individual subjects.
Audition is an important sensory modality for communication. Hearing impairment, a relatively frequent condition1, results in significant limitation to everyday life. In the recent decades cochlear implants (CI) have developed to a level that they can be used to alleviate even congenital or early-age profound hearing loss. However, CI success is restricted by brain developmental critical periods2,3, in that it has a limited time-window for its application. If implantation does not occur very early in life, deaf children do not successfully develop complex auditory skills, and suffer from impaired language processing4,5,6.
This malfunction appears to depend on two factors: the recruitment of the auditory cortex, in the absence of auditory input, for non-auditory functions, such as visual motion processing and sign language7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22, and the dependence of auditory system development on intact sensory auditory experience during development2,5,12. It was also shown that the level of cross-modal plastic recruitment of the auditory cortex in deaf people can predict how much they may benefit from auditory restoration using cochlear implants23,24,25, creating a direct link between cross-modal plasticity and auditory functional retention. Thus, evaluating the recruitment of the auditory cortex during visual tasks using fMRI may be of prognostic medical value. But what is known about the second factor affecting auditory restoration, the genuine role of the auditory cortex and its retained development in the absence of auditory experience?
Although much in-depth research has been conducted in various animal models of deafness, mainly focusing on subcortical auditory nuclei and pathways (reviewed in refs 2 and 12), and showing mixed effects of cross-modal plasticity of visual and somatosensory origin and retained organization26,27,28,29,30, the auditory system of humans bears critical differences from that of other mammals, due to the effects of language processing (e.g.31). Indeed, processing sign language is one of the important causes of cross-modal plasticity in the auditory cortex of deaf people19,32,33,34,35,36. Despite this important difference, the study of auditory organization in deaf humans is more limited, in part due to methodological difficulties. Magnets are commonly used to attach the CI parts, hindering the use of noninvasive measures of fMRI or MEG functional imaging of auditory activity in individuals with CIs. In the absence of a CI, it is impossible to investigate auditory functional organization since there is no way to (non-invasively) provide auditory stimulation to the deaf. Thus, it has proven difficult to map the auditory cortex of the deaf even with regard to its most basic large-scale property, topographic tonotopic organization, which characterizes most processing levels in the auditory pathways37,38.
However, in recent years it has become apparent that functional brain organization can also be investigated without external sensory stimulation. Functional connectivity MRI (fcMRI;39), based on intrinsic slow (<0.1 Hz) BOLD fluctuations in the absence of a task, shows similar spatial patterns to those evident during performance of a task40,41,42,43(though dynamic alterations are also present;44,45). Furthermore, fcMRI is highly correlated with underlying structural connectivity (a combination of direct and indirect polysynaptic connectivity;46,47,48), and may also be used to study brain topography49,50,51. Importantly, resting-state functional connectivity also correlates with functional and structural anatomy changes due to brain plasticity and usage52,53, reflecting ongoing developmental changes in network engagement54,55. Thus, functional connectivity is a sensitive measure to investigate both stable anatomical connectivity as well as plastic changes through life, in normal conditions, atypical development, and disease.
This method allows us to investigate the auditory-based topographic (cochleotopic/tonotopic) network organization of the auditory cortex of the deaf (without CI or auditory experience) even in the absence of auditory stimulation. If auditory experience during postnatal critical periods is necessary for cortex topographical patterns to emerge, or if life-long cross-modal plasticity greatly affects the organization and connectivity patterns of the cortex in the deaf, then topographical fcMRI organization should not be found in the deaf. However, if prenatal factors (which do not necessitate auditory experience) are sufficient for the emergence of tonotopic gradients, and if cross-modal experience throughout life does not cause these to degenerate, then it may be possible to find traces of functional connectivity (FC) replicating tonotopic gradients, even in congenital deafness.
Results
Functional connectivity was computed for a-priori defined seed regions-of-interest (ROIs) located in the core auditory cortex (approximately at the location of A1) using a separate phase-encoded task-based tonotopic mapping localizer (Fig. 1A,B;56) in a separate group of normally hearing controls (n = 10). ROIs were defined in a low-frequency (LF) peak in posterior medial Heschl’s gyrus and in a high frequency (HF) peak in posterior Heschl’s sulcus (Fig. 1C). These seed ROIs were used to compute partial correlation mapping57,58, minimizing the common (correlated) components between the two seeds. This maximizes the differentiation between connectivity patterns of areas processing high-frequency tones and those processing low-frequency tones within A1, and enables a focused investigation of the topographical FC pattern, which would, in a normally hearing brain, correspond to tonotopic gradients. We used this approach to conduct several analyses, including group random-effect GLM analyses (Fig. 1; for each group separately and for their comparison), multivariate analysis of group differences, single subject analyses and across-subject consistency (Fig. 2), quantification of the similarity between tonotopic gradients and the FC patterns, as well as a quantification of the variance between the single subjects’ patterns in the deaf group and its similarity to the hearing group.
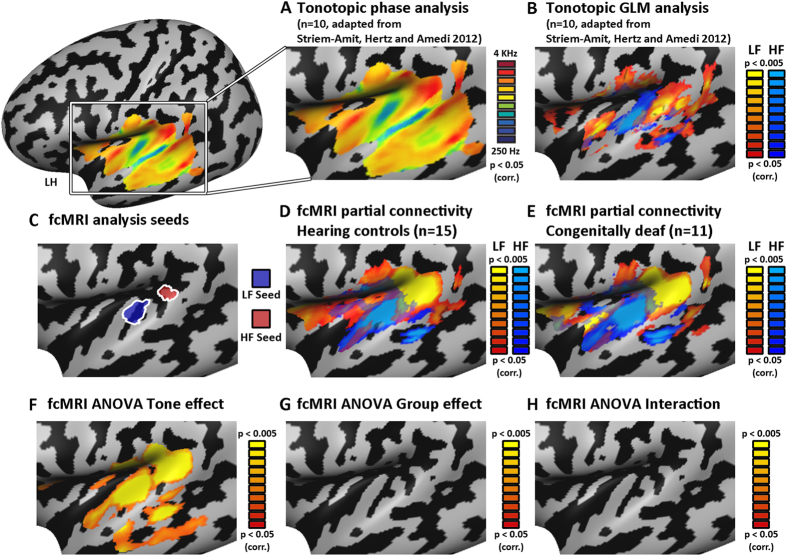
Figure 1. Topographic gradients in fcMRI of the deaf auditory cortex mimic tonotopic organization.
(A) Tonotopic gradients are presented on a left inflated cortical hemisphere, and cover a large extent of the temporal lobe when mapped directly using auditory stimuli analyzed with phase-encoding analyses in normally hearing subjects (adapted with permission from56). (B) Tonotopic peaks are identifiable using a direct binary contrast (HF > LF and vice-versa) in task-based tonotopy experiment. Both the core auditory gradient of HF-LF-HF along the superior temporal plane as well as the belt LF lateral band and STG-STS LF peaks are recognizable in this contrast (see peak marking in Figure S1A,B). (C) For fcMRI analysis, seeds corresponding to high-frequency (HF; marked red) and low-frequency (LF; marked blue) peaks in the core auditory cortex were chosen. These seeds were used in a partial-correlation mapping minimizing the common components between the two seeds, illuminating differential FC preferences for LF- and HF-preferring regions. (D) fcMRI analysis of the hearing control group (random-effect GLM analysis) reveals the topographical organization of functional-connectivity which mimics the tonotopic-organization within the auditory core, belt and beyond. Spatial-similarity-analysis quantitatively supports the significant similarity between the tonotopic-gradients and the FC topographic maps (see results). (E) fcMRI analysis (random-effect GLM analysis) reveals topographical organization in the auditory cortex of the congenitally deaf group, which greatly resembles the tonotopic patterns, extending from the core auditory cortex to speech/voice regions and beyond them. For marking of the comparable peaks between FC and tonotopy and across the groups see Figure S1. A spatial-similarity-analysis quantitatively supports the significant similarity between the tonotopic gradients and the FC topographic maps (see results). This suggests these topographical patterns develop regardless of auditory experience. (F–H) Analysis of variance (ANOVA) of the two groups shows that while most of the auditory cortex of both groups is selective for one seed over the other (F, seed/tone effect; see also MGN; Figure S2), it shows no significant group effect (G) or group X seed interaction (H), suggesting that the FC large-scale topographical patterns are identical across groups. For effects of group and interaction outside the auditory cortex see Figure S8, Tables S1,2.
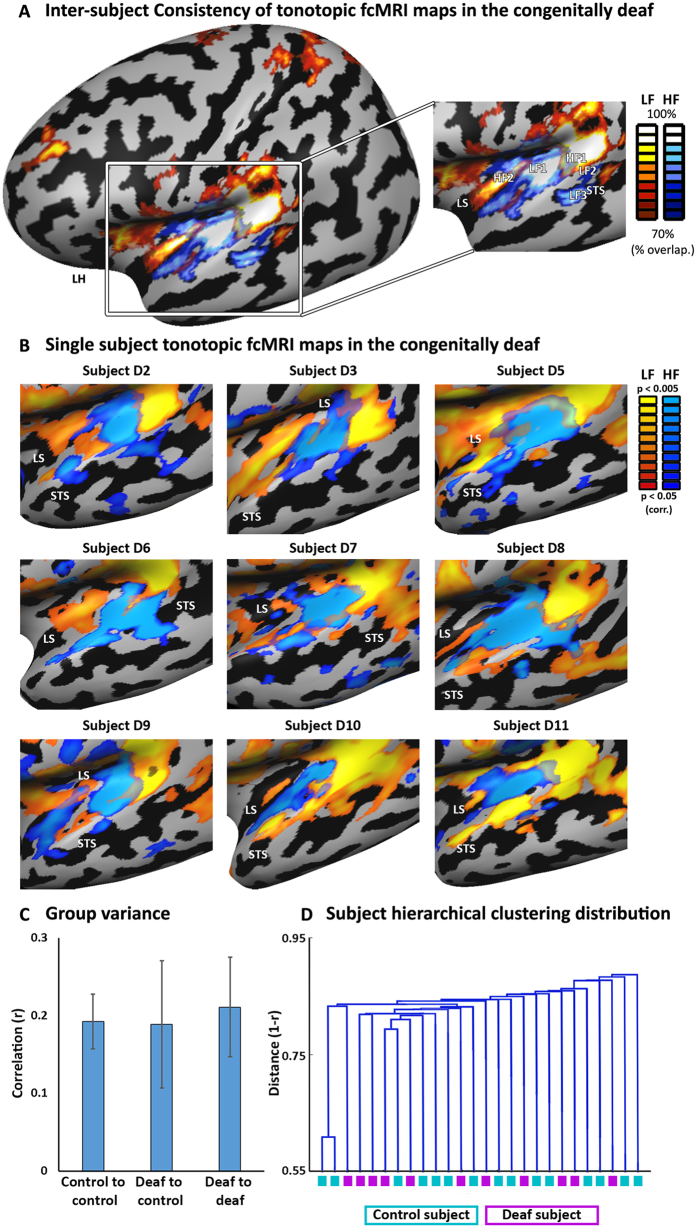
Figure 2. Reproducibility of tonotopic fcMRI maps in the single-subject level.
(A) All single-subject maps for each of the two seeds (each at p < 0.05, corrected) are overlaid and the probability of each voxel to be activated across the subjects is calculated. The resulting map depicts the high inter-subject consistency of the topographical patterns in the deaf. The deaf FC consistently shows not only the core-belt pattern of HF-LF-HF along the temporal plane, but also the posterior-lateral LF band potentially corresponding to speech/voice sensitive regions71,72,73, as well as the more lateral-inferior LF region in the STG-STS. LS – lateral sulcus, STS – superior temporal sulcus. (B) Topographical tonotopic-like fcMRI bands of the core and belt, including those of speech-sensitive regions, can be seen at the single subject level in many of the congenitally deaf individuals. Subjects D2, D3, D5 and D6 have never attempted to use hearing-aids (see Table 1 for all the deaf participant characteristics). (C) The correlation of the single-subject maps to the leave-one-out group average showed similar variability of subjects FC patterns within and between groups, suggesting again the absence of significant group differences. (D) A dendrogram showing the clustering of the subjects based on their FC pattern correlation distances, as computed by a data-driven independent hierarchical clustering analysis. The deaf and hearing controls are intermixed in the clustering, and no clear group distinction can be made.
Group tonotopic organization fcMRI analysis
Resting-state fcMRI random-effect GLM analysis in the hearing control group replicated to a large extent the large-scale tonotopic preference of the core auditory cortex. The tone preference of the core is comprised of topographical mapping pattern of tone-preference shift from high-frequency tones to low-frequency tones and back along the superior temporal plane with Heschl’s gyrus (HG) located within this mirror-symmetric large scale organization38,56,59,60,61,62,63,64,65,66,67,68,69, approximately marking the border between core regions A1 and R69. This is exactly the pattern found also in the FC partial correlation, such that an additional FC-HF preference was observed anteriorly to the FC-LF peak (Fig. 1D; for marking of the peaks see Figure S1D; core peaks are marked as HF1, LF1 and HF2). The increased connectivity between the two HF peaks, anterior and posterior to HG was previously shown in diffusion based anatomical tractography analysis70, forming the anatomical basis for this fcMRI finding.
Beyond the auditory core, tonotopic gradients are harder to identify using only a binary comparison, even for task-based tonotopic experiments. Specifically, when contrasting HF and LF tones in the original tonotopic experiment used to define the seed ROIs (Fig. 1B;56), only a partial pattern of the tonotopic organization can be observed beyond the core. This includes the peak of the lateral LF tone band, possibly reflecting the human homologue of area CL in the macaque (peak LF2 in Figure S1A,B;56,59,67,69) or corresponding to speech/voice sensitive regions71,72,73, shown to prefer low-frequency sounds38,73. Additionally, there is evidence for a HF peak and more dominantly a LF peak in the STG and STS (peaks HF3 and LF3 in Figure S1A,B; potentially reflecting auditory parabelt;56). Importantly, evidence for all these peaks can be seen in the fcMRI analysis of the hearing control group (Fig. 1D; Figure S1D), supporting our use of this analysis for the investigation of tonotopic FC organization.
We then investigated if such tonotopic-based FC patterns can also be found in people profoundly deaf since birth. Using the same seeds ROIs as in the hearing group, a similar pattern of FC can be seen, including both core as well as extra-core peaks (Fig. 1E; for marked peaks see Figure S1E). The tonotopic-like FC patterns extended all the way to the belt and parabelt regions, including the language areas such as the auditory word-form area in the left anterior STG74 and generally the speech/voice sensitive regions71,72,73. Furthermore, although our current dataset may not be of sufficiently high resolution to reveal gradients within the medial geniculate nucleus (MGN), some MGN FC preference was observed in both the deaf and the hearing (Figure S2A,B), suggesting such patterns can be explored further in the future. The cortical topographical fcMRI patterns were also replicated using cortex-based surface alignment rather than volume alignment (see Figure S3; see also single subject data on individual brains; Fig. 2, and unsmoothed data; Figure S4), and applying spatial smoothing only at the surface level (see Figure S5) controlling for any potential spatial coregistration and smoothing artifacts, and confirming the reliability of the findings. Importantly, the observed topographic patterns do not merely depend on spatial local correlations, in which FC changes with the physical distance between the paired areas due to the spatial smoothing and normalization of fMRI data, the spatial properties of the BOLD signal75 and generic local and global cortical effects over the cortex47,76,77. This can be observed clearly from the band pattern of the superior temporal plane: as one moves anteriorly from the left hemisphere HF seed, FC first decreases and then increases again (see plot of FC values within and across hemisphere; Figure S6), in a consistent pattern in both hemispheres, according to the typical HF-LF-HF tonotopic organization of these regions in the hearing.
To quantitatively assess the spatial similarity between the tonotopic selectivity map (resulting from the auditory tonotopic localizer) and the FC selectivity map, we computed the concordance correlation coefficient for each group (CCC; see78). Significant concordance values were observed for both groups (CCC > 0.23 and CCC > 0.30 for hearing and deaf groups, P < 0.0001 for both), supporting the qualitative similarity between the FC preference and tonotopic organization in both the hearing and the deaf (Fig. 1). Thus, topographical tonotopic-like functional connectivity patterns can arise in the absence of auditory experience.
Does deafness cause any substantial changes in FC-based large-scale topographical maps? We applied several analyses to address this question. In order to investigate the potential differences in FC patterns between the groups at the voxel level, we computed an ANOVA model comprised of a preferred seed (within subject; LF seed and HF seed) effect and a group effect. The preferred seed effect showed that most of the left auditory cortex was indeed FC seed-selective (Fig. 1F; as well as the auditory thalamus; Figure S2C), as would be expected in tonotopic areas. Interestingly, although differences between the groups were evident in other parts of the brain (e.g. right anterior insula, dorsolateral prefrontal cortex, see Figures S7, S8 and Table S1), no group effect or interaction (preferred seed X group) were evident in the auditory cortex (Fig. 1G,H). However, when applying a more permissive correction for multiple comparisons, including only the left temporal auditory cortex, a small cluster showing interaction between seed X group effects was found in the medial auditory cortex (Figure S5H), possibly in the core or medial belt. Additionally, a highly significant interaction was seen in the thalamus at the location of the right medial geniculate nucleus (MGN; see Figure S2D), potentially suggesting some effect of FC plasticity.
We additionally conducted a multivariate classifier searchlight analysis (MVPA) to account for any areas whose internal organization or pattern discriminates deaf from hearing. An fMRI searchlight for brain regions discerning FC maps of deaf and control subjects found no significant clusters within the temporal auditory cortex. Therefore, multivariate analyses did not reveal any group differences in FC-based large-scale topographical maps. To compare the overall pattern of network FC, we quantitatively compared the spatial similarity of the maps of the deaf group to those of the hearing controls, using the CCC measure between the group FC maps. Highly significant concordance values were observed for both seed FC-topographic division maps (CCC > 0.69 and 0.75 for LF and HF seeds, CCC > 0.76 for effect size; contrast maps between LF and HF FC, P < 0.0001 for all). In sum, the auditory tonotopic-like functional connectivity networks of the deaf were comparable, across several analyses, to those of the controls.
Cross-subject consistency of tonotopic patterns in the deaf
How reliable and consistent are such FC topographic maps in the deaf? To study this, we examined the patterns of organization emerging in single subjects, as well as across all the subjects (Fig. 2). We computed the FC statistical parametric map of the two seeds in each of the subjects and plotted the cross-subject overlap probability map over all the individual subjects in the deaf group (Fig. 2A). The topographic mapping FC gradients of the auditory core and belt were replicated across a large majority of the subjects (Fig. 2A; the probability map minimal threshold is overlap across 7 of the 11 subjects, but in most of the map the overlap is substantially higher).
Importantly, FC-tonotopic-patterned maps were found at the individual deaf participant level in most subjects (Fig. 2B). Intact FC tonotopic-like patterns were found regardless of the etiology of the tested participant (in participants with hereditary or prenatally-acquired deafness; see Table 1) and regardless of the experience with hearing-aid use (only five of the eleven subjects had ever attempted to use hearing aids, but did not find them helpful; see tonotopic-FC maps of four subjects naïve to hearing-aid use in Fig. 2B). However, the topographic-FC patterns were not found in all subjects, and some variability was also seen in the concordance correlation coefficient between the deaf single-subject maps and group map of the controls (CCC values varied between 0.33 and 0.64 in D4 and D11 respectively).
Table 1. Characteristics of the deaf participants.
| Subject | Age | Gender | Hearing loss age | Deafness etiology | First language | Hearing aid history1 | Hearing ability with aids2 |
|---|---|---|---|---|---|---|---|
| D1 | 21 | F | 0 | Hereditary deafness | Sign | 0 | |
| D2 | 21 | F | 0 | Hereditary deafness | Sign | 0 | |
| D3 | 21 | F | 0 | Maternal disease/drug side effect during pregnancy | Sign | 0 | |
| D4 | 20 | F | 0 | Maternal disease/drug side effect during pregnancy | Sign | 0 | |
| D5 | 20 | F | 0 | Maternal disease/drug side effect during pregnancy | Sign | 0 | |
| D6 | 19 | F | 0 | Ototoxicity | Sign | 0 | |
| D7 | 19 | F | 0 | Dystocia | Oral | 1 | 1 |
| D8 | 18 | M | 0 | Maternal disease/drug side effect during pregnancy | Sign | 1 | 1 |
| D9 | 16 | F | 0 | Maternal disease/drug side effect during pregnancy | Sign & oral | 1 | 1 |
| D10 | 20 | F | 0 | Unknown | Sign & oral | 1 | 1 |
| D11 | 20 | F | 0 | Ototoxicity | Sign | 1 | 1 |
1Hearing aid history:
2. Using now
1. Used in the past
0. Never used
2Hearing ability with aid:
1. Hears something, but difficult to find out where it comes from
2. Distinguishes human voice, but can’t understand what people say
3. Hears and understands what people say well.
Does the absence of auditory inputs create less consistent maps than those of the controls? The variance of the single subject maps between the groups was also computed by applying a leave-one-out analyses to calculate the correlation between the average map (FC maps, z-normalized effect size; HF-FC minus LF-FC average of n-1 subjects) and each subject, and was found to be similar to the variance within each group (Fig. 2C). Additionally, we conducted a hierarchical clustering analysis of the FC maps, computing a dendrogram of the Euclidean distances between all the maps (Fig. 2D). The deaf and hearing subjects were completely distributed across the distance dendrogram, with the most dissimilar outlier subjects belonging to the control group. Therefore, the data-driven clustering algorithm could not discern the deaf and hearing subjects. These analyses suggest that in addition to the overall similarity in average response in each group, the deaf variability in tonotopically-informed FC maps is well within that of the control subjects.
Cross-hemispheric FC connectivity
How clear are the FC-tonotopic patterns across the hemispheres? It seems that while the auditory core shows robust and tone-, or seed-specific functional connectivity across the hemispheres, the pattern is less reliable in the belt and parabelt regions. Using LH seeds generates less robust and less extensive overall differential responsivity in the right hemisphere (e.g. as can be seen in the ANOVA seed effect, compare LH, Fig. 1F, and RH, Figure S7C, when using LH seeds). Furthermore, the pattern of tonotopic-like extra-core bands is less clear in the right hemisphere when using LH seeds (Figure S7A). However, this does not seem to differ much between the two groups, as neither a main group effect nor an interaction between group and seed-effect can be found in the temporal auditory cortex in either hemisphere (although some differences are present in the right anterior insula; Figure S7D,E). Furthermore, directly using RH seeds does show a clear FC-tonotopic patterning in the right hemisphere of both groups (Figure S9), confirming that fcMRI can reveal the topographical structure in both groups also for the right hemisphere. Lastly, using each of the seeds (LH and RH) separately (without applying partial correlation analysis) generates largely bilateral activation, with similar strength on both sides. This suggests the laterality emerges from the absence of selectivity for different FC seeds rather than an overall absence of bilateral FC, in a pattern that is consistent across groups. This effect supports the findings of a recent FC study in typically hearing subjects, which also showed an interhemispheric decrease of frequency-selective FC, especially with regard to the non-core regions50. Therefore, the deaf FC patterns replicate not only the existence of frequency-selective FC of the hearing50, but also its decline between the hemispheres.
Discussion
Our findings suggest that topographic tonotopic-like large-scale functional connectivity patterns can emerge, and are retained through life, in prelingually deaf humans without auditory experience. This is true for the large-scale gradient of the auditory core and belt, and perhaps to some extent also the parabelt, extending to language-related speech/voice sensitive regions69,71,72,73 (Fig. 1D,E). The FC maps showed significant spatial similarity to tonotopic organization in both groups. Interestingly, not only the existence of topographic FC organization is retained, but also its hierarchical relative strength, such that it is most robust in the auditory core regions and less so outside the auditory core (replicating a recent FC study in a hearing group;50), as well as its hemispheric dissociation – such that FC preference patterns are decreased across the hemispheres50, in accordance with their slightly differential functional roles79,80,81,82,83,84,85. The topographical FC patterns could be identified in a vast majority of the deaf (Fig. 2A), including at the single-subject level (Fig. 2B), albeit with some variability among the subjects. Furthermore, our analyses revealed no large-scale differences between the deaf and the normally-hearing controls in their topographical FC patterns in the temporal lobe auditory cortex, in both univariate and multivariate analyses (Fig. 1G,H). In fact, the two groups FC-patterns were significantly spatially correlated (see highly significant CCC analysis), had similar between-subject variability (Fig. 2C), and were not distinguishable in an independent clustering analysis (Fig. 2D), suggesting that at least the large-scale topographical patterns are indeed alike despite life-long auditory deprivation. Moreover, these patterns are found despite cross-modal plasticity evident in the same deaf subjects, both in our results (in the FC between the early auditory cortex seeds and areas outside the temporal lobe, such as the insula; Figure S8; Tables S1 and S2), and in an additional study, which showed visual cross-modal plasticity in the same deaf group14.
These findings add to a large literature dealing with the balance between development of proper auditory organization and plasticity in deafness. These studies reveal preservation of the gross anatomical connectivity patterns (mostly shown in animal studies) alongside extensive functional deficits and reorganization, depending on onset, duration and etiology of deafness (reviewed in refs 5,12). Generally, along with significant evidence for cross-modal plasticity in the connectivity pattern of the auditory cortex of deaf animals, thalamic and cortical auditory cortex connections are largely retained following perinatal deafness26,28,29,30. However, there is inconsistent evidence regarding the topographical mapping in deafened animals’ auditory cortex. In some studies, rudimentary or smeared tonotopic mapping in auditory cortex was found to be grossly maintained in A1 in congenital deaf cats26,29,86, whereas other studies found that long-standing and early-onset deafness (in neonatally deafened animals) diminishes tonotopic gradients87,88. In addition to this inconsistency in the animal literature, the human auditory cortex differs to some extent in its organization from that of other mammals due to the development of language (e.g.31). In humans, non-congenitally deaf cochlear implant patients (who have had auditory experience) show evidence for auditory core tonotopic organization89,90 (although not always:91), and there is also indirect evidence for tonotopic responses in the brainstem of congenitally deaf children92. However, to the best of our knowledge, no direct mapping of the tonotopic, or topographic, organization was conducted in the CI-naïve congenitally deaf human auditory cortex, let alone in its association regions, without any auditory sensory experience. On the other hand, there is evidence for plasticity in this population: there are clear changes in white matter and gray matter volumes and correlation in early auditory cortex93,94,95, and changes in functional connectivity of the auditory cortex9,96,97. Such changes tend to emphasize cross-modal compensatory plasticity, especially with regard to language. Functionally, it is known that cochlear implantation outcome in prelingually (congenitally) deaf children is optimal for early implantation, in the first years of life2,3,98, and that later implantation is far less beneficial for auditory processing skills4,5,6. Therefore, there is grounds for surprise at the extent of topographical organization found in a group of congenitally, profoundly deaf subjects, who use mostly sign-language, have poor oral language skills and do not have a history of efficient use of hearing aids or cochlear implantation. All of these are factors that supposedly interfere with auditory cortex proper organization and promote cross-modal plasticity. Indeed, a recent study in the same subject group found evidence that their auditory cortex contains decodable information about the location of visual stimuli14. Thus, the ability to find retained FC tonotopic organization even in this group suggests that indeed such organization patterns emerge without reliance on auditory sensory inputs during critical periods.
Our findings suggest that some aspects of the tonotopic connectivity organization do not crucially rely upon auditory experience-dependent developmental mechanisms. These connectivity patterns likely develop based on genetic blueprints and activity-dependent mechanisms, by which the primary sensory receptor inner hair cells (IHCs) in the mammalian cochlea spontaneously fire action potentials prior to hearing onset99,100,101,102,103,104. Therefore, many of the auditory projections are already largely present prior to hearing onset105. However, a final refinement of such connectivity is likely to be achieved only as a result of auditory experience5,12,103,106,107. These results resemble those found in the visual modality, where the large-scale functional connectivity topographical architecture (reflecting retinotopic organization) can be found even in congenital blindness49, likely based on prenatal spontaneous patterned retinal firing108,109,110,111,112, although visual experience is needed for the generation of fully functional vision113. Similarly to blindness114, deafness also seems to cause changes in connectivity of the auditory cortex to other regions, such as the limbic and frontal cortex95, while retaining the intra-cortical organization relatively intact, as can be seen in our findings. Thus, similarly to the state of the visual cortex in blindness49, the deaf auditory cortex in humans shows a complex pattern of both plasticity and retained organization115.
While the similarity seen here between the deaf and hearing subjects was reproduced across various analyses, including both univariate and multivariate analyses, it is quite plausible that it may break down to some extent in a higher-resolution mapping of tonotopic gradient peaks, which may be conducted in the future in ultra-high field strength scanners (e.g. 7T;64). Such methodologies may also be able to better discriminate subcortical topographic FC effects (Figure S2A,B) and their plasticity (Figure S2D). The value of high-resolution future studies may be especially important as the absence of group differences cannot serve as evidence for equivalence (despite the highly significant spatial similarity between the maps in the CCC analysis and additional supporting analyses). Thus, a stronger power design may yet reveal significant differences between the groups also in the fine-detailed topographical FC within the auditory cortex (or subcortical structures), which was not detectable by the current methods. Moreover, it was recently discovered that tonotopic preferences even in the hearing population exist only at a relatively global scale as compared to the local cellular level37,116,117, and thus may not reflect directly the factors harmed by long-term or congenital deafness in deciphering auditory information. An additional factor which needs to be assessed is to what extent the intact resting-state FC observed here also represents the dynamic connectivity in the deaf brain, as resting-state FC and dynamic, state- and task-based connectivity show some differences44,45,118,119,120. It may be that the intra-auditory FC patterns manifest less during everyday life, such that the auditory cortex dynamically shifts its connectivity to better connect to the visual cortex and language regions, therefore showing plasticity due to lifelong experience and supporting cross-modal compensatory plasticity in the deaf. However, our finding of the underlying FC organization suggests that even if this is the case, the auditory cortex tonotopic network organization can still be found, and, potentially, utilized (see below). Lastly, a fully informed mapping of the effect of deafness on auditory cortex organization would have to take into account not only functional data and properties (such as tonotopic mapping) in animals and humans, but also the various cytoarchitectonic and myeloarchitectonic mapping models91,121,122,123,124,125,126,127,128, as these are crucial for the full delineation of auditory area borders and determining the areal organization (e.g. as was recently done in hearing subjects;38). Still, the non-invasive use of fcMRI can be useful to delineate functional auditory regions, even without the reliance on auditory stimulation. In the deaf, this method can provide auditory cortex delineation which may be useful in investigating in more depth both the retention of function as well as the different cross-modal changes in the plastic associative auditory cortex (e.g. refs 5 and 21).
Can the current findings and method also be of clinical benefit? It was recently shown that measures of cross-modal plasticity, as indexed by the degree to which the auditory cortex is recruited for non-auditory (mainly visual) processing8,9,10,11,13,15,16,17,18,20,21, may be used as a counter-indication for auditory restoration. Specifically, the degree of auditory cortex hypometabolism, generated by cross-modal reorganization129, (as measured by PET) before the operation was related to the amount of improvement in hearing capability after cochlear implantation, making temporal hypometabolism a possible prognosis factor for implantation24,25. Recently, more direct measures of cross-modal plasticity, auditory cortex activation for visual stimulation, were shown to negatively correlate with cochlear implantation outcome23,130. Therefore, assessing preoperatively the state of the auditory cortex in individual subjects may contribute to the decision to implant a CI, for example, beyond standard cochlear implant therapeutic usefulness ages2,6. This is especially true given that in many cases the cause of deafness or the onset of hearing deficit of any specific individual is not fully clear, and the auditory system organization greatly depends on these factors5,12. Thus, while it remains to be tested directly, it may be plausible that measures of intact auditory cortex organization such as the one tested here (which is reliable at the single-subject level, and yet rather variable and not present in all the subjects), especially at ultra-high field strength scanners might also have prognostic value.
Overall, our findings show that auditory cortex underlying connectivity large-scale patterns seem to develop properly and can be found despite auditory deprivation and cross-modal plasticity. This suggests that the balance of experience-independence and critical developmental periods should be further tested and revisited, for both the early and higher-order sensory areas, such as in the case of auditory language areas. These findings further stress the relevance of anatomical connectivity patterns and innate developmental constraints131 and their robustness in the face of plasticity.
Methods
Participants
15 deaf and 16 hearing subjects participated in the experiment. Participants in the hearing group were between the age of 18 and 22 (mean age = 20.1 years, 3 males), whereas participants in the congenitally deaf group were between 17 and 22 (mean age = 20.4 years, 2 males). Four deaf subjects and one hearing subject were excluded due to excessive head movement (>2 mm or spike-like motion >1 mm), leaving 11 deaf and 15 hearing subjects. All participants had normal or corrected-to-normal vision and had no history of neurological disorder. All experimental protocols were approved by the institutional review board of Beijing Normal University (BNU) Imaging Center for Brain Research in accordance with the Declaration of Helsinki, and all subjects gave written informed consent. The Chinese sign language was the primary language of all deaf individuals, and none could communicate using oral language in more than single words. All deaf subjects had hearing loss above 90 dB binaurally (frequencies tested ranged from 125 to 8000 Hz), and did not benefit from the use of hearing aids (used in the past by 5 of the subjects). The causes of deafness were genetic, pregnancy-related diseases or ototoxic medications, complications at childbirth, or unknown. See Table 1 for detailed characteristics of the deaf participants. The hearing participants reported no hearing impairment or knowledge of Chinese sign language.
Functional Imaging
Images were acquired using a Siemens TRIO 3-T scanner at the Imaging Center for Brain Research, Beijing Normal University. The participants lay supine with their heads snugly fixed with foam pads to minimize head movement. The resting-state functional imaging data were comprised of 200 continuous EPI whole-brain functional volumes: 32 axial slices; 4 mm thickness; TR = 2000 ms; TE = 33 ms; FA = 73°; matrix size = 64 × 64; FOV = 200 × 200 mm; voxel size = 3.125 × 3.125 × 4 mm. During resting-state fMRI scanning, participants were instructed to close their eyes, keep still, and not think about anything systematically or fall asleep. T1-weighted anatomical images were acquired were acquired using a 3D MPRAGE sequence: 144 slices; 1.33 mm thickness; TR = 2530 ms; TE = 3.39 ms; inversion time = 1100 ms; FA = 7°; FOV = 256 × 256 mm; voxel size = 1.0 × 1.0 × 1.33 mm; matrix size = 256 × 256.
fMRI preprocessing
Data analysis was performed using the Brain Voyager QX 2.8 software package (Brain Innovation, Maastricht, Netherlands) and complementary in-house preprocessing in MATLAB (MathWorks, Natick, MA) using standard preprocessing procedures. The first two images of each scan were excluded from the analysis due to non-steady state magnetization. Preprocessing of functional scans included the following steps: 3D motion correction, slice scan time correction, band pass filtering (0.01–0.1 Hz), regression of spurious signals from the ventricles and white matter regions defined using a grow-region function embedded in Brain Voyager on the individual level and spatial smoothing with a 4 mm full-width-at-half-maximum (FWHM) Gaussian kernel. The findings were replicated without spatial smoothing at the volume level, and in applying smoothing at the surface, cortical level, see detail below. Data in which head motion exceeded 2 mm in any given axis, or had spike-like motion of more than 1 mm in any direction (of four deaf subjects and one hearing subject) was excluded from further analysis. The remaining, used data did not differ between the groups in its motion parameters (maximum motion, p = 0.42, maximum motion range, p = 0.56). Data were then normalized to standard Talairach space132. T1-weighted anatomical images were used for surface reconstruction. Anatomical cortical reconstruction procedures included the segmentation of the white matter using a grow-region function embedded in Brain Voyager. The Talairach normalized cortical surface was then inflated and the obtained activation maps were superimposed onto it. Single subject activation maps are presented on the individual cortical sheets, thus preventing any potential misalignments. To further control for any distortions resulting from cortical inflation processes, the unsmoothed individual maps are also presented on individual cortical sheets in which the distances between the nodes on the surface were corrected using BrainVoyager (as done in69), such that they more accurately reflected their actual “native” distance in the original 3D volume (see Figure S4). To further validate that our group results in the deaf group were not in any way affected by the volumetric (Talairach based) spatial coregistration method used (Fig. 1), group analyses were also replicated in surface space. Surface-based alignment was conducted across the subjects according to their cortical curvature (sulci and gyri) patterns. The curvature alignment across the subjects was over 80% subject overlap for the Heschl’s gyrus anterior and posterior borders, showing that 9/11 participants data was fully aligned in this region, validating alignment success (see Figure S3A). As Heschl’s gyrus (regardless of its structure, single or duplicated;65) contains the primary auditory cortex central border between hA1 and R, this overlap verifies that the seed ROIs are sampled appropriately in the single subjects in surface space. The topographic patterns of the auditory cortex organization in the deaf were evident in this surface space control analysis with mild spatial smoothing at the volume level (4 mm FWHM; see Figure S3B,C), and without applying any spatial smoothing at the volume level, and instead using spatial smoothing at the surface level (4 vertex FWHM) following surface-based alignment (see Figure S5), to similar extents as in the main results. In addition to the univariate between-subject analyses, single subject analyses (Fig. 2; unsmoothed data Figure S4), multivariate analysis and spatial similarity analyses (see detail below) also support the high reproducibility of the findings and the appropriateness of the seeds.
Defining a-priori tonotopic regions of interest
In order to define the primary auditory cortex seeds for fcMRI analysis we used data acquired from 10 normally hearing subjects (non-overlapping with the fcMRI hearing group) using a standard phase-encoded tonotopic mapping protocol56. This experiment included a rising logarithmic tone chirp spanning the range of 250–4,000 Hz in 18 seconds, followed by a 12 second baseline period, repeated 15 times. Stimulus intensity was set individually at levels between 86–89 dB SPL in order to optimize hearing on top of scanner noise. This data was analyzed using phase-encoded mapping, revealing the frequency preference of most of the core and high-order human auditory cortex (see Fig. 1A, adapted from56). For the purpose of the current investigation, seed regions-of-interest (ROIs) for fcMRI analyses were defined within the most significantly tone-selective region in the auditory cortex, corresponding to the auditory core (R(299) > 0.3, p < 0.0005 corrected for multiple comparisons;56). Seeds were taken from an area slightly posterior to the peak of preference for low frequency in left Heschl’s gyrus (corresponding to the primary auditory cortex) and from a more posterior area, in left Heschl’s sulcus, supposedly within A1 (which extends posteriorly from Heschl’s gyrus to Heschl’s sulcus;65), showing preference towards high frequency tones (see Fig. 1C). Both ROIs were of a similar size (590 mm3 and 533 mm3 for the LF and HF seeds respectively). The same strategy was used to define seeds in the right hemisphere for a supplementary analysis of interhemispheric topographical differences (Figure S9C).
Analysis of functional connectivity
Functional connectivity was computed for a-priori defined seed regions-of -interest (ROIs) located in the primary auditory cortex (A1), selected according to tonal preference using a separate task-based tonotopic mapping external localizer from normally-hearing controls (see details above). Individual average time courses from each seed ROI were sampled and z-normalized at the individual level. Partial correlation analysis49,57,58 was performed in the following manner: for each seed, the time course was used as an individual predictor while regressing out the time course of the complementary auditory seed component (using it as a nuisance regressor). This was conducted to discard the shared variance and remain solely with the unique variance attributed to the first seed. This was computed both at the individual level, as well as in a group analysis (n = 11 for the deaf, n = 15 for the normally hearing) using a GLM in a hierarchical random effects (RFX) analysis133. This analysis resulted in a group map for each of the 2 ROIs. The two maps from these complementary seeds in A1 were overlaid to reveal the topographic FC pattern for each group separately (Fig. 1D,E). In order to investigate FC only in tonotopic areas, the results are presented in the areas showing significant cortical response to auditory stimulation in the external tonotopic experiment56 (p < 0.05, corrected for multiple comparisons using the Bonferroni correction). The minimum significance level of the findings, unless otherwise specified, was set to p < 0.05 corrected for multiple comparisons, using the spatial extent method based on the theory of Gaussian random fields134, a set-level statistical inference correction. This was done based on the Monte Carlo stimulation approach extended to 3D datasets using the threshold size plug-in for BrainVoyager QX, applied on the entire cortex. For better sensitivity of the multiple comparisons correction, group differences were also inspected within the auditory cortex temporal mask. For thalamus maps (Figure S2) a thalamic anatomical mask (containing the entire thalamus) was used for the multiple comparisons correction. The same analyses were conducted for the curvature alignment control analysis, after transforming the volumetric seed-ROIs to a cortical representation, with mild spatial smoothing done on the volume level (Figure S3) or with spatial smoothing applied only at the surface level (Figure S5), to control for any potential misalignment and smoothing effects. Group comparisons were conducted using ANOVA (Fig. 1F–H) modeling one within subject effect (FC to the two seeds based on tonotopic preferences) and one between subject effect (group). For ANOVA standard seed FC analysis was used in a combined GLM model for both seeds, without using partial correlations (without regressing out the time course of another seed). Group comparison was also replicated with partial correlation (Figure S8). Probability overlap across the individual subjects were computed from partial correlation individual maps, each at p < 0.05 corrected for multiple comparisons. The maps were overlaid and the percent of subjects showing activation at each voxel was calculated. To demonstrate that the topographic organization evident in the findings did not merely arise from spatial local correlations, we sampled the FC (t-values) from the HF seed of sequential spatial regions (10 vertices each) along the left superior temporal plain beginning in the HF seed itself and anteriorly (see sampling points at Figure S6A). T values were sampled for the deaf group FC from the left HF seed and from the right HF seed, to demonstrate that the same pattern of topographic FC (decrease and then increase in FC, in accordance with tonotopic organization) can be found across hemispheres regardless of anatomical distance from the seed (Figure S6B).
Multivariate analysis
Analyses were performed using CoSMoMVPA, an MVPA toolbox in Matlab135. Searchlight pattern classification136 accuracies for discriminating unsmoothed neural FC patterns in the deaf and controls were computed using leave-one-out cross validation, that is, the classifier was trained using the data of 21 patterns and tested on its accuracy at classifying the unseen data from the remaining pattern (data was permuted such that group sizes matched and subjects were included, 1365 iterations). This procedure was carried out using all possible combinations of train and test patterns. The classification accuracies were averaged to give a mean accuracy score per test per neighborhood (sphere sized 4 mm radius). The average classification accuracy’s significance was computed by comparing it to that obtained from data with shuffled labels, permuted 2000 iterations. The resulting accuracy map was z-transformed and corrected for multiple comparisons using the spatial extent method, as described above. No significant effects were found using this analysis.
Analyses of spatial similarity
In order to quantify the spatial similarity between the tonotopic gradients and FC maps, between the deaf and control group FC maps, and assess the discernibility of the two groups we computed several difference measures.
(1) Concordance correlation coefficients (CCC;78) were computed using in-house software written in MATLAB (MathWorks, Natick, MA). Concordance correlation coefficient values range from 1 (perfect spatial similarity) to −1 (perfect spatial dissimilarity). While CCC, similarly to Pearson’s linear correlation coefficient, tests for shared fluctuations in variance of two datasets, it also penalizes for differences in means between the two sets, thus serving as a more sensitive measure for group differences in both spatial patterns and overall FC. CCC values were computed between the tonotopic gradients preference (t-value difference between responses to HF and LF tones in the auditory external localizer; Fig. 1B) and FC map seed preference (t-value difference between FC of HF and LF seeds), to assess the similarity of the FC gradients with tonotopic maps. CCC was also calculated for each seed between the two group maps (e.g. between the low frequency seed map of the deaf and that of the hearing controls), and for the effect size (z-normalized effect size, HF-FC minus LF-FC). A mask of the auditory-responsive cortex (all areas showing auditory responses in the external auditory localizer, p < 0.05, corrected) was used to compute the similarity between the tonotopic gradients and FC maps and between the two group maps only for the relevant regions. The significance level was obtained using a permutation test (100,000 iterations) while randomly shuffling voxels from one group map and convolving the resulting map with a Gaussian kernel based on data smoothness estimation, to account for spatial autocorrelation. These values were then corrected for multiple comparisons using the Bonferroni correction.
(2) Testing subjects’ distribution of spatial FC patterns within and between the groups was conducted by applying a leave-one-out analyses of the z-normalized effect size (HF-FC minus LF-FC) within the auditory cortex (similarly applied in ref. 137). The averaged FC map of all hearing subjects except one was obtained. The correlation of the FC map of the left-out subject to the average was obtained and termed the pattern similarity score. This was repeated in a typical leave-one-out fashion. For the deaf subjects, the FC map for a given subject was correlated with each possible leave-one-out aggregate of the hearing or deaf subjects, and the average correlation across these partitions obtained.
(3) Hierarchical clustering analysis across subjects FC maps (z-normalized effect size, HF-FC minus LF-FC within the auditory cortex) was computed, using the linear shortest Euclidean distance of the correlation (1-r) between all subject pairs, creating a dendrogram of the distances across all the subjects (Fig. 2D). The distribution of the deaf and hearing subjects within the distance dendrogram suggests the deaf variability in tonotopically-informed FC maps is well within that of the hearing controls.
Additional Information
How to cite this article: Striem-Amit, E. et al. Topographical functional connectivity patterns exist in the congenitally, prelingually deaf. Sci. Rep. 6, 29375; doi: 10.1038/srep29375 (2016).
Supplementary Material
Acknowledgments
The current work has received funding from the National Basic Research Program of China (2013CB837300; 2014CB846103) to YB, the National Natural Science Foundation of China (31521063) to YB, the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No. 654837 to ESA, the Israel National Postdoctoral Award Program for Advancing Women in Science to ESA, and the Fundação BIAL grant 112/12 (http://www.bial.com/en/) to JA. We thank Smadar Ovadia-Caro for her help in data analysis.
Footnotes
Author Contributions Q.C., Y.F., Z.H., J.A. and Y.B. planned the experiments, Q.C., Y.F. and Z.H. conducted the experiments, E.S.A. and M.B. conducted the analyses, E.S.A., J.A., A.C. and Y.B. wrote the manuscript.
References
- Stevens G. et al. Global And Regional Hearing Impairment Prevalence: An Analysis Of 42 Studies In 29 Countries. Eur J Public Health. 23, 146–52 (2011). [DOI] [PubMed] [Google Scholar]
- Kral A. Auditory Critical Periods: A Review From System’s Perspective. Neuroscience. 247, 117–33 (2013). [DOI] [PubMed] [Google Scholar]
- Niparko J. K. et al. Spoken Language Development In Children Following Cochlear Implantation. Jama. 303, 1498–506 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R., Macsweeney M. & Woll B. Cochlear Implantation (Ci) For Prelingual Deafness: The Relevance Of Studies Of Brain Organization And The Role Of First Language Acquisition In Considering Outcome Success. Frontiers In Human Neuroscience. 8, 834 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon K. A. et al. Use It Or Lose It? Lessons Learned From The Developing Brains Of Children Who Are Deaf And Use Cochlear Implants To Hear. Brain Topogr. 24, 204–19 (2011). [DOI] [PubMed] [Google Scholar]
- Harrison R. V., Gordon K. A. & Mount R. J. Is There A Critical Period For Cochlear Implantation In Congenitally Deaf Children? Analyses Of Hearing And Speech Perception Performance After Implantation. Dev Psychobiol. 46, 252–61 (2005). [DOI] [PubMed] [Google Scholar]
- Rauschecker J. P. Compensatory plasticity and sensory substitution in the cerebral cortex. Trends Neurosci 18, 36–43 (1995). [DOI] [PubMed] [Google Scholar]
- Sharma A., Gilley P. M., Dorman M. F. & Baldwin R. Deprivation-Induced Cortical Reorganization In Children With Cochlear Implants. Int J Audiol. 46, 494–9 (2007). [DOI] [PubMed] [Google Scholar]
- Shiell M. M., Champoux F. & Zatorre R. J. Reorganization Of Auditory Cortex In Early-Deaf People: Functional Connectivity And Relationship To Hearing Aid Use. Journal Of Cognitive Neuroscience. 27, 1–14 (2014). [DOI] [PubMed] [Google Scholar]
- Bottari D. et al. Visual Change Detection Recruits Auditory Cortices In Early Deafness. Neuroimage. 94, 172–84 (2014). [DOI] [PubMed] [Google Scholar]
- Vachon P. et al. Reorganization Of The Auditory, Visual And Multimodal Areas In Early Deaf Individuals. Neuroscience. 245, 50–60 (2013). [DOI] [PubMed] [Google Scholar]
- Butler B. E. & Lomber S. G. Functional And Structural Changes Throughout The Auditory System Following Congenital And Early-Onset Deafness: Implications For Hearing Restoration. Front Syst Neurosci. 7, 92 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. & Sharma A. Cross-Modal Re-Organization In Adults With Early Stage Hearing Loss. Plos One. 9, E90594 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida J. et al. Decoding Visual Location From Neural Patterns In The Auditory Cortex Of The Congenitally Deaf. Psychological Science. 26, 1771–82 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo Z., Lega C., Cecchetto C. & Papagno C. Auditory Deprivation Affects Biases Of Visuospatial Attention As Measured By Line Bisection. Experimental Brain Research. 232, 2767–73 (2014). [DOI] [PubMed] [Google Scholar]
- Champoux F., Lepore F., Gagne J. P. & Theoret H. Visual Stimuli Can Impair Auditory Processing In Cochlear Implant Users. Neuropsychologia. 47, 17–22 (2009). [DOI] [PubMed] [Google Scholar]
- Doucet M. E., Bergeron F., Lassonde M., Ferron P. & Lepore F. Cross-Modal Reorganization And Speech Perception In Cochlear Implant Users. Brain. 129, 3376–83 (2006). [DOI] [PubMed] [Google Scholar]
- Finney E. M., Fine I. & Dobkins K. R. Visual Stimuli Activate Auditory Cortex In The Deaf. Nat Neurosci. 4, 1171–3 (2001). [DOI] [PubMed] [Google Scholar]
- Bavelier D., Dye M. W. & Hauser P. C. Do Deaf Individuals See Better? Trends Cogn Sci. 10, 512–8 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D. & Neville H. J. Cross-Modal Plasticity: Where And How? Nat Rev Neurosci. 3, 443–52 (2002). [DOI] [PubMed] [Google Scholar]
- Lomber S. G., Meredith M. A. & Kral A. Cross-Modal Plasticity In Specific Auditory Cortices Underlies Visual Compensations In The Deaf. Nat Neurosci. 13, 1421–7 (2010). [DOI] [PubMed] [Google Scholar]
- Neville H. J. et al. Cerebral organization for language in deaf and hearing subjects: Biological constraints and effects of experience. Proceedings of the National Academy of Sciences 95, 922–929 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelnikov et al. Visual Activity Predicts Auditory Recovery From Deafness After Adult Cochlear Implantation. Brain. 136, 3682–95 (2013). [DOI] [PubMed] [Google Scholar]
- Lee D. S. et al. Cross-Modal Plasticity And Cochlear Implants. Nature. 409, 149–50 (2001). [DOI] [PubMed] [Google Scholar]
- Lee H.-J. et al. Cortical Activity At Rest Predicts Cochlear Implantation Outcome. Cerebral Cortex. 17, 909–917 (2007). [DOI] [PubMed] [Google Scholar]
- Stanton S. G. & Harrison R. V. Projections from the medial geniculate body to primary auditory cortex in neonatally deafened cats. J Comp Neurol. 426, 117–29 (2000). [DOI] [PubMed] [Google Scholar]
- Wong C., Chabot N., Kok M. A. & Lomber S. G. Amplified somatosensory and visual cortical projections to a core auditory area, the anterior auditory field, Following early- and late-onset deafness. Journal of Comparative Neurology. 523, 1925–1947 (2015). [DOI] [PubMed] [Google Scholar]
- Chabot N., Butler B. E. & Lomber S. G. Differential Modification of Cortical and Thalamic Projections to Cat Primary Auditory Cortex Following Early- and Late-Onset Deafness. J Comp Neurol. 523, 2297–320 (2015). [DOI] [PubMed] [Google Scholar]
- Barone P., Lacassagne L. & Kral A. Reorganization of the connectivity of cortical field DZ in congenitally deaf cat. PLoS ONE. 8, e60093, doi: 10.1371/journal.pone.0060093 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok M. A., Chabot N. & Lomber S. G. Cross-modal reorganization of cortical afferents to dorsal auditory cortex following early- and late-onset deafness. J Comp Neurol. 522, 654–75 (2014). [DOI] [PubMed] [Google Scholar]
- Fullerton B. C. & Pandya D. N. Architectonic Analysis Of The Auditory-Related Areas Of The Superior Temporal Region In Human Brain. J Comp Neurol. 504, 470–98 (2007). [DOI] [PubMed] [Google Scholar]
- Sadato N. et al. Cross-modal integration and plastic changes revealed by lip movement, random-dot motion and sign languages in the hearing and deaf. Cereb Cortex. 15, 1113–22 (2005). [DOI] [PubMed] [Google Scholar]
- Bavelier D. et al. Impact of early deafness and early exposure to sign language on the cerebral organization for motion processing. J Neurosci. 21, 8931–42 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertz N., Gizewski E. R., De Greiff A. & Forsting M. Cross-modal plasticity in deaf subjects dependent on the extent of hearing loss. Cognitive Brain Research. 25, 884–890 (2005). [DOI] [PubMed] [Google Scholar]
- Macsweeney M. et al. Dispersed activation in the left temporal cortex for speech-reading in congenitally deaf people. Proc Biol Sci. 268, 451–7 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macsweeney M., Capek C. M., Campbell R. & Woll B. The signing brain: the neurobiology of sign language. Trends in Cognitive Sciences. 12, 432–440 (2008). [DOI] [PubMed] [Google Scholar]
- Kanold P. O., Nelken I. & Polley D. B. Local Versus Global Scales Of Organization In Auditory Cortex. Trends In Neurosciences. 37, 502–10 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerel M., De Martino F. & Formisano E. An Anatomical And Functional Topography Of Human Auditory Cortical Areas. Front Neurosci. 8, 225 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B., Yetkin F. Z., Haughton V. M. & Hyde J. S. Functional Connectivity In The Motor Cortex Of Resting Human Brain Using Echo-Planar Mri. Magn Reson Med. 34, 537–41 (1995). [DOI] [PubMed] [Google Scholar]
- Smith S. M. et al. Correspondence Of The Brain’s Functional Architecture During Activation And Rest. Proceedings Of The National Academy Of Sciences. 106, 13040–13045 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G., Jirsa V. K. & Mcintosh A. R. Emerging Concepts For The Dynamical Organization Of Resting-State Activity In The Brain. Nat Rev Neurosci. 12, 43–56 (2011). [DOI] [PubMed] [Google Scholar]
- Fox M. D. & Raichle M. E. Spontaneous Fluctuations In Brain Activity Observed With Functional Magnetic Resonance Imaging. Nat Rev Neurosci. 8, 700–11 (2007). [DOI] [PubMed] [Google Scholar]
- Greicius M. D., Supekar K., Menon V. & Dougherty R. F. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 19, 72–78 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di X., Gohel S., Kim E. H. & Biswal B. B. Task vs. Rest - Different Network Configurations between the Coactivation and the Resting-State Brain Networks. Frontiers in Human Neuroscience 7, 493 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes M., Kelly C., Colcombe S., Castellanos F. X. & Milham M. P. The Extrinsic and Intrinsic Functional Architectures of the Human Brain Are Not Equivalent. Cerebral Cortex 23, 223–229 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux J. S. & Greicius M. D. Greater Than The Sum Of Its Parts: A Review Of Studies Combining Structural Connectivity And Resting-State Functional Connectivity. Brain Struct Funct. 213, 525–33 (2009). [DOI] [PubMed] [Google Scholar]
- Honey C. J. et al. Predicting Human Resting-State Functional Connectivity From Structural Connectivity. Proc Natl Acad Sci USA 106, 2035–40 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J. L. et al. Intrinsic Functional Architecture In The Anaesthetized Monkey Brain. Nature. 447, 83–86 (2007). [DOI] [PubMed] [Google Scholar]
- Striem-Amit E. et al. Functional Connectivity Of Visual Cortex In The Blind Follows Retinotopic Organization Principles. Brain. 138, 1679–95 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha K., Zatorre R. J. & Schönwiesner M. Frequency Selectivity of Voxel-by-Voxel Functional Connectivity in Human Auditory Cortex. Cerebral Cortex. 26, 211–24 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jbabdi S., Sotiropoulos S. N. & Behrens T. E. The Topographic Connectome. Current Opinion In Neurobiology. 23, 207–215 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. et al. How visual is the visual cortex? Comparing connectional and functional fingerprints between congenitally blind and sighted individuals. J Neurosci. 35, 12545–12559 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra-Carrillo B., Mackey A. P. & Bunge S. A. Resting-State Fmri: A Window Into Human Brain Plasticity. Neuroscientist. 20, 522–33 (2014). [DOI] [PubMed] [Google Scholar]
- Dosenbach N. U. et al. Distinct Brain Networks For Adaptive And Stable Task Control In Humans. Proc Natl Acad Sci USA 104, 11073–8 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D. A. et al. Development Of Distinct Control Networks Through Segregation And Integration. Proceedings Of The National Academy Of Sciences. 104, 13507–13512 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striem-Amit E., Hertz U. & Amedi A. Extensive Cochleotopic Mapping Of Human Auditory Cortical Fields Obtained With Phase-Encoding Fmri. Plos One. 6, E17832 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D. et al. Intrinsic Functional Relations Between Human Cerebral Cortex And Thalamus. J Neurophysiol. 100, 1740–8 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies D. S. et al. Resting Developments: A Review Of Fmri Post-Processing Methodologies For Spontaneous Brain Activity. Magma. 23, 289–307 (2010). [DOI] [PubMed] [Google Scholar]
- Petkov C. I., Kayser C., Augath M. & Logothetis N. K. Functional Imaging Reveals Numerous Fields In The Monkey Auditory Cortex. Plos Biol. 4, E215 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz M. & Langers D. R. M. Tonotopic Mapping Of Human Auditory Cortex. Hearing Research. 307, 42–52 (2013). [DOI] [PubMed] [Google Scholar]
- Talavage T. M., Ledden P. J., Benson R. R., Rosen B. R. & Melcher J. R. Frequency-Dependent Responses Exhibited By Multiple Regions In Human Auditory Cortex. Hear Res. 150, 225–44 (2000). [DOI] [PubMed] [Google Scholar]
- Talavage et al. Tonotopic Organization In Human Auditory Cortex Revealed By Progressions Of Frequency Sensitivity. J Neurophysiol. 91, 1282–96 (2004). [DOI] [PubMed] [Google Scholar]
- Woods D. L. & Alain C. Functional Imaging Of Human Auditory Cortex. Curr Opin Otolaryngol Head Neck Surg. 17, 407–11 (2009). [DOI] [PubMed] [Google Scholar]
- Da Costa S., Saenz M., Clarke S. & Van Der Zwaag W. Tonotopic Gradients In Human Primary Auditory Cortex: Concurring Evidence From High-Resolution 7 T And 3 T Fmri. Brain Topography. 28, 66–9 (2014). [DOI] [PubMed] [Google Scholar]
- Da Costa S. et al. Human Primary Auditory Cortex Follows The Shape Of Heschl’s Gyrus. J Neurosci. 31, 14067–75 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formisano E. et al. Mirror-Symmetric Tonotopic Maps In Human Primary Auditory Cortex. Neuron. 40, 859–69 (2003). [DOI] [PubMed] [Google Scholar]
- Humphries C., Liebenthal E. & Binder J. R. Tonotopic Organization Of Human Auditory Cortex. Neuroimage. 50, 1202–11 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langers D. R. M., Krumbholz K., Bowtell R. & Hall D. A. Neuroimaging Paradigms For Tonotopic Mapping (I): The Influence Of Sound Stimulus Type. Neuroimage. 100, 650–62 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver A. M. & Rauschecker J. P. Functional Topography of Human Auditory Cortex. The Journal of Neuroscience 36, 1416–1428 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay J. et al. Function And Connectivity In Human Primary Auditory Cortex: A Combined Fmri And Dti Study At 3 Tesla. Cereb Cortex. 17, 2420–32 (2007). [DOI] [PubMed] [Google Scholar]
- Petkov C. I., Logothetis N. K. & Obleser J. Where Are The Human Speech And Voice Regions, And Do Other Animals Have Anything Like Them? The Neuroscientist. 15, 419–29 (2009). [DOI] [PubMed] [Google Scholar]
- Belin P., Zatorre R. J., Lafaille P., Ahad P. & Pike B. Voice-Selective Areas In Human Auditory Cortex. Nature. 403, 309–12 (2000). [DOI] [PubMed] [Google Scholar]
- Moerel M., De Martino F. & Formisano E. Processing Of Natural Sounds In Human Auditory Cortex: Tonotopy, Spectral Tuning, And Relation To Voice Sensitivity. The Journal Of Neuroscience. 32, 14205–14216 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitt I. & Rauschecker J. P. Phoneme And Word Recognition In The Auditory Ventral Stream. Proceedings Of The National Academy Of Sciences. 109, E505–E514 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel S. A., Glover G. H. & Wandell B. A. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cereb Cortex. 7, 181–192 (1997). [DOI] [PubMed] [Google Scholar]
- Scholvinck M. L., Maier A., Ye F. Q., Duyn J. H. & Leopold D. A. Neural basis of global resting-state fMRI activity. Proc Natl Acad Sci USA 107, 10238–10243 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold D. A., Murayama Y. & Logothetis N. K. Very slow activity fluctuations in monkey visual cortex: implications for functional brain imaging. Cereb Cortex. 13, 422–433 (2003). [DOI] [PubMed] [Google Scholar]
- Lin L. I. A Concordance Correlation Coefficient To Evaluate Reproducibility. Biometrics 45, 255–268 (1989). [PubMed] [Google Scholar]
- Rauschecker J. P. Parallel Processing In The Auditory Cortex Of Primates. Audiol Neurootol 3, 86–103 (1998). [DOI] [PubMed] [Google Scholar]
- Rauschecker J. P. & Tian B. Mechanisms And Streams For Processing Of “What” And “Where” In Auditory Cortex. Proc Natl Acad Sci USA 97, 11800–6 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski L. M. et al. Dual Streams Of Auditory Afferents Target Multiple Domains In The Primate Prefrontal Cortex. Nat Neurosci. 2, 1131–6 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervaniemi M. & Hugdahl K. Lateralization Of Auditory-Cortex Functions. Brain Research Reviews. 43, 231–246 (2003). [DOI] [PubMed] [Google Scholar]
- Zatorre R. J., Belin P. & Penhune V. B. Structure And Function Of Auditory Cortex: Music And Speech. Trends In Cognitive Sciences. 6, 37–46 (2002). [DOI] [PubMed] [Google Scholar]
- Kaas J. H. & Hackett T. A. Subdivisions Of Auditory Cortex And Processing Streams In Primates. Proc Natl Acad Sci USA 97, 11793–9 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomber S. G. & Malhotra S. Double Dissociation Of ‘What’ And ‘Where’ Processing In Auditory Cortex. Nat Neurosci. 11, 609–16 (2008). [DOI] [PubMed] [Google Scholar]
- Hartmann R., Shepherd R. K., Heid S. & Klinke R. Response Of The Primary Auditory Cortex To Electrical Stimulation Of The Auditory Nerve In The Congenitally Deaf White Cat. Hear Res. 112, 115–33 (1997). [DOI] [PubMed] [Google Scholar]
- Raggio M. W. & Schreiner C. E. Neuronal responses in cat primary auditory cortex to electrical cochlear stimulation. III. Activation patterns in short- and long-term deafness. J Neurophysiol. 82, 3506–26 (1999). [DOI] [PubMed] [Google Scholar]
- Fallon J. B., Irvine D. R. F. & Shepherd R. K. Cochlear Implant Use Following Neonatal Deafness Influences The Cochleotopic Organization Of The Primary Auditory Cortex In Cats. The Journal Of Comparative Neurolog. 512, 101–114 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiraud J. et al. Evidence Of A Tonotopic Organization Of The Auditory Cortex In Cochlear Implant Users. J Neurosci. 27, 7838–46 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazeyras F. et al. Functional Mri Of Auditory Cortex Activated By Multisite Electrical Stimulation Of The Cochlea. Neuroimage. 17, 1010–1017 (2002). [PubMed] [Google Scholar]
- Seghier M. L., Boëx C., Lazeyras F., Sigrist A. & Pelizzone M. Fmri Evidence For Activation Of Multiple Cortical Regions In The Primary Auditory Cortex Of Deaf Subjects Users Of Multichannel Cochlear Implants. Cerebral Cortex. 15, 40–48 (2005). [DOI] [PubMed] [Google Scholar]
- Gordon K. A., Salloum C., Toor G. S., Van Hoesel R. & Papsin B. C. Binaural Interactions Develop In The Auditory Brainstem Of Children Who Are Deaf: Effects Of Place And Level Of Bilateral Electrical Stimulation. The Journal Of Neuroscience. 32, 4212–4223 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. M. et al. Morphometric Differences In The Heschl’s Gyrus Of Hearing Impaired And Normal Hearing Infants. Cereb Cortex. 21, 991–8 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. J., Park S. Y., Kim J., Lee D. H. & Park H. J. Alterations Of White Matter Diffusion Anisotropy In Early Deafness. Neuroreport. 20, 1032–6 (2009). [DOI] [PubMed] [Google Scholar]
- Kim E. et al. Morphological brain network assessed using graph theory and network filtration in deaf adults. Hear Res 315, 88–98 (2014). [DOI] [PubMed] [Google Scholar]
- Li Y. et al. Altered Intra- And Inter-Regional Synchronization Of Superior Temporal Cortex In Deaf People. Cereb Cortex. 23, 1988–96 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. et al. Functional reorganizations of brain network in prelingually deaf adolescents. Neural Plast 2016, 9849087, doi: 10.1155/2016/9849087 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral A. & Sharma A. Developmental neuroplasticity after cochlear implantation. Trends Neurosci. 35, 111–22 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship A. G. & Feller M. B. Mechanisms Underlying Spontaneous Patterned Activity In Developing Neural Circuits. Nat Rev Neurosci. 11, 18–29 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch N. X., Yi E., Gale J. E., Glowatzki E. & Bergles D. E. The origin of spontaneous activity in the developing auditory system. Nature. 450, 50–5 (2007). [DOI] [PubMed] [Google Scholar]
- Clause A. et al. The Precise Temporal Pattern Of Prehearing Spontaneous Activity Is Necessary For Tonotopic Map Refinement. Neuron. 82, 822–35 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. L. et al. Position-Dependent Patterning Of Spontaneous Action Potentials In Immature Cochlear Inner Hair Cells. Nat Neurosci. 14, 711–717 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K., Clause A. & Noh J. Tonotopic Reorganization Of Developing Auditory Brainstem Circuits. Nat Neurosci. 12, 711–7 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippe W. R. Relationship Between Frequency Of Spontaneous Bursting And Tonotopic Position In The Developing Avian Auditory System. Brain Res. 703, 205–13 (1995). [DOI] [PubMed] [Google Scholar]
- Cornwell P., Ravizza R. & Payne B. Extrinsic visual and auditory cortical connections in the 4-day-old kitten. J Comp Neurol. 229, 97–120 (1984). [DOI] [PubMed] [Google Scholar]
- Huttenlocher P. R. & Dabholkar A. S. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 387, 167–78 (1997). [DOI] [PubMed] [Google Scholar]
- Kral A., Tillein J., Heid S., Hartmann R. & Klinke R. Postnatal cortical development in congenital auditory deprivation. Cereb Cortex. 15, 552–62 (2005). [DOI] [PubMed] [Google Scholar]
- Ackman J. B. & Crair M. C. Role of emergent neural activity in visual map development. Current Opinion in Neurobiology 24, 166–175 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R. O., Meister M. & Shatz C. J. Transient period of correlated bursting activity during development of the mammalian retina. Neuron 11, 923–938 (1993). [DOI] [PubMed] [Google Scholar]
- Cang J. et al. Development of Precise Maps in Visual Cortex Requires Patterned Spontaneous Activity in the Retina. Neuron 48, 797–809 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin T., Torborg C. L., Feller M. B. & O’Leary D. D. M. Retinotopic Map Refinement Requires Spontaneous Retinal Waves during a Brief Critical Period of Development. Neuron 40, 1147–1160 (2003). [DOI] [PubMed] [Google Scholar]
- Meister M., Wong R., Baylor D. A. & Shatz C. J. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science 252, 939–943 (1991). [DOI] [PubMed] [Google Scholar]
- Maurer D., Lewis T. L. & Mondloch C. J. Missing Sights: Consequences For Visual Cognitive Development. Trends Cogn Sci. 9, 144–51 (2005). [DOI] [PubMed] [Google Scholar]
- Hasson U., Andric M., Atilgan H. & Collignon O. Congenital blindness is associated with large-scale reorganization of anatomical networks. Neuroimage. 128, 362–72 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimler B., Striem-Amit E. & Amedi A. Origins of task-specific sensory-independent organization in the visual and auditory brain: neuroscience evidence, open questions and clinical implications. Curr Opin Neurobiol 35, 169–177 (2015). [DOI] [PubMed] [Google Scholar]
- Rothschild G., Nelken I. & Mizrahi A. Functional Organization And Population Dynamics In The Mouse Primary Auditory Cortex. Nat Neurosci. 13, 353–60 (2010). [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S., Shamma S. A. & Kanold P. O. Dichotomy Of Functional Organization In The Mouse Auditory Cortex. Nat Neurosc. 13, 361–8 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X. et al. Reconfiguration of the Brain Functional Network Associated with Visual Task Demands. Plos ONE 10, e0132518 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andric M. & Hasson U. Global features of functional brain networks change with contextual disorder. NeuroImage 117, 103–113 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussa M. N. et al. Changes in cognitive state alter human functional brain networks. Frontiers in Human Neuroscience 5, 83 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker J. P., Tian B. & Hauser M. Processing Of Complex Sounds In The Macaque Nonprimary Auditory Cortex. Science. 268, 111–4 (1995). [DOI] [PubMed] [Google Scholar]
- Brodmann K. Vergleichende Lokalisationslehre Der Grosshirnrinde In Ihren Prinzipien Dargestellt Auf Grund Des Zellenbaues. (1909).
- Clarke S. & Rivier F. Compartments Within Human Primary Auditory Cortex: Evidence From Cytochrome Oxidase And Acetylcholinesterase Staining. Eur J Neurosci. 10, 741–5 (1998). [DOI] [PubMed] [Google Scholar]
- Galaburda A. & Sanides F. Cytoarchitectonic Organization Of The Human Auditory Cortex. J Comp Neurol. 190, 597–610 (1980). [DOI] [PubMed] [Google Scholar]
- Hackett T. A., Preuss T. M. & Kaas J. H. Architectonic Identification Of The Core Region In Auditory Cortex Of Macaques, Chimpanzees, And Humans. J Comp Neurol. 441, 197–222 (2001). [DOI] [PubMed] [Google Scholar]
- Merzenich M. M., Knight P. L. & Roth G. L. Representation Of Cochlea Within Primary Auditory Cortex In The Cat. J Neurophysiol. 38, 231–249 (1975). [DOI] [PubMed] [Google Scholar]
- Morosan P. et al. Human Primary Auditory Cortex: Cytoarchitectonic Subdivisions And Mapping Into A Spatial Reference System. Neuroimage. 13, 684–701 (2001). [DOI] [PubMed] [Google Scholar]
- Pandya D. N. & Sanides F. Architectonic Parcellation Of The Temporal Operculum In Rhesus Monkey And Its Projection Pattern. Anatomy And Embryology. 139, 127–161 (1973). [DOI] [PubMed] [Google Scholar]
- Catalán-Ahumada M. et al. High Metabolic Activity Demonstrated By Positron Emission Tomography In Human Auditory Cortex In Case Of Deafness Of Early Onset. Brain Research. 623, 287–292 (1993). [DOI] [PubMed] [Google Scholar]
- Sandmann P. et al. Visual Activation Of Auditory Cortex Reflects Maladaptive Plasticity In Cochlear Implant Users. Brain. 135, 555–68 (2012). [DOI] [PubMed] [Google Scholar]
- Mahon B. Z. & Caramazza A. What drives the organization of object knowledge in the brain? Trends Cogn Sci. 15, 97–103 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J. & Tournoux P. Co-Planar Stereotaxic Atlas Of The Human Brain, New York, Thieme (1988). [Google Scholar]
- Friston K. J., Holmes A. P. & Worsley K. J. How Many Subjects Constitute A Study? Neuroimage 10, 1–5 (1999). [DOI] [PubMed] [Google Scholar]
- Friston K. J., Worsley K. J., Frackowiak R. S. J., Mazziotta J. C. & Evans A. C. Assessing The Significance Of Focal Activations Using Their Spatial Extent. Human Brain Mapping. 1, 210–220 (1993). [DOI] [PubMed] [Google Scholar]
- Oosterhof N. N., Connolly A. C. & Haxby J. V. CoSMoMVPA: multi-modal multivariate pattern analysis of neuroimaging data in Matlab/GNU Octave. bioRxiv; doi: 10.1101/047118 (2016). [DOI] [PMC free article] [PubMed]
- Kriegeskorte N., Goebel R. & Bandettini P. Information-based functional brain mapping. Proceedings of the National Academy of Sciences of the United States of America 103, 3863–3868 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt O. H., Benson N. C., Datta R. & Aguirre G. K. The fine-scale functional correlation of striate cortex in sighted and blind people. J Neurosci 33, 16209–16219 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.