1. Introduction
Adolescence is a crucial period for bone acquisition, with nearly half of adult bone mass acquired in the four years surrounding menarche.(1–3) Therefore, modifiable factors contributing to peak bone mass, such as diet and physical activity, may be most influential during this maturational period.(4–6) High impact physical activity, such as gymnastics, is considered particularly osteogenic, yielding greater bone gains than cyclic loading activities, such as distance running, and non-weight bearing activities, such as swimming.(7–10) Work by our group and others suggests that artistic gymnastics exposure is associated with site-specific skeletal benefits in bone mass and geometry, yielding advantages in theoretical bone strength of 4 to 56%.(11–17)
Prospective longitudinal evidence from Bailey et al. has associated higher physical activity participation with greater bone mass gains (16% LSBMC, 11% FNBMC) from 1 year pre- to 1 year post-PHV.(18) Similarly, work by Gunter et al. demonstrated adolescent retention of subtle skeletal advantages 7 years after a 7-month jumping intervention that was administered pre-PHV (retained 1.4% advantage, total hip BMC).(19) In a prior longitudinal analysis, we reported that pre-menarcheal gymnastics exposure for at least 2 years at ≥ 6 hours per week, ceased circum-menarche, was associated with significant advantages over non-gymnasts for radius bone mass, size and areal density over a growth curve that extended to at least 4 years post-menarche.(20) These studies suggest that pre-pubertal and early pubertal activity exposure may yield long-term skeletal advantages.
The primary aim of the present study was to evaluate associations between maturity-specific loading exposure and site-specific bone development over a broad span of childhood and adolescent growth. We analyzed prospective, longitudinal DXA data representing up to 10 years of growth and maturation. We compared girls who were exposed to substantial gymnastics training before and after menarche (GYM) to girls without substantial gymnastics exposure (NON). Our analyses account for variability in biological age, body size and non-bone fat-free mass in a time-varying context, adjusting for age at menarche as an indicator of maturational timing. We hypothesized that girls who participated in gymnastics training would demonstrate site-specific advantages in bone geometry, density and strength indices, over this entire period of bone formation and maintenance, compared to those who did not. Furthermore, we hypothesized that bone geometric benefits would persist after cessation of gymnastic training, whereas bone mineral content benefits would deteriorate subtly over time post-cessation, such that loading advantages averaged over the entire growth curve would remain significant.
2. Methods
2.1. Subjects and Study Design
Study protocol was approved by the Institutional Review Board at SUNY Upstate Medical University (5332F, 2011–2), conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. Subjects and parents/guardians provided written, informed assent and consent, as appropriate for subject age; subjects provided written, informed consent to continue the study after their 18th birthday.
The 44 subjects included in the current analysis are a subset from an existing longitudinal study (1997-present) designed to observe skeletal growth and adaptation in a large group of young female gymnasts (GYM) and non-gymnasts (NON). Subjects were enrolled in 2 cohorts: Cohort 1, 1997–8 (initial GYM n=51, NON n=31; current analysis: GYM=7, NON n=13,) and Cohort 2, 2002–2003 (initial GYM n=12, NON n=28; current analysis: GYM n=6, NON n=18). As subjects in the 1997–8 cohort were originally recruited for an 18-month pilot study, many withdrew from the study after this pilot period.
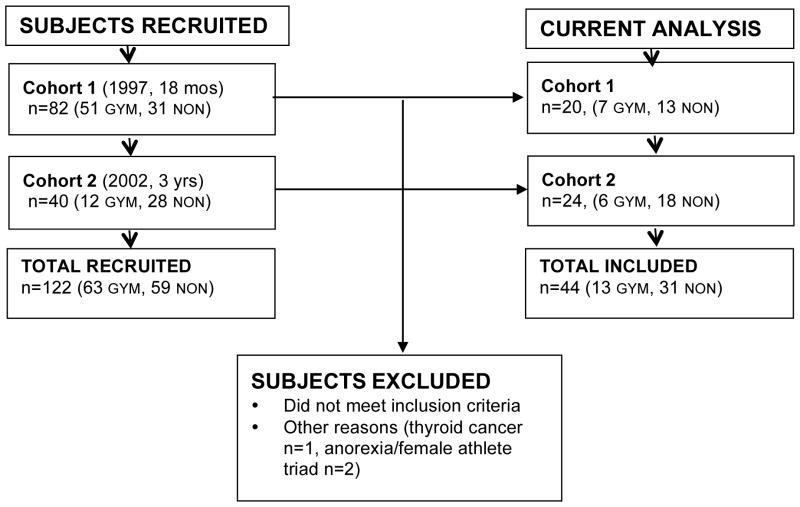
For the present analysis, subjects were included if they provided: ≥ 3 DXA scans with associated data, date of menarche, and date of gymnastics cessation (if applicable). Subjects were excluded if: they ceased gymnastics for ≥ 1 year then resumed participation at a later date, they participated in long-term low level or sporadic gymnastics training (multiple years @1–4 h/wk) and/or they quit gymnastics earlier than 18 months post-menarche (GYNAGE +1.5). One additional non-gymnast was excluded due to thyroid cancer diagnosis, and two non-gymnasts were excluded due to concerns about female athlete triad. Cohort composition is detailed in the CONSORT diagram, Figure 1.
Figure 1. CONSORT Diagram.
Subjects were recruited in two cohorts and included in the current analysis as follows (GYM = gymnast; NON = non-gymnast).
Previous evaluation for the possibility of selection bias, based upon continued study participation, has been completed in our overall study cohort. There were no differences in baseline characteristics for age, height, weight, BMI, sub-head BMC, sub-head lean mass or percent body fat, or age at menarche, between those who were included in these analyses and those who were excluded. Furthermore, there were no differences in these characteristics between included subjects who provided 3 to 6 measurement points versus those who provided 7 to 10 measurement points.
Gymnasts were recruited from local gymnastics clubs; age, size, and maturity-matched non-gymnasts were recruited from local grade schools. Subjects underwent annual DXA scans (whole body, forearm, hip, lumbar spine). Anthropometrics(21), physical activity and maturity were assessed semi-annually until age 18, after which only annual assessments were obtained, coincident with DXA scans.
2.2. Physical Maturity Evaluation
Chronologic age was determined by subtracting measurement date from birthdate (recorded at enrollment) and reported to the nearest 0.1 year. Self-reported age at menarche (queried semi-annually until positive) was used to calculate gynecological age for each measurement point (GYNAGE: years pre- (−) or post- (+) menarche (time zero), calculated as measurement date–menarche date, reported to the nearest 0.1 year). Beginning in 2006, subjects were specifically queried regarding menstrual regularity/irregularity and oral contraceptive use at each measurement session; prior to this date, this information is incomplete.
2.3. Physical Activity Quantification
Investigator-administered questionnaires were used to record average hours per week of organized non-aquatic physical activity (including gymnastics) for the intervals between assessments (semi-annually or annually). A similar methodology was validated in a subset of the overall study cohort via comparison to prospectively recorded coaches’ training logs (r > 0.97, p<0.001).(20) Average hours per week of gymnastics and non-gymnastic organized physical activity were calculated for 6 months prior to the first DXA scan, and for inter-scan intervals thereafter. Subjects with annual mean gymnastics participation of ≥ 6 h/wk for at least 2 consecutive years during the observation period were classified as GYM.(20–22) Subjects with long-term low level (1–4 h/wk) or sporadic gymnastics training were excluded, as they could not be classified as either GYM or NON.
2.4. Densitometry
Postero-anterior (PA) DXA scans were performed using a Hologic QDR4500W DXA scanner (>90%), except for a few later scan assessments that used a cross-calibrated Discovery A scanner. Discovery A scans were similarly distributed across groups (<10%: n=8 NON, n=9 GYM); maximum Discovery A scans per subject= 3). Less than 1% of forearm scans were performed on the Discovery A scanner.
All scans were obtained per study protocol by one of two certified DXA technologists and were re-analyzed by a single investigator (JD) using Apex software (Hologic Discovery A, software v. 12.7.3, Waltham, MA, USA). Sub-head BMC was assessed to represent whole body bone mass. Site-specific bone outcomes were selected for analysis based upon our prior work, choosing measures that demonstrated significant gymnastic loading associations (11, 16, 17) and/or strong agreement with pQCT outcomes (23).
Whole body scans yielded sub-head bone mineral content (subBMC, g), and non-bone lean mass (LM, g). Sub-head measures were used as outcomes, rather than whole-body, in accordance with recommendations by the International Society for Clinical Densitometry regarding pediatric densitometric analyses. PA lumbar spine scans yielded BMC for vertebra 3 (PA L3BMC, g), isolated from the remainder of the lumbar spine to allow comparison with our published analyses evaluating PA and lateral DXA results at L3 (16). Left proximal femur scans yielded femoral neck BMC (FNBMC, g), projected area (FNArea, cm2) and areal BMD (FNBMD, g/cm2), as well as Hip Structural Analysis (HSA) outcomes for the narrow neck. Narrow neck HSA outcomes included: section modulus (NNZ, cm3), periosteal width (NNwidth, cm), endosteal diameter (NNED, cm), cortical thickness (NNCT, cm), and buckling ratio (NNBR).
Non-dominant forearm scans assessed ultra-distal (UD) and 1/3 radius regions of interest, yielding BMC, Area and BMD for both sites. Non-standard, radius-based positioning ensured consistency within and between subjects across development, with the distal reference line positioned at the distal articular surface of the radius (ulnar aspect).(20) Indices of bone geometry and strength were derived from both 1/3 and UD radius results using formulae adapted from Sievänen et al. (23, 24) and validated by our group against pQCT results (23, 25), yielding 1/3 section modulus (1/3 Z, cm3) and UD Index of structural strength in axial compression (UD IBS, g2/cm4).
Lab-specific DXA root mean square error-based coefficients of variation (CVs) were calculated using duplicate scans of approximately 30 middle-aged females for each site. For subhead BMC and non-bone lean mass, CVs were <1.1%. For 1/3 and UD radius, CVs were ≤ 1% for BMC and ≤ 1.3% for projected area. Femoral Neck and PA spine CVs were ≤ 3%. Similar to other reports(26–28), narrow Neck HSA CVs ranged between 2% and 5%, with two exceptions: NNBR (6.0% vs. 4.6% (26)) and NNZ (11.6% vs. 4.0% (26) and 4.8% (27)). Thus, other than those two variables, our lab CVs are similar to those reported in other studies using HSA data. (26–28)
2.5. Statistical Analysis
Linear mixed effects models (29) were used both to explore and present the pattern of bone development over time, and to model the data and test specific hypotheses about bone mass, structure, and strength. Time-varying (Level 1) predictors (or adjustors) included time itself (gynecological age), non-bone sub-head lean mass (LM), and standing height, while time-invariant (Level 2) predictors included group (GYM vs. NON) and age at menarche. The measure of time in all analyses was gynecologic age (GYNAGE), calculated for each girl for each measurement date as the time before (negative) or after (positive) menarche (in years rounded to the nearest 0.1 year). Gynecological, versus chronological, age was used to account for variability in biological age at each measurement point. Standing height and sub-head non-bone lean mass were included to adjust for variation in body size. In this, we take the perspective that, to a large degree, bone adaptations to exercise are mediated by muscle/lean mass, and that our aim is to evaluate the effects of gymnastics participation on bone that are not mediated—or are in excess of those mediated—by muscle (e.g., impact forces). Chronologic age at menarche was entered at Level 2 to adjust for menarche timing as a potential factor in bone growth. All models included random subject-level intercepts to account for correlation between repeated measures on the same subjects.
2.5.1. Bone Development Curves
For each measure of bone growth, we constructed unadjusted exploratory plots by plotting each girl’s bone measure versus GYNAGE, and connecting the points. Light gray was used for the GYM group and dark gray was used for the NON group. These were complemented by mean growth curves estimated separately for each group using smoothing splines as a function of GYNAGE in linear mixed models. Plots adjusted for LM, height and age at menarche were based upon the linear mixed models for each group (GYM and NON) as a smooth function of GYNAGE. Adjusted values were generated by replacing the time-and-subject specific covariate values with their grand means computed across both groups, all subjects, and all times. Individual data points were adjusted by subtracting from each bone measure, the estimated covariate effect for the covariate value for that particular observation, and adding back in the covariate effect at the grand mean value of that covariate.
2.5.2. Effect of Gymnastics Participation
Because exploratory plots strongly indicated that mean growth curves for the GYM and NON groups were roughly parallel when aligned by GYNAGE, we pursued formal modeling and testing under the assumption that bone development curves for GYM and NON groups were parallel. We modeled them with smoothing splines in linear mixed models. Covariate effects were also assumed to be uniform across GYM and NON groups (LM, height, age at menarche).
Stated formally, bone measures yij (for subject i and observation j) were analyzed with the following statistical model:
where ui is a subject-specific random intercept, tij is gynecological age, f(tij) is a smoothing spline curve based on GYNAGE as “time” and eij is a error term. POSTQUIT is described below, and the other three predictors are adjustor variables. In this model, because the effects of the covariates and GYNAGE are uniform across the two groups, the coefficient b1 captures the scientific effect of interest, namely, the difference between the GYM and NON growth curves, “averaged” across GYNAGE.
2.5.3. Effect of Gymnastics Cessation
While our primary hypothesis was that gymnastics exposure during growth would be associated with bone benefits, we also sought to quantify whether post-menarcheal withdrawal from gymnastic training would generate a significant effect on bone outcomes. We formulated and tested the training withdrawal effect as a change in the GYM vs NON contrast as a function of the time since gymnastic cessation. As such, we created a new level 1 variable “POST-QUIT” (calculated as measurement date minus quit date, to 0.1 years). By definition, for subjects in the NON group, the value of POST-QUIT is always 0 (no quit date for NON). In the GYM group, for all time points prior to quitting gymnastics, the value of POST-QUIT is also 0; after quitting gymnastics, the value of POST-QUIT is equal to current date minus quit date. For example, if a subject quits gymnastics 4 years post-menarche (GYNAGE +4), then for all time points before GYNAGE +4, the variable POST-QUIT equals 0, and for the time point GYNAGE +6.5, POST-QUIT equals 2.5 years.
Thus, each gymnast is allowed her own quit date and the statistical effect of POST-QUIT captures average gymnastic withdrawal effects for the GYM group in comparison to the main GYM curve. The effect of POST-QUIT was assumed to be linear in time, starting from the POST-QUIT inflection point. Thus, if the coefficient for POST-QUIT is significant and positive, then at training cessation, slope for that bone outcome increases, indicating either an increase in growth rate or a reduction in the rate of decline, with time after training cessation. If the coefficient for POST-QUIT is significant and negative, at POST-QUIT, growth rate of that bone outcome decreases more rapidly with time or increases at a slower rate, after training cessation. It is important to note here that greater bone outcome values are not necessarily beneficial, so in some cases, an increase or slower rate of decrease is a negative consequence (e.g. buckling ratio).
We report estimates of coefficients for GYM versus NON and for POST-QUIT, with standard errors and p-values. Standardized coefficients were calculated as follows:
Data analyses were conducted using R (version 3.1.2).(30) Model fitting results for the 14 tests of the effect of GYM versus NON and the 14 tests of the effect of POST-QUIT are summarized in Table 4. The 28 reported p-values are unadjusted for multiple comparisons. Interpreted as such, the overall probability of type-I error is inflated. To control the overall family-wise type-I error rate, we apply the “false discovery rate” (FDR)(31, 32) procedure, with 5% FDR. In this multiple comparison adjustment, the 28 p-values are ordered from largest to smallest; the largest value that falls below its assigned threshold yields a test that is significant, as are all tests with still smaller p-values. In our case, the 8th p-value of 0.036 falls below its threshold of 0.0375, yielding 21 significant results at the 5% level, after this multiple comparison adjustment.
3. Results
Bone parameters were evaluated for GYM (n=13) and NON (n=31), representing growth from GYNAGE-2.0 years (pre-menarche) to +8.0 years (post-menarche). The number of annual DXA measurement sessions per subject varied from 3 to 10, with a mean ± standard deviation (SD) of 7.1 ± 1.9 (NON: 3 to 10 scans, mean=6.8 ± 2.1; GYM: 6 to 9 scans, mean=7.7 ± 1.0). Timing of included scans by gynecological age range is detailed by subject and group in Table 1.
Table 1.
Gynecologic Age at DXA Scan
| NON | GYM | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject ID | +2.00 to +1.01 | +1.00 to +0.01 | 0.00 to 0.99 | 1.00 to 1.99 | 2.00 to 2.99 | 3.00 to 3.99 | 4.00 to 4.99 | 5.00 to 5.99 | 6.00 to 6.99 | 7.00 to 7.99 | Total Measurement Sessions |
Subject ID | +2.00 to +1.01 | +1.00 to +0.01 | 0.00 to 0.99 | 1.00 to 1.99 | 2.00 to 2.99 | 3.00 to 3.99 | 4.00 to 4.99 | 5.00 to 5.99 | 6.00 to 6.99 | 7.00 to 7.99 | Total Measurement Sessions |
| 8 | 1 | 1 | 1 | 1 | 1 | 5 | 20 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | |||||||
| 13 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | 21 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | ||||
| 19 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | 29 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | |||
| 24 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | 49 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |||||||
| 31 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 10 | 71 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | ||||
| 46 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | 75 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | |||||
| 47 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | 89 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | |||||
| 50 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | 98 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | |||||
| 53 | 1 | 1 | 1 | 3 | 116 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | |||||||||
| 54 | 1 | 1 | 1 | 3 | 125 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | ||||||||
| 67 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | 174 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |||
| 70 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | 175 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | |||||||
| 82 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | 176 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | |||||
| 86 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | 11 | 10 | 11 | 13 | 11 | 10 | 9 | 7 | 8 | 10 | 100 | |||||
| 88 | 1 | 1 | 1 | 1 | 4 | ||||||||||||||||||
| 100 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | ||||||||||||
| 101 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |||||||||||||||
| 113 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | |||||||||||||
| 122 | 1 | 1 | 1 | 1 | 4 | ||||||||||||||||||
| 132 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | ||||||||||||||||
| 140 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | ||||||||||||||||
| 146 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | ||||||||||||||
| 153 | 1 | 1 | 1 | 1 | 1 | 5 | |||||||||||||||||
| 154 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |||||||||||||||
| 157 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | |||||||||||||
| 180 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |||||||||||||||
| 187 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | |||||||||||||
| 197 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | |||||||||||||
| 202 | 1 | 1 | 1 | 1 | 1 | 1 | 6 | ||||||||||||||||
| 207 | 1 | 1 | 1 | 1 | 1 | 5 | |||||||||||||||||
| 208 | 1 | 1 | 1 | 1 | 4 | ||||||||||||||||||
| 21 | 25 | 24 | 25 | 25 | 22 | 18 | 19 | 19 | 14 | 212 | |||||||||||||
3.1. Subject Characteristics
Table 2 lists subject characteristics and physical activity exposure by the gynecological age (GYNAGE) range in which each scan session fell. Average age at menarche was not significantly different between groups (GYM 13.5 ± 1.2 years, NON 12.9 ± 1.4 years). Height and lean mass were similar between groups across maturity, except at GYNAGE-1, when GYM had significantly more LM than NON, and at +6 years, when NON were significantly taller than GYM (p< 0.05).
Table 2.
Subject Characteristics by Gynecologic Age
| Gynecologic Age | −2 | −1 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| GYM | n | 11 | 10 | 11 | 13 | 11 | 10 | 9 | 7 | 8 | 10 |
| Height (cm) | 145.6 (6.2) | 152.1 (5.6) | 155.9 (9.04) | 157.3 (8.0) | 159.8 (7.4) | 158.8 (8.4) | 159.4 (7.9) | 159.4 (7.8) | 157.9 (7.9) | 158.3 (7.6) | |
| Lean Mass (kg) | 26.47 (3.61) | 31.12 (3.70) | 34.95 (4.78) | 35.99 (4.87) | 36.48 (4.76) | 37.03 (4.71) | 37.00 (5.17) | 37.66 (4.29) | 36.63 (3.48) | 38.38 (4.28) | |
| Gymnasts (n) | 11 | 10 | 11 | 13 | 8 | 5 | 2 | 3 | 1 | 1 | |
| Non-gym PA (hrs/wk) | 0.79 (1.62) | 1.84 (2.35) | 1.35 (1.48) | 1.50 (2.71) | 3.69 (4.15) | 1.38 (1.67) | 4.25 (2.27) | 0.58 (1.01) | |||
| Gymnastics (hrs/wk) | 13.53 (5.80) | 13.43 (4.53) | 14.74 (5.06) | 13.67 (6.04) | 10.19 (5.82) | 13.55 (7.95) | 12.77 (0.64) | 17.92 (1.32) | 15.82 | 15.82 | |
| Ex-gymnasts (n) | 0 | 0 | 0 | 0 | 3 | 5 | 7 | 4 | 7 | 9 | |
| Non-gym PA (hrs/wk) | 1.81 (3.13) | 3.56 (3.92) | 3.02 (2.97) | 6.62 (5.07) | 1.56 (1.11) | 7.04 (6.42) | |||||
| Gymnastics (hrs/wk) | 11.31 (3.92) | 5.72 (4.44) | 0.76 (2.02) | 0.63 (1.26) | 4.26 (6.85) | ||||||
|
| |||||||||||
| NON | n | 22 | 25 | 24 | 25 | 25 | 21 | 17 | 18 | 18 | 13 |
| Height (cm) | 147.9 (9.1) | 152.4 (7.1) | 158.1 (7.0) | 160.9 (6.6) | 162.0 (6.2) | 162.0 (5.7) | 164.0 (8.1) | 162.7 (6.6) | 164.4 (7.1)* | 162.9 (6.9) | |
| Lean Mass (kg) | 24.92 (4.02) | 28.26 (3.41) * | 32.63 (3.51) | 34.46 (3.96) | 35.27 (4.28) | 35.88 (3.14) | 37.02 (4.71) | 37.80 (3.48) | 37.53 (4.55) | 36.70 (2.65) | |
| Non-gym PA (hrs/wk) | 2.74 (2.67) | 3.24 (2.50) | 3.44 (2.70) | 5.15 (3.67) | 4.83 (2.95) | 6.30 (3.39) | 4.77 (3.36) | 5.24 (3.30) | 4.97 (3.62) | 3.65 (3.83) | |
Means and standard deviations (SD) are listed for height, lean mass and physical activity participation at each gynecologic age, separated for gymnasts (GYM) and non-gymnasts (NON). Data were included in a specific whole year gynecologic age x if they were obtained between x and x+0.9 gynecologic age. Gymnasts are further subdivided into gymnast and ex-gymnast categories for the presentation of activity participation (gymnastic and non-gymnastic physical activity), based upon subject classification at each gynecologic age. A subject was categorized as an ex-gymnast for the gynecologic age interval in which she ceased gymnastics participation.
PA=physical activity, non-gym=non-gymnastics
significant difference from GYM group (p<0.05)
By study design, all GYM continued to participate in gymnastics through GYNAGE 1.5. However, all but one gymnast ceased gymnastics participation before their final measurement date, with their gymnastics quit dates ranging from GYNAGE +1.9 to +8.5 years (mean 4.1 ± 1.9). From GYNAGE +3 years onwards, <50% of GYM were still participating in gymnastics training. Also, many gymnasts reported injuries during this phase of maturity, possibly leading to training cessation.
Many subjects reported the use of oral contraceptive medication during the study period. Four subjects (3 NON, 1 GYM) used oral contraceptive pills prior to GYNAGE +4, for the reported purpose of improving menstrual regularity. An additional 15 NON and 10 GYM used oral contraceptives beginning at GYNAGE ≥ 4. This information was not recorded for 4 NON. In total, 66% of subjects used oral contraceptives, or may have used them (considering unrecorded information), at some point in the study period (61% of NON, 77% of GYM).
3.2. Gymnast Advantages
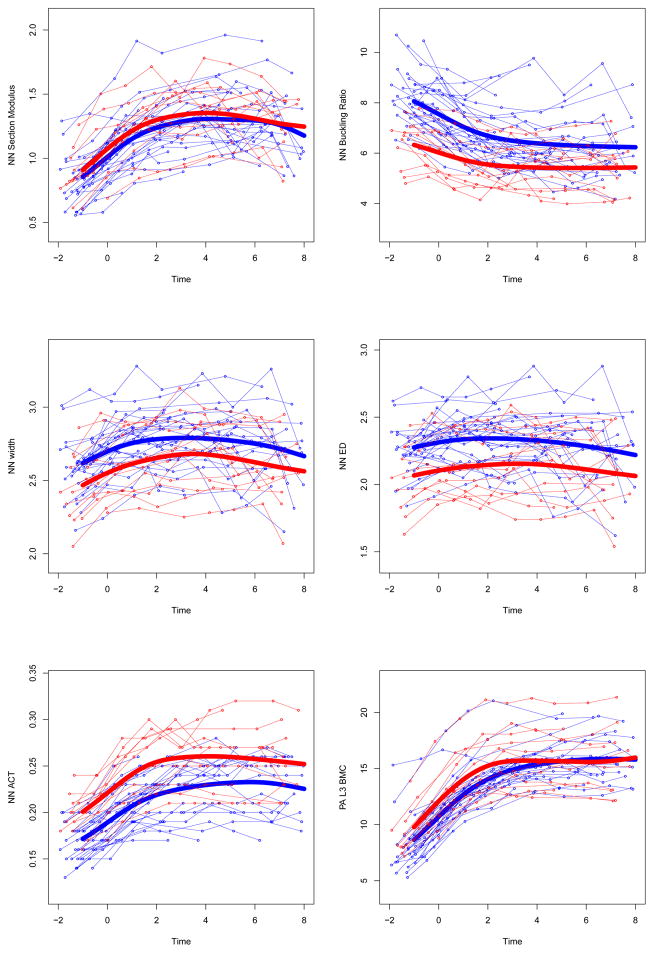
After incorporating and testing for the potential effect of training cessation as a variable in our models, mean growth curves for the GYM group were significantly different than those for the NON group for all bone outcomes after multiple testing adjustment, except for NN section modulus (p=0.08) and NN width (p=0.09). Table 3 presents coefficients for group membership, with standard errors and significance. Growth curves for bone parameters, divided by GYM and NON groups, are presented in Figure 2.
Table 3.
Key Linear Mixed Effect Model Statistics
| Variable | GYM vs NON | POST-QUIT (PER YEAR) | ||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | Standardized Coefficient | (standard error) | p | Coefficient | Standardized Coefficient | (standard error) | p | |
| Sub BMC (g) | 82.41 | 0.664 | (39.08) | 0.036 | −5.893 | −0.048 | (6.010) | 0.328 |
| 1/3 Area (cm2) | 0.334 | 1.539 | (0.072) | <0.0001 | 0.018 | 0.083 | (0.007) | 0.010 |
| 1/3 BMC (g) | 0.339 | 2.042 | (0.054) | <0.0001 | 0.019 | 0.114 | (0.006) | 0.002 |
| 1/3 Z (cm3) | 0.196 | 1.677 | (0.037) | <0.0001 | 0.025 | 0.214 | (0.005) | 0.000 |
| UD Area (cm2) | 0.281 | 1.546 | (0.059) | <0.0001 | 0.011 | 0.061 | (0.007) | 0.120 |
| UD BMC (g) | 0.386 | 2.375 | (0.051) | <0.0001 | −0.056 | −0.345 | (0.008) | 0.000 |
| UD IBS (g2/cm4) | 0.093 | 1.871 | (0.015) | <0.0001 | −0.022 | −0.443 | (0.003) | 0.000 |
| FN BMC (g) | 0.361 | 0.86 | (0.133) | 0.007 | −0.046 | −0.11 | (0.019) | 0.017 |
| NN Width (cm) | −0.092 | −0.521 | (0.054) | 0.089 | 0.003 | 0.017 | (0.010) | 0.735 |
| NN Z (cm3) | 0.091 | 0.539 | (0.052) | 0.080 | −0.015 | −0.089 | (0.009) | 0.113 |
| NN ED (cm) | −0.154 | −0.768 | (0.062) | 0.013 | 0.011 | 0.055 | (0.010) | 0.275 |
| NN CT (cm) | 0.033 | 1.163 | (0.009) | <0.0001 | −0.004 | −0.141 | (0.001) | 0.001 |
| NN BR | −1.354 | −1.139 | (0.383) | <0.0001 | 0.145 | 0.122 | (0.047) | 0.002 |
| PA L3 BMC (g) | 1.380 | 0.789 | (0.561) | 0.015 | −0.284 | −0.162 | (0.074) | 0.000 |
Sub= sub-head, 1/3= 1/3 radial diaphysis region of interest, BMC= bone mineral content, Z= section modulus, UD= ultradistal radial metaphysis region of interest, IBS= index of bone strength in axial compression, FN= femoral neck, NN= narrow neck, Z= section modulus, ED= endosteal diameter, CT= cortical thickness, BR= buckling ratio, PA L3= postero-anterior lumbar spine vertebra 3.
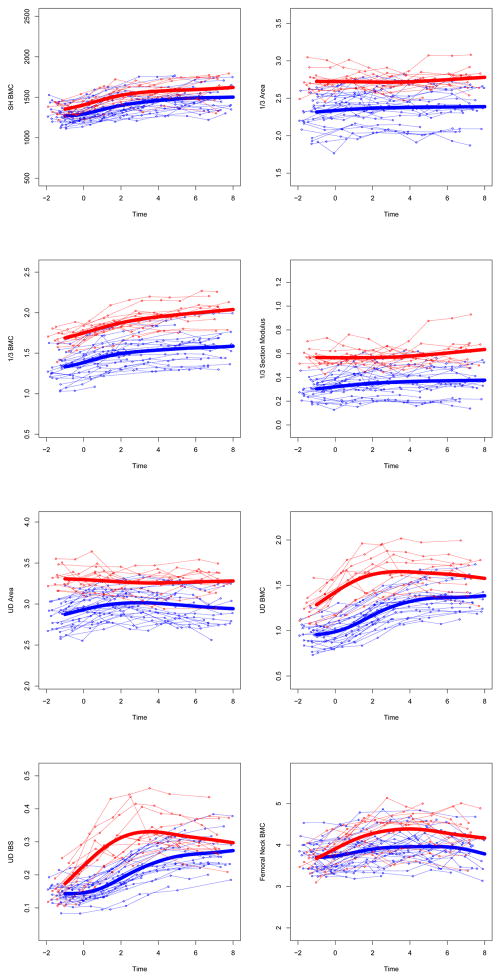
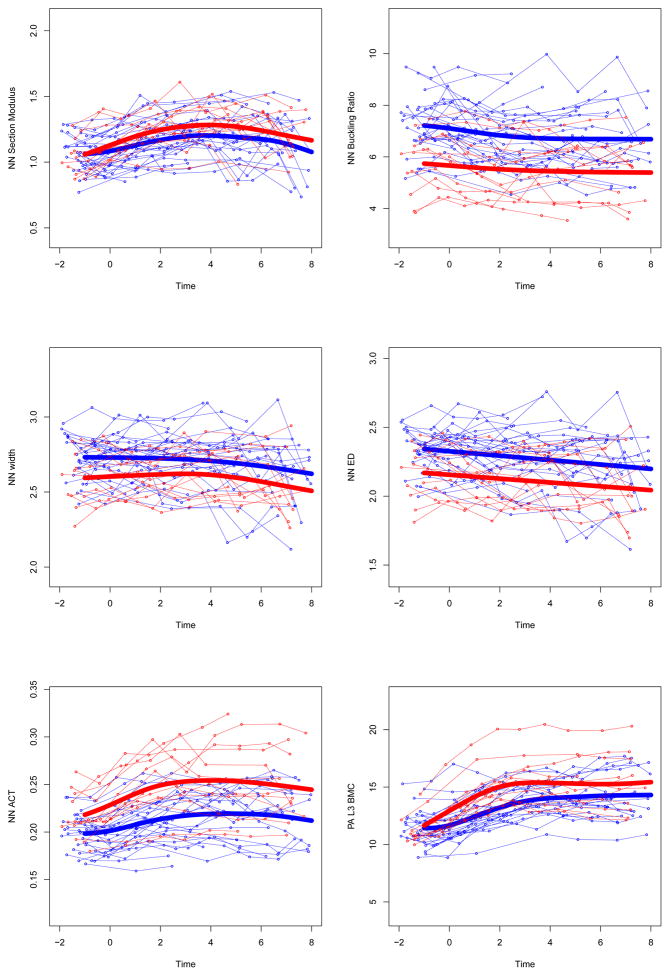
Figure 2. Adjusted individual time-series and group mean growth curves for bone outcomes.
Adjusted data points are computed as described in the statistical methods section (GYM=light gray, NON= dark gray). Adjusted points and curves remove effects of chronologic age at menarche, height, and non-bone lean mass by adjusting them to their grand mean values. Units for bone outcome measures are given in Table 3.
UD=ultra-distal, BMC=bone mineral content, IBS=index of bone strength in axial compression, NN=narrow neck, PA=postero-anterior, L3= 3rd lumbar vertebrae
At both 1/3 and ultradistal radius regions (diaphysis and metaphysis, respectively), growth curves for bone area and mass were elevated in GYM relative to NON, yielding GYM advantages in 1/3 Z and UD IBS (p<0.0001). In contrast, at the femoral neck, growth curves demonstrated greater GYM BMC (p<0.01) combined with narrower GYM endosteal diameter (p<0.02) and similar periosteal width (p=0.09), yielding GYM advantages in narrow neck cortical thickness and buckling ratio (p<0.001, lower BR indicates lower fracture risk). At the lumbar spine, GYM BMC was greater than NON BMC across growth (p<0.02). Similarly, sub-head BMC was, on average, greater in GYM than NON across growth (p<0.036).
3.3 Withdrawal Effects After Quitting Gymnastics
Following gymnastics cessation, GYM bone outcomes increased at a faster rate (or decreased at a slower rate) relative to their prior bone trajectories for 1/3 distal radius Area, BMC and section modulus (positive coefficients, p<0.01). In contrast, a POST-QUIT increase in slope for narrow neck buckling ratio at the training cessation transition indicated a subtle increase in fracture risk (or slowing in the rate of decreasing fracture risk) (positive coefficient, p=0.002). GYM slopes decreased POST-QUIT for ultradistal BMC, ultradistal IBS, femoral neck BMC, narrow neck cortical thickness, and PA L3 BMC, indicating either slowed growth rate or increased rate of decline compared to growth rates during training (negative coefficient, p<0.036). There were no significant training withdrawal effects on growth rates for sub-head BMC, ultradistal Area, narrow neck width, narrow neck endosteal diameter, or narrow neck section modulus.
3.4. Secondary Results
As would be expected, in models for all variables except NN width and NN endosteal diameter (p>0.37), sub-head lean mass exhibited a significant coefficient, indicating that greater lean mass is associated with advantages in indices of bone mass, geometry and strength (p≤0.002; specific results not shown).
4. Discussion
In this group of young girls, evaluated longitudinally over a period spanning from pre-menarche to up to 8 years post-menarche, we identified advantages in bone parameters at multiple sites for gymnasts compared to non-gymnasts. Specifically, after aligning data by biological age (gynecologic age), adjusting for variability in body size (height and lean mass), accounting for variability in maturational timing (age at menarche) and adjusting for the effect of training withdrawal (post-quit), girls exposed to gymnastics from pre-menarche to more than 1.5 yrs. post-menarche demonstrated bone mass advantages at the distal radius metaphysis and diaphysis, lumbar spine, femoral neck and sub-head regions of interest. While our prior data (20) and those of Jackowski et al. (33) demonstrate distal radius benefits associated with gymnastic training early in growth and up to menarche, the present study is the first to longitudinally demonstrate loading-related advantages in bone parameters at multiple sites over the entire span of adolescent growth.
Our data confirm site-specific variation in loading-associated bone growth. Bone width was greater for gymnasts compared to non-gymnasts at both the distal radius metaphysis and diaphysis (area). Combined with greater bone mass, periosteal expansion at the distal radius yielded a theoretical advantage in diaphysis bending strength and metaphysis compressive strength. This combination of loading-related adaptations in the distal radius has been previously noted in gymnasts and other athletes in cross-sectional and longitudinal analyses by our group and others. (7–9, 20, 34–36) In contrast, at the femoral narrow neck, gymnastic exposure was not associated with greater periosteal expansion, but with similar narrow neck periosteal width and smaller endosteal diameter, compared to non-gymnasts. Combined with greater overall femoral neck bone mass, this compact geometry was associated with gymnast advantages in narrow neck cortical thickness, yielding lower mean buckling ratio (low fracture risk). This distinctive pattern has been identified in three cross-sectional studies demonstrating diminished narrow neck and endosteal diameter (17, 28, 37) and enhanced cortical thickness (17, 28) for pre- and post-menarcheal gymnasts, including a large group of similar subjects from our own cohort.(17)
In a similar, excellent, mixed longitudinal study, Nurmi-Lawton et al. demonstrate gymnast advantages in bone mass accrual patterns, pieced together using 1–4 annual assessments in 45 gymnasts and 52 non-gymnasts over a shorter interval surrounding peak height velocity (spanning up to 3 years) (38). Our findings reflect longer growth periods in a smaller number of subjects (6–9 years, 13 gymnasts; 2–9 years, 31 non-gymnasts). Thus, the current study is the first to demonstrate gymnast advantages on a longitudinal basis, across a broad growth curve, in the same individuals, spanning from childhood into young adulthood. Our observational longitudinal data provide strong evidence for loading-associated advantages in bone acquisition and geometric patterning that result in improved bone strength over the course of adolescent growth and into young adulthood.
Although we did not specifically tailor this analysis to evaluate maintenance of benefits acquired from adolescent exercise, we did evaluate the effect of gymnastics cessation on site-specific bone parameters. By design, our GYM group was comprised of subjects who participated in gymnastics from pre-menarche until at least 1.5 years post-menarche; all but one gymnast had quit by the time of the final follow-up assessment. Time between quit and final follow-up ranged from 1.1 to 5.1 years (mean 3.2 ± 1.4). Our statistical design allowed us to account for this variability in cessation timing by evaluating the associated change in growth rate for each girl, compiling those data to test a group-specific withdrawal effect. As the quit dates were all POST-menarcheal, this statistical effect specifically relates to POST-menarcheal training cessation, in contrast to our prior work limited to the distal radius that evaluated PERI-menarcheal training cessation.(20)
At the predominantly cortical distal radius diaphysis, where strong GYM advantages were noted during gymnastics participation, growth rates in bone mass, area and strength either increased or exhibited a reduced rate of decrease after gymnastics cessation (1/3 BMC, area and strength). We identified no effect of training withdrawal on growth in bone geometry at the ultradistal radius or femoral narrow neck sites. This was not surprising, as recent work by others supports the concepts that bone geometry is less malleable post-menarche (36, 39) and that geometric adaptations are sustained into adulthood (20, 40). In contrast, GYM growth rates decreased post-quit for metabolically sensitive bone parameters at the distal radius metaphysis, lumbar spine and femoral neck (ultradistal, femoral neck and lumbar spine BMC; narrow neck cortical thickness; ultradistal compressive strength). As expected, these corticocancellous sites, comprised of a large percentage of trabecular bone, and therefore high metabolic activity, were susceptible to a decrease in bone mass growth rate following loading withdrawal. GYM advantages in narrow neck cortical thickness were based upon the combination of narrower bone width and narrower endosteal diameter associated with gymnastic loading. Although post-quit changes in narrow neck width and endosteal diameter growth were not significant, we surmise that withdrawal of loading post-menarche results in lower prioritization of BMC to the endosteum, allowing cortical thickness growth rate to decline post-quit. Similarly, a decrease in mechanical loading at the distal radius metaphysis would reduce prioritization of bone mass at that site, reducing compressive strength growth rate. Continued longitudinal data collection in larger numbers of ex/gymnasts and non-gymnasts will enable us to specifically evaluate long-term retention of skeletal benefits beyond loading cessation, over an extended period of bone growth and maintenance.
The existing literature, consisting primarily of cross-sectional analyses in adult former gymnasts, demonstrates gymnast advantages in aBMD at the forearm, lumbar spine, femoral neck and total body up to 24 years post-retirement.(41–44) Two cross-sectional studies using pQCT have identified significant theoretical strength advantages at the distal radius of young adult, former female gymnasts: at the metaphysis, 6–14 yrs. post-retirement(44); at the diaphysis, 3–18 years post-retirement(45). In a longitudinal analysis of ex-gymnasts from our own cohort, loading-associated benefits in bone mass and area for distal radius metaphysis and diaphysis sites were evident across growth, including data extending 4–9 years post-retirement.(15) These studies and our current results provide evidence that gymnast skeletal advantages at multiple sites, acquired during childhood and adolescent participation, are likely maintained after gymnastics cessation and into adulthood. Further study, evaluating a larger number of subjects with variable loading profiles at different maturity phases will provide additional information regarding loading-related bone acquisition and maintenance.
4.1. Limitations
Our study is observational, not interventional in design; group membership was assigned based upon sport participation at study outset. Thus, it is theoretically possible that group differences reflect inherent bias related to group selection. However, Laing et al. previously evaluated bone properties in pre-pubertal females; they noted lower bone mineral and smaller body size in a group of 65 girls who enrolled in gymnastics compared to a group of 78 girls who did not enroll.(46) Over two years, gymnasts demonstrated greater rate of gain in mean forearm bone area and lumbar spine aBMD compared to non-gymnasts. This work disputes the notion that selection bias is responsible for greater bone mineral in gymnasts than non-gymnasts.
Three-dimensional strength parameters at the radius were derived from two-dimensional DXA outcomes using simplified geometric models. However, these outcomes were specifically chosen for analysis because they had previously demonstrated strong agreement with three-dimensional pQCT measurements in similar subjects from our longitudinal study cohort (R=0.97, DXA IBS vs. pQCT IBS; R=0.96, DXA 1/3 Z v pQCT SSI).(23) Nonetheless, three-dimensional assessments, such as those provided by pQCT and MRI, which distinguish between trabecular and cortical bone, are necessary to corroborate our findings.
Only PA DXA data were available for analysis of lumbar spine adaptations, limiting our ability to evaluate vertebral geometry. Our prior work in a similar cohort, evaluating PA and lateral lumbar spine scans, has demonstrated that PA BMC advantages are mainly attributable to cortical adaptation of the posterior elements, not trabecular advantages which would contribute to vertebral strength.(16) Gymnast strength advantages at the spine are related to greater geometric cross-sectional growth, specifically vertebral width, which cannot be isolated without combining PA scans and lateral lumbar spine scans.(16) Therefore, future longitudinal analyses of paired PA and lateral DXA data are necessary for the appropriate evaluation of maturity-specific adaptation to gymnastics loading at the lumbar spine.
Our models included coefficients for subhead lean mass, and these coefficients indicated a significant association between greater lean mass and advantageous bone properties for all but NN width and endosteal diameter. Thus, our gymnast growth curve advantages may be considered conservative, if lean mass is a key link in loading adaptation. Future analyses in the expanded sample across the expanded growth curve will allow for more in depth analysis of the relationship between lean mass and bone in the context of mechanical loading exposure during growth.
The sample size available for this analysis was governed by our stringent inclusion criteria, which were purposefully employed to optimize the integrity of our analyses. We excluded subjects who provided fewer than 3 data points, and limited the gynecologic age range of our analyses to the portion of the growth curve for which the largest number of subjects had provided data. All but one subject provided multiple data points across or during the peri-menarcheal phase (Table 1). With these strategies, we minimized undue influence from subjects who had only provided data on the extremes of the curve (minimum sample size was ≥ 10 per group at curve extremes). Despite a relatively small sample size, our sample size was adequate to detect significant differences between group growth curves across the represented growth phases for most variables. With continued data collection in our younger cohort of subjects, greater sample size and statistical power will allow evaluation of advantages in a wider variety of bone outcomes.
Finally, these analyses did not evaluate potential influence from oral contraceptive use or nutritional variation, as these data were not complete for all subjects. The inability to evaluate these variables is a limitation of the study.
4.2. Conclusions
This longitudinal study demonstrates skeletal advantages in gymnasts vs. non-gymnasts, spanning development from childhood to early adulthood. It provides longitudinal evidence that loading-associated bone acquisition and adaptation vary uniquely by skeletal site. At the radius and proximal femur, gymnastics participation was associated with advantages in bone geometry, mass, and strength. In contrast to the radius, site-specific proximal femur narrow neck strength advantages were a product of narrower width and endosteal diameter, yielding greater cortical thickness and strength. Loading-associated advantages in bone mass were also noted at the lumbar spine and sub-head regions. Finally, geometric skeletal loading advantages acquired during growth did not appear to diminish after gymnastics cessation. As greater bone mass, geometry, and strength reduce osteoporosis and fracture risk, our results indicate that mechanical loading during pre-, peri- and post-menarcheal growth yields a persistent decrement in risk of morbidity and mortality associated with osteoporotic fracture.
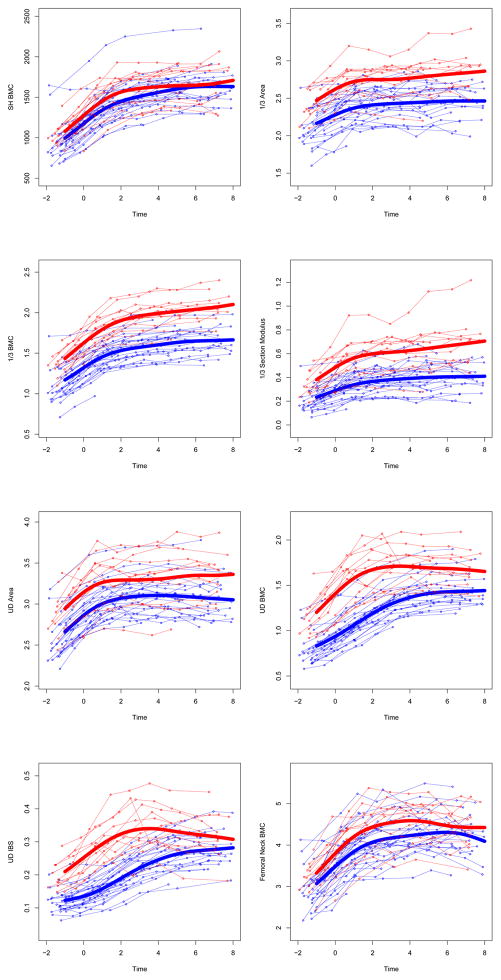
Appendix 1. Unadjusted individual time-series and group mean growth curves for bone outcomes.
Unadjusted raw data points are plotted and connected for each subject (GYM=light gray, NON= dark gray). Unadjusted group mean growth curves (thick lines) are plotted as a cubic spline function of gynecological age. Units for bone outcome measures are given in Table 3.
UD=ultra-distal, BMC=bone mineral content, IBS=index of bone strength in axial compression, NN=narrow neck, PA=postero-anterior, L3= 3rd lumbar vertebrae
Distal radius and femoral neck strength increase with peri-menarcheal loading.
Narrow endosteal diameter, greater cortical thickness produce femoral neck strength.
Lumbar spine and subhead BMC are greater with peri-menarcheal loading.
When loading ceases, cortical bone may improve, corticocancellous bone may diminish.
Acknowledgments
The project described was funded by grants from the Orthopedic Research and Education Foundation, SUNY Upstate Medical University, the National Institute of Arthritis, Musculoskeletal and Skin Diseases (R03AR047613; R01AR054145). This publication was made possible by bridge funding from the University of Wisconsin-Madison, through both the Department of Orthopedics and Rehabilitation and School of Medicine and Public Health. Biostatistics funding support was provided by the University of Wisconsin Institute for Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Services (NCATS), grant UL1TR000427. These funding sources had no involvement in the study design, in the collection, analysis and interpretation of data, in the writing of the manuscript or in the decision to submit it for publication.
Footnotes
All authors have no conflict of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sabatier JP, Guaydier-Souquieres G, Benmalek A, Marcelli C. Evolution of lumbar bone mineral content during adolescence and adulthood: A longitudinal study in 395 healthy females 10–24 years of age and 206 premenopausal women. Osteoporos Int. 1999;9(6):476–82. doi: 10.1007/s001980050173. [DOI] [PubMed] [Google Scholar]
- 2.Cadogan J, Blumsohn A, Barker ME, Eastell R. A longitudinal study of bone gain in pubertal girls: Anthropometric and biochemical correlates. J Bone Miner Res. 1998 Oct;13(10):1602–12. doi: 10.1359/jbmr.1998.13.10.1602. [DOI] [PubMed] [Google Scholar]
- 3.Bailey DA. The Saskatchewan pediatric bone mineral accrual study: Bone mineral acquisition during the growing years. Int J Sports Med. 1997 Jul;18( Suppl 3):S191–4. doi: 10.1055/s-2007-972713. [DOI] [PubMed] [Google Scholar]
- 4.Bernardoni B, Scerpella T, Rosenbaum P, Kanaley JA, Raab L, Li Q, Wang S, Dowthwaite JN. The influence of organized physical activity (including gymnastics) on young adult skeletal traits: Is maturity phase important? Pediatric exercise science. 2015 May;27(2):285–96. doi: 10.1123/pes.2014-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackelvie KJ, McKay HA, Khan KM, Crocker PR. A school-based exercise intervention augments bone mineral accrual in early pubertal girls. J Pediatr. 2001 Oct;139(4):501–8. doi: 10.1067/mpd.2001.118190. [DOI] [PubMed] [Google Scholar]
- 6.Heinonen A, Sievanen H, Kannus P, Oja P, Pasanen M, Vuori I. High-impact exercise and bones of growing girls: A 9-month controlled trial. Osteoporos Int. 2000;11(12):1010–7. doi: 10.1007/s001980070021. [DOI] [PubMed] [Google Scholar]
- 7.Duncan CS, Blimkie CJ, Cowell CT, Burke ST, Briody JN, Howman-Giles R. Bone mineral density in adolescent female athletes: Relationship to exercise type and muscle strength. Med Sci Sports Exerc. 2002 Feb;34(2):286–94. doi: 10.1097/00005768-200202000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Courteix D, Lespessailles E, Peres SL, Obert P, Germain P, Benhamou CL. Effect of physical training on bone mineral density in prepubertal girls: A comparative study between impact-loading and non-impact-loading sports. Osteoporos Int. 1998;8(2):152–8. doi: 10.1007/BF02672512. [DOI] [PubMed] [Google Scholar]
- 9.Cassell C, Benedict M, Specker B. Bone mineral density in elite 7- to 9-yr-old female gymnasts and swimmers. Med Sci Sports Exerc. 1996 Oct;28(10):1243–6. doi: 10.1097/00005768-199610000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Grimston SK, Willows ND, Hanley DA. Mechanical loading regime and its relationship to bone mineral density in children. Med Sci Sports Exerc. 1993 Nov;25(11):1203–10. [PubMed] [Google Scholar]
- 11.Dowthwaite JN, Flowers PPE, Spadaro JA, Scerpella TA. Bone geometry, density and strength indices of the distal radius reflect loading via childhood gymnastic activity. J Clin Densitom. 2007;10:65, 65–75. doi: 10.1016/j.jocd.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowthwaite JN, Scerpella TA. Skeletal geometry and indices of bone strength in artistic gymnasts. J Musculoskelet Neuronal Interact. 2009 Oct-Dec;9(4):198–214. [PMC free article] [PubMed] [Google Scholar]
- 13.Ward KA, Roberts SA, Adams JE, Mughal MZ. Bone geometry and density in the skeleton of pre-pubertal gymnasts and school children. Bone. 2005 Jun;36(6):1012–8. doi: 10.1016/j.bone.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Dyson K, Blimkie CJR, Davison KS, Webber CE, Adachi JD. Gymnastic training and bone density in pre-adolescent females. Med Sci Sports Exerc. 1997;29:443, 443–50. doi: 10.1097/00005768-199704000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Dowthwaite JN, Scerpella TA. Distal radius geometry and skeletal strength indices after peripubertal artistic gymnastics. Osteoporos Int. 2011 Jan;22(1):207–16. doi: 10.1007/s00198-010-1233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dowthwaite JN, Rosenbaum PF, Scerpella TA. Mechanical loading during growth is associated with plane-specific differences in vertebral geometry: A cross-sectional analysis comparing artistic gymnasts vs. non-gymnasts. Bone. 2011 Nov;49(5):1046–54. doi: 10.1016/j.bone.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dowthwaite JN, Rosenbaum PF, Scerpella TA. Site-specific advantages in skeletal geometry and strength at the proximal femur and forearm in young female gymnasts. Bone. 2012 May;50(5):1173–83. doi: 10.1016/j.bone.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bailey DA, McKay HA, Mirwald RL, Crocker PR, Faulkner RA. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: The University of Saskatchewan bone mineral accrual study. J Bone Miner Res. 1999 Oct;14(10):1672–9. doi: 10.1359/jbmr.1999.14.10.1672. [DOI] [PubMed] [Google Scholar]
- 19.Gunter K, Baxter-Jones AD, Mirwald RL, Almstedt H, Fuchs RK, Durski S, Snow C. Impact exercise increases BMC during growth: An 8-year longitudinal study. J Bone Miner Res. 2008 Jul;23(7):986–93. doi: 10.1359/JBMR.071201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scerpella TA, Dowthwaite JN, Rosenbaum PF. Sustained skeletal benefit from childhood mechanical loading. Osteoporos Int. 2011 Sep 14;22:2205–10. doi: 10.1007/s00198-010-1373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scerpella TA, Davenport M, Morganti CM, Kanaley JA, Johnson LM. Dose related association of impact activity and bone mineral density in pre-pubertal girls. Calcif Tissue Int. 2003 Jan;72(1):24–31. doi: 10.1007/s00223-001-1131-x. [DOI] [PubMed] [Google Scholar]
- 22.Dowthwaite JN, DiStefano JG, Ploutz-Snyder RJ, Kanaley JA, Scerpella TA. Maturity and activity-related differences in bone mineral density: Tanner I vs. II and gymnasts vs. non-gymnasts. Bone. 2006 Oct;39(4):895–900. doi: 10.1016/j.bone.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Dowthwaite JN, Flowers PPE, Scerpella TA. Agreement between pQCT and DXA-derived indices of bone geometry, density and theoretical strength in females of varying age, maturity and physical activity. J Bone Miner Res [Internet] 2011;26:1349–57. doi: 10.1002/jbmr.322. Published online Dec 28, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sievanen H, Kannus P, Nieminen V, Heinonen A, Oja P, Vuori I. Estimation of various mechanical characteristics of human bones using dual energy X-ray absorptiometry: Methodology and precision. Bone. 1996 Jan;18(1 Suppl):17S–27S. doi: 10.1016/8756-3282(95)00376-2. [DOI] [PubMed] [Google Scholar]
- 25.Dowthwaite JN, Hickman RM, Kanaley JA, Ploutz-Snyder RJ, Spadaro JA, Scerpella TA. Distal radius strength: A comparison of DXA-derived vs pQCT-measured parameters in adolescent females. J Clin Densitom. 2009 Jan-Mar;12(1):42–53. doi: 10.1016/j.jocd.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Khoo BC, Beck TJ, Qiao QH, Parakh P, Semanick L, Prince RL, Singer KP, Price RI. In vivo short-term precision of hip structure analysis variables in comparison with bone mineral density using paired dual-energy X-ray absorptiometry scans from multi-center clinical trials. Bone. 2005 Jul;37(1):112–21. doi: 10.1016/j.bone.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Nikander R, Sievanen H, Heinonen A, Kannus P. Femoral neck structure in adult female athletes subjected to different loading modalities. J Bone Miner Res. 2005 Mar;20(3):520–8. doi: 10.1359/JBMR.041119. [DOI] [PubMed] [Google Scholar]
- 28.Maimoun L, Coste O, Mariano-Goulart D, Galtier F, Mura T, Philibert P, Briot K, Paris F, Sultan C. In peripubertal girls, artistic gymnastics improves areal bone mineral density and femoral bone geometry without affecting serum OPG/RANKL levels. Osteoporos Int. 2011 Feb 26; doi: 10.1007/s00198-011-1541-1. [DOI] [PubMed] [Google Scholar]
- 29.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. Hoboken, NJ: John Wiley & Sons; 2012. [Google Scholar]
- 30.Wood SN. Generalized Additive Models: An Introduction with R. Chapman and Hall/CRC Press; 2006. [Google Scholar]
- 31.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Royal Stat Soc. 1995;57:289–300. [Google Scholar]
- 32.Storey JD, Tayler JE, Siegmund D. Strong control, conservative point estimation and simultaneous conservative consistency of false discovery rates: A unified approach. J Royal Stat Soc. 2004;66:187–205. [Google Scholar]
- 33.Jackowski SA, Baxter-Jones AD, Gruodyte-Raciene R, Kontulainen SA, Erlandson MC. A longitudinal study of bone area, content, density, and strength development at the radius and tibia in children 4–12 years of age exposed to recreational gymnastics. Osteoporos Int. 2015 Jun;26(6):1677–90. doi: 10.1007/s00198-015-3041-1. [DOI] [PubMed] [Google Scholar]
- 34.Bass SL, Saxon L, Daly RM, Turner CH, Robling AG, Seeman E, Stuckey S. The effect of mechanical loading on the size and shape of bone in pre-, peri-, and postpubertal girls: A study in tennis players. J Bone Miner Res. 2002 Dec;17(12):2274–80. doi: 10.1359/jbmr.2002.17.12.2274. [DOI] [PubMed] [Google Scholar]
- 35.Heinonen A, Sievanen H, Kannus P, Oja P, Vuori I. Site-specific skeletal response to long-term weight training seems to be attributable to principal loading modality: A pQCT study of female weightlifters. Calcif Tissue Int. 2002 Jun;70(6):469–74. doi: 10.1007/s00223-001-1019-9. [DOI] [PubMed] [Google Scholar]
- 36.Kontulainen S, Sievanen H, Kannus P, Pasanen M, Vuori I. Effect of long-term impact-loading on mass, size, and estimated strength of humerus and radius of female racquet-sports players: A peripheral quantitative computed tomography study between young and old starters and controls. J Bone Miner Res. 2003 Feb;18(2):352–9. doi: 10.1359/jbmr.2003.18.2.352. [DOI] [PubMed] [Google Scholar]
- 37.Faulkner RA, Forwood MR, Beck TJ, Mafukidze JC, Russell K, Wallace W. Strength indices of the proximal femur and shaft in prepubertal female gymnasts. Med Sci Sports Exerc. 2003 Mar;35(3):513–8. doi: 10.1249/01.MSS.0000053724.33480.8B. [DOI] [PubMed] [Google Scholar]
- 38.Nurmi-Lawton JA, Baxter-Jones AD, Mirwald RL, Bishop JA, Taylor P, Cooper C, New SA. Evidence of sustained skeletal benefits from impact-loading exercise in young females: A 3-year longitudinal study. J Bone Miner Res. 2004 Feb;19(2):314–22. doi: 10.1359/JBMR.0301222. [DOI] [PubMed] [Google Scholar]
- 39.Ireland A, Maden-Wilkinson T, Ganse B, Degens H, Rittweger J. Effects of age and starting age upon side asymmetry in the arms of veteran tennis players: A cross-sectional study. Osteoporos Int. 2014 Apr;25(4):1389–400. doi: 10.1007/s00198-014-2617-5. [DOI] [PubMed] [Google Scholar]
- 40.Warden SJ, Hurst JA, Sanders MS, Turner CH, Burr DB, Li J. Bone adaptation to a mechanical loading program significantly increases skeletal fatigue resistance. J Bone Miner Res. 2005 May;20(5):809–16. doi: 10.1359/JBMR.041222. [DOI] [PubMed] [Google Scholar]
- 41.Erlandson MC, Kontulainen SA, Chilibeck PD, Arnold CM, Faulkner RA, Baxter-Jones AD. Higher premenarcheal bone mass in elite gymnasts is maintained into young adulthood after long-term retirement from sport: A 14-year follow-up. J Bone Miner Res. 2012 Jan;27(1):104–10. doi: 10.1002/jbmr.514. [DOI] [PubMed] [Google Scholar]
- 42.Pollock NK, Laing EM, Modlesky CM, O’Connor PJ, Lewis RD. Former college artistic gymnasts maintain higher BMD: A nine-year follow-up. Osteoporos Int. 2006;17(11):1691–7. doi: 10.1007/s00198-006-0181-3. [DOI] [PubMed] [Google Scholar]
- 43.Bass S, Pearce G, Bradney M, Hendrich E, Delmas PD, Harding A, Seeman E. Exercise before puberty may confer residual benefits in bone density in adulthood: Studies in active prepubertal and retired female gymnasts. J Bone Miner Res. 1998 Mar;13(3):500–7. doi: 10.1359/jbmr.1998.13.3.500. [DOI] [PubMed] [Google Scholar]
- 44.Erlandson MC, Kontulainen SA, Chilibeck PD, Arnold CM, Faulkner RA, Baxter-Jones AD. Former premenarcheal gymnasts exhibit site-specific skeletal benefits in adulthood after long-term retirement. J Bone Miner Res. 2012 Nov;27(11):2298–305. doi: 10.1002/jbmr.1689. [DOI] [PubMed] [Google Scholar]
- 45.Eser P, Hill B, Ducher G, Bass SL. Skeletal benefits after long-term retirement in former elite female gymnasts. J Bone Miner Res. 2009;24:1981–8. doi: 10.1359/jbmr.090521. [DOI] [PubMed] [Google Scholar]
- 46.Laing EM, Wilson AR, Modlesky CM, O’Connor PJ, Hall DB, Lewis RD. Initial years of recreational artistic gymnastics training improves lumbar spine bone mineral accrual in 4- to 8-year-old females. J Bone Miner Res. 2005 Mar;20(3):509–19. doi: 10.1359/JBMR.041127. [DOI] [PubMed] [Google Scholar]