Abstract
Aim
Coinfecting bacterial pathogens are a major cause of morbidity and mortality in influenza. However, there remains a paucity of literature on the magnitude of coinfection in influenza patients.
Method
A systematic search of MeSH, Cochrane Library, Web of Science, SCOPUS, EMBASE, and PubMed was performed. Studies of humans in which all individuals had laboratory confirmed influenza, and all individuals were tested for an array of common bacterial species, met inclusion criteria.
Results
Twenty‐seven studies including 3215 participants met all inclusion criteria. Common etiologies were defined from a subset of eight articles. There was high heterogeneity in the results (I 2 = 95%), with reported coinfection rates ranging from 2% to 65%. Although only a subset of papers were responsible for observed heterogeneity, subanalyses and meta‐regression analysis found no study characteristic that was significantly associated with coinfection. The most common coinfecting species were Streptococcus pneumoniae and Staphylococcus aureus, which accounted for 35% (95% CI, 14%–56%) and 28% (95% CI, 16%–40%) of infections, respectively; a wide range of other pathogens caused the remaining infections. An assessment of bias suggested that lack of small‐study publications may have biased the results.
Conclusions
The frequency of coinfection in the published studies included in this review suggests that although providers should consider possible bacterial coinfection in all patients hospitalized with influenza, they should not assume all patients are coinfected and be sure to properly treat underlying viral processes. Further, high heterogeneity suggests additional large‐scale studies are needed to better understand the etiology of influenza bacterial coinfection.
Keywords: antibiotic resistance, bacterial coinfection, influenza, meta‐analysis, MRSA, Streptococcus Pneumoniae
What this adds to existing literature
Clinical treatment of influenza presents difficulties because of significant uncertainty regarding the probability of bacterial coinfection. Despite this uncertainty, the frequency of overall coinfection in influenza patients is still poorly characterized. This meta‐analysis increases understanding of the likelihood that patients hospitalized with influenza also have a bacterial coinfection; however, the variability in results suggests that physicians should ensure that appropriate cultures are taken to minimize the overuse of antibiotics.
Introduction
Influenza causes widespread annual epidemics infecting up to 20% of the population and resulting in significant morbidity and mortality.1 Coinfecting bacterial pathogens are a major cause of that morbidity and mortality and are associated with both pandemic and seasonal influenza virus illness.2 Lung tissue samples from the 1918 influenza pandemic suggest that the majority of the estimated 20–60 million deaths were from bacterial infections rather than from direct effects of the virus.3 In seasonal epidemics, influenza bacterial coinfection is associated with increases in hospital admissions,4, 5 more severe symptoms,6 and increases in mortality.7 Viral damage to the epithelial lining of the respiratory tract is believed to facilitate establishment of bacterial infections.8, 9, 10 However, other factors, such as changes in airway function, up‐regulation and exposure of receptors, dampening of the immune response, or enhancement of inflammation may also play a role.11
Clinically, it can be difficult to identify influenza patients experiencing bacterial coinfections, given the substantial symptom overlap of influenza and bacterial infections. Identification of coinfected patients and coinfecting pathogen enables clinicians to initiate appropriate antibiotic therapy and improve patient outcomes.12 While prior studies have examined the frequency of select bacterial species in influenza cases,13, 14 particularly the presence of methicillin‐resistant S. aureus (MRSA),15, 16, 17, 18 the frequency of overall coinfection in influenza patients is still poorly characterized. We undertook a systematic review to determine the frequency of bacterial coinfections in patients with laboratory confirmed influenza and to identify the most common coinfecting bacterial species.
Methods
We conducted a systematic review, which is reported in accordance with PRISMA guidelines,19 to determine the frequency of bacterial coinfection among individuals with laboratory confirmed influenza. Inclusion was restricted to studies of humans in which all individuals had laboratory confirmed influenza, and all individuals were tested for an array of common bacterial species. Studies reanalyzing prior published data were excluded. There were no limitations based on participant age or the location of participant recruitment (i.e., community, outpatient, hospital). Coinfection was assumed to be any acute bacterial infection identified in respiratory secretions, sputum, or sterile site (e.g., bacteremia). We restricted results to publications in English published after January 1982. To avoid analyses of historic samples, particularly related to the influenza pandemic of 1968, studies using data collected prior to 1972 were excluded. Case reports, defined as studies with a sample size of fewer than 10 individuals, were excluded, but no other limitations based on study design were imposed.
Literature search
We performed a systematic search of MeSH, Cochrane Library, Web of Science, SCOPUS, EMBASE, and PubMed for publications in August 2014. The search terms included influenza, bacterial infection, bacterial coinfection, bacterial pathogens, bacteremia, bacterial–viral infection, coinfection, secondary infection, mixed infection, concomitant infection, H1N1, swine influenza, bird flu, gripe, pandemic influenza, seasonal influenza, influenza virus A H1N1, and avian influenza. The complete search strategy, which was completed in consultation with a research librarian, is detailed in Table S1.
Selection of studies
Two authors (BM, AG) independently screened the title and abstract of all the search‐returned publications to determine whether they met study criteria. The full text of all studies meeting the criteria and those for which a conclusion could not be made were reviewed independently by the two authors. Disagreements were resolved through consultation with a third party.
Data extraction
A structured data extraction form was used to collect data elements of each study into a Microsoft Excel worksheet. Two authors (BM, AG) extracted study data from all included publications independently, and results were then compared. Differences were resolved by consensus. Information extracted included: study design (i.e., prospective, retrospective), location of the study, study size, year of enrollment, study enrollment setting (i.e., intensive care unit [ICU], hospital, or emergency department [ED]), influenza strain (A, A pH1N1, B, all), participant age, bacterial collection method (sputum, blood, bronchial alveolar lavage [BAL]), method of bacterial detection (stain, culture, polymerase chain reaction [PCR], antibody), bacterial species evaluated, and bacterial species identified. In cases where only a percentage or subject number was published, its counterpart was calculated for analysis in the current review. Sources of data were carefully reviewed by BM, AG, EK, and studies reporting already included data were excluded (the study with the earlier publication date was considered the primary study, and all others excluded).
Assessment of bias
The potential bias of each study was assessed using the Quality Assessment Tool for Quantitative Studies developed by the National Collaborating Centre for Methods and Tools.20 This tool was selected for its comprehensive ability to assess the methodological quality of non‐randomized studies and has shown good reliability and validity.21, 22 A 3‐point scale was used for the following criteria: selection bias, study design, confounders, blinding, data collection methods, and study withdrawals. A global rating of “strong” was awarded for 4 “strong” ratings and no “weak” ratings, “moderate” for less than four “strong” and one “weak,” and “weak” for two or more “weak” ratings. Each study was independently evaluated by two authors, and discrepancies regarding bias assessment were resolved by consensus. Funnel plots and calculation of Egger's test of asymmetry were also used to assess biases such as publication and small‐study effects.23
Data analysis
The primary outcome was the proportion of bacterial coinfection. Coinfection was defined as the number of cases with a confirmed bacterial coinfection in all tested cases of patients with laboratory confirmed influenza. Because of differences between studies, we analyzed combined data on coinfection frequencies using the DerSimonian‐Laird method in the metaphor package,24 a meta‐analysis package for R.25 Heterogeneity was quantified using the I 2 statistic.26 Least‐squares meta‐regressions were performed to investigate the effect of differences in a priori defined trial‐level characteristics on the frequency of coinfection.27 These included: (i) age of the participants; (ii) study enrollment setting; (iii) year of enrollment; (iv) retrospective or prospective study design; (v) study size; (vi) bacterial collection method (BAL versus other); and (vii) method of bacterial detection. For bacterial detection, we examined the types of tests used to detect bacteria individually as well as the total number of tests used. To investigate the heterogeneity between studies and the influence of studies on the results, we performed a leave‐one‐out analysis as well as used Cook's distances to group the most heterogeneous studies. For species‐level analysis, only studies providing the numbers or percentages of each bacterial coinfecting pathogen were included. We included all pathogens in cases where more than one bacterial pathogen was found.
Results
Study screening and selection
The initial literature search yielded 1122 references, which was reduced to 1034 after removing duplicates. Following initial abstract review, 101 articles remained. The full‐text review resulted in the exclusion of an additional 74 articles. A total of 27 articles encompassing 3215 patients met all the inclusion criteria and were included in the final analysis.28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54 Of those 27, only eight studies, with 334 patients, provided the numbers or percentages of each bacterial coinfecting pathogen.28, 32, 33, 34, 38, 43, 46, 47 Thus, these eight studies formed the basis for identifying the most common coinfecting bacterial pathogens (Figure 1).
Figure 1.
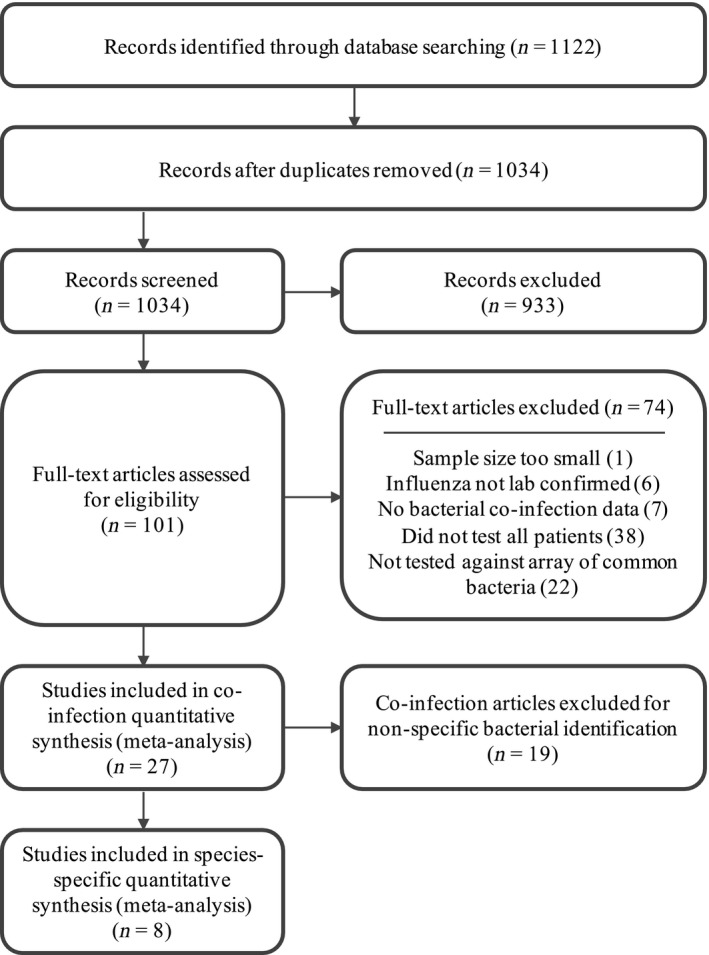
Flow diagram of study selection.
Study characteristics
All 27 included studies were observational, of which 13 (1218 patients) were prospective studies,30, 32, 33, 36, 40, 41, 43, 44, 48, 49, 50, 51, 52 and 14 (1997 patients) were retrospective analyses (Table 1). The majority (21) of studies were cohort studies, and the rest were case–control studies. Fifteen of the studies (1885 patients) began enrollment in 2009 or later,28, 32, 33, 34, 35, 37, 39, 41, 42, 44, 46, 48, 49, 53, 54 and of these, all but one was specifically focused on the 2009 H1N1 pandemic strain. Most studies enrolled only adults, although the lower age cutoffs varied (e.g., some studies considered adults older than 14 while others used 21). Only seven studies (1214 patients) focused on young children or newborns,29, 31, 38, 46, 49, 51, 54 and two studies (504 patients) included both children and adults.42, 53 Severity of illness varied among studies, but all were focused on hospitalized patients, suggesting a greater than average severity. Eleven of the studies (1007 patients) focused exclusively on patients initially enrolled in an intensive care unit (ICU),32, 34, 35, 38, 39, 41, 42, 44, 46, 47, 51 10 enrolled non‐ICU hospitalized patients (973 patients),30, 33, 36, 37, 40, 43, 45, 49, 53, 54 3 enrolled patients in the emergency department (ED; 135 patients),28, 48, 50 and 2 enrolled patients in a mix of inpatient and outpatient settings (1100 patients).29, 52 The median sample size was 51 (IQR, 18·5–101), and the mean was 119 (SD: 203), which was driven by three large studies29, 44, 53 that contributed 1958 (61%) of the total number of patients included in this analysis.
Table 1.
Study characteristics
| Study | Country (study) | Enrollment Year | Study Design | Setting | Participant Age Class | Sample Size | Bacterial CoInfection (%) |
|---|---|---|---|---|---|---|---|
| Ahn S, et al., 201128 | South Korea | 2009 | Retrospective (cohort) | ED | Adult | 60 | 26·7 |
| Bender JM, et al., 201029 | USA | 2004 | Retrospective (cohort) | Mixed | Pediatric | 833 | 1·9 |
| Bjarnason A, et al., 201230 | Iceland | 2008 | Prospective (cohort) | Hospital | Adult | 22 | 13·6 |
| Carr SB, et al., 201231 | USA | 2002 | Retrospective (cohort) | Mixed | Pediatric | 107 | 2·8 |
| Choi S‐H, et al., 201232 | South Korea | 2010 | Prospective (cohort) | ICU | Adult | 12 | 33·3 |
| Cordero E, et al., 201133 | Spain | 2009 | Prospective (cohort) | Hospital | Adult | 51 | 11·8 |
| Cuquemelle E, et al., 201134 | France | 2009 | Retrospective (case–control) | ICU | Adult | 103 | 46·6 |
| Dave BM, 201435 | India | 2012 | Retrospective (cohort) | ICU | Adult | 34 | 35·3 |
| Falsey AR, et al., 201236 | USA | 2008 | Prospective (cohort) | Hospital | Adult | 90 | 12·2 |
| Guervilly C, et al., 201037 | France | 2009 | Retrospective (cohort) | Hospital | Adult | 99 | 11·1 |
| Hon KL, et al., 200838 | China | 2003 | Retrospective (cohort) | ICU | Pediatric | 13 | 15·4 |
| Ingram PR, et al., 201039 | Australia | 2009 | Retrospective (case–control) | ICU | Adult | 17 | 5·9 |
| Johansson N, et al., 201040 | Sweden | 2004 | Prospective (cohort) | Hospital | Adult | 14 | 50·0 |
| Lopez‐Delgado J, et al., 201341 | Spain | 2009 | Prospective (cohort) | ICU | Adult | 60 | 25·0 |
| Malato L, et al., 201142 | France | 2009 | Retrospective (cohort) | ICU | Both | 24 | 25·0 |
| Marcos MA, et al., 200643 | Spain | 2003 | Prospective (cohort) | Hospital | Adult | 16 | 25·0 |
| Martin‐Loeches I, et al., 201144 | Spain | 2009 | Prospective (cohort) | ICU | Adult | 645 | 17·5 |
| Mermond S, et al., 201045 | France | 2006 | Prospective (cohort) | Hospital | Adult | 26 | 65·4 |
| Nguyen T, et al., 201246 | USA | 2009 | Retrospective (cohort) | ICU | Pediatric | 66 | 51·5 |
| Schnell D, et al., 201347 | France | 2007 | Retrospective (cohort) | ICU | Adult | 13 | 15·4 |
| Sohn CH, et al., 201348 | South Korea | 2009 | Prospective (cohort) | ED | Adult | 59 | 25·4 |
| Torres JP, et al., 201249 | Chile | 2009 | Prospective (cohort) | Hospital | Pediatric | 27 | 25·9 |
| Van Gageldonk‐Lafeber AB, et al., 201350 | Netherlands | 2007 | Prospective (cohort) | ED | Adult | 16 | 18·8 |
| Vieira RA, et al., 200351 | Brazil | 1999 | Prospective (case–control) | ICU | Pediatric | 20 | 30·0 |
| von Baum H, et al., 201152 | Germany | 2002 | Prospective (case–control) | Mixed | Adult | 160 | 21·3 |
| Yan XX, et al., 201153 | China | 2009 | Retrospective (case–control) | Hospital | Both | 480 | 19·0 |
| Zhang Q, et al., 201254 | China | 2009 | Retrospective (case–control) | Hospital | Pediatric | 148 | 25·7 |
ED, Emergency Department; ICU, Intensive Care Unit.
Assessment of bias
The average quality of the studies was moderate, with the most common quality issues being related to study design, selection biases, and data collection (Table S2). While studies were generally representative of the targeted population, most studies did not report the percentage of patients that agreed to participate. This lack of detail did not allow us to eliminate the possibility of selection bias in the study cohorts. Studies were also not fully clear on the reliability of the tools used for data collection, which made it difficult to eliminate this as a potential source of bias in the strains detected. However, results from the bias assessment suggest that the largest potential source of bias was the fact that all the studies were observational studies, and most were small cohort studies. A funnel plot appeared asymmetrical (Figure 2), suggesting statistical heterogeneity; in particular, there seems to have been a lack of smaller studies with lower rates of bacterial coinfection. Egger's test of asymmetry was also significant for bias (P = 0·0004).
Figure 2.
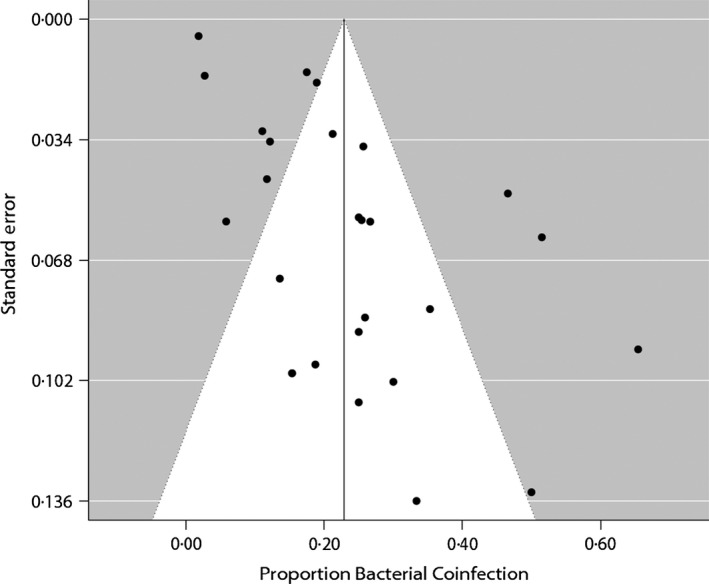
Funnel plot of each study's standard error (y‐axis) against each study's frequency of bacterial coinfection in laboratory confirmed hospitalized patients. Because small studies have less precision and large studies have more, scatter should form an inverted funnel when there are no systematic missing studies. The line indicates the overall mean frequency of coinfection (23%). The funnel plot appears asymmetric. Egger's test of asymmetry was significant for bias (P = 0·0004).
Bacterial coinfection
Among the individual studies, the proportion of bacterial coinfection ranged from 2% (newborns born in USA) to 65% (immunocompetent adults in France) (Figure 3). High statistical heterogeneity of the included studies (I 2 = 95%) removed confidence in reporting an estimate of the pooled mean proportion of bacterial coinfection through meta‐analysis. Using Cook's distances to identify studies that most greatly affected the heterogeneity and results, we found that seven studies contributed more than 50% of the heterogeneity.29, 31, 34, 39, 40, 45, 46 The proportion of bacterial coinfection among the remaining 20 studies, representing 64% of all patients, was between 11% and 35% (I 2 = 37%). Subanalyses and meta‐regression of age, setting, year of enrollment, study type, study size, and bacterial collection method were unable to determine the main sources of heterogeneity. Although heterogeneity was greater in the pediatric studies (I 2 = 98%) than in the adult studies (I 2 = 89%), coinfection frequency was statistically the same (P = 0·47). No significant trend was seen when stratifying the studies by mean or median age (Figure S1). Patients enrolled in the ICU had a slightly higher frequency of coinfection than patients enrolled elsewhere, although this was also not statistically significant (P = 0·14). Despite the fact that the study period included the 2009 H1N1 pandemic, we observed no significant effect of enrollment year on the frequency of coinfection (P = 0·19, Figure S2). The largest study contributed greatly to the heterogeneity of the results; however, study size did not significantly contribute to the heterogeneity in the results (P = 0·06 with the largest study, while P = 0·41 excluding this study). Finally, study design (P = 0·47) and bacterial coinfection collection and detection methods (each was tested independently and all P‐values were greater than 0·05) were also not significant. A multivariate analysis also found no significant correlation between any of the variables and overall rates of coinfection.
Figure 3.
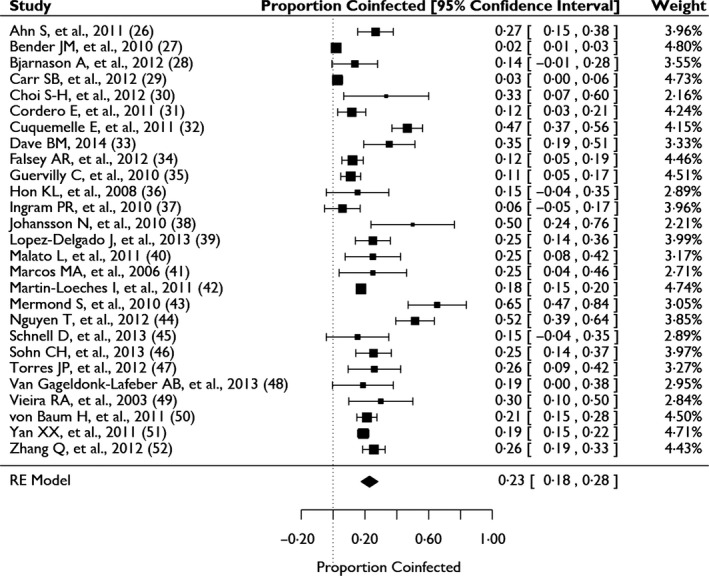
Frequency of bacterial coinfection in hospitalized patients with laboratory confirmed influenza.
Several factors may have contributed to heterogeneity between the selected studies that we were unable to quantify. Only four studies explicitly measured and reported on comorbidities of patients with coinfection and found that older age,28, 44, 53 a higher APACHE II (Acute Physiology and Chronic Health Evaluation II) score,44, 53 diabetes,33 and sepsis33 were risk factors for coinfection. Further, some studies only included severely immunocompromised patients who had concurrent malignancy or organ transplant,31, 33, 49 while others excluded immunocompromised patients. Severity of illness however was not a factor that could be standardized among the studies.
Antibiotic use at the time of, or prior to enrollment, was another factor that may have contributed to significant heterogeneity, but could not be systematically assessed. Only three studies excluded participants based on antibiotic use and reported coinfection rates of 12·2%, 26·7%, and 46·6%.28, 34, 36 An additional six studies reported on participant antibiotic use at the time of enrollment and found that 12–50% of patients had preceding antibiotic treatment which may have led to an underestimation of the true frequency of bacterial coinfection in their sample population.30, 33, 40, 45, 47, 52 For example, Bjarnason and colleagues found that none of the patients using antibiotics were in the coinfected group and when they excluded antibiotic users from the study, the prevalence of coinfection increased from 14% to 45%.30 One study found that previous antibiotic use made patients more likely to acquire atypical bacterial infections.50
Species‐level analysis
Streptococcus pneumoniae and Staphylococccus aureus were the most common pathogens accounting for 35% (95% CI, 14%–56%) and 28% (95% CI, 16%–40%) of identified coinfecting bacteria, respectively (Figure 4). A number of other pathogens were also identified as causing coinfections: Pseudomonas aeruginosa, Streptococcus pyogenes, Haemophilus influenzae, Klebsiella pneumoniae, and Mycoplasma pneumoniae. In addition, other Staphylococcal pathogens such as S. epidermidis, and Gram‐negative bacteria, such as Escherichia coli and Moraxella catarrhalis, were also frequently found.
Figure 4.
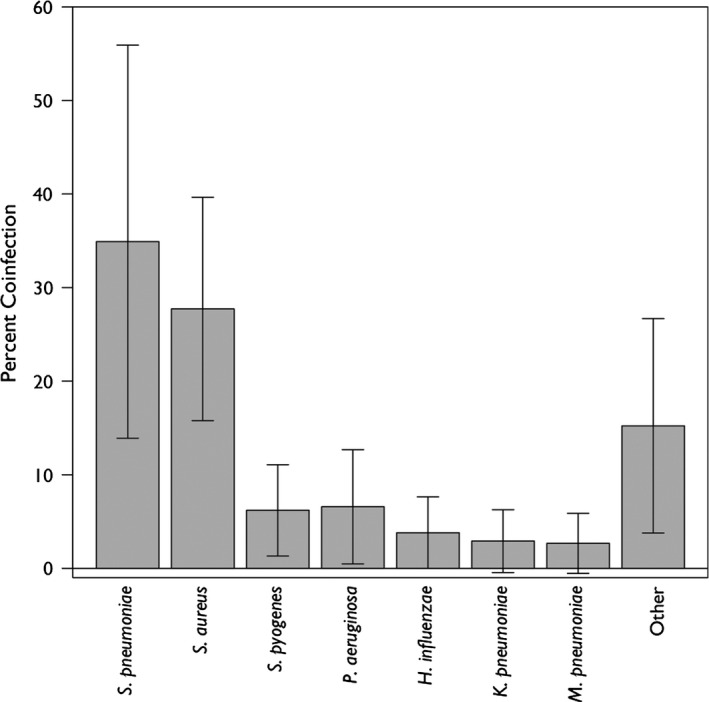
Percent of laboratory confirmed influenza infections that were coinfected by each bacterial species.
Discussion
Despite the long historical understanding of the risk posed by bacterial coinfections in influenza patients,2 the extent of coinfection has not been systematically examined.55 Understanding the risk of bacterial coinfection in hospitalized patients with influenza can help clinicians balance the need to minimize patient morbidity and mortality due to bacterial infection as well as the individual and societal risks of unnecessary antibiotic use.12 To assess the frequency of bacterial coinfection in laboratory confirmed influenza patients, we performed a systematic review and meta‐analysis of papers published since 1982. We found 27 studies covering 3215 patients. The results from these studies were highly variable, ranging from 2% to 65%. Although the majority of studies ranged between 11% and 35%, no specific characteristics of the studies were associated with variability in coinfection frequency. However, there was some suggestion that negative findings or low levels of bacterial coinfection were not published.
Differentiating viral from bacterial infection remains a challenge for clinicians. This diagnostic uncertainty has contributed to a widely recognized overuse of antibiotics in patients with viral illness.56, 57 The CDC recommends simultaneous antiviral and antibiotic use in the event of influenza‐related pneumonia or suspected bacterial coinfection in patients with influenza.58 However, as previous observational studies have shown, patients admitted to the hospital with influenza are more likely to receive antibiotics than antiviral medications.59, 60 Our findings suggest that while patients hospitalized with moderate to severe influenza may be coinfected with both viral and bacterial pathogens, many patients will likely not be coinfected. Thus, although recognition and treatment of potential bacterial coinfections is important, particularly community‐acquired pneumonia in which pathogens are difficult to detect,61 clinicians should consider treatment of potential underlying viral processes as well, particularly for high‐risk patients.60 Furthermore, to avoid overuse of antibiotics, our study suggests that routine cultures are advisable in patients hospitalized with influenza, particularly those started on antibiotic therapy empirically. Antibiotic therapy may then be de‐escalated as necessary based on microbiological results.
Consistent with the prior literature,55, 62 we found that S. pneumoniae was the most frequent bacterial coinfection; however, both S. aureus and other bacterial coinfections were also quite common. This diverse profile of coinfecting pathogens confirms current Infectious Disease Society of America (IDSA) recommendations for broad‐spectrum antibiotic coverage for influenza‐related pneumonia.63 However, although there have been significant increases in the incidence of MRSA infections in the last decade, particularly community‐associated MRSA (CA‐MRSA),64 there was not enough data to draw any inferences regarding temporal changes in the etiology of coinfecting pathogens. Given that over 25% of identified isolates were S. aureus, and that approximately 50% of hospital S. aureus isolates are MRSA,64 our study supports IDSA recommendations for empiric coverage of CA‐MRSA in influenza‐related pneumonia patients.63
The lack of a statistically significant study covariate may be due to some of the limitations of the study. First of all, although our final sample size included 27 studies and more than 3000 patients, these are relatively small numbers compared to annual estimates of up to 200 000 influenza‐related hospitalizations.65 Second, studies included only patients who were hospitalized for influenza and thus represent a population with moderate to severe influenza. Although a few studies enrolled patients in the outpatient or ED setting, all required hospitalization. The study does not, therefore, represent the vast majority of influenza patients, including asymptomatic patients, who are not hospitalized. This identifies a gap in the current literature as the frequency of bacterial coinfection in outpatients with confirmed influenza remains unknown. Third, studies included in this analysis detected bacterial pathogens in a number of different ways, which have varying sensitivity for different organisms and potential coinfecting sites (e.g., sputum versus blood). Some difficult‐to‐detect bacteria may, therefore, be underdiagnosed. Hence, these results may underrepresent the actual number of bacterial coinfections and the distribution of the pathogens of those coinfections. Results for bacterial distribution and likelihood of coinfection may also be affected by colonization rather than infection; however, studies were selected that specifically looked for bacterial coinfection and thus the issue of colonization should be minimal. Finally, we were unable to explain the significant heterogeneity among studies. It was not accounted for by differences in patient age, year, study enrollment setting, study design, study size, or method of bacterial sample collection or detection. This lack of statistically significant variability may be due to unrecorded differences in the studies, such as genetic differences in the populations, local differences in either the severity of viral or bacterial illness, unrecorded patient comorbidities, variation in treatment, or as noted above, antibiotic use (either current or past).
The high heterogeneity and lack of statistically significant covariates also points to the need for additional studies aimed at better understanding rates of bacterial coinfection, outcomes by pathogen, the effect of increased testing for both bacterial and viral pathogens, and the efficacy of interventions, such as increased use of antiviral drugs. These are particularly important in light of recent findings that viral pathogens were more commonly found than bacterial pathogens in suspected community‐acquired pneumonia infections.61
Conclusion
We found that bacterial coinfection of hospitalized patients with influenza is often common, although results were highly heterogeneous. The predominant coinfecting organism in the studies was S. pneumoniae followed by S. aureus, but many other organisms were also found to cause infections. Providers should consider possible bacterial coinfection in patients hospitalized with influenza, and bacterial cultures should be taken to avoid patient exposure to the risks of prolonged unnecessary antibiotic use. If antibiotic treatment is started, possible coinfection with MRSA should be considered, particularly for community‐acquired pneumonia infections, when selecting appropriate antibiotics, and therapy should be discontinued or de‐escalated as indicated by microbiological results. Finally, the frequency of coinfection should be better characterized in the entire influenza patient population, including outpatients, in future analyses.
Supporting information
Table S1 Search terms.
Table S2 Quality assessment.
Figure S1 Meta‐regression analysis of patient age on co‐infection frequency.
Figure S2 Meta‐regression analysis of enrollment year on co‐infection frequency.
Acknowledgements
We thank Hellen Gelband for her helpful comments. This work was supported by the Cooperative Agreement IDSEP130014‐01‐00 from The Assistant Secretary for Preparedness and Response within the US Department of Health and Human Services. Contents do not necessarily represent the official views of the US Department of Health and Human Services. Use of trade names and commercial sources is for identification only and does not imply endorsement by the US Department of Health and Human Services. The authors declare that there are no conflict of interests. Competing interests: The authors have no competing interests. EYK had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Klein et al (2016) The frequency of influenza and bacterial coinfection: a systematic review and meta‐analysis. Influenza and Other Respiratory Viruses 10(5), 394–403.
References
- 1. Thompson WW, Shay DK, Weintraub E et al Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179–186. [DOI] [PubMed] [Google Scholar]
- 2. Metersky ML, Masterton RG, Lode H, File TM Jr, Babinchak T. Epidemiology, microbiology, and treatment considerations for bacterial pneumonia complicating influenza. Int J Infect Dis 2012; 16:e321–e331. [DOI] [PubMed] [Google Scholar]
- 3. Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008; 198:962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Masiá M, Padilla S, Antequera P, Ramos JM, Ruiz M, Gutiérrez F. Predictors of pneumococcal co‐infection for patients with pandemic (H1N1) 2009. Emerg Infect Dis 2011; 17:1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blyth CC, Webb SA, Kok J et al The impact of bacterial and viral co‐infection in severe influenza. Influenza Other Respir Viruses 2013; 7:168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cillóniz C, Ewig S, Menéndez R et al Bacterial co‐infection with H1N1 infection in patients admitted with community acquired pneumonia. J Infect 2012; 65:223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paddock CD, Liu L, Denison AM et al Myocardial injury and bacterial pneumonia contribute to the pathogenesis of fatal influenza B virus infection. J Infect Dis 2012; 205:895–905. [DOI] [PubMed] [Google Scholar]
- 8. Wilson WJ, Steer P. Bacteriological and pathological observations on influenza as seen in France during 1918. Br Med J 1919; 1:634–635. [Google Scholar]
- 9. Louria DB, Blumenfeld HL, Ellis JT, Kilbourne ED, Rogers DE. Studies on influenza in the pandemic of 1957‐1958. II. Pulmonary complications of influenza. J Clin Invest 1959; 38:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harford CG, Leidler V, Hara M. Effect of the lesion due to influenza virus on the resistance of mice to inhaled pneumococci. J Exp Med 1949; 89:53–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McCullers JA. Insights into the Interaction between Influenza Virus and Pneumococcus. Clin Microbiol Rev 2006; 19:571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Van‐Tam J, Sellwood C. Pandemic Influenza. Boston: CABI, 2012. [Google Scholar]
- 13. Chertow DS, Memoli MJ. Bacterial coinfection in influenza: a grand rounds review. JAMA 2013; 309:275–282. [DOI] [PubMed] [Google Scholar]
- 14. Wang X‐Y, Kilgore PE, Lim KA et al Influenza and bacterial pathogen coinfections in the 20th century. Interdiscip Perspect Infect Dis 2011; 2011:146376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Centers for Disease Control Prevention . Severe methicillin‐resistant Staphylococcus aureus community‐acquired pneumonia associated with influenza–Louisiana and Georgia, December 2006‐January 2007. MMWR Morb Mortal Wkly Rep 2007; 56:325–329. [PubMed] [Google Scholar]
- 16. Centers for Disease Control Prevention . Severe coinfection with seasonal influenza A (H3N2) virus and Staphylococcus aureus–Maryland, February‐March 2012. MMWR Morb Mortal Wkly Rep 2012; 61:289–291. [PubMed] [Google Scholar]
- 17. Randolph AG, Vaughn F, Sullivan R et al Critically ill children during the 2009–2010 influenza pandemic in the United States. Pediatrics 2011; 128:e1450–e1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reed C, Kallen AJ, Patton M et al Infection with community‐onset Staphylococcus aureus and influenza virus in hospitalized children. Pediatr Infect Dis J 2009; 28:572–576. [DOI] [PubMed] [Google Scholar]
- 19. Liberati A, Altman DG, Tetzlaff J et al The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. National Collaborating Centre for Methods and Tools. Quality Assessment Tool for Quantitative Studies. Vol (Updated 13 April . Hamilton. ON: McMaster University, 2010; 2008. [Google Scholar]
- 21. Thomas BH, Ciliska D, Dobbins M, Micucci S. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs 2004; 1:176–184. 179p. [DOI] [PubMed] [Google Scholar]
- 22. Deeks J, Dinnes J, D'Amico R, Sowden A, Sakarovitch C. Evaluating non‐randomised intervention studies. Health Technol Assess 2003; 7:186. [DOI] [PubMed] [Google Scholar]
- 23. Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997; 315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models. New York: Springer, 2005. [Google Scholar]
- 25. R: A language and environment for statistical computing [computer program]. Vienna, Austria: R Foundation for Statistical Computing; 2013.
- 26. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thompson SG, Higgins JPT. How should meta‐regression analyses be undertaken and interpreted? Stat Med 2002; 21:1559–1573. [DOI] [PubMed] [Google Scholar]
- 28. Ahn S, Kim WY, Kim SH et al Role of procalcitonin and C‐reactive protein in differentiation of mixed bacterial infection from 2009 H1N1 viral pneumonia. Influenza Other Respi Viruses 2011; 5:398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bender JM, Ampofo K, Gesteland P et al Influenza virus infection in infants less than three months of age. Pediatr Infect Dis J 2010; 29:6–9. [DOI] [PubMed] [Google Scholar]
- 30. Bjarnason A, Thorleifsdottir G, Love A et al Severity of influenza A 2009 (H1N1) pneumonia is underestimated by routine prediction rules. Results from a prospective, population‐based study. PLoS ONE 2012; 7:e46816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carr SB, Adderson EE, Hakim H, Xiong X, Yan X, Caniza M. Clinical and demographic characteristics of seasonal influenza in pediatric patients with cancer. Pediatr Infect Dis J 2012; 31:e202–e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Choi SH, Hong SB, Ko GB et al Viral infection in patients with severe pneumonia requiring intensive care unit admission. Am J Respir Crit Care Med 2012; 186:325–332. [DOI] [PubMed] [Google Scholar]
- 33. Cordero E, Perez‐Romero P, Moreno A et al Pandemic influenza A(H1N1) virus infection in solid organ transplant recipients: impact of viral and non‐viral co‐infection. Clin Microbiol Infect 2012; 18:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cuquemelle E, Soulis F, Villers D et al Can procalcitonin help identify associated bacterial infection in patients with severe influenza pneumonia? A multicentre study. Intensive Care Med 2011; 37:796–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dave BM. Clinical data of h1n1 infected patients, 2012–2013. Indian J Crit Care Med 2014; 18(Suppl 13):S25. [Google Scholar]
- 36. Falsey AR, Becker KL, Swinburne AJ et al Bacterial complications of respiratory tract viral illness: a comprehensive evaluation. J Infect Dis 2013; 208:432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guervilly C, Coisel Y, Bothelo‐Nevers E et al High levels of procalcitonin and (greater‐than or equal to) reactive protein during H1N1 illness. Intensive Care Med 2010; 36(Suppl 2):S138. [Google Scholar]
- 38. Hon KL, Leung E, Tang J et al Premorbid factors and outcome associated with respiratory virus infections in a pediatric intensive care unit. Pediatr Pulmonol 2008; 43:275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ingram PR, Inglis T, Moxon D, Speers D. Procalcitonin and C‐reactive protein in severe 2009 H1N1 influenza infection. Intensive Care Med 2010; 36:528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Johansson N, Kalin M, Annika TL, Giske CG, Hedlund J. Etiology of Community‐Acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis 2010; 50:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lopez‐Delgado J, Rovira A, Esteve F et al Thrombocytopenia as a mortality risk factor in acute respiratory failure in H1N1 influenza. Swiss Med Wkly 2013; 143:w13788. [DOI] [PubMed] [Google Scholar]
- 42. Malato L, Llavador V, Marmier E et al Pandemic influenza A(H1N1)2009: molecular characterisation and duration of viral shedding in intensive care patients in Bordeaux, south‐west France, May 2009 to January 2010. Euro Surveill 2011; 16:19776. [PubMed] [Google Scholar]
- 43. Marcos M, Camps M, Pumarola T et al The role of viruses in the aetiology of community‐acquired pneumonia in adults. Antivir Ther 2006; 11:351–359. [PubMed] [Google Scholar]
- 44. Martin‐Loeches I, Sanchez‐Corral A, Diaz E et al Community‐acquired respiratory coinfection in critically iii patients with pandemic 2009 Influenza A(H1N1) Virus. Chest 2011; 139:555–562. [DOI] [PubMed] [Google Scholar]
- 45. Mermond S, Berlioz‐Arthaud A, Estivals M, Baumann F, Levenes H, Martin PM. Aetiology of community‐acquired pneumonia in hospitalized adult patients in New Caledonia. Trop Med Int Health 2010; 15:1517–1524. [DOI] [PubMed] [Google Scholar]
- 46. Nguyen T, Kyle UG, Jaimon N et al Coinfection with Staphylococcus aureus increases risk of severe coagulopathy in critically ill children with influenza A (H1N1) virus infection. Crit Care Med 2012; 40:3246–3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schnell D, Gits‐Muselli M, Canet E et al Burden of respiratory viruses in patients with acute respiratory failure. J Med Virol 2014; 86:1198–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sohn CH, Ryoo SM, Yoon JY et al Comparison of clinical features and outcomes of hospitalized adult patients with novel influenza A (H1N1) pneumonia and other pneumonia. Acad Emerg Med 2013; 20:46–53. [DOI] [PubMed] [Google Scholar]
- 49. Torres JP, Labrana Y, Ibanez C et al Frequency and clinical outcome of respiratory viral infections and mixed viral‐bacterial infections in children with cancer, fever and neutropenia. Pediatr Infect Dis J 2012; 31:889–893. [DOI] [PubMed] [Google Scholar]
- 50. Van Gageldonk‐Lafeber AB, Wever PC, Van DL et al The aetiology of community‐acquired pneumonia and implications for patient management. Neth J Med 2013; 71:418–425. [PubMed] [Google Scholar]
- 51. Vieira RA, Diniz EM, Vaz FA. Clinical and laboratory study of newborns with lower respiratory tract infection due to respiratory viruses. J Matern Fetal Neonatal Med 2003; 13:341–350. [DOI] [PubMed] [Google Scholar]
- 52. von Baum H, Schweiger B, Welte T et al How deadly is seasonal influenza‐associated pneumonia? The German Competence Network for Community‐Acquired Pneumonia. Eur Respir J 2011; 37:1151–1157. [DOI] [PubMed] [Google Scholar]
- 53. Yan XX, Xu HB, Liu Y et al Influenza a (H1n1) virus complicated with bacteria infection. Respirology 2011; 16(Suppl 2):60. [Google Scholar]
- 54. Zhang Q, Ji W, Guo Z, Bai Z, MacDonald NE. Risk factors and outcomes for pandemic H1N1 influenza compared with seasonal influenza in hospitalized children in China. Can J Infect Dis Med Microbiol 2012; 23:199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Brundage JF. Interactions between influenza and bacterial respiratory pathogens: implications for pandemic preparedness. Lancet Infect Dis 2006; 6:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gonzales R, Bartlett JG, Besser RE et al Principles of appropriate antibiotic use for treatment of acute respiratory tract infections in adults: background, specific aims, and methods. Ann Emerg Med 2001; 37:690–697. [PubMed] [Google Scholar]
- 57. Metlay JP, Camargo CA, MacKenzie T et al Cluster‐randomized trial to improve antibiotic use for adults with acute respiratory infections treated in emergency departments. Ann Emerg Med 2007; 50:221–230. [DOI] [PubMed] [Google Scholar]
- 58. Fiore AE, Fry A, Shay D, et al. Antiviral agents for the treatment and chemoprophylaxis of influenza—Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60:1–24. [PubMed] [Google Scholar]
- 59. Dugas AF, Monteforte B, Puri A, Awad M, Hsieh Y‐H, Rothman R. ED compliance with influenza antiviral recommendations. Am J Emerg Med 2014; 32:1550–1552. [DOI] [PubMed] [Google Scholar]
- 60. Dugas AF, Valsamakis A, Atreya MR et al Clinical diagnosis of influenza in the ED. Am J Emerg Med 2015; 33:770–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jain S, Self WH, Wunderink RG et al Community‐acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015; 373:415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. McCullers JA. The co‐pathogenesis of influenza viruses with bacteria in the lung. Nat Rev Micro 2014; 12:252–262. [DOI] [PubMed] [Google Scholar]
- 63. Harper SA, Bradley JS, Englund JA et al Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:1003–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Klein EY, Sun L, Smith DL, Laximarayan R. The changing epidemiology of methicillin‐resistant Staphylococcus aureus in the United States: A national observational study. Am J Epidemiol 2013; 177:666–674. [DOI] [PubMed] [Google Scholar]
- 65. Thompson WW, Shay DK, Weintraub E et al Influenza‐associated hospitalizations in the United States. JAMA 2004; 292:1333–1340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Search terms.
Table S2 Quality assessment.
Figure S1 Meta‐regression analysis of patient age on co‐infection frequency.
Figure S2 Meta‐regression analysis of enrollment year on co‐infection frequency.


