Abstract
Purpose
We examined the neuroprotective effects of exogenous brain-derived neurotrophic factor (BDNF), which provides protection to retinal ganglion cells (RGCs) in rodents, in a model of transient intraocular pressure (IOP) elevation using a mutant (triple Y-F) self-complementary adeno-associated virus type 2 vector encoding BDNF (tm-scAAV2-BDNF).
Methods
The tm-scAAV2-BDNF or control vector encoding green fluorescent protein (GFP; tm-scAAV2-GFP) was intravitreally administered to rats, which were then divided into four groups: control, ischemia/reperfusion (I/R) injury only, I/R injury with tm-scAAV2-GFP, and tm-scAAV2-BDNF. I/R injury was then induced by transiently increasing IOP, after which the rats were euthanized to measure the inner retinal thickness and cell counts in the RGC layer.
Results
Intravitreous injection of tm-scAAV2-BDNF resulted in high levels of BDNF expression in the neural retina. Histological analysis showed that the inner retinal thickness and cell numbers in the RGC layer were preserved after transient IOP elevation in eyes treated with tm-scAAV2-BDNF but not in the other I/R groups. Significantly reduced glial fibrillary acidic protein (GFAP) immunostaining after I/R injury in the rats that received tm-scAAV2-BDNF indicated reduced retinal stress, and electroretinogram (ERG) analysis confirmed preservation of retinal function in the tm-scAAV2-BDNF group.
Conclusions
These results demonstrate the feasibility and effectiveness of neuroprotective gene therapy using tm-scAAV2-BDNF to protect the inner retina from transiently high intraocular pressure. An in vivo gene therapeutic approach to the clinical management of retinal diseases in conditions such as glaucoma, retinal artery occlusion, hypertensive retinopathy, and diabetic retinopathy thus appears feasible.
Introduction
Glaucoma is a collection of neurodegenerative diseases that result in retinal ganglion cell (RGC) axon degeneration and death, ultimately leading to irreversible blindness. It is estimated that glaucoma will affect about 80 million people worldwide by 2020 [1]. Glaucoma is associated with structural damage at the optic nerve head, thinning of the neuroretinal rim, excavation (cupping), and sectoral retinal nerve fiber layer defects. Elevated pressure within the eye is the most important known risk factor for glaucoma, but lowering intraocular pressure (IOP) is not always sufficient to halt the progression of the optic neuropathy. Therefore, glaucoma is thought to be a neurodegenerative disease, possibly amenable to therapies other than IOP reduction [2]. Consequently, considerable effort has been made to develop neuroprotective strategies that prevent or delay disease progression [3]. For example, RGC neuroprotection was previously demonstrated using brain-derived neurotrophic factor (BDNF) [4-6], ciliary neurotrophic factor [6-8], glial cell line–derived neurotrophic factor [9], and pigment-epithelium derived factor [10,11] in rodent models of ocular hypertension, retinal ischemia, and ganglion cell axotomy.
Among neurotrophic factors, BDNF stands out for its potent protective effect on injured RGCs [3]; BDNF is essential for RGC survival during development [12,13] and in adults [14,15]. BDNF synthesized in the superior colliculus binds to the tyrosine kinase receptor B (trkB) receptor and undergoes retrograde transport in microsomal vesicles via RGC axons to the cell bodies [16]. Exogenous BDNF protein has been administered through intraocular injection [12,17-20], as well as gene transfer using an adenovirus vector [21] or an adeno-associated virus (AAV) vector [22,23]. For example, Ren et al. investigated whether BDNF expressed from a single-strand (ss) AAV2 vector could provide retinal neuronal protection in a rat transient IOP elevation model in which the elevated IOP caused retinal ischemia/reperfusion (I/R) injury [23]. They found that whereas injection of the BDNF protein into the vitreous (used as a positive control) provided retinal protection, no such acute phase protection was obtained through administration of an ssAAV2 vector encoding BDNF.
AAV vectors are considered optimal for ocular gene therapy because of their efficiency, persistence, and low immunogenicity [24]. Moreover, intravitreous injection of ssAAV2 leads to transduction of the RGCs lining the vitreous. Until now, however, the transduction efficacy has been low. Despite thousands of publications describing the protection of neurons in the laboratory, no beneficial treatment for human patients has yet been achieved. Therefore, we speculated that the lack of successful ocular gene therapy in humans is due to poor retinal transduction efficiency. We tested that idea in the present study by assessing the RGC neuroprotection provided by a mutant (triple Y-F) self-complementary AAV2 vector (tm-scAAV2), a vector with reportedly high transduction efficiency, in an experimental rat model of transient IOP elevation.
Methods
Animals
All experiments were performed using 7-week-old Sprague-Dawley rats weighing 200–250 g. For most experiments, the rats were divided into four groups: control, I/R injury without treatment, I/R injury with tm-scAAV2-green fluorescent protein (GFP) and I/R injury with tm-scAAV2-BDNF (n = 6 in each group). All animals were treated in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. The studies were approved by the Animal Care and Use Committee of Nippon Medical School.
Generation of AAV vectors
After plasmid pscAAV-CB-BDNF harboring cDNA encoding human BDNF (Cat#; RC212722, OriGene, Rockville, MD) was constructed, a 0.9 kbp BamHI-FseI fragment containing the BDNF gene (Gene ID: 627, OMIM 113505) was excised, treated with klenow, and ligated into the HincII site of pscAAV2-GFP that encodes green fluorescent protein (provided by Dr. Arun Srivastava) [25-27]. pACG2-3M (pAAV2-Y730+500+444F; provided by Dr. Arun Srivastava) served as the packaging plasmid. AAV vectors were generated using an adenovirus-free system. The virions were purified with continuous iodixanol gradient centrifugation, as described previously [28,29], after which the particle titers of the purified virions were determined using real-time PCR. The titers of tm-scAAV2-BDNF and tm-scAAV2-GFP were 5×1012 vector genomes (v.g.)/ml.
Expression pattern of tm-scAAV2-GFP in the rat control retina and the I/R injured retina
To compare the patterns of GFP expression in healthy and I/R-injured retinas, the rats were anesthetized with an intraperitoneal injection of pentobarbital (50 mg/kg), and their pupils were dilated using topical phenylephrine hydrochloride and tropicamide (Santen, Osaka, Japan). After topical application of 0.4% oxybuprocaine hydrochloride (Santen), 3 μl of tm-scAAV2-GFP were intravitreally injected using a 33-gauge Hamilton needle and syringe, as described previously [30]. Three weeks after administration, the eyes were enucleated, sectioned, and immunostained using an anti-GFP antibody, as described in the Immunohistochemistry section.
Injection of AAV vectors and induction of rat I/R injury
The rats were anesthetized with an intraperitoneal injection of pentobarbital (50 mg/kg), and their pupils were dilated using topical phenylephrine hydrochloride and tropicamide (Santen). After topical application of 0.4% oxybuprocaine hydrochloride (Santen), 3 μl of tm-scAAV2-BDNF or tm-scAAV2-GFP were intravitreally injected using a 33-gauge Hamilton needle and syringe, as described previously [30]. The same vectors were injected into both eyes. Two weeks later, retinal I/R injury was induced essentially as described previously [31]. The anterior chamber was cannulated with a 30-gauge infusion needle connected to a reservoir containing normal saline. The IOP was then raised to 110 mmHg for 60 min by elevating the saline reservoir. Retinal ischemia was confirmed by whitening of the iris and fundus. After 60 min of ischemia, the needle was withdrawn from the anterior chamber, and the IOP was normalized. We also set up a control non-transduced group that was defined as “no vehicle.”
Semiquantitative real-time PCR in vivo
To measure BDNF gene expression in the rat retina (n = 6), samples were transduced in the same manner described for the enzyme-linked immunosorbent assays (ELISAs). BDNF mRNA was then measured using a previously described method [32] with primers obtained from OriGene (Cat#; HK201084). As a control, GAPDH was used. PCR conditions were as follows: 95 °C for 10 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 34 s.
ELISA in vitro and in vivo
An ELISA was used to assess expression of tm-scAAV2-BDNF in vitro. C2C12 cells (ATCC® CRL-1772™, Manassas, VA) maintained in Dulbecco’s modified Eagle’s medium (DMEM, Sigma-Aldrich, St. Louis, MO) supplemented with 8% fetal bovine serum were seeded to a density of 3×104 cells/well in a 24-well plate and allowed to adhere for 6 h, after which they were transduced with 5 μl of tm-scAAV2-BDNF. Twenty-four hours later, the cells were washed 3 times to remove any BDNF that originated from the C2C12 cells before transduction. After incubation for an additional 3 days, the supernatants were collected and preserved at −80 °C until analyzed (n = 3 in each group). The BDNF levels were determined using a human BDNF Quantikine ELISA kit according to the manufacturer’s protocol (R&D Systems, Minneapolis, MN).
To measure retinal BDNF production, the sclera-choroid-RPE complexes and neurosensory retinas were isolated from the rats 7 days after the induction of I/R. Thereafter, samples of the sclera-choroid-RPE complex and neurosensory retina were placed in 200 μl of saline and homogenized. The resultant lysate was centrifuged at 45,000 ×g for 5 min at 4 °C, and the BDNF levels in 50 μl aliquots of supernatant were determined using a human BDNF Quantikine ELISA kit. In addition, the total protein concentrations were measured using a protein assay system (Bio-Rad Protein Assay Dye Reagent Concentrate; Bio-Rad Laboratories, Hercules, CA). The final results are expressed as picograms of BDNF per milligrams of total retinal protein in the in vivo experiment.
Thickness of the inner retina and cell counts in the RGC layer
Thinning of the inner retina is apparent after retinal I/R injury [31]. To measure retinal thickness, the rats (n=6) were anesthetized with an intraperitoneal injection of pentobarbital (50 mg/kg) 7 days after injury, after which sections were prepared as previously described [31]. In brief, The eyes were enucleated and fixed for 1 h in 1% glutaraldehyde (GA) and 4% paraformaldehyde (PFA) in 0.1 M phosphate-buffered saline (PBS; 0.1 g/l MgCl2 • 6H2O, 0.2 g/l KCI, 0.2 g/l KH2PO4, 8.0 g/l NaCl, 1.15 g/l Na2HPO4, pH7.2, D5773, Sigma-Aldrich), after which the anterior segments were removed, and the corneas and lenses were discarded. The entire eye cups were then further fixed overnight in 1% GA and 4% PFA at 4 °C and then sequentially transferred in a stepwise manner to PBS with 10% sucrose for 3 h, 20% sucrose for 6 h, and 30% sucrose overnight. The eyes were then frozen in optimum cutting temperature (OCT) compound on dry ice, and 10-μm cryostat sections were cut in a plane parallel to the vertical meridian of the eye. Sections were then stained with hematoxylin and eosin (H&E). Retinal thickness, defined as the total width between the inner limiting membrane and the interface of the inner nuclear layer, was then measured. These measurements were made in an area 1 mm from the optic disc using a light microscope, and the thicknesses measured in three sections were averaged. Simultaneously with the retinal thickness, the cell density in the RGC layer was determined in three sections per eye at a final magnification of 400× using a light microscope. All measurements and cell counts were performed using image analysis software (Photoshop; Adobe Systems, San Jose, CA).
Immunohistochemistry
Eyes were enucleated 7 days after reperfusion and fixed for 1 h in 1% glutaraldehyde (GA) and 4% paraformaldehyde (PFA) in 0.1 M PBS, after which the anterior segments were removed, and the corneas and lenses were discarded. The entire eye cups were then further fixed overnight in 1% GA and 4% PFA at 4 °C and then sequentially transferred in a stepwise manner to PBS with 10% sucrose for 3 h, 20% sucrose for 6 h, and 30% sucrose overnight. The eyes were then frozen in optimum cutting temperature (OCT) compound on dry ice, and 10-μm cryostat sections were cut in a plane parallel to the vertical meridian of the eye. After the eyes were frozen in OCT compound on dry ice, 10-μm cryostat sections were cut in a plane parallel to the vertical meridian of the eye. Three sections from each eye were analyzed immunohistochemically, as described previously [33,34]. The primary antibodies used were rabbit anti-GFP (1:1,000; Santa Cruz Biotechnology, Heidelberg, Germany), goat anti-Brn-3a (1:400; sc-31984, Santa Cruz Biotechnology), and rabbit anti-cow glial fibrillary acidic protein (GFAP; 1:500; Dako, Copenhagen, Denmark). The secondary antibodies used were Alexa Fluor 488-conjugated goat anti-rabbit IgG (1:500; Invitrogen, Life Technology Japan, Tokyo, Japan) for GFP, Alexa Fluor 568-conjugated donkey anti-goat immunoglobulin G (IgG, 1:300; Invitrogen) for Brn-3a, and biotinylated anti-rabbit IgG (Vector Laboratories, Burlingame, CA) and Cy3-conjugated streptavidin (Jackson ImmunoResearch Laboratories, West Grove, PA). Sections were then mounted using medium containing 4’,6’-diamidino-2-phenylindole (DAPI, Vector Laboratories) and observed under a fluorescence microscope (Olympus DP50, Tokyo, Japan).
Electroretinograms
Six days after I/R injury, full-field electroretinogram (ERG) responses from the experimental and control eyes were simultaneously recorded using a synchronized trigger and summing amplifier (Primus; Mayo, Nagoya, Japan) with a stimulation device (LS-W; Mayo), as described in our previous report [30]. In brief, rats were dark-adapted overnight, then anesthetized with an intraperitoneal injection of ketamine (90 mg/kg) and xylazine (10 mg/kg). Using topical 0.4% oxybuprocaine hydrochloride, the cornea was anesthetized, and the pupils were dilated with 0.5% tropicamide and 0.5% phenylephrine hydrochloride. Electroretinograms (ERGs) were recorded using a synchronized trigger and summing amplifier (Primus; Mayo, Nagoya, Japan) with a stimulation device (LS-W; Mayo). Dark-adapted ERG responses were recorded using a white light–emitting diode with built-in corneal contact-type bipolar electrodes, which were placed on each cornea. Subcutaneous needle electrodes were also placed in the forehead and earlobe as the negative and ground electrodes. After overnight dark-adaptation, dilated pupil and scotopic responses were examined. ERG responses were measured according to the International Society for Clinical Electrophysiology of Vision guidelines. Scotopic-adapted standard white flash stimuli were set at 1000 cd/m2 for 3 ms. At least three ERG readings were collected from each eye. Experimental and control eyes from each rat were compared at each time point to minimize the effect of individual conditions.
Intensity index of GFAP expression
Levels of GFAP expression in the four groups were analyzed using ImageJ software (Version 1.44, NIH, Bethesda, MD), as previously described [30,35]. Each image was captured using the same camera settings for gain and time (400×, 1/10 s). Data were obtained for each region of interest (ROI) based on pixel intensity and averaged across three images for each eye. Quantitation was performed in a blinded manner.
Statistical analysis
Morphometric data from different regions in each eye were averaged to provide one value per eye. The mean and standard deviation (SD) for these measurements were calculated for each group, and comparisons between groups were made by using the Student–Newman–Keuls (SNK) method (Excel; Microsoft, Tokyo, Japan). A p value of less than 0.05 was considered statistically significant.
Results
Pattern of tm-scAAV2-GFP expression in normal and I/R-injured retinas
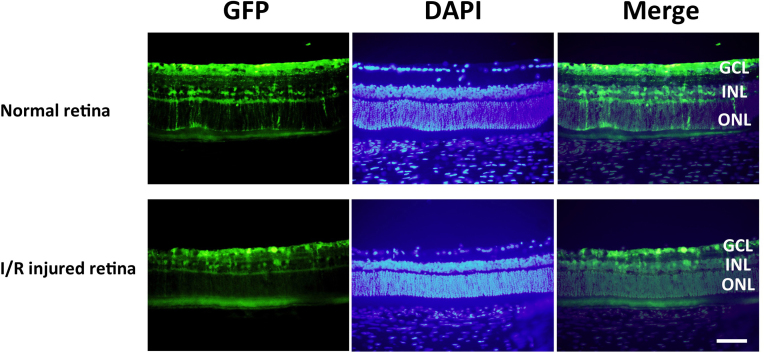
Petrs-Silva et al. reported that the tm-scAAV2 vector exhibited high transduction efficiency following intravitreous injection [26] and that this vector enhanced the efficiency of ganglion cell transduction by >30-fold after intravitreal injection [25]. Upon intravitreal administration, tm-scAAV2-GFP transduced numerous ganglion cell layer (GCL) neurons, Müller cells, and inner nuclear layer (INL) cells in healthy and I/R-injured retinas (Figure 1). In subsequent experiments, therefore, the tm-scAAV2 vector was used to express BDNF in the rat retina.
Figure 1.
Transduction of rat retina with tm-scAAV2-GFP. The upper row shows the green fluorescent protein (GFP) expression pattern for a healthy retina, and the bottom row shows the GFP expression pattern for the ischemia/reperfusion (I/R) injured retina. After injection of mutant (triple Y-F) self-complementary adeno-associated virus type 2 vector encoding green fluorescent protein (tm-scAAV2-GFP) into the rat vitreous cavity, GFP expression was detected in the ganglion cell layer (GCL), Müller, and inner nuclear layer (INL) cells. Scale bar, 50 μm.
BDNF expression
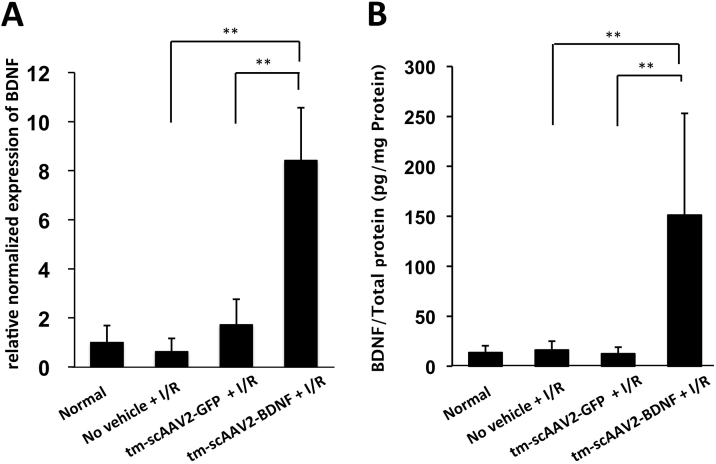
Using semiquantitative real-time PCR to analyze BDNF gene expression, we found that the BDNF/GAPDH ratio for the tm-scAAV2-BDNF group was significantly higher than in the tm-scAAV2-GFP group (Figure 2A, 8.42±2.15 versus 1.73±1.03; p<0.01). In addition, a specific ELISA showed that there was a corresponding increase in the levels of the BDNF protein (Figure 2B). Furthermore, the BDNF of the tm-scAAV2-BDNF-transduced C2C12 cells were significantly higher than the BDNF of the non-transduced cells (16.6±0.85 ng/ml versus 0.175±0.05 ng/ml; p<0.01; data not shown). The BDNF levels in the neurosensory retina from rats treated with tm-scAAV2-BDNF were also significantly higher than in retinas treated with tm-scAAV2-GFP (Figure 2B. 151.2±101.7 pg/mg protein versus 12.4±6.7 pg/mg protein; p<0.01).
Figure 2.
BDNF gene and protein expression. Relative levels of the brain-derived neurotrophic factor (BDNF) mRNA (ratio of brain-derived BDNF/GAPDH) (A) and protein (B) were significantly increased in the neurosensory retinas of rats treated with mutant (triple Y-F) self-complementary adeno-associated virus type 2 vector encoding BDNF (tm-scAAV2-BDNF). n = 6 in each group. Bars depict means ± standard deviation (SD).
Effect of AAV vector–mediated BDNF expression on histopathological changes, cell density in the RGC layer, and Brn-3a-positivity in retinal cross sections
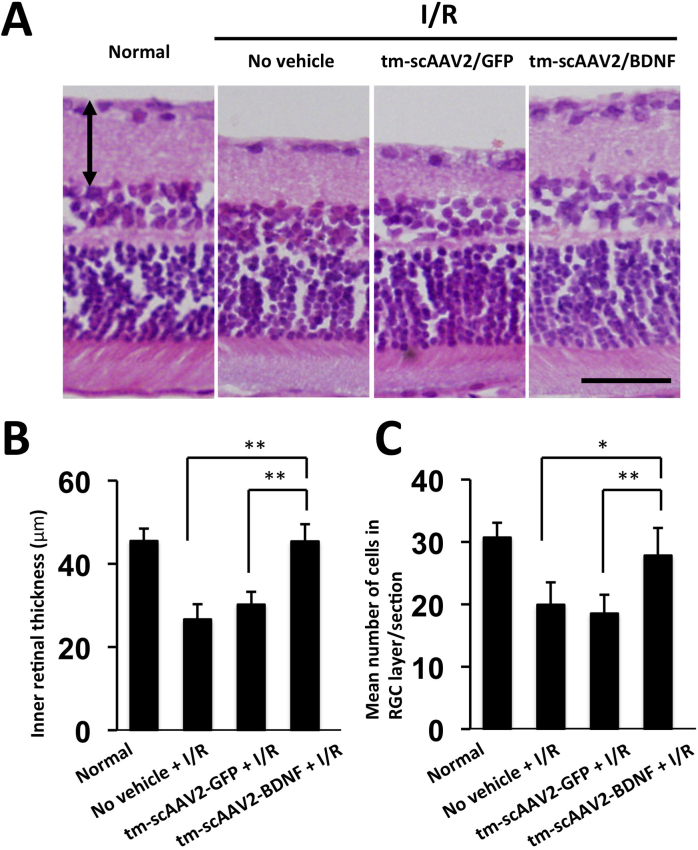
To evaluate the protective effect of tm-scAAV2-BDNF, we examined histopathologic and morphometric changes 7 days after retinal I/R injury. The retinal thickness of the normal group was 45.5±2.9 μm. In the tm-scAAV2-BDNF-treated group, the retinal structure was thicker and nearly normal, whereas retinas in the tm-scAAV2-GFP-treated group exhibited marked thinning and atrophy, as did retinas in the no vehicle I/R-injured group (26.6±3.7 μm; Figure 3A). Using quantitative morphometry, we also determined that the I/R-injured retinas treated with tm-scAAV2-BDNF (45.4±4.2 μm) were significantly thicker than the retinas treated with tm-scAAV2-GFP (30.2±3.0 μm; Figure 3B; p<0.01; n = 6 in each group).
Figure 3.
Thickness of the inner retina and cell counts in the ganglion cell layer. A: Images of representative slices of healthy retinas and ischemia/reperfusion (I/R)-injured retinas with no vehicle, or with mutant (triple Y-F) self-complementary adeno-associated virus type 2 vector encoding green fluorescent protein (tm-scAAV2-GFP), or tm-scAAV2- brain-derived neurotrophic factor (BDNF) treatment. Thickness is defined as the total width between the inner limiting membrane and the interface of the inner nuclear layer (double arrow). Scale bar, 50 μm. B: Inner retinal thicknesses in each treatment group. n = 6 in each group. Bars depict means ± standard deviation (SD). The thickness of the I/R-injured retinas treated with the tm-scAAV2-BDNF vector was significantly greater than that of the no vehicle (**p<0.01) retinas and the retinas treated with the tm-scAAV2-GFP vector (**p<0.01). C: Number of cells in the retinal ganglion cell (RGC) layer or section. A significantly greater number of cells was retained after I/R injury in the RGC layer of retinas treated with tm-scAAV2-BDNF than in the no vehicle (*p<0.05) retinas or those treated with the tm-scAAV2-GFP vector (**p<0.01). Bars depict means ± standard deviation (SD).
GCL cell counts were made on cross-sectional slides stained with H&E [36,37]. No attempt was made to distinguish RGCs from displaced amacrine cells or other neuron-like cells, but morphologically distinguishable glial cells and vascular endothelial cells were excluded. The mean number of cells in the RGC layer or section in rats treated with tm-scAAV2-BDNF was significantly greater than in rats treated with tm-scAAV2-GFP (Figure 3C; p<0.01; n = 6 in each group; normal, 30.7±2.4 cells; no vehicle + I/R, 19.9±3.6 cells; tm-scAAV2-GFP, 18.5±3.0 cells; tm-scAAV2-BDNF, 27.8±4.4 cells).
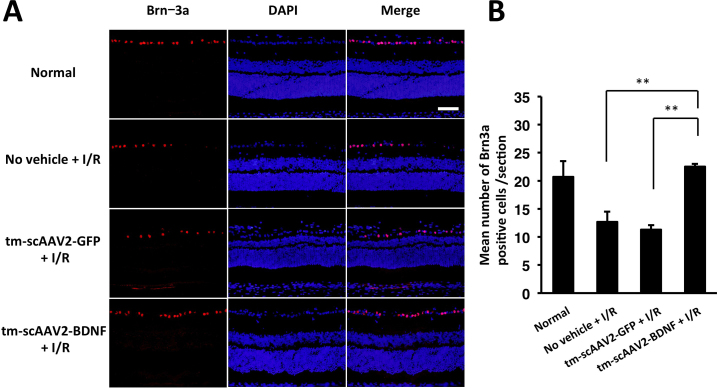
The photographs in Figure 4A show representative retinal cross sections with cells stained positive for Brn-3a (ganglion cell marker), while Figure 4B shows the average number of Brn-3a-positive cells counted under normal (20.8±2.8 cells), no vehicle (12.7±1.8 cells), tm-scAAV2-GFP (11.2±0.8 cells), and tm-scAAV2-BDNF (22.6±0.5 cells) conditions. The tm-scAAV2-BDNF treatment provided significant protection to ganglion cells, compared to the no vehicle (p<0.01) and tm-scAAV2-GFP (p<0.01) treatments.
Figure 4.
Immunohistochemical analysis of Brn-3a. A: Representative slices showing Brn-3a expression in healthy and ischemia/reperfusion (I/R)-injured retinas. Scale bar, 50 μm. B: Brn-3a-positive cells were counted in each section. Following I/R injury, significantly greater numbers of cells were retained in the retinal ganglion cell (RGC) layer of retinas treated with mutant (triple Y-F) self-complementary adeno-associated virus type 2 vector encoding brain-derived neurotrophic factor (tm-scAAV2-BDNF) than in those treated with no vehicle (**p<0.01) or with the tm-scAAV2-GFP vector (**p<0.01; each, n = 3). Bars depict means ± standard deviation (SD).
Electroretinograms
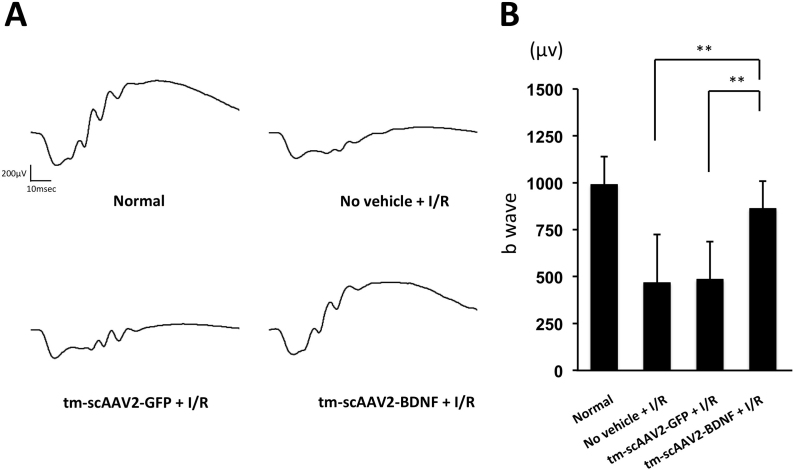
Scotopic ERG results are summarized in Figure 5. Figure 5A contains representative ERG recordings; Figure 5B shows the b-wave amplitudes. There was no statistical difference in b-waves between the normal (990.4±148.2 μv) and tm-scAAV2-BDNF (862.6±146.6 μv) groups. The tm-scAAV2-BDNF treatment provided significant protection to retinas, compared to the no vehicle (467.1±256.7 μv; p<0.01) and tm-scAAV2-GFP (484.8±201.6 μv; p<0.01) treatments. In addition, the absence of a significant difference between the no vehicle- and tm-scAAV2-GFP groups indicates AAV2 transduction causes no obvious toxicity.
Figure 5.
ERGs for healthy and I/R-injured retinas. A: Representative electroretinograms (ERGs) from healthy retinas and ischemia/reperfusion (I/R)-injured retinas treated with no vehicle, mutant (triple Y-F) self-complementary adeno-associated virus type 2 vector encoding green fluorescent protein (tm-scAAV2-GFP), or tm-scAAV2-brain-derived neurotrophic factor (BDNF). B: b-wave amplitudes 6 days after ischemia. Bars depict means ± standard deviation (SD). There was no statistical difference in the b-waves between the normal retinas (n = 8) and the tm-scAAV2-BDNF-treated retinas (n = 6; p>0.05). The tm-scAAV2-BDNF-treated retinas were significantly protected compared to the no vehicle (n = 8; **p<0.01) and tm-scAAV2-GFP (n = 3; **p<0.01) groups.
Transduction of retinal neurons and expression of GFAP in I/R-injured retina
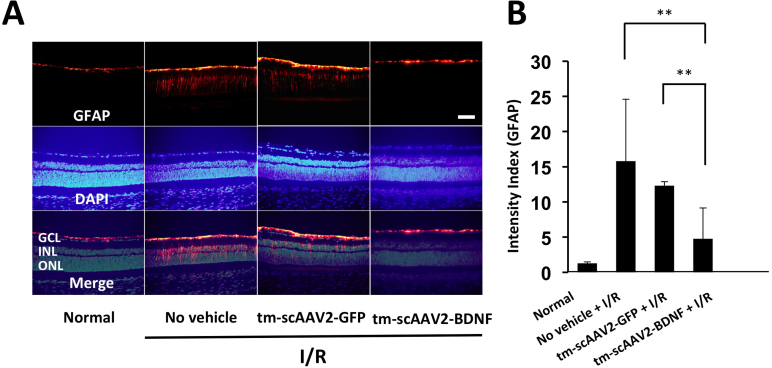
GFAP staining is a widely used molecular indicator of retinal stress [38]. We investigated the immunohistochemical changes in GFAP 7 days after retinal I/R injury. Shown in Figure 6A are representative images of normal and I/R-injured retinas. GFAP immunostaining was significantly diminished in the tm-scAAV2-BDNF (4.8±4.4) group compared to the no vehicle (15.8±8.8; p<0.01) and tm-scAAV2-GFP (12.3±0.6; p<0.01; Figure 6B) groups.
Figure 6.
Immunohistochemical analysis of GFAP. A: Representative slice showing glial fibrillary acidic protein (GFAP) expression in healthy (n = 4) and ischemia/reperfusion (I/R)-injured retinas. Scale bar, 50 μm. B: Intensity index (GFAP) calculations revealed significantly lower GFAP levels in I/R-injured retinas following mutant (triple Y-F) self-complementary adenovirus-associated virus type 2 vector encoding brain-derived neurotrophic factor (tm-scAAV2- BDNF) treatment (n = 4) than in the no vehicle (n = 3; **p<0.01) retinas or the retinas treated with tm-scAAV2-GFP (n = 3; **p<0.01). Bars depict means ± standard deviation (SD).
Discussion
In this study, we used the tm-scAAV2 vector to achieve high transduction efficiency in retinas after a single intravitreous injection. The ELISA results showed that high levels of BDNF were produced in the rat neural retina after tm-scAAV2 administration, and histological analysis demonstrated that the BDNF exerted protective effects at the inner retina resulting in the presence of a greater number of cells in the RGC layer after transient IOP elevation. Functionally, ERG analyses confirmed the preservation of retinal function after transient IOP elevation. Collectively, these results indicate that tm-scAAV2-BDNF is potentially useful for neuroprotective gene therapy.
We used the rat transient IOP elevation model, which induces retinal I/R injury and has the characteristics of retinal artery occlusion [39] and glaucoma [40]. In an earlier study [23], supplemental BDNF protein and ssAAV2-BDNF were administered 6 h after retinal ischemia/reperfusion. The supplemented BDNF protein was effective for the acute phase of retinal artery occlusion, and the slow-onset AAV-mediated BDNF expression was effective for the later phase, which is probably more similar to the gradual loss of RGCs observed in chronic glaucoma models [8,41]. In the present experiment, the tm-scAAV2-BDNF vector was administered 2 weeks before retinal ischemia-reperfusion. We reasoned that 2 weeks should be sufficient to induce gene expression using the tm-scAAV2 vector. An intravitreally delivered tm-scAAV2 vector was previously reported to have marginally higher transduction efficiency than ssAAV2 and to mediate rapid expression in the inner retinal cells [42]. Similarly, we observed tm-scAAV2-GFP expression 2 days after intravitreous injection, and the intensity of the tm-scAAV2-GFP expression gradually increased thereafter (data not shown). These results suggest that at the time of retinal I/R, the tm-scAAV2 vector was mediating sufficient BDNF expression to provide a neuroprotective effect.
To evaluate retinal damage due to I/R injury, tissue sections were immunostained for GFAP, a widely used molecular indicator of retinal stress. Rats administered tm-scAAV2-GFP showed substantial GFAP expression following I/R injury, whereas tm-scAAV2-BDNF suppressed GFAP expression, presumably reflecting amelioration of the I/R injury. In healthy retinas, the GFAP-positive cells are astrocytes. In injured retinas, Müller cells (retina-specific glial cells) react with the anti-GFAP antibody across the retinal layers [43]. Similar GFAP activation has also been observed in the neuronal retina in human glaucoma [44] and in an experimental glaucoma model [45]. Our findings suggest tm-scAAV2-BDNF reduces the retinal damage caused by transient IOP elevation.
BDNF plays a key role in the central and peripheral nervous systems, supporting the survival of existing neurons and encouraging the growth and differentiation of new neurons and synapses [46,47]. Compared to the retinas of healthy mice, the retinas in mice lacking BDNF have smaller retinal ganglion cell axons, reduced hypomyelination, and lower density of GCL cells and horizontal, bipolar, and amacrine cells [48,49]. Administration of BDNF to mice lacking BDNF increased the density of the GCL, horizontal, bipolar, and amacrine cells [48]. We found that tm-scAAV2-GFP has the capacity to directly transduce GCL and Müller cells. BDNF derived from tm-scAAV2-transduced GCL cells may act directly to protect neuronal cells, as tyrosine kinase receptor B (trkB), the BDNF receptor, is expressed in GCL and Müller cells [50]. TrkB mediates the effect of BDNF, which includes neuronal differentiation and survival. In addition, it was also recently reported that TrkB plays a role in indirect neuronal protection and is indispensable for the renewal of neuronal cells [51]. It may be crucial that GCL and Müller cells are transduced with BDNF for neuronal protection to be achieved.
Although the present findings suggest strong BDNF expression could play an important role in the treatment of various retinal diseases, traditional therapeutic approaches remain essential. These therapies include reduction of IOP in glaucoma [52], thrombolytic therapy for retinal artery occlusion [53], reduction of blood pressure in hypertensive retinopathy [54], and photocoagulation in diabetic retinopathy [55]. We suggest BDNF gene therapy may be useful in combination with these traditional approaches.
In conclusion, we have shown that gene therapy with tm-scAAV2-BDNF is an effective means of preventing retinal ischemic damage in rats. This finding demonstrates the feasibility of using an in vivo gene therapeutic approach to the clinical management of retinal diseases such as glaucoma.
Acknowledgments
This work was supported in part by Grant-in-Aid for Scientific Research (C; No. 23792014 and 26462650) from MEXT (Ministry of Education, Culture, Sports, Science and Technology). We thank Dr. Arun Srivastava at the University of Florida for providing the pACG2–3M (pAAV2-Y730+500+444F) packaging plasmid and scAAV-GFP vector plasmid. Author Disclosure Statement: Igarashi T, Miyake K, Takahashi T and Okada T receive research support through the Nippon Medical School from Teika Pharmaceutical Co.,Ltd.
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schumer RA, Podos SM. The nerve of glaucoma! Arch Ophthalmol. 1994;112:37–44. doi: 10.1001/archopht.1994.01090130047015. [DOI] [PubMed] [Google Scholar]
- 3.Wilson AM, Di Polo A. Gene therapy for retinal ganglion cell neuroprotection in glaucoma. Gene Ther. 2012;19:127–36. doi: 10.1038/gt.2011.142. [DOI] [PubMed] [Google Scholar]
- 4.Martin KR, Quigley HA, Zack DJ, Levkovitch-Verbin H, Kielczewski J, Valenta D, Baumrind L, Pease ME, Klein RL, Hauswirth WW. Gene therapy with brain-derived neurotrophic factor as a protection: retinal ganglion cells in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2003;44:4357–65. doi: 10.1167/iovs.02-1332. [DOI] [PubMed] [Google Scholar]
- 5.Mo X, Yokoyama A, Oshitari T, Negishi H, Dezawa M, Mizota A, Adachi-Usami E. Rescue of axotomized retinal ganglion cells by BDNF gene electroporation in adult rats. Invest Ophthalmol Vis Sci. 2002;43:2401–5. [PubMed] [Google Scholar]
- 6.Unoki K, LaVail MM. Protection of the rat retina from ischemic injury by brain-derived neurotrophic factor, ciliary neurotrophic factor, and basic fibroblast growth factor. Invest Ophthalmol Vis Sci. 1994;35:907–15. [PubMed] [Google Scholar]
- 7.Cui Q, Harvey AR. CNTF promotes the regrowth of retinal ganglion cell axons into murine peripheral nerve grafts. Neuroreport. 2000;11:3999–4002. doi: 10.1097/00001756-200012180-00019. [DOI] [PubMed] [Google Scholar]
- 8.Ji JZ, Elyaman W, Yip HK, Lee VW, Yick LW, Hugon J, So KF. CNTF promotes survival of retinal ganglion cells after induction of ocular hypertension in rats: the possible involvement of STAT3 pathway. Eur J Neurosci. 2004;19:265–72. doi: 10.1111/j.0953-816x.2003.03107.x. [DOI] [PubMed] [Google Scholar]
- 9.Koeberle PD, Ball AK. Neurturin enhances the survival of axotomized retinal ganglion cells in vivo: combined effects with glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor. Neuroscience. 2002;110:555–67. doi: 10.1016/s0306-4522(01)00557-7. [DOI] [PubMed] [Google Scholar]
- 10.Takita H, Yoneya S, Gehlbach PL, Duh EJ, Wei LL, Mori K. Retinal neuroprotection against ischemic injury mediated by intraocular gene transfer of pigment epithelium-derived factor. Invest Ophthalmol Vis Sci. 2003;44:4497–504. doi: 10.1167/iovs.03-0052. [DOI] [PubMed] [Google Scholar]
- 11.Vigneswara V, Berry M, Logan A, Ahmed Z. Pigment epithelium-derived factor is retinal ganglion cell neuroprotective and axogenic after optic nerve crush injury. Invest Ophthalmol Vis Sci. 2013;54:2624–33. doi: 10.1167/iovs.13-11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma YT, Hsieh T, Forbes ME, Johnson JE, Frost DO. BDNF injected into the superior colliculus reduces developmental retinal ganglion cell death. J Neurosci. 1998;18:2097–107. doi: 10.1523/JNEUROSCI.18-06-02097.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weibel D, Kreutzberg GW, Schwab ME. Brain-derived neurotrophic factor (BDNF) prevents lesion-induced axonal die-back in young rat optic nerve. Brain Res. 1995;679:249–54. doi: 10.1016/0006-8993(95)00238-l. [DOI] [PubMed] [Google Scholar]
- 14.Mansour-Robaey S, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci USA. 1994;91:1632–6. doi: 10.1073/pnas.91.5.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takano M, Horie H, Iijima Y, Dezawa M, Sawada H, Ishikawa Y. Brain-derived neurotrophic factor enhances neurite regeneration from retinal ganglion cells in aged human retina in vitro. Exp Eye Res. 2002;74:319–23. doi: 10.1006/exer.2001.1118. [DOI] [PubMed] [Google Scholar]
- 16.DiStefano PS, Friedman B, Radziejewski C, Alexander C, Boland P, Schick CM, Lindsay RM, Wiegand SJ. The neurotrophins BDNF, NT-3, and NGF display distinct patterns of retrograde axonal transport in peripheral and central neurons. Neuron. 1992;8:983–93. doi: 10.1016/0896-6273(92)90213-w. [DOI] [PubMed] [Google Scholar]
- 17.Chen H, Weber AJ. BDNF enhances retinal ganglion cell survival in cats with optic nerve damage. Invest Ophthalmol Vis Sci. 2001;42:966–74. [PubMed] [Google Scholar]
- 18.Klocker N, Kermer P, Weishaupt JH, Labes M, Ankerhold R, Bahr M. Brain-derived neurotrophic factor-mediated neuroprotection of adult rat retinal ganglion cells in vivo does not exclusively depend on phosphatidyl-inositol-3′-kinase/protein kinase B signaling. J Neurosci. 2000;20:6962–7. doi: 10.1523/JNEUROSCI.20-18-06962.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mey J, Thanos S. Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res. 1993;602:304–17. doi: 10.1016/0006-8993(93)90695-j. [DOI] [PubMed] [Google Scholar]
- 20.Peinado-Ramon P, Salvador M, Villegas-Perez MP, Vidal-Sanz M. Effects of axotomy and intraocular administration of NT-4, NT-3, and brain-derived neurotrophic factor on the survival of adult rat retinal ganglion cells. A quantitative in vivo study. Invest Ophthalmol Vis Sci. 1996;37:489–500. [PubMed] [Google Scholar]
- 21.Di Polo A, Aigner LJ, Dunn RJ, Bray GM, Aguayo AJ. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Muller cells temporarily rescues injured retinal ganglion cells. Proc Natl Acad Sci USA. 1998;95:3978–83. doi: 10.1073/pnas.95.7.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leaver SG, Cui Q, Plant GW, Arulpragasam A, Hisheh S, Verhaagen J, Harvey AR. AAV-mediated expression of CNTF promotes long-term survival and regeneration of adult rat retinal ganglion cells. Gene Ther. 2006;13:1328–41. doi: 10.1038/sj.gt.3302791. [DOI] [PubMed] [Google Scholar]
- 23.Ren R, Li Y, Liu Z, Liu K, He S. Long-term rescue of rat retinal ganglion cells and visual function by AAV-mediated BDNF expression after acute elevation of intraocular pressure. Invest Ophthalmol Vis Sci. 2012;53:1003–11. doi: 10.1167/iovs.11-8484. [DOI] [PubMed] [Google Scholar]
- 24.Daya S, Cortez N, Berns KI. Adeno-associated virus site-specific integration is mediated by proteins of the nonhomologous end-joining pathway. J Virol. 2009;83:11655–64. doi: 10.1128/JVI.01040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petrs-Silva H, Dinculescu A, Li Q, Deng WT, Pang JJ, Min SH, Chiodo V, Neeley AW, Govindasamy L, Bennett A, Agbandje-McKenna M, Zhong L, Li B, Jayandharan GR, Srivastava A, Lewin AS, Hauswirth WW. Novel properties of tyrosine-mutant AAV2 vectors in the mouse retina. Mol Ther. 2011;19:293–301. doi: 10.1038/mt.2010.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrs-Silva H, Dinculescu A, Li Q, Min SH, Chiodo V, Pang JJ, Zhong L, Zolotukhin S, Srivastava A, Lewin AS, Hauswirth WW. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol Ther. 2009;17:463–71. doi: 10.1038/mt.2008.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, Cooper M, Herzog RW, Zolotukhin I, Warrington KH, Jr, Weigel-Van Aken KA, Hobbs JA, Zolotukhin S, Muzyczka N, Srivastava A. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci USA. 2008;105:7827–32. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurai T, Hisayasu S, Kitagawa R, Migita M, Suzuki H, Hirai Y, Shimada T. AAV1 mediated co-expression of formylglycine-generating enzyme and arylsulfatase a efficiently corrects sulfatide storage in a mouse model of metachromatic leukodystrophy. Mol Ther. 2007;15:38–43. doi: 10.1038/sj.mt.6300012. [DOI] [PubMed] [Google Scholar]
- 29.Miyake K, Miyake N, Yamazaki Y, Shimada T, Hirai Y. Serotype-independent method of recombinant adeno-associated virus (AAV) vector production and purification. Journal of Nippon Medical School = Nippon Ika Daigaku Zasshi. 2012;79:394–402. doi: 10.1272/jnms.79.394. [DOI] [PubMed] [Google Scholar]
- 30.Igarashi T, Miyake K, Asakawa N, Miyake N, Shimada T, Takahashi H. Direct comparison of administration routes for AAV8-mediated ocular gene therapy. Curr Eye Res. 2013;38:569–77. doi: 10.3109/02713683.2013.779720. [DOI] [PubMed] [Google Scholar]
- 31.Oharazawa H, Igarashi T, Yokota T, Fujii H, Suzuki H, Machide M, Takahashi H, Ohta S, Ohsawa I. Protection of the retina by rapid diffusion of hydrogen: administration of hydrogen-loaded eye drops in retinal ischemia-reperfusion injury. Invest Ophthalmol Vis Sci. 2010;51:487–92. doi: 10.1167/iovs.09-4089. [DOI] [PubMed] [Google Scholar]
- 32.Igarashi T, Fujimoto C, Suzuki H, Ono M, Iijima O, Takahashi H, Takahashi H. Short-time exposure of hyperosmolarity triggers interleukin-6 expression in corneal epithelial cells. Cornea. 2014;33:1342–7. doi: 10.1097/ICO.0000000000000256. [DOI] [PubMed] [Google Scholar]
- 33.Igarashi T, Miyake K, Hayakawa J, Kawabata K, Ishizaki M, Takahashi H, Shimada T. Apoptotic cell death and regeneration in the newborn retina after irradiation prior to bone marrow transplantation. Curr Eye Res. 2007;32:543–53. doi: 10.1080/02713680701389333. [DOI] [PubMed] [Google Scholar]
- 34.Igarashi T, Miyake K, Masuda I, Takahashi H, Shimada T. Adeno-associated vector (type 8)-mediated expression of soluble Flt-1 efficiently inhibits neovascularization in a murine choroidal neovascularization model. Hum Gene Ther. 2010;21:631–7. doi: 10.1089/hum.2009.153. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi H, Kanesaki H, Igarashi T, Kameya S, Yamaki K, Mizota A, Kudo A, Miyagoe-Suzuki Y, Takeda S, Takahashi H. Reactive gliosis of astrocytes and Muller glial cells in retina of POMGnT1-deficient mice. Mol Cell Neurosci. 2011;47:119–30. doi: 10.1016/j.mcn.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 36.You Y, Gupta VK, Li JC, Al-Adawy N, Klistorner A, Graham SL. FTY720 protects retinal ganglion cells in experimental glaucoma. Invest Ophthalmol Vis Sci. 2014;55:3060–6. doi: 10.1167/iovs.13-13262. [DOI] [PubMed] [Google Scholar]
- 37.Yun H, Lathrop KL, Yang E, Sun M, Kagemann L, Fu V, Stolz DB, Schuman JS, Du Y. A laser-induced mouse model with long-term intraocular pressure elevation. PLoS ONE. 2014;9:e107446. doi: 10.1371/journal.pone.0107446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shahar J, Zemel E, Perlman I, Loewenstein A. Physiological and toxicological effects of cefuroxime on the albino rabbit retina. Invest Ophthalmol Vis Sci. 2012;53:906–14. doi: 10.1167/iovs.11-8053. [DOI] [PubMed] [Google Scholar]
- 39.Kuroiwa S, Katai N, Shibuki H, Kurokawa T, Umihira J, Nikaido T, Kametani K, Yoshimura N. Expression of cell cycle-related genes in dying cells in retinal ischemic injury. Invest Ophthalmol Vis Sci. 1998;39:610–7. [PubMed] [Google Scholar]
- 40.Huang Y, Cen LP, Luo JM, Wang N, Zhang MZ, van Rooijen N, Pang CP, Cui Q. Differential roles of phosphatidylinositol 3-kinase/akt pathway in retinal ganglion cell survival in rats with or without acute ocular hypertension. Neuroscience. 2008;153:214–25. doi: 10.1016/j.neuroscience.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Sawada A, Neufeld AH. Confirmation of the rat model of chronic, moderately elevated intraocular pressure. Exp Eye Res. 1999;69:525–31. doi: 10.1006/exer.1999.0732. [DOI] [PubMed] [Google Scholar]
- 42.Mowat FM, Gornik KR, Dinculescu A, Boye SL, Hauswirth WW, Petersen-Jones SM, Bartoe JT. Tyrosine capsid-mutant AAV vectors for gene delivery to the canine retina from a subretinal or intravitreal approach. Gene Ther. 2014;21:96–105. doi: 10.1038/gt.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Block F, Grommes C, Kosinski C, Schmidt W, Schwarz M. Retinal ischemia induced by the intraluminal suture method in rats. Neurosci Lett. 1997;232:45–8. doi: 10.1016/s0304-3940(97)00575-2. [DOI] [PubMed] [Google Scholar]
- 44.Wang L, Cioffi GA, Cull G, Dong J, Fortune B. Immunohistologic evidence for retinal glial cell changes in human glaucoma. Invest Ophthalmol Vis Sci. 2002;43:1088–94. [PubMed] [Google Scholar]
- 45.Tanihara H, Hangai M, Sawaguchi S, Abe H, Kageyama M, Nakazawa F, Shirasawa E, Honda Y. Up-regulation of glial fibrillary acidic protein in the retina of primate eyes with experimental glaucoma. Arch Ophthalmol. 1997;115:752–6. doi: 10.1001/archopht.1997.01100150754011. [DOI] [PubMed] [Google Scholar]
- 46.Acheson A, Conover JC, Fandl JP, DeChiara TM, Russell M, Thadani A, Squinto SP, Yancopoulos GD, Lindsay RM. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374:450–3. doi: 10.1038/374450a0. [DOI] [PubMed] [Google Scholar]
- 47.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arango-Gonzalez B, Cellerino A, Kohler K. Exogenous brain-derived neurotrophic factor (BDNF) reverts phenotypic changes in the retinas of transgenic mice lacking the BDNF gene. Invest Ophthalmol Vis Sci. 2009;50:1416–22. doi: 10.1167/iovs.08-2244. [DOI] [PubMed] [Google Scholar]
- 49.Cellerino A, Carroll P, Thoenen H, Barde YA. Reduced size of retinal ganglion cell axons and hypomyelination in mice lacking brain-derived neurotrophic factor. Mol Cell Neurosci. 1997;9:397–408. doi: 10.1006/mcne.1997.0641. [DOI] [PubMed] [Google Scholar]
- 50.Rickman DW, Bowes Rickman C. Suppression of trkB expression by antisense oligonucleotides alters a neuronal phenotype in the rod pathway of the developing rat retina. Proc Natl Acad Sci USA. 1996;93:12564–9. doi: 10.1073/pnas.93.22.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harada C, Guo X, Namekata K, Kimura A, Nakamura K, Tanaka K, Parada LF, Harada T. Glia- and neuron-specific functions of TrkB signalling during retinal degeneration and regeneration. Nat Commun. 2011;2:189. doi: 10.1038/ncomms1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sambhara D, Aref AA. Glaucoma management: relative value and place in therapy of available drug treatments. Ther Adv Chronic Dis. 2014;5:30–43. doi: 10.1177/2040622313511286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agarwal N, Gala NB, Karimi RJ, Turbin RE, Gandhi CD, Prestigiacomo CJ. Current endovascular treatment options for central retinal arterial occlusion: a review. Neurosurg Focus. 2014;36:E7. doi: 10.3171/2013.11.FOCUS13331. [DOI] [PubMed] [Google Scholar]
- 54.Gupta R, Thomas AJ, Masih A, Horowitz MB. Treatment of extracranial carotid artery pseudoaneurysms with stent grafts: case series. J Neuroimaging. 2008;18:180–3. doi: 10.1111/j.1552-6569.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- 55.Stefansson E, Machemer R, de Juan E, Jr, McCuen BW, 2nd, Peterson J. Retinal oxygenation and laser treatment in patients with diabetic retinopathy. Am J Ophthalmol. 1992;113:36–8. doi: 10.1016/s0002-9394(14)75750-2. [DOI] [PubMed] [Google Scholar]