| Patent Application Title: |
Naphthyl Sulfonamide Pyrrolidine Derivatives as Keap-1 Modulators for the Treatment of Diabetes, Obesity, Dyslipidemia, and Related Disorders |
| Patent Application Number: |
EP 2997966A1 |
Publication date: |
March 23, 2016 |
| Inventors: |
Szill, H.; Ruf, S.; Glombik, H.; Kallus, C.; Engel, K−C.; Guessregen, S.; Schmoll, D.; Kannt, A.; Dudda, A.; Monecke, P.; Elshorst, B. |
| Applicant: |
Sanofi 75008 Paris (FR) |
| Disease Area: |
Diabetes, obesity, dyslipidemia, and related disorders |
Biological Target: |
The Kelch-like ECH-associated protein 1 (Keap1) |
| Summary: |
The invention in this patent application is related to naphthyl sulfonamide pyrrolidine derivatives represented generally by formula (I). These compounds are Keap-1 modulators and may be useful for the prevention and/or treatment of diabetes, obesity, dyslipidemia, and related disorders as well as neuroinflammatory and neurodegenerative diseases such as multiple sclerosis, Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease. |
| The transcription factor nuclear factor erythroid 2 p45 (NF-E2)-related factor (Nrf2) is a cellular sensor of oxidative and electrophilic stress. It is a member of the Cap ‘n’ collar family of basic leucine zipper transcription factors, and it is critical for the expression of several cytoprotective genes. Nrf2 is regulated by the Kelch-like ECH-associated protein 1 (Keap-1), which is an adaptor protein of the cullin 3-dependent ubiquitin E3 ligase complex. Keap-1 contains functional domains, including a broad complex/tramtrack/Bric-a-Brac (BTB) domain, an intervening region (IVR) domain, and a Kelch domain. |
| Ubiquitin is the original member of a family of small proteins, which are found in most cellular tissues in the human body that help in regulating the processes of other cellular proteins. Ubiquitin and other relative molecules add covalently to cellular proteins in a process known as ubiquitinylation that alters their activities and the functions. This process plays a critical role in apoptosis of proteins as well as in several other cellular processes related to the regulation of proteins. Keap-1 mediates the ubiquitinylation and subsequent degradation of Nrf2 by the 26S proteasome. In this interaction, the Kelch domain on a Keap-1 homodimer binds to the DLG and ETGE sequence motives of Nrf2 molecule. The Keap-1 BTB domain contains a cysteine residue at the 151 position, while the IVR domain contains two cysteine residues, Cys273 and Cys288. While Keap-1 has other cysteine residues, these three residues are highly reactive and play critical roles in stress sensing. They are modified in response to oxidative stress or electrophilic reagents, and that alters the conformation of Keap-1, inhibits its function, and prevents the degradation of Nrf2. This leads to the translocation of Nrf2 into the nucleus and its subsequent activation. Nrf2 binds to the antioxidant response element (ARE) in the nucleus and drives the expression of Nrf2 target genes such as NAD(P)H quinone oxidoreductase 1 (NQO1) and heme oxygenase 1 (HMOX1), which are upregulated by the activation of Nrf2. |
| The activation of Nrf2 may provide a potential therapy for the prevention and treatment of several diseases associated with increased oxidative and inflammatory stress. For example, diabetes is associated with impaired activation of Nrf2, which causes a reduced oxidative-stress defense. Diabetes is also associated with low-grade inflammation. Inflammatory processes and excessive oxidative stress induce insulin resistance, endothelial dysfunction, and diabetic nephropathy. Therefore, pharmacological activation of Nrf2 may potentially improve glucose control and lipid handling as well as micro- and macrocardiovascular disease in diabetics. In addition, the activation of Nrf2 reduces cellular stress and damage in response to xenobiotics, which could be useful for the prevention of cancer. Since excessive production of reactive oxidative species is implicated in neurodegenerative diseases such as multiple sclerosis, Huntington’s and Parkinson’s disease; therefore, the activation of Nrf2 may also provide a potential therapeutic strategy for these diseases. Therefore, targeting Nrf2 may also be useful in treating chronic inflammatory diseases such as chronic obstructive pulmonary disease, arthritis, and sepsis. |
| The modulators of KEAP-1, particularly those with antagonistic activities, such as the compounds of formula (I) described in this patent application can lead to activation of Nrf2 and may potentially provide therapies for the prevention and/or treatment of diabetes, obesity, dyslipidemia, and other related disorders. |
| Summary (continued): |
Note: It is important to point out a dual role of activated Nrf2 in cancer treatment. As mentioned above, the activation of Nrf2 is a major defense mechanism in the cell that protects against diabetes, neuroinflammatory diseases, neurodegenerative diseases and cancer. However, recent studies have implicated activated Nrf2 in promoting cancer cell survival. Accumulation of Nrf2 in cancer cells supports their growth and protects them against oxidative stress, chemotherapeutic agents, and radiotherapy (see refs 1 and 2 below for more information and detailed discussions). |
| Important Compound Classes: |
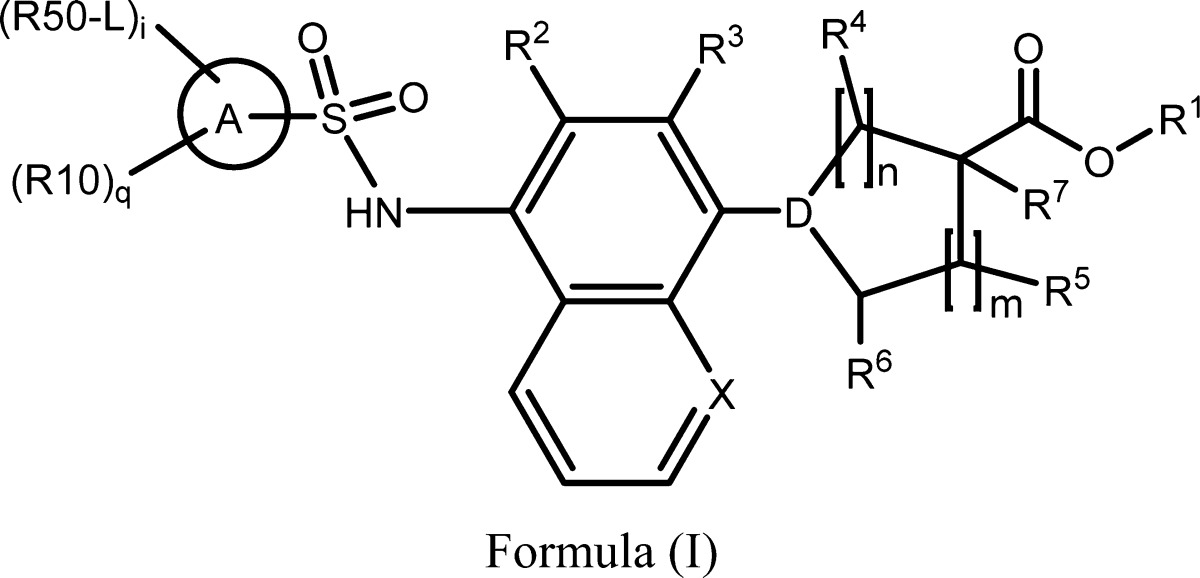 |
| Key Structures: |
The inventors reported the structures of 78 compounds of formula (I) including the following representative examples: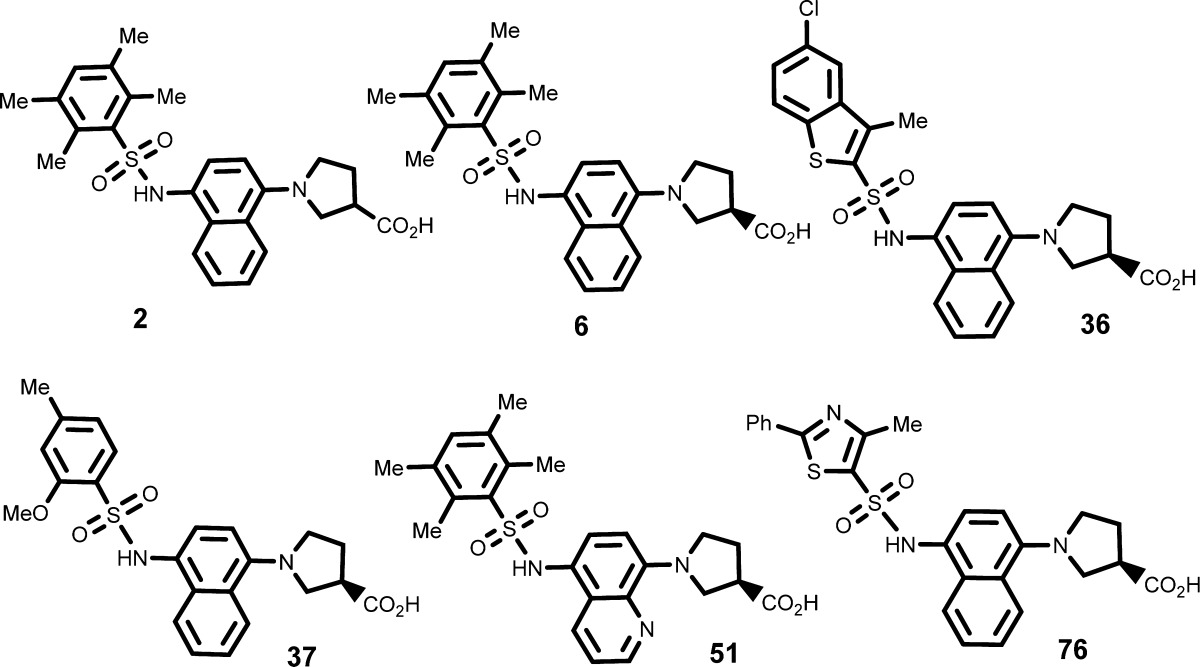
|
| Biological Assay: |
|
| Biological Data: |
The IC50 values obtained from testing the above representative examples in the Keap1-Nrf2 peptide binding assay are listed in the following table.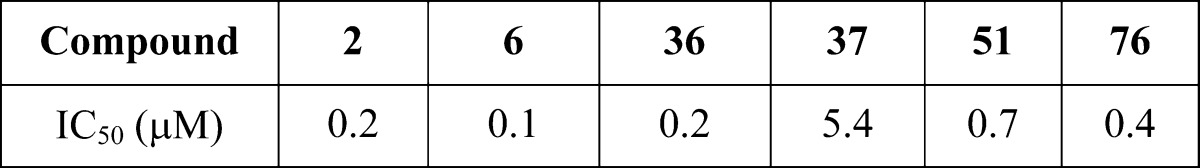
|
| Recent Review Articles: |
Jaramillo M. C.; Zhang D. D.. Genes Dev. 2013, 27, 2179−2191. |
| Sporn M. B.; Liby K. T.. Nat. Rev. Cancer. 2012, 12 ( (8), ), 564−571. |
| Calkins M. J.; Johnson D. A.; Townsend J. A.; Vargas M. R.; Dowell J. A.; Williamson T. P.; Kraft A. D.; Lee J. M.; Li J.; Johnson J. A.. Antioxid Redox Signal. 2009, 11 ( (3), ), 497−508. |





