Abstract
Two 1-(4-aryl-5-alkyl-pyridin-2-yl)-3-methylurea glucokinase activators were identified with robust in vivo efficacy. These two compounds possessed higher solubilities than the previously identified triaryl compounds (i.e., AM-2394). Structure–activity relationship studies are presented along with relevant pharmacokinetic and in vivo data.
Keywords: Type 2 diabetes, glucokinase activator, GKA, methylurea-substituted pyridines
Glucokinase (GK) is a hexokinase isozyme that phosphorylates glucose in the presence of ATP to generate glucose-6-phosphate. It is expressed predominantly in the liver, pancreas, brain, and enterocytes.1 In the pancreas, it is the rate-limiting step in glucose metabolism and thus controls glucose-stimulated insulin secretion. In the liver, it regulates the rate of glucose metabolism and glycogen synthesis. Human genetic mutations in GK underscore the important role this enzyme plays in maintaining proper glucose homeostasis. Loss of function mutations cause maturity-onset diabetes of the young type 2 and gain of function mutations cause hyperinsulinemia and hypoglycemia.2
Because glucokinase controls key steps regulating glucose homeostasis, it has been the focus of considerable attention as a potential target for treating type 2 diabetes. In 2003, Grimsby et al. published the first report of a synthetic small molecule activator of glucokinase.3 The compound, referred to as a GK activator (GKA), binds to a site separate (i.e., allosteric) from the glucose binding site and increases both the affinity of the enzyme for glucose and the maximal velocity. Consequently, activator-bound GK is active at lower glucose concentrations and, when administered to animals, improves plasma glucose levels in both fasting states and following an oral glucose challenge. Several GKAs have advanced to clinical trials, and although robust glucose lowering efficacy has been observed, potential liabilities have also been documented, including hypoglycemia, elevated triglycerides, and blood pressure.4−6
The present report details our efforts to develop novel GKAs for treating type 2 diabetes. Three kinetic parameters were used to characterize our GKAs. The S0.5 is the affinity of the enzyme for glucose, approximately 8 mM in the absence of activator. The maximal velocity, or Vmax, is the rate of glucose phosphorylation at saturating glucose concentrations, defined as 100% in the absence of activator. We also measured the EC50 of the activator in the presence of 5 mM glucose. In addition, the EC50 assay was run in the presence of human serum albumin (HSA, 4% final concentration), and the shift in the EC50 was compared to the value in the absence of HSA. The resulting comparison was used as a functional measure of protein binding.
As described previously in Dransfield et al.,7 the identification of AM-2394 (1) provided a molecule with good potency and in vivo efficacy in various rodent glucose models, however, with poor to moderate solubility (Figure 1). Solubility issues for this compound were believed to be detrimental for further development of this molecule as an orally administered drug. Our goal for the current work was to maintain the favorable potency and kinetic parameters (EC50, S0.5, and Vmax) of AM-2394 (1) while increasing the solubility of the GKAs for further development. The kinetic parameters of AM-2394 were selected to deliver an acceptable balance between efficacy and risk of hypoglycemia, a known potential side effect of GKAs.1
Figure 1.
Properties of AM-2394 (1).
We hypothesized that decreasing the number of aromatic rings might improve solubility by disrupting the packing in the crystal lattice of the compounds.8 Previous structure-guided studies9 showed that the protein pocket in which the C5-alkoxypyridine binds is very flexible, accommodating both small and large groups. We anticipated that a variety of modifications to the C5-alkoxypyridine of AM-2394 (1) would be tolerated. We thus focused our SAR efforts on exploring this region of the molecule, incorporating various alkyl groups to replace the C5 alkoxypyridine to impart desired physicochemical properties into the GKA molecules. Factors such as molecular weight (<500) and log P of these molecules are generally within acceptable range of oral drugs, so the reduction of aromaticity was the focus to improve solubility of these molecules.8,10
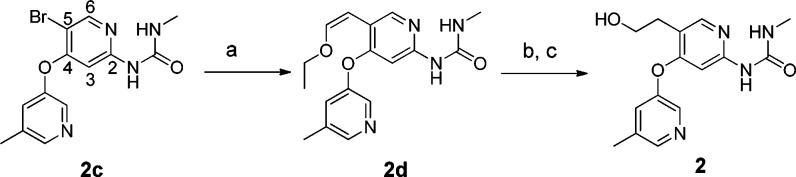
The syntheses of some of the key compounds are shown in Schemes 1–3 and also outlined in the previous manuscript.7 (Z)-1-Ethoxy-2-(tributylstannyl)ethene was coupled to bromo methylurea 2c to provide the ethoxyvinyl urea compound 2d. Then acid-mediated hydrolysis of the ethoxyvinyl moiety followed by sodium borohydride reduction led to the primary alcohol 2 (Scheme 1).
Scheme 1.
Reagents and conditions: (a) Pd(PPh3)4, (Z)-1-ethoxy-2-(tributylstannyl)ethene, toluene, 80%; (b) 4 N HCl(aq), THF; (c) NaBH4, MeOH, 35%.
Scheme 3.
Reagents and conditions: (a) 2-(5,6-dihydro-2H-pyran-3-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, Pd2dba3, PCy3, K3PO4, dioxane, 51%; (b) H2, Pd/C, MeOH, EtOAc, SFC chromatography (see Supporting Information for conditions), 32%.
Synthesis of compound 7 (Scheme 2) started from the lithiation of bromo methylurea 2c. Addition of the lithiated intermediate to 3-(benzyloxy)cyclobutanone generated intermediate 7a, which was hydrogenated under acidic conditions to provide 7 as a single isomer. Compound 7 has a cis relationship between the hydroxyl group and the aromatic substitution on the cyclobutane ring, which was confirmed through a NOESY experiment.
Scheme 2.
Reagents and conditions: (a) MeLi, n-BuLi, 3-(benzyloxy)cyclobutanone, THF, 26%; (b) 10% MeSO3H, EtOH, MeOH, Pd/C, H2, 3 days, 77%.
Suzuki coupling of bromo methylurea 17a with 2-(5,6-dihydro-2H-pyran-3-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane generated compound 20. It was hydrogenated to produce compound 21 after SFC chiral chromatography.
Table 1 describes the SAR of some of the acyclic alkyl groups substituted at the 5-position of the methylurea pyridine. Despite the generally low S0.5 values of these compounds, changing from the bulkier aromatic substitution at C5 to small alkyl alcohol and ethers, generally produced analogues with excellent potency. The exception was the more highly alpha-substituted compound 5 that lost approximately 5-fold in potency. These compounds do not have much potency shift (one to 3-fold) in the presence of 4% HSA, indicating that these compounds are not highly protein-bound. Compounds 3 and 5 also have similar Vmax as AM-2394. Furthermore, both alcohols (2–4) and ethers (6) exhibited much improved (approximately 10-fold increase) solubility in PBS buffer (pH 7.4) over AM-2394.
Table 1. Exploration of the Acyclic Groups at the 5-Position.

The estimated coefficients of variation for the EC50, S0.5, and Vmax are 30% (n = 383), 17% (n = 297), and 4% (n = 329), respectively, based on the performance of a reference compound. Also, human recombinant GK was used in all the assays.
Sol: solubility in PBS buffer at pH 7.4.
This prompted us to explore additional C5 alkyl alcohols and ethers toward identifying analogues with a higher S0.5 to reduce hypoglycemia risk while maintaining similar potency and Vmax as AM-2394. An exercise to constrain the alcohols from Table 1 into a ring is shown in Table 2. Compound 7 showed a more desirable S0.5 of 0.66 and an excellent Vmax of 0.97 while having good potency. Its trans isomer 8 showed improved potency but a lower S0.5 of 0.55. A couple of analogues of 7 (compounds 9–11) were synthesized to see if further improvement in the kinetic parameters could be achieved. However, all of them showed lower Vmax values. A second hydroxyl substitution at the benzylic position (12) decreased the potency dramatically. We then moved to synthesizing alcohols with different ring sizes. Compound 13, with a cyclopropyl methyl alcohol, also had a similar Vmax and potency to 7. However, the S0.5 was less favorable. Dimethyl substitution at the carbon alpha to hydroxyl group (14) resulted in a decrease in Vmax ratio as in previous analogues (10 vs 9). The five- and six-membered ring alcohols (15, 16) have similar potency and Vmax values as those of 7; however, both had less satisfactory S0.5 values.
Table 2. Exploration of the Cyclic Group at the 5-Position.

The estimated coefficients of variation for the EC50, S0.5, and Vmax are 30% (n = 383), 17% (n = 297), and 4% (n = 329), respectively, based on the performance of a reference compound. Also, human recombinant GK was used in all the assays
Compound 7 has very good solubility in PBS buffer (332 μM, pH 7.4) and low plasma protein binding (32% Fu in rat, 9.4% in mouse, and 25% in human). In addition, 7 had low intrinsic clearance in rat and human liver microsomes (24 μL/(min·mg) and <14 μL(min·mg), respectively). Compound 7 also demonstrated moderate clearance in vivo in mice and rats (0.87 and 1.3L/h/kg, respectively) and good oral bioavailability (F = 20% and 39%) (Table 4). Based on its overall favorable biochemical, physiochemical, and pharmacokinetic profiles, compound 7 was tested in the ob/ob mouse diabetic model. When evaluated at doses 3, 10, and 30 mg/kg, compound 7 demonstrated a dose-proportional decrease in blood glucose levels during an oral glucose tolerance test, with a 32% OGTT-AUC reduction at 30 mg/kg (Figure 2).
Table 4. Pharmacokinetic Properties of 7 and 21 (AM-9074).
| IVa |
POa |
|||||
|---|---|---|---|---|---|---|
| compd | species | CL (L/h/kg) | Vss (L/kg) | t1/2 (h) | F (%) | AUC (μM·h) |
| 1 | rat | 1.8 | 2.7 | 2.3 | 60 | 2.70 |
| 7 | rat | 1.3 | 1.2 | 3.0 | 39 | 1.84 |
| 21 | rat | 1.7 | 0.66 | 0.3 | 20 | 3.64 |
| 7 | mouse | 0.87 | 0.65 | 1.2 | 20 | 3.69 |
| 21 | mouse | 9.4 | 3.3 | 0.3 | 5 | 0.13 |
Rat IV dose: 0.5 mg/kg in 100% DMSO, n = 3; PO dose: 2.0 mg/kg in 98.5% water, 1% Tween 80, 0.5% methyl cellulose, n = 3. Mouse IV dose: 1.0 mg/kg in 100% DMSO, n = 3; PO dose: 5.0 mg/kg in 49.55% water, 30% propylene glycol 10% ethanol, 10% dimethylacetamide, 0.45% NaCl, n = 3.
Figure 2.

In vivo results of dosing compound (7) in a diabetes model in mice. Statistical significance compared to vehicle treatment is denoted by *(p < 0.05), **(p < 0.01), ***(p < 0.001), and ****(p < 0.0001), as determined by two-way ANOVA, and is color-coded to the treatment in the figure legends.
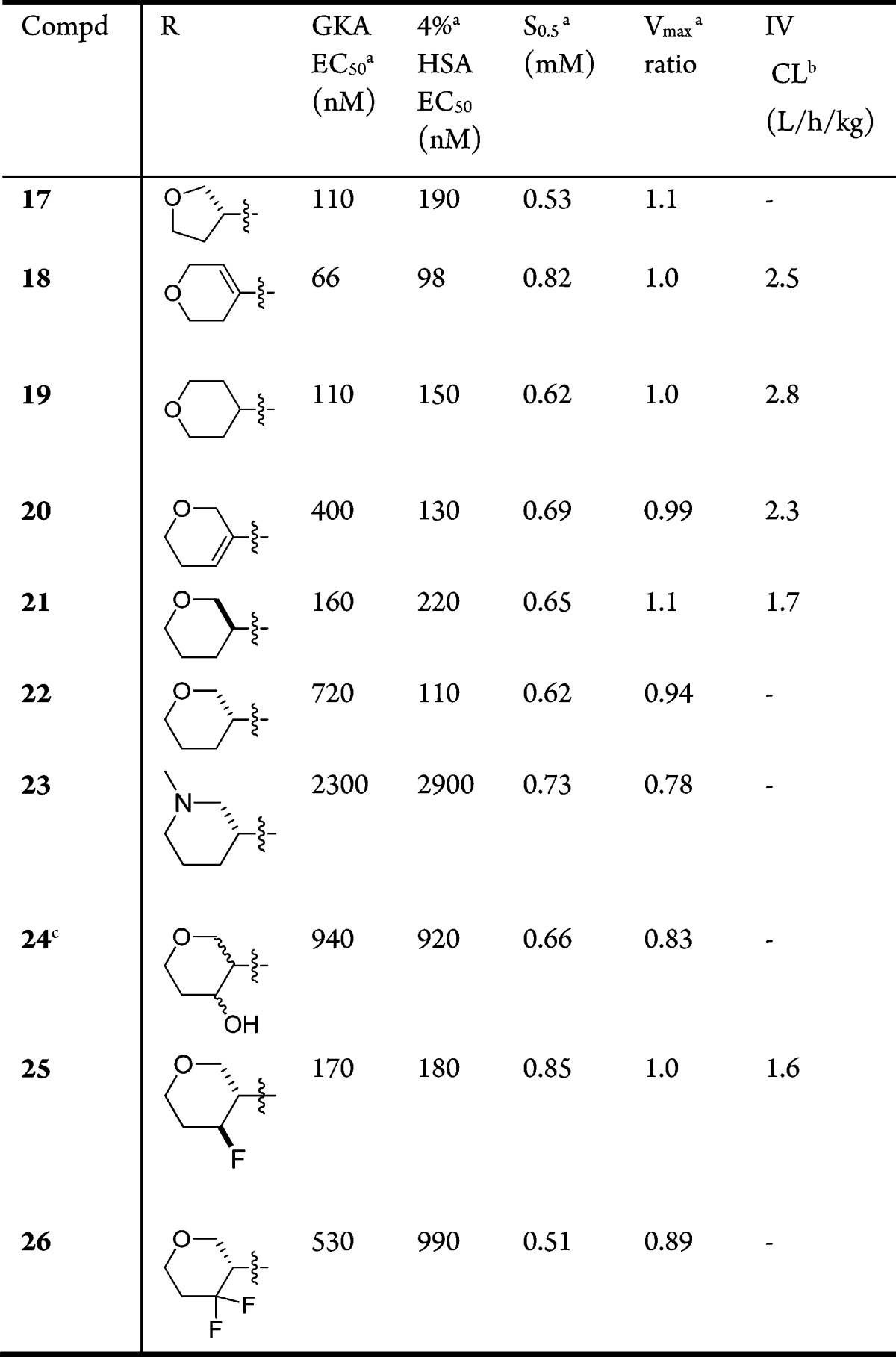
We continued to explore structural diversity at C5 by investigating cyclic ether substitutions (Table 3). A 3-methoxypyridine instead of a 3-methylpyridine at the C4 pyridine position was used due to interchangeability and a slight improvement of potency and kinetics described in our previous manuscript.7 An initial THF analogue 17 had good Vmax and potency but low S0.5. The 4-DHP analogue 18 had a significant boost in S0.5 and favorable potency and Vmax. The 3-DHP analogue 20 also had a favorable kinetic profile. Both compounds were not pursued further because of their lack of in vivo efficacy. This was due to their high IV clearance in rodents that did not enable a durable response over an appropriate time course of an experiment.
Table 3. Exploration of the Cyclic Ether Group at the 5-Position.

The estimated coefficients of variation for the EC50, S0.5, and Vmax are 30% (n = 383), 17% (n = 297), and 4% (n = 329), respectively, based on the performance of a reference compound. Also, human recombinant GK was used in all the assays.
Rat IV dose: 0.5 mg/kg, n = 3.
Racemic, a pair of cis isomers; in addition, all stereochemistry was assigned arbitrarily.
Similar to 7, compound 21 has low plasma protein binding (28% Fu in rat, 14% in mouse, and 16% in human) and good solubility in PBS buffer (303 μM, pH 7.4). Compound 21 had high in vivo clearance in mouse but moderate clearance and acceptable oral bioavailability in rat (Table 4). Metabolic identification studies were performed on 21 that identified the THP ring as one site of oxidation (see Supporting Information). Additional modifications on the 3-THP ring, such as increasing polarity or addition of electron-withdrawing groups (23, 24, 25, and 26), were made to potentially lower the clearance further by possibly blocking this metabolic pathway. These tended to adversely impact potency, although not in the case of compound 25. However, as 25 did not show improved in vivo clearance, it was not pursued further. The 4-THP analogue (compound 19) of compound 21 also had higher in vivo clearance.
The ability of compound 21 (AM-9074) to lower fed blood glucose levels was tested in male Sprague–Dawley rats. When evaluated at doses 3, 10, 30, and 100 mg/kg, compound 21 demonstrated a dose-proportional decrease in blood glucose levels during a glucose tolerance test, with a 40% reduction in AUC at 100 mg/kg (Figure 3).
Figure 3.

(top) AM-9074 fed blood glucose levels. (bottom) In vivo results of dosing compound AM-9074 (21) in normal rat model. Statistical significance compared to vehicle treatment is denoted by *(p < 0.05), **(p < 0.01), and ***(p < 0.001), as determined by ANOVA, and is color-coded to the treatment in the figure legends.
In conclusion, through systematic exploration of acyclic and cyclic alcohol and ether substitutions at the C5 position, we have identified multiple compounds with improved solubility compared to AM-2394, while maintaining similar potency and kinetic parameters. This increase in solubility should aid in the drug development of this GKA class of urea compounds,11 including the ability to achieve high exposures in vivo with maximal dosing across several species. Compounds 7 and 21 (AM-9074) exhibited acceptable clearance and oral bioavailability in rodents and effectively lowered plasma glucose levels in the appropriate model studies.
Acknowledgments
The authors would like to acknowledge the analytical and separations group at ASF, particularly Brent Murphy for large scale chiral separation of compound 21.
Glossary
ABBREVIATIONS
- Compd
compound
- OGTT
oral glucose tolerance test
- PBS
phosphate buffered saline
Biography
Todd J. Kohn received his BS with Honors in Chemistry from the University of Wisconsin−Madison where he performed undergraduate research in the laboratories of Prof. Steven D. Burke. Following graduation from Wisconsin, he was employed at Eli Lilly in various associate scientist positions for almost 14 years working on a number of projects including identification of novel thrombin and BACE inhibitors. He then moved to Amgen in 2006, where he has since achieved the level of Scientist. During his time at Amgen, he has worked on several projects including PI3K-delta and GKA inhibitors exemplified in this publication.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.6b00145.
Experimental procedures, data for compounds, and in vivo procedures (PDF)
Author Contributions
‡ These authors contributed equally to this work. The manuscript was written through contributions of all authors All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Matschinsky F. M. Assessing the potential of glucokinase activators in diabetes therapy. Nat. Rev. Drug Discovery 2009, 8, 399–416. 10.1038/nrd2850. [DOI] [PubMed] [Google Scholar]
- Miller S. P.; Anand G. R.; Karschnia E. J.; Bell G. I.; LaPorte D. C.; Lange A. J. Characterization of glucokinase mutations associated with maturity-onset diabetes of the young type 2 (MODY-2): different glucokinase defects lead to a common phenotype. Diabetes 1999, 48, 1645–1651. 10.2337/diabetes.48.8.1645. [DOI] [PubMed] [Google Scholar]
- Grimsby J.; Sarabu R.; Corbett W. L.; Haynes N. E.; Bizzarro F. T.; Coffey J. W.; Guertin K. R.; Hilliard D. W.; Kester R. F.; Mahaney P. E.; Marcus L.; Qi L.; Spence C. L.; Tengi J.; Magnuson M. A.; Chu C. A.; Dvorozniak M. T.; Matschinsky F. M.; Grippo J. F. Allosteric activators of glucokinase: potential role in diabetes therapy. Science 2003, 301, 370–373. 10.1126/science.1084073. [DOI] [PubMed] [Google Scholar]
- Bonadonna R. C.; Heise T.; Arbet-Engels C.; Kapitza C.; Avogaro A.; Grimsby J.; Zhi J.; Grippo J. F.; Balena R. Piragliatin (RO4389620), a novel glucokinase activator, lowers plasma glucose both in the postabsorptive state and after a glucose challenge in patients with type 2 diabetes mellitus: a mechanistic study. J. Clin. Endocrinol. Metab. 2010, 95, 5028–5036. 10.1210/jc.2010-1041. [DOI] [PubMed] [Google Scholar]
- Meininger G. E.; Scott R.; Alba M.; Shentu Y.; Luo E.; Amin H.; Davies M. J.; Kaufman K. D.; Goldstein B. J. Effects of MK-0941, a novel glucokinase activator, on glycemic control in insulin-treated patients with type 2 diabetes. Diabetes Care 2011, 34, 2560–2566. 10.2337/dc11-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bue-Valleskey J. M.; Schneck K. B.; Sinha V. P.; Wonddmagegnehu E. T.; Kapitza C.; Miller J.. W.LY2599506, a novel glucokinase activator (GKA), improves fasting and postprandial glucose in patients with type 2 diabetes mellitus (T2DM). Presented at the 71st American Diabetes Association Meeting, San Diego, CA, 2011.
- Dransfield P.; Pattaropong V.; Lai S.; Fu Z.; Kohn T.; Du X.; Cheng A.; Xiong Y.; Komorowski R.; Jin L.; Conn M.; Tien E.; DeWolf W.; Hinklin R.; Aicher T.; Kraser C.; Boyd S.; Voegtli W.; Condroski K.; Veniant M.; Medina J.; Houze J.; Coward P. A novel series of potent glucokinase activators leading to discovery of AM-2394. ACS Med. Chem. Lett. 2016, 10.1021/acsmedchemlett.6b00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovering F.; Bikker J.; Humblet C. Escape from flatland: Increasing saturation as an approach to improving clinical success. J. Med. Chem. 2009, 52, 6752–6756. 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- Hinklin R. J.; Boyd S. A.; Chicarelli M. J.; Condroski K. R.; Dewolf W. E. Jr.; Lee P. A.; Lee W.; Singh A.; Thomas L.; Voegtli W. C.; Williams L.; Aicher T. D. Identification of a new class of glucokinase activators through structure-based design. J. Med. Chem. 2013, 56, 7669–7678. 10.1021/jm401116k. [DOI] [PubMed] [Google Scholar]
- Lipinski C. A.; Lombardo F.; Dominy B. W.; Feeney P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Delivery Rev. 1997, 23, 3–25. 10.1016/S0169-409X(96)00423-1. [DOI] [PubMed] [Google Scholar]
- Amidon G. L.; Lennernas H.; Shah V. P. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12, 413–420. 10.1023/A:1016212804288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.