Summary
Enhancing the immune system is a validated strategy to combat infectious disease, cancer and allergy. Nevertheless, the development of immune adjuvants has been hampered by safety concerns. Agents that can stimulate the immune system often bear structural similarities with pathogen‐associated molecular patterns found in bacteria or viruses and are recognized by pattern recognition receptors (PRRs). Activation of these PRRs results in the immediate release of inflammatory cytokines, up‐regulation of co‐stimulatory molecules, and recruitment of innate immune cells. The distribution and duration of these early inflammatory events are crucial in the development of antigen‐specific adaptive immunity in the forms of antibody and/or T cells capable of searching for and destroying the infectious pathogens or cancer cells. However, systemic activation of these PRRs is often poorly tolerated. Hence, different strategies have been employed to modify or deliver immune agonists in an attempt to control the early innate receptor activation through temporal or spatial restriction. These approaches include physicochemical manipulation, covalent conjugation, formulation and conditional activation/deactivation. This review will describe recent examples of discovery and optimization of synthetic immune agonists towards clinical application.
Keywords: adjuvants, drug discovery, immuno‐oncology, Toll‐like receptor, vaccine
Abbreviations
- BCG
bacillus Calmette–Guérin
- HBV
hepatitis B virus
- MPLA
monophosphoryl lipid A
- PEG
polyethylene glycol
- PRR
pattern recognition receptors
- ssRNA
single‐stranded RNA
- STING
stimulator of interferon gene
- Th2
T helper type 2
- TLR
Toll‐like receptors
Introduction
Therapeutic use of immune potentiators holds great promise where the enhancement of the immune response to either foreign or endogenous antigens is the desired outcome. Beginning with the work of Jenner, vaccinology has been a proven science, and vaccines have eliminated many deadly diseases such as smallpox.1 As vaccine developers have begun to move away from live‐attenuated microorganisms to subunit vaccines, they have also stripped away many of the immune‐stimulating agents naturally embedded within pathogenic bacteria or viruses, ultimately resulting in reduced vaccine efficacy. Hence, subunit prophylactic vaccines often require the addition of exogenous adjuvants.2 Immune activation also has the potential to treat established infectious diseases. Most of the antiviral and antibacterial drugs used today target pathogens. However, in many situations, pathogens can mutate and become resistant to these drugs. Alternatively, pathogens can evade or even blunt the host immune response. Raising or restoring host immunity could be a solution to treat chronic infections that are difficult to cure.3 Furthermore, vaccination can be extended beyond protection against infectious pathogens to priming of an immune response against self‐antigens that have become transformed or malignant.4 Over a century ago, the famous experiment by Coley demonstrated the potential of immune therapy by injecting bacteria into cancer patients.5 Today, many investigational cancer vaccines incorporate powerful adjuvants to overcome the immune suppressive tumour microenvironment or to break self‐tolerance. Lastly, the hygiene hypothesis suggests that the lack of childhood exposure to infectious microorganisms increases the susceptibility to atopic allergic disorders, because the immune system suffers from a lack of tolerance induction to the environment during early development.6 One experimental approach to suppress the allergic T helper type 2 (Th2) response is to raise the opposing Th1 response through the appropriate type of immune potentiators. Collectively, although immune potentiators could be used in many beneficial ways to treat infectious disease, cancer and allergy, immune activation is a double‐edged sword, and the development of novel immune adjuvants has been hampered historically by poor safety and tolerability. Concepts or technologies to uncouple immune‐mediated efficacy from toxicity are therefore key to novel immune agonist design (Fig. 1).
Figure 1.
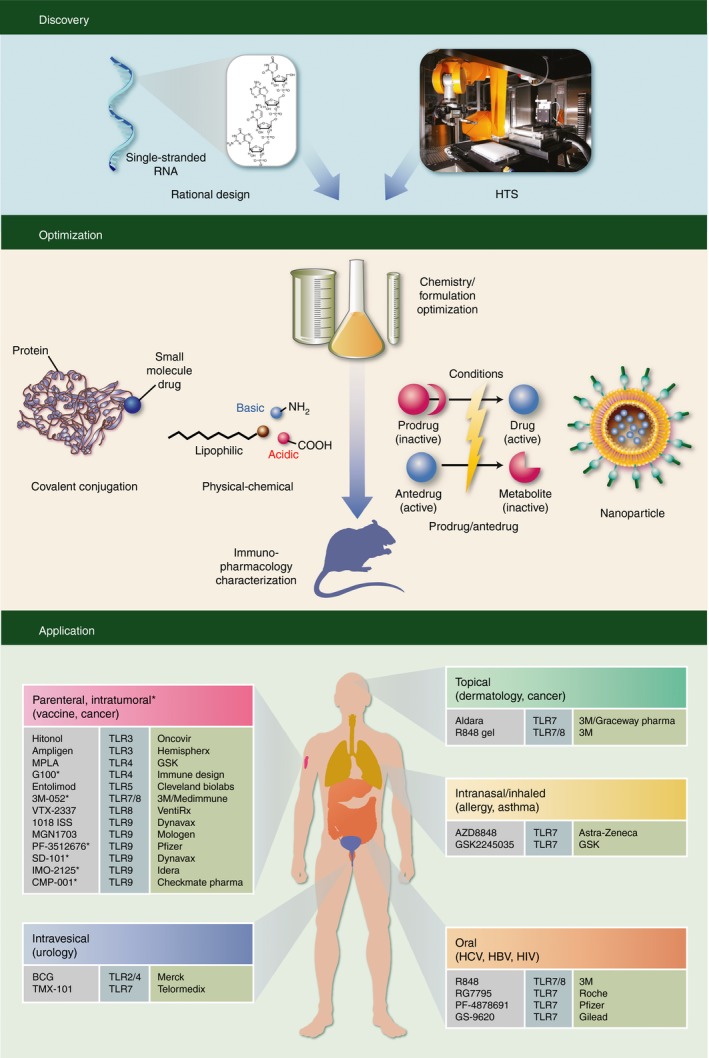
Discovery, optimization and application of synthetic immune agonists. Discovery of novel immune agonists can come from rational design based on pathogen‐associated molecular patterns or from un‐biased high‐throughput screening of diverse compound libraries. Chemistry/formulation optimization can follow a number of strategies with the common goal of eliciting antigen‐specific immune response. Co‐delivery of antigen and adjuvant can be achieved through covalent conjugation or nanoparticle formulation. The physical–chemical properties of synthetic immune agonists can be engineered to allow preferential tissue distribution. Conditional activation/deactivation by designed prodrug/antedrug attempts to restrict the immune activation within certain tissues. Optimized agonists are then characterized in various immuno‐pharmacological animal models of their intended clinical applications. Different routes of administration are required for local delivery to afford the greatest benefit‐to‐risk ratio (therapeutic index). Vaccine adjuvants are administered parenterally, either through subcutaneous or intramuscular injection, together with the antigens. Autologous vaccination injects the immune agonists directly into the tumour to generate an immune response against tumour antigens in situ. Topical applications are used to treat dermatological diseases. Intranasal administration and inhalation of immune agonists are investigated for their ability to suppress allergy or asthma. Oral TLR7 agonists have been explored for treatment of hepatitis C virus and hepatitis B virus. Intravesical delivery of bacillus Calmette–Guèrin (BCG) directly into the bladder is currently the standard of care for non‐invasive bladder cancer.
Immune agonists often structurally resemble bacterial or viral components that are recognized by pattern recognition receptors (PRR), the most studied being Toll‐like receptors (TLRs).7 TLR1/2 and TLR2/6 recognize the N‐terminus of lipoproteins from Gram‐positive bacteria and other microbial sources. TLR4 is stimulated by lipopolysaccharide and its derivatives, produced by Gram‐negative bacteria. Bacterial flagellin is a ligand of TLR5. Of the viral recognition receptors, TLR3 senses double‐stranded RNA, whereas TLR7 and TLR8 sense single‐stranded RNA (ssRNA). TLR9 is stimulated by unmethylated CpG motifs commonly found in bacterial DNA. Besides TLRs, nucleotide‐binding oligomerization domain‐like receptors are stimulated by bacterial products.8 Retinoic acid‐inducible gene‐1‐like receptors detect the presence of viral infection.9 C‐type lectin receptors recognize a variety of carbohydrate derivatives.10 The most recently discovered PRR, stimulator of interferon gene (STING), is a sensor of cytosolic DNA.11
Although all PRR ligands can be derived from microbial sources, many of these natural product derivatives have undefined structures (e.g. complex glycolipids) that are often polymeric, which make manufacturing on a commercial scale a daunting task. The mechanism of action is often complicated, and these natural products exhibit poor physicochemical properties, which presents formulation challenges compared with modern small‐molecule drug standards. Progressively, with a better understanding of innate receptor biology, researchers have explored different ways to simplify or synthesize pathogen‐associated molecular pattern mimetics using chemistry to improve pharmacological properties. New adjuvant chemotypes often bear structural resemblance to the natural ligands, but are derivatized in ways that increase and/or maintain the adjuvant potency, while reducing the structural complexity for ease of manufacture and improving other important characteristics.
Most modern small‐molecule drugs have gone through iterative rounds of optimization, often with the goal of increasing systemic bioavailability and extending in vivo half‐life for maximum therapeutic effects. Immune adjuvants, however, follow different design principles. Adjuvants function by stimulating antigen‐presenting cells to more effectively uptake and present antigens through inflammatory cytokines and co‐stimulatory molecules. This enhanced process leads to better adaptive humoral and cellular responses. Without the intended antigen(s) in proximity, adjuvants induce non‐productive immune activation, or wasted inflammation, which contributes little to adaptive immunity. Literature reports have demonstrated that systemic distribution of vaccine adjuvants is not required to elicit antigen‐specific immune responses.12, 13 Moreover, repeated systemic administration of TLR agonists has been shown to blunt the innate response, possibly through receptor desensitization or other mechanisms of immune tolerance induction.14, 15 Hence, for better efficacy and improved tolerability, a key attribute of adjuvant distribution should be co‐localization with the antigen(s) it serves to boost immunity against. Systemic distribution and systemic immune activation are not needed nor desired to induce an antigen‐specific adaptive immune response.
Chemistry functionalization can be used to enhance the biological activities and drug‐like properties of small molecules. Strategies of adjuvant optimization often involve tuning selectivity and reducing off‐target activity, simplifying structural complexity, increasing stability, enhancing injection site retention, improving antigen/adjuvant co‐delivery, or engineering conditional activation/deactivation. Cumulative knowledge gained from systematic adjuvant optimization has provided the guiding principles for designing safe and effective adjuvants.16, 17, 18, 19, 20 This review will illustrate strategies of synthetic immune agonist discovery and optimization toward clinical application.
Discovery from rational design (inspired by nature)
Among the known innate immune receptors, TLR7 was the first receptor reported to be activated by small molecules; hence, it has been the target for which most small‐molecule agonists have been generated and researched. TLR7 recognizes viral ssRNA, which consists of repeating units of ribonucleotides.21, 22 However, even before the discovery of TLR7, small heterocyclic molecules bearing nucleoside‐like structure were reported to exhibit therapeutic effects through immune potentiation. As early as the 1970s, Nichol et al.23 reported the interferon‐inducing activity of a substituted pyrimidine, a scaffold shared by nucleic acid bases cytosine and thymidine. During the 1980s, Goodman and Weigle24 first reported the lymphocyte activation activity of a purine nucleoside analogue. In the late 1980s, Bernstein et al. and Chen et al.25, 26 reported antiviral activity of an imidazoquinoline named R837/S26308 (later named imiquimod) in guinea‐pig models of herpes simplex virus and cytomegalovirus. Building on the earlier findings, Hirota et al.27 described the structural activity relationship of a series of non‐nucleoside adenine analogues as potent interferon inducers without knowing the target. It was not until 2002 that Hemmi et al.28 identified the target of imiquimod to be TLR7. A year later, Lee et al.29 demonstrated that purine nucleoside analogues also function through TLR7. Relative to complex natural products and biologics, small synthetic molecules can be more easily made into defined drug candidates using controlled and reproducible manufacturing processes. Hence, the discovery that low‐molecular‐weight agonists can stimulate TLR7 in a manner comparable to the natural ligand, ssRNA, prompted biotech and pharmaceutical companies to search for novel and proprietary chemical space. Expanding on the earlier chemotypes, a series of deazapurine,30 pyrimidine,31 pteridinone,32 benzonaphthyridine,33 and other scaffolds have been disclosed in publications and patents.34 It is of interest to note that most of these TLR7 chemotypes share a common 2‐aminopyridine or 2‐aminopyrimidine core, which could be a critical nucleic acid recognition motif for the receptor. Although the structure of TLR7 has yet to be solved, researchers have built homology models based on the X‐ray crystal structure of the TLR8 ectodomain in an attempt to rationalize the observed structure activity relationship and guide future medicinal chemistry optimization.32
Similar to TLR7, TLR8 is also an endosomal TLR that recognizes ssRNA. The main differences between the two RNA recognition receptors lie in their expression and functional activity across different species. Whereas TLR7 is predominantly expressed in plasmacytoid dendritic cells, TLR8 is expressed in myeloid dendritic cells, monocytes and macrophages.35, 36 Interestingly, rodents lack a functional TLR8, making it challenging to characterize TLR8 agonists in vivo.37, 38 CL075 is an imidazoquinoline TLR7/8 agonist reported to have more TLR8‐biased activity.39 Extending from the imidazoquinoline series, Kokatla et al.40 identified a series of furoquinolines that displays much improved selectivity over TLR7. Recently, the X‐ray co‐crystal structure of TLR8 has been solved with a number of small‐molecule ligands.41, 42 Guided by this information, structure‐based ligand design led to the identification of several additional TLR8 agonists.43, 44
Bacterial lipoproteins and lipopeptides are known to stimulate the innate immune system through TLR2.45, 46 Even before the discovery of TLR2, N‐acyl‐S‐diacylglyceryl cysteine motifs were known simply as macrophage‐activating lipopeptides.47 X‐ray crystallography revealed that the number of acyl chains determines selectivity between TLR1/2 versus TLR2/6 heterodimerization. Tri‐acylated lipopeptides activate TLR1/2 by insertion of the N‐acyl chain to TLR1 and the remaining two glyceryl acyl chains to TLR2.48 In contrast, di‐acylated lipopeptides, with the N‐acyl chain missing, activate TLR2/6.49 Detailed structure activity relationship studies of the cysteine‐glycerol core showed the importance of stereochemistry, the thioether bridge, and ester connections.50 The peptide region can tolerate more functional group manipulation. Many synthetic lipopeptides have introduced polyethylene glycol (PEG) or sugar moieties at the peptide region with the aim of improving solubility and/or amphiphilic properties of these otherwise poorly soluble and lipophilic molecules.51, 52, 53 Further simplifying the structure, a series of monoacyl lipopeptides was discovered to be human‐specific TLR2 agonists.54, 55 Another simplified class of lipoamino acid agonist was identified by maintaining the minimal structure requirement for TLR2 activity.56
The natural ligand of TLR4 is bacterial lipopolysaccharide.57 X‐ray crystal structure studies have shown that the recognition element lies in the lipid A portion through the adaptor protein MD2.58 Mono‐phosphoryl lipid A (MPLA) is a TLR4 agonist produced from alkaline hydrolysis of salmonella‐derived lipopolysaccharide.59 The structure of MPLA is not synthetically defined, as the product contains a mixture of derivatives with five and six alkyl chains. It is clinically approved as an adjuvant in hepatitis B virus (HBV) and human papillomavirus vaccines. Apart from the semi‐synthetic MPLA, fully synthetic derivatives of lipid A have been reported including glucopyranosyl lipid A,60 aminoalkyl glucosaminide 4‐phosphate61 and E6020.62
Discovery from high throughput screening
With the advent of new screening technologies with the ability to assay large collections of small‐molecule compound libraries in miniaturized cellular assays, both industrial and academic institutions have turned to high‐throughput screening as a modern way of identifying novel small‐molecule immune potentiators. VentiRx has reported a series of benzoazepine TLR8 agonists identified from screening.63 The lead candidate, VTX‐2337, has been shown to activate natural killer cells and enhance antibody‐dependent cell‐mediated cytotoxicity in human cells in vitro, and is being developed for cancer indications.64 Another benzoazepine derivative, VTX‐294, was reported to activate newborn and adult leucocytes better than other TLR7/8 agonists R848 and CL075.65 Other human‐specific small‐molecule agonists discovered from high‐throughput screening have been reported for TLR2,66 TLR467, 68 and STING.69 Interestingly, given that these screenings were conducted in human cells, many of these agonists were reported to exhibit significantly reduced activity in mouse cells.
Physicochemical manipulation
Most low‐molecular‐weight drugs are designed with the intent for oral bioavailability and systemic delivery. In contrast, immune adjuvants should be co‐localized to the antigen(s) against which the immune response is intended. Restricting the spatial distribution of adjuvants prevents systemic activation of peripheral immune cells, and thereby minimizes inflammatory cytokine production that is not contributing to the antigen‐specific immune response. As vaccines are often given parenterally, one way to provide localized immune activation is through increasing local injection site retention. This could be achieved by manipulating the physicochemical properties of the small molecule adjuvant. Towards this end, Smirnov et al.12 installed long lipophilic alkyl moiety for slow dissemination from the site of application. Upon subcutaneous injection, 3M‐052 drives a strong Th1 response to haemagglutinin and serum neutralization of viable H1N1 virus in the absence of circulating tumour necrosis factor‐α or the induction of Th1 cytokines. Intratumoral administration of 3M‐052 in the B6.F10 melanoma model generated systemic antitumour immunity and suppressed both injected and non‐injected (distal) tumour growth.70 Similarly, Chan et al.71, 72 described the synthesis and characterization of adenine TLR7 agonists modified with PEG and/or phospholipid for improved pharmacokinetics and biodistribution. Installation of a phospholipid onto the benzylic region of 8‐oxo‐adenine increased in vitro potency 100‐fold over the unmodified TLR7 agonist and induced both Th1 and Th2 antigen‐specific immune responses in an ovalbumin model.71 The phospholipid–adenine conjugate was further demonstrated to reduce cancer growth in a B16c‐ovalbumin melanoma model via intralesional administration.73 Shukla et al.74 designed TLR7‐agonistic dendrimers with three to six units of the active imidazoquinoline pharmacophore. These high‐molecular‐weight dendrimers retain potent TLR7 activity and were shown to induce high‐affinity antibodies to bovine α‐lactalbumin. Taken together, there are multiple ways in which synthetic immune agonists can be localized through physical chemical parameters, such as increasing molecular weight and lipophilicity (logP), or reducing polarity (polar surface area) and solubility.
Covalent conjugation
In order to elicit a more efficient and specific immune response, an effective vaccine delivery system would function to target the immune agonist (adjuvant) and antigen to the same antigen‐presenting cells. To this end, researchers have explored various approaches to covalently conjugate adjuvant and antigen. Wille‐Reece et al.75, 76 have demonstrated that covalent linking of an imidazoquinoline TLR7/8 agonist to HIV Gag protein dramatically enhanced the magnitude and altered the quality of the Th1 response, compared with animals co‐immunized with HIV Gag protein (non‐conjugated) and the TLR7/8 agonist or CpG‐oligodeoxynucleotide. Follow‐up mechanistic studies revealed that the TLR agonist–antigen conjugate elicits CD8+ T‐cell responses based not on the capacity to induce dendritic cell maturation or antigen persistence and uptake, but on the engagement of dendritic cell cross‐presentation pathways.77 Vecchi et al.78 described the conjugation of a TLR7 agonist to Streptococcus pneumoniae antigen, which resulted in dose‐sparing of both antigen and adjuvant, and it also protected mice from a lethal challenge. No adjuvant effect was observed when equimolar unconjugated TLR7 agonist was co‐administered together with the non‐conjugated antigen. Other examples of TLR agonist conjugation to peptide antigens have been extensively reviewed.79
In addition to vaccine antigens, conjugation of small‐molecule immune agonists to other macromolecules such as proteins, carbohydrates or antibodies can be used to achieve special properties. Wu et al.80 showed that covalent attachment of an adenine TLR7 agonist to mouse serum albumin can increase local immune activation and reduce systemic inflammation. When administered into mouse lung in vivo, the TLR7 agonist–mouse serum albumin conjugate induced 10‐fold higher local release of cytokines relative to the unconjugated TLR7 agonist. The functional efficacy of the conjugate was demonstrated in an anthrax and flu challenge model to delay mortality. Leveraging non‐covalent affinity, Liu et al.81 modified a TLR9‐activating CpG‐oligonucleotide with lipid moieties capable of physical binding to albumin. Hitch‐hiking on endogenous serum albumin, lipid‐modified CpG effectively accumulated in lymph nodes, which resulted in better T‐cell priming, enhanced antitumour efficacy in a B16F10 tumor model, and reduced systemic toxicity. Shinchi et al.82 conjugated an adenine TLR7 agonist onto polysaccharides to improve aqueous solubility. The resulting conjugate appeared to be a more potent adjuvant, and the authors hypothesized that this was due to the enhanced uptake by the antigen‐presenting cells. Lastly, to mimic the combination effect of multiple types of TLR agonists found naturally in microorganisms, Esser‐Kahn and co‐workers have conjugated agonists of TLR2 and TLR9, and also agonists of TLR4, TLR7 and TLR9, respectively. These spatially defined di‐ and tri‐agonists were shown to exhibit higher activity synergistically over the unconjugated mixtures.83, 84
Delivery of small‐molecule drug via antibodies through covalent conjugation is a validated technology, as several antibody–drug conjugates have been approved for clinical treatment of cancer.85 Extending this concept, several groups have attempted to deliver immune agonists by conjugation to antibodies targeting tumour cells. Sharma et al.86 conjugated CpG‐oligodeoxynucleotide (TLR9 agonist) to anti‐Her‐2/neu monoclonal antibody and demonstrated that the conjugate can bind to Her‐2/neu+ tumours, activate dendritic cells, and induce antitumour responses. Li et al.87 described the conjugation of CpG to clinically approved monoclonal antibodies rituximab and trastuzumab. Recently, Gadd et al.88 have extended the antibody conjugation approach to TLR7 agonists as the payload. Instead of targeting tumour cells, Kreutz et al.89 reported an antibody–antigen–adjuvant conjugate designed to target DEC205+ dendritic cells. The antibody–antigen–adjuvant triple conjugate was demonstrated to be superior to the antibody‐free antigen–adjuvant double conjugate in priming of cytotoxic T‐lymphocyte responses and efficiently induced anti‐tumour immunity in a B16 model, although the authors had noted possible non‐specific delivery to cells that are independent of the DEC205.
Formulation‐assisted delivery
Although covalent conjugation and increased lipophilicity are strategies that have led to good tool adjuvants through localized immune activation, there are practical considerations for commercial development. Covalent attachment of immune agonist requires careful control of conjugation chemistry to afford homogeneous protein production. Each covalently modified antigen is a new biological entity that requires separate manufacturing and characterization protocols. Co‐administering a vaccine antigen with a locally retained adjuvant offers the advantage of stockpiling one adjuvant formulation to be used with multiple vaccines. However, highly lipophilic and poorly soluble adjuvants often present formulation challenges, resulting in inconsistent dosing and prolonged residence time at the injection site, sometimes lasting longer than the antigens.
To better understand the ideal properties of immune adjuvant, Wu et al.13 investigated the minimal essential temporal and spatial distribution required for effective adjuvanticity using structurally similar TLR7 agonists with differential physicochemical properties. Gene expression and cytokine profiling revealed that most of the immediate inflammatory activity is needed only at the injection site, and that increased inflammation in the serum does not necessarily contribute to better adjuvanticity. To address the poor developability of insoluble adjuvants, the authors subsequently designed soluble analogues that can be adsorbed onto alum via phosphonate/Al(OH)3 ligand exchange. Alum has been used in human vaccines for decades.90 It is thought that one attribute linked to its adjuvanticity is increased antigen deposition, via non‐covalent adsorption. This is argued to be mediated through electrostatic, hydrophobic and ligand exchange interactions.91 As alum behaves like a particulate at the injection site, alum adsorption of adjuvant and antigen results in a highly co‐localized vaccine formulation with good development potential. Once dissociated from alum, these soluble TLR7‐phosphonates are readily eliminated from the injected muscle and undergo rapid systemic clearance, resulting in minimal wasted inflammation. TLR7/alum adjuvant formulations were tested to effectively enhance a vaccine against Neisseria meningitidis serotype B, increasing both the depth and breadth of serum bactericidal antibody coverage against 17 Neisseria meningitidis serotype B strains. Furthermore, the authors demonstrated that the modification of TLR7 agonists with phosphonate to afford alum adsorption was a generalizable approach to enhance vaccine adjuvanticity in several other TLR7 chemotypes.
Polymer‐based encapsulation has also been extensively investigated for adjuvant delivery.92, 93 Kasturi et al.94 demonstrated that immunization of mice with poly(lactic‐co‐glycolic acid) nanoparticles containing antigens plus TLR7 and TLR4 ligands induced synergistic increases in antigen‐specific, neutralizing antibodies. Moreover, immunization protected completely against lethal avian and swine influenza virus strains in mice, and induced robust immunity against pandemic H1N1 influenza in rhesus macaques. Ilyinskii et al.95 demonstrated that co‐delivery of an antigen with a TLR7/8 or TLR9 agonist in synthetic vaccine particles resulted in a strong augmentation of humoral and cellular immune responses with minimal systemic production of inflammatory cytokines. Dranoff, Mooney and co‐workers have reported the incorporation of granulocyte–macrophage colony‐stimulating factor (a dendritic cell enhancement factor) and CpG (a dendritic cell activating factor) in biomaterials based on poly(lactic‐co‐glycolic acid),96 mesoporous silica rods97 and cryogel.98 The goal of this approach is to induce a coordinated regulation of a dendritic cell network to enhance host immunity against added tumour lysate or in situ tumour antigens. DeMuth et al.99 generated microneedle arrays coated with biodegradable cationic poly(β‐amino ester) and negatively charged interbilayer‐cross‐linked multilamellar lipid vesicles. These interbilayer‐cross‐linked multilamellar lipid vesicles were loaded with protein antigen and MPLA, and the vaccination promoted a robust humoral response compared with the soluble components. Hanson et al.100 demonstrated that encapsulation of STING agonist cdGMP using PEGylated lipid nanoparticle can block systemic dissemination and enhance draining lymph node accumulation, leading to increased CD8 T‐cell response and antitumour immunity. Lynn et al.101 investigated the physicochemical properties of polymer‐linked TLR7/8 agonists by varying different linkers with agonist densities. Interestingly, improved local retention is necessary but not sufficient for enhancing T‐cell immunity, as low‐density unimolecular polymer coils showed reduced immunogenicity compared with high‐density submicron polymer particles.
Conditional activation through prodrug and antedrug
Chemical modification of TLR agonists can be used to elicit tissue‐specific immune activation through controlled drug cleavage. Fletcher et al.102 designed masked oral prodrugs of TLR7 agonist that can bypass immune stimulation in the gastrointestinal tissues, thereby reducing gastrointestinal intolerances. Fosdick et al.103 showed that oral administration of a pteridinone agonist (GS‐9620) with high first‐pass hepatic clearance induced more interferon than intravenous administration, while achieving similar systemic exposure; therefore, the majority of interferon is generated pre‐systemically from the gut‐associated lymphoid tissue. At low doses, GS‐9620 activates interferon‐stimulated genes without inducing systemic interferon and related adverse effects, providing a potential therapeutic window for inducing an anti‐HBV immune response. Recently, Ryu et al.104 have reported an imidazoquinoline TLR7 agonist prodrug that can be activated by light. Light‐activated TLR7 agonists can be used in combination with radiation therapy, where localized irradiation of tumour can kill the cancer cells and release cancer antigens, while simultaneously activating the TLR7 prodrug for enhanced antigen uptake and presentation.
Although prodrugs are activated under certain biological conditions, antedrugs are deactivated to limit the activity within certain tissues. Administration of low‐dose TLR7 agonist to the upper airway has the potential to treat various allergic diseases by skewing the immune microenvironment from Th2 to Th1.105 To prevent undesired systemic activity, Kurimoto et al.106 applied the antedrug concept to an adenine TLR7 agonist to restrict innate immune activation in the airway. The TLR7 antedrug is designed to be metabolically deactivated by plasma esterase to avoid systemic spillover. Even with transient activity in the lung, the adenine antedrug was demonstrated to effectively inhibit allergen‐induced airway inflammation without inducing systemic cytokines.107
Clinical application
Though many potential utilities have been demonstrated in pre‐clinical models, a key clinical translation hurdle for all immune adjuvants is safety. Successful clinical applications for TLR agonists have been so far limited to local delivery. R837 (imiquimod) is currently the only approved TLR7 agonist for use in humans to topically treat basal cell carcinoma.108 Despite being effective against these skin lesions, there are significant adverse effects associated with excessive inflammation at the treated sites, which is sometimes accompanied with fever and flu‐like symptoms.109 We now know that R837 acts, at least in part, through activation of TLR7. But because it was developed before knowledge of the molecular target, it is likely that the drug was identified solely based on phenotypic activities without fully understanding the selectivity against a broader panel of proteins and receptors. Many reports have described R837 having TLR7‐independent effects, which could contribute either to the efficacy and/or adverse effects.110, 111, 112 Recently, a non‐imidazoquinoline‐based TLR7 agonist was reported to be equally effective in a guinea‐pig herpes simplex virus model through intravaginal administration, but without the side‐effects of weight loss and fever.113 This raises the possibility that perhaps not all of the TLR7‐independent effects are required for R837's efficacy. In additional to dermatological diseases, topical R848 (resiquimod) gel is also being investigated as cancer vaccine adjuvant.114
Parenteral TLR agonists are administered as single agents (subcutaneous), together with vaccine antigens (intramuscular), or more recently, directly into the tumour where tumour‐associated antigens reside (intratumoral). AS04 is an adjuvant that contains the TLR4 agonist MPLA and alum.115 It is approved for intramuscular injection as adjuvant in human HBV and human papillomavirus vaccines. Bacillus Calmette–Guérin (BCG) is a live‐attenuated bacteria used for the treatment of non‐invasive bladder cancer.116 Its mechanism of action has been attributed to TLR2/4 activation. TMX‐101 is a soluble formulation of R837 for intravesical delivery directly into the bladder.117 In addition to being synthetic, R837 also has the advantage of being non‐infectious compared to BCG. TLR3 agonists Ampligen™ and Hiltonol™ have been used for the treatment of chronic fatigue syndrome and various types of cancer, as well as vaccine adjuvants.118, 119, 120 The TLR5 agonist entolimod has been implicated in radiation countermeasures and cancer therapy.121, 122 A number of TLR9 agonists have been investigated as adjuvants for human vaccines, the most advanced being 1018 ISS in HBV vaccine.123 More recently, in situ vaccination through intratumoral injection of TLR9 agonist (PF‐3512676) has been demonstrated to induce systemic anti‐tumour immunity in murine models and in patients with B‐cell lymphomas.124, 125 Interestingly, in other trials the same CpG molecule given systemically through subcutaneous administration reached dose‐limiting toxic levels before any therapeutic benefits were observed.126, 127 The critical abscopal response, where intratumoral administration diminishes tumour growth at both treated and non‐treated sites, has also been observed with TLR7/8 and STING agonists in murine models.70, 128 Hence, several companies are currently exploring intratumoral injection of TLR4 (G100), TLR7/8 (3M‐052 aka MEDI9197), or TLR9 agonists (SD‐101, IMO‐2125, CMP‐001) in clinical trials as single agents or in combination with checkpoint inhibitors. VTRX‐2337 is a TLR8 agonist also being investigated for cancer treatment in combination with chemotherapy.129
With regard to infectious diseases, oral TLR7 agonists such as R848, ANA773 (now RG‐7795) and PF‐4878691 have been explored for treatment of hepatitis C virus.130, 131, 132 All of these investigational drugs showed adverse effects with symptoms reminiscent of interferon induction. Low doses of the TLR7 agonist GS‐9620 are currently being investigated for treatment of chronic HBV,133 which has been shown to be efficacious in the woodchuck hepatitis viral model and in HBV‐infected chimps.134, 135 GS‐9620 has also shown promise in reversing HIV latency.136 In addition, intranasal TLR agonists have been investigated for allergen immunotherapy. In a phase IIa trial, AZD8848 (TLR7 antedrug) dosed intranasally was associated with sustained reduction in allergen responsiveness, although it also produced interferon‐related effects.137 AZD8848 was also tested in humans for tolerability via inhalation.138 GSK2245035 is yet another intranasal TLR7 agonist being investigated to treat respiratory diseases.139, 140
Conclusion
Stimulation of innate immune receptors has been implicated in the treatment of many diseases. Even before the discovery of TLRs, there were numerous reports of synthetic molecules capable of inducing cytokines and activating lymphocytes. With better understanding of the TLR structure and biology, discoveries of novel synthetic adjuvants have come from rational design or high‐throughput screening. Optimization of adjuvants has aimed at increasing potency and reducing structural complexity, but most importantly, improving safety and tolerability. Strategies such as manipulation of physicochemical properties, conjugation to macromolecules, formulation‐assisted delivery, and prodrug/antedrug all serve to localize the immune activation to the intended antigen(s). So far, clinical applications of immune agonists have been mainly limited to local delivery to minimize immune‐related toxicities. Non‐antigen‐specific, systemic activity is not desired for adjuvanticity and should be minimized to avoid wasted inflammation. The ability to tune the properties of synthetic agonists through chemistry or formulation may allow broader clinical utilities of these immune agonists to benefit more patients as we understand more about them.
Disclosures
None.
Acknowledgement
TW would like to thank Nicholas Valiante, Andrew Miller and Jonathan Deane for scientific advice.
References
- 1. Kennedy RB, Ovsyannikova IG, Jacobson RM, Poland GA. The immunology of smallpox vaccines. Curr Opin Immunol 2009; 21:314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O'Hagan DT, De Gregorio E. The path to a successful vaccine adjuvant – ’the long and winding road’. Drug Discov Today 2009; 14:541–51. [DOI] [PubMed] [Google Scholar]
- 3. Isorce N, Lucifora J, Zoulim F, Durantel D. Immune‐modulators to combat hepatitis B virus infection: from IFN‐α to novel investigational immunotherapeutic strategies. Antiviral Res 2015; 122:69–81. [DOI] [PubMed] [Google Scholar]
- 4. Banday AH, Jeelani S, Hruby VJ. Cancer vaccine adjuvants – recent clinical progress and future perspectives. Immunopharmacol Immunotoxicol 2015; 37:1–11. [DOI] [PubMed] [Google Scholar]
- 5. Wiemann B, Starnes CO. Coley's toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol Ther 1994; 64:529–64. [DOI] [PubMed] [Google Scholar]
- 6. Sheikh A, Strachan DP. The hygiene theory: fact or fiction? Curr Opin Otolaryngol Head Neck Surg 2004; 12:232–6. [DOI] [PubMed] [Google Scholar]
- 7. Kawai T, Akira S. The role of pattern‐recognition receptors in innate immunity: update on Toll‐like receptors. Nat Immunol 2010; 11:373–84. [DOI] [PubMed] [Google Scholar]
- 8. Motta V, Soares F, Sun T, Philpott DJ. NOD‐like receptors: versatile cytosolic sentinels. Physiol Rev 2015; 95:149–78. [DOI] [PubMed] [Google Scholar]
- 9. Yoo JS, Kato H, Fujita T. Sensing viral invasion by RIG‐I like receptors. Curr Opin Microbiol 2014; 20:131–8. [DOI] [PubMed] [Google Scholar]
- 10. Drickamer K, Taylor ME. Recent insights into structures and functions of C‐type lectins in the immune system. Curr Opin Struct Biol 2015; 34:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barber GN. STING‐dependent cytosolic DNA sensing pathways. Trends Immunol 2014; 35:88–93. [DOI] [PubMed] [Google Scholar]
- 12. Smirnov D, Schmidt JJ, Capecchi JT, Wightman PD. Vaccine adjuvant activity of 3M‐052: an imidazoquinoline designed for local activity without systemic cytokine induction. Vaccine 2011; 29:5434–42. [DOI] [PubMed] [Google Scholar]
- 13. Wu TY, Singh M, Miller AT, De Gregorio E, Doro F, D'Oro U et al Rational design of small molecules as vaccine adjuvants. Sci Transl Med 2014; 6:263ra160. [DOI] [PubMed] [Google Scholar]
- 14. Bourquin C, Hotz C, Noerenberg D, Voelkl A, Heidegger S, Roetzer LC et al Systemic cancer therapy with a small molecule agonist of toll‐like receptor 7 can be improved by circumventing TLR tolerance. Cancer Res 2011; 71:5123–33. [DOI] [PubMed] [Google Scholar]
- 15. Koga‐Yamakawa E, Murata M, Dovedi SJ, Wilkinson RW, Ota Y, Umehara H et al TLR7 tolerance is independent of the type I IFN pathway and leads to loss of anti‐tumor efficacy in mice. Cancer Immunol Immunother 2015; 64:1229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang X, Smith C, Yin H. Targeting Toll‐like receptors with small molecule agents. Chem Soc Rev 2013; 42:4859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mancini RJ, Stutts L, Ryu KA, Tom JK, Esser‐Kahn AP. Directing the immune system with chemical compounds. ACS Chem Biol 2014; 9:1075–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Irvine DJ, Hanson MC, Rakhra K, Tokatlian T. Synthetic Nanoparticles for Vaccines and Immunotherapy. Chem Rev 2015; 115:11109–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu H, Irvine DJ. Guiding principles in the design of molecular bioconjugates for vaccine applications. Bioconjug Chem 2015; 26:791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hotaling NA, Tang L, Irvine DJ, Babensee JE. Biomaterial Strategies for Immunomodulation. Annu Rev Biomed Eng 2015; 17:317–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S et al Species‐specific recognition of single‐stranded RNA via toll‐like receptor 7 and 8. Science 2004; 303:1526–9. [DOI] [PubMed] [Google Scholar]
- 22. Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C.. Innate antiviral responses by means of TLR7‐mediated recognition of single‐stranded RNA. Science 2004; 303:1529–31. [DOI] [PubMed] [Google Scholar]
- 23. Nichol FR, Weed SD, Underwood GE. Stimulation of murine interferon by a substituted pyrimidine. Antimicrob Agents Chemother 1976; 9:433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goodman MG, Weigle WO. Activation of lymphocytes by brominated nucleoside and cyclic nucleotide analogues: implications for the “second messenger” function of cyclic GMP. Proc Natl Acad Sci U S A 1981; 78:7604–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bernstein DI, Harrison CJ. Effects of the immunomodulating agent R837 on acute and latent herpes simplex virus type 2 infections. Antimicrob Agents Chemother. 1989; 33:1511–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen M, Griffith BP, Lucia HL, Hsiung GD. Efficacy of S26308 against guinea pig cytomegalovirus infection. Antimicrob Agents Chemother 1988; 32:678–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hirota K, Kazaoka K, Niimoto I, Kumihara H, Sajiki H, Isobe Y et al Discovery of 8‐hydroxyadenines as a novel type of interferon inducer. J Med Chem 2002; 45:5419–22. [DOI] [PubMed] [Google Scholar]
- 28. Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K et al Small anti‐viral compounds activate immune cells via the TLR7 MyD88‐dependent signaling pathway. Nat Immunol 2002; 3:196–200. [DOI] [PubMed] [Google Scholar]
- 29. Lee J, Chuang TH, Redecke V, She L, Pitha PM, Carson DA et al Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll‐like receptor 7. Proc Natl Acad Sci U S A 2003; 100:6646–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tran TD, Pryde DC, Jones P, Adam FM, Benson N, Bish G et al Design and optimisation of orally active TLR7 agonists for the treatment of hepatitis C virus infection. Bioorg Med Chem Lett 2011; 21:2389–93. [DOI] [PubMed] [Google Scholar]
- 31. Bennett N, McInally T, Pimm A, Thom S, Isobe Y. International PCT Application 2010:WO2010133882.
- 32. Roethle PA, McFadden RM, Yang H, Hrvatin P, Hui H, Graupe M et al Identification and optimization of pteridinone Toll‐like receptor 7 (TLR7) agonists for the oral treatment of viral hepatitis. J Med Chem 2013; 56:7324–33. [DOI] [PubMed] [Google Scholar]
- 33. Wu TY, Li Y, Cortez A, Zou Y, Mishra P, Zhang X et al International PCT Application 2009:WO2009111337.
- 34. Hussein WM, Liu TY, Skwarczynski M, Toth I. Toll‐like receptor agonists: a patent review (2011–2013). Expert Opin Ther Pat 2014; 24:453–70. [DOI] [PubMed] [Google Scholar]
- 35. Gorden KB, Gorski KS, Gibson SJ, Kedl RM, Kieper WC, Qiu X et al Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol 2005; 174:1259–68. [DOI] [PubMed] [Google Scholar]
- 36. Ishii N, Funami K, Tatematsu M, Seya T, Matsumoto M. Endosomal localization of TLR8 confers distinctive proteolytic processing on human myeloid cells. J Immunol 2014; 193:5118–28. [DOI] [PubMed] [Google Scholar]
- 37. Jurk M, Heil F, Vollmer J, Schetter C, Krieg AM, Wagner H et al Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R‐848. Nat Immunol 2002; 3:499. [DOI] [PubMed] [Google Scholar]
- 38. Gorden KK, Qiu XX, Binsfeld CC, Vasilakos JP, Alkan SS. Cutting edge: activation of murine TLR8 by a combination of imidazoquinoline immune response modifiers and polyT oligodeoxynucleotides. J Immunol 2006; 177:6584–7. [DOI] [PubMed] [Google Scholar]
- 39. Spranger S, Javorovic M, Burdek M, Wilde S, Mosetter B, Tippmer S et al Generation of Th1‐polarizing dendritic cells using the TLR7/8 agonist CL075. J Immunol 2010; 185:738–47. [DOI] [PubMed] [Google Scholar]
- 40. Kokatla HP, Sil D, Malladi SS, Balakrishna R, Hermanson AR, Fox LM et al Exquisite selectivity for human toll‐like receptor 8 in substituted furo[2,3‐c]quinolines. J Med Chem 2013; 56:6871–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tanji H, Ohto U, Shibata T, Miyake K, Shimizu T. Structural reorganization of the Toll‐like receptor 8 dimer induced by agonistic ligands. Science 2013; 339:1426–9. [DOI] [PubMed] [Google Scholar]
- 42. Tanji H, Ohto U, Shibata T, Taoka M, Yamauchi Y, Isobe T et al Toll‐like receptor 8 senses degradation products of single‐stranded RNA. Nat Struct Mol Biol 2015; 22:109–15. [DOI] [PubMed] [Google Scholar]
- 43. Kokatla HP, Sil D, Tanji H, Ohto U, Malladi SS, Fox LM et al Structure‐based design of novel human Toll‐like receptor 8 agonists. ChemMedChem 2014; 9:719–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Beesu M, Malladi SS, Fox LM, Jones CD, Dixit A, David SA. Human Toll‐like receptor 8‐selective agonistic activities in 1‐alkyl‐1H‐benzimidazol‐2‐amines. J Med Chem 2014; 57:7325–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, Bleharski JR et al Host defense mechanisms triggered by microbial lipoproteins through toll‐like receptors. Science 1999; 285:732–6. [DOI] [PubMed] [Google Scholar]
- 46. Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD et al Cell activation and apoptosis by bacterial lipoproteins through toll‐like receptor‐2. Science 1999; 285:736–9. [DOI] [PubMed] [Google Scholar]
- 47. Baschang G. Muramylpeptides and lipopeptides: studies toward immunostimulatns. Tetrahedron 1989; 45:6331–60. [Google Scholar]
- 48. Jin MS, Kim SE, Heo JY, Lee ME, Kim HM, Paik SG et al Crystal structure of the TLR1‐TLR2 heterodimer induced by binding of a tri‐acylated lipopeptide. Cell 2007; 130:1071–82. [DOI] [PubMed] [Google Scholar]
- 49. Kang JY, Nan X, Jin MS, Youn SJ, Ryu YH, Mah S et al Recognition of lipopeptide patterns by Toll‐like receptor 2‐Toll‐like receptor 6 heterodimer. Immunity 2009; 31:873–84. [DOI] [PubMed] [Google Scholar]
- 50. Wu W, Li R, Malladi SS, Warshakoon HJ, Kimbrell MR, Amolins MW et al Structure‐activity relationships in toll‐like receptor‐2 agonistic diacylthioglycerol lipopeptides. J Med Chem 2010; 53:3198–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Prajeeth CK, Jirmo AC, Krishnaswamy JK, Ebensen T, Guzman CA, Weiss S et al The synthetic TLR2 agonist BPPcysMPEG leads to efficient cross‐priming against co‐administered and linked antigens. Eur J Immunol 2010; 40:1272–83. [DOI] [PubMed] [Google Scholar]
- 52. Spanedda MV, Heurtault B, Weidner S, Baehr C, Boeglin E, Beyrath J et al Novel powerful water‐soluble lipid immunoadjuvants inducing mouse dendritic cell maturation and B cell proliferation using TLR2 pathway. Bioorg Med Chem Lett 2010; 20:1869–72. [DOI] [PubMed] [Google Scholar]
- 53. Thomann JS, Monneaux F, Creusat G, Spanedda MV, Heurtault B, Habermacher C et al Novel glycolipid TLR2 ligands of the type Pam2Cys‐α‐Gal: synthesis and biological properties. Eur J Med Chem 2012; 51:174–83. [DOI] [PubMed] [Google Scholar]
- 54. Salunke DB, Shukla NM, Yoo E, Crall BM, Balakrishna R, Malladi SS et al Structure‐activity relationships in human Toll‐like receptor 2‐specific monoacyl lipopeptides. J Med Chem 2012; 55:3353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Salunke DB, Connelly SW, Shukla NM, Hermanson AR, Fox LM, David SA. Design and development of stable, water‐soluble, human Toll‐like receptor 2 specific monoacyl lipopeptides as candidate vaccine adjuvants. J Med Chem 2013; 56:5885–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Abdel‐Aal AB, Al‐Isae K, Zaman M, Toth I. Simple synthetic toll‐like receptor 2 ligands. Bioorg Med Chem Lett 2011; 21:5863–5. [DOI] [PubMed] [Google Scholar]
- 57. Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X et al Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 1998; 282:2085–8. [DOI] [PubMed] [Google Scholar]
- 58. Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4‐MD‐2 complex. Nature 2009; 458:1191–5. [DOI] [PubMed] [Google Scholar]
- 59. Tagliabue A, Rappuoli R. Vaccine adjuvants: the dream becomes real. Hum Vaccin 2008; 4:347–9. [DOI] [PubMed] [Google Scholar]
- 60. Lambert SL, Yang CF, Liu Z, Sweetwood R, Zhao J, Cheng L et al Molecular and cellular response profiles induced by the TLR4 agonist‐based adjuvant Glucopyranosyl Lipid A. PLoS ONE 2012; 7:e51618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Baldridge JR, Cluff CW, Evans JT, Lacy MJ, Stephens JR, Brookshire VG et al Immunostimulatory activity of aminoalkyl glucosaminide 4‐phosphates (AGPs): induction of protective innate immune responses by RC‐524 and RC‐529. J Endotoxin Res 2002; 8:453–8. [DOI] [PubMed] [Google Scholar]
- 62. Ishizaka ST, Hawkins LD. E6020: a synthetic Toll‐like receptor 4 agonist as a vaccine adjuvant. Expert Rev Vaccines 2007; 6:773–84. [DOI] [PubMed] [Google Scholar]
- 63. Lu H, Dietsch GN, Matthews MA, Yang Y, Ghanekar S, Inokuma M et al VTX‐2337 is a novel TLR8 agonist that activates NK cells and augments ADCC. Clin Cancer Res 2012; 18:499–509. [DOI] [PubMed] [Google Scholar]
- 64. Stephenson RM, Lim CM, Matthews M, Dietsch G, Hershberg R, Ferris RL. TLR8 stimulation enhances cetuximab‐mediated natural killer cell lysis of head and neck cancer cells and dendritic cell cross‐priming of EGFR‐specific CD8+ T cells. Cancer Immunol Immunother 2013; 62:1347–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dowling DJ, Tan Z, Prokopowicz ZM, Palmer CD, Matthews MA, Dietsch GN et al The ultra‐potent and selective TLR8 agonist VTX‐294 activates human newborn and adult leukocytes. PLoS ONE 2013; 8:e58164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Guan Y, Omueti‐Ayoade K, Mutha SK, Hergenrother PJ, Tapping RI. Identification of novel synthetic toll‐like receptor 2 agonists by high throughput screening. J Biol Chem 2010; 285:23755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chan M, Hayashi T, Mathewson RD, Nour A, Hayashi Y, Yao S et al Identification of substituted pyrimido[5,4‐b]indoles as selective Toll‐like receptor 4 ligands. J Med Chem 2013; 56:4206–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nour A, Hayashi T, Chan M, Yao S, Tawatao RI, Crain B et al Discovery of substituted 4‐aminoquinazolines as selective Toll‐like receptor 4 ligands. Bioorg Med Chem Lett 2014; 24:4931–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sali TM, Pryke KM, Abraham J, Liu A, Archer I, Broeckel R et al Characterization of a novel human‐specific STING agonist that elicits antiviral activity against emerging alphaviruses. PLoS Pathog 2015; 11:e1005324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Singh M, Khong H, Dai Z, Huang XF, Wargo JA, Cooper ZA et al Effective innate and adaptive antimelanoma immunity through localized TLR7/8 activation. J Immunol 2014; 193:4722–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chan M, Hayashi T, Kuy CS, Gray CS, Wu CC, Corr M et al Synthesis and immunological characterization of toll‐like receptor 7 agonistic conjugates. Bioconjug Chem 2009; 20:1194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chan M, Hayashi T, Mathewson RD, Yao S, Gray C, Tawatao RI et al Synthesis and characterization of PEGylated toll like receptor 7 ligands. Bioconjug Chem 2011; 22:445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hayashi T, Chan M, Norton JT, Wu CC, Yao S, Cottam HB et al Additive melanoma suppression with intralesional phospholipid‐conjugated TLR7 agonists and systemic IL‐2. Melanoma Res 2011; 21:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Shukla NM, Salunke DB, Balakrishna R, Mutz CA, Malladi SS, David SA. Potent adjuvanticity of a pure TLR7‐agonistic imidazoquinoline dendrimer. PLoS ONE 2012; 7:e43612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wille‐Reece U, Flynn BJ, Lore K, Koup RA, Kedl RM, Mattapallil JJ et al HIV Gag protein conjugated to a Toll‐like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc Natl Acad Sci U S A 2005; 102:15190–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wille‐Reece U, Wu CY, Flynn BJ, Kedl RM, Seder RA. Immunization with HIV‐1 Gag protein conjugated to a TLR7/8 agonist results in the generation of HIV‐1 Gag‐specific Th1 and CD8+ T cell responses. J Immunol 2005; 174:7676–83. [DOI] [PubMed] [Google Scholar]
- 77. Oh JZ, Kedl RM. The capacity to induce cross‐presentation dictates the success of a TLR7 agonist‐conjugate vaccine for eliciting cellular immunity. J Immunol 2010; 185:4602–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Vecchi S, Bufali S, Uno T, Wu T, Arcidiacono L, Filippini S et al Conjugation of a TLR7 agonist and antigen enhances protection in the S. pneumoniae murine infection model. Eur J Pharm Biopharm 2014; 87:310–7. [DOI] [PubMed] [Google Scholar]
- 79. Zom GG, Khan S, Filippov DV, Ossendorp F. TLR ligand‐peptide conjugate vaccines: toward clinical application. Adv Immunol 2012; 114:177–201. [DOI] [PubMed] [Google Scholar]
- 80. Wu CC, Hayashi T, Takabayashi K, Sabet M, Smee DF, Guiney DD et al Immunotherapeutic activity of a conjugate of a Toll‐like receptor 7 ligand. Proc Natl Acad Sci U S A 2007; 104:3990–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B et al Structure‐based programming of lymph‐node targeting in molecular vaccines. Nature 2014; 507:519–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shinchi H, Crain B, Yao S, Chan M, Zhang SS, Ahmadiiveli A et al Enhancement of the Immunostimulatory Activity of a TLR7 Ligand by Conjugation to Polysaccharides. Bioconjug Chem 2015; 26:1713–23. [DOI] [PubMed] [Google Scholar]
- 83. Mancini RJ, Tom JK, Esser‐Kahn AP. Covalently coupled immunostimulant heterodimers. Angew Chem Int Ed Engl 2014; 53:189–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tom JK, Dotsey EY, Wong HY, Stutts L, Moore T, Davies DH et al Modulation of Innate Immune Responses via Covalently Linked TLR Agonists. ACS Cent Sci 2015; 1:439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Jerjian TV, Glode AE, Thompson LA, O'Bryant CL. Antibody‐Drug Conjugates: A Clinical Pharmacy Perspective on an Emerging Cancer Therapy. Pharmacotherapy 2016; 36:99–116. [DOI] [PubMed] [Google Scholar]
- 86. Sharma S, Dominguez AL, Manrique SZ, Cavallo F, Sakaguchi S, Lustgarten J. Systemic targeting of CpG‐ODN to the tumor microenvironment with anti‐neu‐CpG hybrid molecule and T regulatory cell depletion induces memory responses in BALB‐neuT tolerant mice. Cancer Res 2008; 68:7530–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Li Z, Jang JK, Lechner MG, Hu P, Khawli L, Scannell CA et al Generation of tumor‐targeted antibody‐CpG conjugates. J Immunol Methods 2013; 389:45–51. [DOI] [PubMed] [Google Scholar]
- 88. Gadd AJ, Greco F, Cobb AJ, Edwards AD. Targeted Activation of Toll‐Like Receptors: Conjugation of a Toll‐Like Receptor 7 Agonist to a Monoclonal Antibody Maintains Antigen Binding and Specificity. Bioconjug Chem 2015; 26:1743–52. [DOI] [PubMed] [Google Scholar]
- 89. Kreutz M, Giquel B, Hu Q, Abuknesha R, Uematsu S, Akira S et al Antibody‐antigen‐adjuvant conjugates enable co‐delivery of antigen and adjuvant to dendritic cells in cis but only have partial targeting specificity. PLoS ONE 2012; 7:e40208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Oleszycka E, Lavelle EC. Immunomodulatory properties of the vaccine adjuvant alum. Curr Opin Immunol 2014; 28:1–5. [DOI] [PubMed] [Google Scholar]
- 91. Hem SL, Hogenesch H. Relationship between physical and chemical properties of aluminum‐containing adjuvants and immunopotentiation. Expert Rev Vaccines 2007; 6:685–98. [DOI] [PubMed] [Google Scholar]
- 92. Jewell CM, Lopez SC, Irvine DJ. In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant‐releasing polymer particles. Proc Natl Acad Sci U S A 2011; 108:15745–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Moon JJ, Suh H, Li AV, Ockenhouse CF, Yadava A, Irvine DJ. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc Natl Acad Sci U S A 2012; 109:1080–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI et al Programming the magnitude and persistence of antibody responses with innate immunity. Nature 2011; 470:543–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ilyinskii PO, Roy CJ, O'Neil CP, Browning EA, Pittet LA, Altreuter DH et al Adjuvant‐carrying synthetic vaccine particles augment the immune response to encapsulated antigen and exhibit strong local immune activation without inducing systemic cytokine release. Vaccine 2014; 32:2882–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ali OA, Emerich D, Dranoff G, Mooney DJ. In situ regulation of DC subsets and T cells mediates tumor regression in mice. Sci Transl Med 2009; 1:8ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kim J, Li WA, Choi Y, Lewin SA, Verbeke CS, Dranoff G et al Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. Nat Biotechnol 2015; 33:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bencherif SA, Warren Sands R, Ali OA, Li WA, Lewin SA, Braschler TM et al Injectable cryogel‐based whole‐cell cancer vaccines. Nat Commun 2015; 6:7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. DeMuth PC, Moon JJ, Suh H, Hammond PT, Irvine DJ. Releasable layer‐by‐layer assembly of stabilized lipid nanocapsules on microneedles for enhanced transcutaneous vaccine delivery. ACS Nano 2012; 6:8041–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hanson MC, Crespo MP, Abraham W, Moynihan KD, Szeto GL, Chen SH, et al Nanoparticulate STING agonists are potent lymph node‐targeted vaccine adjuvants. J Clin Invest 2015; 125:2532‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lynn GM, Laga R, Darrah PA, Ishizuka AS, Balaci AJ, Dulcey AE et al In vivo characterization of the physicochemical properties of polymer‐linked TLR agonists that enhance vaccine immunogenicity. Nat Biotechnol 2015; 33:1201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Fletcher S, Steffy K, Averett D. Masked oral prodrugs of toll‐like receptor 7 agonists: a new approach for the treatment of infectious disease. Curr Opin Investig Drugs 2006; 7:702–8. [PubMed] [Google Scholar]
- 103. Fosdick A, Zheng J, Pflanz S, Frey CR, Hesselgesser J, Halcomb RL et al Pharmacokinetic and pharmacodynamic properties of GS‐9620, a novel Toll‐like receptor 7 agonist, demonstrate interferon‐stimulated gene induction without detectable serum interferon at low oral doses. J Pharmacol Exp Ther 2014; 348:96–105. [DOI] [PubMed] [Google Scholar]
- 104. Ryu KA, Stutts L, Tom JK, Mancini RJ, Esser‐Kahn AP. Stimulation of innate immune cells by light‐activated TLR7/8 agonists. J Am Chem Soc 2014; 136:10823–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Aryan Z, Rezaei N. Toll‐like receptors as targets for allergen immunotherapy. Curr Opin Allergy Clin Immunol 2015; 15:568–74. [DOI] [PubMed] [Google Scholar]
- 106. Kurimoto A, Hashimoto K, Nakamura T, Norimura K, Ogita H, Takaku H et al Synthesis and biological evaluation of 8‐oxoadenine derivatives as toll‐like receptor 7 agonists introducing the antedrug concept. J Med Chem 2010; 53:2964–72. [DOI] [PubMed] [Google Scholar]
- 107. Biffen M, Matsui H, Edwards S, Leishman AJ, Eiho K, Holness E et al Biological characterization of a novel class of toll‐like receptor 7 agonists designed to have reduced systemic activity. Br J Pharmacol 2012; 166:573–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Oldfield V, Keating GM, Perry CM. Imiquimod: in superficial basal cell carcinoma. Am J Clin Dermatol 2005; 6:195–200; discussion 1–2. [DOI] [PubMed] [Google Scholar]
- 109. Cantisani C, Lazic T, Richetta AG, Clerico R, Mattozzi C, Calvieri S. Imiquimod 5% cream use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov 2012; 6:65–9. [DOI] [PubMed] [Google Scholar]
- 110. Schon MP, Schon M, Klotz KN. The small antitumoral immune response modifier imiquimod interacts with adenosine receptor signaling in a TLR7‐ and TLR8‐independent fashion. J Invest Dermatol 2006; 126:1338–47. [DOI] [PubMed] [Google Scholar]
- 111. Walter A, Schafer M, Cecconi V, Matter C, Urosevic‐Maiwald M, Belloni B et al Aldara activates TLR7‐independent immune defence. Nat Commun 2013; 4:1560. [DOI] [PubMed] [Google Scholar]
- 112. Hwang H, Min H, Kim D, Yu SW, Jung SJ, Choi SY et al Imiquimod induces a Toll‐like receptor 7‐independent increase in intracellular calcium via IP(3) receptor activation. Biochem Biophys Res Commun 2014; 450:875–9. [DOI] [PubMed] [Google Scholar]
- 113. Bernstein DI, Cardin RD, Bravo FJ, Earwood J, Clark JR, Li Y et al Topical SMIP‐7.7, a toll‐like receptor 7 agonist, protects against genital herpes simplex virus type‐2 disease in the guinea pig model of genital herpes. Antivir Chem Chemother 2014; 23:189–96. [DOI] [PubMed] [Google Scholar]
- 114. Sabado RL, Pavlick A, Gnjatic S, Cruz CM, Vengco I, Hasan F et al Resiquimod as an immunologic adjuvant for NY‐ESO‐1 protein vaccination in patients with high‐risk melanoma. Cancer Immunol Res 2015; 3:278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Garcon N, Wettendorff M, Van Mechelen M. Role of AS04 in human papillomavirus vaccine: mode of action and clinical profile. Expert Opin Biol Ther 2011; 11:667–77. [DOI] [PubMed] [Google Scholar]
- 116. LaRue H, Ayari C, Bergeron A, Fradet Y. Toll‐like receptors in urothelial cells – targets for cancer immunotherapy. Nat Rev Urol 2013; 10:537–45. [DOI] [PubMed] [Google Scholar]
- 117. Arends TJ, Lammers RJ, Falke J, van der Heijden AG, Rustighini I, Pozzi R et al Pharmacokinetic, Pharmacodynamic, and Activity Evaluation of TMX‐101 in a Multicenter Phase 1 Study in Patients With Papillary Non‐Muscle‐Invasive Bladder Cancer. Clin Genitourin Cancer 2015; 13:204–9 e2. [DOI] [PubMed] [Google Scholar]
- 118. Strayer DR, Carter WA, Stouch BC, Stevens SR, Bateman L, Cimoch PJ et al A double‐blind, placebo‐controlled, randomized, clinical trial of the TLR‐3 agonist rintatolimod in severe cases of chronic fatigue syndrome. PLoS ONE 2012; 7:e31334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Glavan TM, Pavelic J. The exploitation of Toll‐like receptor 3 signaling in cancer therapy. Curr Pharm Des 2014; 20:6555–64. [DOI] [PubMed] [Google Scholar]
- 120. Martins KA, Bavari S, Salazar AM. Vaccine adjuvant uses of poly‐IC and derivatives. Expert Rev Vaccines 2015; 14:447–59. [DOI] [PubMed] [Google Scholar]
- 121. Krivokrysenko VI, Toshkov IA, Gleiberman AS, Krasnov P, Shyshynova I, Bespalov I et al The Toll‐Like Receptor 5 Agonist Entolimod Mitigates Lethal Acute Radiation Syndrome in Non‐Human Primates. PLoS ONE 2015; 10:e0135388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Brackett CM, Kojouharov B, Veith J, Greene KF, Burdelya LG, Gollnick SO et al Toll‐like receptor‐5 agonist, entolimod, suppresses metastasis and induces immunity by stimulating an NK‐dendritic‐CD8+ T‐cell axis. Proc Natl Acad Sci U S A 2016; 113:E874–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Janssen JM, Jackson S, Heyward WL, Janssen RS. Immunogenicity of an investigational hepatitis B vaccine with a toll‐like receptor 9 agonist adjuvant (HBsAg‐1018) compared with a licensed hepatitis B vaccine in subpopulations of healthy adults 18‐70 years of age. Vaccine 2015; 33:3614–8. [DOI] [PubMed] [Google Scholar]
- 124. Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH et al In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol 2010; 28:4324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Marabelle A, Kohrt H, Sagiv‐Barfi I, Ajami B, Axtell RC, Zhou G et al Depleting tumor‐specific Tregs at a single site eradicates disseminated tumors. J Clin Invest 2013; 123:2447–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Hirsh V, Paz‐Ares L, Boyer M, Rosell R, Middleton G, Eberhardt WE et al Randomized phase III trial of paclitaxel/carboplatin with or without PF‐3512676 (Toll‐like receptor 9 agonist) as first‐line treatment for advanced non‐small‐cell lung cancer. J Clin Oncol 2011; 29:2667–74. [DOI] [PubMed] [Google Scholar]
- 127. Manegold C, van Zandwijk N, Szczesna A, Zatloukal P, Au JS, Blasinska‐Morawiec M et al A phase III randomized study of gemcitabine and cisplatin with or without PF‐3512676 (TLR9 agonist) as first‐line treatment of advanced non‐small‐cell lung cancer. Ann Oncol 2012; 23:72–7. [DOI] [PubMed] [Google Scholar]
- 128. Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE et al Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep 2015; 11:1018–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Northfelt DW, Ramanathan RK, Cohen PA, Von Hoff DD, Weiss GJ, Dietsch GN et al A phase I dose‐finding study of the novel Toll‐like receptor 8 agonist VTX‐2337 in adult subjects with advanced solid tumors or lymphoma. Clin Cancer Res 2014; 20:3683–91. [DOI] [PubMed] [Google Scholar]
- 130. Pockros PJ, Guyader D, Patton H, Tong MJ, Wright T, McHutchison JG et al Oral resiquimod in chronic HCV infection: safety and efficacy in 2 placebo‐controlled, double‐blind phase IIa studies. J Hepatol 2007; 47:174–82. [DOI] [PubMed] [Google Scholar]
- 131. Bergmann JF, de Bruijne J, Hotho DM, de Knegt RJ, Boonstra A, Weegink CJ et al Randomised clinical trial: anti‐viral activity of ANA773, an oral inducer of endogenous interferons acting via TLR7, in chronic HCV. Aliment Pharmacol Ther 2011; 34:443–53. [DOI] [PubMed] [Google Scholar]
- 132. Fidock MD, Souberbielle BE, Laxton C, Rawal J, Delpuech‐Adams O, Corey TP et al The innate immune response, clinical outcomes, and ex vivo HCV antiviral efficacy of a TLR7 agonist (PF‐4878691). Clin Pharmacol Ther 2011; 89:821–9. [DOI] [PubMed] [Google Scholar]
- 133. Gane EJ, Lim YS, Gordon SC, Visvanathan K, Sicard E, Fedorak RN et al The oral toll‐like receptor‐7 agonist GS‐9620 in patients with chronic hepatitis B virus infection. J Hepatol 2015; 63:320–8. [DOI] [PubMed] [Google Scholar]
- 134. Menne S, Tumas DB, Liu KH, Thampi L, AlDeghaither D, Baldwin BH et al Sustained efficacy and seroconversion with the Toll‐like receptor 7 agonist GS‐9620 in the Woodchuck model of chronic hepatitis B. J Hepatol 2015; 62:1237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Lanford RE, Guerra B, Chavez D, Giavedoni L, Hodara VL, Brasky KM et al GS‐9620, an oral agonist of Toll‐like receptor‐7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology 2013; 144:1508–17, 17 e1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Cohen J. Drug flushes out hidden AIDS virus. Science 2015; 347:1056. [DOI] [PubMed] [Google Scholar]
- 137. Greiff L, Ahlstrom‐Emanuelsson C, Alenas M, Almqvist G, Andersson M, Cervin A et al Biological effects and clinical efficacy of a topical Toll‐like receptor 7 agonist in seasonal allergic rhinitis: a parallel group controlled phase IIa study. Inflamm Res 2015; 64:903–15. [DOI] [PubMed] [Google Scholar]
- 138. Delaney S, Biffen M, Maltby J, Bell J, Asimus S, Aggarwal A et al Tolerability in man following inhalation dosing of the selective TLR7 agonist, AZD8848. BMJ Open Respir Res 2016; 3:e000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Tsitoura D, Ambery C, Price M, Powley W, Garthside S, Biggadike K et al Early clinical evaluation of the intranasal TLR7 agonist GSK2245035: use of translational biomarkers to guide dosing and confirm target engagement. Clin Pharmacol Ther 2015; 98:369–80. [DOI] [PubMed] [Google Scholar]
- 140. Biggadike K, Ahmed M, Ball DI, Coe DM, Dalmas Wilk DA, Edwards CD et al Discovery of 6‐Amino‐2‐{[(1S)‐1‐methylbutyl]oxy}‐9‐[5‐(1‐piperidinyl)pentyl]‐7,9‐dihydro‐8H‐pu rin‐8‐one (GSK2245035), a Highly Potent and Selective Intranasal Toll‐Like Receptor 7 Agonist for the Treatment of Asthma. J Med Chem 2016; 59:1711–26. [DOI] [PubMed] [Google Scholar]


