Summary
Vitamins A and E and select flavonoids in the family of catechins are well‐defined small molecules that, if proven to possess immunomodulatory properties, hold promise as vaccine adjuvants and various therapies. In an effort to determine the in vivo immunomodulatory properties of these molecules, we found that although mucosal and systemic vaccinations with a recombinant HIV‐1BaLgp120 with either a catechin, epigallo catechin gallate (EGCG) or pro‐vitamin A (retinyl palmitate) alone in a vegetable‐oil‐in‐water emulsion (OWE) suppressed antigen‐specific responses, the combination of EGCG and vitamin A or E in OWE (Nutritive Immune‐enhancing Delivery System, NIDS) synergistically enhanced adaptive B‐cell, and CD4+ and CD8+ T‐cell responses, following induction of relatively low local and systemic innate tumour necrosis factor‐α (TNF‐α), interleukin‐6 (IL‐6) and IL‐17, but relatively high levels of early systemic IL‐15 responses. For induction of adaptive interferon‐γ and TNF‐α responses by CD4+ and CD8+ T cells, the adjuvant effect of NIDS was dependent on both IL‐15 and its receptor. In addition, the anti‐oxidant activity of NIDS correlated positively with higher expression of the superoxide dismutase 1, an enzyme involved in reactive oxygen species elimination but negatively with secretion of IL‐1β. This suggests that the mechanism of action of NIDS is dependent on anti‐oxidant activity and IL‐15, but independent of IL‐1β and inflammasome formation. These data show that this approach in nutritive vaccine adjuvant design holds promise for the development of potentially safer effective vaccines.
Keywords: adjuvant, flavonoid, HIV, vaccine, vegetable oil, vitamin A, vitamin E
Introduction
The immunomodulatory roles of the various vitamins and flavonoids remain controversial as although some studies suggest that some vitamins and flavonoids enhance immunity, other studies, using the same compounds, show that they in fact suppress immunity.1, 2 Specifically, immunomodulation by vitamins A and D includes both up‐ and down‐regulation of certain immune responses, varying by the type of immune response, pathogen and host.1, 2, 3, 4, 5, 6, 7
For over a century, the focus on designing immune‐enhancing vaccine adjuvants and vaccines in general has been on live attenuated viruses and bacteria, toxins and molecules and delivery systems that induce strong innate pro‐inflammatory responses that may cause moderate to severe adverse events and side effects.8, 9, 10 Notwithstanding, it is generally accepted that innate immune responses dictate the development and nature of adaptive immune responses.11, 12, 13 For instance, early pro‐inflammatory cytokine responses such as interleukin‐12 (IL‐12) and tumour necrosis factor‐α (TNF‐α) or chemokine responses such as macrophage inflammatory protein‐1α (MIP‐1α) and MIP‐1β have been implicated to lead to strong adaptive B‐cell and T‐cell immune responses.11, 12, 13
Several cytokines have been implicated in playing important roles in the innate to adaptive response transitions. Interleukin‐15 is a pleiotropic cytokine, which leads to strong adaptive B‐cell and T‐cell responses, including T helper and cytotoxic T‐cell responses, and it has been proposed to act as a vaccine adjuvant.14, 15, 16, 17, 18, 19, 20 Equally important in designing a safe vaccine is to measure IL‐17, which in various closely related forms, is elaborated by T helper type 17 (Th17) CD4+ T cells during innate and adaptive immune responses. The role of IL‐17 in vaccine design, however, has been controversial in that although it has been deemed important in protection against select microbial infections, it has also been deemed as a factor in generation and maintenance of auto‐immune responses.21, 22, 23, 24, 25, 26 These data suggest that it is important to measure IL‐15 and IL‐17 in the innate and adaptive phases of the immune response in vaccine design.
Signal transduction pathways for vitamins A and E and flavonoids and their derivatives also indicate a link between these nutritional components, induction of metabolic pathways and immune response elements or vice versa.27, 28, 29 This suggests that common gene clusters, involving immune response elements, are affected by vitamins, flavonoid and lipids. Because the Nutritive Immune‐enhancing Delivery System (NIDS) contains lipids (vegetable oil), vitamins A and E and a flavonoid, the NIDS may exert its immunomodulatory effects via metabolic to immune response pathways.
Previously, we demonstrated the adjuvant effect of an emulsion containing retinoic acid, catechin hydrate and vitamin E in mustard seed oil used as a vaccine adjuvant.30 In the present study, innate and adaptive immunomodulatory properties of vitamin A palmitate, vitamin E and a catechin, in a vegetable oil, were tested as a vaccine against HIV‐1, using recombinant gp120BaL as antigen. As a mucosal adjuvant control, synthetic double‐stranded RNA (Poly (I:C), a Toll‐like receptor 3 (TLR3) agonist and Th1 inducer) was used.31, 32, 33 The systemic adjuvant controls were Alum or a squalene oil‐in‐water emulsion, both of which are licensed for human vaccines and are well‐known inducers of Th2 type responses.34, 35
Materials and methods
Immunomodulators, vaccine preparations and vaccinations
The HIV‐1BaL gp120 protein was obtained from the NIH AIDS Research and Reference Reagents Program and was used at 5 μg per dose for the combined sublingual/intranasal, and 2·5 μg per dose for intramuscular (i.m.) vaccinations. Retinoic acid (Cat. no. R2625, and R3375; Sigma‐Aldrich, St Louis, MO) and catechin hydrate (Cat. no. C1251; Sigma‐Aldrich) were prepared as described previously.30 Retinyl palmitate (RP) (Cat. no. R3375; Sigma‐Aldrich) was in oil form. Epigallocatechin‐3‐gallate (EGCG) (Cat. no. 70935; Cayman Chemicals, Ann Arbor, MI) was dissolved in water at 10 mg/ml. Vitamin E, (Sigma Aldrich; α‐tocopherol, Cat. no. T3251) was in oil form. Mustard seed oil (MO) was purchased from Botanic Oil Innovations, Inc. (Spooner, WI). Allyl isothiocyanate (Cat. no. W203408‐500G‐K; Sigma Aldrich) was used at 1% volume/volume for the mucosal vaccinations only. The in vitro and in vivo doses were 30 μg for retinoic acid and RP, 120 μg for catechin hydrate and EGCG, 2 mg for vitamin E, and 49% volume/volume for MO. Sterile Dulbecco's PBS (Cat. no. 21‐030‐CV; Sigma‐Aldrich) was used to adjust the final volume for each dose. All components were tested for endotoxin with a Genscript kit (Cat. no. L00350; Piscataway, NJ) and the endotoxin content in each component was found to be < 0·005 EU/ml. All vaccines were prepared in endotoxin‐free 2·0‐ml tubes (Eppendorf biopur safe‐lock microcentrifuge tubes). All other vaccine preparations and vaccination protocols were performed as described previously.30
Mice
The studies were carried out using female BALB/c or C57BL/6, mice that were 6–8 weeks old at the onset of the studies, purchased from Charles River Laboratories (Wilmington, MA). For IL‐15‐related studies, wild‐type (WT) C57BL/6 mice were purchased from the National Cancer Institute‐Charles River (Fredericksburg, MD). Interlukin‐15 knockout (KO) mice36 were maintained in the facility at University of Connecticut Health Center. The IL‐15/IL‐15R−/− double KO mice were generated by intercrossing IL‐15 KO and IL‐15R−/− KO mice.37 All studies were performed in accordance with the Institutional Animal Care and Use Committee of UConn Health and Murigenics, Inc. at their respective AAALAC‐approved and/or USDA approved vivarium.
The murine Air‐pouch model
Sterile air pouches were produced in the shaved lower backs of BALB/c mice as described elsewhere.38 Briefly, this was performed by blowing 5 ml sterile air intra‐dermally three times over a 24‐hr period, after which the various immunomodulators were injected at seven times higher doses than the vaccination doses, in a volume of 0·7 ml each. Six hours later, 0·5 ml of sterile saline solution was injected into each of the air‐pouches and withdrawn, centrifuged to separate cells and debris and frozen at –80°.
Cell cultures and measurement of cytokines and antibodies in supernatants by ELISA and Millipore/Merck Luminex Assays
At 1 week after the fourth and final vaccination the mice were killed. Spleens, cervical lymph nodes and iliac lymph nodes were removed aseptically from individual mice and single‐cell suspensions were prepared as described in detail.30 The various cytokines and chemokines in the supernatants, sera or air‐pouch lavages were measured either individually by the ELISA Max Deluxe kits (Biolegend, San Diego, CA), or alternatively, by using magnetic‐based multiplex Luminex assay kits (Millipore/Merck, Billerica, MA) according to the manufacturer's protocols using a MAGPIX instrument (MAGPIX, Millipore Luminex instrument; Darmstadt, Germany).
Fluorescence‐activated cell sorter flow cytometry analysis
Single cell suspensions from spleen and lymph nodes were prepared and incubated overnight at 5 × 106 cells per 250 μl per well, in the presence or absence of 4 μg/well recombinant gp120BaL. Following the overnight incubation for 16 hr, the cells were spun down at 600 g for 5 min and the supernatants were removed. One millilitre of Brefeldin A in PBS containing 1% fetal bovine serum (FBS) was added to each well together with 4 μg/well of gp120BaL and incubated at 4° in the dark for 5 hr. The cells were centrifuged at 600 g for 5 min and supernatants were removed, following which 1 ml of permeability buffer (Millipore) was added and the cells were incubated for 4–5 min, and washed with PBS containing 1% FBS. For the intracellular stainings, rat anti‐mouse interferon‐γ (IFN‐γ), TNF‐α or IL‐17 antibodies were added (1 μl/sample) into the permeabilization buffer (1 ml/sample) and the cells were incubated for 1 hr on ice. The cells were centrifuged at 600 g for 5 min, the supernatant (SN) were removed and the cells were re‐suspended in PBS containing 1% FBS. Next, surface staining was performed by adding rat anti‐mouse CD4, CD8 or IL‐17R (Millipore) at 1 μl/sample in PBS containing 1% FBS and the cells were incubated on ice for 1 hr. The cells were centrifuged at 600 g for 5 min, the supernatant was removed and the cells were re‐suspended in the fixation buffer (Millipore). The intracellular and surface fluorescent stains were analysed by flow cytometry using a FACS‐Calibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ).
Measurement of anti‐oxidant activity
Antioxidant capacity was determined using the Total Antioxidant Capacity Assay from Cell BioLabs Inc. (San Diego, CA; product number: STA‐360). Samples were prepared in methanol according to the manufacturer's protocol. The data shown have been adjusted for dilutions. Vitamin E, vitamin A and EGCG have uric acid equivalencies of 10·84 mm, 1·33 mm and 83·1 mm, respectively. Upon combining the three substances, a uric acid equivalency of 118·2 mm was found. Vitamin C had a value of 34·1 mm of uric acid.
Gene expression analysis by quantitative real‐time PCR
Groups of five mice each were either given NIDS daily or not treated for 30 days. Total RNA was extracted from individual spleens using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Reverse transcription and quantitative real‐time PCR analysis were performed as previously described.39, 40
Statistical analysis
The data from all the tests were analysed to show statistically significant differences between the means of vaccination or treatment groups, using either the analysis of variance (anova) test or Student's t‐test as appropriate. The P‐values are indicated in the figures, and related tables.
Results
Vitamin A and/or E combined with a catechin in vegetable oil synergistically enhance adaptive antibody responses
We sought to determine whether a catechin, EGCG and vitamins A and E, in a MO emulsion, alone or together would exert any immune‐enhancing or immunosuppressive effects. In several preclinical studies, mucosal priming and systemic boosting vaccinations have been shown to induce optimal mucosal and systemic responses.8 Therefore, mice were vaccinated twice mucosally, through combined sublingual and intranasal routes, followed by once systemically (i.m.) with HIV‐1BaL gp120 alone in PBS (no adjuvant) or with a combination of a pro‐vitamin A, RP, with or without vitamin E, and, emulsified in MO. As controls, Poly (I:C) was used through the same vaccination routes as the NIDS components. As an additional control, alum [aluminium and magnesium hydroxide; Imject Alum (Pierce; Rockford, IL)] was used for i.m. vaccinations. Combinations of RP, with or without vitamin E, with EGCG in MO synergistically enhanced serum IgG1 responses against HIV‐1BaL gp120, which was significantly higher than Poly (I:C) following two mucosal vaccinations (Fig. 1a), and following two mucosal and one i.m. vaccination (Fig. 1b, Table 1). Furthermore, combinations of RP, with or without VE, with EGCG in MO synergistically enhanced serum IgA responses against HIV‐1BaL gp120, which was significantly higher than Poly (I:C) following two mucosal and one i.m. vaccination (Fig. 1b, Table 1). Importantly, mucosal and systemic vaccinations with EGCG in MO and RP in MO significantly suppressed serum IgG1 responses compared with vaccinations with the combinations of vitamin A and EGCG in MO or even no adjuvant (Fig. 1b, Table 1). Furthermore, the combined two mucosal followed by two systemic vaccinations with NIDS also significantly enhanced serum IgA (Fig. 1b) compared with the same vaccination routes with Poly (I:C), or four i.m. vaccinations with Imject Alum (Fig. 1b, Table 1). Interestingly, two mucosal priming and one systemic boosting vaccination with Alum and Poly (I:C), but not with various NIDS formulations, significantly enhanced serum anti‐gp120 IgG2a antibody responses compared with the same vaccinations with no adjuvant (Fig. 1b, Table 1). Of note, a single mucosal priming vaccination did not induce detectable antigen‐specific IgM responses and two mucosal priming followed by two systemic boosting vaccinations did not induce any detectable antigen‐specific IgE responses. These data show that vitamins A and/or E synergize with EGCG in an oil‐in‐water emulsion to enhance adaptive serum IgG1 and IgA antibody responses.
Figure 1.
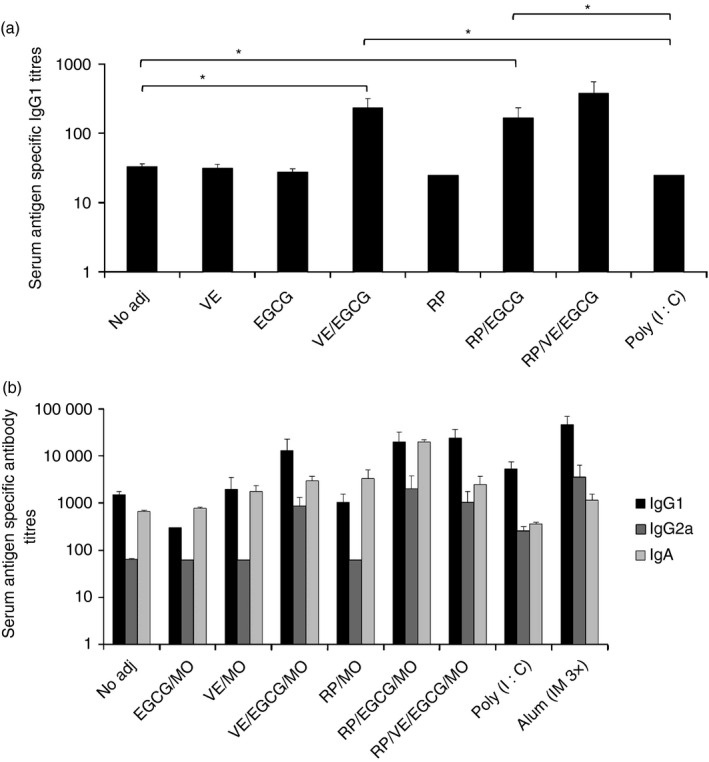
Effects of Nutritive Immune‐enhancing Delivery System (NIDS) versus other adjuvants on antibody responses following combination of mucosal and systemic vaccinations. (a) Serum antibody titres against HIV‐1BaL gp120 following two mucosal vaccinations. Combinations of retinyl palmitate, with or without vitamin E, with epigallo catechin gallate in mustard seed oil significantly enhanced serum IgG1 responses against HIV‐1BaL gp120 compared with Poly (I:C). (b) Serum antibody titres against HIV‐1BaL gp120 following two mucosal and one systemic vaccination. The data presented represent one study of two with similar data. Compared with all the other groups, significantly enhanced IgG1 and IgA responses following vaccinations with NIDS were measured in sera at 2 weeks after the third vaccination (2WP3). The data are presented as mean values from six mice per group ± SEM (error bars) and P values are displayed as *P ≤ 0·05, **P < 0·01 and ***P < 0·001. One representative study of two with similar results is shown.
Table 1.
Statistical P values; represents the P values for Fig. 1(b)
| t‐test (two‐tailed, two‐sample equal variance) | |||
|---|---|---|---|
| IgG1 | IgG2a | IgA | |
| RP/EGCG:No Adj | ** | ***** | |
| RP/EGCG:EGCG | *** | ***** | |
| RP/EGCG:VE | ** | **** | |
| RP/EGCG:RP | ** | ** | |
| RP/EGCG:Poly (I:C) | **** | ||
| Poly (I:C):No Adj | *** | **** | *** |
| RP/EGCG:Alum | * | ||
| Alum:No Adj | *** | *** | |
| VE/RP/EGCG:No Adj | ** | * | |
| VE/RP/EGCG:EGCG | **** | * | |
| VE/RP/EGCG:VE | *** | * | |
| VE/RP/EGCG:RP | *** | * | |
P values are displayed as *P ≤ 0·1, **P ≤ 0·05, ***P < 0·01, ****P < 0·001 and *****P < 0·00001.
NIDS enhancement of adaptive Th1 and Th2, but not Th17 responses
We next determined CD4+ and CD8+ T‐cell responses in local draining lymph nodes and systemically in spleens, after two mucosal priming followed by two i.m. boosting vaccinations. The prime/boost vaccinations with NIDS significantly enhanced local Th1 (IL‐2 and TNF‐α) and Th2 (IL‐5) cytokine responses in iliac lymph nodes compared with vaccinations with antigen alone (Fig. 2a). Of note, prime/boost vaccinations with Poly (I:C) or i.m. vaccination with Alum also induced significantly higher levels of the Th1 and Th2 cytokines compared with prime/boost vaccinations with no adjuvant (Fig. 2a). In addition, similar response for IL‐2 and TNF‐α was obtained in spleen with the combined mucosal and systemic vaccination with NIDS versus Poly (I:C) (data not shown).
Figure 2.

Cellular responses in lymph nodes following two mucosal and two systemic vaccinations with Nutritive Immune‐enhancing Delivery System (NIDS) versus other adjuvants. (a) Cytokine responses in iliac lymph nodes (iLN). Vaccinations with NIDS significantly enhanced local T helper type 1 (Th1) [interleukin‐2 (IL‐2) and tumour necrosis factor‐α (TNF‐α)] and Th2 (IL‐5) cytokine responses in iliac lymph nodes (iLN) compared with no adjuvant. (b) Cellular interferon‐γ (IFN‐γ) responses by CD4+ T helper and CD8+ T cells in iLN. Vaccinations with NIDS significantly enhanced percentage of local CD4+ IFN‐γ + and CD8+ IFN‐γ + cells in iLN, compared with mucosal/systemic vaccinations with Poly (I:C), or systemic vaccination with Alum. (c) Cellular IL‐17 responses by CD4+ T helper cells and IFN‐γ responses by CD4+ T helper and CD8+ T cells in cervical lymph nodes (CLN). Vaccinations with NIDS significantly diminished the percentage of CD4+ IL‐17+ cells in CLN, compared with mucosal/systemic vaccinations with Poly (I:C), or systemic vaccination with Alum. The data are presented as mean values from six mice per group ± SEM (error bars) and P values are displayed as *P ≤ 0·05, **P < 0·01 and ***P < 0·001. The iLN and CLN from two mice per group were pooled, i.e. n = 3. One representative study of two with similar results is shown.
Because IFN‐γ responses are of particular interest in Th1‐mediated immunity, we next measure IFN‐γ responses by both CD4+ and CD8+ T cells. The combined prime/boost vaccinations induced significantly higher percentages of CD4+ IFN‐γ + and CD8+ IFN‐γ + cells in iliac lymph nodes following vaccinations with NIDS compared with vaccinations with no adjuvant (Fig. 2b). In contrast, prime/boost vaccinations with Poly (I:C) or i.m. vaccinations with Alum compared with prime/boost vaccinations with no adjuvant did not result in significantly increased percentages of CD4+ IFN‐γ + and CD8+ IFN‐γ + cells compared with vaccinations with no adjuvant (Fig. 2b). Importantly, vaccinations with NIDS not only did not enhance local CD4+ IL‐17+ responses, but in fact significantly diminished such responses compared with vaccinations with no adjuvant, Poly (I:C) or Alum (Fig. 2c). Taken together, these results show that vaccinations with NIDS induced higher CD4+ IFN‐γ + and CD8+ IFN‐γ + responses compared with vaccinations with no adjuvant, in the absence of IL‐17 responses.
NIDS induction of strong adaptive responses occurs in the absence of strong local and systemic pro‐inflammatory innate responses
To address an important aspect of localized adverse events, i.e. site of injection pro‐inflammatory immune responses, we measured various innate immunity cytokines and chemokines in the murine air‐pouch model.41, 42 Seven‐fold higher doses than the vaccination doses of sterile PBS, NIDS, Poly (I:C) or Imject Alum were injected into air pouches in the lower backs of mice and select cytokines and chemokines were measured by a multiplex Luminex assay in the air‐pouch lavages collected 6 hr later. Injection of NIDS significantly enhanced local, site of injection, levels of IL‐12p70 and granulocyte–macrophage colony‐stimulating factor, but decreased levels of TNF‐α, IL‐5 and IL‐13 compared with injection of PBS (Fig. 3a, Table 2). Importantly, injection of Poly (I:C) significantly enhanced site of injection IFN‐γ, IL‐6, IL‐12p70, IL‐5, IFN‐γ inducible protein 10 (IP‐10), monocyte chemoattractant protein‐1, Keratinocyte Chemoattractant‐like (KC), MIP‐1α and ‐β, MIP‐2, regulated on activation, normal T cell expressed and secreted (RANTES) and granulocyte colony‐stimulating factor, compared with injection of NIDS. Furthermore, injection of Alum significantly enhanced the same cytokines and chemokines as Poly (I:C), but in addition also TNF‐α and granulocyte–macrophage colony‐stimulating factor compared with injection with NIDS (Fig. 3a, Table 2).
Figure 3.

Effects of Nutritive Immune‐enhancing Delivery System (NIDS) versus other adjuvants on early innate and pro‐inflammatory cytokine and chemokine responses. (a) In vivo, early innate cytokine and chemokine responses using the mouse air‐pouch model. The data presented represent one study of two with similar data. Compared with NIDS, significantly enhanced pro‐inflammatory cytokines and chemokines following injection of Poly (I:C) and Alum were measured. Note, the limit of detection for various analytes was different and an analyte that is below the limit of detection for all samples is presented without an error bar. (b) Comparison of early pro‐inflammatory cytokine and chemokine responses in serum following a single intramuscular (i.m.) administration. Significantly higher serum concentrations of interleukin‐5 (IL‐5), KC and granulocyte colony‐stimulating factor (G‐CSF) were observed following i.m. vaccination with NIDS compared with no adjuvant. Compared with no adjuvant or NIDS, Poly (I:C) induced significantly higher serum interferon‐γ‐inducible protein 10 (IP‐10) levels. (c) Sixteen hours following a single i.m. vaccination with no adjuvant, NIDS, Poly (I:C) and Alum, serum IL‐15 levels were found to be significantly higher following vaccination with NIDS compared with no adjuvant, Alum or Poly (I:C). The data are presented as mean values from three to eight mice per group ± SEM (error bars) and P values are displayed as *P ≤ 0·05, **P < 0·01 and ***P < 0·001.
Table 2.
Statistical P values; represents the P values for Fig. 3(a)
| t‐test (two‐tailed, two sample equal variance) | |||||
|---|---|---|---|---|---|
| IFN‐γ | IL‐6 | TNF‐α | IL‐2 | IL‐17 | |
| NIDS:No Adj | ** | * | |||
| NIDS:Poly(I:C) | ** | ***** | * | ||
| Poly(I:C):No Adj | *** | * | |||
| NIDS:Alum | **** | ***** | ***** | ***** | |
| Alum:No Adj | *** | **** | *** | ||
| IL‐12 p70 | IL‐12 p40 | IL‐4 | IL‐5 | IL‐13 | |
| NIDS:No Adj | ***** | ** | *** | ** | |
| NIDS:Poly(I:C) | *** | ** | |||
| Poly(I:C):No Adj | *** | ** | *** | ||
| NIDS:Alum | ***** | * | *** | ***** | |
| Alum:No Adj | ***** | ** | ** | ***** | *** |
| IP‐10 | KC | MCP‐1 | MIP‐1α | MIP‐1β | |
| NIDS:No Adj | * | ||||
| NIDS:Poly(I:C) | *** | *** | **** | ** | *** |
| Poly(I:C):No Adj | ***** | * | *** | *** | ***** |
| NIDS:Alum | * | ***** | **** | **** | **** |
| Alum:No Adj | *** | **** | *** | ***** | ***** |
| MIP‐2 | RANTES | G‐CSF | GM‐CSF | ||
| NIDS:No Adj | ** | *** | ** | ||
| NIDS:Poly(I:C) | ** | **** | **** | ||
| Poly(I:C):No Adj | ** | **** | ** | ** | |
| NIDS:Alum | **** | ***** | ***** | *** | |
| Alum:No Adj | ***** | ***** | ***** | **** | |
Abbreviations: IFN‐γ, interferon‐γ; IL‐6, interleukin‐6; IP‐10, IFN‐γ‐inducible protein 10; KC, ; MCP‐1, monocyte chemoattractant protein 1; MIP‐1α, macrophage inflammatory protein‐1α; TNF‐α, tumour necrosis factor‐α;
P values are displayed as *≤ 0·1, **≤ 0·05, ***< 0·01, ****< 0·001 and *****< 0·00001.
To determine innate responses following a common route of systemic vaccination, next, we measured serum innate cytokine and chemokine responses at 16 hr following a single i.m. vaccination with no adjuvant (PBS), NIDS, Poly (I:C) or Alum. Whereas serum concentrations of IL‐5, KC and granulocyte colony‐stimulating factor were significantly higher following i.m. vaccination with NIDS compared with PBS, Poly (I:C) induced significantly higher serum IP‐10 compared with vaccination with no adjuvant or with NIDS (Fig. 3b). Moreover, vaccination with Poly (I:C) induced significantly higher IP‐10, compared with no adjuvant (Fig. 3b). Hence, although there were differences in induction of local (air pouch) versus systemic (serum) innate cytokines and chemokines following vaccinations with no adjuvant compared with NIDS, Poly (I:C) and Alum, in general, vaccinations with NIDS induced significantly lower pro‐inflammatory cytokines and chemokines compared with vaccination with Poly (I:C) and/or Alum. Innate serum cytokines and chemokines were also measured in serum after a single sublingual/intranasal administration of NIDS, Poly (I:C) or no adjuvant. However, no significant differences in serum concentrations of several innate cytokines or chemokines were discernible (data not shown).
Early innate production of IL‐15 has been linked to qualitatively and quantitatively better adaptive cellular responses.36, 37 Sixteen hours following a single i.m. vaccination with PBS, NIDS, Poly (I:C) or Alum, serum IL‐15 levels were found to be significantly higher following vaccination with NIDS compared with PBS, Alum or Poly (I:C) (Fig. 3c). These data show that while several innate responses were not elevated following i.m. vaccination with NIDS, the innate IL‐15 responses were preserved and enhanced.
Induction of local CD4+ IL‐17R+ by Poly (I:C) but not by NIDS following i.m. administration
To determine any potential role of Th17 responses in the innate responses, we next performed flow cytometric analysis of inguinal lymph node cells prepared 10 hr after a single i.m. administration of NIDS, Poly (I:C) or no adjuvant. There was a significantly higher percentage of CD4+ IL‐17R+ cells in inguinal lymph nodes of mice given Poly (I:C) compared with mice given NIDS (Fig. 4), further supporting the notion that the NIDS formulation generally suppresses local Th17 and pro‐inflammatory chemokines and cytokines locally and systemically.
Figure 4.
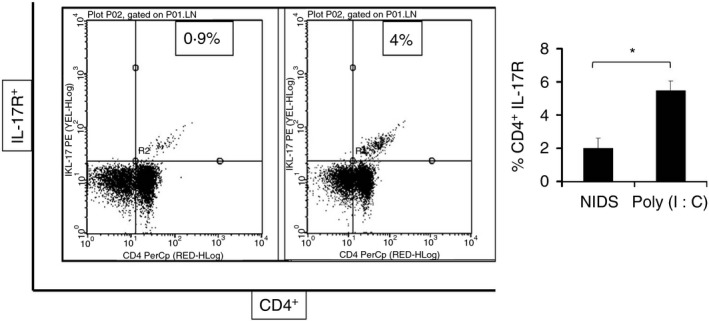
Flow cytometric analysis (FACS, fluorescence activated cell sorter) of interleukin‐17 receptor (IL‐17R) expression by CD4+ T cells. Significantly higher percentages of CD4+ IL‐17R+ cells were measured from inguinal lymph node cells after a single i.m. administration of Poly (I:C) compared with Nutritive Immune‐enhancing Delivery System. The data are presented as mean values from eight mice per group, where the lymph nodes from two mice per group were pooled ± SEM (error bars) and P values are displayed as *P ≤ 0·05, **P < 0·01 and ***P < 0·001.
Action of NIDS adjuvant depends on IL‐15 and IL‐15R and correlates positively with anti‐oxidant activity and superoxide dismutase 1 and negatively with IL‐1β production
The finding that innate IL‐15 responses were enhanced following a single i.m. vaccination with NIDS (Fig. 3c) prompted us to determine whether IL‐15 is essential for differentiation of adaptive B‐cell and particularly T‐cell responses following vaccinations with NIDS. To this end, we vaccinated C57BL/6 wild‐type (WT), and IL‐15/IL‐15R−/− (DKO) mice twice i.m. with HIV‐1BaL gp120 with no adjuvant, or mixed with NIDS, and measured antigen‐specific B‐cell and T‐cell responses in serum and spleen (SP), respectively. In WT mice, as expected, NIDS induced significantly higher serum gp120‐specific IgG1 compared with no adjuvant (Fig. 5a). The adjuvant effect of NIDS for induction of serum antigen‐specific IgG1 responses, however, was intact in DKO mice (Fig. 5a).
Figure 5.
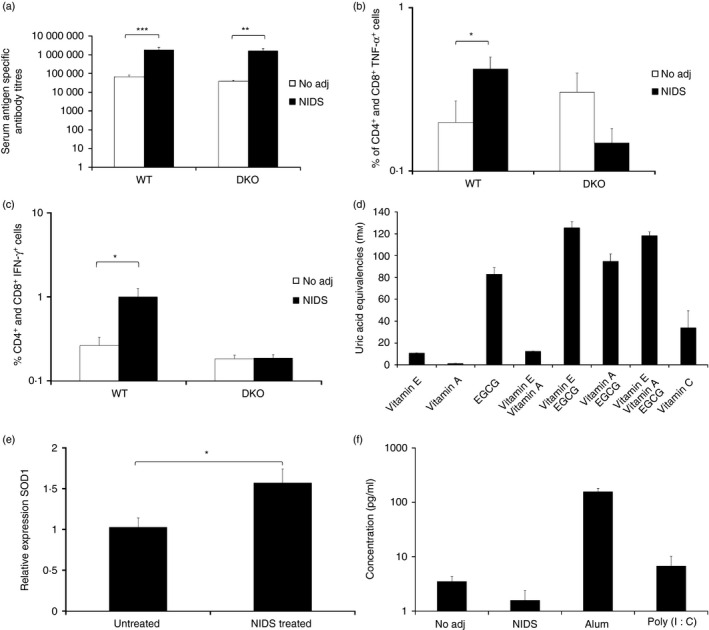
Adjuvant effects of Nutritive Immune‐enhancing Delivery System (NIDS) versus no adjuvant and Poly (I:C) on B‐cell and T‐cell responses in wild‐type (WT), and interleukin‐15 (IL‐15)/IL‐15 receptor (IL‐15R)−/− (DKO) mice following two systemic vaccinations as well as potential mechanisms of action of NIDS based on anti‐oxidant activity, superoxide dismutase 1, and IL‐1β production. (a) Serum antibody titres against HIV‐1BaL gp120. Compared with no adjuvant, significantly higher serum anti‐HIV‐1BaL gp120 IgG1 responses were induced in vaccination with NIDS. (b, c) Cellular response of CD4+/CD8+ cells expressing tumour necrosis factor‐α (TNF‐α) (b) and interferon‐γ (IFN‐γ) (c) in SP. Percentage of CD4+ and CD8+ cells expressing TNF‐α and IFN‐γ were significantly enhanced in SP followed by vaccination with NIDS in WT but not in DKO mice. The data are presented as mean values from five to six individual mice per group ± SEM (error bars) and P values are displayed as *P ≤ 0·05, **P < 0·01 and ***P < 0·001. (d) Anti‐oxidant activity of NIDS components plus vitamin C. The anti‐oxidant activity of vitamins A and E and epigallo catechin gallate, as components of the NIDS formulation were measured alone or in combinations and compared with a strong anti‐oxidant, vitamin C. (e) Gene expression of superoxide dismutase 1 (SOD1) following daily oral administration of NIDS for 30 days. (f) IL‐1β production in air pouches of mice vaccinated with PBS (no adjuvant), NIDS, Alum or Poly (I:C).
We next addressed the role of IL‐15 on the development of adaptive T‐cell responses following vaccinations with NIDS versus no adjuvant of WT and DKO mice. We found significantly enhanced percentages of TNF‐α + (Fig. 5b) and IFN‐γ + (Fig. 5c) responses by CD4+ and CD8+ cells in the spleens of WT but not DKO mice. Hence, the adjuvant effect of NIDS appeared to depend on IL‐15 and its receptor for generation of important effector functions by T cells, but not B cells.
In an effort to understand further the mechanism of action of NIDS we also measured the anti‐oxidant activity of the NIDS components, i.e. vitamin A, vitamin E and EGCG. Relatively strong anti‐oxidant activity of the NIDS components was detected (Fig. 5d). We next determined whether prolonged feeding of NIDS to mice for 30 days would induce expression of any enzymes that correlate with strong anti‐oxidant activity. We found that the expression of superoxide dismutase 1, an enzyme that binds copper and zinc ions and converts superoxide radicals to free oxygen, was significantly enhanced in NIDS‐treated mice versus untreated mice (P < 0·05) (Fig. 5e). These data show that there is a positive correlation between the anti‐oxidant activity of NIDS, with enhanced expression of superoxide dismutase 1 and innate IL‐15 responses.
The adjuvant action of several TLR agonists, including Poly (I:C), a TLR3 agonist,43 and bacterial lipopolysaccharide, a TLR 4 agonist,44 depend on the activation of the inflammasome protein complex, which leads to enhanced IL‐1β production. Hence, in the air‐pouch model, we measured production of IL‐1β, 6 hr following activation with PBS (no adjuvant), NIDS, Poly (I:C) and Alum. We found that IL‐1β production in the air pouches was significantly enhanced following activation with Alum (P <0·01) and Poly (I:C) (P < 0·05), compared with activation with no adjuvant or with NIDS (Fig. 5f). These data provide indirect evidence that the NIDS does not induce formation of inflammasomes.
Discussion
While several studies have shown various degrees of immune‐enhancing properties of select vitamins and flavonoids, others have indeed shown immunosuppressing effects of such compounds.1 These discrepancies may be due to differences in in vitro, ex vivo and in vivo studies, differences in species tested, routes of administration, presence of other active compounds, and the dose and purity of the tested compounds, all of which play important roles in the observed differences in the immunomodulatory properties of vitamins and flavonoids in various models.
This is the first study in which the combination of vitamins A and E and a catechin synergistically enhanced not only B‐cell responses, but also Th1, Th2 and CD8+ T‐cell responses, in the absence of select innate, pro‐inflammatory responses. Studies on the immunomodulatory properties of vitamins and flavonoids are gaining momentum.1, 2 Although in a previous publication we had used retinoic acid and catechin hydrate to generate NIDS,30 in the current study, we successfully replaced catechin hydrate with EGCG, and retinoic acid with RP, because of their low cost for large‐scale manufacturing. Vitamins A and E and EGCG are small molecules obtainable as GMP grade, and several batches of the seed oil from different harvests showed no differences in the adjuvant effect of NIDS.
The enhancement of early local and systemic IL‐15 responses following administration of NIDS, and the fact that IL‐15 and IL‐2 partially share the same receptor, led us to the hypothesis that IL‐15 may be important for the adjuvant action of NIDS. Interestingly, because the NIDS induced strong local IL‐15 responses, this may have played a role in the suppression of innate and adaptive IL‐17 responses.45
Induction and maintenance of mucosal immunological memory is important for vaccines against HIV and other mucosally transmitted pathogens.46 Moreover, it has been shown that long‐term antigen‐specific responses following mucosal vaccinations may be maintained by IgM‐secreting and CD45R+ B cells.47, 48, 49 Although essential in vaccine design, by far most vaccine studies do not address whether vaccine candidates induce long‐term immune responses or immunity.46 While our preliminary data indicate that serum antigen‐specific antibody responses following vaccinations using NIDS adjuvant persist for several months, the issue of life‐long immunological memory needs to be addressed in future studies.
Whether vaccinations with NIDS can induce protective responses against select pathogens is an important question. In a previous study, we demonstrated that mucosal priming followed by systemic boosting vaccinations with gp120CN54 in NIDS induced serum neutralizing antibodies. Furthermore, our preliminary studies have demonstrated that systemic vaccinations with NIDS mixed with recombinant haemagglutinin from an H1N1 influenza virus strain, induced relatively very high haemagglutination inhibition titres and protection against infection, as measured by lung plaque‐forming unit titres in a mouse model (data not shown). These data suggest that vaccinations with NIDS hold promise to protect against infection or disease.
Elucidation of mechanism of action of vaccine adjuvants is a complex task that has traditionally taken decades to accomplish. This has been the case for Alum, squalene oil‐based adjuvants, toxins and toxin mutants, and TLR agonists. Given the novelty of the NIDS, it is expected that understanding the complete mechanism of action of this adjuvant may take many years. However, in the current study, we have shed some light on the potential mechanism of action of NIDS. This includes the role of IL‐15 in T‐cell responses, the metabolic to immune pathway of the anti‐oxidant activity, and the lack of IL‐1β and the classic inflammasome complex formation. There is increasing evidence that metabolic pathways, such as anti‐oxidant activity, correlate with innate responses.50 Further studies to determine the mechanism of action of NIDS are underway.
Disclosures
Michael Vajdy owns shares of EpitoGenesis, Inc. Sapna Patel, Archana Akalkotkar and Mingke Yu are former employees of EpitoGenesis, Inc. Sara Colpitts has no conflict of interest.
Acknowledgements
This study was in part supported by grants from the NIAID, 1R43AI084690‐01, NIAID 1R41AI096706‐01A1, the Treasury Department's Therapeutic Discovery Award and the State of Connecticut's Department of Economic Development. We thank Murigenics for performing the air‐pouch model studies and for providing a vivarium and related services for several of the reported studies. We gratefully acknowledge Millipore/Merck for providing the multiplexing and flow cytometry kits.
References
- 1. Vajdy M. Immunomodulatory properties of vitamins, flavonoids and plant oils and their potential as vaccine adjuvants and delivery systems. Expert Opin Biol Ther 2011; 11:1501–13. [DOI] [PubMed] [Google Scholar]
- 2. Patel S, Vajdy M. Induction of cellular and molecular immunomodulatory pathways by vitamin A and flavonoids. Expert Opin Biol Ther 2015;15:1411–28. doi:10.1517/14712598.2015.1066331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Duriancik DM, Hoag KA. Vitamin A deficiency alters splenic dendritic cell subsets and increases CD8+ Gr‐1+ memory T lymphocytes in C57BL/6J mice. Cell Immunol 2010; 265:156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sirisinha S. The pleiotropic role of vitamin A in regulating mucosal immunity. Asian Pac J Allergy Immunol 2015; 33:71–89. [PubMed] [Google Scholar]
- 5. Chattha KS, Kandasamy S, Vlasova AN, Saif LJ. Vitamin A deficiency impairs adaptive B and T cell responses to a prototype monovalent attenuated human rotavirus vaccine and virulent human rotavirus challenge in a gnotobiotic piglet model. PLoS One 2013; 8:e82966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vlasova AN, Chattha KS, Kandasamy S, Siegismund CS, Saif LJ. Prenatally acquired vitamin A deficiency alters innate immune responses to human rotavirus in a gnotobiotic pig model. J Immunol 2013; 190:4742–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spencer SP, Wilhelm C, Yang Q, Hall J a., Bouladoux N, Boyd a et al Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science 2014; 343:432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vajdy M, Srivastava I, Polo J, Donnelly J, O'Hagan D, Singh M. Mucosal adjuvants and delivery systems for protein‐, DNA‐ and RNA‐based vaccines. Immunol Cell Biol 2004; 82:617–27. [DOI] [PubMed] [Google Scholar]
- 9. Hopkins RJ, Daczkowski NF, Kaptur PE, Muse D, Sheldon E, LaForce C et al Randomized, double‐blind, placebo‐controlled, safety and immunogenicity study of 4 formulations of Anthrax Vaccine Adsorbed plus CPG 7909 (AV7909) in healthy adult volunteers. Vaccine 2013; 31:3051–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cooper CL, Davis HL, Morris ML, Efler SM, Krieg AM, Li Y et al Safety and immunogenicity of CPG 7909 injection as an adjuvant to Fluarix influenza vaccine. Vaccine 2004; 22:3136–43. [DOI] [PubMed] [Google Scholar]
- 11. Pulendran B, Artis D. New paradigms in type 2 immunity. Science 2012; 337:431–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Desmet CJ, Ishii KJ. Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nat Rev Immunol 2012; 12:479–91. [DOI] [PubMed] [Google Scholar]
- 13. Levitz SM, Golenbock DT. Beyond empiricism: informing vaccine development through innate immunity research. Cell 2012; 148:1284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stevceva L, Moniuszko M, Ferrari MG. Utilizing IL‐12, IL‐15 and IL‐7 as mucosal vaccine adjuvants. Lett Drug Des Discov 2006; 3:586–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stonier SW, Schluns KS. Trans‐presentation: a novel mechanism regulating IL‐15 delivery and responses. Immunol Lett 2010; 127:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by γ(c) family cytokines. Nat Rev Immunol 2009; 9:480–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perera P‐Y, Lichy JH, Waldmann TA, Perera LP. The role of interleukin‐15 in inflammation and immune responses to infection: implications for its therapeutic use. Microbes Infect 2012; 14:247–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sui Y, Gagnon S, Dzutsev A, Zhu Q, Yu H, Hogg A et al TLR agonists and/or IL‐15 adjuvanted mucosal SIV vaccine reduced gut CD4+ memory T cell loss in SIVmac251‐challenged rhesus macaques. Vaccine 2011; 30:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Herndler‐Brandstetter D, Landgraf K, Jenewein B, Tzankov A, Brunauer R, Brunner S et al Human bone marrow hosts polyfunctional memory CD4+ and CD8+ T cells with close contact to IL‐15‐producing cells. J Immunol 2011; 186:6965–71. [DOI] [PubMed] [Google Scholar]
- 20. Pulle G, Vidric M, Watts TH. IL‐15‐dependent induction of 4‐1BB promotes antigen‐independent CD8 memory T cell survival. J Immunol 2006; 176:2739–48. [DOI] [PubMed] [Google Scholar]
- 21. Mele DA, Salmeron A, Ghosh S, Huang H‐R, Bryant BM, Lora JM. BET bromodomain inhibition suppresses TH17‐mediated pathology. J Exp Med 2013; 210:2181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koga T, Hedrich CM, Mizui M, Yoshida N, Otomo K, Lieberman LA et al CaMK4‐dependent activation of AKT/mTOR and CREM‐α underlies autoimmunity‐associated Th17 imbalance. J Clin Invest 2014; 124:2234–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khader SA, Cooper AM. IL‐23 and IL‐17 in tuberculosis. Cytokine 2008; 41:79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sonnenberg GF, Weiner DB. Manipulation of T H 17 responses in pulmonary immunity and disease through vaccination. Hum Vaccin 2014; 5:510–9. [DOI] [PubMed] [Google Scholar]
- 25. Michel M‐L, Keller AC, Paget C, Fujio M, Trottein F, Savage PB et al Identification of an IL‐17‐producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med 2007; 204:995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marwaha AK, Leung NJ, McMurchy AN, Levings MK. TH17 cells in autoimmunity and immunodeficiency: protective or pathogenic? Front Immunol 2012; 3:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu Y, Chen Q, Pai T, Ross AC. All‐trans‐retinoic acid and Erk1/2 signaling synergistically regulate the expression of CD300B in human monocytic cells. Cell Immunol 2011; 268:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gohil K, Oommen S, Vasu VT, Aung HH, Cross CE. Tocopherol transfer protein deficiency modifies nuclear receptor transcriptional networks in lungs: modulation by cigarette smoke in vivo . Mol Aspects Med 2007; 28:453–80. [DOI] [PubMed] [Google Scholar]
- 29. Park JW, Choi YJ, Suh SI, Kwon TK. Involvement of ERK and protein tyrosine phosphatase signaling pathways in EGCG‐induced cyclooxygenase‐2 expression in raw 264.7 cells. Biochem Biophys Res Commun 2001; 286:721–5. [DOI] [PubMed] [Google Scholar]
- 30. Yu M, Vajdy M. A novel retinoic acid, catechin hydrate and mustard oil‐based emulsion for enhanced cytokine and antibody responses against multiple strains of HIV‐1 following mucosal and systemic vaccinations. Vaccine 2011; 29:2429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jacobs BL, Langland JO. When two strands are better than one: the mediators and modulators of the cellular responses to double‐stranded RNA. Virology 1996; 219:339–49. [DOI] [PubMed] [Google Scholar]
- 32. Le Bon A, Schiavoni G, D'Agostino G, Gresser I, Belardelli F, Tough DF. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo . Immunity 2001; 14:461–70. [DOI] [PubMed] [Google Scholar]
- 33. Subramanya S, Kim S‐S, Abraham S, Yao J, Kumar M, Kumar P et al Targeted delivery of small interfering RNA to human dendritic cells to suppress dengue virus infection and associated proinflammatory cytokine production. J Virol 2010; 84:2490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lindblad EB. Aluminium compounds for use in vaccines. Immunol Cell Biol 2004; 82:497–505. [DOI] [PubMed] [Google Scholar]
- 35. Valensi JP, Carlson JR, Van Nest GA. Systemic cytokine profiles in BALB/c mice immunized with trivalent influenza vaccine containing MF59 oil emulsion and other advanced adjuvants. J Immunol 1994; 153:4029–39. [PubMed] [Google Scholar]
- 36. Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M et al Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15‐deficient mice. J Exp Med 2000; 191:771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S et al IL‐15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity 1998; 9:669–76. [DOI] [PubMed] [Google Scholar]
- 38. Sedgwick AD, Moore AR, Al‐Duaij AY, Edwards JC, Willoughby DA. The immune response to pertussis in the 6‐day air pouch: a model of chronic synovitis. Br J Exp Pathol 1985; 66:455–64. [PMC free article] [PubMed] [Google Scholar]
- 39. Ku CS, Pham TX, Park Y, Kim B, Shin MS, Kang I et al Edible blue‐green algae reduce the production of pro‐inflammatory cytokines by inhibiting NF‐κB pathway in macrophages and splenocytes. Biochim Biophys Acta 2013; 1830:2981–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang Y, Seo JM, Nguyen A, Pham TX, Park HJ, Park Y et al Astaxanthin‐rich extract from the green alga Haematococcus pluvialis lowers plasma lipid concentrations and enhances antioxidant defense in apolipoprotein E knockout mice. J Nutr 2011; 141:1611–7. [DOI] [PubMed] [Google Scholar]
- 41. Ashton JC. Cannabinoids for the treatment of inflammation. Curr Opin Investig Drugs 2007; 8:373–84. [PubMed] [Google Scholar]
- 42. Teixeira CR, Cavassani KA, Gomes RB, Teixeira MJ, Roque‐Barreira M‐C, Cavada BS et al Potential of KM+ lectin in immunization against Leishmania amazonensis infection. Vaccine 2006; 24:3001–8. [DOI] [PubMed] [Google Scholar]
- 43. Yu M, Levine SJ. Toll‐like receptor 3, RIG‐I‐like receptors and the NLRP3 inflammasome: key modulators of innate immune responses to double‐stranded RNA viruses. Cytokine Growth Factor Rev 2011; 22:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stowe I, Lee B, Kayagaki N. Caspase‐11: arming the guards against bacterial infection. Immunol Rev 2015; 265:75–84. [DOI] [PubMed] [Google Scholar]
- 45. Pandiyan P, Yang X‐P, Saravanamuthu SS, Zheng L, Ishihara S, O'Shea JJ et al The role of IL‐15 in activating STAT5 and fine‐tuning IL‐17A production in CD4 T lymphocytes. J Immunol 2012; 189:4237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vajdy M. Generation and maintenance of mucosal memory B cell responses? Curr Med Chem 2006; 13:3023–37. [DOI] [PubMed] [Google Scholar]
- 47. Vajdy M, Lycke N. Mucosal memory B cells retain the ability to produce IgM antibodies 2 years after oral immunization. Immunology 1995; 86:336–42. [PMC free article] [PubMed] [Google Scholar]
- 48. Soenawan E, Srivastava I, Gupta S, Kan E, Janani R, Kazzaz J et al Maintenance of long‐term immunological memory by low avidity IgM‐secreting cells in bone marrow after mucosal immunizations with cholera toxin adjuvant. Vaccine 2004; 22:1553–63. [DOI] [PubMed] [Google Scholar]
- 49. Yu M, Goodsell A, Zhou F, Vajdy M. Maintenance of long‐term immunological memory by Ig+CD45R+ non‐plasma B cells following mucosal immunizations. Immunol Lett 2011; 138:63–70. [DOI] [PubMed] [Google Scholar]
- 50. Peden DB. The role of oxidative stress and innate immunity in O(3) and endotoxin‐induced human allergic airway disease. Immunol Rev 2011; 242:91–105. [DOI] [PubMed] [Google Scholar]


