Abstract
Sutures and synchondroses, the fibrous and cartilaginous articulations found in the skulls of vertebrates, have been studied for many biological applications at the morphological scale. However, little is known about these articulations at the microscopic scale in non‐mammalian vertebrates, including extant archosaurs (birds and crocodilians). The major goals of this paper were to: (i) document the microstructure of some sutures and synchondroses through ontogeny in archosaurs; (ii) compare these microstructures with previously published sutural histology (i.e. that of mammals); and (iii) document how these articulations with different morphological degrees of closure (open or obliterated) appear histologically. This was performed with histological analyses of skulls of emus, American alligators, a fossil crocodilian and ornithischian dinosaurs (hadrosaurids, pachycephalosaurids and ceratopsids). Emus and mammals possess a sutural periosteum until sutural fusion, but it disappears rapidly during ontogeny in American alligators. This study identified seven types of sutural mineralized tissues in extant and extinct archosaurs and grouped them into four categories: periosteal tissues; acellular tissues; fibrous tissues; and intratendinous tissues. Due to the presence of a periosteum in their sutures, emus and mammals possess periosteal tissues at their sutural borders. The mineralized sutural tissues of crocodilians and ornithischian dinosaurs are more variable and can also develop via a form of necrosis for acellular tissues and metaplasia for fibrous and intratendinous tissues. It was hypothesized that non‐avian dinosaurs, like the American alligator, lacked a sutural periosteum and that their primary mode of ossification involved the direct mineralization of craniofacial sutures (instead of intramembranous ossification found in mammals and birds). However, we keep in mind that a bird‐like sutural microstructure might have arisen within non‐avian saurichians. While synchondroseal histology is relatively similar in archosaurs and mammals, the microstructural differences between the sutures of these two clades are undeniable. Moreover, the current results suggest that the degree of sutural closure can only accurately be known via microstructural analyses. This study sheds light on the microstructure and growth of archosaurian sutures and synchondroses, and reveals a unique, undocumented histological diversity in non‐avian dinosaur skulls.
Keywords: Alligator mississippiensis, Dinosauria, Dromaius novaehollandiae, histology, Mammalia, ontogeny, skull, sutures, synchondroses
Introduction
Sutures are the unossified tissues that unite the membrane bones of the skulls of vertebrates (Kokich, 1986; Herring, 2000; Opperman, 2000), while the junctions between the endochondral bones of the skull‐base are known as synchondroses (Opperman et al. 2005). Sutures and synchondroses are two different types of articulations, fibrous for the former and cartilaginous for the latter (Marieb, 2014). When these articulations are unossified, they facilitate the growth of the skull during ontogeny (Herring, 2000). They may close by fusion of the sutural bone fronts in sutures, or by the replacement of cartilage by endochondral bone in synchondroses. These articulations have been thoroughly investigated for the understanding of craniosynostosis in human infants (the premature fusion of the bones of the skull; Kokich, 1986; Opperman et al. 2005) and for applications in orthodontology (Kokich, 1976). They have also been described anatomically and in regards to skull biomechanics in various vertebrate clades (Herring, 1972; Herring & Mucci, 1991; Sun et al. 2004; Rayfield, 2005; Holliday & Witmer, 2008; Curtis et al. 2013). In the field of paleontology, they have been used for decades as a proxy to assess the maturity of fossils, including non‐avian dinosaurs (i.e. sutural patency indicates juvenescence while sutural closure/obliteration indicates maturity; Bakker & Williams, 1988; Chinsamy‐Turan, 2001; Sereno et al. 2009; Longrich & Field, 2012).
The way that sutures and synchondroses form and fuse has only been reported in mammals (Pritchard et al. 1956; Kokich, 1976; Persson et al. 1978; Herring, 2000; Opperman et al. 2005) and, unfortunately, very little is known about those structures in non‐mammalian vertebrates at the microscopic scale, including in archosaurs. For this reason, the main goal of this study was to document sutural and synchondroseal microstructure in non‐avian dinosaurs and their extant phylogenetic bracket (Witmer, 1995). This was investigated by means of histological analyses in growth series of skulls: in the emu, Dromaius novaehollandiae; in the American alligator, Alligator mississippiensis, in some non‐avian dinosaurs and a fossil crocodilian. Both cranial and facial sutures were analyzed, as well as some braincase synchondroses (Tables 1 and 2). The results were compared with what is available in the literature concerning the histology of articulations in other vertebrates (which is strongly biased towards mammals; Herring, 2000). Even though sutures and synchondroses are present in all vertebrates, it is not implausible to hypothesize that archosaurian and mammalian articulations (as well as their associated mineralized tissues) have considerably different histologies, due to the phylogenetic distance between these two clades.
Table 1.
List of the extant specimens sectioned in the present study
| Taxon | Species | Specimen number | Other numerotation | Skull length (cm) | Provenance/D or W | Estimated age | Ontogenetic category | Skull/head and embedding media |
|---|---|---|---|---|---|---|---|---|
| Aves | Dromaius novaehollandiae | MOR OST 1799 | Emu 1 | 5.5 | Montana/D | Few weeks | Hatchling | Dry skull/Epoxy |
| MOR OST 1800 | Emu 2 | 9.7 | Montana/D | Few weeks | Juvenile | Dry skull/Epoxy | ||
| MOR OST 1801 | Emu 3 | ~ 12.8 | Montana/D | 8–10 monthsa | Juvenile | Head/PMMA | ||
| MOR OST 1802 | Emu 4 | 12.8 | Montana/D | 8–10 monthsa | Juvenile | Dry skull/Epoxy | ||
| MOR OST 232 | Emu 5 | 15.8 | ? | > 18 months | Sexually/skeletally mature | Dry skull/Epoxy | ||
| MOR OST 1803 | Emu 6 | 15.2 | Montana/D | 20 years‐old (male)a | Sexually/skeletally mature | Dry skull/PMMA | ||
| Crocodylia | Alligator mississippiensis | MOR OST 1647 | Alligator 1 | 2.54 | Louisiana/? | A few days | Hatchling | Head/PMMA |
| MOR OST 1796 | Alligator 2 | 4.19 | Florida/D | A few weeks | Juvenile | Dry skull/Epoxy | ||
| MOR OST 1797 | Alligator 3 | 15.5 | Louisiana/? | 4–5 years | Sub‐adult | Head/PMMA | ||
| MOR OST 1798 | Alligator 4 | 28.5 | Louisiana/W | 9–12 years | Sexually mature | Head/PMMA | ||
| MOR OST 1795 | Alligator 5 | 35.5 | Louisiana/W | 10 years if male | Sexually mature if male | Dry skull/Epoxy | ||
| 21–23 years if female | Skeletally mature if female | |||||||
| MOR OST 1789 | Alligator 6 | 42.6 | Louisiana/W | 15 years (male) | Sexually mature | Dry skull/Epoxy |
The same articulations were systematically chosen and sectioned in each extant specimens: (i) the fronto‐parietal suture (cranial); (ii) the internasal suture (facial); and (iii) the basioccipital‐exoccipital synchondrosis (skull‐base). Ages and ontogenetic categories were estimated based on skull length and on the literature available on the growth trajectories of these species (for the emu, Minnaar & Minnaar, 1992; Davies, 2002; and for the American alligators, Chabreck & Joanen, 1979; Woodward et al. 1995). Some of these specimens were previously analyzed morphologically and histologically, and placed into these different ontogenetic categories in another study (Bailleul et al. in press).
D, domestic; W, wild.
Age data (and sex data in one case only) were known. Sexual dimorphism was taken into account when assessing the age/categories of American alligators. Note that Alligator 6 can only be a male (based on Chabreck & Joanen, 1979).
Table 2.
List of the fossil specimens and the articulations sectioned in the present study
| Taxon | Genus | Geological formation | Specimen number | Estimated ontogenetic stage | Sutures/synchondroses |
|---|---|---|---|---|---|
| Crocodylia | cf Brachychampsa | Two medicine | MOR 552 | ? | Fronto‐postorbital (cranial) |
| Basioccipital‐exoccipital (cranial base) | |||||
| Basisphenoid‐basioccipital (cranial Base) | |||||
| Hadrosauria | Brachylophosaurus | Judith River | MOR 1071 | Juvenile | Fronto‐parietal (cranial) |
| Hypacrosaurus | Two medicine | MOR 548a | Hatchling | ||
| MOR 548 | Hatchling | Supraoccipital‐exoccipital (cranial base) | |||
| Gryposaurus | Two medicine | MOR 553Ma | Adult‐not fully grown | Fronto‐parietal (cranial) | |
| Judith River | MOR 2573 | Adult‐not fully grown | |||
| Prosaurolophus | Two medicine | MOR 447a | Sub‐adult | Basisphenoid‐basioccipital (cranial base) | |
| Ceratopsia | Triceratops | Hell Creek | MOR 2587 | Sub‐adult | Internasal (facial) |
| Nasal‐premaxilla (facial) | |||||
| MOR 8661 | At least an adult‐not fully grown | Interpremaxilla (facial) | |||
| MOR 2570 | Young adult | Jugal‐epijugal (facial) | |||
| UCMP 174838 | At least an adult‐not fully grown | Jugal‐quadratojugal (facial) | |||
| MOR 1110 | Juvenile | Basioccipital‐exoccipital (cranial base) | |||
| MOR 8657 | At least sub‐adult (young adult?) | ||||
| MOR 8658 | At least sub‐adult | ||||
| MOR 8659 | At least a juvenile | Basisphenoid‐basioccipital (cranial base) | |||
| Pachycephalosauria | Stegoceras | Judith River | UCMP 130049 | Juvenile | Fronto‐parietal (cranial) |
| Pachycephalosauridae indet. | Judith River | MOR 2555a | ? | ||
| Pachycephalosaurus | Hell Creek | UCMP 128383 | Juvenile | ||
| Pachycephalosauridae indet. | Judith River | TMP 1974.10.74 | ? | ||
| Hell Creek | MOR 2915 | ? | Parietal‐postorbital (cranial) | ||
| Hell Creek | UCMP 154919 | ? | Interfrontal (cranial) | ||
| Pachycephalosaurus | Hell Creek | VRD 13 | Adult‐not fully grown | Parietal‐postorbital (cranial) |
The petrographic thin‐sections that show diagenetically altered structures and, for this reason, they are not shown in the present study.
This study also documents how sutures and synchondroses with different morphological degrees of closure appear at the microscopic scale, because external morphology and histology can often show contradicting results (for example, closure may be evident morphologically but fusion might not be present at the histological scale; Brochu, 1996; Herring, 2000; Cole et al. 2003). It is tested whether archosaurian articulations that are morphologically established as obliterated are obliterated histologically as well, and vice versa, if articulations that are considered ‘open’ morphologically are also open histologically. This part of the investigation will be meaningful for the re‐evaluation of the method of maturity assessment using sutures in archosaur paleontology (see Bailleul et al. in press for further discussion on the matter). To frame methodology and analyses, a brief review of the basic biology and histology of sutures and synchondroses is necessary.
Biology and histology of sutures
Human anatomy textbooks and neontologists describe ‘sutures’ as the fibrous connective tissues between two membrane bones (Fig. 1; Kokich, 1986; Herring, 2000; Marieb, 2014). The borders of the bone in contact with a suture have been described by various terms, such as ‘juxta‐sutural bone’ (Enlow, 1990), ‘sutural bony margins’ (Kokich, 1986) or ‘sutural bone fronts’ (Opperman, 2000). In the present study, they are called sutural bone or sutural mineralized tissues (Fig. 1). It is common in paleontological studies to use a more global definition for the term ‘suture’, which often includes the cartilaginous articulations between endochondral bones (e.g. the neurocentral ‘sutures’ of the vertebrae, the ‘sutures’ of the skull‐base, those between the scapula and coracoid, or even in the epiphyses of long bones; see Cole et al. 2003). The proper biological and medical term for these cartilaginous articulations is ‘synchondroses’ (Fig. 2; in the skull‐base and vertebrae) and ‘epiphyseal growth plates’ (in limb bones). It is important that the distinction between these two terms be maintained because the embryological origins and histologies of these articulations are completely different.
Figure 1.
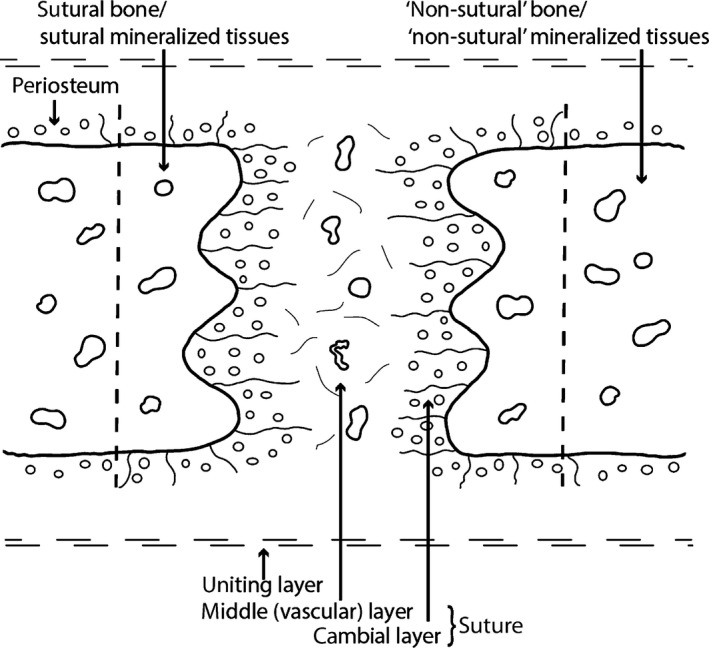
Schematic representation of a mammalian suture. Sutures are composed of a cambial layer with numerous osteoblasts and a vascular middle layer (non‐osteogenic). The sutural cambial layer is continuous with the periosteum of the bones. In the present study, the mineralized tissues directly bordering the sutures are referred to as sutural bone or sutural mineralized tissues. The mineralized tissues that are more distant from the sutural borders are referred to as ‘non‐sutural’ bone (or ‘non‐sutural’ mineralized tissues). Skull bones and their sutures are united together by the uniting layers, dense regular connective tissues mostly composed of collagen fibers. This figure is a re‐interpretation of the drawings of Pritchard et al. (1956) and Persson (1973).
Figure 2.
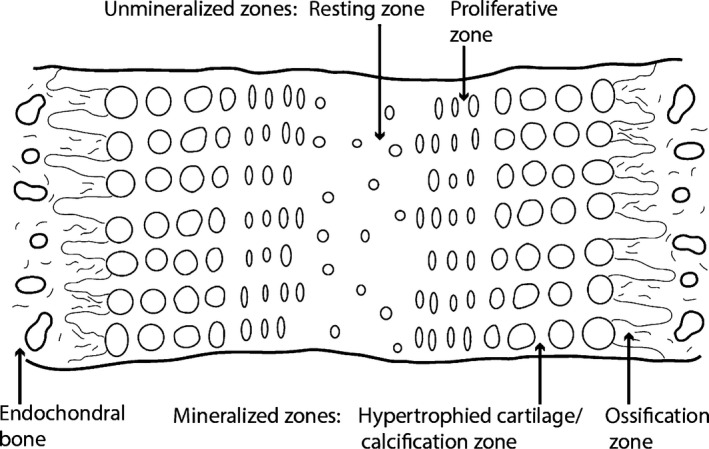
Schematic representation of a mammalian synchondrosis. Synchondroses are composed of different cellular zones: once central resting zone and mirroring proliferative, calcification and ossification zones. Due to this back‐to‐back organization, synchondroseal growth is bidirectional. The resting and proliferative zones are unmineralized (formed of hyaline cartilage), and the calcification/hypertrophied cartilage and ossification zones are mineralized. Endochondral bone is present on the two borders of synchondroses. This figure is a re‐interpretation of a schematic representation in Opperman et al. (2005).
While sutures are patent, they function as compensatory growth sites during craniofacial development (Opperman, 2000). They allow bone formation at the edges of the sutural bone fronts in response to their soft‐tissue environment, which is mostly the expanding brain in mammals (Kokich, 1986). Bone formation (and resorption) at the sutural margins is not a primary growth force of the skull, but it is secondary as it occurs as a response (also known as an adaptive force; Hall, 2005). The preservation of the overall shape and dimensions of bones during the growth of the skull (at least in mammals) is maintained by remodeling apposition and resorption of the periosteal and endosteal surfaces (Enlow, 1990). Sutural obliteration occurs by fusion of the bone fronts across the suture (Opperman, 2000); however, obliteration is optional as sutures often never fuse throughout the ontogeny of many species (e.g. those of some carnivoran mammals; Goswami et al. 2013; some ruminants; Bärmann & Sánchez‐Villagra, 2012; rhynchocephalians and lizards; Evans, 2008; Jones et al. 2011; snakes; Herring, 2000; or American alligators; Bailleul et al. in press). After fusion, the ‘suture’ is called a synostosis (Marieb, 2014). It is commonly assumed that sutural obliteration marks the cessation of any growth, but this assumption is incorrect, growth is still possible after fusion of cranio‐facial sutures (Cohen, 1993, 2000). Despite this, the relationship between closure (seen morphologically), fusion (seen histologically) and the overall growth of the skull remains ambiguous and is undoubtedly not simple. Studies concerning the precise microscopic structure of cranio‐facial sutures through ontogeny remain rare and exclusively concern humans (Kokich, 1976; Persson & Thilander, 1977) and other mammals (mostly rats and rabbits; Pritchard et al. 1956; Moss, 1958; Persson, 1973).
The fibrous tissues in sutures are known as the sutural ligament. The major differences between facial and cranial sutures are that the former originate from the mesenchyme, while the latter differentiate in a preformed fibrous membrane (the ectomeninx) surrounding the brain (Pritchard et al. 1956). Sutures are composed of: (i) the periosteum of the two separated bones (each having a cambial layer containing collagenous fibers, osteoprogenitor cells and osteoblasts, and a rarely observed capsular layer composed mostly of collagenous fibers, small blood vessels and fibroblasts); and (ii) a middle layer that is non‐osteogenic and composed mostly of collagens fibers, mesenchymal cells and fibroblasts (Fig. 1; Pritchard et al. 1956). They are encapsulated in dense fibrous connective tissues called uniting layers (Fig. 1; Pritchard et al. 1956). Some authors have, however, noted that the differentiation between all these sutural layers is sometimes difficult, and that it is not uncommon to observe only one single osteogenic layer throughout the whole suture (Persson, 1973, see Fig. 1, p. 10 to compare all the sutural layers reported by different authors).
The middle layer is first called the ‘presumptive suture mesenchyme’, and then differentiates into a ‘suture’ proper, more fibrous and more cellular (Opperman, 2000). The periosteum is continuous with the ectomeninx (preformed in cranial sutures, but formed secondarily in facial sutures; Pritchard et al. 1956). Once cranial and facial sutures are fully formed, it is thought that they grow in the same way. However, recent studies suspect that the different tissues underlying these two types of sutures (i.e. cartilage of the nasal capsules in the face vs. the dura mater in the calvaria) might play different roles during their growth; Opperman, 2000).
Sutural bone is usually woven early in development, but is resorbed and replaced by lamellar bone later during ontogeny (Pritchard et al. 1956). Sutures have three distinct shapes: flat (or straight), interdigitated and beveled (or overlapping) (Herring, 1972, 2000; but see alternative names reviewed in Jones et al. 2011). Through ontogeny it is common for sutures to start flat and to develop increasing degrees of interdigitations with age, with high degrees of interdigitations seen in locations that are heavily loaded (Herring, 2000). Interdigitations seem to be associated with compression and flat sutures with tensile stresses (Herring, 1972, Fig. 10, p. 238; Herring & Mucci, 1991; Rafferty & Herring, 1999; Sun et al. 2004; Markey et al. 2006). After the bone fronts fuse, interdigitations disappear, and the suture is often replaced by bone marrow and blood vessels (Pritchard et al. 1956). The mode of sutural fusion (i.e. interpreted from the type of bone/mineralized tissue bridging the suture) is thought to be mostly made via ‘normal’ intramembranous ossification (Pritchard et al. 1956; Kokich, 1976; Persson & Thilander, 1977; Hinton et al. 1984) or via chondroid bone formation, an intermediate tissue between bone and cartilage (Goret‐Nicaise et al. 1984, 1988; Manzanares et al. 1988; Rafferty & Herring, 1999). The latter has been reported as the ‘major vector of sutural growth’ during early development (i.e. embryonic and early postnatal), but it is resorbed rapidly a few months after birth (Goret‐Nicaise et al. 1988).
A type of cartilaginous tissue, called secondary cartilage, is sometimes found in the sutures of mammals. It arises after bone formation on membrane bones, and is restricted to fetal and/or juvenile specimens (Persson, 1973; Kokich, 1986; Vinkka‐Puhakka, 1991). By definition, secondary cartilage cannot be found in synchondroses (contra Ikejiri, 2012) because the latter form in between endochondral bones, not membrane bones. In birds (Hall, 1967, 1968) and non‐avian dinosaurs (Bailleul et al. 2012, 2013), it has been found at many jaw articulations, but not in the sutures per se. This suggests that it would be unlikely to find secondary cartilage in the specimens analyzed here (especially in specimens of relatively late ontogenetic stages; Tables 1 and 2).
Biology and histology of synchondroses
Unlike sutures, synchondroses are cartilaginous and have an intrinsic growth potential (recall that bone growth occurs in response to extrinsic factors in sutures; Opperman et al. 2005). At the histological level, synchondroses are unique: they can be described as two epiphyseal growth plates positioned back to back with one common resting zone and mirroring proliferative zones (where cellular divisions occur), hypertrophied cartilage/calcification zones and ossification zones (Fig. 2; Opperman et al. 2005; Cendekiawan et al. 2010). As a consequence of these mirroring layers, synchondroseal growth is bidirectional (Fig. 2), as opposed to that of an epiphyseal growth plate that is unidirectional (Barreto et al. 1993). Unfortunately, even in mammals, little is known about the biology and histology of synchondroses (in both the cranial base and the vertebrae). Some authors argued that this is due to the common assumption that synchondroses function like the growth plates of long bones (Dixon & Gakunga, 1993; Opperman et al. 2005). Nevertheless, it has been shown in the fetal rat that synchondroses start out as ‘presumptive synchondroses’ composed of undifferentiated mesenchymal cells (Dorenbos, 1973). At this time the adjacent cartilaginous models of some endochondral elements of the braincase are already formed (i.e. chondrocytes are already present in the basioccipital, but not in the presumptive basioccipital‐basisphenoid synchondrosis; Dorenbos, 1973). Only at the end of embryonic development are the different cell layers of the synchondroses differentiated into chondrocytes and organized, and only then can this union be called a true synchondrosis (Dorenbos, 1973). The postnatal ontogeny of the spheno‐occipital synchondrosis of humans has also been studied (Thilander & Ingervall, 1973). Obliteration of this synchondrosis is made via endochondral ossification. The hyaline cartilage of the synchondrosis can also be replaced by fibro‐cartilage in some instances (Thilander & Ingervall, 1973).
Materials and methods
Specimens
The extant archosaurs consist of two growth series of six skulls each: of the emu, D. novaehollandiae, and the American alligator, A. mississippiensis (Table 1). All of the skulls examined are from the osteology collections (OST) of the Museum of the Rockies (MOR) and all were originally obtained from animals that had died of natural causes. Five of the emu specimens came from the Montana Emu Ranch (MER, Kalispell, Montana). MOR OST 232 had been previously purchased online from a legal commercial website (Skulls Unlimited). Fleshy heads from MER were sent frozen to the MOR and then defleshed by a dermestid beetles colony (Deer Lodge, Montana). MOR OST 1801 was not defleshed and its soft‐tissues were included in the histological slides. The estimated age of these specimens is shown in Table 2. Only three specimens had a precise known age (MOR OST 1801, MOR OST 1802 and MOR OST 1803) provided directly by the emu rancher (D. Collis, MER). Specimens were placed into ontogenetic categories according to data available in the literature about the growth of this species (Minnaar & Minnaar, 1992; Davies, 2002). Some of these specimens had previously been examined morphologically for another study (Bailleul et al. in press).
Three of the American alligator skulls (MOR OST 1796, MOR OST 1795, MOR OST 1789) previously defleshed by dermestid beetles were also purchased online from legal commercial websites (Skulls Unlimited and Atlantic Coral Enterprise). The other three alligator heads were from the Rockefeller Wildlife Refuge (Louisiana), and their soft‐tissues were kept for histological analyses (MOR OST 1647, MOR OST 1797, MOR OST 1798). Their age (and estimated ontogenetic categories) were calculated/evaluated by using data previously published on the growth curve of this species, and using their geographic provenance and their skull length as a proxy (Chabreck & Joanen, 1979; Woodward et al. 1995). Note that all these emu and alligator skulls (not the heads) were prepared by dermestid beetles, because this is thought to be the best method to preserve bony articulations.
Most of the fossil specimens came from the collections of the MOR, and had been previously collected during various field seasons in Montana (Table 2). Some specimens belong to the University of California Museum of Paleontology (UCMP), the Tyrell Museum of Paleontology (TMP) and Sierra College in California (VRD). Fossil taxa include members of the Crocodylia and Ornithischia (Hadrosauridae, Ceratopsidae, Pachycephalosauridae). Their estimated ontogenetic stage is also shown (E. Freedman‐Fowler and J. Scannella, personal communications; J. Horner, personal observation). They were all isolated bony elements containing sutures and do not come from articulated skulls.
Histological and paleohistological preparation
The same three sutures were systematically chosen and sectioned in the extant specimens. These include the fronto‐parietal suture (cranial), the internasal suture (facial) and the exoccipital‐basioccipital synchondrosis (in the skull‐base). The authors are aware that, to be completely accurate, only homologous articulations should be compared between extant and fossil specimens. However, in some fossil specimens, it was not possible to cut these specific, homologous sutures and, therefore, the most adjacent sutures were sectioned (e.g. jugal‐epijugal and interfrontal sutures; Table 2). Note, however, that when non‐homologous articulations were compared, they always belonged to the same anatomical region (i.e. facial, cranial or skull‐base complex). All ground sections were undecalcified and have a final thickness of 80–120 μm (ground sections of the extant specimens being generally thinner than the fossils, between 80 and 100 μm).
Preparation of extant specimens
Small fragments of bone possessing the sutures/synchondroses of interest were extracted from previously defleshed skulls using a dremel and a diamond blade. These fragments were then fixed in 10% buffered formalin solution for 3–4 days (with one solution change per day), then transferred to a solution of 70% ethanol (EtOH) and serially dehydrated in graded solutions of 80%, 95% and finally 100% EtOH. The time spent in each of these solutions varied from 24 h to 3 days depending on the size of the extracted fragments, but specimens were never in 100% EtOH for more than 24–48 h (according to methods suggested by A. Lee, personal communication). The cranial and facial fragments of alligator bone took considerably longer than any of the emu fragments.
When the fleshy heads were relatively small, they were entirely fixed in formalin, and the bony fragments with their associated soft‐tissues were extracted and transferred to EtOH separately (MOR OST 1801 and MOR OST 1797). One fleshy head was too large to be fixed entirely in formalin, so the fragments were extracted from it while it was still frozen (MOR OST 1798). The head was put back into the freezer after extraction. All the bony fragments that have associated soft‐tissues (i.e. from the fleshy heads) were embedded in poly‐methyl‐methacrylate (PMMA) because this resin allows better visualization than that of the resins generally used for paleohistological embedding (e.g. epoxy or polyester resins; A. Lee and E.‐T. Lamm, personal communications). Almost all the bony fragments coming from dry skulls were embedded in epoxy resin in a vacuum chamber for 5 min (Epothin 2™, Buehler™; see the embedding media of each specimen in Table 1). Prior to epoxy embedding, fragments were cleared in xylene following dehydration. After 2 days (time for the resin to set), they were treated in the same manner as small fossils (according to the techniques established for small fossil thin‐sectioning by Lamm, 2013, see following paragraph). Two sections were made for each suture/synchondrosis, leaving one slide unstained and the other one stained with toluidine‐blue. Three of those unstained slides were stained with Masson's trichrome. Slides coming from frozen heads gave more details about the unmineralized parts of the sutures and synchondroses than those of the skulls defleshed by dermestid beetles, which showed some soft‐tissue degradation. However, the latter slides still showed well‐preserved structures at the bony sutural borders (and even in the sutures and synchondroses in some instances).
Preparation of fossil specimens
Molds and casts were made of all the bones before sectioning to retain morphological data. A few important casts were painted and restored into the real bone. Bones were embedded in either polyester (for large specimens) or epoxy resin (for small specimens, with Epothin 2™, Buehler™). Small specimens were sectioned with a Norton 5″ or 7″ diamond‐edge blade on an Isomet precision saw (Buehler™). Thick‐sections (between 1.0 and 1.3 mm) were mounted on plastic (Plexiglas) slides with cyanoacrylate glue. Large specimens were sectioned with a tile saw, and the thick‐sections (from 2.5 to 3.5 mm) were mounted on glass slides with 2‐ton epoxy resin (on the side previously frosted with aluminum powder). All mounted thick‐sections were then ground by hand on a Buehler Ecomet™ grinder with water and silicon carbide paper of decreasing grit sizes: 60, 120, 180, 320, 400, 600 and 800. For the small slides, the rougher papers (60, 120 and 180) were not used. Sections were then polished on the grinder with 800 grit paper and 5 μm aluminum oxide powder (with the paper slightly wet). Finally, they were polished by hand with wet polishing cloths and more aluminum oxide powder (5 μm then 1 μm). Finished petrographic thin‐sections were studied by light microscopy under normal and polarized light with a Nikon Optiphot‐Pol polarizing microscope. Photographs were taken with a Nikon DS‐Fi1 digital sight camera and the nis elements br 4.13 software. Whole slide images (of the fossils only) were digitized on a Canon Canoscan 8800F flatbed scanner. One picture (Fig. 4H) was taken with a Jenoptik ProgRes C14Plus camera, with the software progres capture pro on a Zeiss Axio Scope A1. Note that many of the petrographic thin‐sections of the pachycephalosaurs were already made and published in Goodwin & Horner (2004) and Horner & Goodwin (2009).
In the following section, both unmineralized soft‐tissues (in the extant specimens only) and mineralized tissues (in the extant and fossil specimens) were described. Soft‐tissues were not preserved in the fossils, and the original structures are filled in with sediments, minerals and/or broken fragments of bone. The soft‐tissues that are described include: (i) the sutures; and (ii) the unmineralized parts of the synchondroses (Figs 1 and 2). Descriptions of these structures are made in the extant specimens who were embedded with their soft‐tissues and are avoided in the dry skulls (due to some soft‐tissue deterioration, unless preservation was exceptional or the dry specimens showed very important features). The extant specimens will not be designated by their MOR OST numbers, but as Emu 1–6 and Alligator 1–6 (Table 1). The described mineralized tissues include: (i) the sutural mineralized tissues; and (ii) the mineralized parts of the synchondroses (Figs 1 and 2). Emphasis will not be put on the non‐sutural mineralized tissues, or on those bordering the edges of the synchondroses unless they show unique characteristics.
Results
Cranial and facial sutures
Extant archosaurs
Cranial sutures
Figure 3 shows selected specimens of D. novaehollandiae stained with toluidine‐blue (Emu 1, Fig. 3A; Emu 3, Fig. 3B,D,E; Emu 6, Fig. 3C,F). The suture is beveled and does not form any interdigitations throughout ontogeny. In Emu 1, the sutural borders are composed of woven bone with many simple vascular canals typical of an actively growing bone (Fig. 3A). Lamellar bone starts to form at the stage of Emu 3 and 4 (8–10 months), and the suture is still completely open during this stage (Fig. 3B). In Emu 5 and 6, the fronto‐parietal suture is completely obliterated, and the elements are composed of trabeculae of lamellar bone (Fig. 3C). No osteonal tissue is present even in these late stages of ontogeny. The suture is replaced by bony trabeculae and bone marrow spaces (Fig. 3F). In Emu 3, the suture is encapsulated in a uniting layer composed of a dense regular connective tissue (Fig. 3D, double arrows). A periosteal cambial layer with numerous osteoblasts can be seen right next to the sutural bone (Fig. 3E, double arrows), and it appears to be continuous with the periosteum located on the ectocranial side of this element (Fig. 3E). The suture is also composed of a middle layer that is less cellular (Fig. 3E, white asterisk). Pritchard et al. (1956) reported a periosteal capsular layer surrounding the cambial layer in the sutures of some mammals, but it is not observed here in the emu or in any other suture of this sample.
Figure 3.

Parasagittal sections of the fronto‐parietal suture of emus stained with toluidine‐blue. (A) Fronto‐parietal suture of Emu 1. (B) Fronto‐parietal suture of Emu 3. (C) Whole section of the fronto‐parietal suture of Emu 6. (D) Close‐up of the red box in (B). The double black arrows show the uniting layer. Bone and cell nuclei are stained deep blue. (E) Close‐up of the red box in (D), showing the suture divided into two cambial layers (double black arrows) and one middle layer (white asterisk). The cambial layers are continuous with the periosteum. (F) Close‐up of the red box in (C), showing trabeculae of lamellar bone. Images (A–C) are modified from Bailleul et al. (in press). Image (A) was reversed for consistent orientation. P, periosteum; Ro, rostral.
The fronto‐parietal suture in A. mississippiensis has a very different histology (Fig. 4). Selected specimens stained with toluidine‐blue and Masson's trichrome are shown: Alligator 1 (Fig. 4A); Alligator 3 (Fig. 4B,D,E); and Alligator 6 (Fig. 4C,F–K). This suture stays open during ontogeny in the American alligator (i.e. the sutural mineralized tissues never meet; Fig. 4). Through ontogeny, interdigitations increase in number and in size (Type‐A interdigitations in Jones et al. 2011), and the sutural mineralized tissues become much more fibrous (Fig. 4A–C). Alligator 1 shows two thin struts of woven bone (the frontal on the top and the parietal on the bottom; Fig. 4A). In the suture, a loose irregular connective tissue can be seen (Fig. 4A), and it is not comparable to the dense fibrous tissue that was observed in the emu (Fig. 3). Perhaps this loose connective tissue represents a presumptive suture (i.e. an incompletely formed suture). In Alligator 3 and 4, the sutural bone is still woven with very little vascularization, but the non‐sutural bone has a few primary and secondary osteons. The suture is now formed of a dense connective tissue (Fig. 4D,E; asterisks). Only one uniform layer can be discerned in the suture, with collagenous fibers and a few fibroblasts (see the elongated cell nuclei in blue; Fig. 4D,E, black asterisks). Unlike the sutures of emus (Fig. 3E), the crocodilian sutures do not show any periosteal cambial layer. In some areas, fibers are thick and continuous between the sutural bone and the suture itself (Fig. 4E, arrow). In the most mature alligators (Alligator 5 and 6), two important features can be seen: (i) a very high fibrous content (Fig. 4C); and (ii) an enigmatic sutural mineralized tissue (Fig. 4F). Due to the absence of periosteum in the sutures and the high fibrous content of the sutural mineralized tissues, it is difficult to characterize the fronto‐parietal bones of these specimens as ‘regular’ intramembranous bone. Toluidine‐blue reveals this enigmatic sutural mineralized tissue with a light purple stain that clearly demarcates it from the darker woven bone (Fig. 4F,G). It also possesses simple vascular canals (Fig. 4F, arrows). At higher magnification, the entire tissue lacks the characteristics of regular bone (Fig. 4G). The vascular canal has irregular internal borders, and the tissues surrounding this canal can be differentiated into cellular, acellular, fibrous and non‐fibrous zones. The cellular zones show cellular lacunae resembling plump osteocytes with canaliculi. At higher magnification (Fig. 4H), acellular zones have globular structures with arc‐shaped borders (black arrows) and dark spaces of many different shapes (yellow arrows). While these dark spaces could potentially be cellular lacunae, they do not have the appearance of regular osteocyte lacunae. In an adjacent section of the same suture, Masson's trichrome reveals that this tissue is present almost everywhere along the sutural borders (Fig. 4I; black arrows). It does not stain like the core of the interdigitations (see the clear junctions labeled with white arrows). Higher magnification reveals that this tissue stains with blue and red mottles (Fig. 4J), while the core of the interdigitations only stain red. Unfortunately the authors have not been able to identify the meaning of these differences in staining. In rare instances, some Howship's lacunae can be seen that indicate that it is a mineralized tissue (Fig. 4K).
Figure 4.
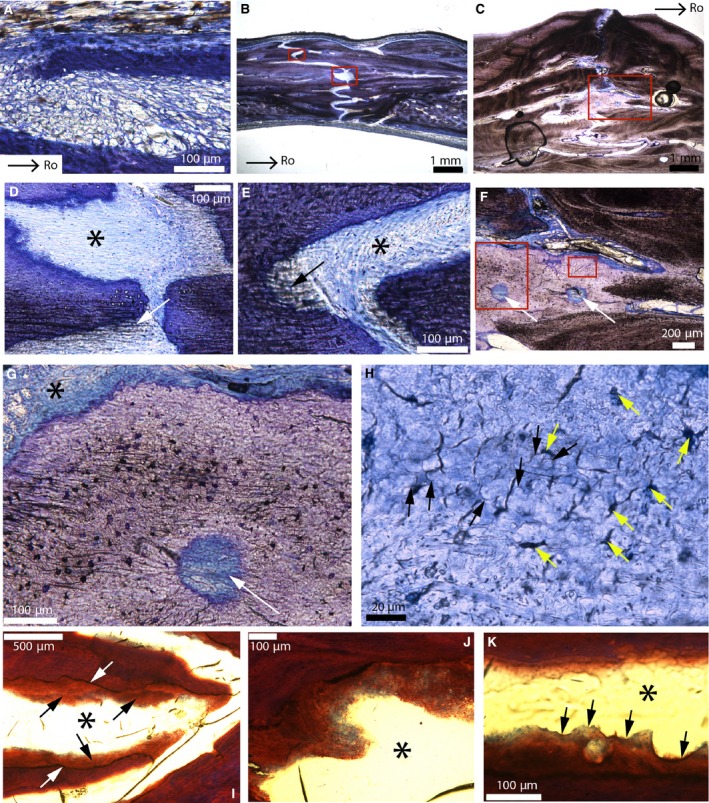
Parasagittal sections of the fronto‐parietal suture of American alligators stained with toluidine‐blue (A–H) and Masson's trichrome (I–K). (A) Fronto‐parietal suture of Alligator 1. It appears to be formed of mesenchyme. (B) Fronto‐parietal suture of Alligator 3. (C) Ectocranial part of the fronto‐parietal suture of Alligator 6. (D) Close‐up of the lower red box in (B). Thick collagen fiber bundles are continuous between the bone and the suture (white arrow). The black asterisk shows a uniform dense fibrous tissue (with blue fibroblast nuclei). This suture lacks a periosteum. (E) Close‐up of the upper red box in (B). Once more, thick collagenous fibers can be seen (black arrow), as well as a uniform sutural layer (black asterisk). (F) Close‐up of the red box in (C). A light purple tissue is found right at the sutural borders. It possesses two vascular canals (white arrows). (G) Close‐up of the right red box in (F), showing a vascular canal (white arrow) bordered by different zones: cellular, acellular, fibrous and non‐fibrous. The cellular zones show cellular lacunae resembling plump osteocytes with canaliculi. (H) Close‐up of the left red box in (F). In areas that appear acellular, this tissue shows globular structures (black arrows) and dark spaces of various shapes (yellow arrows). These spaces do not have the appearance of regular osteocyte lacunae and they show no evidence of canaliculi. (I) Adjacent section of this same suture in Alligator 6 (black asterisk) stained with Masson's trichrome. Interdigitations are ‘covered’ by this same tissue (black arrows). The core of the interdigitations does not stain like this tissue (see the clear limits indicated by the white arrows). (J) Close‐up of this tissue from another area in this same section. It shows shades of blue and red, while the core of the interdigitations only stains red. The suture is indicated by the black asterisk. (K) Another area in this section shows that this tissue is mineralized as it shows Howship's lacunae (black arrows). The suture is indicated by the black asterisk. Images (A and B) are modified from Bailleul et al. (in press). Images (C, F–H) were reversed for consistent orientation. Abbreviations: same as previous figure.
Facial sutures
The internasal suture of the emu stained with toluidine‐blue is shown in Fig. 5 (Emu 3, Fig. 5A,C–E and Emu 6, Fig. 5B). The cartilage of the nasal capsule is stained purple (Fig. 5A). As opposed to the fronto‐parietal suture of the same species, this suture never fuses during ontogeny (i.e. it is still open in Emu 6, a 20 year old male; Fig. 5B). Through ontogeny, both the sutural bone and the non‐sutural bone transition from being woven with simple vascular canals (Fig. 5C,D) to a compacta completely reworked with secondary osteons (Fig. 5B). The internasal suture is composed of different layers: (i) a thick cambial layer with numerous osteoblasts, continuous with the periosteum located more ectocranially (see the nuclei stained in blue, double white arrows); and (ii) a middle layer (white asterisk). The latter is a vascularized loose irregular connective tissue (see veins and arteries, black arrows; Fig. 5C). A mineralized tissue with cartilage‐like cell lacunae embedded in a bone matrix was seen in the sutural areas of Emu 3 (Fig. 5E). It contains the features of chondroid bone, a tissue that has already been reported in the sutural areas of the chick, some mammals and a hadrosaur (Bailleul et al. 2015).
Figure 5.

Cross‐sections of the internasal and nasal‐premaxilla sutures of emus stained with toluidine‐blue. (A) Internasal and nasal‐premaxilla sutures of Emu 3. The bone stains deep blue and the cartilage of the nasal capsules is purple. (B) Internasal and nasal‐premaxilla sutures of Emu 6. (C) Close‐up of the middle red box in (A). The suture is composed of a cambial layer (double white arrows) and a middle layer (white asterisk) formed of loose irregular connective tissues and some vessels (black arrows). (D) Close‐up of the right box in (A) showing an actively growing bone and the formation of vascular canals. (E) Close‐up of the left red box in (A), showing chondroid bone (up) and hyaline cartilage (down, in purple). Cells of chondroid bone are cartilage‐like (round) but are embedded in a bone matrix. Hyal. C, hyaline cartilage; Na, nasal; Na. caps. c., Nasal capsule cartilage; Pm, premaxilla.
The ontogeny of the internasal suture of the American alligator is shown in Fig. 6. It is shown in Alligator 1 (Fig. 6A), Alligator 3 (Fig. 6B,D,E), Alligator 4 (Fig. 6F) and Alligator 6 (Fig. 6C,G). It is extremely similar to that of the fronto‐parietal suture of this same species (Fig. 4). In Alligator 1, the suture is beveled and the sutural bone is composed of two small struts (Fig. 6A). The suture is composed of two layers: a dense connective tissue closest to the sutural bone; and a middle layer that looks similar to the loose connective tissue reported previously (Fig. 4A). It is unclear if the layer closest to the sutural bone represents a periosteum. Through ontogeny, the interdigitations increase in number and extent (Type‐A interdigitations in Jones et al. 2011), whereas the sutural bone becomes much more fibrous (Fig. 6B,C). In the suture of Alligator 3, the central loose connective tissue layer has disappeared and a uniform layer made of a dense fibrous connective tissue with fibroblasts can be seen (see the elongated cell nuclei stained in blue; Fig. 6D,E). Once again, this suture appears to lack a periosteum. The collagen fibers can be either parallel or perpendicular to the suture (Fig. 6D, black arrow and Fig. 6E). The ectocranial part of this suture in Alligator 4 is filled with numerous circular structures resembling nerves (Fig. 6F, black arrow). A neurovascular bundle is found within a depression on the ectocranial side of this same bone, and perhaps these nerves could be part of a neuronal network. In the most mature alligators, the sutural mineralized tissues are extremely fibrous and show dark lamellae that are parallel to the suture and separated from each other by brighter‐colored zones (Fig. 6G). This organization is similar to lamellar‐zone bone (Francillon‐Vieillot et al. 1990), but these brighter zones do not appear to be lines of arrested growth (LAGs).
Figure 6.
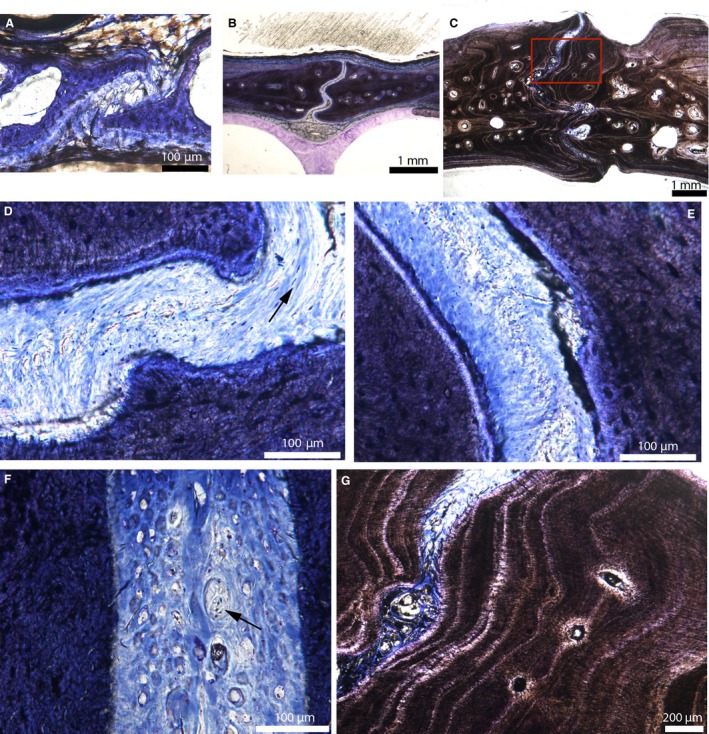
Cross‐sections of the internasal suture of American alligators stained with toluidine‐blue. (A) Internasal suture of Alligator 1. (B) Internasal suture of Alligator 3. (C) Internasal suture of Alligator 6. (D) Close‐up of the suture in (B), showing one uniform fibrous layer. Fibers can be oriented perpendicular or parallel to the sutural borders (black arrow). This suture lacks a periosteal cambial layer. (E) Close‐up of another area in the suture in (B), showing fibers that are perpendicular to the sutural borders. The suture is composed of a uniform dense fibrous connective tissue with fibroblast nuclei stained in blue. Again it lacks a periosteum. (F) Ectocranial part of the internasal suture of Alligator 4. The suture appears to be filled in with many nerves (black arrow). (G) Close‐up of the red box in (C). The sutural borders are composed of lamellae of fibrous bone, all parallel to the suture. This resembles lamellar‐zonal bone. Images (A and B) are modified from Bailleul et al. (in press).
Fossil archosaurs/non‐avian dinosaurs
Cranial sutures
Crocodilian
The fronto‐postorbital suture of a fossil crocodilian (MOR 552; cf Brachychampsa) is shown in Fig. 7 (red arrows). Like the fronto‐parietal and the internasal sutures of modern American alligators, this suture is open and interdigitated (Fig. 7A,B). The fibrous suture is not preserved and is filled in by minerals (Fig. 7B). As it was observed in the most mature alligators (Fig. 6G), the mineralized sutural tissues are composed of alternating lamellae resembling lamellar‐zonal bone (Francillon‐Vieillot et al. 1990; Fig. 7C). However, as it was observed in the modern alligators, the limit between each lamella does not appear to represent a complete arrest of growth, but only a slowing of growth (compare with Fig. 7E that shows clear LAGs, white arrows). Polarized light indicates that these lamellae are made of woven tissues (Fig. 7D). All of these empirical observations suggest a relatively rapid growth of the sutural mineralized tissues compared with non‐sutural tissues.
Figure 7.
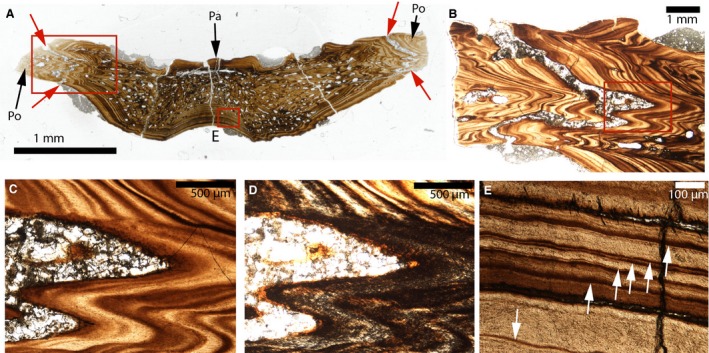
Paleohistological cross‐sections in the parietal‐postorbital sutures of a fossil crocodilian (cf Brachychampsa). (A) Whole cross‐section of the parietal‐postorbital bones of MOR 552. The ectocranial and endocranial parts of the parietal‐postorbital sutures are indicated by the red arrows. (B) Close‐up of the left red box in (A). (C) Close‐up of the red box in (B). The sutural borders are composed of bone lamellae that are all parallel to the suture. (D) Close‐up of the red box in (B) shown under polarized light. Polarized light indicates that this tissue is woven. (E) Close‐up of the right red box in (A), showing lamellar‐zonal bone. The white arrows indicate lines of arrested growth. Pa, parietal; Po, postorbital.
Hadrosaurs
Four hadrosaur fronto‐parietal bones were sectioned, but only two of them are shown here, a juvenile Brachylophosaurus (Fig. 8A–D), and an adult Gryposaurus (Fig. 8E–H), as the remainder were diagenetically altered too greatly to permit histological analysis (Table 2). In Brachylophosaurus (MOR 1071) and Gryposaurus (MOR 2573), the frontoparietal suture is completely open and filled in with sediment (Fig. 8C). It is interdigitated (Fig. 8A,E, red arrows), but the interdigitations are not as sharp as the ones present in the modern and fossil crocodilians described previously (Figs 4, 6 and 7). In Brachylophosaurus, part of the parietal‐postorbital suture can also be seen (black arrow). The sutural mineralized tissues found in these two specimens are identical: the sutural borders are composed of a tissue that looks extremely fibrous and cellular at low magnification (Fig. 8B,F). A compacta composed of secondary osteons can be seen bordering this tissue (Fig. 8B). The latter forms entire mineralized struts in the parietal‐postorbital suture of Brachylophosaurus (Fig. 8C). At higher magnification, this tissue shows globular structures (Fig. 8D,H, white arrows) separated from each other by dark spaces of different shapes, many of which are arcuate‐shaped (Fig. 8D,H, blue arrows). This organization is similar to that found in modern alligators (Fig. 4H).
Figure 8.
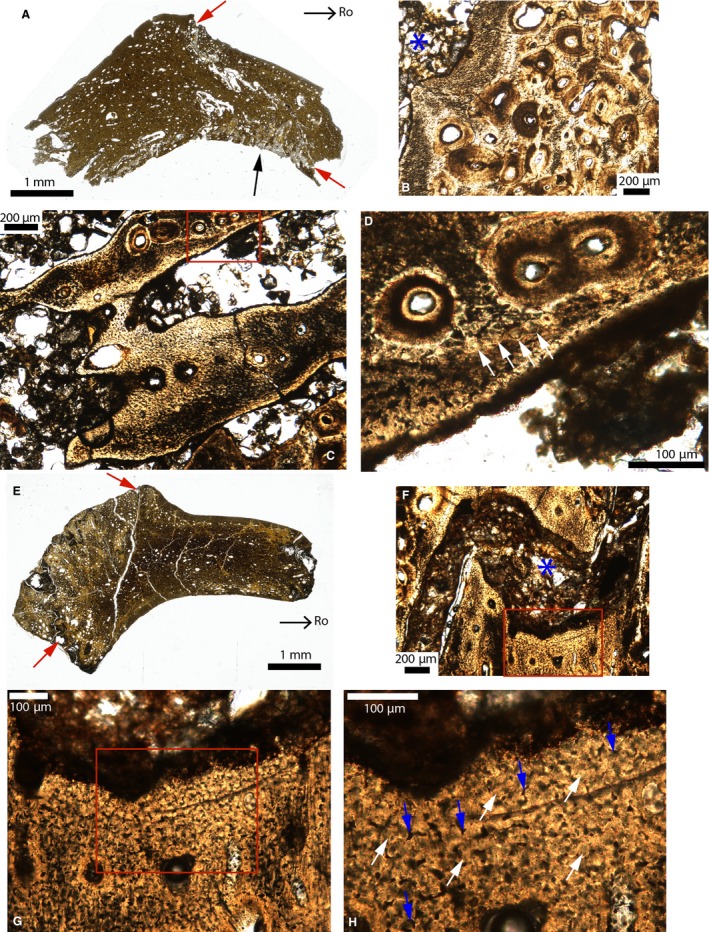
Paleohistological cross‐sections in the fronto‐parietal sutures of Brachylophosaurus (A–D) and Gryposaurus (E–H). (A) Whole section of MOR 1071. The red arrows indicate the ectocranial and endocranial sides of the fronto‐parietal suture. The parietal‐postorbital suture is indicated by the black arrow. (B) Close‐up of the fronto‐parietal suture in (A). The sutural borders are composed of a tissue that appears highly fibrous. Many secondary osteons are adjacent to this tissue. The suture is indicated by the blue asterisk. (C) Close‐up of the same suture in another area showing interdigitations entirely composed of this same fibrous tissue. (D) Close‐up of the red box in (C). This tissue shows some globular structures (white arrows). (E) Whole section of MOR 2573. The red arrows indicate the ectocranial and endocranial sides of the fronto‐parietal suture. (F) Close‐up of this suture (indicated by the blue asterisk). (G) Close‐up of the red box in (F). (H) Close‐up of the red box in (G), showing globular structures (white arrows) separated from each other by spaces of different shapes (including arcuate shapes; blue arrows). Images (E and F) were reversed for consistent orientation. Abbreviations: same as previous figures.
Pachycephalosaurs
The cranial sutures of some Pachycephalosauridae are shown in Figs 9 and 10, including the fronto‐parietal sutures of a juvenile Stegoceras (UCMP 130049; Fig. 9A,C,D), a juvenile Pachycephalosauridae indet (TMP 1974.10.74; Fig. 9B,E,F), the interfrontal suture in a Pachycephalosauridae indet (UCMP 154919; Fig. 9G–J), and the parietal‐postorbital sutures of a Pachycephalosauridae indet (MOR 2915; Fig. 10A–F) and Pachycephalosaurus (VRD 13; Fig. 10G–K). Two more specimens were sectioned, but they are not shown here because their histology is similar to other specimens (for UCMP 128383), or because they were diagenetically altered beyond reasonable limits for histological analysis (for MOR 2555). The two small fronto‐parietal domes show obliterated sutures on the ectocranial side, but open sutures on the endocranial side (Fig. 9A–C, red arrows). On the endocranial side the suture is slightly interdigitated (Fig. 9C), but ectocranially it is completely obliterated and replaced by a mineralized tissue displaying the characteristics of intramembranous bone (Fig. 9D). In TMP 1974.10.74, the majority of the mineralized sutural borders are composed of a highly fibrous tissue with cellular and acellular zones (Fig. 9E). This same tissue bridges the suture in some areas (Fig. 9F).
Figure 9.
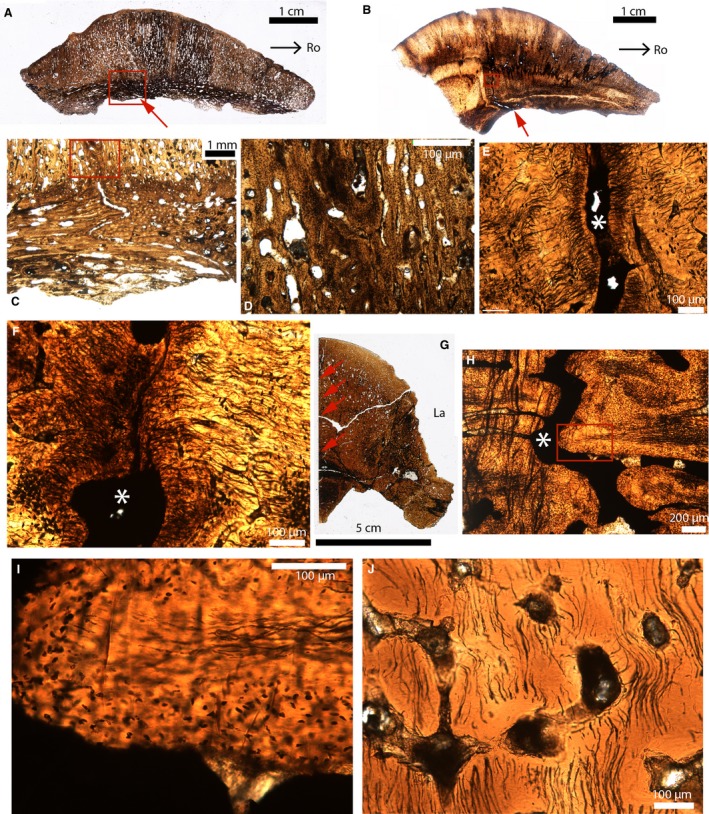
Paleohistological parasagittal sections (A–F) and cross‐sections (G–J) in the cranial domes of some pachycephalosaurids. (A) Whole section of the fronto‐parietal dome of Stegoceras (UCMP 130049). The endocranial part of the suture is indicated by the red arrow. The ectocranial part of this suture is obliterated. (B) Whole section of the fronto‐parietal dome of a Pachycephalosauridae indet (TMP 1974.10.74). The endocranial part of the suture is indicated by the red arrow. The ectocranial part of this suture is obliterated. (C) Close‐up of the red box in (A). (D) Close‐up of the red box in (C). (E) Close‐up of the red box in (B). The suture is indicated by the white asterisk. (F) Close‐up of another area in (B), showing an obliterated suture with two fusing fronts composed of a highly fibrous tissue. The white asterisk indicates the suture. (G) Whole section of the frontals of a Pachycephalosauridae indet (UCMP 154919). The interfrontal suture is indicated by the red arrows. (H) Close‐up of the interfrontal suture in (G). (I) Close‐up of the red box in (H). The interdigitation is composed of an acellular fibrous tissue in the center and a cellular tissue in the periphery. (J) Section showing acellular bone from a ‘non‐sutural’ area. Images (A–E) were reversed for consistent orientation. La, lateral; Ro, rostral.
Figure 10.
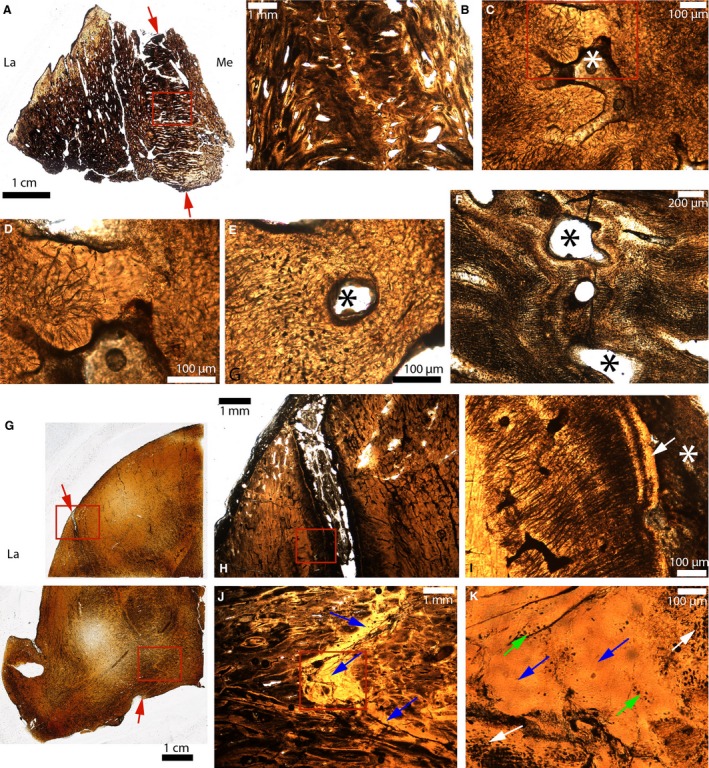
Paleohistological cross‐sections in the parietal‐postorbital sutures of some pachycephalosaurids. (A) Whole section of a broken dome fragment of a Pachycephalosauridae indet (MOR 2915). The ectocranial and endocranial sides of the parietal‐postorbital suture are indicated by the red arrows. (B) Close‐up of the red box in (A). (C) Close‐up of (B) showing an obliterated suture. The fused bony fronts are fibrous and acellular. The white asterisk indicates what might be bone marrow and/or vascularized spaces. (D) Close‐up of the red box in (C). (E) Close‐up of another obliterated area of this same suture. Here, the tissue looks fibrous and cellular. The black asterisk indicates what might be bone marrow and/or vascularized spaces. (F) Another area of this same suture where the sutural fronts are extremely fibrous and parallel to the sutural borders. The black asterisk indicates what might be bone marrow and/or vascularized spaces. (G) Whole sections of half of a Pachycephalosaurus dome (VRD 13). The ectocranial and endocranial sides of the parietal‐postorbital suture are indicated by the red arrows. (H) Close‐up of the upper red box in (G). The ectocranial part of this suture is open. (I) Close‐up of the red box in (H). The sutural borders are composed of an acellular tissue (white arrow). The suture is indicated by the white asterisk. (J) Close‐up of the lower red box in (G), showing an interdigitated suture completely obliterated by a bright yellow acellular tissue (blue arrows). (K) Close‐up of the red box in (J). At higher magnification, this tissue has zones with regular and numerous osteocyte lacunae (white arrows), zones with lacunae that are less numerous and less regular (green arrows) and acellular areas (blue arrows). La, lateral; Me, medial.
In the interfrontal suture of UCMP 154919 (Fig. 9G, red arrows), the sutural borders are slightly undulating (Fig. 9H). This suture is open and the sutural borders are formed of another type of fibrous tissue (compare Fig. 9E with Fig. 9H). At higher magnification one of these struts shows a central acellular core with fibers surrounded by a tissue containing numerous dark spaces. These dark spaces are too wide to be fibers cut in a transverse plane. They also do not look like ostecyte lacunae as they are irregularly shaped and do not present canaliculi. Two options are possible: they are the cellular lacunae of another cell type (e.g. fibroblasts or fibrocytes); or they are osteocyte lacunae that were mineralized and underwent ‘acellularization’. Acellularization of cellular bone has been described in teleosts, and this process involves osteocytes becoming pyknotic (or necrotic) and subsequently mineralizing by ‘intracellular calcification’ (Moss, 1961, 1963; Meunier, 1989). The fact that these cellular lacunae: (i) have different sizes and shapes; and (ii) that some tissues are completely acellular in many ‘non‐sutural’ areas (Fig. 9J; Goodwin & Horner, 2004) suggest that a similar acellularization process is occurring here. Perhaps newly formed sutural mineralized tissues are cellular but they undergo intracellular calcification later during ontogeny (i.e. once relocated to the ‘non‐sutural’ areas; Fig. 9J).
The parietal‐postorbital suture of MOR 2915 is straight (red arrows, Fig. 10A). The mineralized struts composing the sutural borders have a different orientation to that of the non‐sutural mineralized tissues (i.e. they are perpendicular to the suture; Fig. 10A). Higher magnification shows that this suture is obliterated and replaced by vascular and/or marrow spaces (Fig. 10B,C). Three different types of tissues bridge the suture in this specimen: an acellular fibrous tissue (Fig. 10C,D); a cellular fibrous tissue (Fig. 10E, similar to that present in Fig. 9E,I); and an extremely fibrous tissue (Fig. 9F). The organization of this last tissue (i.e. with bony lamellae parallel to the suture) looks rather similar to that found in the most mature alligator (Fig. 6G) and fossil crocodile (Fig. 7C) specimens.
In the adult Pachycephalosaurus (VRD‐13), the parietal‐postorbital suture is open on the ectocranial side and closed on the endocranial side (Fig. 10G,H,J). It is straight ectocranially but becomes slightly interdigitated endocranially (Fig. 10G). On the ectocranial side, the mineralized sutural borders are formed of bright acellular bands directly bordering the suture (Fig. 10I, arrow) and a fibrous tissue more internally (Fig. 10I). These fibers are similar to those described above (Figs 9E,F and 10F). On the endocranial side, the suture is synostosed and replaced by a bright acellular tissue (Fig. 10J,K, blue arrows). Once more, it appears that some sort of acellularization occurred, as higher magnification reveals cellular lacunae with different shapes, perhaps all undergoing different levels of intracellular calcification (Fig. 10K): some areas have numerous large cell lacunae (white arrows), while others only show a few irregular lacunae (green arrows), and finally some are completely acellular (blue arrows). In this adult specimen, it appears that the parietal‐postorbital suture was replaced by acellular tissue.
Facial sutures
Triceratops
The interpremaxillary and the nasal‐premaxillary sutures were sectioned in an adult Triceratops (MOR 8661; Fig. 11B, red arrows). An isolated nasal of a younger specimen (MOR 2587) is shown for anatomical purposes (Fig. 11A). The nasal‐premaxillary suture in MOR 8661 is completely synostosed, and replaced by trabeculae of intramembranous bone (perhaps endosteal bone) and bone marrow/vascular spaces (Fig. 11C). The interpremaxillary suture is also synostosed, but its former position is indicated by a large canal along its length. The function of this canal is unclear, but it undoubtedly belongs to the morphologically observed tube‐like structure on the ventral side of these elements (Fig. 11E). Even though this ‘tube’ obscured the sutural histology of this specimen, it appears that sutural closure in the interpremaxillary and the nasal‐premaxillary sutures of Triceratops was made via intramembranous ossification.
Figure 11.
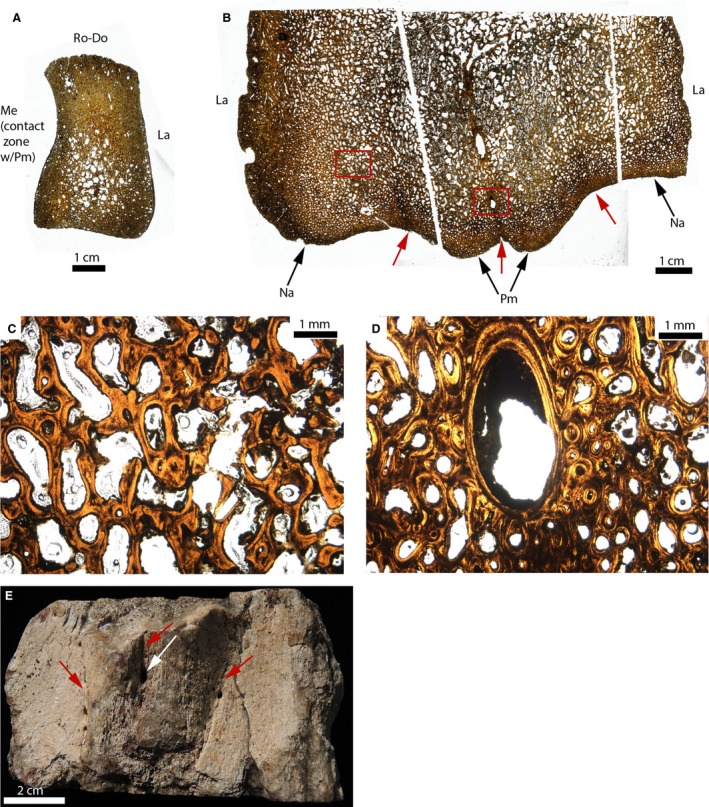
Paleohistological cross‐sections of an isolated nasal (A), and of the fused nasals and premaxillae of Triceratops (B–E). (A) Whole section of an unfused nasal (MOR 2587). (B) Whole sections of the ventral part of fused premaxillae and nasals (MOR 8661). The ventral part of the two nasal‐premaxilla and the interpremaxillary sutures are indicated by the red arrows. (C) Close‐up of the left red box in (B). It shows no trace of the suture. It is obliterated and replaced by lamellar bone trabeculae, and vascular and/or bone marrow spaces. (D) Close‐up of the right red box in (B), showing a canal present at the interpremaxillary suture. The suture is also obliterated here. (E) Photograph of the morphology of these elements in ventral view. The canal is indicated by the white arrow. The three sutures are obliterated morphologically but their general locations are indicated by the red arrows. La, lateral; Me, medial; Na, nasal; Pm, premaxilla; Ro‐Do, rostro‐dorsal.
The jugal‐epijugal and jugal‐quadratojugal sutures of two adult Triceratops are shown in Fig. 12 (UCMP 174838; Fig. 12A,C–F; and MOR 2570; Fig. 12B,G,H). These sutures are open in UCMP 174838 (Fig. 12A) and MOR 2570 (Fig. 12B), except for the lateral side of the jugal‐epijugal of UCMP 174838 (Fig. 12A). They do not show any interdigitations. In UCMP 174838, two types of sutural mineralized tissues can be found. First, a tissue with highly disorganized fibers (Fig. 12C) is seen that looks exactly like the tissue found at the periosteal borders of this same element (e.g. see the periosteal borders of the epijugal; Fig. 12D). This fibrous tissue presents the characteristics of the ‘metaplastic bone’ that has been found in the parietal frill of this same species (Horner & Lamm, 2011; see their figs 3C and 6B,C, and see discussion for further elaboration). It was also involved in the sutural obliteration of the lateral part of the jugal‐epijugal suture of this specimen (data not shown). The second mineralized sutural tissue that was found shows mineralized lamellae parallel to the suture (Fig. 12E, white arrows). At higher magnification, this arrangement into lamellae is less visible (Fig. 12F) and instead it is composed of numerous black spaces resembling cellular lacunae (Fig. 12F, white arrows). These lacunae, however, do not look like regular osteocyte lacunae (compare with the osteocytes of the adjacent lamellar bone; blue arrows, Fig. 12F), and yet they are different from the other enigmatic cellular lacunae observed in UCMP 154919 (Fig. 9I). Horner & Lamm (2011) reported these same structures and hypothesized that they are not osteocyte lacunae, but rather are spaces fitted between bundles of collagen fibers cut in a transverse direction (see their Fig. 8E, and discussion for further elaboration). In MOR 2570, a third sutural mineralized tissue was found (Fig. 12G,H). It presents the general characteristics of the acellular tissue previously described in the pachycephalosaurs (Figs 9 and 10), with areas containing irregularly shaped cellular lacunae (white arrows, Fig. 12H) and other completely acellular areas.
Figure 12.
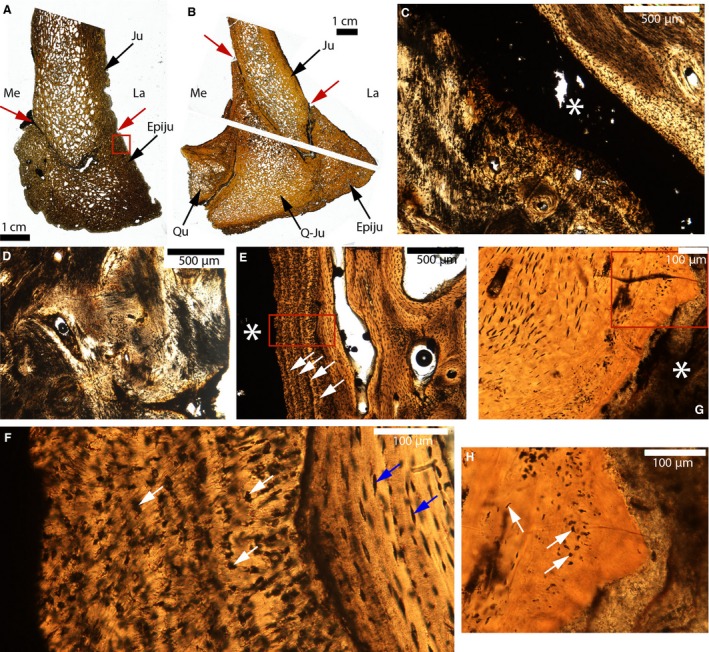
Paleohistological cross‐sections of the jugal‐epijugal and the jugal‐quadratojugal sutures of Triceratops. (A) Whole section of UCMP 174838. The lateral and medial parts of the suture are indicated by the red arrows. (B) Whole sections of MOR 2570. The lateral and medial parts of the suture are indicated by the red arrows. (C) Close‐up of the suture in (A). The sutural borders on the lower part of the picture are composed of a very fibrous tissue. The suture is indicated by the white asterisk. (D) Close‐up of the red box in (A). The same fibrous tissue as that presented in (C) is also found at many external borders. It has previously been designated as ‘metaplastic bone’. (E) Close‐up of another part of this same suture, showing lamellar bone on the right and some lamellae that are parallel to the suture (white arrows). The suture is indicated by the white asterisk. (F) Close‐up of the red box in (E), showing the internal lamellar bone with regular osteocyte lacunae (blue arrows) and a tissue composed of irregular dark spaces (white arrows) near the suture. (G) Close‐up of the suture in (B). The sutural borders are composed of an acellular tissue. The white asterisk indicates the suture. (H) Close‐up of the red box in (G). The white arrows indicate cellular lacunae of various shapes and sizes. Abbreviations: same as previous figures; Epiju, epijugal; Ju, jugal; Q‐Ju, quadratojugal; Qu, quadrate.
Synchondroses of the skull‐base
Extant archosaurs
The basioccipital‐exoccipital synchondrosis is shown in a growth series consisting of Emu 1 (Fig. 13A,D), Emu 3 (Fig. 13B,E–G), Emu 4 (Fig. 13H,I) and Emu 6 (Fig. 13C) stained with toluidine‐blue. The bone stains blue, the hyaline cartilage light purple and the calcified cartilage dark purple. The synchondrosis is straight in Emu 1 (Fig. 13A), slightly undulating in Emu 3 (Fig. 13B) and completely ossified in Emu 6 (Fig. 13C). In this 20‐year‐old emu the synchondroseal cartilage has been eroded and replaced by endochondral ossification (Fig. 13C). Emu 3 and 4 show that ossification starts from the lateral side of the synchondrosis (Fig. 13B,E, yellow arrows).
Figure 13.

Cross‐sections in the basioccipital‐exoccipital synchondrosis of emus stained with toluidine‐blue. (A) Section of the left basioccipital‐exoccipital synchondrosis of Emu 1. The bone stains blue, the hyaline cartilage light purple and the calcified cartilage dark purple. (B) Whole section of the occipital condyle of Emu 3. (C) Whole section of the occipital condyle of Emu 6. (D) Close‐up of the red box in (A). (E) Close‐up of the right box in (B). The yellow arrows indicate where the synchondrosis is being replaced by bone via endochondral ossification. (F) Close‐up of the middle red box in (B). The resting zone can be seen in the middle, as well as the mirroring proliferative zones, hypertrophied cartilage zones and ossification zones (double yellow arrows). (G) Close‐up of the left red box in (A). The white arrows indicate proliferative chondroblasts. The yellow arrows indicate some collagen fiber aggregates (‘asbestos transformation’) in the hyaline resting zone. (H) Most lateral part of the right basioccipital‐exoccipital synchondrosis of Emu 4. This lateral edge shows a disorganization of the chondrocytes (i.e. no organization into resting and proliferative zones). Even though this section comes from a dry skull, the hyaline cartilage is still very well preserved and some cellular divisions can be seen (white arrows). (I) Close‐up of the red box in (H). Cellular divisions are indicated by white arrows. The yellow arrows indicate collagen fiber aggregates (‘asbestos transformation’). hcz, hypertrophied cartilage zone; oz, ossification zone; pz, proliferative zone; rz, resting zone.
In the youngest emu the differentiation between the resting and the proliferative zone is difficult, but in Emu 3 and 4 all the different synchondroseal zones are easily recognizable (Fig. 13F; except in the most lateral parts of the synchondrosis, where no cellular organization into resting and proliferating zones can be seen; Fig. 13H,I). In Emu 3 and 4 the proliferative zone is composed of six–12 cells oriented into columns (white arrows, Fig. 13G). According to Trueta & Trias (1961), the more numerous the cells are in this layer, the higher the growth rate. The number of hypertrophic cartilage cells is usually 10–15 (Fig. 13F). It appears that the unmineralized hyaline cartilage of the resting zone becomes fibrocartilage and/or undergoes ‘asbestos’ transformation (i.e. thick collagen fiber aggregates; Thilander & Ingervall, 1973; Kuehnel, 2003; Fig. 13G–I, yellow arrows). Note that the sections of Emu 4 are exquisitely preserved (see the cellular divisions; white arrows, Fig. 13H,I) even though they were obtained from a dry skull.
The same synchondrosis stained with toluidine‐blue is shown in Alligator 1 (Fig. 14A), Alligator 3 (Fig. 14B–D,E), Alligator 4 (Fig. 14E) and Alligator 6 (Fig. 14F–H). In Alligator 1, the basioccipital‐exoccipital synchondrosis is not formed yet and appears to be a synovial joint (red arrow, Fig. 14A), which makes it a presumptive synchondrosis. In Alligator 3, 4 and 5 the synchondrosis is slightly undulating (Fig. 14B,C,E), and it becomes interdigitated in Alligator 6 (Fig. 14F). The different synchondroseal zones are easily identifiable in Alligator 3, 4 and 5, and are similar to those reported by Ikejiri (2012) in the vertebral column of this species (Fig. 14D). The number of cells in the proliferative and the hypertrophic zones of the alligator are very low compared with those of the emu, averaging two–three (Fig. 14D, white arrows) and four–eight cells (Fig. 14D), respectively. In Alligator 6 (a dry skull), the unmineralized hyaline cartilage seems to have been replaced with fibrocartilage (Fig. 14G, yellow arrow). The hypertrophied cartilage zone is still present, but the resting and proliferative zones are no longer visible in the fibrocartilage. The most astonishing characteristic featured in this specimen is the apparent transformation of the most medial and lateral parts of this synchondrosis into a suture‐like structure (see the medial part, Fig. 14H). Even though the soft‐tissues have been slightly deteriorated in this dry skull, the most internal part of the synchondrosis appears to be made of fibrocartilage (yellow arrow, Fig. 14H), but the most external part shows thick bundles of collagen fibers bridging the two bone fronts (red arrow, Fig. 14H). No cartilage can be seen in this fibrous area (Fig. 14H, red arrow). To the authors' knowledge, this type of transformation or replacement (i.e. from a synchondrosis to a suture) has not been reported before.
Figure 14.
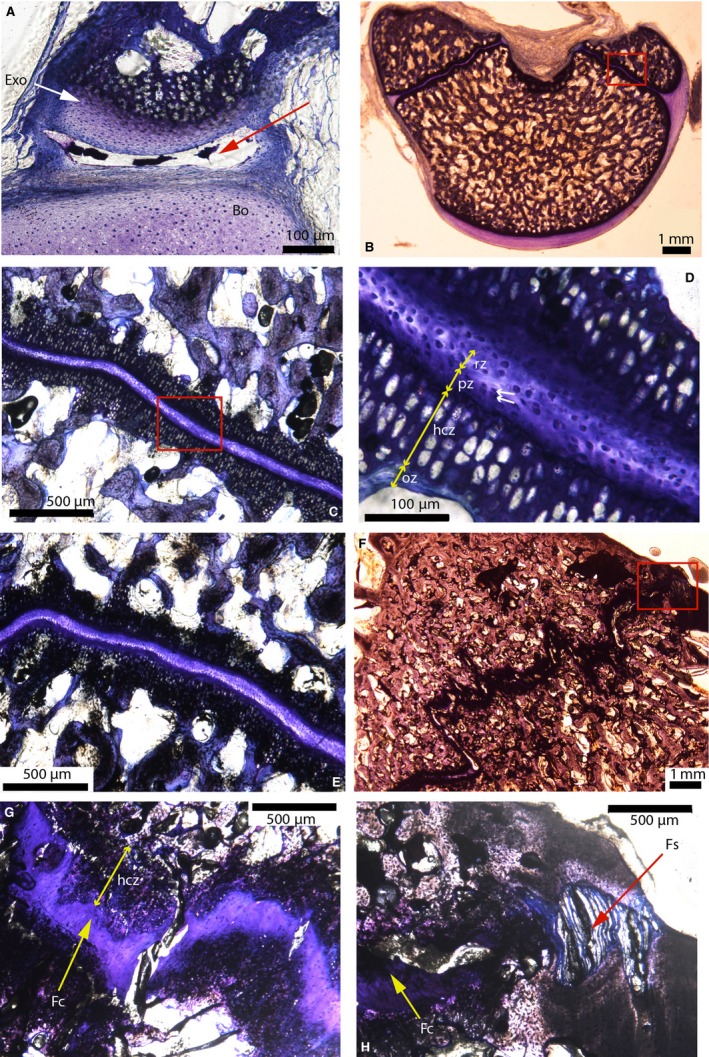
Cross‐sections in the basioccipital‐exoccipital synchondrosis in American alligators stained with toluidine‐blue. (A) Right ‘presumptive’ basioccipital‐exoccipital synchondrosis of Alligator 1. This synchondrosis is not formed yet and appears to be a synovial joint (see the synovial cavity indicated by the red arrow). (B) Whole section showing the occipital condyle of Alligator 3. (C) Close‐up of the red box in (B). (D) Close‐up of the red box in (C), showing the middle resting zone and the mirroring proliferative zones, hypertrophied cartilage zones and ossification zones (double yellow arrows). The proliferative zone is composed of two–three cells (white arrows). (E) Right basioccipital‐exoccipital synchondrosis of Alligator 4. (F) Left basioccipital‐exoccipital synchondrosis of Alligator 6. This synchondrosis is interdigitated. (G) Close‐up of the right basioccipital‐exoccipital synchondrosis of Alligator 6. The hyaline cartilage has been replaced by fibrocartilage (yellow arrow). The mirroring hypertrophied cartilage zones are still present (double yellow arrows). (H) Close‐up of the red box in (F). The most internal part of the synchondrosis is composed of fibrocartilage (yellow arrow), while the most external (medial) part is composed of a fibrous tissue (red arrow), giving this portion the appearance of a suture. Image (A) is modified from Bailleul et al. (in press). Abbreviations: same as the previous figure; Bo, basioccipital; Exo, exoccipital; Fc, fibrocartilage; Fs, fibrous suture.
Fossil archosaurs/non‐avian dinosaurs
Crocodilian
The basioccipital‐exoccipital and the basisphenoid‐basioccipital synchondroses (see red and green arrows) in the fossil crocodilian (MOR 552; cf Brachychampsa) are shown in Fig. 15A–G. The basioccipital‐exoccipital synchondrosis shows two mirroring layers of calcified cartilage (Fig. 15C, white arrows) and a gap between them filled with minerals (Fig. 15C, double black arrows). These two cartilaginous layers were undoubtedly the hypertrophied cartilage zones and the gap used to host the unmineralized resting and proliferative zones. Laterally, the calcified cartilage zones end abruptly (see the limit between cartilage and bone; yellow arrows, Fig. 15D), and eventually no trace of hypertrophied cartilage can be seen in the synchondrosis (Fig. 15E). This suggests that, just as it was observed in Alligator 6 (Fig. 14H), the lateral margin turned into or was replaced by a suture. It was hypothesized that the gap between the two elements was filled with fibers and not by unmineralized cartilage (Fig. 15E). The entire basisphenoid‐basioccipital synchondrosis shares the same histology as that presented in Fig. 15E, where no calcified cartilage was found anywhere at its borders (Fig. 15F). These observations are very enigmatic, but it was hypothesized that the entire basisphenoid‐basioccipital synchondrosis had been ‘replaced’ by a fibrous suture (see Discussion for further elaboration). The borders of this same articulation are composed of a mineralized tissue not resembling endochondral bone or intramembranous bone (Fig. 15G). It shows globular structures (white arrows, Fig. 15G) comparable to the ones previously found in the modern alligators (Fig. 4H) and the hadrosaurs (Fig. 8D,H). These globular structures resemble chondrocytes at low magnification, but higher magnification reveals that they are not (see Discussion for further description).
Figure 15.

Paleohistological cross‐sections of the basioccipital‐exoccipital‐basisphenoid complex of a fossil crocodilian, cf. Brachyhampsa (A–G) and parasagittal sections of the basisphenoid‐basioccipital complex of Prosaurolophus (H, I). (A) Whole section of MOR 552. The basioccipital‐exoccipital synchondroses are shown by the red arrows. The basisphenoid‐basioccipital synchondroses are shown by the green arrows. (B) Close‐up of the upper red box in (A). (C) Close‐up of the upper red box in (B), with a middle zone filled in with minerals (double black arrows) and two mirroring hypertrophied cartilage zones (white arrows). (D) Close‐up of the lower red box in (B). The hypertrophied cartilage of the synchondrosis disappears abruptly (yellow arrows) and the remainder of the ‘synchondrosis’ appears to be a ‘suture’. (E) Close‐up of the right red box in (A), showing a histology comparable to that of a suture. (F) Close‐up of the lower red box in (A). In this basioccipital‐basisphenoid junction, no hypertrophied calcified cartilage can be seen, which suggests again that it could be a suture. (G) Close‐up of the red box in (F). The sutural borders are composed of a tissue with globular structures (white arrows). (H) Whole section of the basisphenoid‐basioccipital bones of MOR 447. The two ends of the basioccipital‐basisphenoid synchondrosis are indicated by the red arrows. (I) Close‐up of the red box in (H). The middle resting and proliferative zones are not preserved (double black arrows). Two mirroring hypertrophied cartilage zones can be seen. Abbreviations: same as previous figure; Bs, basisphenoid.
Hadrosaurs
Some hadrosaur synchondroses were also sectioned, but their preservation was very poor (Hypacrosaurus MOR 548, data not shown; and Prosaurolophus, MOR 447; Fig. 15H,I). The basisphenoid‐basioccipital synchondrosis of Prosaurolophus is shown (red arrows, Fig. 15H), but the entire element is diagenetically altered beyond histological resolution and only a few areas show relatively decent preservation (Fig. 15I). The two hypertrophic zones can be seen as well as an empty middle zone, which was undoubtedly the unmineralized resting and proliferative zones (double arrows, Fig. 15I). Because the two hadrosaur specimens were diagenetically altered, it was not possible to extract much data from the synchondroses in this group of non‐avian dinosaurs.
Triceratops
The ontogeny of the basioccipital‐exoccipital synchondrosis was interpreted from the analyses of three specimens of Triceratops: a juvenile in MOR 1110 (Fig. 16A,D); and two sub‐adults in MOR 8657 (Fig. 16B,E,F) and MOR 8658 (Fig. 16C,G). In the juvenile, the occipital condyle is not fused yet (only one of the two exoccipitals is shown here). The elements are composed of cancellous lamellar trabeculae resembling normal endochondral bone (perhaps endosteal bone) with islands of calcified cartilage that are remnants of their primary cartilage model (Fig. 16D, black arrows). The periosteal borders of these two bones were completely obscured by a black mineral (possibly hematite, D. Varricchio, personal communication), and therefore the synchondrosis was not observable. In MOR 8657 and MOR 8658, the occipital condyles are partially fused (Fig. 16B,C). The histology of these condyles is the same so they are described together. The basioccipital‐exoccipital and the exoccipital‐exoccipital synchondroses are open on the outside of the condyles (Fig. 16B,C, red arrows and Fig. 16E,G), but they become completely obliterated deeper within the cortex, made of a dense compacta formed of secondary osteons (Fig. 16F). All the synchodroses transition into cracks (Fig. 16B,C; possibly post mortem cracks). These two condyles do not show any remnant of calcified cartilage (whether synchondroseal or from the primary cartilage models, e.g. Fig. 16E,G). This suggests that parts of these synchondroses were transformed into fibrous sutures, perhaps in a similar fashion to that observed in the extant and fossil crocodilians (Figs 14 and 15).
Figure 16.
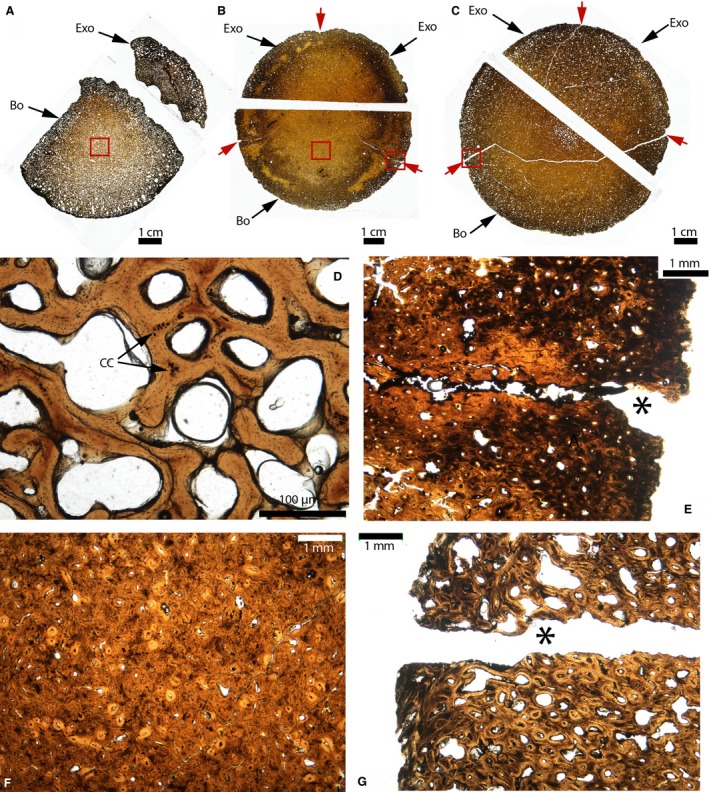
Paleohistological cross‐sections of the occipital condyles of Triceratops. (A) Whole sections of the unfused occipital condyle of MOR 1110. (B) Whole sections of the fused occipital condyle of MOR 8657. (C) Whole sections of the fused occipital condyle of MOR 8658. Synchondroses are indicated by the red arrows in (B and C). (D) Close‐up of the red box in (A). Trabeculae of lamellar bone can be seen, as well as some islands of calcified cartilage, remnants of the primary cartilage model (black arrows). (E) Close‐up of the right red box in (B). The right basioccipital‐exoccipital synchondrosis is open externally (black asterisk) and does not present any calcified cartilage. It presents the histological charateristics of a suture. (F) Close‐up of the left red box in (B). The bone is a compacta with numerous secondary osteons. (G) Close‐up of the red box in (C). It presents the same histological characteristics as (E). Abbreviations: same as previous figures; CC, calcified cartilage.
The basisphenoid‐basioccipital synchondrosis of a Triceratops (MOR 8659) was also sectioned, but it was completely obliterated and replaced by trabeculae of lamellar bone. No calcified cartilage was found anywhere in this element. The overall histology of MOR 8659 resembles that presented in Figs 11 and 16. Therefore, it was not found to be necessary to show these sections in the present study.
Morphological vs. histological degree of closure
For obvious reasons, sutures are often analyzed solely at the macroscopic scale in paleontology without any microstructural investigation. The morphological and histological degrees of closure were compared for multiple sutures/synchondroses. The results show that most of the time, when these articulations are morphologically open, they are also open histologically. This is the case for all the open sutures/synchondroses of emus (e.g. compare Fig. 17A with Fig. 3A), all the open sutures/synchondroses of American alligators and the fossil crocodilian (e.g. compare Fig. 17B with Fig. 4), and the open fronto‐parietal suture of Gryposaurus and Brachylophosaurus (e.g. compare Fig. 17C with Fig. 8A). Many of the morphologically obliterated sutures are obliterated histologically as well. This is the case for all the fused sutures/synchondroses of emus (e.g. compare Fig. 17D with Fig. 3C), the interpremaxillary and nasal‐premaxillary sutures of Triceratops (compare Fig. 11E with Fig. 11B), and the basisphenoid‐basioccipital synchondroses of Triceratops (MOR 8659).
Figure 17.
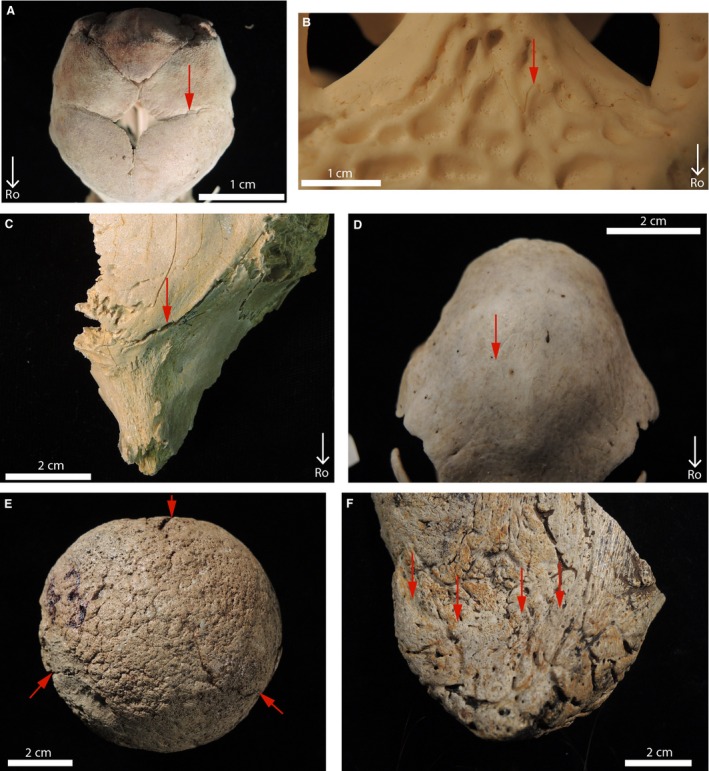
Morphological characteristics of the sutures and synchondroses of extant and fossil archosaurs. (A) Right fronto‐parietal suture of Emu 1 in dorsal view (red arrow). It presents an ‘open’ morphology (with a suture line still visible). (B) Open fronto‐parietal suture of Alligator 5 in dorsal view (red arrow). (C) Open fronto‐parietal suture of MOR 1071 (Brachylophosaurus) in dorsal view (red arrow). (D) Obliterated fronto‐parietal suture of Emu 6 in dorsal view (red arrow). (E) Open synchondroses in the occipital condyle of MOR 8657 (Triceratops) in caudal view (red arrows). (F) Obliterated jugal‐epijugal suture of UCMP 173848 (Triceratops) in lateral view (red arrows).
However, in two specimens histology and morphology show contradicting results. This is the case for the occipital condyles of two Triceratops (MOR 8657 and MOR 8658), which show open synchondroses morphologically (Fig. 17E), but obliterated synchondroses more internally (Fig. 16). Moreover, because sutures and synchondroses have two openings (e.g. an endocranial and an ectocranial side), two types of morphologies are indicated by the same articulation in some instances. This is the case in some pachycephalosaurs (Figs 9A,B and 10G) and in the skull‐base of emus (Fig. 13B). The jugal‐epijugal suture of Triceratops (UCMP 174838) shows an obliterated suture on its lateral side (Fig. 17F), but due to taphonomical alterations it is impossible to identify the degree of patency of this suture on its medial side. Histological analyses show that this side is open (Fig. 12A, left red arrow).
Discussion
Although sutural and synchondroseal histology are extremely well documented in Mammalia, they remain largely unexamined in archosaurs. Microscopic investigations are almost entirely unsurveyed in the sutures and the cranial base synchondroses of lepidosaurs as well, the extant sister taxa of archosaurs (Payne et al. 2011; Mezzasalma et al. 2014).
This section summarizes and categorizes the archosaurian sutural and synchondroseal tissues that were found in the samples, and compares them with those that have been previously reported in mammals, i.e. the only point of comparison available in this present study, as sutural and synchondroseal histology through ontogeny is essentially known in this taxon. The modes of ossification by which they might have been formed are discussed and hypothesized.
Comparative sutural histology: Archosauria vs. Mammalia
A total of seven sutural mineralized tissues were found in the current samples, and they are summarized in Table 3. They are subdivided into four groups: periosteal tissues; acellular tissues; fibrous tissues; and intratendinous tissues (Table 3).
Table 3.
Summary of the sutural mineralized tissues found in archosaurs and mammals
| Sutural mineralized tissues | Mammals | D. novaehollandiae | A. mississippiensis and cf Brachychampsa | Non‐avian dinosaurs | |
|---|---|---|---|---|---|
| Periosteal tissues | Intramembranous bonea | Present (e.g. Persson et al. 1978) | Present (Figs 3 and 5C) | Present (not directly observed for both) | Present (Pachycephalosauridae: Fig. 9D; Triceratops: Figs 11 and 16; Hadrosauridae: not directly observed) |
| Sutural secondary cartilage | Present (e.g. Vinkka‐Puhakka, 1991) | Absent | Absent | Absent | |
| Chondroid bonea | Present (e.g. Manzanares et al. 1988) | Present (Fig. 5E) | Absent | Present (Hadrosauridae: Bailleul et al. 2015) | |
| Acellular tissues | Acellular/fibrous tissuesa | Absent | Absent | Absent | Present (Pachycephalosauridae: Fig. 10C,D) |
| Acellular necrotic tissuea | Absent | Absent | Absent | Present (Pachycephalosauridae: Fig. 10I–K; Triceratops: Fig. 12G,H) | |
| Fibrous tissues | Sutural‐fiber‐rich tissuea | Absent | Absent | Present (in A. mississippiensis: Figs 4D,E and 6G) | Present (Pachycephalosauridae: Fig. 10F,I) |
| ‘Metaplastic bone’a | Absent | Absent | Absent | Present (Triceratops: Fig. 12C) | |
| Intratendinous tissues | Intratendinous mineralized tissue | Absent | Absent | Present (in A. mississippiensis: Fig. 4H; in cf Brachychampsa: Fig. 15G) | Present (Hadrosauridae: Fig. 8D,H; Triceratops?: Fig. 12F)? |
| Total number of tissues per taxa | 3 | 2 | 3 | 7 | |
This summary is based on the results of the present study and those of other previously published investigations.
The tissues were involved in sutural obliteration (either directly observed in the present study, or reported in the literature).
Periosteal tissues
Two types of periosteal tissues are reported in the sutural borders of archosaurs: intramembranous bone and chondroid bone. These two types of tissues are known to be deposited by the osteogenic cells originating from the periosteum (Lengelé, 1997; Marieb, 2014), hence the term ‘periosteal tissues’.
The identification of intramembranous bone was based on comparisons with the ‘standard’ appearance of mammalian intramembranous bone and/or on the presence of a periosteum (i.e. the sutural cambial layer). This mineralized tissue was found in the sutural areas of emus (Figs 3E and 5C), pachycephalosaurs (Fig. 9D) and Triceratops (Figs 11 and 16). It was involved in sutural obliteration in these three taxa. However, because intramembranous ossification is plesiomorphic and is the main mode of formation of the membrane bones of vertebrates, the safe assumption can be made that at least at some point during their ontogeny, intramembranous bone was a constituent of the mineralized sutural borders of all the archosaurs of the current sample (even though this type of bone and/or a periosteum was not directly observed; Table 3).
Chondroid bone was found exclusively in the emu and only in the internasal sutural areas of an actively growing specimen (Fig. 5E). Chondroid bone had previously been reported in the sutural areas of mammals, chick embryos (Lengelé et al. 1996) and hadrosaurs (Bailleul et al. 2015). It was also found in the embryonic skull of A. mississippiensis (Vickaryous & Hall, 2008). While it is highly plausible that American alligators have chondroid bone in their sutural areas, this tissue was not confidently identified in the current samples. Like intramembranous ossification, it appears that chondroid bone is plesiomorphic within the vertebrate tree. Indeed, it was reported in species of many different clades in the Vertebrata: mammals, birds, hadrosaurs and fossil agnathans (see more complete review in Bailleul et al. 2015). It is hypothesized that the absence of chondroid bone in all of the fossilized specimens is perhaps due to their relatively late ontogenetic stages and/or to the absence of a sutural periosteum.
These two periosteal tissues have been previously reported in mammals (Pritchard et al. 1956; Persson, 1973; Kokich, 1976; Persson & Thilander, 1977; Persson et al. 1978; Hinton et al. 1984; Cohen, 2000 for intramembranous bone; Goret‐Nicaise et al. 1988; Manzanares et al. 1988; Lengelé et al. 1990, 1996; Sun et al. 2004 for chondroid bone). Secondary cartilage, another periosteal tissue, is also commonly found in the sutures of mammals (Sitsen, 1933; Pritchard et al. 1956; Bloore et al. 1969; Friede, 1973; Persson, 1973; Hinton et al. 1984; Kokich, 1986; Manzanares et al. 1988; Vinkka‐Puhakka, 1991), but it was not found in any suture or sutural area in the archosaurs of the present study (Table 3).
The next three categories of tissues that are exposed have not been reported in mammals and, in fact, many of them have not been observed in any extant vertebrate. Understanding this, caution was taken when hypothesizing and deducting their modes of formation.
Acellular tissues
Acellular tissues are often found in the skeleton of teleosts (Moss, 1961, 1963; Ekanayake & Hall, 1988; Meunier, 1989), but are uncommon in the skeletons of tetrapods. It is therefore counterintuitive to observe acellular tissues with such a high abundance in marginocephalians (pachycephalosaurs and ceratopsids). An acellular fibrous tissue was found that obliterated the parietal‐postorbital suture of a pachycephalosaurid (Fig. 10C,D). It shows irregular and ‘globular’ mineralized borders that contain radiating ‘fibers’. This acellular tissue and its ‘fibers’ will not be discussed further because they will be the subject of another study. An acellular non‐fibrous tissue was found bordering the sutures of Pachycephalosaurus (Fig. 10I, white arrow) and Triceratops (Fig. 12G,H). It was also involved in the complete synostosis/obliteration of a Pachycephalosaurus suture (Fig. 10J,K). These tissues have previously been reported in pachycephalosaurids by Goodwin & Horner (2004) and Horner & Goodwin (2009), but not directly in sutural areas. As mentioned in the Results, due to the different shapes and sizes that osteocyte lacunae can have in the vicinity of this tissue, it was hypothesized that it started out as a cellular tissue and then progressively underwent acellularization (with cellular pyknosis and intracellular mineralization; Moss, 1961), hence the term ‘acellular necrotic tissue’ was proposed. A ‘bionecrotic’ mineralized tissue has been reported in the sutures of humans and rabbits but, in these cases, it was much less abundant and appeared as free‐nodules in the suture or as masses attached to short spicules extending from the sutural borders (see Fig. 7 in Persson et al. 1978). It is not comparable to the potential necrotic sutural tissue found in marginocephalians. The function and advantage (if there are any) of this acellularization are unknown, but a potential cause of this process could be mechanical. This could be analogous to the acellularization of mammalian periodontal ligaments that may occur when the ligaments receive stresses above a certain threshold causing their fibroblasts to undergo apoptosis, resulting in a ligament with completely acellular zones. This process is called ‘hyalinization’ (Jónsdóttir et al. 2012). Even though this example does not relate directly to a mineralized tissue, it shows the possibility that some mechanical stresses in the sutures (or sutural mineralized borders) may be the cause of acellularization. This is a plausible hypothesis considering that sutures are known to be shock absorbers and protect the non‐sutural mineralized tissues from fracture (Jaslow & Biewener, 1995). On that same note, it is important to mention that because open sutures act as shock absorbers, and because many sutures were obliterated in the pachycephalosaurs of the current sample, the previously proposed hypothesis of a head‐butting behavior in these dinosaurs (Galton, 1970) does not seem plausible. Nevertheless, further investigation of this acellular necrotic tissue obliterating the sutures is underway and will be part of a future study.
Fibrous tissues
Two different types of sutural mineralized ‘fibrous’ tissues were identified. The first type shows numerous fibers that are all perpendicular to the suture. It was found in the modern alligators (Fig. 6G) and the pachycephalosaurids (Figs 9E and 10I). In the latter taxa it was also found obliterating the sutures (Figs 9F and 10F). In some instances these mineralized tissues are composed of lamellae that are all parallel to the suture (Figs 6G and 10F). These lamellae undoubtedly represent the earlier sutural borders. It was hypothesized that these fibers were collagenous fibers initially located in the sutures that became incorporated in the mineralized sutural tissues. Similar fibers were observed in the fronto‐parietal suture of the American alligator (Fig. 4D, white arrow, and E, black arrow). This type of mineralized tissue is referred to as a ‘sutural‐fiber‐rich tissue’. In some instances, the sutural fibers are so numerous that it is not clear if the tissue is cellular or acellular. The second type of fibrous tissue was found exclusively in the sutural areas of Triceratops (Fig. 12C). Its fibers are also very numerous, but unlike the previous fibrous tissue they are disorganized and not always found perpendicular to the suture (Fig. 12C). This type of tissue has already been reported and named ‘metaplastic bone’ in the osteoderms of ankylosaurs (Scheyer & Sander, 2004) and stegosaurs (Main et al. 2005). Metaplasia is the direct transformation of soft‐tissues in situ without the intervention of a periosteum (Vickaryous & Hall, 2008). The term ‘metaplastic bone’ was created by Haines & Mohuiddin (1968) to describe a hard skeletal tissue formed in the absence of osteoblasts. Vickaryous & Hall (2008) reported that the osteoderms of A. mississippiensis form partially via metaplasia, which suggested that the previous assessments of Main et al. (2005) and Scheyer & Sander (2004) were correct. However, Vickaryous & Hall (2008) only examined decalcified sections, and it is still unclear if their metaplastic tissue corresponds to the ‘metaplastic bone’ observed previously by Scheyer & Sander (2004) and Main et al. (2005) in thick undecalcified sections. More recently, this same ‘metaplastic bone’ was found very abundantly in the parietal frill of Triceratops (Horner & Lamm, 2011). Two lines of evidence suggest that the ‘sutural‐fiber‐rich tissue’ and the ‘metaplastic bone’ found in the current sample were indeed formed by metaplasia: (i) they do not present osteocyte lacunae with canaliculi; and (ii) they are in direct contact with soft‐tissues (i.e. the suture). Moreover, unlike mammalian sutures that present a periosteal cambial layer containing numerous osteoblasts (Fig. 1), the sutures of American alligators appear to lack a periosteum (they are formed of a uniform layer with fibers and a few fibroblasts; Figs 4D,E and 6D,E). It is therefore not implausible to hypothesize that some archosaurian sutural tissues mineralize directly from the soft‐tissues of their sutures. As mentioned above, because fibrous tissues presenting these same characteristics in undecalcified sections of extant animals have never reported, it is yet impossible to confirm this hypothesis.
In the last category of sutural mineralized tissues (intratendinous tissues), considerably stronger evidence was provided that some metaplastic transformations were involved during the formation of these tissues.
Intratendinous tissues
This last category is characterized by mineralized tissues that show globular structures separated by arcuate‐shaped spaces. They were found in the modern alligators (Fig. 4H), the fossil crocodilian (Fig. 15G) and two species of hadrosaurs (Fig. 8D,G,H). The histology of these ‘globular’ tissues is virtually identical to that of the ossified tendons in hadrosaurs (Adams & Organ, 2005) and extant birds (Fig. 18). Avian mineralized tendons do not form via normal intramembranous ossification, but via metaplastic transformations (Horner et al. 2015). This mode of ossification was previously designated as ‘intratendinous ossification’ (Rooney, 1994; Berge & Storer, 1995), hence the term ‘intratendinous tissues’ used here. Figure 18 is modified from Horner et al. (2015) and shows the microstructure of a mineralized tendon of Bubo virginianus (Great horned owl) stained with toluidine‐blue. Mineralized tendons are manifested as vascular canals (black arrow) with indistinct boundaries (red arrows). These types of canals do not have the regular characteristics of Haversian systems (Fig. 18A) and were therefore named ‘secondary reconstruction’ by Horner et al. (2015). The collagen fiber fascicles forming the ‘young’ unmineralized tendons calcify in situ. This process produces globular structures in undecalcified sections (i.e. the mineralized collagen fiber fascicles, yellow arrows) separated from each other by dark spaces of different shapes (white arrows). When these shapes are arcuate, they represent the endotenons of the fascicles while the other irregular spaces may host osteogenic cells (Horner et al. 2015). No evidence of osteocyte lacunae and/or canaliculi was observed in avian tendons, and therefore it was hypothesized that some non‐osteogenic cells transformed into and played the role of osteoblasts (perhaps a population of fibroblasts transforming directly into osteoblasts; M. Vickaryous, personal communication in Horner et al. 2015).
Figure 18.
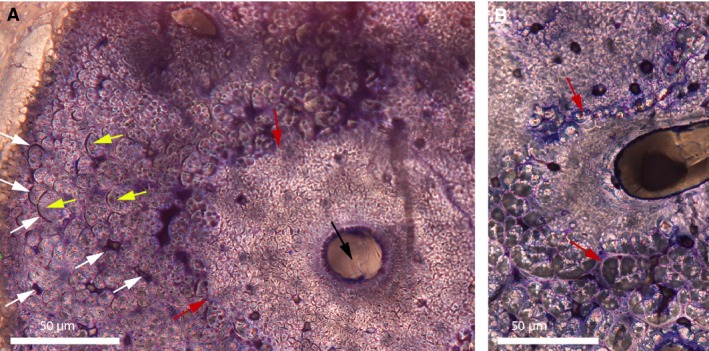
Cross‐sections of a mineralized tendon from M. extensor carpi radialis of Bubo virginianus (Great Horned Owl). (A) Part of a mineralized tendon of MOR.H.2001‐10R. A vascular canal can be seen (black arrow). It does not show the typical characteristics of a primary osteon or a haversian system, and therefore was named ‘secondary reconstruction’ (SR) by Horner et al. (2015). The boundary between this SR and the rest of the tissue is ragged and undistinct (red arrows). It appears to be made of small collagen fiber bundles and fascicles tightly packed together. Further away from this SR, the tissue is composed of larger fiber fascicles (yellow arrows), separated from each other by spaces of different shapes, many of which are arc‐shaped (white arrows). These arc‐shaped spaces are the endotenons of the fiber fascicles. No regular osteocyte lacunae (nor canaliculi) can be seen anywhere in this tendon. (B) Section of another area in this same tendon showing the boundary of a SR where large fascicles are deconstructed into smaller fascicles (red arrows). This figure is modified from Horner et al. (2015).
The histological similarities between mineralized avian tendons and the sutural mineralized tissues of A. mississippiensis (Fig. 4H), Brachylophosaurus (Fig. 8D) and Gryposaurus (Fig. 8H) are undeniable. Additionally, it appears that large fiber fascicles in avian mineralized tendons are able to undergo destruction or a rearrangement into smaller fiber bundles (Fig. 18B, red arrows). The globular structures shown by the fossil crocodilian that resembled chondrocytes at first sight (Fig. 15G) are very similar to these ‘rearranging’ fiber bundles when observed at higher magnification. Based on the histological similarities with avian mineralized tendons known to be formed by metaplasia, it was hypothesized that the intratendinous tissues found in the American alligator, the fossil crocodilian and the hadrosaurs formed via metaplasia, and undoubtedly involved the direct mineralization of their sutures in the absence of a sutural periosteum.
Another ceratopsian tissue presents some unusual dark spaces that do not have the morphology of osteocyte lacunae (Fig. 12F). As mentioned earlier, this same type of tissue was found in the parietal frill of Triceratops and it was hypothesized that the dark structures are spaces fitted between fiber fascicles (Horner & Lamm, 2011). Due to the lack of a known extant analogue presenting these structures, it is still unclear if this ceratopsian tissue (Fig. 12F) is a form of intratendinous tissue as well.
Comparative synchondroseal histology: Archosauria vs. Mammalia
This discussion is essentially based on the synchondroses of the extant archosaurs because the fossils had incompletely preserved synchondroses (only their calcified parts). Archosaurian and mammalian synchondroses are very similar. They all show four synchondroseal zones (resting, proliferative, calcification and ossification zones). The number of cells in the proliferative zones in 8–10‐month‐old emus (six–12 cells; Fig. 13G) is similar to that reported in 4‐day‐old rats (eight–12 cells; Roberts & Blackwood, 1983). This is much higher than what was found in American alligators (two–three cells; Fig. 14D). These differences likely relate to physiological regimes (i.e. extant mammals and birds are endotherms, while alligators are ectotherms and grow much more slowly; Lee & Werning, 2008). The fates of synchondroseal hyaline cartilage seem to be the same in mammals and archosaurs as well: it can be replaced by fibrocartilage, undergo ‘asbestos’ transformation (Fig. 13G; Thilander & Ingervall, 1973; Cendekiawan et al. 2010) or be replaced by endochondral ossification (Fig. 13E; Opperman et al. 2005). Endochondral ossification was not observed, however, in American alligators as their synchondroses never obliterate (i.e. there is still cartilage in the most mature alligator; Fig. 14G). The fact that the histological characteristics of mammalian and archosaurian synchondroses are very similar suggests a conservation of synchondroseal cellular organization through evolution. The current results suggest that synchondroseal architecture is more conserved than sutural architecture (see the spectrum of different tissues in Table 3).
Only one important dissimilarity was found between archosaurian and mammalian synchondroses. During the late phases of ontogeny, it appears that the medial and lateral parts of the basioccipital‐exoccipital synchondrosis of A. mississippiensis can turn into a suture (Fig. 14H). This was found in the exact same synchondrosis in the fossil crocodilian, suggesting that it is a crocodilian characteristic (Fig. 15D,E). More surprisingly, the entire basisphenoid‐basioccipital synchondrosis of this fossil lacks calcified cartilage and has the appearance of a suture (Fig. 15F). This suggests that even though some articulations are embryonically ‘designed’ to be synchondroses, they may be able to show the histological characteristics of a suture (late during ontogeny). In a similar fashion the synchondroses of the occipital condyle in the Triceratops adults did not show any trace of calcified cartilage, and their most external openings also had the appearance of sutures (Fig. 16). This suggests that the ability of synchondroses to show the morphological characteristics of sutures is perhaps also shared by some non‐avian dinosaurs. The mode of formation of these ‘sutures’ is not clear; it could involve neoplasia or a form of metaplasia (perhaps the synchondroseal chondrocytes transforming into fibroblasts, followed by the secretion of sutural fibers).
Further histological investigations of synchondroses in the skull‐base of extant crocodilians must be made to answer these questions.
Morphology vs. histology
It was attempted to provide a preliminary assessment of the relationship between the morphological and the histological degree of sutural closure in archosaurs. The results show that, overall, the histological degree of closure matches its associated morphology relatively well for both extant and fossil archosaurs at a qualitative level (at least for this sample). Such a correlation between morphology and histology has also been found in a previous study in American alligators with quantitative histomorphometric analyses (Bailleul et al. in press). All of the morphologically obliterated sutures and synchondroses of emus are obliterated histologically. Obliterated sutures were not investigated in American alligators because only two sutures obliterate completely (Bailleul et al. in press). All morphologically open sutures and synchondroses of American alligators are open histologically (with fibers in the sutures and fibrocartilage in the synchondroses regardless of specimen age).
However, some of the current results show morphologies and histologies that are opposite, and when one suture presents two different morphologies at opposite ends (e.g. endocranial vs. ectocranial side, or lateral vs. medial side), the accurate degree of closure of a suture can only be known via histological analyses. This observation is consistent with what previous authors have already suggested and/or found (Brochu, 1996; Herring, 2000; Cole et al. 2003; Sun et al. 2004; Irmis, 2007). These results confirm that the degree of closure of a suture or synchondrosis can only accurately be known via microstructural methods. The latter should be employed more often in paleontology, for example, when examining sutures and then estimating the ontogenetic stage of non‐avian dinosaur specimens (even though it was previously showed that this method of maturity assessment is still ambiguous; Bailleul et al. in press).
The qualitative examination is, however, imperfect due to the methods employed: each suture and each synchondrosis was sampled at two specific points along its length (i.e. two cuts per articulation). Therefore, the histological sections only show the degree of closure in specific points, and do not necessarily reflect the rest of the articulation. Cohen (1993) stated that obliteration begins at one point and spreads along the suture, without any way to predict where the point of fusion will start (at least it is the case in mammals). According to this statement, and if this holds true for archosaurs as well, serial sections along the entire length of sutures are necessary to assess their correct and complete degree of closure. Moreover, the methods here are qualitative and not quantitative. Persson & Thilander (1977) proposed that the use of an ‘obliteration index’, i.e. the ratio between the fused suture length and the entire suture length, could be used to quantitatively define closure. While this ratio is much more informative than the qualitative methods, it is only meaningful if it is calculated in each serial section along the entire length of a suture. Other techniques have also been employed in the past to visualize sutures, such as X‐ray computed tomography and micro‐tomography (Schott et al. 2011). These methods are relatively rapid and can be used on extremely rare specimens; however, their end results do not show the details provided by paleohistological analyses (e.g. they cannot provide a precise obliteration index shown by a histological slide). A comparison between results obtained by histological, computed tomography and micro‐tomography analyses should be made to find the best method to clearly and rapidly visualize the skull articulations of both extant and fossil archosaurs.
Conclusions
In summary, it has been shown that the sutures of mammals and archosaurs grow and fuse with different mechanisms: the sutural mineralized tissues of archosaurs and mammals are completely different, and the diversity of archosaurian tissues is higher than that of mammalian tissues (seven tissues for archosaurs as a whole and three for mammals). All the tissues found in the sutural areas of mammals are deposited via a periosteum, while the tissues of archosaurs involve other modes of ossification (a form of bionecrosis and metaplastic transformations). It appears that metaplasia was the prevalent mode of ossification in the sutural areas of archosaurs. This echoes the findings of Horner et al. (2015), who suggest that metaplastic transformations are more abundant than previously thought in the soft‐tissues of non‐avian dinosaurs. It was hypothesized that these transformations are more rapid and more efficient than regular intramembranous ossification, as they start from pre‐formed precursor tissues (i.e. the sutures in this case).
Enigmatically, the sutures of emus are more similar to those of mammals than those of other archosaurs. Emus also show the lowest diversity of sutural mineralized tissues in this sample (with only two tissues). It was hypothesized that this low histological diversity will also be observed in other species of extant birds. This may be due to the increase of avian growth rate that occurred during evolution (Padian et al. 2001). Because extant birds attain skeletal maturity so rapidly, usually in < 1 year (Lee & Werning, 2008), they probably do not have sufficient time to develop many different tissue types (e.g. tissues that were present in their archosaurian ancestors and that appear late during ontogeny). It is important to note that the current sample did not include saurischian dinosaurs, and it is plausible to hypothesize that bird‐like sutural features might have arisen within non‐avian saurichians. This hypothesis should be tested in the future. Moreover, histological analyses with both decalcified and undecalcified sections of other extant archosaurs and their extant sister taxa will have to be made to fully understand the process by which non‐periosteal archosaurian sutural tissues form, and whether this alternative mode of ossification is plesiomorphic or apomorphic in archosaur sutures.
This study significantly widens the taxonomic breadth of suture research and therefore enhances general appreciation of suture biology in all vertebrates. The authors hope it represents an important step forward in expanding the spectrum of surveyed vertebrates, which will facilitate a more complete analysis of the evolution of suture microstructure. Moreover, as skeletal pathologies are often the expression of reverted plesiomorphic conditions of amniotes (Gaengler, 2000), a greater understanding of non‐mammalian amniotes is likely to inform research into craniosynostosis, the premature fusion of the bones of the skulls of humans (Cohen, 2000).
Acknowledgements
The authors thank the MOR and the Gabriel Lab for Cellular and Molecular Paleontology for providing the facilities to process the petrographic thin‐sections. This work was funded by Whitney McMillan, the Jurassic Foundation, Sigma‐Xi, the Evolving Earth Foundation and the Geological Society of America. AMB would like to thank Holly Woodward‐Ballard, Christian Heck, Ellen‐T Lamm, Bob Harmon and Carrie Ancell for their help and for various trainings. Technical help was also provided by Christian Heck, Cary Woodruff, Jacob Gardner, Holley Flora, Brian Baziak and Jamie Jette. Ellen‐T Lamm previously had made many of the pachycephalosaur slides. Tresa Goins stained some slides with Masson's trichrome, Damien Laudier embedded specimens in PMMA. For providing frozen specimens, the authors greatly thank Don Collins at Montana Emu Ranch and Ruth Elsey at the Rockefeller Wildlife Refuge. Mark Goodwin gave permission to section the UCMP specimen. Patrick Bannon prepared emu skulls at Skull Taxidermy (Deer Lodge, MT). Nick Atwood edited earlier versions of this manuscript. Discussions and/or insights on various aspects of histology and paleontology were provided by Brian K. Hall, Susan Gibson, David Varricchio, Holly Woodward‐Ballard, Mark Goodwin, Elizabeth Freedman‐Fowler, Matthew Vickaryous, Maurits Persson, Stephen Smith and Andrew Lee. Finally, the authors thank two anonymous reviewers, and their editors Julia Clarke and Edward Fenton for their helpful comments on this manuscript.
References
- Adams JS, Organ CL (2005) Histologic determination of ontogenetic patterns and processes in hadrosaurian ossified tendons. J Vertebr Paleontol 25, 614–622. [Google Scholar]
- Bailleul AM, Hall BK, Horner JR (2012) First evidence of dinosaurian secondary cartilage in the post‐hatching skull of Hypacrosaurus stebingeri (Dinosauria, Ornithischia). PLoS ONE 7, e36112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailleul AM, Hall BK, Horner JR (2013) Secondary cartilage revealed in a non‐avian dinosaur embryo. PLoS ONE 8, e56937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailleul AM, Nyssen‐Behets C, Lengelé B, et al. (2015) Chondroid bone in dinosaur embryos and nestlings (Ornithischia: Hadrosauridae): insights into the growth of the skull and the evolution of skeletal tissues. CR Palevol 15, 49–64. [Google Scholar]
- Bailleul AM, Scannella JB, Horner JR, Evans DC (2016) Fusion Patterns in the Skulls of Modern Archosaurs Reveal That Sutures Are Ambiguous Maturity Indicators for the Dinosauria. PLoS ONE 11(2), e0147687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker RT, Williams M (1988) Nanotyrannus, a new genus of pygmy tyrannosaur, from the latest Cretaceous of Montana. Hunteria 1, 1–30. [Google Scholar]
- Bärmann EV, Sánchez‐Villagra MR (2012) A phylogenetic study of late growth events in a mammalian evolutionary radiation – the cranial sutures of terrestrial artiodactyl mammals. J Mamm Evol 19, 43–56. [Google Scholar]
- Barreto C, Albrecht RM, Bjorling DE, et al. (1993) Evidence of the growth‐plate and the growth of long bones in juvenile dinosaurs. Science 262, 2020–2023. [DOI] [PubMed] [Google Scholar]
- Berge JCV, Storer RW (1995) Intratendinous ossification in birds: a review. J Morphol 226, 47–77. [DOI] [PubMed] [Google Scholar]
- Bloore JA, Furstman L, Bernick S (1969) Postnatal development of the cat palate. Am J Orthod 56, 505–515. [DOI] [PubMed] [Google Scholar]
- Brochu CA (1996) Closure of neurocentral sutures during crocodilian ontogeny: implications for maturity assessment in fossil archosaurs. J Vertebr Paleontol 16, 49–62. [Google Scholar]
- Cendekiawan T, Wong RW, Rabie ABM (2010) Relationships between cranial base synchondroses and craniofacial development: a review. Open Anat J 2, 67–75. [Google Scholar]
- Chabreck RH, Joanen T (1979) Growth rates of American alligators in Louisiana. Herpetologica 35, 51–57. [Google Scholar]
- Chinsamy‐Turan A (2001) Dinosaur growth: egg to adult In: Encyclopedia of Life Sciences, pp. 1–9. [Google Scholar]
- Cohen MM (1993) Sutural biology and the correlates of craniosynostosis. Am J Med Genet 47, 581–616. [DOI] [PubMed] [Google Scholar]
- Cohen M Jr (2000) Merging the old skeletal biology with the new. I. Intramembranous ossification, endochondral ossification, ectopic bone, secondary cartilage, and pathologic considerations. J Craniofac Genet Dev Biol 20, 84–93. [PubMed] [Google Scholar]
- Cole A, Fedak T, Hall B, et al. (2003) Sutures joining ontogeny and fossils. Palaeontol Assoc Newsl 52, 29–32. [Google Scholar]
- Curtis N, Jones M, Evans S, et al. (2013) Cranial sutures work collectively to distribute strain throughout the reptile skull. J R Soc Interface 10, 20130442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S (2002) Ratites and Tinamous: Tinamidae, Rheidae, Dromaiidae, Casuariidae, Apterygidae, Struthionidae (Bird Families of the World). New York, NY: Oxford University Press. [Google Scholar]
- Dixon AD, Gakunga PT (1993) Morphometric changes in growth of the rat pelvis after papain administration. Anat Rec 235, 312–318. [DOI] [PubMed] [Google Scholar]
- Dorenbos J (1973) Morphogenesis of the spheno‐occipital and the presphenoidal synchondrosis in the cranial base of the fetal Wistar rat. Acta Morphol Neerl Scand 11, 63–74. [PubMed] [Google Scholar]
- Ekanayake S, Hall BK (1988) Ultrastructure of the osteogenesis of acellular vertebral bone in the Japanese medaka, Oryzias latipes (Teleostei, Cyprinidontidae). Am J Anat 182, 241–249. [DOI] [PubMed] [Google Scholar]
- Enlow DH (1990) Facial Growth. University of Michigan: SPCK Publishing. [Google Scholar]
- Evans S (2008) The skull of lizards and tuatara In: The Skull of Lepidosauria, Biology of the Reptilia. (eds Gans C, Gaunt A, Alder K.), pp. 1–347. Ithaca, NY: Society for the Study of Amphibians and Reptiles. [Google Scholar]
- Francillon‐Vieillot H, De Buffrénil V, Castanet J, et al. (1990) Microstructure and mineralization of vertebrate skeletal tissues In: Skeletal Biomineralization: Patterns, Processes and Evolutionary Trends. (ed. Carter JG.), pp. 175–234. Washington, D.C.: American Geophysical Union. [Google Scholar]
- Friede H (1973) Histology of the premaxillary‐vomerine suture in a bilateral cleft case. Cleft Palate J 10, 14–22. [PubMed] [Google Scholar]
- Gaengler P (2000) Evolution of tooth attachment in lower vertebrates to tetrapods In: Development, Function and Evolution of Teeth. (eds Teaford MF, Smith MM, Ferguson WFM.), pp. 173–185. New York: Cambridge University Press. [Google Scholar]
- Galton PM (1970) Pachycephalosaurids: dinosaurian battering rams. Discovery 6, 22–32. [Google Scholar]
- Goodwin MB, Horner JR (2004) Cranial histology of pachycephalosaurs (Ornithischia: Marginocephalia) reveals transitory structures inconsistent with head‐butting behavior. Paleobiology 30, 253–267. [Google Scholar]
- Goret‐Nicaise M, Lengele B, Dhem A (1984) The function of Meckel's and secondary cartilages in the histomorphogenesis of the cat mandibular symphysis. Arch Anat Microsc Morphol Exp 73, 291–303. [PubMed] [Google Scholar]
- Goret‐Nicaise M, Manzanares M, Bulpa P, et al. (1988) Calcified tissues involved in the ontogenesis of the human cranial vault. Anat Embryol 178, 399–406. [DOI] [PubMed] [Google Scholar]
- Goswami A, Foley L, Weisbecker V (2013) Patterns and implications of extensive heterochrony in carnivoran cranial suture closure. J Evol Biol 6, 1294–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines RW, Mohuiddin A (1968) Metaplastic bone. J Anat 103, 527–538. [PMC free article] [PubMed] [Google Scholar]
- Hall BK (1967) The distribution and fate of the adventitious cartilage in the skull of the eastern rosella, Platycerus eximius (Aves: Psittaciformes). Aust J Zool 15, 685–698. [Google Scholar]
- Hall BK (1968) The fate of adventitious and embryonic articular cartilage in the skull of the common fowl, Gallus domesticus (Aves: Phasianidae). Aust J Zool 16, 795–805. [Google Scholar]
- Hall B (2005) Bones and Cartilage: Developmental and Evolutionary Skeletal Biology. London: Elsevier Academic Press. [Google Scholar]
- Herring S (1972) Sutures – a tool in functional cranial analysis. Cells Tissues Organs 83, 222–247. [DOI] [PubMed] [Google Scholar]
- Herring S (2000) Sutures and craniosynostosis: a comparative, functional, and evolutionary perspective In: Craniosynostosis: Diagnosis, Evaluation, and Management, 2nd edn (eds Cohen MM, MacLean RE.), pp. 3–10. New York, NY: Oxford University Press. [Google Scholar]
- Herring SW, Mucci RJ (1991) In vivo strain in cranial sutures: the zygomatic arch. J Morphol 207, 225–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton DR, Becker LE, Muakkassa KF, et al. (1984) Lambdoid synostosis: Part 1: the lambdoid suture: normal development and pathology of “synostosis”. J Neurosurg 61, 333–339. [DOI] [PubMed] [Google Scholar]
- Holliday CM, Witmer LM (2008) Cranial kinesis in dinosaurs: intracranial joints, protractor muscles, and their significance for cranial evolution and function in diapsids. J Vertebr Paleontol 28, 1073–1088. [Google Scholar]
- Horner JR, Goodwin MB (2009) Extreme cranial ontogeny in the Upper Cretaceous dinosaur Pachycephalosaurus . PLoS ONE 4, e7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner JR, Lamm E‐T (2011) Ontogeny of the parietal frill of Triceratops: a preliminary histological analysis. CR Palevol 10, 439–452. [Google Scholar]
- Horner JR, Woodward HN, Bailleul AM (2015) Mineralized tissues in dinosaurs interpreted as having formed through metaplasia: a preliminary evaluation. CR Palevol 15, 176–196. [Google Scholar]
- Ikejiri T (2012) Histology‐based morphology of the neurocentral synchondrosis in Alligator mississippiensis (Archosauria, Crocodylia). Anat Rec 295, 18–31. [DOI] [PubMed] [Google Scholar]
- Irmis RB (2007) Axial skeleton ontogeny in the Parasuchia (Archosauria: Pseudosuchia) and its implications for ontogenetic determination in archosaurs. J Vertebr Paleontol 27, 350–361. [Google Scholar]
- Jaslow C, Biewener A (1995) Strain patterns in the horncores, cranial bones and sutures of goats (Capra hircus) during impact loading. J Zool 235, 193–210. [Google Scholar]
- Jones ME, Curtis N, Fagan MJ, et al. (2011) Hard tissue anatomy of the cranial joints in Sphenodon (Rhynchocephalia): sutures, kinesis, and skull mechanics. Palaeontol Electronica 14, 17A. [Google Scholar]
- Jónsdóttir S, Giesen E, Maltha J (2012) The biomechanical behaviour of the hyalinized periodontal ligament in dogs during experimental orthodontic tooth movement. Eur J Orthod 34, 542–546. [DOI] [PubMed] [Google Scholar]
- Kokich VG (1976) Age changes in the human frontozygomatic suture from 20 to 95 years. Am J Orthod 69, 411–430. [DOI] [PubMed] [Google Scholar]
- Kokich V (1986) The biology of sutures In: Craniosynostosis: Diagnosis, Evaluation, and Management. (ed. Cohen MM.), pp. 81–103. [Google Scholar]
- Kuehnel W (2003) Color Atlas of Cytology, Histology, and Microscopic Anatomy. Stuttgart: Thieme. [Google Scholar]
- Lamm E‐T (2013) Preparation and Sectioning of Specimens In: Bone Histology of Fossil Tetrapods: Advancing Methods, Analysis, and Interpretation, pp. 55–160. University of California Press. [Google Scholar]
- Lee AH, Werning S (2008) Sexual maturity in growing dinosaurs does not fit reptilian growth models. Proc Natl Acad Sci USA 105, 582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengelé B (1997) Le tissu chondroïde dans le squelette en croissance, pp. 224 Louvain: Université Catholique de Louvain. [Google Scholar]
- Lengelé B, Dhem A, Schowing J (1990) Early development of the primitive cranial vault in the chick embryo. Journal of Craniofacial Genetics and Developmental Biology 10, 103–112. [PubMed] [Google Scholar]
- Lengelé B, Schowing J, Dhem A (1996) Embryonic origin and fate of chondroid tissue and secondary cartilages in the avian skull. Anat Rec 246, 377–393. [DOI] [PubMed] [Google Scholar]
- Longrich NR, Field DJ (2012) Torosaurus is not Triceratops: ontogeny in chasmosaurine ceratopsids as a case study in dinosaur taxonomy. PLoS ONE 7, e32623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main RP, de Ricqlès A, Horner JR, et al. (2005) The evolution and function of thyreophoran dinosaur scutes: implications for plate function in stegosaurs. Paleobiology 31, 291–314. [Google Scholar]
- Manzanares M, Goret‐Nicaise M, Dhem A (1988) Metopic sutural closure in the human skull. J Anat 161, 203–215. [PMC free article] [PubMed] [Google Scholar]
- Marieb EN (2014) Essentials of Human Anatomy & Physiology. New York: Pearson Education. [Google Scholar]
- Markey MJ, Main RP, Marshall CR (2006) In vivo cranial suture function and suture morphology in the extant fish Polypterus: implications for inferring skull function in living and fossil fish. J Exp Biol 209, 2085–2102. [DOI] [PubMed] [Google Scholar]
- Meunier F (1989) The acellularisation process in osteichthyan bone In: Trends in Vertebrate Morphology. (eds Splechtna Heinz, Hilgers Helge.), pp. 443–446. Stuttgart: Fischer. [Google Scholar]
- Mezzasalma M, Maio N, Guarino FM (2014) To move or not to move: cranial joints in European gekkotans and lacertids, an osteological and histological perspective. Anat Rec 297, 463–472. [DOI] [PubMed] [Google Scholar]
- Minnaar P, Minnaar M (1992) The Emu Farmer's Handbook. Groveton, Texas: Induna Company. [Google Scholar]
- Moss ML (1958) Fusion of the frontal suture in the rat. Am J Anat 102, 141–165. [DOI] [PubMed] [Google Scholar]
- Moss ML (1961) Osteogenesis of acellular teleost fish bone. Am J Anat 108, 99–109. [Google Scholar]
- Moss ML (1963) The biology of acellular teleost bone. Ann N Y Acad Sci 109, 337–350. [DOI] [PubMed] [Google Scholar]
- Opperman LA (2000) Cranial sutures as intramembranous bone growth sites. Dev Dyn 219, 472–485. [DOI] [PubMed] [Google Scholar]
- Opperman LA, Gakunga PT, Carlson DS (2005) Genetic factors influencing morphogenesis and growth of sutures and synchondroses in the craniofacial complex. Semin Orthod 11, 199–208. [Google Scholar]
- Padian K, de Ricqlès AJ, Horner JR (2001) Dinosaurian growth rates and bird origins. Nature 412, 405–408. [DOI] [PubMed] [Google Scholar]
- Payne SL, Holliday CM, Vickaryous MK (2011) An osteological and histological investigation of cranial joints in geckos. Anat Rec 294, 399–405. [DOI] [PubMed] [Google Scholar]
- Persson M (1973) Structure and growth of facial sutures: histologic, microangiographic and autoradiographic studies in rats and a histologic study in man. Odontologisk Revy 24(Supplement 26), 1–146.4514061 [Google Scholar]
- Persson M, Thilander B (1977) Palatal suture closure in man from 15 to 35 years of age. Am J Orthod 72, 42–52. [DOI] [PubMed] [Google Scholar]
- Persson M, Magnusson B, Thilander B (1978) Sutural closure in rabbit and man: a morphological and histochemical study. J Anat 125, 313–321. [PMC free article] [PubMed] [Google Scholar]
- Pritchard J, Scott J, Girgis F (1956) The structure and development of cranial and facial sutures. J Anat 90, 73–86. [PMC free article] [PubMed] [Google Scholar]
- Rafferty KL, Herring SW (1999) Craniofacial sutures: morphology, growth, and in vivo masticatory strains. J Morphol 242, 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayfield EJ (2005) Using finite element analysis to investigate suture morphology: A case study using large carnivorous dinosaurs. The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology 283, 349–365. [DOI] [PubMed] [Google Scholar]
- Roberts G, Blackwood H (1983) Growth of the cartilages of the mid‐line cranial base: a radiographic and histological study. J Anat 136, 307–320. [PMC free article] [PubMed] [Google Scholar]
- Rooney P (1994) Intratendinous ossification. Bone 8, 47–83. [Google Scholar]
- Scheyer TM, Sander PM (2004) Histology of ankylosaur osteoderms: implications for systematics and function. J Vertebr Paleontol 24, 874–893. [Google Scholar]
- Schott RK, Evans DC, Goodwin MB, et al. (2011) Cranial ontogeny in Stegoceras validum (Dinosauria: Pachycephalosauria): a quantitative model of pachycephalosaur dome growth and variation. PLoS ONE 6, e21092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno PC, Tan L, Brusatte SL, et al. (2009) Tyrannosaurid skeletal design first evolved at small body size. Science 326, 418–422. [DOI] [PubMed] [Google Scholar]
- Sitsen A (1933) Zur Entwicklung der Nähte des Schädeldaches. Anat Embryol 101, 121–152. [Google Scholar]
- Sun Z, Lee E, Herring SW (2004) Cranial sutures and bones: growth and fusion in relation to masticatory strain. Anat Rec A Discov Mol Cell Evol Biol 276, 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thilander B, Ingervall B (1973) The human spheno‐occipital synchondrosis II. A histological and microradiographic study of its growth. Acta Odontol 31, 323–334. [DOI] [PubMed] [Google Scholar]
- Trueta J, Trias A (1961) The vascular contribution to osteogenesis IV. The effect of pressure upon the epiphysial cartilage of the rabbit. J Bone Joint Surg Br 43, 800–813. [DOI] [PubMed] [Google Scholar]
- Vickaryous MK, Hall BK (2008) Development of the dermal skeleton in Alligator mississippiensis (Archosauria, Crocodylia) with comments on the homology of osteoderms. J Morphol 269, 398–422. [DOI] [PubMed] [Google Scholar]
- Vinkka‐Puhakka H (1991) Craniofacial secondary cartilages in the hamster and rat In: Fundamentals of Bone Growth: Methodology and Applications. (eds Dixon AD, Sarnat BG, Hoyte DA.), pp. 131–137. Boca Raton, FL: CRC Press. [Google Scholar]
- Witmer LM (1995) The extant phylogenetic bracket and the importance of reconstructing soft tissues in fossils In: Functional Morphology in Vertebrate Paleontology. (ed. Thomason J.), pp. 19–33. New York, NY: Cambridge University Press. [Google Scholar]
- Woodward AR, White JH, Linda SB (1995) Maximum size of the alligator (Alligator mississippiensis). J Herpetol 29, 507–513. [Google Scholar]


