Abstract
Digital methodologies for rendering the gross morphology of the brain from X‐ray computed tomography data have expanded our current understanding of the origin and evolution of avian neuroanatomy and provided new perspectives on the cognition and behavior of birds in deep time. However, fossil skulls germane to extracting digital endocasts from early stem members of extant avian lineages remain exceptionally rare. Data from early‐diverging species of major avian subclades provide key information on ancestral morphologies in Aves and shifts in gross neuroanatomical structure that have occurred within those groups. Here we describe data on the gross morphology of the brain from a mid‐to‐late Paleocene penguin fossil from New Zealand. This most basal and geochronologically earliest‐described endocast from the penguin clade indicates that described neuroanatomical features of early stem penguins, such as lower telencephalic lateral expansion, a relatively wider cerebellum, and lack of cerebellar folding, were present far earlier in penguin history than previously inferred. Limited dorsal expansion of the wulst in the new fossil is a feature seen in outgroup waterbird taxa such as Gaviidae (Loons) and diving Procellariiformes (Shearwaters, Diving Petrels, and allies), indicating that loss of flight may not drastically affect neuroanatomy in diving taxa. Wulst enlargement in the penguin lineage is first seen in the late Eocene, at least 25 million years after loss of flight and cooption of the flight stroke for aquatic diving. Similar to the origin of avian flight, major shifts in gross brain morphology follow, but do not appear to evolve quickly after, acquisition of a novel locomotor mode. Enlargement of the wulst shows a complex pattern across waterbirds, and may be linked to sensory modifications related to prey choice and foraging strategy.
Keywords: avian neuroanatomy, penguins, waterbirds, wing‐propelled diving, wulst
Introduction
Current understanding of the evolution of gross avian neuroanatomy in deep time was notably expanded by modeling of external brain structure from X‐ray computed tomography (CT) of fossil avian skulls, providing a novel approach for investigating the evolution of avian sensory systems and behavior (Balanoff et al. 2015a; Wood & De Pietri, 2015). In spite of their rarity, studies of avian and other maniraptoran endocasts elucidated the complicated early evolution, modification, and modernization of the avian brain in the Mesozoic (Walsh & Milner, 2010; Balanoff et al. 2013, 2014, 2015b) as well as evolution of the neuroanatomy of modern avian clades and genera (Milner & Walsh, 2009; Ksepka et al. 2012; Paulina‐Carabajal et al. 2015; Tambussi et al. 2015). In addition, several authors utilized digital methods to investigate variation in the structure and evolution of the avian brain endocasts across multiple related extant and extinct taxa (Ashwell & Scofield, 2008; Corfield et al. 2008; Scofield & Ashwell, 2009; Ksepka et al. 2012; Smith & Clarke, 2012; Walsh et al. 2013; Kawabe et al. 2014; Carril et al. 2015), providing insight into avian neuroanatomical variation as it relates to phylogeny, sensory function, and ecology.
Several of those comparative works focused on the neuroanatomy of wing‐propelled diving birds within Charadriiformes and the waterbird clade (Ksepka et al. 2012; Smith & Clarke, 2012; Kawabe et al. 2014; Tambussi et al. 2015). Those authors were interested in attempting to identify neuroanatomical changes that may be associated with the acquisition of that novel diving behavior (Ksepka et al. 2012; Smith & Clarke, 2012; Kawabe et al. 2014; Paulina‐Carabajal et al. 2015; Tambussi et al. 2015). Penguins in particular have provided a greater sample of brain endocasts than most lineages due to a robust fossil record that was the focus of considerable recent research (e.g. Clarke et al. 2003, 2007, 2010; Tambussi et al. 2005, 2015; Slack et al. 2006; Acosta Hospitaleche et al. 2007; Ksepka & Clarke, 2010; Fordyce & Thomas, 2011; Ksepka et al. 2012; Jadwiszczak et al. 2013; Chavez‐Hoffmeister, 2014). Within the course of their evolution, penguins underwent a radical shift in locomotor ecology from using the wing in aerial locomotion to the loss of aerial flight and cooption of the flight stroke for deep‐diving behaviors (Simpson, 1946). The nature of that shift and relative availability of fossil specimens makes this evolutionary transition a compelling system for examining the relationship between neuroanatomical evolution, sensory systems, and locomotor behaviors in birds.
Our understanding of the evolution of gross neuroanatomy of penguins in deep time has been informed by study of an array of digital endocasts sourced from fossil and extant penguins. For one extant genus (Pygoscelis), fossil endocasts indicate that the gross neuroanatomy in this clade may have been conserved since the Miocene (Paulina‐Carabajal et al. 2015). Indeed, examination of a late Miocene fossil stem lineage penguin, Paraptenodytes, showed that many of the features shared by extant penguin species were in place prior to the radiation of the crown clade (Ksepka et al. 2012). However, Paraptenodytes also possesses attributes such as an interaural pathway that are consistent with potential differences in sound localization in stem taxa and changes in skeletal ontogeny in the penguin lineage (Ksepka et al. 2012). Recently, several endocasts from isolated skulls of stem penguins from the late Eocene of Antarctica (~ 34.2–36 Ma; Dingle & Lavelle, 1998) were described (Tambussi et al. 2015). Those specimens showed that several shifts in gross neuroanatomy as reflected in cranial endocasts occurred in the course of penguin evolution, including an increase in overlap of the cerebellum by the telencephalon, loss of cerebellar folds impressions on the endocranium, lateral expansion of the telencephalon, caudal expansion of the wulst, reduction in the size of the olfactory bulb, reduction in intracranial pneumatization, and reduction in diameter of the semicircular canals (Ksepka et al. 2012; Tambussi et al. 2015). Although this recent work has closed important gaps in our knowledge of the endocranial anatomy of Paleogene stem penguins, endocrania of many taxa, even those with well‐preserved crania, have not been described. Here we report on the earliest known cranial endocast of a stem penguin. Data were acquired from a skull with associated postcranial material from the middle to late Paleocene of New Zealand (~ 56–61.5 Ma; Raine et al. 2015). This specimen provides a window into early gross neuroanatomical evolution in penguins that is approximately twice as old as the oldest previously described digital endocast in the clade (Tambussi et al. 2015). In addition, we discuss its implications for understanding penguin neuroanatomical evolution in relation to flightless wing‐propelled diving, penguin sensory ecology, and waterbird neuroanatomical variation.
Neuroanatomy
Materials and methods
Target specimen
A well‐preserved fossil of a stem penguin, CM 2013.27.1, housed at the Canterbury Museum (CM) in Christchurch, New Zealand, was CT‐scanned (Fig. 1). This fossil comes from a pyritized concretion in the middle to late Paleocene Waipara Greensand of the South Island of New Zealand. The horizon from which the concretion was collected is approximately 30 m above the contact between the Waipara Greensand and mudstones of the Loburn Formation [New Zealand Fossil Record Site Number M34/f0655 (‐43.058169,172.593796)], just above a prominent Waiparaconus bed. This fossil is from the same deposits that produced specimens of the oldest known stem penguin, Waimanu, which is the most basally diverging known lineage of penguins (Slack et al. 2006). The exact stratigraphic relationships of sites within the Waipara Greensand are poorly understood (Morgans et al. 2005), although this site is significantly higher in the section than the type locality of Waimanu manneringi. Its position approximately 30 m above the Loburn formation is consistent with a Teurian age, ~ 56–66 Ma (Hollis et al. 2012; Raine et al. 2015), shared with the other known Waimanu remains (Slack et al. 2006).
Figure 1.

Lateral and dorsal views of the skull block of CM 2013.27.1 (A) and 3D‐rendering of the brain endocast visible in lateral and dorsal aspect (B). CM 2013.27.1 possesses a minutely expanded wulst relative to other sampled penguins and waterbird taxa. In addition, it possesses a large floccular lobe like other penguins, and lacks cerebellar folds. Scale bar: 2.5 cm. ce, cerebellum; el, endosseus labyrinth; fl, floccular lobe; ol, optic lobe; os, occipital sinus impression; pb, pituitary bulb; t, telencephalon; w, wulst.
CM 2013.27.1 comprises a mostly complete skull, including the rostrum and mandible, both quadrates, two cervical vertebrae adhered to the caudal margin of the skull, and right scapula (Fig. 1). Additional material is prepared separately from the skull block, including five thoracic vertebrae, multiple ribs, right ilium and partial right ischium, complete right coracoid, partial right humerus, and complete tarsometatarsus. The braincase is in good condition but has been dorsoventrally crushed, deforming the ethmoidal region and displacing the frontal caudally, resulting in intrusion of the frontal into the endocranial cavity. Contrast between the matrix and the skull provides a good view of overall endocranial anatomy, though finer details such as cranial nerve canals and the endosseus labyrinth are less apparent and so were not digitally reconstructed.
CM 2013.27.1 is referred to the stem sphenisciform taxon Waimanu by a unique combination of characters proposed to diagnose that taxon relative to all other named Sphenisciformes: a scapular blade that does not expand distally, strongly laterally excavated thoracic vertebrae, and an elongate, waisted tarsometatarsus with a slightly plantarly deflected trochlea metatarsal II, prominent medial hypotarsal crest with two low but distinct lateral and intermediate crests, and shallow intermetatarsal grooves on the dorsal aspect (Slack et al. 2006; Ksepka & Clarke, 2010). The humerus is flattened and thickened relative to volant taxa – both features indicative of a flightless wing‐propelled diving ecology (Ksepka & Clarke, 2010). Assessment of the taxonomic status of the new specimen relative to the two named species of Waimanu and a full description is in preparation as a separate work. CM 2013.27.1 represents one of the oldest examples of the penguin lineage and is only the third stem penguin skull to be recovered from the Paleocene (Slack et al. 2006; Ksepka & Clarke, 2010).
The paratype specimens of Waimanu tuatahi (CM Zfa 33; CM Zfa 34) also include skull material (Slack et al. 2006). Although not reconstructed in 3D, we provide comparisons between the new specimen and these paratype specimens where possible. Pilot scans of CM Zfa 33 show that the braincase is moderately crushed and is significantly laterally deformed (see Supporting Information Video S3). Partial preservation of the telencephalon is apparent, although low‐resolution scan data makes preservation of other regions of the brain difficult to assess. This is in contrast to CM Zfa 34, where pilot scans show that the skull is considerably deformed mediolaterally, particularly in the region of the telencephalon, with little of the morphology of the braincase preserved (see Supporting Information Video S4).
Methods
We scanned CM 2013.27.1 using an NSI scanner at the UTCT facility at The University of Texas at Austin. Scans consisted of 2165 total slices scanned at 200 kV and 0.27 mA. Inter‐slice spacing was 0.071 mm in the z dimension (coronal), 0.67 mm in the x and y dimensions, with a maximum fei Software field of view of 68.2 mm. Resulting data consisted of a series of 16‐bit TIFF files. We used avizo v.6.1 (http://www.fei.com/software/avizo3d/) to process these scans and produce digital endocasts of the endocranium (Fig. 1) by digital segmentation of the endocranial cavity. We utilized avizo to post‐process and smooth the endocast mesh. We implemented meshlab v. 1.3.3 (http://meshlab.sourceforge.net/), avizo, and mimics v.17.0 (Materialise Software, http://biomedical.materialise.com/mimics) for anatomical description and comparison of the endocast with those described from other birds. We also employed imagej v. 1.50a (http://imagej.nih.gov/ij/) to examine individual 2D slices along the three anatomical planes to aid description and validate that features observed on the endocast are actually preserved in the fossil and are not the result of post‐processing (Fig. 2). Although the braincase is crushed dorsoventrally, we estimated endocranial volume by utilizing a previously published regression correlating endocranial volume with maximum width of the endocast, −log y = 2.913 × log x−0.495, where y equals endocranial volume in cubic centimeters and x equals maximum width of the telencephalon in centimeters (Kawabe et al. 2009). This allows for limited quantitative comparisons with previously published endocranial volumes in spite of an incomplete endocast. Low contrast between bone and matrix, as well as moderate crushing to the ear area prevented complete segmentation of the semicircular canals, although we attempted qualitative description of their anatomy from 2D slice images (Fig. 2). We also utilized 2D slice images for comparisons with the paratype specimens of Waimanu tuatahi.
Figure 2.
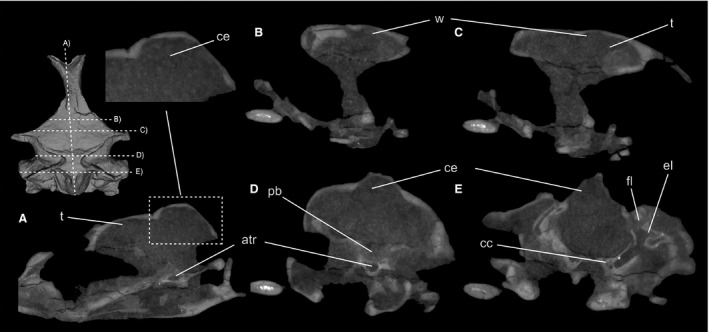
Two‐dimensional slice data in coronal view from CT scans of CM 2013.27.1, viewed in the rostral aspect. Major features of the endocast, including the relatively minute wulst and lack of cerebellar folding, are apparent in two‐dimensional slice data. An anterior tympanic recess is also present, as in other sampled stem penguins. atr, anterior tympanic recess; cc, carotid canal; ce, cerebellum; el, endosseus labyrinth; fl, floccular lobe; pb, pituitary bulb; t, telencephalon; w, wulst.
When discussing anatomical features of the brain, we refer to externally evident features of the brain endocast, following the example of previous workers (see Milner & Walsh, 2009; Ksepka et al. 2012; Smith & Clarke, 2012; Kawabe et al. 2014; Tambussi et al. 2015). In addition to anatomical description, we constructed a supertree (branch lengths = 1) of exemplar waterbird taxa that have been sampled for endocast data in previous studies (Fig. 3) (Ksepka et al. 2012; Kawabe et al. 2014; Tambussi et al. 2015). Placement of extant taxa follows recent molecular analyses (Hackett et al. 2008; Jarvis et al. 2014; Prum et al. 2015) that recover the same relationships among the major waterbird clades. The placement of plotopterids follows Olson (1989), Smith (2010), and Mayr et al. (2015), whereas the placement of stem penguins follows Ksepka & Clarke (2010). We then scored discrete endocranial characters for each taxon (characters 4–18 of Smith & Clarke, 2012), utilizing parsimony‐based ancestral state reconstruction in mesquite (version 3.04 build 725; Maddison & Maddison, 2015) to model endocranial character histories in waterbirds (see Supporting Information).
Figure 3.
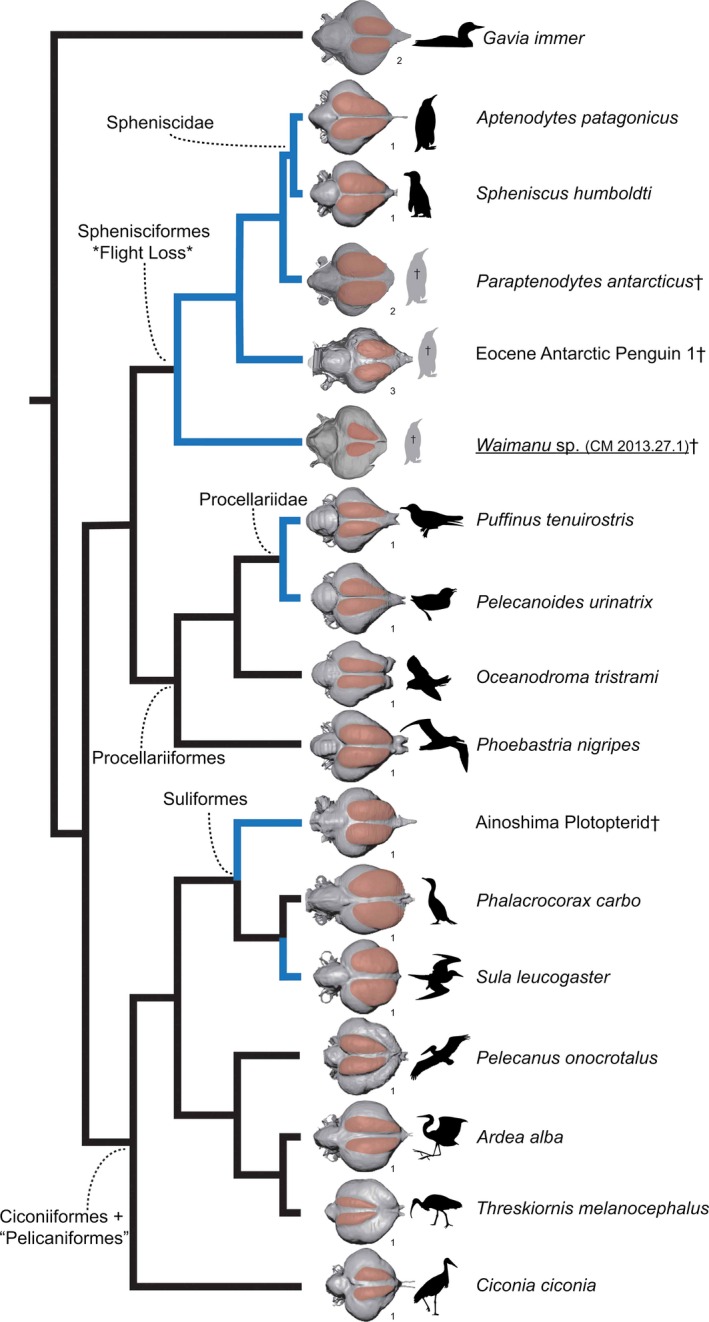
Comparative representation of waterbird endocasts in dorsal view mapped onto a supertree of waterbirds. Tree topology follows Hackett et al. (2008), Jarvis et al. (2014), and Prum et al. (2015), with the position of plotopterids corresponding to previous hypotheses proposed by Olson (1989), Smith (2010), and Mayr et al. (2015). The external distribution of the wulst is highlighted on each endocast. Blue branches identify taxa that utilize wing‐propelled diving. Endocasts are numbered according to their source: (i) Kawabe et al. (2014); (ii) Ksepka et al. (2012); (iii) Tambussi et al. (2015).
Results
Description
The long‐axis of the brain is parallel to the long‐axis of the skull, a condition observed in all crown penguins, fossil stem penguins such as Paraptenodytes antarcticus, and Gavia (Ksepka et al. 2012; Tambussi et al. 2015). The endocast resembles Gavia, Puffinus, and Eocene Antarctic stem penguins, more than Paraptenodytes, crown penguins, and other Procellariiformes with regard to overlap between the cerebral hemispheres and the cerebellum in lateral view (see Ksepka et al. 2012; Kawabe et al. 2014; Tambussi et al. 2015; Fig. 1). In other waterbird taxa such as storks, herons, cormorants, sulids, and plotopterids, the cerebellum and cerebral hemispheres show little overlap, whereas in taxa such as ibises and pelicans overlap is particularly marked (Kawabe et al. 2014). We estimate endocranial volume at 8.16 cm3, derived from a maximum telencephalic width of 3.04 cm. In comparison, other described stem penguins possess endocranial volumes in the range of 27.34–278.78 cm3 (Tambussi et al. 2015), whereas crown penguins possess endocranial volumes in the range of 9.79–26.27 (Kawabe et al. 2014; Tambussi et al. 2015). This volume estimate should be treated with caution given the significant crushing of the endocranial space in the new specimen.
Telencephalon
The olfactory bulbs manifest in penguin endocasts as relatively thin midline lobes extending rostrally toward the narial cavity (Ksepka et al. 2012; Paulina‐Carabajal et al. 2015). Preserved olfactory bulbs are known from endocasts of extinct penguins (Ksepka et al. 2012; Paulina‐Carabajal et al. 2015; Tambussi et al. 2015). All are similar in size to extant penguins, with the exception of the Eocene Antarctic Fossil 1 of Tambussi et al. 2015, which is larger than in extant penguins. Unfortunately, the olfactory bulbs of CM 2013.27.1 are not preserved due to intrusion of the frontal into the endocranium and overall crushing of the narial cavity.
More caudally, the cerebral hemispheres expand laterally (Fig. 1), producing a ‘heart‐shaped’ appearance in dorsal view, similar in extent to that of Eocene Antarctic stem penguins, most Procellariiformes, and Gavia. Relative to Paraptenodytes, crown penguins, and Phoebastria, the telencephalon is less laterally expanded compared with the width of the cerebellum, presenting a narrower profile (Ksepka et al. 2012; Kawabe et al. 2014; Tambussi et al. 2015).
The endocast of CM 2013.27.1 shows a distinct difference from other described stem and crown penguin endocasts (Ksepka et al. 2012; Kawabe et al. 2014; Tambussi et al. 2015) in that the wulst is not well‐developed (Figs 1 and 2). Although the skull has undergone a degree of dorsoventral crushing, examination of the dorsal aspect of the interior braincase in 2D indicates that the dorsal extent of the wulst – as comparable among endocasts– could not have been much larger than that currently preserved (Fig. 2; see also Supporting Information Video S1). Inspection of scan data from CM Zfa 33 indicates that the wulst shows similarly limited development in this Waimanu tuatahi paratype specimen (see Supporting Information Video S3). In the development of this structure, Waimanu most closely resembles the condition observed in Gavia, the wing‐propelled diving Procellariiformes Puffinus, and Pelecanoides in terms of its dorsal and anterior extent and position close to the cranial midline (Fig. 3) (Ksepka et al. 2012; Kawabe et al. 2014).
Described Antarctic stem penguin endocasts also possess a wulst that is less dorsally expanded than more crownward taxa, though it is not nearly as small as in CM 2014.27.1 (Tambussi et al. 2015). Procellariiformes such as Phoebastria and Oceanodroma, as well as other waterbird taxa including plotopterids, possess distinctly a more dorsally prominent and rostrally extensive wulst (Fig. 3) (Ksepka et al. 2012; Kawabe et al. 2014). Suliformes and plotopterids in particular possess rostral wulsts that protrude noticeably beyond the lateral margin of the cerebral hemispheres in dorsal aspect (Fig. 3), contrasting sharply with the limited rostral expansion of the wulst in the new basal penguin endocast (Kawabe et al. 2014). The wulst in CM 2013.27.1 is smaller than in most charadriiform wing‐propelled divers, but similar to murrelets in the taxa Brachyramphus and Synthliboramphus (Smith & Clarke, 2012).
Like previously described Antarctic Eocene stem penguins (Tambussi et al. 2015), the caudal margin of the wulst in CM 2013.27.1 does not approach the cerebellum as closely as it does in more crownward penguins (Fig. 3). Like the Miocene penguin Paraptenodytes and Eocene Antarctic stem penguins, CM 2013.27.1 possesses a wider interhemispheral fissure than crown penguins and most other waterbirds, including plotopterids (Fig. 3) (Ksepka et al. 2012; Kawabe et al. 2014; Tambussi et al. 2015).
Diencephalon
Compared with other parts of the brain, the endocast of the diencephelon is poorly preserved due to crushing of the ventral portion of the braincase. Consequentially, little can be said of its anatomy, though certain observations are possible. The primary portion of the endocast of the diencephalon preserved in CM 2013.27.1 is the caudal part of the pituitary fossa. As in Paraptenodytes and Eocene Antarctic Fossil 1, the pituitary fossa appears larger and more oblong than in extant penguins (Fig. 1), a conformation that has been ascribed to the confluence of the cranial carotid arteries with the pituitary fossa (Ksepka et al. 2012; Tambussi et al. 2015).
Mesencephalon
Only the right optic lobe of CM 2013.27.1 is preserved, though it has been subject to deformation. The lobe appears more caudally positioned relative to the telencephalon than in crown penguins, making it partially visible in dorsal view (Fig. 1; also see Supporting Information Fig. S1). This is similar to the condition observed in stem penguins as well as certain waterbird taxa such as Gavia and Phalacrocorax (Ksepka et al. 2012; Kawabe et al. 2014; Tambussi et al. 2015). Plotopterids also appear to possess caudally positioned optic lobes, though their dorsal visibility is difficult to judge due to deformation; that the cerebral hemispheres ‘considerably overlie the optic lobes’ was noted by Kawabe et al. (2014, pp. 484).
Metencephalon
The cast of the cerebellum of CM 2013.27.1 is well preserved, although medially deformed on the left side. The endocast does not appear to preserve cerebellar folds (Fig. 2), and a weak occipital sinus impression is present. Close examination of 2D scan data shows there is a lack of any laterally continuous grooves that might indicate the presence of cerebellar folds (Fig. 2). The absence of prominent cerebellar folds on endocasts is a condition observed in other wing‐propelled diving birds such as other penguins, plotoperids, and alcids (Ksepka et al. 2012; Smith & Clarke, 2012; Kawabe et al. 2014; Tambussi et al. 2015). Within Charadriiformes, indistinct cerebellar folds are optimized as apomorphies of wing‐propelled diving alcids (Smith & Clarke, 2012). The absence of cerebellar fold impressions on endocasts may be linked to the relative size of the occipital sinus and thickness of the meningeal tissue (Ksepka et al. 2012). Penguins, for example, possess elaborated cerebellar folds (Iwaniuk et al. 2006, 2007), although these folds do not preserve impressions on endocasts.
Elongate floccular sinuses are preserved in CM 2013.27.1, though their dorsoventral orientation appears distorted by deformation (Fig. 2; see Supporting Information Videos S1 & S2). A similarly developed floccular recess is also visible in scan data of Waimanu tuatahi CM Zfa 34 (see Supporting Information Video S4). Like stem Eocene Antarctic penguins, Paraptenodytes, other crown penguins, Procellariiformes, Gavia, and plotopterids, flocullar recesses are prominent and caudo‐laterally directed, whereas in other taxa they are not nearly as laterally well‐projected or as visible in dorsal view (Ksepka et al. 2012; Kawabe et al. 2014; Tambussi et al. 2015). In CM 2013.27.1 the lobes appear to be slightly dorsally deflected in comparison with other described penguins, and also appear relatively longer and more laterally compressed, though this may be due to deformation. The direction of floccular lobe projection is variable in extant penguins, because some taxa display more ventrally deflected floccular lobes than others (Tambussi et al. 2015).
Endosseus labyrinth
The robust semicircular canals of CM 2013.27.1 have a proportionally similar diameter to Eocene Antarctic stem penguins (Fig. 2; also see Supporting Information Fig. S2). Although they could only be partially reconstructed, the short crus communis and laterally originating horizontal semicircular canals are also similar to the morphologies in Eocene forms (Fig. 2) (Tambussi et al. 2015). A similar morphology is present in scan data of Waimanu tuatahi CM Zfa 34 (see Supporting Information Video S4).
Cranial vasculature
The carotid anastamosis and canals for the carotid arteries are preserved in CM 2013.27.1, though their caudal extent cannot be determined, particularly in the right canal. As in Eocene Antarctic Fossil 1 and Paraptenodytes, the carotid arteries converge to form a short anastamosis directly caudal to the pituitary fossa, conforming to an X‐type anastamosis (Baumel & Gerchman, 1968; Ksepka et al. 2012; Tambussi et al. 2015). This condition is also reported in the Spheniscus clade, Phoebastria, and Gavia (Ksepka et al. 2012).
The occipital sinus impression of CM 2013.27.1, although not well‐preserved, appears most similar in development to the diminutive impression in Spheniscus and Paraptenodytes (Ksepka et al. 2012). By contrast, in plotoperids, Suliformes, Pelecanus, or Ciconiiformes, it is more greatly developed, and in Procellariiformes and most crown penguins it is absent (Fig. 3) (Kawabe et al. 2014). Eocene Antarctic stem penguins do not appear to preserve a conspicuous occipital sinus, though this may be due to deformation (Tambussi et al. 2015). Indistinct occipital sinuses have also been reported in the Alcidae, another clade of wing‐propelled diving birds (Smith & Clarke, 2012).
Cranial pneumaticity
CM 2013.27.1 possesses an anterior tympanic recess (Fig. 2), a structure that is significantly reduced in extant penguins but present in Eocene and Miocene stem penguins (Ksepka et al. 2012; Tambussi et al. 2015). In CM 2013.27.1, the two tracks of the anterior tympanic recess meet slightly rostral to the carotid artery anastamosis and continue rostrally as a single cavity into the parasphenoid, which is largely fragmentary. Although its rostral‐most extent is obscured, the presence of the anterior tympanic recess supports the notion that minor reduction in cranial pneumaticity occurred later in penguin evolution (Ksepka et al. 2012). Minor pneumatization of the frontals, parietals, and postorbital bar in CM 2013.27.1 is similar to other stem penguins (Ksepka et al. 2012; Tambussi et al. 2015).
Discussion
One of the most significant aspects of investigating gross neuroanatomy in extinct taxa is their potential to inform our understanding of tempo and mode of neuroanatomical evolution in response to changes in behavior and ecology. New data sourced from late Paleocene penguin diversity increase our understanding of timing of evolutionary shifts in neuroanatomy relative to skeletal morphology following the evolution of wing‐propelled diving and loss of flight in penguins. Data from the new fossil endocast reveal that the relative proportions and orientation of the telencephalon and cerebellum were present in penguins at least 25 million years earlier than previously inferred from Eocene stem penguin endocasts. Telencephalic expansion and increase in overlap of the telencephalon and cerebellum obscuring the optic lobe, both notable features of crown penguin neuroanatomy, are present by the Miocene but are notably lacking in sampled Paleogene taxa (Ksepka et al. 2012; Tambussi et al. 2015). The new fossil endocast also indicates that a well‐developed floccular recess was also present early in penguin evolution. Prominent flocculi appear to be a general feature of diving taxa within waterbirds (Early et al. 2012), though flocculus size does not appear to be correlated with the capacity for maneuvering in three‐dimensional space, as in flight (Walsh et al. 2013) or wing‐propelled diving (Smith & Clarke, 2012).
Data from the new endocast indicate that distinct cerebellar fold impressions have been absent in the penguin lineage since the Paleocene. Correlation between a lack of cerebellar fold impressions and diving has been proposed (Elzanowski & Galton, 1991), and deep‐diving waterbird taxa consistently lack these impressions (Early et al. 2012), as do stem and crown members of the diving Alcidae (Smith & Clarke, 2012). However, there are diving taxa that possess cerebellar fold impressions, and non‐diving taxa that do not (Ksepka et al. 2012; Kawabe et al. 2014; Tambussi et al. 2015). Data from the new Paleocene endocast support a scenario where loss of fold impressions occurred close to loss of aerial flight in penguins. Within waterbirds, only Procellariiformes possess notable cerebellar fold impressions (Ksepka et al. 2012; Kawabe et al. 2014; Tambussi et al. 2015; Fig. 3).
Data from the new Paleocene specimen indicate enlarged semicircular canals – a feature remarked upon in stem penguins (Ksepka et al. 2012; Tambussi et al. 2015) – were also present early in penguin history. Enlarged horizontal canals might provide extra sensitivity to yaw motion, though functional explanations for their expanded diameter in stem penguins remain elusive (Tambussi et al. 2015). The semicircular canals of stem and crown wing‐propelled diving Alcidae within Charadriiformes are highly compressed and do not show enlargement as in stem penguins (Smith & Clarke, 2012). Although the significance of this morphology is unknown, the striking difference in the evolution of the semicircular canals within these two clades merits further study.
Multiple endocasts of extinct stem penguin taxa provide an unprecedented window into how aspects of neuroanatomy have changed with time within a single major avian subclade. Data from the new endocast in concert with other Paleogene stem penguins show that the wulst has undergone notable dorsal and caudal expansion in the course of penguin evolution. Smaller wulst size in stem avian taxa relative to their crown clade relatives has been noted in several aquatic taxa such as Prophaethon, a stem tropicbird, and Odontopteryx, a pelagornithid (Milner & Walsh, 2009; Ksepka et al. 2012). Lithornis, an extinct paleognath, possessed a notably smaller wulst than extant paleognaths, with the exception of the Kiwi (Ashwell & Scofield, 2008; Zelenintsky et al. 2011). In contrast to the new fossil and the taxa named above, Halcyornis, an Eocene Neoavian, possessed a wulst similar in size to many extant taxa (Walsh & Milner, 2010). Implications of the size of the wulst in Halcyornis are difficult to discern because it has highly uncertain taxonomic affinities, although its endocranial similarity to Charadriiformes, particularly Laridae & Alcidae, was noted by Smith & Clarke (2012). Data on the size of the wulst in Mesozoic stem birds are deficient – a small dorsal indentation on the telencephalon of Archaeopteryx may demarcate a minutely developed wulst (Balanoff et al. 2013). Marsh (1880) illustrated the brain endocasts of Hesperornis and Ichthyornis and did not reconstruct the presence of a wulst. However, Ichthyornis material has been considered to be too fragmentary to reconstruct brain morphology accurately (Edinger, 1951; Clarke, 2004) and Hesperornithine endocasts have not yet been published. The new penguin endocast and other stem avian taxa demonstrate that enlargement of the wulst occurred multiple times within different avian lineages. Taken together with data from stem members of extant avian clades and outgroup taxa, these data indicate that a diminutive wulst may be the plesiomorphic condition within crown Aves.
Various aspects of somatosensory and visual processing are known to occur in the wulst (Karten et al. 1973; Pettigrew & Konishi, 1976; Pettigrew, 1979; Pettigrew & Frost, 1985; Wild, 1987; Deng & Wang, 1993; Nieder, 2002; Baron et al. 2007; Harmening & Wagner, 2011). As a consequence, interpreting the expansion of the wulst within the penguin lineage is not straightforward. In the evolution of penguins, visual and somatosensory challenges of wing‐propelled diving have been invoked as a factor affecting wulst enlargement (Ksepka et al. 2012). In Caprimulgiformes and other extant birds, wulst size has been linked to binocular field size and orbit orientation (Iwaniuk & Wylie, 2006; Iwaniuk et al. 2008). Binocular field size and visual field orientation appears to be primarily linked to use of the bill in foraging, including in diving birds (Martin, 2007, 2014). Evidence from skull morphology indicates that penguins appear to have undergone a shift in prey choice in their evolution, from larger swimming prey in stem Paleogene taxa to smaller invertebrates and fish in more crownward taxa (Zusi, 1975; Livezey, 1989; Clarke et al. 2007; Haidr & Acosta Hospitaleche, 2014). This shift in prey choice may have resulted in changes to their visual fields, which may be reflected in changes in wulst development in the penguin lineage. Prey choice is known to affect visual field morphology in extant diving taxa such as alcids (Martin & Wanless, 2015). Although this is speculative and additional comparative data on waterbird brain morphology, feeding ecology, and visual systems from unsampled taxa such as loons and diving petrels are needed, the link between wulst morphology, the visual system, and foraging strategies may provide a novel direction for research.
As a stem member of an extant waterbird clade, the new penguin endocast also provides valuable context for examining gross neuroanatomical evolution in waterbirds and for assessing phylogenetic signal of endocast characters in this group. Phylogenetically informed studies of gross neuroanatomical characters remain uncommon – neuroanatomical apomorphies have been detected in two major avian clades: Charadriiformes (Smith & Clarke, 2012) and Psittaciformes (Carril et al. 2015). Comparative study of the endocranial features of the Paleognathae has also been examined, supporting the presence of endocranial apomorphies in this clade as well (Corfield et al. 2008). No analogous study has been published for waterbirds, although research assessing morphometric variation in endocast morphology to glean phylogenetic information has been published (Kawabe et al. 2014). That work diagnosed a basal split between Sphensciformes+Procellariiformes and Ciconiiformes+‘Pelicaniformes’ on the basis of a cerebellum width and floccular length and allied the extinct plotopterid clade to Sphensciformes+Procellariiformes (Kawabe et al. 2014). We find little discrete character evidence to support this relationship (see Supporting Information), recovering similarities between plotopterids and Suliformes such as rostrolaterally expanded wulsts and weak dorsa projection of the cerebellum. Although the relationship of plotopterids to penguins has been proposed (Mayr, 2005), recent analyses place plotopterids as the sister taxon to Suliformes – excluding Fregatidae (Mayr et al. 2015) – which is broadly consistent with previous phylogenetic hypotheses (Olson, 1989; Smith, 2010).
Within waterbirds, ancestral state reconstruction for discrete neuroanatomical characters reveals several gross neuroanatomical shifts that may be diagnostic of major subclades and show a phylogenetic signal. Specifically, Ciconiiformes+‘Pelecaniformes’ appears to be characterized by a rounded, blunt rostral margin of the forebrain region of the endocast and prominent occipital sinus impressions (Fig. 3). In contrast, Sphenisciformes+Procellariiformes share a rostrally tapering endocast. Given the presence of this tapered morphology in Gavia and tropicbirds (Milner & Walsh, 2009; Ksepka et al. 2012), this morphology may be the plesiomorphic condition in waterbirds.
Plotopterids are similar to Suliformes in that they share rostrolaterally expanded wulsts, as well as weak dorsal projection of the cerebellum. This constitutes a major difference between penguins and plotopterids, and the new specimen indicates that wulst evolution in the penguin lineage proceeded differently from Plotopterids and Suliformes, consisting of dorsal and caudal expansion rather than rostral expansion. Plotopterids also display less overlap between the cerebellum and telencephalon, as in Suliformes, than dp crown penguins or Procellariiformes. This condition may be plesiomorphic for waterbirds, as it is shared with Gavia, tropicbirds, and stem penguins, although it is less exaggerated in these taxa. An additional feature that appears plesiomorphic for waterbirds is the curve‐shaped contact between the telencephalon and the cerebellum (Fig. 3), which is apomorphic in most Procellariiformes, crown penguins, and ‘Pelicaniformes’, where it is v‐shaped (character 10 of Smith & Clarke, 2012) (Milner & Walsh, 2009; Ksepka et al. 2012; Kawabe et al. 2014; Tambussi et al. 2015).
Several noticeable similarities exist between the endocasts of Gavia, Puffinus, and stem penguins, specifically less dorsally projected wulsts, curve‐shaped junctions between the telencephalon and cerebellum, a wider cerebellum relative to the telencephalon width, and prominent flocculi. Furthermore, the diving petrel Pelecanoides shares a relatively diminutive wulst with all of these taxa (Ksepka et al. 2012; Kawabe et al. 2014). Similarities between outgroup tropicbirds and these taxa are also of note (Milner & Walsh, 2009), indicating that these features may reflect plesiomorphy in waterbird endocranial anatomy. Of additional interest are shared features between crown penguins and Phoebastria. Although phylogeny may largely explain these patterns of variation, ecological similarities between these taxa, namely diving behavior, may also be explanatory, in which case acquisition of diving behavior may shape gross neuroanatomy more than loss of flight in the penguin lineage. Little is known of the diving behavior of many procellariiform taxa, and diving behavior in taxa such as albatross (Prince et al. 1994) and tropicbirds (Le Corre, 1997) remain largely ignored in conversations surrounding diving bird evolution, which may lead to unwarranted assumptions about the relationship between gross neuroanatomy and ecology. Ultimately, additional comparative study of avian endocranial anatomy, along with behavioral and functional studies, are needed to examine the relationship of gross neuroanatomy, phylogeny, sensory function, and behavioral ecology in birds.
Conclusions
The oldest known penguin brain endocast, from the Paleocene of New Zealand, reveals that many features of stem penguin taxa were present very early in the evolution in this clade, and that modern penguin neuroanatomy is not linked to the evolution of flightless wing‐propelled diving itself. This novel locomotor shift shows a similarity with the acquisition of flight, as many gross features of extant avian brain morphology appear to arise long after the origin of flight. In addition, similarity between stem penguins and diving taxa such as loons, diving Procellariiformes, and outgroup tropicbirds support that the evolution of diving behavior may be more important in shaping waterbird neuroanatomy compared with the loss of flight. Alternatively, similarities between these taxa may indicate plesiomorphy in waterbird brain anatomy, indicating the need for greater comparative phylogenetic study of endocranial characters in waterbirds and Aves in general. Furthermore, the new specimen supports a complex pattern of wulst enlargement and reduction in Aves. Enlargement in penguins begins in the Eocene more than 25 million years after the gain of wing‐propelled diving and loss of flight. Correlated with changes in skull morphology, wulst enlargement may be linked to shifts in sensory tuning related to foraging for smaller prey. However, there are many gaps in our understanding of comparative waterbird neuroanatomy and its relationship to phylogeny, as well as to variations in sensory systems and behavior. If gross neuroanatomy is to serve as a window into the evolution of these anatomical and behavioral systems in deep time, increased collaborative study on extant avian taxa is warranted.
Supporting information
Fig. S1 . Lateral and anterior views of the 3D‐rendered endocast.
Fig. S2. Rostral to caudal (A–D) 2D coronal slices of the skull from CT scans of 2009.99.1, viewed from the rostral aspect. Labels indicate visible structures of the endosseus labyrinth.
Video S1. Rostral to caudal 2D coronal slice data from CT scans of 2009.99.1, viewed from the rostral aspect.
Video S2. Sagittal 2D slice data from CT scans of 2009.99.1, viewed from the left.
Video S3. 2D CT slice data of Waimanu tuatahi paratype CM Zfa 33 in coronal view, proceeding from caudal to rostral with respect to the cranial material.
Video S4. 2D CT slice data of Waimanu tuatahi paratype CM Zfa 34 in coronal view, proceeding from caudal to rostral with respect to the cranial material.
Acknowledgements
This project was funded by the National Science Foundation (DEB 0949897; GRFP DGE 1110007). We thank two anonymous reviewers and the Editor for their comments, which significantly improved this manuscript. We thank Taylor Watts for assistance in digital endocast rendering. We additionally thank Federico Degrange, Daniel Ksepka and Soichiro Kawabe for graciously providing permission to utilize figures of other waterbird endocasts. We also would like to acknowledge the assistance of Matt Colbert, UTCT, for scanning the specimen and for advice in endocast production and interpretation. This manuscript was also aided by the constructive comments of Chris Bell, Robert Burroughs, Chad Eliason, Lauren English, Chris Torres, Zhiheng Li, and Xia Wang.
References
- Acosta Hospitaleche C, Tambussi C, Donato M, et al. (2007) A new Miocene penguin from Patagonia and its phylogenetic relationships. Acta Palaeontol Pol 52, 299–314. [Google Scholar]
- Ashwell KWS, Scofield RP (2008) Big birds and their brains: paleoneurology of the New Zealand Moa. Brain Behav Evol 71, 151–166. [DOI] [PubMed] [Google Scholar]
- Balanoff AM, Bever GS, Rowe TB (2013) Evolutionary origins of the avian brain. Nature 501, 93–96. [DOI] [PubMed] [Google Scholar]
- Balanoff AM, Bever GS, Norell MA (2014) Reconsidering the avian nature of the oviraptorosaur brain (Dinosauria: Theropoda). PLoS One 9, e113559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balanoff AM, Bever GS, Colbert MW, et al. (2015a) Best practices for digitally constructing endocranial casts: examples from birds and their dinosaurian relatives. J Anat Early View. doi:10.1111/joa.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balanoff AM, Smaers JB, Turner AH (2015b) Brain modularity across the theropod‐bird transition: testing the influence of flight on neuroanatomical variation. J Anat Early View. doi:10.1111/joa.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron J, Pinto L, Dias MO, et al. (2007) Directional responses of the visual wulst neurons to grating and plaid patterns in the awake owl. Eur J Neurosci 26, 1950–1968. [DOI] [PubMed] [Google Scholar]
- Baumel JJ, Gerchman L (1968) The avian intercarotid anastomosis and its homologue in other vertebrates. Am J Anat 122, 1–18. [DOI] [PubMed] [Google Scholar]
- Carril J, Tambussi CP, Degrange FJ, et al. (2015) Comparative brain morphology of neotropical parrots (Aves, Psittaciformes) inferred from virtual 3D endocasts. J Anat Early View. doi:10.1111/joa.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez‐Hoffmeister M (2014) Phylogenetic characters in the humerus and tarsometatarsus of penguins. Pol Polar Res 35, 469–496. [Google Scholar]
- Clarke JA (2004) Morphology, phylogenetic taxonomy, and systematics of Ichthyornis and Aptornis (Avialae: Ornithurae). Bull Am Mus Nat Hist 286, 5–179. [Google Scholar]
- Clarke JA, Olivero EB, Puerta P (2003) Description of the earliest fossil penguin from South America and first Paleogene vertebrate locality of Tierra Del Fuego, Argentina. Am Mus Novit 3423, 1–18. [Google Scholar]
- Clarke JA, Ksepka DT, Stucchi M, et al. (2007) Paleogene equatorial penguins challenge the proposed relationship between biogeography, diversity, and Cenozoic climate change. Proc Natl Acad Sci U S A 104, 11545–11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JA, Ksepka DT, Salas‐Gismondi R, et al. (2010) Fossil evidence for evolution of the shape and color of penguin feathers. Science 330, 954–957. [DOI] [PubMed] [Google Scholar]
- Corfield JR, Wild JM, Hauber ME, et al. (2008) Evolution of brain size in the paleognath lineage, with an emphasis on New Zealand ratites. Brain Behav Evol 71, 87–99. [DOI] [PubMed] [Google Scholar]
- Deng C, Wang B (1993) Convergence of somatic and visual afferent impulses in the wulst of pigeon. Exp Brain Res 96, 287–290. [DOI] [PubMed] [Google Scholar]
- Dingle RV, Lavelle M (1998) Antarctic peninsular cryosphere: early Oligocene (c. 30 Ma) initiation and a revised glacial chronology. J Geol Soc (London, UK) 155, 433–437. [Google Scholar]
- Early CM, Sclafani M, Balanoff AM, et al. (2012) Comparative neuroanatomy of fossil and living waterbirds. Programs & Abstracts Society of Vertebrate Paleontology, 72nd Annual Meeting, Raleigh, pp.89–90.
- Edinger T (1951) The brains of the Odontognathae. Evolution 5, 6–24. [Google Scholar]
- Elzanowski A, Galton PM (1991) Braincase of Enaliornis, an Early Cretaceous bird from England. J Vertebr Paleontol 11, 90–107. [Google Scholar]
- Fordyce RE, Thomas DB (2011) Kaiika maxwelli, a new Early Eocene archaic penguins (Sphensicoformes, Aves) from Waihao Valley, South Canterbury, New Zealand. N Z J Sci Technol Sect B 54, 43–51. [Google Scholar]
- Hackett SJ, Kimball RT, Reddy S, et al. (2008) A phylogenomic study of birds reveals their evolutionary history. Science 320, 1763–1768. [DOI] [PubMed] [Google Scholar]
- Haidr N, Acosta Hospitaleche C (2014) Miocene Patagonian penguins: craniomandibular morphology and functional mechanics. Alcheringa 38, 273–280. [Google Scholar]
- Harmening WM, Wagner H (2011) From optics to attention: visual perception in barn owls. J Comp Physiol A 197, 1031–1042. [DOI] [PubMed] [Google Scholar]
- Hollis CJ, Taylor KWR, Handley L, et al. (2012) Early Paleogene temperature history of the Southwest Pacific Ocean: reconciling proxies and models. Earth Planet Sci Lett 349–350, 53–66. [Google Scholar]
- Iwaniuk AN, Wylie DRW (2006) The evolution of stereopsis and the wulst in caprimulgiform birds: a comparative analysis. J Comp Physiol A 192, 1313–1326. [DOI] [PubMed] [Google Scholar]
- Iwaniuk AN, Hurd PL, Wylie DRW (2006) Comparative morphology of the avian cerebellum: I. degree of foliation. Brain Behav Evol 68, 45–62. [DOI] [PubMed] [Google Scholar]
- Iwaniuk AN, Hurd PL, Wylie DRW (2007) Comparative morphology of the avian cerebellum: II. size of folia. Brain Behav Evol 69, 196–219. [DOI] [PubMed] [Google Scholar]
- Iwaniuk AN, Heesy CP, Hall MI, et al. (2008) Relative wulst volume is correlated with orbit orientation and binocular visual field in birds. J Comp Physiol A 194, 267–282. [DOI] [PubMed] [Google Scholar]
- Jadwiszczak P, Krajewski KP, Pushina Z, et al. (2013) A first record of fossil penguins from East Antarctica. Antarct Sci 25, 397–408. [Google Scholar]
- Jarvis ED, Mirarab S, Aberer AJ, et al. (2014) Whole‐genome analyses resolve early branches in the tree of life of modern birds. Science 346, 1320–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karten HJ, Hodos W, Nauta WJH, et al. (1973) Neural connections of the ‘visual wulst’ of the avian telencephalon. Experimental studies in the pigeon (Columba livia) and owl (Speotyto cunicularia). J Comp Neurol 150, 253–277. [DOI] [PubMed] [Google Scholar]
- Kawabe S, Shimokowa T, Miki H, et al. (2009) A simple and accurate method for estimating the brain volume of birds: possible application in paleoneurology. Brain Behav Evol 74, 295–301. [DOI] [PubMed] [Google Scholar]
- Kawabe S, Ando T, Endo H (2014) Enigmatic affinity in the brain morphology between plotopterids and penguins, with a comprehensive comparison among water birds. J Linn Soc London, Zool 170, 467–493. [Google Scholar]
- Ksepka DT, Clarke JA (2010) The basal penguin (Aves: Sphenisciformes) Perudyptes devriesi and a phylogenetic evaluation of the penguin fossil record. Bull Am Mus Nat Hist 337, 3–77. [Google Scholar]
- Ksepka DT, Balanoff AM, Walsh S, et al. (2012) Evolution of the brain and sensory organs in Sphenisciformes: new data from the stem penguin Paraptenodytes antarcticus . J Linn Soc London, Zool 166, 202–219. [Google Scholar]
- Le Corre M (1997) Diving depths of two tropical Pelecaniformes: the Red‐tailed Tropicbird and the Red‐footed Booby. Condor 99, 1004–1007. [Google Scholar]
- Livezey BC (1989) Morphometric patterns in recent and fossil penguins (Aves, Sphenisciformes). J Zool (1987) 219, 269–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison WP, Maddison DR (2015) Mesquite: a modular system for evolutionary analysis. Version 3.04 http://mesquiteproject.org.
- Marsh OC (1880) Odontornithes: A Monograph on the Extinct Toothed Birds of North America. Memoirs of the Peabody Museum of Yale College, Vol. 1. [Google Scholar]
- Martin GR (2007) Visual fields and their functions in birds. J Ornithol 148, S547–S562. [Google Scholar]
- Martin GR (2014) The subtlety of simple eyes: the tuning of visual fields to perceptual challenges in birds. Philos Trans R Soc Lond B Biol Sci 369, 20130040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GR, Wanless S (2015) The visual fields of Common Guillemot Uria aalge and Atlantic Puffin Fratercula arctica: foraging, vigilance, and collision vulnerability. Ibis 157, 798–807. [Google Scholar]
- Mayr G (2005) Tertiary plotopterids (Aves, Plotopteridae) and a novel hypothesis on the phylogenetic relationships of penguins (Spheniscidae). J Zool Syst Evol Res 43, 61–71. [Google Scholar]
- Mayr G, Goedert JL, Vogel O (2015) Oligocene plotopterid skulls from western North America and their bearing on the phylogenetic affinities of these penguin‐like seabirds. J Vertebr Paleontol 35, e943764. [Google Scholar]
- Milner AC, Walsh SA (2009) Avian brain evolution: new data from Paleogene birds (Lower Eocene) from England. . Zool J Linn Soc 155, 198–219. [Google Scholar]
- Morgans HEG, Jones CM, Crouch EM, et al. (2005) Upper Cretaceous to Eocene stratigraphy and sample collections, mid‐Waipara River section, North Canterbury. Institute of Geological and Nuclear Sciences Science Report 2003/08, 1–101.
- Nieder A (2002) Seeing more than meets the eye: processing of illusory contours in animals. J Comp Physiol A 188, 249–260. [DOI] [PubMed] [Google Scholar]
- Olson SL (1989) A new genus of penguin‐like pelecaniform bird from the Oligocene of Washington (Pelecaniformes: Plotopteridae). Contributions in Science, Natural History Museum of Los Angeles County 330, 51–57. [Google Scholar]
- Paulina‐Carabajal A, Acosta‐Hospitaleche C, Yury‐Yañez RE (2015) Endocranial morphology of Pygoscelis calderensis (Aves, Spheniscidae) from the Neogene of Chile and remarks on brain morphology in modern Pygoscelis. Hist Biol 27, 571–582. [Google Scholar]
- Pettigrew JD (1979) Visual processing in the owl's telencephalon. Proc R Soc Lond B 204, 435–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew JD, Frost BJ (1985) A tactile fovea in the Scolopacidae?. Brain Behav Evol 26, 185–195. [PubMed] [Google Scholar]
- Pettigrew JD, Konishi M (1976) Neurons selective for orientation and binocular disparity in the visual wulst of the Barn Owl (Tyto alba). Science 193, 675–678. [DOI] [PubMed] [Google Scholar]
- Prince PA, Huin N, Weimerskirch H (1994) Diving depths of albatrosses. Antarct Sci 6, 353–354. [Google Scholar]
- Prum RO, Berv JS, Dornburg A, et al. (2015) A comprehensive phylogeny of birds (Aves) using targeted next‐generation DNA sequencing. Nature 526, 569–573. [DOI] [PubMed] [Google Scholar]
- Raine JI, Beau AG, Boyes AF, et al. (2015) Revised calibration of the New Zealand Geological Timescale: NZGT2015/1. GNS Science Report 2012/39.
- Scofield RP, Ashwell KWS (2009) Rapid somatic expansion causes the brain to lag behind: the case of the brain and behavior of New Zealand's Haast's Eagle (Harpagornis moorei). J Vertebr Paleontol 29, 637–649. [Google Scholar]
- Simpson GG (1946) Fossil penguins. Bull Am Mus Nat Hist 87, pp. 7–99. [Google Scholar]
- Slack KE, Jones CM, Ando T, et al. (2006) Early penguin fossils, plus mitochondrial genomes, calibrate avian evolution. Mol Biol Evol 23, 1144–1155. [DOI] [PubMed] [Google Scholar]
- Smith ND (2010) Phylogenetic analysis of Pelecaniformes (Aves) based on osteological data: implications for waterbird phylogeny and fossil calibration studies. PLoS One 5, e13354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NA, Clarke JA (2012) Endocranial anatomy of the Charadriiformes: sensory system variation and the evolution of wing‐propelled diving. PLoS One 7, e49584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambussi CP, Reguero MA, Marensii SA, et al. (2005) Crossvallia unienwillia, a new Spheniscidae (Sphenisciformes, Aves) from the Late Paleocene of Antarctica. Geobios 38, 667–675. [Google Scholar]
- Tambussi CP, Degrange FJ, Ksepka DT (2015) Endocranial anatomy of Antarctic Eocene stem penguins: implications for sensory system evolution in Sphenisciformes (Aves). J Vertebr Paleontol 35, e981635. doi:10.1080/02724634.2015.981635. [Google Scholar]
- Walsh S, Milner A (2010) Halcyornis toliapicus (Aves: Lower Eocene, England) indicates advanced neuromorphology in Mesozoic Neornithes. J Syst Paleontol 9, 173–181. [Google Scholar]
- Walsh SA, Iwaniuk AN, Knoll MA, et al. (2013) Avian cerebellar floccular fossa size is not a proxy for flying ability in birds. PLoS One 8, e67176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild JM (1987) The avian somatosensory system: connections of regions of body representation in the forebrain of the pigeon. Brain Res 412, 205–223. [DOI] [PubMed] [Google Scholar]
- Wood JR, De Pietri VL (2015) Next‐generation paleornithology: technological and methodological advances allow new insights into the evolutionary and ecological histories of living birds. Auk 132, 486–506. [Google Scholar]
- Zelenintsky DR, Therrien F, Ridgely RC, et al. (2011) Evolution of olfaction in non‐avian theropod dinosaurs and birds. Proc R Soc B 278, 3625–3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zusi RL (1975) An interpetation of skull structure in penguins In: The Biology of Penguins. (ed. Stonehouse B.), 59–84, Baltimore: University Park Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 . Lateral and anterior views of the 3D‐rendered endocast.
Fig. S2. Rostral to caudal (A–D) 2D coronal slices of the skull from CT scans of 2009.99.1, viewed from the rostral aspect. Labels indicate visible structures of the endosseus labyrinth.
Video S1. Rostral to caudal 2D coronal slice data from CT scans of 2009.99.1, viewed from the rostral aspect.
Video S2. Sagittal 2D slice data from CT scans of 2009.99.1, viewed from the left.
Video S3. 2D CT slice data of Waimanu tuatahi paratype CM Zfa 33 in coronal view, proceeding from caudal to rostral with respect to the cranial material.
Video S4. 2D CT slice data of Waimanu tuatahi paratype CM Zfa 34 in coronal view, proceeding from caudal to rostral with respect to the cranial material.


