Abstract
Psittaciformes are a very diverse group of non‐passerine birds, with advanced cognitive abilities and highly developed locomotor and feeding behaviours. Using computed tomography and three‐dimensional (3D) visualization software, the endocasts of 14 extant Neotropical parrots were reconstructed, with the aim of analysing, comparing and exploring the morphology of the brain within the clade. A 3D geomorphometric analysis was performed, and the encephalization quotient (EQ) was calculated. Brain morphology character states were traced onto a Psittaciformes tree in order to facilitate interpretation of morphological traits in a phylogenetic context. Our results indicate that: (i) there are two conspicuously distinct brain morphologies, one considered walnut type (quadrangular and wider than long) and the other rounded (narrower and rostrally tapered); (ii) Psittaciformes possess a noticeable notch between hemisphaeria that divides the bulbus olfactorius; (iii) the plesiomorphic and most frequently observed characteristics of Neotropical parrots are a rostrally tapered telencephalon in dorsal view, distinctly enlarged dorsal expansion of the eminentia sagittalis and conspicuous fissura mediana; (iv) there is a positive correlation between body mass and brain volume; (v) psittacids are characterized by high EQ values that suggest high brain volumes in relation to their body masses; and (vi) the endocranial morphology of the Psittaciformes as a whole is distinctive relative to other birds. This new knowledge of brain morphology offers much potential for further insight in paleoneurological, phylogenetic and evolutionary studies.
Keywords: 3D brain reconstructions, Aves, character mapping, endocranial morphology, geometric morphometrics
Introduction
Among non‐passeriform birds, Psittaciformes are one of the most species‐diverse clades (Mayr, 2010). They are characterized by their advanced cognitive abilities, and highly developed locomotor and feeding behaviours (Iwaniuk et al. 2005). Psittaciformes differ morphologically in many respects from other birds. The orbital rings, peculiarities in the shape of the beak and mandible, and the shape of the quadrate are some of the exceptional characters of the skull. Moreover, the few anatomical studies on the brain of Psittaciformes have identified a number of features that provide a general characterization: relatively larger brains and telencephala than other non‐passerines birds (Iwaniuk et al. 2005), which is possibly related to their advanced cognitive abilities, including vocal learning and communication (Iwaniuk & Hurd, 2005; Iwaniuk et al. 2005). In comparison with other birds, Psittaciformes show a high degree of cerebellum foliation (Iwaniuk et al. 2006, 2007), and smaller diencephala, myelencephala, mesencephala, optic tecta and cerebellum (Mlíkovský, 1989; Iwaniuk et al. 2004, 2005). In only one species, the Kakapoo (Strigops habroptilus; Gray, 1845), the olfactory bulbs are large (Corfield et al. 2011).
Using computed tomography (CT) and three‐dimensional (3D) visualization software, we studied the endocranial anatomy of representatives of Neotropical parrots whose monophyly has been supported by morphological and molecular studies (Smith, 1975; Sibley & Alquist, 1990; de Kloet & de Kloet, 2005; Tavares et al. 2006). Neotropical parrots comprise a total of 167 species (Forshaw, 2010), which vary greatly in body mass, but have homogeneous morphological characters and behavioural habits (Collar, 1997).
The use of CT scans has gained in importance as an exploratory tool in the field of biology. The study of 3D models generated from CT scans opens a range of possibilities never before investigated in the field. This methodology presents obvious advantages. Materials usually do not need treatment before entering the scanner, no deterioration occurs during the scan, and the results allow the reconstruction of high‐quality 3D models. This includes modelling of both hard and soft tissues that can be used for different purposes, such as anatomical and biomechanical studies. The use of this technology has been a breakthrough in studies of various types, both in fossil (Cunningham et al. 2014; Sutton et al. 2014) and extant organisms, resulting in both descriptive (Lautenschlager et al. 2013; Cox & Faulkes, 2014; Quayle et al. 2014; Racicot & Rowe, 2014) and/or ontogenetic studies (Picasso et al. 2010; Fisher et al. 2014; Rücklin et al. 2014). The datasets obtained by the scans are long‐lasting and easily portable, facilitating the comparison and study of the 3D models obtained, which are easily manipulated, sectioned and redescribed. More importantly, this methodology provides data on extant taxa that are characterized as ‘very rare’, endangered or rare in the fossil record.
Although it has been stated that endocranial morphology varies across Aves, variation among closely related species remains largely unknown (Smith & Clarke, 2012). The aim of this work is to explore the brain morphology of Neotropical parrots and to identify potential differences and similarities with other Psittaciformes. Moreover, we intended to provide a database of brain anatomy of living Neotropical Psittaciformes that will contribute to functional, behavioural, evolutionary and/or paleoneurological studies. For these purposes, we employed 3D digital endocast reconstructions, and we explored endocast shape variation using 3D geometric morphometric methods. The latter approach has recently been used by other researchers (Kawabe et al. 2014), but this is the first time it has been applied to Psittaciformes. We also trace the evolution of morphological traits over a molecular phylogeny to infer the characteristics at ancestral nodes. The strength of this analysis lies in the tree, the taxa included and the evolutionary liability of the characters analysed. This study represents an excellent opportunity to examine in detail the history of certain important neurological characteristics. Previous hypotheses about the evolution of brain morphology are scarce, and primarily focused on the initial transformations at the origin of birds and not within a particular clade of Neornithes. We expected that the morphology of Neotropical parrots (according to their ecological and behavioural similarities) would be more conservative in comparison with non‐Neotropical Psittaciformes. We also predicted that, as noted for other birds (Jerison, 1973; Iwaniuk et al. 2005; Balanoff et al. 2013), there would be a positive correlation between body mass and brain volume and, given their complex cognitive capacities (Iwaniuk et al. 2005), the Neotropical Psittaciformes would have high encephalization quotient (EQ) values.
Materials and methods
Dry skulls of 14 specimens of extant Neotropical Psittaciformes at the osteological collections of the Museo de La Plata (MLP) and of the Museo Argentino de Ciencias Naturales ‘Bernardino Rivadavia’ (MACN; Argentina) were scanned. The species were: Amazona aestiva, Amazona vinacea, Anodorhynchus hyacinthinus, Ara chloropterus, Ara sp., Aratinga leucophthalma, Cyanoliseus patagonus, Enicognathus ferrugineus, Myiopsitta monachus, Nandayus nenday, Pionopsitta pileata, Pionus maximiliani, Primolius auricollis and Pyrrhura frontalis (Table 1). Also, in our analysis, a Cacatua galerita endocast reconstruction was included in order to compare the shape of Neotropical parrots with a representative of a non‐Neotropical Psittaciformes.
Table 1.
Endocranial volume, body mass and EQ of the brains of Neotropical parrots
| Species | Specimen No. | ECV (cm3) | BM (g) | EQ |
|---|---|---|---|---|
| Amazona aestiva | MLP 621 | 7.237 | 451 | 1.642 |
| Amazona vinacea | MACN 1032 | 8.399 | 254 | 2.640 |
| Anodorhynchus hyacinthinus | MACN 2355a | 23.715 | 1331 | 2.909 |
| Ara chloropterus | MACN 1479a | 23.418 | 1214 | 3.027 |
| Ara sp.a | MLP 943 | 15.906 | 923 | 2.402 |
| Aratinga leucophthalma | MLP 407 | 2.932 | 158 | 1.207 |
| Cyanoliseus patagonus b | MLP 65 | 6.383 | 278 | 1.906 |
| Enicognathus ferrugineus | MACN 1426a | 4.911 | 160 | 2.007 |
| Myiopsitta monachus | MLP 79 | 3.258 | 120 | 1.568 |
| Nandayus nenday | MLP 405 | 4.008 | 128 | 1.859 |
| Pionopsitta pileata | MLP 67 | 2.769 | 119 | 1.339 |
| Pionus maximiliani | MLP 343 | 5.257 | 293 | 1.523 |
| Primolius auricollis | MACN 2518 | 7.780 | 245 | 2.496 |
| Pyrrhura frontalis b | MLP 351 | 2.394 | 80 | 1.450 |
| Cacatua galerita | MLP 755 | 11.604 | 723.5 | 2.012 |
BM, body mass; ECV, endocranial volume; EQ, encephalization quotient.
Average between body mass of all species of Ara.
Average between female and male body mass.
Scans were taken in the transverse plane using a General Electric Bright Speed HiSpeed CT scanner at 140 kV and 300 mA with 0.62 mm slice thickness at the Hospital San Juan de Dios, La Plata, Argentina. The DICOM images were processed and reconstructions of the 3D models of the endocast were made with the open source software 3d‐slicer (Fedorov et al. 2012; Fig. 1).
Figure 1.
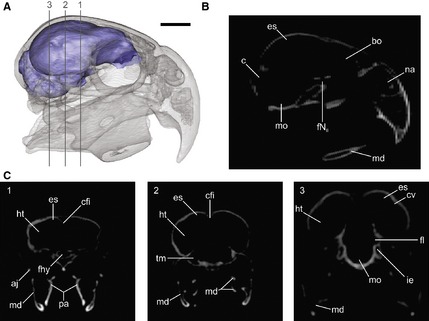
Three‐dimensional reconstruction of Cyanoliseus patagonus endocast, generated from CT scans. (A) Lateral view of the skull in transparence showing the endocast; (B, C) tomographs of the skull in (B) sagittal and (C) coronal planes (1–3) showing the spaces occupied by the brain and its different regions/structures. aj, arcus jugalis; bo, bulbus olfactorius; c, cerebellum; cfi, crista frontalis interna; cv, crista vallecularis; es, eminentia sagittalis; fhy, fossa hypohysialis; fl, floculus; fNII, foramen nervus opticum; ht, hemisphaeria telencephali; ie, inner ear; md, mandible; mo, medulla oblongata; na, apertura nasi ossea; pa, ossa palatina; tm, tectum mesencephali. Scale bar: 1 cm.
Additionally, in order to corroborate the relationship of endocast features to brain structures, we dissected out the brain of a monk parakeet Myiopsitta monachus (Fig. 2). Specifically, we evaluated the foliation of the surface of the brain and the cerebellum. In extant birds, the cranial cavity is almost completely filled by the brain, so the internal surface of the cranial bones closely reflects brain anatomy (Edinger, 1951; Elzanowski & Galton, 1991; Iwaniuk & Nelson, 2002; Osmólska, 2004; Evans, 2005; Picasso et al. 2010). This makes it possible to build casts of the endocranial volume (i.e. endocasts), and study the development of different traits and regions of this ‘brain’.
Figure 2.
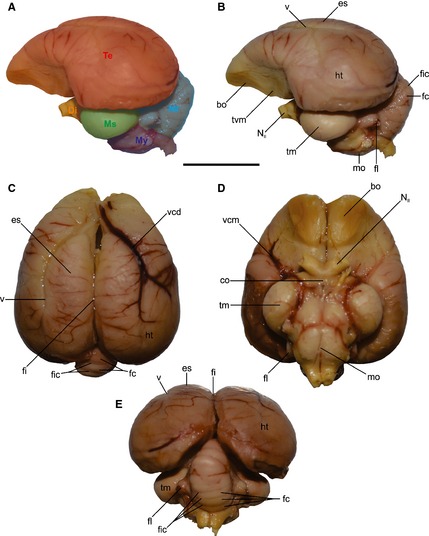
Dissection of Myiopsitta monachus brain. (A) Regions of the brain in lateral view, (B) left lateral, (C) dorsal, (D) ventral and (E) caudal views showing the main structures of the brain. bo, bulbus olfactorius; co, chiasma opticum; Di, diencephalon; es, eminentia sagittalis; fc, folia cerebeli; fi, fissura interhemispherica; fic, fissure cerebeli; fl, floculus; ht, hemisphaeria telencephalica; mo, medulla oblongata; Ms, mesencephalon; Mt, metencephalon; My, myelencephalon; NII, nervus opticum; Te, telencephalon; tm, tectum mesencephali; tvm, tuber ventromediale; v, vallecula; vcd, vena cerebralis dorsorostralis; vcm, vena cerebralis media. Scale bar: 1 cm.
For descriptions of the endocasts, the osteological nomenclature proposed by Baumel & Witmer (1993) is used, and brain anatomical terminology for the central nervous system follows Breazile & Kuenzel (1993).
The EQ (Jerison, 1973) for each specimen was calculated according to the following equation: EQ = ECV/0.137 W 0.568 (Smith & Clarke, 2012), where ECV is the endocranial volume (cm3) calculated using the 3d‐slicer software and W is the body mass (g) obtained from Dunning (2008). Species with endocranial volumes larger than expected for their body mass have EQ > 1, whereas species with endocranial volumes smaller than expected have EQ values < 1.
We used past 3.02a software (Hammer et al. 2001 ) to test a linear regression between log10‐transformed body mass and brain volume.
For the 3D geomorphometric analysis, 25 homologous landmarks were digitized from the endocast (Fig. 3; Table 2) using amira (v.5.1 trial version; Mercury Computer Systems, San Diego, CA, USA). The dataset was analysed by applying generalized least‐squares Procrustes analysis (Rohlf & Slice, 1990a,b) using the morphoj software package (Klingenberg, 2011). This process works by translating, rotating and scaling all specimens to a common reference, minimizing the distance between homologous landmarks. As a consequence, the effects of size, position and orientation are eliminated, and the remaining information reflects shape disparity alone (Procrustes shape coordinates). The absolute size of the specimen is preserved as centroid size, which is calculated as the square root of the sum of squared distances of landmarks from their centroids (Bookstein, 1991). To explore the patterns of major morphological variation of the brains, we applied a principal component (PC) analysis to the Procrustes shape coordinates using morphoj. morphologika software was used to illustrate 3D shape change.
Figure 3.
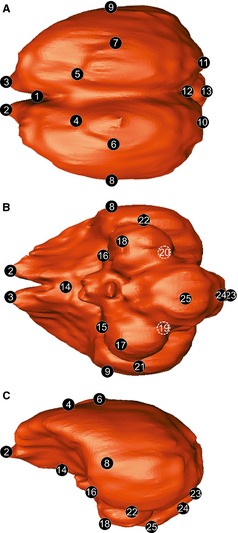
The 3D brain landmarks used for shape analysis shown in Enicognathus ferrugineus brain. (A) Dorsal, (B) ventral, (C) lateral view. See Table 2 for landmark description.
Table 2.
Landmarks used and anatomical description (Fig. 3)
| Landmark | Anatomical description |
|---|---|
| 1 | Most rostral point of the fissura interhemispherica |
| 2–3 | Most rostral point of the hemisphaeria telencephalica |
| 4–5 | Most rostral point of the eminentia sagittalis |
| 6–7 | Most lateral point of the eminentia sagittalis |
| 8–9 | Most lateral point of the widest part of the telencephalona |
| 10–11 | Most caudal point of the hemisphaeria telencephalica |
| 12 | Median junction between telencephalon and cerebelluma |
| 13 | Most caudal point of the cerebellum |
| 14 | Most caudal point of the tuber ventromediale |
| 15–16 | Intersection of telencephalon, tectum mesencephali and diencephalona |
| 17–18 | Most rostral point of the optic lobes (tectum mesencephali) |
| 19–20 | Intersection of telencephalon, tectum mesencephali and cerebelluma |
| 21–22 | Most lateral point of the optic lobes (tectum mesencephali) |
| 23 | Median dorsal point of foramen magnuma |
| 24 | Median ventral point of foramen magnuma |
| 25 | Most ventral point of the medulla oblongata |
To evaluate the evolutionary history of morphological characters that have been proposed as diagnostic (the dorsal shape of the telencephalon, the dorsal expansion of the eminentia sagittalis, the relative size of the optic lobes and the conspicuity of the fissura mediana; characters 4, 5, 11 and 21 of Smith & Clarke, 2012 respectively), we reconstructed ancestral states using the mesquite package Version 3.01 (Maddison & Maddison, 2014) and a molecular phylogeny (Tavares et al. 2006). Characters were considered as unordered. Parsimony and Maximum Likelihood (Markov‐K‐state1, with equal probability for any particular character change) approaches were applied.
Results
Brain–body relationship
As predicted, we found a strong positive correlation between body mass and brain volume (r 2 = 0.92, P < 0.05; Fig. 4). All the Neotropical Psittaciformes studied here had an EQ higher than 1 (Table 1). A. leucophthalma showed the smallest value of EQ (1.207), and A. chloropterus reached the highest EQ values (3.027). Five species, A. vinacea, A. hyacinthinus, Ara sp., E. ferrugineus and P. auricollis had EQs higher than 2.
Figure 4.
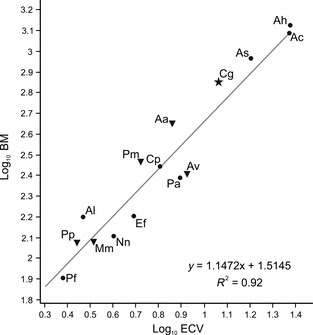
Scatterplot of log‐transformed brain volume vs. body mass in parrots. Solid star indicates cockatoos; solid dots indicate Macaws and allies; and inverted solid triangles indicate Amazons and allies, according to Tavares et al. (2006). Aa, Amazona aestiva; Av, Amazona vinacea; Ah, Anodorhynchus hyacinthinus; Ac, Ara chloropterus; As, Ara sp.; Al, Aratinga leucophthalma; Cg, Cacatua galerita; Cp, Cyanoliseus patagonus; Ef, Enicognathus ferrugineus; Mm, Myiopsitta monachus; Nn, Nandayus nenday; Pp, Pionopsitta pileata; Pm, Pionus maximiliani; Pa, Primolius auricollis; Pf, Pyrrhura frontalis.
Description of the endocasts
General morphology
Fresh material studies confirm the similarity between the actual brain and the virtual endocast (Fig. 2). Virtual cranial endocasts are hereafter referred to as brain models or simply brains. In the brain of Psittaciformes, the telencephalon conspicuously overlaps the cerebellum, and therefore the brain is high and short in general appearance (Figs 2B,C and 5A,C,F,H). All brains showed a noticeable notch located rostrally between hemisphaeria, originating two expansions of the most rostral portions of the telencephalon and dividing the bulbus olfactorius (Fig. 5).The overall shape of the brain is either quadrangular (A. hyacinthinus and Ara), or more rounded and rostrally tapered (C. patagonus, P. auricollis, N. nenday, P. maximiliani, Amazona, E. ferrugineus, P. pileata, P. frontalis, A. leucophthalma and M. monachus). In the former, the brain is similar in shape to a walnut when viewed dorsally (Fig. 5A), the notch aforementioned wider and more pronounced, the fissura interhemispherica broader, and the telencephalon more rostrally and caudally expanded (Fig. 5F). In the rounded morphotype (Fig. 5F), the notch is less excavated, the fissura interhemispherica narrower, and the telencephalon rostrally and caudally less expanded. Another difference between the brain morphotypes is the lateral extension of the tectum mesencephali: rounder brains present more laterally expanded tecta. A significant difference between both morphotypes is that walnut brains present a more longitudinally linear arrangement, while rounder brains are folded more cranio‐caudally, giving them an S‐shape in which the optic lobes are more rostrally positioned than in the walnut brain. The cerebellum is more caudally positioned in the rounded brain.
Figure 5.
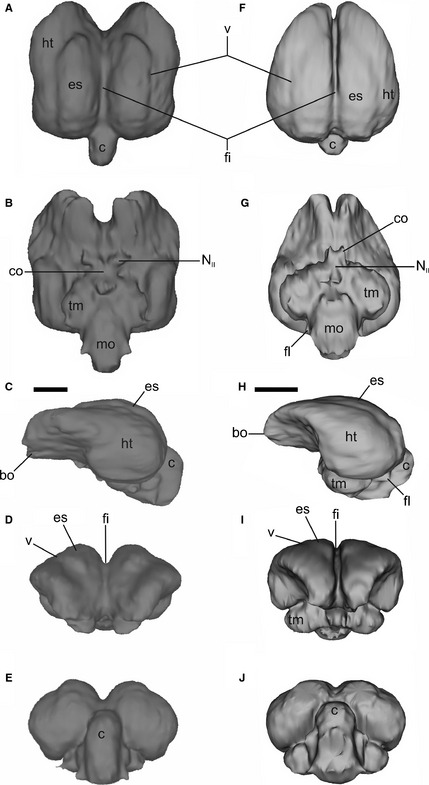
Endocast of adult Anodorhynchus hyacinthinus (A–E) and Pionus maximiliani (F–J) in dorsal, ventral, left lateral, cranial and caudal views. bo, bulbus olfactorius; c, cerebellum; co, chiasma opticum; es, eminentia sagittalis; fi, fissura interhemispherica; fl, floculus; ht, hemispherium telencephali; mo, medulla oblongata; NII, nervus opticum; tm, tectum mesencephali; v, vallecula. Scale bar: 1 cm.
Telencephalon
The most noticeable feature of the telencephalon was the pronounced fissura interhemispherica (Figs 2C,E and 5A,D,F,I). In all brains compared, the eminentia sagittalis originates behind the notch, extending to the cerebellum and occupying more than half of the total length of the brain (Fig. 5A,F). The caudally positioned eminentia sagittalis corresponds to type B of Stingelin (1957; in type A, the eminentia sagittalis is rostrally positioned; Milner & Walsh, 2009). The eminentia sagittalis is prominent except in P. maximiliani and Amazona (Fig. 5F,H). In all specimens, the bulbus olfactorius was simple, corresponding to the one‐lobe type of Cobb (1959), being small and ventrally oriented (Fig. 5C,D,H,I).
Diencephalon
In just a few cases, CT data allowed us to reconstruct the exit of the optic nerve (Fig. 5B,G). The nerves are transversely thick and diverge widely in a rostral direction. The chiasma opticum, where the optic nerves partially cross, is not clearly delimited in the CT images but is easily observed in the fresh material (Fig. 2D).
Mesencephalon
The tecta mesencephali (optic lobes) are prominent, but small relative to the telencephalon. They are proportionally larger in rounded brains. They have a crescent shape, and they are noticeable in lateral and caudal views (Fig. 5C,E,H,J). They are covered by the telencephalum in dorsal view (Fig. 5A,F).
Metencephalon
The boundary between the cerebellum and telencephalon is easily distinguishable on the dorsal surface, although in P. frontalis and A. leucophthalma the cerebellum is partially covered by the telencephalon. The cerebellum shows a cranio‐caudal elongation, and no folia cerebelli can be distinguished in the endocast (Fig. 5A,E,F,J). This is because the sinus/meningial tissue surrounding the cerebellum is sufficiently thick to prevent the cerebellum from contacting the endocranium. Like other living birds, parrots have folia cerebelli (Fig. 2) and, in fact, they show a high degree of cerebellar foliation (Iwaniuk et al. 2006). The sinus occipitalis could not be differentiated in the endocast. Regrettably, the extension and development of the floculi could not be established in most of the 3D reconstructions. However, the dissection of Myiopsitta (Fig. 2) shows that the floculi are well developed, with a wide base, directed and tapering caudally.
Myelencephalon
The medulla oblongata is elongated (Fig. 5B,G) and wider in the rounded brains. It shows a poorly marked fissura mediana.
Geomorphometric analysis
More than half of the variation is contained in the first three components (PC1, 35.68%; PC2, 21.09%; PC3, 8.4%).
The plot of PC1 and PC2 (Fig. 6A) shows that most taxa are grouped together with negative values for PC1. Separate from the majority are A. hyacinthinus and C. galerita, with positive values for PC1, and E. ferrugineus, with positive values for PC2, although the latter shows lower values for PC1 and higher values for PC2 than the first two taxa. Also, A. aestiva and A. chloroptera are separated by positive values for PC1 and negative values for PC2.
Figure 6.
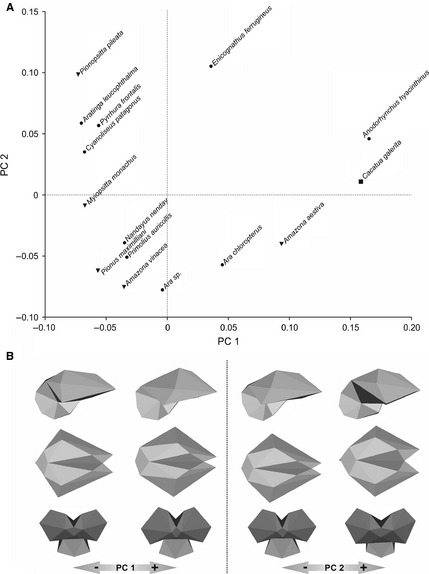
Principle component (PC) analysis of the brain shape of parrots. (A) Plot of PC1 against PC2. (B) Brain shape variation along PC1 and PC2 axis. Solid dots indicate Macaws and allies; inverted solid triangles indicate Amazons and allies; and solid squares indicate cockatoos, according to Tavares et al. (2006).
The PC2 vs. PC3 plot does not allow groups to be discriminated, while the PC1 vs. PC3 plot separates two groups: most of the taxa are located in the positive space for PC1, whereas species with negative values of PC1 include E. ferrugineus, A. aestiva, A. chloroptera, A. hyacinthinus and C. galerita.
Principal component 1 is strongly influenced by brain width, the length and width of the fissura interhemispherica, the rostral arrangement of the tectum mesencephali and the caudal extension of the cerebellum (Fig. 6B). Positive values correspond to rostrally wide brains, with deep and wide fissura interhemispherica, rostrally directed tectum mesencephali and caudally extended cerebellum. Also, both hemispheres are rostrally directed. Linear regression between PC1 and the body mass logarithm (r = 0.68; r 2 = 0.46; P = 0.005) shows that PC1 is poorly correlated with size. Only 46% of the form change in CP1 is explained by size. PC2 is influenced by the height and width of eminentia sagittalis, the width of fissura interhemispherica, the dorsoventral development of the cerebellum and the location where maximum brain width occurs (Fig. 6B). Positive values correspond to brains with narrower and higher eminentia sagittalis, narrower fissura interhemispherica and higher cerebellum. Maximum brain width is more rostrally located. Lastly, PC3 is influenced by the width and length of the fissura interhemispherica, the dorso‐ventral development of the hemispheres in the lateral view, the width of the cerebellum, the caudal extension of the medulla oblongata, and the orientations of the foramen magnum and the tectum mesencephali. Positive values indicate narrow fissura interhemispherica, greater ventral extension of the hemispheres, narrower cerebellum, shorter medulla oblongata, ventro‐caudally directed foramen magnum and more caudo‐ventrally directed tectum mesencephali.
Character plotting and ancestral state reconstruction
Brain morphological character states are traced onto the Psittaciformes phylogenetic hypothesis of Tavares et al. (2006) in Fig. 7. From the 28 characters of Smith & Clarke (2012), features 18–20 (floculi), 22 (hypophysis), 23 (carotid artery) and 24–28 (inner ear) could not be encoded here and are therefore not plotted into a cladogram. Strigops was excluded from this analysis because we lacked an endocast for this species. All Neotropical parrot endocasts showed: (i) reduced, simple and ventrally positioned bulbus olfactorius; (ii) eminentia sagittalis caudally positioned, not laterally extended; (iii) large tectum mesencephali (but small relative to the telencephalon), not visible in dorsal view, with a curved contact with the telencephalon; (iv) expanded telencephalon; (v) short and narrow cerebellum, with a v‐shaped contact with the telencephalon and indistinct fissura cerebelli; and (vi) not distinguishable sinus occipitalis. However, some variations among Neotropical parrots can be diagnosed in the eminentia sagitalis (characters 4 and 5 of Smith & Clarke, 2012), the size of the optic lobes relative to the rhombencephalon (character 11) and recognition of the fissura mediana (character 21).
Figure 7.
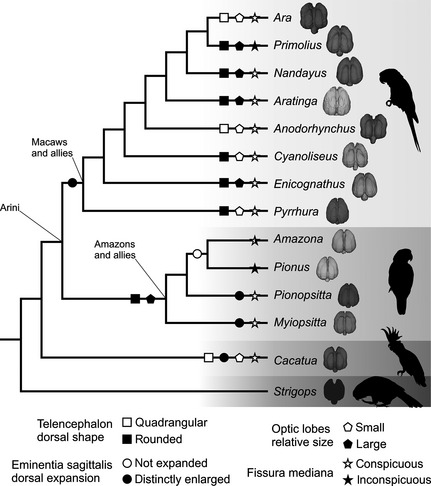
Phylogenetic diagram plotting the occurrence of some brain morphological traits in Neotropical parrots. Phylogenetic hypothesis modified from Tavares et al. (2006). Characters from Smith & Clarke (2012).
Figure 8 shows ancestral morphological states inferred from reconstructions of character history. In all but one of the characters (optic lobes, Fig. 8C), Parsimony and Maximum Likelihood models have no conflicts in the consequent interpretations. Mapping of endocranial character changes on a phylogeny of Psittaciformes allowed for inferences regarding morphological changes of the endocranium in the Neotropical parrots (Arini) relative to the remainder Psittaciformes.
Figure 8.
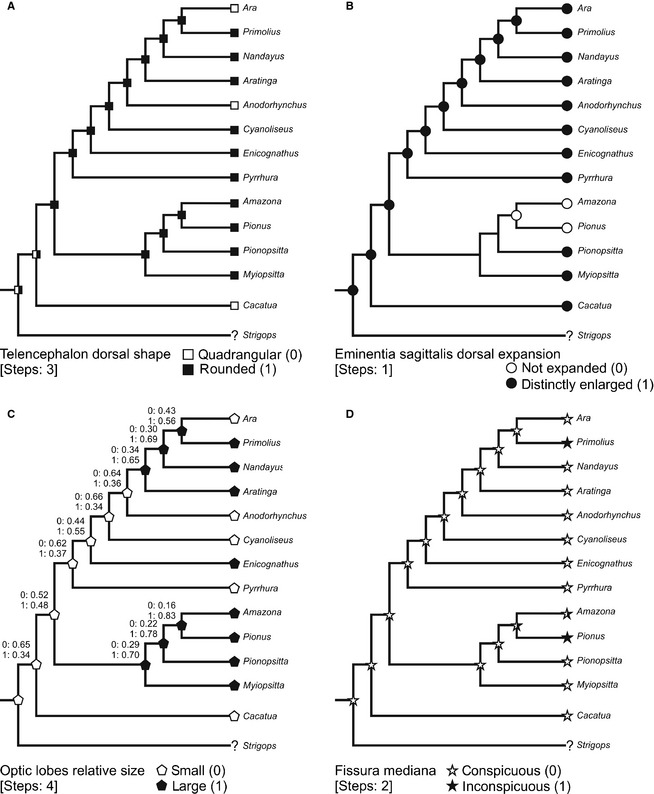
Ancestral‐state reconstruction of endocast in Arini (Psittaciformes) based on Parsimony analysis (steps are indicated between brackets). Additionally in (C), Maximum Likelihood state inferences are indicated by numbers. Characters were taken from Smith & Clarke (2012) and considered as unordered. Phylogenetic proposal modified from Tavares et al. (2006).
The reconstruction of character evolution revealed that the rounded telencephalon in dorsal view is the ancestral condition for Neotropical psittaciforms (Fig. 8A), and it is maintained in most Arini studied here. Several previous studies have shown that the rounded condition is likely plesiomorphic for Aves (Corfield et al. 2008; Kawabe et al. 2009; Smith & Clarke, 2012). Both Ara and Anodorhynchus are the only Arini that change to the quadrangular condition, and the same state is present in Cacatua. It appears that a potential parallelism or convergent evolution happened at least once.
According to the ancestral reconstruction, the distinctly enlarged dorsal expansion of the eminentia sagittalis is the plesiomorphic condition (Fig. 8B). This trait is maintained in all the Arini, except in the cluster formed by Amazona and Pionus that have an unexpanded condition. There is considerable variation in the dorsal expansion of the eminentia sagittalis within birds. Birds with different ecological or behavioural attributes show similar expansion of the eminentia sagittalis (Walsh & Milner, 2011), whereby the relationship between both is not clear.
Concerning the question about the evolution of the size of the optic lobes, the situation is not resolved. A parsimony model (Fig. 8C) proposes that small optic lobes is the ancestral state that evolved to the large condition in Primolius, Nandayus, Aratinga, Enicognathus and the clade of all Amazon parrots. Reversals happened in Ara, and remain in the ancestral state in Anodorhynchus, Cyanoliseus, Pyrrhura and Cacatua. The likelihood approach reconstructed the evolutionary history of this trait with greater vagueness. Small or large optic lobes have about the same chance (0.52 and 0.48, respectively) of being the ancestral condition of Neotropical parrots, pointing to the need of further detailed investigations on this structure. Optic lobes are relatively small for example in the kiwi, owls, some charadriiforms and several songbirds (Corfield et al. 2012; Smith & Clarke, 2012) and, for the moment, there is no biological or ecological correlation that would help to understand the evolutionary history shown in our models.
Conspicuous fissura mediana is the ancestral state (Fig. 8D). Most taxa conserved this trait that evolves into inconspicuous fissura only in Primolius and Pionus. Potential advantages of one or another state remain unclear at this time.
Discussion
Brain morphology often reflects the influence of ecological, behavioural or phylogenetic factors (Lefebvre et al. 2002, 2004). Because, in extant birds, the cranial cavity is almost completely filled by the brain, studying brain endocasts allows us to study brain morphology, and to test correlations between sensorial or cognitive capabilities and the development of some brain regions (Edinger, 1951; Elzanowski & Galton, 1991; Iwaniuk & Nelson, 2002; Osmólska, 2004; Evans, 2005; Picasso et al. 2010).
Both qualitative (descriptions) and quantitative (3D geomorphometrics analysis; Fig. 6) methods differentiate two conspicuously distinct brain morphologies, mainly based on the maximum width of the hemisphaeria. Walnut brains are associated with a higher and/or narrower eminentia sagittalis, a more extended cerebellum and wider fissura interhemispherica than rounded brains. For example, A. hyacinthinus is characterized by a walnut brain shape with a high and narrow eminentia sagittalis, a high and caudally extended cerebellum, and a wide and deep fissura interhemispherica (Fig. 5A–E). In contrast, P. maximiliani has a more rounded brain, with a low and wide eminentia sagittalis, a low cerebellum, and a narrow and shallow fissura interhemispherica (Fig. 5F–J). Moreover, brain characters traced onto a cladogram show that brain morphologies in the Neotropical parrots are heterogeneously distributed in the clade (Fig. 7). We find no functional or phylogenetic explanation for this heterogeneity. However, it seems plausible that the primitive condition for brain morphology is the walnut type, as more basal Psittaciformes such as Nestor notabilis and Cacatua galerita exhibit this morphology (Corfield et al. 2011; Gsell, 2012). The walnut type could be then synapomorphic for the clade Psittaciformes as it is distinctive relative to other birds. According to this hypothesis, the rounded type seems to be the primitive condition of the Neotropical Arini, and the walnut brain type has appeared more than once in the Neotropical parrots (Figs 7 and 8).
Except for P. maximiliani and Amazona, the eminentia sagittalis occupies a caudal position of the telencephalon in Psittaciformes. This feature, formed by the hyperpallium, is mostly involved in integrating and processing the visual signals (Reiner et al. 2004; Reiner, 2005; Walsh & Milner, 2011) and somatosensory inputs (Medina & Reiner, 2004; Mouritsen et al. 2005). The caudal position of this structure (also seen in Anseriformes and Apterygiformes) could be related to the increase in the rostral‐most portions of the telencephalon (Walsh & Milner, 2011).
The noticeable notch between hemisphaeria that separates the two lobes of the bulbi olfactorius is related to the presence of a septum osseum fossae bulbi, a bony septum present only in parrots, albatrosses and kiwis (Baumel & Witmer, 1993). This notch, termed the ‘interhemispheric septum’ sensu Iwaniuk et al. (2001), varies in size and shape within psittaciforms, depending on body size and the size of the novel ethmomandibularis muscle (which attaches to the septum), and it could play a role in protecting the brain during feeding and locomotion, which often involve strong bite forces (Iwaniuk et al. 2001). Interestingly, according to our observations, the walnut type only occurs in large parrots, such as Strigops, Cacatua, Anodorhynchus and Ara (i.e. taxa with body mass ranging 800–2000 g). The walnut‐shaped brains of these taxa reflect the presence of a very pronounced interhemispheric sulcus coinciding with an interhemispheric bony septum. To our knowledge, the ethmomandibularis muscle (that elevates the lower jaw) is particularly noticeable in two of these larger taxa, Ara and Anodorhynchus (Porto, 2004). It could be assumed that the size of the muscle associated with the shape of the septum would sculpture the brain. Unfortunately, we have no information on how this muscle is in the other two taxa. For now, this may not be more than an assumption. On the contrary, the m. ethmomandibularis is also big in smaller parrots such as Myiopsitta (Carril, Degrange & Tambussi, in press), whose brain is rounded. The potential relationship between brain types with behavioural variables is a question that remains to be explored.
Except for the kiwis, vultures and seabirds, the bulbus olfactorius of most living birds (including parrots) are small (Walsh & Milner, 2011), mainly because their herbivorous diet allows them to locate food by sight (Fernandez et al. 1997). Bulbus with a relatively small size had been previously observed for parrots and passerines in the classical work of Cobb (1959).
The tecta mesencephali are prominent in Neotropical parrots, as has been described for other diurnal Psittaciformes (eg. kea and cockatoo; Corfield et al. 2011; Gsell, 2012). In the only parrot with nocturnal habits, the kakapoo Strigops habroptilus, they are extremely small and partially obscured by the lateral aspect of the cerebral hemisphaeria (Corfield et al. 2011; Gsell, 2012). Strikingly, the kakapoo shows a relatively large bulbi olfactorius. Only few Neotropical parrots (such as A. hyacinthinus) show relatively large bulbi compared with the rest of the Psittaciformes, but they are as large of those of C. galerita (or smaller).
Parrots have a relatively small cerebellum but significantly more folia than other groups of birds (Iwaniuk et al. 2005, 2006). Unfortunately, the development of the folia cerebelli could not be distinguished in the 3D reconstructions, but they can be seen in the fresh brain (Fig. 2E). It has been suggested that the morphology of the endocranial cavity containing the cerebellum could be constrained in size and shape in Psittaciformes by the large jaw adductor muscles attached to the braincase surrounding this cavity (Iwaniuk et al. 2006). Studies on the mandibular muscles in Neotropical parrots show a well‐developed adductor mandibulae complex, including the m. adductor mandibulae externus profundus, origin of which is located on a deep fossa temporalis (Carril, Degrange & Tambussi, in press).
The EQ values of Neotropical parrots (Table 1) suggest they possess brain volumes higher than expected for their body masses, reaffirming that Psittaciformes has significantly larger brains than other birds (Iwaniuk et al. 2005). Large brain size could play an important role in the evolution of advanced cognitive abilities (Iwaniuk et al. 2005), and it has been demonstrated that species with larger brains have greater behavioural flexibility (Gsell, 2012), resulting in more successfully adapting to changing environments. However, it is important to consider brain organization as well as brain size in assessing the effect of brain morphology on cognitive abilities (Iwaniuk et al. 2005). Despite this caveat, it can be assumed that larger regions of the brain are associated with more complexity in the functions controlled by or related to that region (Jerison, 1973; Healy & Rowe, 2007). Parrots have a large tecta mesencephali and small bulbi olfactori, which could be related to their highly versatile behaviour during arboreal locomotion. Arboreal locomotion involves perching, climbing, hanging and moving easily among trees using both the head (jaws, tongue and neck) and hindlimbs (Carril et al. 2014). All these activities demand a big compromise between visual acuity and gaze stabilization.
Conclusions
Based on our CT scan data, Neotropical parrots show two conspicuously distinct brain morphologies (walnut type and rounded), mainly based on the shape and maximum width of the hemisphaeria, with a striking rostral notch. The functional interpretation of this distinction is pending.
Given a phylogeny and an evolutionary hypothesis, it is possible to reconstruct histories of character change and to infer models of character evolution. Rounded telencephalon in dorsal shape, distinctly enlarged dorsal expansion of the eminentia sagittalis and conspicuous fissura mediana are the ancestral conditions and retained throughout most Neotropical parrots.
This new knowledge of brain morphology provides a basis for further insights regarding paleoneurological, phylogenetic and evolutionary studies.
Acknowledgements
The authors are pleased to acknowledge to Amy Balanoff, Daniel Kspeka and Adam Smith for the invitation to participate to the workshop ‘A Deeper Look into the Avian Brain: Using Modern Imaging to Unlock Ancient Endocasts’ (North Carolina, USA). Attendance to the workshop for two of the authors (CPT, FJD) was supported by the National Evolutionary Synthesis Center (NESCent, National Science Foundation EF‐0905606). The authors wish to thank two anonymous reviewers and the editor, Adam Smith, for greatly improving this manuscript. Special thanks are due to the authorities of the Diagnostic Imaging Service from San Juan de Dios Hospital from La Plata city, Argentina. For assistance with CT scanning, the authors thank Graciela Alcuaz and Juan Ignacio Cuesta. The authors also thank Yolanda Davies and Pablo Tubaro from Museo Argentino de Ciencias Naturales ‘Bernardino Rivadavia’ from Buenos Aires for the loan of materials under their care. The authors appreciate the improvements in English usage made by Rebecca Cramer through the Association of Field Ornithologists’ program of editorial assistance. Support was received from CONICET PIP 0437 and UNLP N671.
References
- Balanoff AM, Bever GS, Rowe TB, et al. (2013) Evolutionary origins of the avian brain. Nature 501, 93–97. [DOI] [PubMed] [Google Scholar]
- Baumel JJ, Witmer LM (1993) Osteologia In: Handbook of Avian Anatomy: Nomina Anatomica Avium, No. 23. (eds Baumel J, King A, Breazile J, Evans H, Vanden Berge J.), pp. 45–132. Cambridge, MA: Publications of the Nuttall Ornithological Club. [Google Scholar]
- Bookstein FL (1991) Morphometric Tools for Landmark Data: Geometry and Biology. New York, NY: Cambridge University Pres. [Google Scholar]
- Breazile J, Kuenzel W (1993) Systema nervosum centrale In: Handbook of Avian Anatomy: Nomina Anatomica Avium, No. 23. (eds Baumel J, King A, Breazile J, Evans H, Vanden Berge J.), pp. 493–554. Cambridge, MA: Publications of the Nuttall Ornithological Club. [Google Scholar]
- Carril J, Degrange FJ, Tambussi CP (2015) Jaw myology and bite force of the monk parakeet (Aves, Psittaciformes) Journal of Anatomy: in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carril J, Mosto MC, Picasso MBJ, et al. (2014) Hindlimb myology of the monk parakeet (Aves, Psittaciformes). J Morphol 275, 732–744. [DOI] [PubMed] [Google Scholar]
- Cobb S (1959) A note on the size of the avian olfactory bulbs. Epilepsia 1, 394–402. [DOI] [PubMed] [Google Scholar]
- Collar NJ(1997) Family Psittacidae (Parrots) In: Handbook of the Birds of the World, Vol. 4: Sandgrouse to Cookoos. (eds del Hoyo J, Elliott A, Sargatal J.), pp. 280–477. Barcelona: Lynx Editions. [Google Scholar]
- Corfield JR, Gsell AC, Brunton D, et al. (2011) Anatomical specializations for nocturnality in a critically endangered parrot, the Kakapo (Strigops habroptilus). PLoS ONE 6, e22945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfield JR, Wild JM, Hauber ME, et al. (2008) Evolution of brain size in the Palaeognath lineage, with an emphasis on New Zealand ratites. Brain Behav Evol 71, 87–99. [DOI] [PubMed] [Google Scholar]
- Corfield JR, Wild JM, Parsons S, et al. (2012) Morphmetric analysis of telencephalic structure in a variety of Neognath and Paleognath bird species reveals regional differences associated with specific behavioral traits. Brain Behav Evol 80, 181–195. [DOI] [PubMed] [Google Scholar]
- Cox PG, Faulkes CG (2014) Digital dissection of the masticatory muscles of the naked mole‐rat, Heterocephalus glaber (Mammalia, Rodentia). PeerJ 2, e448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham JA, Rahmna IA, Lautenschlager S, et al. (2014) A virtual world of paleontology. Trends Ecol Evol 29, 347–357. [DOI] [PubMed] [Google Scholar]
- de Kloet RS, de Kloet SR (2005) The evolution of the splindlin gene in birds: sequence analysis of an intron of the spindling W and Z gene reveals four major divisions of the Psittaciformes. Mol Phylogenet Evol 36, 706–721. [DOI] [PubMed] [Google Scholar]
- Dunning JB (2008) Handbook of Avian Body Masses, 2nd edn Boca Raton, FL: CRC Press. [Google Scholar]
- Edinger T (1951) The brains of the Odonthognathae. Evolution 5, 6–25. [Google Scholar]
- Elzanowski A, Galton PM (1991) Braincase of Enaliornis, an early cretaceous bird from England. J Vertebr Paleontol 11, 90–107. [Google Scholar]
- Evans DC (2005) New evidence on brain‐endocranial cavity relationships in ornithischian dinosaurs. Acta Palaeontol Pol 50, 617–622. [Google Scholar]
- Fedorov A, Beichel R, Kalpathy‐Cramer J, et al. (2012) 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging 30, 1323–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez P, Carezzano F, Bee de Speroni N (1997) Análisis cuantitativo encefálico e índices cerebrales de Aratinga acuticaudata y Myiopsitta monachus de Argentina (Aves: Psittacidae). Rev Chil Hist Nat 70, 269–275. [Google Scholar]
- Fisher DC, Shirley EA, Whalen CD (2014) X‐ray computed tomography of two mammoth calf mummies. J Paleontol 88, 664–675. [Google Scholar]
- Forshaw JM (2010) Parrots of the World. Princeton, NJ: Princeton University Press. [Google Scholar]
- Gsell AC (2012) The ecology and anatomy of scent in the critically endangered kakapo (Strigops habroptilus). PhD Thesis. Auckland, New Zealand: Massey University. [Google Scholar]
- Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electronica 4, 9. [Google Scholar]
- Healy SD, Rowe C (2007) A critique of comparative studies of brain size. Proc R Soc Lon B Biol Sci 274, 453–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaniuk AN, Nelson JE (2002) Can endocranial volume be used as an estimate of brain size in birds? Can J Zool 80, 16–23. [Google Scholar]
- Iwaniuk AN, Dean KM, Nelson JE (2005) Interspecific allometry of the brain and brain regions in parrots (Psittaciformes): comparisons with other birds and primates. Brain Behav Evol 65, 40–59. [DOI] [PubMed] [Google Scholar]
- Iwaniuk AN, Hurd PL (2005) The evolution of cerebrotypes in birds. Brain Behav Evol 65, 215–230. [DOI] [PubMed] [Google Scholar]
- Iwaniuk AN, Hurd PL, Wylie DRW (2006) Comparative morphology of the avian cerebellum: I. Degree of foliation. Brain Behav Evol 68, 45–62. [DOI] [PubMed] [Google Scholar]
- Iwaniuk AN, Hurd PL, Wylie DRW (2007) Comparative morphology of the avian cerebellum: II. Size of folia. Brain Behav Evol 69, 196–219. [DOI] [PubMed] [Google Scholar]
- Iwaniuk AN, Nelson JE, James HF, et al. (2004) A comparative test of the correlated evolution of flightlessness and relative brain size in birds. J Zool 263, 317–327. [Google Scholar]
- Iwaniuk AN, Nelson JE, O'Leary S (2001) Big brains need big protection: the ‘interhemispheric septum’ in the Psittaciform braincase. J Morphol Sixth International Congress of Vertebrate Morphology, Jena, Germany, July 21–26. [Google Scholar]
- Jerison H (1973) Evolution of the Brain and Intelligence. New York, NY: Academic Press. [Google Scholar]
- Kawabe S, Shimokawa T, Miki H, Matsuda S, Endo H (2013) Variation in avian brain shape: relationship with size and orbital shape. Journal of Anatomy 223, 495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe S, Ando T, Endo H (2014) Enigmatic affinity in the brain morphology between plotopterids and penguins, with a comprehensive comparison among water birds. Zool J Linn Soc 170, 467–493. [Google Scholar]
- Kawabe S, Shimokawa T, Miki H, et al. (2009) A simple and accurate method for estimating the brain volume of birds: possible application in paleontology. Brain Behav Evol 74, 295–301. [DOI] [PubMed] [Google Scholar]
- Klingenberg CP (2011) MORPHOJ: an integrated software package for geometric morphometrics. Mol Ecol Res 11, 353–357. [DOI] [PubMed] [Google Scholar]
- Lautenschlager S, Bright JA, Rayfield EJ (2013) Digital dissection – using contrast‐enhanced computed tomography scanning to elucidate hard‐ and soft‐tissue anatomy in the Common Buzzard Buteo buteo . J Anat 224, 412–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre L, Nicolakakis N, Boire D (2002) Tools and brains in birds. Behaviour 139, 939–973. [Google Scholar]
- Lefebvre L, Reader S, Sol D (2004) Brains, innovations and evolution in birds and primates. Brain Behav Evol 63, 233–246. [DOI] [PubMed] [Google Scholar]
- Maddison WP, Maddison DR (2014) Mesquite: a modular system for evolutionary analysis. Version 3.01. http://mesquiteproject.org
- Mayr G (2010) Parrot interrelationships – morphology and the new molecular phylogenies. Emu 110, 348–357. [Google Scholar]
- Medina L, Reiner A (2004) Do birds possess homologues of mammalian primary visual, somatosensory and motor cortices? Trends Neurosci 23, 1–12. [DOI] [PubMed] [Google Scholar]
- Milner AC, Walsh SA (2009) Avian brain evolution: new data from Palaeogene birds (Lower Eocene) from England. Zool J Linn Soc 155, 198–219. [Google Scholar]
- Mlíkovský J (1989) Brain size in birds: 3. Columbiformes through Piciformes. Vést Cs Spolec Zool 53, 252–264. [Google Scholar]
- Mouritsen H, Feenders G, Liedvogl M, et al. (2005) Night‐vision brain area in migratory songbirds. PNAS 102, 8339–8344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmólska H (2004) Evidence on relation of brain to endocranial cavity in oviraptorid dinosaurs. Acta Palaeontol Pol 49, 321–324. [Google Scholar]
- Picasso MBJ, Tambussi CP, Degrange FJ (2010) Virtual reconstructions of the endocranial cavity of Rhea americana (Aves, Palaeognathae): postnatal anatomical changes. Brain Behav Evol 76, 176–184. [DOI] [PubMed] [Google Scholar]
- Porto M (2004) Anatomia comparada do esqueleto da cabeça e da musculatura da mastigação de Anodorhynchus Spix, 1824, Ara Lacépède, 1799, Diopsittaca Ridgway, 1912, Prophyrrura Miranda‐Ribeiro, 1920 e Orthopsittaca Ridgway, 1912 (Aves: Psittaciformes: Arinae). PhD Thesis. Seropédica, Brazil: Universidade Federal do Rio de Janeiro. [Google Scholar]
- Quayle MR, Barnes DG, Kaluza OL, et al. (2014) An interactive three dimensional approach to anatomical description – the jaw musculature of the Australian laughing kookaburra (Dacelo novaeguineae). PeerJ 2, e355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racicot RA, Rowe T (2014) Endocranial anatomy of a new fossil porpoise (Odontoceti, Phocoenidae) from the Pliocene San Diego formation of California. J Paleontol 88, 652–663. [Google Scholar]
- Reiner A, Perkel DJ, Bruce LL, et al. (2004) Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol 473, 377–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A (2005) A new avian brain nomenclature: why, how and what. Brain Res Bull 66, 317–331. [DOI] [PubMed] [Google Scholar]
- Rohlf FJ, Slice D (1990a) Extensions of the procrustes method for the optimal superimposition of landmarks. Syst Zool 39, 40–59. [Google Scholar]
- Rohlf FJ, Slice D (1990b) GRF: A Program for Generalized Rotational Fitting. Stony Brook, NY: Department of Ecology and Evolution, State University of New York at Stony Brook. [Google Scholar]
- Rücklin M, Donoghue PCJ, Cunningham JA, et al. (2014) Developmental paleobiology of the vertebrate skeleton. J Paleontol 88, 676–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley CG, Alquist JE (1990) Phylogeny Classification of Birds: A Study in Molecular Evolution. New Haven, CT: Yale University Press. [Google Scholar]
- Smith GA (1975) Systematics of parrots. Ibis 117, 18–68. [Google Scholar]
- Smith NA, Clarke JA (2012) Endocranial anatomy of the charadriiformes: sensory system variation and the evolution of wing‐propelled diving. PLoS ONE 7, e49584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingelin W (1957) Vergleichend morphologische untersuchungen am vorderhirn der Vögelauf cytologischer und cytoarchitektonischer grundlage. Basel: Verlag Helbing and Lichtenhahn. [Google Scholar]
- Sutton M, Rahman I, Garwood R (2014) Techniques for Virtual Palaeontology. Chichester, UK: Wiley‐Blackwell. [Google Scholar]
- Tavares ES, Baker AJ, Pereira SL, et al. (2006) Phylogenetic relationships and historical biogeography of Neotropical parrots (Psittaciformes: Psittacidae: Arini) inferred from mitochondrial and nuclear DNA sequences. Syst Biol 55, 454–470. [DOI] [PubMed] [Google Scholar]
- Walsh S, Milner A (2011) Halcyornis toliapicus (Aves: Lower Eocene, England) indicates advanced neuromorphology in Mesozoic Neornithes. J Syst Palaeontol 9, 173–181. [Google Scholar]


