Abstract
Background
Globozoospermia is a rare syndrome with an incidence of less than 0.1% among infertile men. Researchers have recently identified a large deletion, about 200 kbp, encompassing the whole length of DPY19L2 or mutations in SPATA16 and PICK1 genes associated with globozoospermia. The aim of this study was to analyze the DPY19L2 gene deletion using polymerase chain reaction technique for the exons 1, 48, 11 and 22 as well as break point (BP) “a” in globozoospermic men.
Materials and Methods
In this experimental study, genome samples were collected from 27 men with globozoospermia (cases) and 36 fertile individuals (controls), and genomic analysis was carried out on each sample.
Results
Deletion of DPY19L2 gene accounted for 74% of individuals with globozoospermia. DPY19L2 gene deletion was considered as the molecular pathogenic factor for the onset of globozoospermia in infertile men. By quantitative real-time polymerase chain reaction (qPCR), we genotyped DPY19L2 deletion and identified carriers within the population.
Conclusion
This technique may be considered as a method for family counseling and has the potential to be used as a pre-implantation genetic diagnosis, especially in ethnic community with high rate of consanguineous marriages.
Keywords: Gene Expression, Genotyping, Globozoospermia
Introduction
Globozoospermia is a rare autosomal recessive genetic syndrome with an incidence of less than 0.1%. In this syndrome, due to defect in the process of acrosome biogenesis, the sperm contains a round head shape, con- sequently leading to no penetration into the oocyte during fertilization. Thus, direct intra- cytoplasmic sperm insemination (ICSI) along with artificial oocyte activation is the only so- lution to gain pregnancy at couples suffering this abnormality (1). Genetic pedigree assess- ment of these individuals indicates the con- genital origin of globozoospermia. To define molecular defects involved in this disorder, several autosomal genes have been identified in knockout mice models including: Csnk2a2, Hrb, Gopc, Pick1, Gba2, Vps54, Zpbp1 and Hsp90b1 (2-9). Defect of these genes in mouse models represented phenotypically similar abnormalities to human globozoospermia. However, among the aforementioned genes, only PICK1 mutation was yet detected in hu- man. PICK1 protein is involved in subcellular trafficking in brain, pancreas and testis. The respective gene is located on human chromosome 22, and contains 13 exons. In spermatogenesis, PICK1 is involved in trafficking of pro-acrosomal vesicles from golgi apparatus to acrosome. Liu et al. (10) showed a ho- mozygous missense mutation (G198A) at the C-terminal domain of PICK1 which disrupted PvuII site, culminating in formation of sperms with round head shape in human. Other human autosomal genes involved in globozoospermia are SPATA16 and DPY19L2 (11-13). SPATA16 is a testis specific gene, translating a protein which is localized in the golgi apparatus and plays a role in the transportation of pro-acro- somal vesicles from golgi to the acrosome in the round and elongated spermatids (14). Dam et al. (11) found a homozygous sequence vari- ation in the last nucleotide of exon 4 (G848A) of this gene which impaired NciI or HpaII recognition site, in three infertile brothers of a Jewish family with globozoospermia.
However, the most likely considered gene to have a pivotal role in globozoospermia is DPY19L2. This gene is expressed primarily in spermatids with a specific localization limited to the inner nuclear membrane, facing the acroso- mal vesicle. Lack of the relevant protein causes instability of acrosome vesicles and thereby loss of acrosome (15). It has been demonstrated that complete deletion of DPY19L2 by non-allelic homologous recombination (NAHR) results in globozoospermia (12, 13). Recent studies have revealed that DPY19L2 gene function could be eliminated at nine possible breakpoints covering three regions, known as breakpoints “a, b and c” in two low copy flanking repeats (LCRs) of DPY19L2 gene. High incidence (96.5%) of LCR sequences facilitates the occurrence of NAHR in this region (16).
Considering the role of aforementioned genes in globozoospermia and in line with our per- vious study (16), the aim of this study was to evaluate the prevalence of missense mutations, G848A, in exon 4 of SPATA16 gene and G198A in exon 13 of PICK1, as well as DPY19L2 de- letion in Iranian infertile individuals with globozoospermia referring to Isfahan fertility and infertility center (IFIC). Herein, we observed complete deletion of DPY19L2 gene in 20 out of 27 globozoospermic individuals, but no mutation was detected in SPATA16 or PICK1 gene. We also performed quantitative real-time polymerase chain reaction (qPCR) assay to identify individuals with homo/hemizygous deletion of DPY19L2 gene.
Materials and Methods
Mutational analysis of SPATA16, PICK1 and DPY19L2 genes
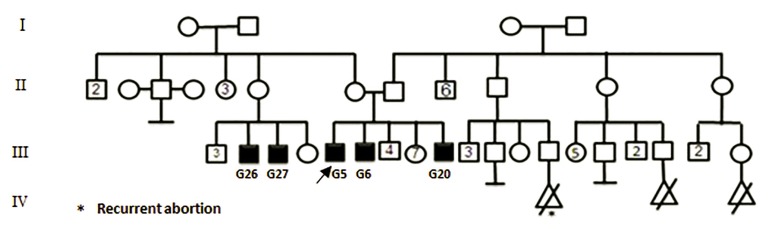
This experimental study was approved by Institutional Review Board (IRB) of Royan Institute. In this case-control study, 27 male with globozoospermia from Iranian population were contributed. An arbitrary number was assigned to each globozoospermic individual (G1 to G29), out of whole two individuals, G11 and G17, were omitted due to missing. We assessed the mutations for SPATA16 and PICK1 genes and provided pedigrees for two families with complete deletion of DPY19L2 and one family with deletion of exon 5, 6 and 7 in DPY19L2 gene.
In this process, blood samples were taken from 27 individuals, with globozoospermia with round-headed spermatozoa who referred to IFIC, as well as their family members after completing a consent form. Two out of 27 persons with more than 50% acrosomeless spermatozoa in their normal and round-headed sperm samples were considered to have partial globozoospermia, while the rest of individuals were suffering from total globozoospermia. Peripheral blood samples were also taken from 30 fertile men as well as the parents of three individuals with globozoospermia (G8, 14 and 21). In the sample group, except three brothers (G21, 22, and 23) and two cases of five (G5, 6, 20, 26, and 27) and two (G9 and 29) cousin subjects, the remaining 17 individuals with globozoospermia belonged to unrelated families (Table 1).
Table 1.
Features of 27 individuals with globozoospermia
| Patient | Type of globozoospermia | Consanguinity | Deficiency in DPY19L2 gene | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| Complete | Partial | No deletion | Complete deletion | Partial deletion (exous 5, 6, 7) | ||||
| Unknown break point | Break point "a" | |||||||
| G1, 4, 7, 12, 13, 15 | ✓ | Non-familial | ✓ | (16) | ||||
| G2, 8, 10, 14,16 | ✓ | Non-familial | ✓ | (16) | ||||
| G3, 18 | ✓ | Non-familial | ✓ | (16) | ||||
| G19 | ✓ | Non-familial | ✓ | (16) | ||||
| G5, 6, 20 | ✓ | familial | ✓ | (16) | ||||
| G9 | ✓ | familial(G29) | ✓ | (16) | ||||
| G21, 22, 23 | ✓ | familial | ✓ | Current study | ||||
| G24 | ✓ | Non-familial | ✓ | Current study | ||||
| G25, 28 | ✓ | Non-familial | ✓ | Current study | ||||
| G26, 27 | ✓ | Familial (G5) | ✓ | Current study | ||||
| G29 | ✓ | Familial (G9) | ✓ | Current study | ||||
Genomic DNA was extracted from individuals’ peripheral blood samples using standard salting out procedure and kept at -20 C until usage (17). Specific primers for identification of G848A, in exon 4 of SPATA16 gene and G198A in exon 13 of PICK1 gene were designed by oligo7 primer designing software (Molecular Biology Insights, CO, USA) according to the respective sequences obtained from National Center for Biotechnology Information (NCBI) database, whereas primer sequences (Table 2) for assessment of DPY19L2 deletion were ordered according to previous report (16). Missense mutations of SPATA16 and PICK1 genes were assessed using Restriction Fragment Length Polymorphism PCR (RFLP-PCR) assay, due to ability of their PCR products digestion by NciI and PvuII restriction enzymes, respectively. Indeed, G848A nucleotide variation in SPATA16 gene causes disruption of NciI site in this location. Thus, a partial PCR product (635 bp) of this gene encompassing G848A could not be cut to produce 283 and 352 bp fragments. Similarly, mutation of G198A region in PICK1 gene disrupts one of two PvuII restriction sites located in this 548 bp PCR product. Thus, G198A mutation produces two bands after PvuII cut, lack of which could cause production of three bands after PvuII digestion. In this study, we did not evaluate the other mutations in these two genes.
Table 2.
List of primers used for polymerase chain reaction and real time PCR analysis
| Genes | Amplified sequences | Primer sequence (5'→3') | Annealing temperature (°C) | Product length (bp) | |
|---|---|---|---|---|---|
| Conventional PCR | β-ACTIN | - | F: CGTGACATTAAGGAGAAGCTGTGC | 55 | 375 |
| R: CTCAGGAGGAGCAATGATCTTGAT | |||||
| DPY19L2 | Exon 1 | F: GGCCAACTTCTTTCTACTCGGAC | 65 | 504 | |
| R: GACCCAGCTCCACCATACTCCTT | |||||
| Exon 4 | F: CAAAATAGCGAGAAGTGATTAG | 54 | 414 | ||
| R: TTCTACTCAACTATAAGGATACAC | |||||
| Exon 5 | F: AGCTTCATCCATGTCACTAT | 60 | 432 | ||
| R: AGCCTTCTCAGAAAACTATTTT | |||||
| Exon 6 | F: GGGTAAATAATTAAACACAGCA | 57 | 462 | ||
| R: AAACAACAGAATAAAAGGGAT | |||||
| Exon 7 | F: AATTTATACGTACACTTTTTAGAATTA | 55 | 420 | ||
| R: ATTTAAACATTTCAATCAACATGC | |||||
| Exon 8 | F: TGGACATGGTAGTTAATTGCTG | 55 | 371 | ||
| R: TCCCAAAGTGCTGAATTGAA | |||||
| Exon 11 | F: AACCTCCTCAAGTGACTTAG | 53 | 516 | ||
| R: TTGGCCAAGAGTCATT | |||||
| Exon 22 | F: GTGTCTGTTATTAAAGCTTGTG | 59 | 313 | ||
| R: ATTGTCTCTAGACAGCAATACAT | |||||
| Break point “a” | - | F: ATGCCATGTTGCCTGCT | 62 | 1700 | |
| R: TCTTCTGGGAAAGGTATTATCGTAG | |||||
| SPATA16 | Exon 4 | F: AATTCTTTGCCATTGTCATATC | 58 | 635 | |
| R: GGTCAAGCGCATTTCTATTAC | |||||
| PICK1 | Exon 13 | F: TGGGCTGCCATCCATGATC | 66 | 568 | |
| R: GCTCCCAGGCTCCGTCCTC | |||||
| PROTAMINE1 | - | F: CCCCTGGCATCTATAACAGGCCGC | 60 | 530 | |
| R: TCAAGAACAAGGAGAGAAGAGTGG | |||||
| Real-time PCR | β-ACTIN | - | F: AGATGCGTTGTTACAGGAAG | 60 | 92 |
| R: TGTGTGGACTTGGGAGAG | |||||
| DPY19L2 | - | F: GACCCAGCTCCACCATACTCCTT | 60 | 144 | |
| R: TTCCATCTCCTCCTCTACCTCCG | |||||
Following identification of three exons (5, 6 and 7) deletion in one of the affected Iranian individual (G9) which was previously reported by Elinati et al. (16), and due to the history of infertility in his family, blood samples of several volunteer family members were obtained and the target of interest was analyzed in their DNA samples.
For detection of DPY19L2 deletion, a multiplex PCR assay was performed for exons 1, 5, 6, 7, 11 and 22 of this gene, together with a part of β-ACTIN or PROTAMIN 1 genes, as internal control using specific primers (Table 2). Lack of amplification for all or some DPY19L2 exons indicates respectively total or partial deletion of this gene in the studied cases. To confirm complete deletion of this gene, specific breakpoint “a” amplification was performed in the samples with lack of amplification for all DPY19L2 exons.
Quantitative assessment of mutated DPY19L2 alleles
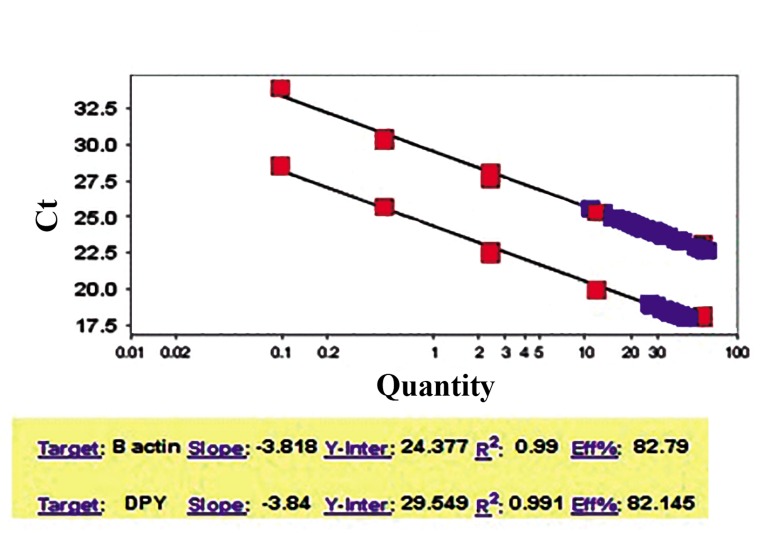
qPCR was implemented by two alternative methods, to analyze the genotyping of DPY19L2 gene for the family members of three cases (G8, 14 and 21) in terms of homo/hemizygosity deletion or normal state of DPY19L2 gene. Specific DPY19L2 and β-ACTIN primer pairs were designed to quantify both the target and reference genes (Table 2). Of note, primer efficiencies for target gene (DPY19L2) and reference gene (β-ACTIN) were almost equal (Fig .1).
Fig.1.
A standard threshold cycle (Ct) curve was drawn to calculate the allele quantities of DPY19L2 and β-ACTIN for individuals who are suspected to be carrier for pathogenic allele of DPY19L2. As described in materials and methods, a standard Ct curve was drawn using different amounts of DNA from a fertile donor (60, 12, 2.5, 0.5 and 0.1 ng, red squares) through qPCR. Then, the quantity of the target gene (DPY19L2, lower curve) and the reference gene (β-ACTIN, upper curve) of each tested sample, for individuals who were suspected carriers of the pathogenic allele of DPY19L2, was calculated based on their Ct on the standard curve. The primer efficiency for both genes was almost similar. Meanwhile, regression coefficients (R2) and the slope of Ct curves were mostly equal (approximately 0.99, and-3.8 respectively).
In the first method, samples were quantified absolutely, using a control blood sample obtained from a healthy fertile donor, who voluntarily participated in this study. After genomic DNA extraction by standard salting out procedure, 60 ng of standard genomic DNA was used as a template for further serial dilution preparations. Different amounts of DNA (60, 12, 2.5, 0.5, 0.1 ng) from this fertile donor were used as template in each PCR reaction in three set of PCR to draw a standard threshold cycle (Ct) curve (red squares shown in the Fig.1). Then, 60 ng of sample tests were subjected to PCR reactions (blue squares shown in the Fig.1). The quantity of the target gene (DPY19L2, lower curve shown in the Fig.1) and the reference gene (β-ACTIN, upper curve shown in the Fig.1) of each subject was calculated based on their Ct in the standard curve which was drawn with different amounts of DNA from the fertile (control) sample in ABI step one plus real-time PCR system (Life Technologies, CA, USA). Proportion of PCR products of DPY19L2 to β-ACTIN quantities was considered for further analyses. This proportion for fertile was considered between 0.8-1, for carrier and patient cases was approximately 0.5 (ranged 0.3-0.7) and 0 respectively, as reported earlier (18). Additionally, to assess the accuracy of this method, equal volume of DNA extracts from blood samples taken from the fertile individual (control sample) and a patient with globozospermia (G14) were mixed and the resulting mixture was used as a heterozygous (hetero) sample.
In the second method, conventional relative quantification (RQ, using 2-ΔΔCt equation) method was used with the same samples, utilizing 60 ng of DNA templates to quantify PCR-products of DPY19L2 relative to β-ACTIN. In this study, RQ level was considered 0.8-1 for normal cases, while this level was approximately 0.5 (ranged 0.3-0.7) and 0 in carrier and patients respectively, as previously reported (18). All PCR reactions contained 5 µl SYBR Green (TaKaRa, Japan), 0.2 µl Rox and 5 µM of each specific primer (0.2 µl) for DPY19L2 or for β-ACTIN (0.5 µl) in a 10 µl final volume of PCR reaction.
Results
Clinical characteristics of the patients with globozoospermia
Clinical parameters of the patients who participated in this study are depicted in the Table 3. Analyses showed lower sperm motility of the patients, compared to the highlighted standard criteria by World Health Organization (WHO). Regarding the round-headed shape of the sperms, in this study, ICSI technique was used to obtain successful fertilization culminated in three healthy births (Table 3). In this survey, three pedigree members that suffered from globozospermia were further studied.
Table 3.
Clinical parameters of patients with globozoospermia
| Patient | Consanguinity of the parents | Sperm parameters | ICSI attempts and results | |||||
|---|---|---|---|---|---|---|---|---|
| Round-headed sperm(%) | Volume (mL) | Sperm concentration (106/mL) | Progressive motility (%) | Number of ICSI (ET cycles) | Clinical pregnancy (Abortion) | Live delivery (Sexuality) | ||
| G1 | Non-familial | 100 | 3 | 80 | 10 | 2 | No (-) | - |
| No (-) | ||||||||
| G2 | NA | 100 | 3 | 80 | 30 | 1 | No (-) | - |
| G3 | NA | 100 | 4 | 64 | 15 | ND | - | - |
| G4 | Familial | 100 | 3 | 20 | 5 | ND | - | - |
| G5 | Familial | 100 | 3.5 | 40 | 2 | ND | - | - |
| G6 | Familial | 100 | 4 | 66 | 10 | ND | - | - |
| G7 | Non-familial | 100 | 1 | 65 | 25 | ND | - | - |
| G8 | Non-familial | 100 | 4 | 66 | 10 | 1 | Yes (-) | Ongoing |
| G9 | Familial | 100 | 4 | 50 | 20 | 1 | No (-) | - |
| G10 | Familial | 100 | 2.5 | 40 | 20 | 3 | No (-) | 1 Singleton (Girl) |
| yes (+) | ||||||||
| Yes (-) | ||||||||
| G12 | Non-familial | 100 | 1.5 | 70 | 20 | 1 | Yes (-) | 1 Singleton (Boy) |
| G13 | Non-familial | 100 | 0.5 | 67 | 25 | 2 | No (-) | 1 Singleton (Girl) |
| Yes (-) | ||||||||
| G14 | Familial | 100 | 1 | 2 | 25 | ND | - | - |
| G15 | Non-familial | 100 | 2 | 74 | 0 | ND | - | - |
| G16 | Non-familial | 100 | 3 | 30 | 15 | 1 | No (-) | - |
| (1) | No (-) | |||||||
| G18 | NA | 100 | 4 | 80 | 10 | 1 | No (-) | - |
| (1) | No (-) | |||||||
| G19 | NA | 98 | 4 | 80 | 0 | 1 | Yes (-) | Ongoing |
| G20 | Familial | 100 | 3 | 40 | 15 | 1 | No (-) | - |
| (1) | No (-) | |||||||
| G21 | Non-familial | 100 | 3.1 | 10 | 5 | ND | - | - |
| No (-) | ||||||||
| G22 | Non-familial | 100 | 6.7 | 60 | 35 | 2 | No (-) | - |
| G23 | Non-familial | 100 | 2.1 | 10 | 5 | ND | - | - |
| G24 | Familial | 100 | 2 | 18 | 5 | 1 | No (-) | - |
| G25 | Familial | 96 | 2.9 | 90 | 40 | 1 | No (-) | - |
| G26 | Familial | 100 | 2.3 | 40 | 10 | ND | - | - |
| G27 | Familial | 100 | 1 | 40 | 15 | 1 | No (-) | - |
| (1) | No (-) | |||||||
| G28 | Familial | 98 | 2.3 | 28 | 10 | ND | - | - |
| G29 | Familial | 100 | 3 | 45 | 40 | 1 | No (-) | - |
Two samples of G11 and G17 were lost, thus they were deleted. ET; Freeze-thawed embryo transfer, ICSI; Intra-cytoplasmic sperm inseminaton, NA; Not assigned, ND; Not done, +; Stands for successful pregnancy, -; Stands for abortion, and *; Globo 23 is single and not married.
Mutational analysis in SPATA16 and PICK1 genes
In this study, 27 cases with globozoospermia and 30 fertile men as control group were analyzed for detection of nucleotide variation (Table 1). In our first screening, regarding that digestion of the PCR products resulted in similar pattern to the fertile cases (data not shown), we did identify missense mutations of neither G848A in exon 4 of SPATA16 gene (Fig .2, left panel) nor G198A in exon 13 of PICK1 gene in the studied cases (Fig .2, right panel).
Fig.2.
Assessment of missense mutations of G848A in the exon 4 of SPATA16 (left panel) and G198A in the exon 13 of PICK1 (right panel) genes using NciI and PvuII restriction endonuclease enzymes, respectively. No mutation was observed due to complete digestion of amplified fragments as described in materials and methods. M; 50 bp DNA ladder.
Analysis of DPY19L2 deletion in the cases with globozoospermia
We have previously reported that DPY19L2 gene deletion leads to globozoospermia (16). In this study, further to 14 (out of 18) individuals who had shown some deletion in DPY19L2 gene, six (out of nine) new cases with globozoospermia, missed the entire length of DPY19L2 gene (G21, 22, 23, 24, 26 and 27, Table 1). One of these six individuals was unrelated (G24), while the remaining individuals were originated from two different pedigrees (Table 1).
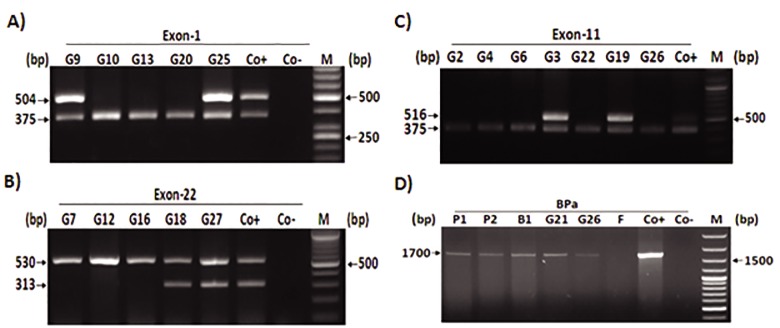
This experiment was carried out by several multiplex PCR on exon 1 (Fig .3A), exon 11 (Fig .3B) and exon 22 of DPY19L2 gene (Fig .3C) with a part of β-ACTIN or PROTAMINE1 gene. Two new individuals (G25 and 28), with partially globozoospermia demonstrations, showed a wild-type condition for DPY19L2 gene (Table 1). Furthermore, data indicated the presence of breakpoint “a” (BPa) in most of the new cases (five out of six) with entire DPY19L2 gene deletion (Fig .3D, Fig respective lanes for G21 and 26).
Fig.3.
Analysis of DPY19L2 gene deletion in exons 1, 11 and 22 as well as identification of breakpoint “a” (BPa) in a number of in- dividuals with globozoospermia (G#). Multiplex PCR products of A. Exon 1(504 bp, upper band), B. Multiplex PCR products of exon 22 of DPY19L2 gene (313 bp, lower band) and part of PROTAMINE1 gene (530 bp, upper band). Co+ or positive control in A, B and C is a fertile specimen and Coor negative control is no template sample, C. Exon 11 (516 bp, upper band) of DPY19L2 gene together with a part of β-ACTIN gene (375 bp, lower band) and D. PCR analysis of BPa. P1 and P2 are parents of globozoospermia patient (G21) and B1 is his fertile brother and negative control is a fertile specimen, F, and positive control is a case with globozoospermia, which has been confirmed to have BPa. M; 50 bp DNA ladder in panel A and 100 bp DNA ladder for the rest of the panels and PCR; Polymerase chain reaction.
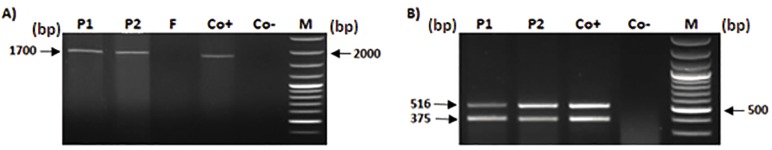
Moreover, DPY19L2 gene hemizygosity (complete deletion of one DPY19L2 gene allele) was evaluated in parents of one case (G8), who has previously been recognized to suffer from deletion of entire length of DPY19L2 gene (16). Here we confirmed hemizygosity of DPY19L2 for both parents of G8, by amplification of BPa (Fig .4A) and exon 11 (Fig .4B). Of note that other siblings of this family were fertile.
Fig.4.
Detection of exon 11 and BPa in parents of one case with globozoospermia (G8). A. Amplification of BPa implied hemizygosity state for DPY19L2 gene in the parents. DNA sample of fertile individual (F) was not amplified for BPa as expected, Co+; Globozoospermia who previously proved to have BPa, , P1, P2; Parents of G8, Co-; No template sample and B. Multiplex PCR products for exon 11 of DPY19L2 gene (516 bp, upper band) together with a part of β-ACTIN gene (375 bp, lower band), Co+; Sample from a fertile man, Co-; No DNA template, M; 100 bp DNA ladder and P1, P2; Parents of G8.
Evaluation of familial globozoospermia
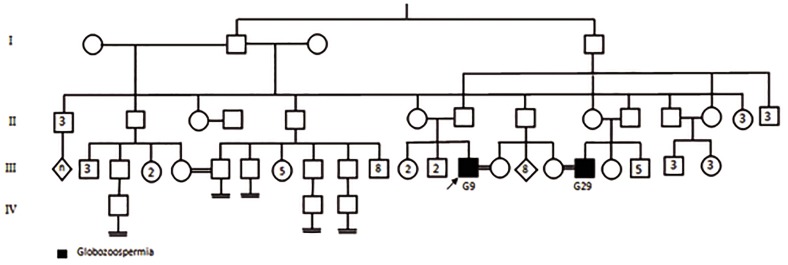
In this experiment study, two cases (G5 and 9) were selected for sibling analysis. As shown in the Figure 5, the genetic pedigree belongs to family of G5 (with the history of reproductive failure and miscarriage) revealed that all of five members (G5, 6, 20, 26, and 27) had globozoospermia associated with complete deletion of DPY19L2 gene. We have previously demonstrated (16) a partial deletion of DPY19L2 including exons 5, 6 and 7 in one case (G9, Table 1). Due to infertility history of his family (Fig .6) and access to DNA samples of all family members, multiplex PCR of the aforementioned exons was performed. Curiously, we determined similar mutations pattern of DPY19L2 gene to G9 patient, in the cousin with complete globozoospermia (G29). Indeed, detection of exons 4 and 8 by PCR confirmed this partial deletion (data not shown).
Fig.5.
Pedigree of one case with globozoospermia (G5) and repeated pregnancy loss. There is more consanguineous marriage in this fam- ily but for simplicity detailed data are not depicted in the pedigree. Polymerase chain reaction (PCR) analysis showed G26, 27, 5, 6 and 20 suffering from globozoospermia due to complete deletion of DPY19L2 gene. II2, III11 and III15 are infertile individuals with performing no genetic analysis. The inset numbers which are shown in the squares/circles represent the numbers of healthy (fertile) siblings who were not shown in this pedigree.
Fig.6.
Consanguineous pedigree of the G9 family, with partial deletion of DPY19L2 gene. Only one cousin (G29) who was also suffering from globozoospermia had deletion of exons 5, 6 and 7. The inset numbers which are shown in the squares/circles represent the numbers of healthy (fertile) siblings who were not shown in this pedigree. n; The sexuality and numbers of siblings were not determined.
DPY19L2 genotyping analysis
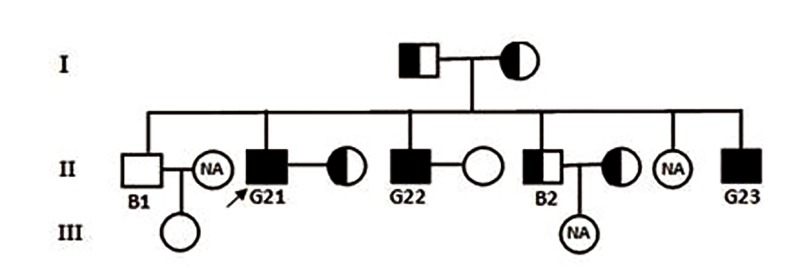
To set a reliable method for homo/hemizygosity state of DPY19L2 deletion, we performed qPCR based genotyping analysis for family members of one case who showed whole DPY19L2 gene deletion, G21. The DPY19L2 deletion consan- guineous pedigree for G21 patient is shown in the Figure 7. Data analyses demonstrated whole DPY19L2 gene deletion of one allele for the G21 parents. Quantity proportion values of DPY19L2 to β-ACTIN for carriers of DPY19L2 deletion were almost between 0.40.6, while in normal cases, it ranged between 0.8-1 based on two calculated methods (quantities ratio and 2-∆∆Ct ratio presented in the Table 3). In addition, no amplification plot was detected for G21, 22 and 23 indicating lack of the mentioned gene in these subjects. In addition, detection of BPa in hemizygote individuals of the family confirmed the outcomes of qPCR assay (Fig .3D, Fig where P1 and P2 are parents of G21 patient, B1 is the hemizygote fertile brother. G21 is proband person) (Table 4).
Table 4.
DPY19L2 genotype achieved by quantitative real-time PCR for the family members of Globo 21 and Globo 22
| Subject | Quantities ratio | 2-∆∆Ct ratio |
|---|---|---|
| Father (P1) | 0.522924 | 0.4 |
| Mother (P2) | 0.408959 | 0.4 |
| Brother-1 (B1)* | 1.006982 | 1 |
| B1 sibling | 0.957183 | 0.9 |
| Brother-2 (B2)* | 0.499332 | 0.4 |
| B2 wife | 0.414017 | 0.4 |
| Globo22 wife | 1.037303 | 1 |
| Globo21 wife | 0.488268 | 0.4 |
*; B1 and B2 are 2 fertile patient’s (Globo21) brothers, Ct; Threshold cycle, and PCR; Polymerase chain reaction.
Fig.7.
Consanguineous pedigree of G21 with complete deletion of DPY19L2 gene in BPa. DPY19L2 genotyping analysis in this pedigree indicated that B2 and wives of G21 and B2 are hemizygote. Three pedigree members (B1 wife, G21 sister and B2 daughter) did not par- ticipate in the analysis and their zygousity state remained undefined (NA)
To extend the application of previously suggest- ed method (quantities ratio) for identification of gene homo/hemizygosity at different individuals, we performed further analyses on the G8 and 14 patients’ parents, besides of the hemizygote sam- ple (hetero) as notified in materials and methods. Data affirmed the hemizygote status of the parents and hetero case by two alternatively implicated calculation methods (Table 5).
Table 5.
| Subject | Quantities ratio | 2-∆∆Ct ratio |
|---|---|---|
| Globo 14 father | 0.58377 | 0.5 |
| Globo 14 mother | 0.40623 | 0.4 |
| Globo 8 father (P1) | 0.47476 | 0.5 |
| Globo 8 mother (P2) | 0.424836 | 0.4 |
| Hetero | 0.560374 | 0.4 |
| Control-1 | 0.973175 | 0.9 |
| Control-2 | 1.381863 | 1.3 |
Ct; Threshold cycle and PCR; Polymerase chain reaction.
Discussion
In the recent years, there have been an increasing amounts of literatures proposing the molecular mechanisms of globozoospermia (7,9,13,15,16,19,21). Our previous studies have described DPY19L2 gene as a basic factor required for development of normal acrosome biogenesis. Partial or complete deletion of the DPY19L2 gene is pivotal factor in globozoospermia (16).
Therefore, we investigated complete deletion of DPY19L2 gene effects to reaffirm the potential association of DPY19L2 gene and globozoospermia. In addition, deletion of this gene was evaluated in the family members of three globozoospermic individuals. Thus, deletion analysis of DPY19L2 gene (12q14.2) was carried out in three exons 1, 11, 22 of DPY19L2 gene, using multiplex PCR, compared to β-ACTIN or PROTAMINE1 genes as internal controls. Briefly, all of three assessed exons of DPY19L2 gene (1,11,22) were missed in 20 out of 27 cases (74%) suggesting total absence of DPY19L2 gene in these cases.
It should be noted that identification of total deletion of DPY19L2 gene with BPa in 18 cases, out of 27, has previously been reported by Elinati et al. (16). Overall, six out of nine new individuals showed complete deletion of DPY19L2 gene, five of whom carried BPa and the remaining may have unknown BP. Also, one new patient (G29) harbored a partial deletion of this gene and two others (G25 and G28) with partial globozoospermia had two wild type alleles. Previous studies have also demonstrated molecular mutations in DPY19L2 gene (19,21). Deletion of the DPY19L2 gene is a common genomic rearrangement that occurs due to LCRs flanking the gene by NAHR. Concurrent with the cases, family members of three globozoospermic patients were investigated in this study. In this regard, two pedigrees (G5 and 9 pedigrees) from different geographically accommodation regions, similar ethnicity and high rate of consanguineous marriages showed the history of reproductive failure due to globozoospermia. Regarding high tincidence of this rare abnormality among tribal races, diagnosis of carrier individuals could help them, in terms of genetic management, for future family planning.
Several studies have previously detected heterozygosity of the other genes, like SMN1 and DYSTROPHIN, through quantitative real-time PCR based on comparative Ct method (18,22,23). In this article, we identified the carriers in one pedigree (G21 pedigree) by this method and also proposed a modified method, quantities ratio. Thus, we designed qPCR assay for family members of G21. Analyses were performed based on proportion of DPY19L2 to β-ACTIN quantities. After providing the standard curve based on serial diluted DNA samples of a fertile man, quantities of the reference and target gene were estimated. Quantitative analysis of DPY19L2 gene for G21 family members led us to identify individuals with hemizygosity at this gene. We determined that parents with a quantity ratio ranging between 0.4-0.6 are carrier. One of the fertile brothers (B2) as well as partners of G21 and B2, were hemizygote for deletion of DPY19L2 gene. Quantity ratio for normal cases, consisting one of the fertile brother, the grand daughter and partner of G22, were ranging from 0.95-1.3. These results were similar to previously reported threshold cycle method verifying our conclusion to determine the individuals with no gene deletion or carriers (18,22). Considering non-consanguinity of parents, the incidence of the abnormality in this family could be attributed to their accommodation in the same geographical area. To validate our calculation method on the allele hemizygosity, we extended experiments on the G8 and G14 patients’ parents who kindly accepted to participate voluntarily in this survey.
These findings are in agreement with previous studies, indicating a strong relationship between DPY19L2 gene and globozoospermia. However, molecular cause of few cases remains yet unclear, requiring further investigations to identify genetic defect(s) in the other gene(s) affecting globozoospermia.
Regarding the other genes, in the present study mutation screening of the SPATA16 and PICK1 genes were also carried out on 27 cases with globozoospermia and 30 fertile men. Our data revealed that PICK1 and SPATA16 genes were intact in all of studied individuals.
Conclusion
Our result revealed that qPCR analysis can be used for genotyping of DPY19L2 deletion and this may help genetic consolers in family planning. In future, it might also help prevent occurrence of this syndrome in carrier families through pre-implantation genetic diagnosis, especially in ethnic community with high consanguineous marriages.
Acknowledgments
This study was supported by Royan Institute and we would like to express our gratitude to staff of IFIC for their fully supports. There is no conflict of interest in this study.
References
- 1.Dam AH, Feenstra I, Westphal JR, Ramos L, van Golde RJ, Kremer JA. Globozoospermia revisited. Hum Reprod Update. 2007;13(1):63–75. doi: 10.1093/humupd/dml047. [DOI] [PubMed] [Google Scholar]
- 2.Xu X, Toselli PA, Russell LD, Seldin DC. Globozoospermia in mice lacking the casein kinase II alpha catalytic subunit. Nat Genet. 1999;23(1):118–121. doi: 10.1038/12729. [DOI] [PubMed] [Google Scholar]
- 3.Kang-Decker N, Mantchev GT, Juneja SC, McNiven MA, Van Deursen JM. Lack of acrosome formation in Hrb-deficient mice. Science. 2001;294(5546):1531–1533. doi: 10.1126/science.1063665. [DOI] [PubMed] [Google Scholar]
- 4.Yao R, Ito C, Natsume Y, Sugitani Y, Yamanaka H, Kuretake S, et al. Lack of acrosome formation in mice lacking a Golgi protein, GOPC. Proc Natl Acad Sci USA. 2002;99(17):11211–11216. doi: 10.1073/pnas.162027899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao N, Kam C, Shen C, Jin W, Wang J, Lee KM, et al. PICK1 deficiency causes male infertility in mice by disrupting acrosome formation. J Clin Invest. 2009;119(4):802–812. doi: 10.1172/JCI36230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yildiz Y, Matern H, Thompson B, Allegood JC, Warren RL, Ramirez DM, et al. Mutation of beta-glucosidase 2 causes glycolipid storage disease and impaired male fertility. J Clin Invest. 2006;116(11):2985–2994. doi: 10.1172/JCI29224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paiardi C, Pasini ME, Gioria M, Berruti G. Failure of acrosome formation and globozoospermia in the wobbler mouse, a Vps54 spontaneous recessive mutant. Spermatogenesis. 2011;1(1):52–62. doi: 10.4161/spmg.1.1.14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin YN, Roy A, Yan W, Burns KH, Matzuk MM. Loss of zona pellucida binding proteins in the acrosomal matrix disrupts acrosome biogenesis and sperm morphogenesis. Mol Cell Biol. 2007;27(19):6794–6805. doi: 10.1128/MCB.01029-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Audouard C, Christians E. Hsp90beta1 knockout targeted to male germline: a mouse model for globozoospermia. Fertil Steril. 2011;95(4):1475–1477. doi: 10.1016/j.fertnstert.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Liu G, Shi QW, Lu GX. A newly discovered mutation in PICK1 in a human with globozoospermia. Asian J Androl. 2010;12(4):556–560. doi: 10.1038/aja.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dam AH, Koscinski I, Kremer JA, Moutou C, Jaeger AS, Oudakker AR, et al. Homozygous mutation in SPATA16 is associated with male infertility in human globozoospermia. Am J Hum Genet. 2007;81(4):813–820. doi: 10.1086/521314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harbuz R, Zouari R, Pierre V, Ben Khelifa M, Kharouf M, Coutton C, et al. A Recurrent deletion of DPY19L2 causes infertility in man by blocking sperm head elongation and acrosome formation. Am J Hum Genet. 2011;88(3):351–361. doi: 10.1016/j.ajhg.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koscinski I, Elinati E, Fossard C, Redin C, Muller J, Velez de la Calle J, et al. DPY19L2 deletion as a major cause of globozoospermia. Am J Hum Genet. 2011;88(3):344–350. doi: 10.1016/j.ajhg.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu M, Xiao J, Chen J, Li J, Yin L, Zhu H, et al. Identification and characterization of a novel human testis-specific Golgi protein, NYD-SP12. Mol Hum Reprod. 2003;9(1):9–17. doi: 10.1093/molehr/gag005. [DOI] [PubMed] [Google Scholar]
- 15.Pierre V, Martinez G, Coutton C, Delaroche J, Yassine S, Novella C, et al. Absence of Dpy19l2, a new inner nuclear membrane protein, causes globozoospermia in mice by preventing the anchoring of the acrosome to the nucleus. Development. 2012;139(16):2955–2965. doi: 10.1242/dev.077982. [DOI] [PubMed] [Google Scholar]
- 16.Elinati E, Kuentz P, Redin C, Jaber S, Vanden Meerschaut F, Makarian J, et al. Globozoospermia is mainly due to DPY19L2 deletion via non-allelic homologous recombination involving two recombination hotspots. Hum Mol Genet. 2012;21(16):3695–3702. doi: 10.1093/hmg/dds200. [DOI] [PubMed] [Google Scholar]
- 17.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee TM, Kim SW, Lee KS, Jin HS, Koo SK, Jo I, et al. Quantitative analysis of SMN1 gene and estimation of SMN1 deletion carrier frequency in Korean population based on real-time PCR. J Korean Med Sci. 2004;19(6):870–873. doi: 10.3346/jkms.2004.19.6.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coutton C, Zouari R, Abada F, Ben Khelifa M, Merdassi G, Triki C, et al. MLPA and sequence analysis of DPY19L2 reveals point mutations causing globozoospermia. Hum Reprod. 2012;27(8):2549–2558. doi: 10.1093/humrep/des160. [DOI] [PubMed] [Google Scholar]
- 20.Kuentz P, Vanden Meerschaut F, Elinati E, Nasr-Esfahani MH, Gurgan T, Iqbal N, et al. Assisted oocyte activation overcomes fertilization failure in globozoospermic patients regardless of the DPY19L2 status. Hum Reprod. 2013;28(4):1054–1061. doi: 10.1093/humrep/det005. [DOI] [PubMed] [Google Scholar]
- 21.Zhu F, Gong F, Lin G, Lu G. DPY19L2 gene mutations are a major cause of globozoospermia: identification of three novel point mutations. Mol Hum Reprod. 2013;19(6):395–404. doi: 10.1093/molehr/gat018. [DOI] [PubMed] [Google Scholar]
- 22.Akbari MT, Noruzinia M, Mozdarani H, Hamid M. Determination of exon 7 SMN1 deletion in Iranian patients and heterozygous carriers by quantitative real-time PCR. J Genet. 2011;90(1):133–136. doi: 10.1007/s12041-011-0038-1. [DOI] [PubMed] [Google Scholar]
- 23.Maksimovic N, Andjelkovic A, Rasic VM, Stojanovic VR, Kotlica BK, Brankovic S, et al. Quantitative analysis of the dystrophin gene by real-time PCR. Arch Biol Sci. 2012;64(2):787–792. [Google Scholar]