Abstract
Background
Metals can cause male infertility through affection of spermatogenesis and sperm quality. Strong evidences confirm that male infertility in metal-exposed humans is mediated via various mechanisms such as production of reactive oxygen species (ROS). Flavonoids have antioxidant and metal chelating properties which make them suitable candidates for neutralizing adverse effects of metals on semen quality. In the current study, we have evaluated the effects of five types of flavonoids (rutin, naringin, kaempferol, quercetin, and catechin) on recovery of sperm motility and prevention of membrane oxidative damage from aluminum chloride (AlCl3), cadmium chloride (CdCl2), and lead chloride (PbCl4).
Materials and Methods
In this experimental study, motility and lipid peroxidation of metalexposed sperm was investigated in the presence of different concentrations of five kinds of flavonoids. Malondialdehyde (MDA) production was assessed as a lipid peroxidation marker.
Results
Aluminum chloride (AlCl3), cadmium chloride (CdCl2), and lead chloride (PbCl4) diminished sperm motility. Treatment of metal-exposed sperm with rutin, naringin, and kaempferol attenuated the negative effects of the metals on sperm motility. Quercetin and catechin decreased the motility of metal-exposed sperm.
Conclusion
Based on the MDA production results, only AlCl3 significantly induced lipid peroxidation. Treatment with rutin, naringin, and kaempferol significantly decreased MDA production.
Keywords: Metal Toxicity, Sperm Motility, Lipid Peroxidation, Flavonoids, Semen Quality
Introduction
Metals are one of the main constituents of an industrialized lifestyle that have a wide range of applications. Metals such as lead (Pb), aluminum (Al) and cadmium (Cd) induce toxicity in humans and other living organisms by impacting enzyme activity and generation of free radical production. However, in terms of their unique characteristics, their applications are expansive, even in medical and drug industries (1,2).
Metals can affect male and female fertility by induction of reactive oxygen species (ROS) production. Therefore, antioxidant therapy that inhibits metal-induced toxicity is under active investigation (3). Flavonoids are a broad group of natural antioxidant compounds with flavan nucleus and a benzo-ƴ-pyrone structure. These compounds are low molecular weight polyphenols ubiquitously synthesized by green plants that may show various pharmacological attributes according to their chemical structures (4). Direct antioxidant effects and the ability of flavonoids to chelate metal ions have been previously researched (5,7). Researchers report the existence of a cardioprotective role (8,9) and free radical scavenging potential of flavonoids (10). Until now, over 4000 natural flavonoids have been identified in leaves, seeds, barks, and flowers of different plants (11). Protection against ultraviolet (UV) light, pathogens, herbivores, and the attraction of pollinating insects are major proposed roles for flavonoids in various plants (12,14). Flavonoids can occur both in the free form and as glycosides. Their structure is composed of a basic C6-C3-C6phenyl-benzopyran backbone (Fig .1). The position of the phenyl ring relative to the benzopyran moiety, oxidation of central ring, hydroxylation profile, and degree of polymerization determine chemical properties of a flavonoid (15).
Fig.1.
Chemical structure of flavonoids. A. Basic structure of a flavonoid with two benzene rings and a heterocyclic pyran ring as the linker. Chemical structures of: B. Rutin, C. Naringin, D. Kaempferol, E. Quercetin, and F. Catechin
ROS induce cellular membrane instability (16), destruction of DNA structures, and promotion of transformation, (17) ultimately resulting in cellular aging (18), mutagenesis (17), carcinogenesis (19), induction of coronary heart disease (CHD) (4), and infertility (20). In addition to ROS, nitrogen reactive species (NOS) can cause cardiovascular diseases (CVD) through oxidation of LDL particles (21,22) and increased release of matrix metalloproteinase-2 (MMP-2) in the coronary effluent (23). Based on the scientific findings, a flavonoid-rich diet is highly recommended to decrease CVD and other ROS-/ NOS-induced myocardial injuries (4).
Recent interest in flavonoids arises from the potential health benefits attributed to the antioxidant activities of these polyphenolic compounds. Functional hydroxyl groups in flavonoids mediate their antioxidant effects by scavenging free radicals and/ or by chelating metal ions (4,11). The chelating of metals can be crucial in prevention of radical generation which damage target biomolecules (11). In the current study, we have evaluated the effects of five types of flavonoids (rutin, naringin, kaempferol, quercetin, and catechin) on recovery of sperm motility and prevention of membrane oxidative damage from aluminum chloride (AlCl3), cadmium chloride (CdCl2 ), and lead chloride (PbCl4).
Materials and Methods
Materials
For this experimental study, AlCl3 , CdCl2 , PbCl4 , naringin, kaempferol, and quercetin were obtained from Merck (Darmstadt, Germany). Rutin, catechin and the remainder of chemicals and reagents used in this research were purchased from SigmaAldrich (St. Louis, MO, USA).
Sample collection and preparation of sperm suspension
Sperm samples considered compatible to the world health organization (WHO) reference value for human semen (volume ≥3.0, sperm concentration/ ml ≥50×106, forward motility ≥60%, and atypical forms ≤40%) (24) were collected and pooled from 40 healthy, non-smoking volunteers, that resided in Ahvaz, Khuzestan Province, Iran. We compared the effects of flavonoides on motility and lipid peroxidation of metal-exposed sperms using laboratory studies. The Institutional Ethics Committee of Ahvaz University of Medical Sciences reviewed and approved the protocol. All participants in the current study signed informed consents. Collected sperm samples were separated from semen plasma for assessment of clinical attributes by washing three times with an equal volume of M 6solution and subsequent centrifugation for 10 minutes at 1600 g (25). M6 solution contained (per liter, pH=7.4): 0.55% NaCl, 0.03% KCl, 0.019% CaCl2 , 0.016% K3PO4, 0.029% MgSO4, 0.031% NaHCO3, 0.496% HEPES, 0.26% sodium lactate, 36×10-4% sodium pyruvate, 0.11% glucose, 0.4% bovine serum albumin, 60×10-4% penicillin, and 50×10-4% streptomycin. Separated pellets were suspended in M 6solution at a density of 100 million sperm/ml and freshly were used. Sperm counts were performed by a MMC-SK Sperm Counting Chamber (Saint Petersburg, Russia).
Incubation of sperm samples with aluminum chloride, cadmium chloride, and lead chloride
We evaluated the effects of AlCl3 , CdCl2 , and PbCl4 on sperm motility and lipid peroxidation of sperm cells at different concentrations (125 µM, 250 µM, 500 µM, 1 mM, and 5 mM) of the metal salts. The metal salt solutions were prepared in M 6solution. Sperm samples were incubated in the presence of defined concentrations of these metals for 2 hours at 37˚C. From the examined concentrations of metals, we selected those that significantly impacted sperm motility for additional experiments with the flavonoids (P≤0.05).
Effects of flavonoids on the motility of metalexposed sperm
Sperm samples were treated for 2 hours at 37˚C with AlCl3 (1.0 mM), CdCl2 (500 µM) or PbCl4 (250 µM) in the presence of various concentrations (25, 50, 100, 200, 500, and 1000 µM) of rutin, naringin, kaempferol, quercetin, and catechin. Subsequently, we assessed sperm mobility by MMC Sperm. In order to increase solubility, all flavonoids were solvated in a 1:1 (v/v) of Dimethyl sulfoxide (DMSO): M6solution prior to their treatment of the sperm cells.
Effects of flavonoids on lipid peroxidation of metal-exposed sperm
Induction of lipid peroxidation was evaluated in sperm samples in the presence of various concentrations of AlCl3 , CdCl2 , and PbCl4 . Between treated groups, sperm samples treated with 20 mM of AlCl3 were simultaneously incubated with 25 µM, 50 µM, 100 µM, 200 µM, 500 µM, and 1 mM each of rutin, naringin, kaempferol, quercetin, and catechin for 2 hours at 37˚C. After incubation, we assessed for lipid peroxidation of the sperm cells according to the indicated approach.
Analytical methods
Assessment of sperm motility
Evaluation of sperm motility was performed by MMC Sperm (MultiMedia Catalog Sperm). MMC Sperm is an automated image analysis software package for sperm quality analysis according to parameters recommended by the WHO laboratory manual (26).
Measurement of lipid peroxidation
Lipid peroxidation was measured using malondialdehyde (MDA) and thiobarbituric acid-reactivity (27,28). Briefly, 50 µl of 0.2% butylated hydroxytoluene (dissolved in ethanol) and 1.0 ml of 15% aqueous trichloroacetic acid were successively added to 2.0×107sperm. The mixture was then centrifuged at 4000 g for 15 minutes at 4˚C. An aliquot of 500 μl of the deproteinized supernatant was added to 1.0 ml thiobarbituric acid (0.375% in 0.25 M HCl) and the mixture was heated at 100˚C for 20 minutes. After cooling, the solution was analyzed by a spectrophotometer at 532 nm.
Statistical analysis
All treatments were performed in triplicate. Each experiment was run at least three times. Results were expressed as mean ± SE. Significance of difference between treatment groups was determined by the student’s t test. P<0.05 was considered statistically significant.
Results
Effects of aluminum chloride, cadmium chloride, and lead chloride on sperm motility
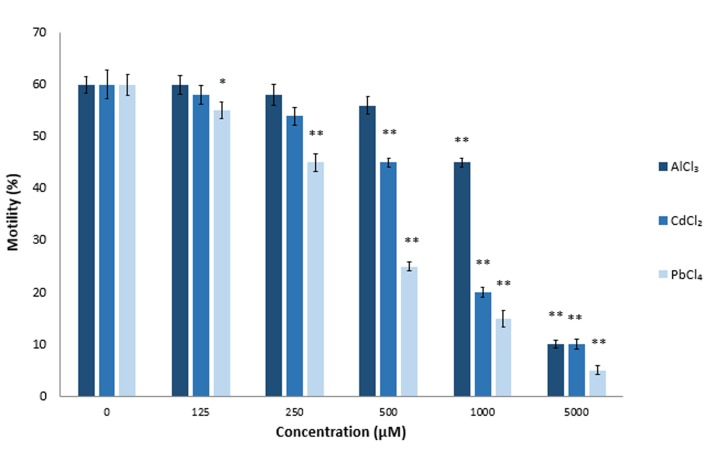
AlCl3 is an abundant metal in the earth which has toxic effects. High concentrations of AlCl3 induce free radical-mediated cytotoxicity and can be toxic for the male reproductive system (29,30). In previous studies, it has been shown that treatment with AlCl3 could decrease ejaculate volume, sperm concentration, and sperm motility (31). CdCl2 is a wellknown nephrotoxin and carcinogen (32,33) that can induce ROS production. Exposure to CdCl2 may result in decreased sperm concentration, diminished sperm motility, creation of abnormal forms of sperm following long-term exposure to CdCl2 (3,34), and infertility in treated male mice (35). PbCl4 poisoning can result in decreased sperm motility. A number of reports discuss DNA fragmentation in sperm cells exposed to this metal in vitro (36). Our in vitro studies have confirmed the above mentioned findings where different concentrations of AlCl3 , CdCl2 and PbCl4 significantly decreased sperm motility (P≤0.05, Fig .2). Mean sperm motility after a 2-hour incubation period in the presence of 5.0 mM AlCl3 , CdCl2, and PbCl4 were 93% (AlCl3), 75% (CdCl2 ), and 41% (PbCl4) less than the control groups. As seen in Figure 2, the effect of Pb on sperm motility was higher at the same concentrations of the three tested metals AlCl3 , at the 1.0 mM concentration, significantly affected sperm motility (P≤0.0013). The 500 µM concentration of CdCl2 significantly affected sperm motility (P≤0.032), whereas PbCl4 significantly affected motility at the 250 µM (P≤0.0005) concentration (Fig .2). The adverse effects of all three metals on sperm motility were completely dose-dependent.
Fig.2.
Effects of aluminum chloride (AlCl3), cadmium chloride (CdCl2), and lead chloride (PbCl4) on sperm motility. We evalu- ated the effects of these compounds on sperm motility at differ- ent concentrations (125 μM, 250 μM, 500 μM, 1 mM, and 5 mM) of metal salts. Sperm samples were incubated in the presence of the defined concentrations of metals for 2 hours at 37˚C. *; P<0.05 and **; P<0.01 compared to the untreated control.
Effects of flavonoids on motility of aluminum chloride-exposed sperm
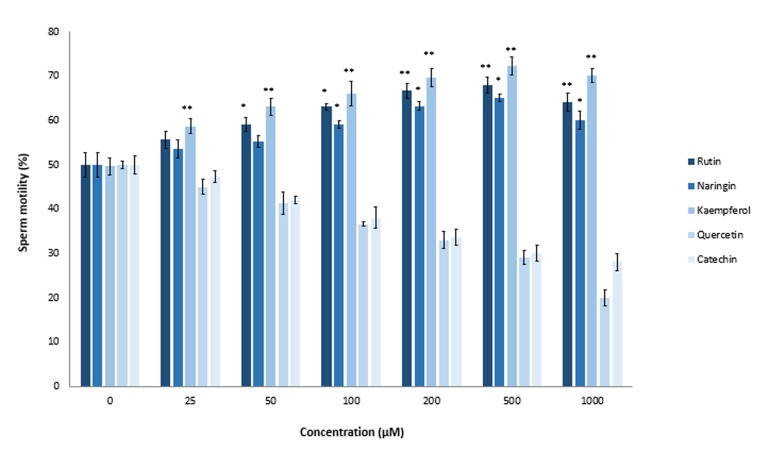
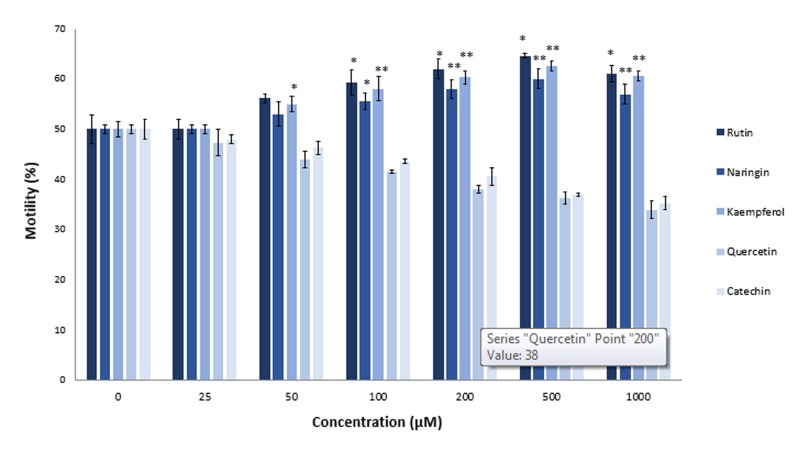
Previous studies reported an in vitro protective effect of ascorbic acid (vitamin C) and tocopherol (vitamin E) on AlCl3 -treated sperm (31,37). As seen in Figure 2, 1000 µM of AlCl3 significantly decreased sperm motility by 15% (P≤0.0013). Therefore, we used this concentration for additional studies with flavonoids. We used different concentrations of rutin, naringin, kaempferol, quercetin, and catechin for motility recovery of AlCl3 -exposed sperm. Compared to the untreated control group, rutin increased sperm motility by 9% at the 50 µM concentration and 18% at the 200 µM concentration. Naringin, at a final concentration of 100 µM, significantly increased sperm motility by 9% (P≤0.038). There was a gradual increase in recovery of sperm motility when the concentration of naringin increased to 500 µM (Fig .3). Kaempferol showed the most protective effect of all the tested flavonoids. There was 10% recovery of sperm motility at the kaempferol concentration of 25 µM. On the other hand, effects of quercetin and catechin on the sperm mobility completely differed from the other tested flavonoids rutin, naringin and kaempferol. The antioxidants, quercetin and catechin did not protect sperm cells from heavy metal-mediated damages; rather, they showed inhibitory effects on sperm motility. When we increased the concentrations of quercetin and catechin from 0 to 1000 µM, there was a gradual decrease in sperm motility compared to the untreated control group. Mean motility of AlCl3 -exposed sperm after a 2 hours incubation period in the presence of 1000 µM quercetin was 22% and for catechin, it was 28%.
Fig.3.
Effects of rutin, naringin, kaempferol, quercetin, and catechin on aluminum chloride (AlCl3)-exposed sperm. Sperm samples were treated for 2 hours at 37˚C with AlCl3 (1.0 mM) in the presence of various concentrations (25, 50, 100, 200, 500, and 1000 μM) of rutin, naringin, kaempferol, quercetin, and catechin. Sperm mobility was assessed by MMC Sperm. *; P<0.05 and **; P<0.01 compared to the flavonoid untreated control.
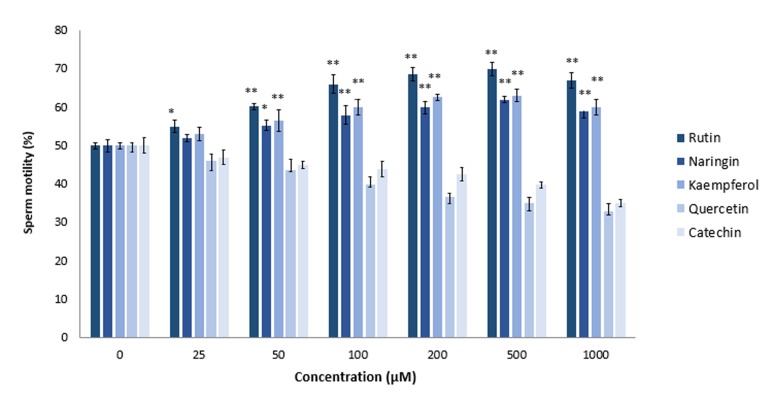
Effects of flavonoids on motility of cadmium chloride-exposed sperm
Previous studies by El-Demerdash et al. (3) in male rats showed beneficial effects of vitamin E and β-carotene in reducing the toxic effects of CdCl2 on the male reproductive system. In the current study, we observed that treatment with rutin, naringin and kaempferol resulted in recovery of motility in CdCl2 exposed sperm cells. Our results showed that rutin, naringin, and kaempferol at 25-500 μM significantly increased (P≤0.05) motility of CdCl2 -exposed sperm cells in a dose-dependent manner (Fig .4). In contrast, quercetin and catechin did not induce any protective effect against CdCl2 toxicity; they reduced the motility of CdCl2 -exposed sperm compared to the untreated control samples (Fig .4). These results disagreed with an in vivo study by Farombi et al. (38) about the antioxidative nature of quercetin. They showed that administration of the biflavonoid, kolaviron, or quercetin prevented Cd-mediated decreased sperm motility in adult male rats. Other researchers reported the positive effects of quercetin on sperm capacity under both in vitro and in vivo conditions (39). Supplementation of quercetin restored the decrease in glutathione (GSH) level, and superoxide dismutase (SOD) and GSH peroxidase activities in Cd-exposed mice. This discrepancy between in vitro and in vivo results might be attributed to the difference in quercetin exposure time or to in situ metabolic alteration of quercetin (40).
Fig.4.
Effects of rutin, naringin, kaempferol, quercetin, and catechin on cadmium chloride (CdCl2 )-exposed sperm. Sperm samples were treated for 2 hours at 37˚C with CdCl2 (500 μM) in the presence of various concentrations (25, 50, 100, 200, 500, and 1000 μM) of rutin, naringin, kaempferol, quercetin, and catechin. Sperm mobility was assessed by MMC Sperm. *; P<0.05 and **; P<0.01 compared to the flavonoid untreated control.
Effects of flavonoids on motility of lead chlorideexposed sperm
Toxic effects of PbCl4 on sperm quality, motility, DNA fragmentation, and acrosome reaction have been investigated extensively in mice and humans (36,41,44). According to our results (Fig .2), PbCl4 compared to AlCl3 and CdCl2 had more adverse effects on sperm motility at the 0.125 to 5.0 mM concentrations. We used the 250 µM concentration of PbCl4 for additional experiments with flavonoids. Quercetin and catechin decreased motility of PbCl4 -exposed sperm cells in a dose-dependent manner. However, as seen in Figure 5, the 500 µM concentration of rutin, naringin, and kaempferol significantly increased sperm motility to 65% (rutin), 60% (naringin) and 63% (kaempferol). Rutin was more efficient in fortifying sperm cells against PbCl4-induced harmful attacks.
Fig.5.
Effects of rutin, naringin, kaempferol, quercetin, and catechin on lead chloride (PbCl4)-exposed sperm. Sperm samples were treated for 2 hours at 37˚C with PbCl4 (250 μM) in the presence of various concentrations (25, 50, 100, 200, 500, and 1000 μM) of rutin, naringin, kaempferol, quercetin, and catechin. Sperm mobility was assessed by MMC Sperm. *; P<0.05 and **; P<0.01 compared to flavonoid untreated control.
Sperm lipid peroxidation in the presence of aluminum chloride, cadmium chloride and lead
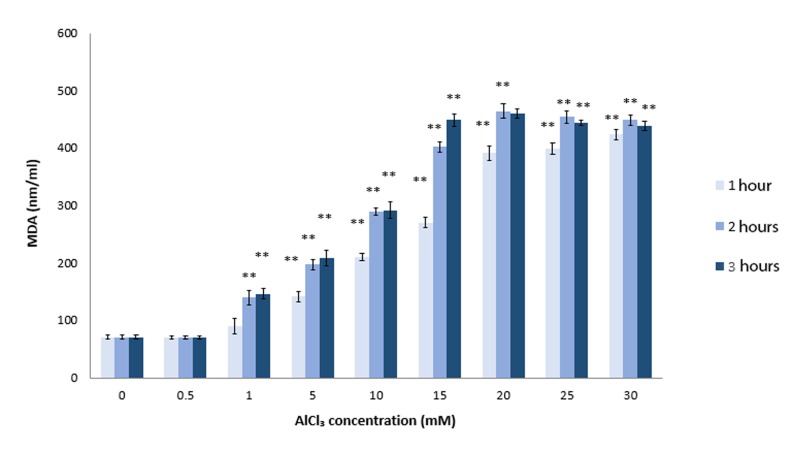
chloride Sperm membranes are rich in polyunsaturated fatty acids (PUFAs) (45). Previous in vivo studies have demonstrated that Al could increase peroxidation of PUFAs in sperm samples (31,46). The presence of a high level of PUFA in the sperm plasma membrane is required for membrane fusion events associated with fertilization. Loss of fluidity as a result of lipid peroxidation can diminish the rates of sperm-oocyte fusion (47). Our in vitro studies have shown that AlCl3 at concentrations higher than 0.5 mM significantly induced MDA production after 1 hour of incubation (P≤0.0008, Fig .6). MDA is an end-product of enzymatic and oxygen radical-initiated oxidative decomposition of PUFAs and most frequently used as an indicator of lipid peroxidation. We have shown that the effect of AlCl3 on sperm lipid peroxidation was doseand time-dependent (Fig .6). There were no significant changes in sperm MDA formation observed following incubation with 0.5-30 mM of CdCl2 or PbCl4 (data not shown). Therefore, we only investigated the effects of flavonoids on MDA formation in AlCl3 -exposed sperm cells.
Fig.6.
Sperm lipid peroxidation in the presence of aluminum chloride (AlCl3). Sperm samples were treated with AlCl3 (20 mM) for 2 hours at 37˚C. After incubation, we assessed the amount of lipid peroxidation of the sperm cells with MDA. **; P<0.01 compared to the untreated control group and MDA; Malondialdehyde.
Effects of flavonoids on lipid peroxidation of aluminum chloride-exposed sperm
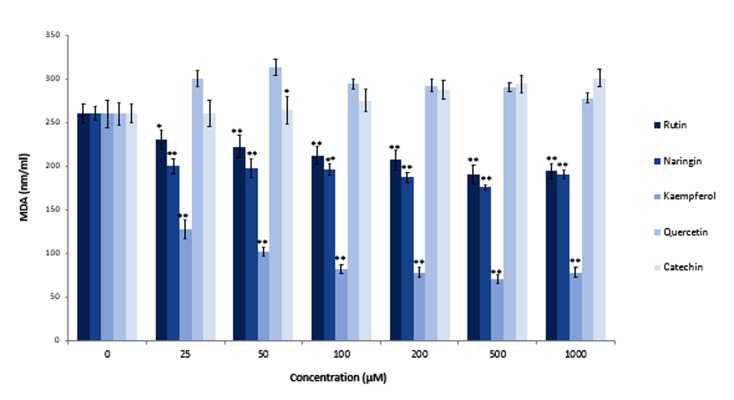
Researchers previously reported the protective effect of ascorbic acid as an antioxidant against induction of lipid peroxidation by AlCl3 in sperm cells (46). However, to the best of our knowledge there was no report about the protective effect of flavonoids against lipid peroxidation in Alexposed sperm cells. Moretti et al. showed that quercetin, rutin and, to a lesser extent, naringenin, significantly decreased tert-butyl hydroperoxide induced lipid peroxidation in human sperm (48). Their studies indicated that epicatechin was not efficacious as an antioxidant to protect sperm cells against oxidants. Our investigations showed that kaempferol was the most effective amongst the tested products in protection of sperm cells against AlCl3 -induced lipid peroxidation (Fig .7). Kaempferol, at a concentration of 100 µM, reduced MDA production from 250 nmol/ml (in untreated cells) to approximately 80 nmol/ml. Naringin and rutin were less effective in protection of AlCl3 -exposed sperm cells against lipid peroxidation compared to kaempferol. We observed that quercetin and catechin did not protect sperm. Quercetin, as an antioxidant, did not protect sperm cells against lipid peroxidation; rather, it had inhibitory effects on sperm motility. Khanduja et al. (49) have reported a significant decrease in sperm Ca 2+-ATPase activity following quercetin treatment. Ca 2+-ATPase is the responsible enzyme that provides energy for progressive movement of sperm cells. Inhibition of Ca 2+-ATPase activity has been shown to result in Ca 2+accumulation in the cells and blockage of the sperm motility apparatus (50).
Fig.7.
Effects of rutin, naringin, kaempferol, quercetin, and catechin on lipid peroxidation of aluminum chloride (AlCl3)-exposed sperm. Sperm samples were treated with AlCl3 (20 mM) and simultaneously incubated with different concentrations of rutin, naringin, kaempferol, quercetin, and catechin for 2 hours at 37˚C. After incubation, we assessed the lipid peroxidation of sperm cells with MDA. *; P<0.05, **; P<0.01 compared to the flavonoid untreated control group and MDA; Malondialdehyde.
Discussion
The impact of heavy metal toxicity, even at low concentrations, on the male reproductive system has been extensively investigated and confirmed (51,54). Sperm motility depends on the synchronized actions of proteins, sugars, ions, and small organic molecules. It is one of the main factors that facilitates the journey of sperm toward the egg and the subsequent fertilization process (55). Defects in sperm motility are a common reason for infertility in humans (56). In the current study we have shown that AlCl3 , CdCl2 and PbCl4 significantly affected sperm motility. PbCl4 had the most toxic effect.
Infertility due to metal toxicity usually occurs as a result of ROS induction (57). Therefore, antioxidant therapy is a promising strategy for treatment of individuals with heavy metal poisoning (58). Among natural antioxidants, flavonoids are more likely to exert protective activities against metal toxicity compared to carotenoids and vitamin E (37,59). Based on our results, three flavonoids, rutin, naringin, and kaempferol have been shown to restore motility of AlCl3 -, CdCl2 -, and PbCl4 exposed sperm cells. The other two flavonoids, catechin and quercetin, had no positive effects on motility of metal-exposed sperm; rather, they decreased sperm motility compared to untreated control samples.
We conducted additional research on the protective effects of flavonoids as antioxidant agents against heavy metal-induced lipid peroxidation. MDA formation was assessed in AlCl3 -exposed sperm cells treated with the five above mentioned flavonoids. Among flavonoids, quercetin due to its free radical scavenging and metal chelating abilities has been extensively investigated (60). However, according to the obtained results, quercetin and catechin did not protect sperm cells from ROS-mediated damages. They adversely affected sperm motility. Inhibition of sperm motility without considerable effects on peroxidation of PUFAs would indicate involvement of other inhibitory mechanisms. In contrast, increased motility of Alexposed sperm cells treated with rutin, naringin and kaempferol was accompanied by decreased levels of MDA formation. We have concluded that antioxidant or chelating properties were not sufficient to protect sperm cells against the harmful damages of heavy metals. Flavonoids, as naturally occurring compounds may have some inhibitory effects on enzyme activities (49) or exert their growth inhibitory activities through binding to human receptors (61). Therefore, it is essential to know the exact mechanisms of metal-induced toxicity and the properties of flavonoids before prescribing medications to combat the adverse effects of heavy metals on infertility.
Acknowledgments
This work was financially supported by a grant from the Cellular and Molecular Research Center of Ahvaz Jundishapur University of Medical Sciences (Ahvaz, Iran), project number CMRC-003. All authors have no conflicts of interest for any portion of this manuscript.
References
- 1.Nordberg GF, Fowler BA, Nordberg M, Friberg L. Handbook on the toxicology of metals. 3rd ed. Oxford: Academic; 2007. pp. 117–145. [Google Scholar]
- 2.Cadmium in the human environment: toxicity and carcinogenicity. Symposium proceedings; Gargnano, Italy. September 1991; 1992. pp. 1–464. [PubMed] [Google Scholar]
- 3.El-Demerdash FM, Yousef MI, Kedwany FS, Baghdadi HH. Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: protective role of vitamin E and betacarotene. Food Chem Toxicol. 2004;42(10):1563–1571. doi: 10.1016/j.fct.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13(10):572–584. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 5.Plaza M, Pozzo T, Liu J, Gulshan Ara KZ, Turner C, Nordberg Karlsson E. Substituent effects on in vitro antioxidizing properties, stability and solubility in flavonoids. J Agric Food Chem. 2014;62(15):3321–3333. doi: 10.1021/jf405570u. [DOI] [PubMed] [Google Scholar]
- 6.Rice-Evans C. Flavonoid antioxidants. Curr Med Chem. 2001;8(7):797–807. doi: 10.2174/0929867013373011. [DOI] [PubMed] [Google Scholar]
- 7.Flora SJ. Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxid Med Cell Longev. 2009;2(4):191–206. doi: 10.4161/oxim.2.4.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazur A, Bayle D, Lab C, Rock E, Rayssiguier Y. Inhibitory effect of procyanidin-rich extracts on LDL oxidation in vitro. Atherosclerosis. 1999;145(2):421–422. doi: 10.1016/s0021-9150(99)00115-x. [DOI] [PubMed] [Google Scholar]
- 9.Kondo K, Hirano R, Matsumoto A, Igarashi O, Itakura H. Inhibition of LDL oxidation by cocoa. Lancet. 1996;348(9040):1514–1514. doi: 10.1016/s0140-6736(05)65927-2. [DOI] [PubMed] [Google Scholar]
- 10.Korkina LG, Afanas'ev IB. Antioxidant and chelating properties of flavonoids. Adv Pharmacol. 1997;38:151–163. doi: 10.1016/s1054-3589(08)60983-7. [DOI] [PubMed] [Google Scholar]
- 11.Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55(6):481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 12.Hammerstone JF, Lazarus SA, Schmitz HH. Procyanidin content and variation in some commonly consumed foods. J Nutr. 2000;130(8S Suppl):2086S–2092S. doi: 10.1093/jn/130.8.2086S. [DOI] [PubMed] [Google Scholar]
- 13.Carando S, Teissedre PL, Pascual-Martinez L, Cabanis JC. Levels of flavan-3-ols in French wines. J Agric Food Chem. 1999;47(10):4161–4166. doi: 10.1021/jf9810564. [DOI] [PubMed] [Google Scholar]
- 14.Prior RL, Cao G. Antioxidant capacity and polyphenolic components of teas: implications for altering in vivo antioxidant status. Proc Soc Exp Biol Med. 1999;220(4):255–261. doi: 10.1046/j.1525-1373.1999.d01-44.x. [DOI] [PubMed] [Google Scholar]
- 15.Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. ScientificWorldJournal. 2013;2013:162750–162750. doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mora A, Payá M, Ríos JL, Alcaraz MJ. Structure-activity relationships of polymethoxyflavones and other flavonoids as inhibitors of non-enzymic lipid peroxidation. Biochem Pharmacol. 1990;40(4):793–797. doi: 10.1016/0006-2952(90)90317-e. [DOI] [PubMed] [Google Scholar]
- 17.Takabe W, Niki E, Uchida K, Yamada S, Satoh K, Noguchi N. Oxidative stress promotes the development of transformation: involvement of a potent mutagenic lipid peroxidation product, acrolein. Carcinogenesis. 2001;22(6):935–941. doi: 10.1093/carcin/22.6.935. [DOI] [PubMed] [Google Scholar]
- 18.Sastre J, Pallardó FV, Viña J. Mitochondrial oxidative stress plays a key role in aging and apoptosis. IUBMB Life. 2000;49(5):427–435. doi: 10.1080/152165400410281. [DOI] [PubMed] [Google Scholar]
- 19.Kawanishi S, Hiraku Y, Oikawa S. Mechanism of guaninespecific DNA damage by oxidative stress and its role in carcinogenesis and aging. Mutat Res. 2001;488(1):65–76. doi: 10.1016/s1383-5742(00)00059-4. [DOI] [PubMed] [Google Scholar]
- 20.Sheweita SA, Tilmisany AM, Al-Sawaf H. Mechanisms of male infertility: role of antioxidants. Curr Drug Metab. 2005;6(5):495–501. doi: 10.2174/138920005774330594. [DOI] [PubMed] [Google Scholar]
- 21.Thomas SR, Davies MJ, Stocker R. Oxidation and antioxidation of human low-density lipoprotein and plasma exposed to 3-morpholinosydnonimine and reagent peroxynitrite. Chem Res Toxicol. 1998;11(5):484–494. doi: 10.1021/tx970173a. [DOI] [PubMed] [Google Scholar]
- 22.Moore KP, Darley-Usmar V, Morrow J, Roberts LJ 2nd. Formation of F2-isoprostanes during oxidation of human low-density lipoprotein and plasma by peroxynitrite. Circ Res. 1995;77(2):335–341. doi: 10.1161/01.res.77.2.335. [DOI] [PubMed] [Google Scholar]
- 23.Wang W, Sawicki G, Schulz R. Peroxynitrite-induced myocardial injury is mediated through matrix metalloproteinase-2. Cardiovasc Res. 2002;53(1):165–174. doi: 10.1016/s0008-6363(01)00445-x. [DOI] [PubMed] [Google Scholar]
- 24.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 25.Farrell PB, Foote RH, Simkin ME, Clegg ED, Wall RJ. Relationship of semen quality, number of sperm inseminated, and fertility in rabbits. J Androl. 1993;14(6):464–471. [PubMed] [Google Scholar]
- 26.World Health Organization. [Laboratory manual of the WHO for the examination of human semen and spermcervical mucus interaction] Ann Ist Super Sanita. 2001;37(1):I-XII, 1-123. [PubMed] [Google Scholar]
- 27.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 28.Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1990;9(6):515–540. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- 29.Dawson EB, Ritter S, Harris WA, Evans DR, Powell LC. Comparison of sperm viability with seminal plasma metal levels. Biol Trace Elem Res. 1998;64(1-3):215–219. doi: 10.1007/BF02783337. [DOI] [PubMed] [Google Scholar]
- 30.Yousef MI, Salama AF. Propolis protection from reproductive toxicity caused by aluminium chloride in male rats. Food Chem Toxicol. 2009;47(6):1168–1175. doi: 10.1016/j.fct.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Yousef MI, El-Morsy AM, Hassan MS. Aluminium-induced deterioration in reproductive performance and seminal plasma biochemistry of male rabbits: protective role of ascorbic acid. Toxicology. 2005;215(1-2):97–107. doi: 10.1016/j.tox.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 32.Waalkes MP, Anver M, Diwan BA. Carcinogenic effects of cadmium in the noble (NBL/Cr) rat: induction of pituitary, testicular, and injection site tumors and intraepithelial proliferative lesions of the dorsolateral prostate. Toxicol Sci. 1999;52(2):154–161. doi: 10.1093/toxsci/52.2.154. [DOI] [PubMed] [Google Scholar]
- 33.Waalkes MP, Anver MR, Diwan BA. Chronic toxic and carcinogenic effects of oral cadmium in the Noble (NBL/ Cr) rat: induction of neoplastic and proliferative lesions of the adrenal, kidney, prostate, and testes. J Toxicol Environ Health A. 1999;58(4):199–214. doi: 10.1080/009841099157296. [DOI] [PubMed] [Google Scholar]
- 34.Oliveira H, Spanò M, Santos C, Pereira Mde L. Adverse effects of cadmium exposure on mouse sperm. Reprod Toxicol. 2009;28(4):550–555. doi: 10.1016/j.reprotox.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Monsefi M, Alaee S, Moradshahi A, Rohani L. Cadmiuminduced infertility in male mice. Environ Toxicol. 2010;25(1):94–102. doi: 10.1002/tox.20468. [DOI] [PubMed] [Google Scholar]
- 36.Gomes M, Gonçalves A, Rocha E, Sá R, Alves A, Silva J, et al. Effect of in vitro exposure to lead chloride on semen quality and sperm DNA fragmentation. Zygote. 2015;23(3):384–393. doi: 10.1017/S0967199413000671. [DOI] [PubMed] [Google Scholar]
- 37.Yousef MI, Kamel KI, El-Guendi MI, El-Demerdash FM. An in vitro study on reproductive toxicity of aluminium chloride on rabbit sperm: the protective role of some antioxidants. Toxicology. 2007;239(3):213–223. doi: 10.1016/j.tox.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 38.Farombi EO, Adedara IA, Akinrinde SA, Ojo OO, Eboh AS. Protective effects of kolaviron and quercetin on cadmiuminduced testicular damage and endocrine pathology in rats. Andrologia. 2012;44(4):273–284. doi: 10.1111/j.1439-0272.2012.01279.x. [DOI] [PubMed] [Google Scholar]
- 39.Gibb Z, Butler TJ, Morris LH, Maxwell WM, Grupen CG. Quercetin improves the postthaw characteristics of cryopreserved sex-sorted and nonsorted stallion sperm. Theriogenology. 2013;79(6):1001–1009. doi: 10.1016/j.theriogenology.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 40.Metodiewa D, Jaiswal AK, Cenas N, Dickancaite E, Segura-Aguilar J. Quercetin may act as a cytotoxic prooxidant after its metabolic activation to semiquinone and quinoidal product. Free Radic Biol Med. 1999;26(1-2):107–116. doi: 10.1016/s0891-5849(98)00167-1. [DOI] [PubMed] [Google Scholar]
- 41.Zribi N, Chakroun NF, Elleuch H, Abdallah FB, Ben Hamida AS, Gargouri J, et al. Sperm DNA fragmentation and oxidation are independent of malondialdheyde. Reprod Biol Endocrinol. 2011;9:47–47. doi: 10.1186/1477-7827-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rafique M, Khan N, Perveen K, Naqvi A. The effects of lead and zinc on the quality of semen of albino rats. J Coll Physicians Surg Pak. 2009;19(8):510–513. [PubMed] [Google Scholar]
- 43.Graça A, Ramalho-Santos J, de Lourdes Pereira M. Effect of lead chloride on spermatogenesis and sperm parameters in mice. Asian J Androl. 2004;6(3):237–241. [PubMed] [Google Scholar]
- 44.Mushina EV. Study of the combined effects of lead and cadmium on experimental animals. Gig Sanit. 1989;(9):89–90. [PubMed] [Google Scholar]
- 45.Sikka SC. Relative impact of oxidative stress on male reproductive function. Curr Med Chem. 2001;8(7):851–862. doi: 10.2174/0929867013373039. [DOI] [PubMed] [Google Scholar]
- 46.Ige SF, Akhigbe RE. The role of Allium cepa on aluminuminduced reproductive dysfunction in experimental male rat models. J Hum Reprod Sci. 2012;5(2):200–205. doi: 10.4103/0974-1208.101022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aitken RJ. Free radicals, lipid peroxidation and sperm function. Reprod Fertil Dev. 1995;7(4):659–668. doi: 10.1071/rd9950659. [DOI] [PubMed] [Google Scholar]
- 48.Moretti E, Mazzi L, Terzuoli G, Bonechi C, Iacoponi F, Martini S, et al. Effect of quercetin, rutin, naringenin and epicatechin on lipid peroxidation induced in human sperm. Reprod Toxicol. 2012;34(4):651–657. doi: 10.1016/j.reprotox.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Khanduja KL, Verma A, Bhardwaj A. Impairment of human sperm motility and viability by quercetin is independent of lipid peroxidation. Andrologia. 2001;33(5):277–281. doi: 10.1046/j.1439-0272.2001.00432.x. [DOI] [PubMed] [Google Scholar]
- 50.Breitbart H, Rubinstein S, Nass-Arden L. The role of calcium and Ca2+-ATPase in maintaining motility in ram spermatozoa. J Biol Chem. 1985;260(21):11548–11553. [PubMed] [Google Scholar]
- 51.Iavicoli I, Fontana L, Bergamaschi A. The effects of metals as endocrine disruptors. J Toxicol Environ Health B Crit Rev. 2009;12(3):206–223. doi: 10.1080/10937400902902062. [DOI] [PubMed] [Google Scholar]
- 52.Pizent A, Tariba B, Živković T. Reproductive toxicity of metals in men. Arh Hig Rada Toksikol. 2012;63(Suppl 1):35–46. doi: 10.2478/10004-1254-63-2012-2151. [DOI] [PubMed] [Google Scholar]
- 53.Ghaffari MA, Motlagh B. In vitro effect of lead, silver, tin, mercury, indium and bismuth on human sperm creatine kinase activity: a presumable mechanism for men infertility. Iran Biomed J. 2011;15(1-2):38–43. [PMC free article] [PubMed] [Google Scholar]
- 54.Järup L. Hazards of heavy metal contamination. Br Med Bull. 2003;68:167–182. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- 55.Yoshida M, Kawano N, Yoshida K. Control of sperm motility and fertility: diverse factors and common mechanisms. Cell Mol Life Sci. 2008;65(21):3446–3457. doi: 10.1007/s00018-008-8230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McLaren JF. Infertility evaluation. Obstet Gynecol Clin North Am. 2012;39(4):453–463. doi: 10.1016/j.ogc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 57.Lavranos G, Balla M, Tzortzopoulou A, Syriou V, Angelopoulou R. Investigating ROS sources in male infertility: a common end for numerous pathways. Reprod Toxicol. 2012;34(3):298–307. doi: 10.1016/j.reprotox.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 58.Niederberger C. Re: the role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. J Urol. 2012;187(4):1377–1377. doi: 10.1016/j.juro.2011.12.130. [DOI] [PubMed] [Google Scholar]
- 59.Mansuri ML, Parihar P, Solanki I, Parihar MS. Flavonoids in modulation of cell survival signalling pathways. Genes Nutr. 2014;9(3):400–400. doi: 10.1007/s12263-014-0400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu JP, Calomme M, Lasure A, De Bruyne T, Pieters L, Vlietinck A, et al. Structure-activity relationship of flavonoids with superoxide scavenging activity. Biol Trace Elem Res. 1995;47(1-3):327–331. doi: 10.1007/BF02790134. [DOI] [PubMed] [Google Scholar]
- 61.Garrett SD, Lee HA, Morgan MR. A nonisotopic estrogen receptor-based assay to detect estrogenic compounds. Nat Biotechnol. 1999;17(12):1219–1222. doi: 10.1038/70773. [DOI] [PubMed] [Google Scholar]