Abstract
CoA (coenzyme A) is an essential cofactor that is involved in many metabolic processes. CoA is derived from pantothenate in five biosynthetic reactions. The CoA biosynthetic pathway is regulated by PanKs (pantothenate kinases) and four active isoforms are expressed in mammals. The critical physiological functions of the PanKs are revealed by systematic deletion of the Pank genes in mice.
Keywords: coenzyme A (CoA), mitochondriom, pantothenate kinase
Coenzyme A
CoA (coenzyme A) is an essential cofactor in many metabolic reactions, including the synthesis and oxidation of fatty acids, complex lipid synthesis and, notably, the oxidation of pyruvate in the citric acid cycle. CoA-substrates participate in over 100 biochemical reactions and CoA also donates the phosphopantetheine prosthetic group that activates fatty acid synthase and carrier proteins. The CoA pool is largely made up of free, unacylated CoA and acetyl-CoA is the largest component of the acyl-CoA pool. The acetyl-CoA thioester is an allosteric regulator in intermediary metabolism: it inhibits the PDH (pyruvate dehydrogenase) reaction and stimulates pyruvate carboxylase. Acetyl-CoA is strategically positioned at the crossroads of energy metabolism and it is required for the metabolism of fatty acids, carbohydrates, amino acids and ketone bodies.
CoA is derived from pantothenate, which is a required vitamin (B5) in mammals. Pantothenate can be obtained from the diet and from intestinal bacteria. CoA synthesis occurs in a series of five reactions, the first of which is catalysed by PanK (pantothenate kinase). Most prokaryotes and lower metazoans have one PanK, but there are four active PanK proteins in mammals, encoded by three distinct genes. The activities of the PanK proteins are regulated by feedback inhibition by CoA or CoA thioesters, with the exception of type II and III PanKs in bacteria. Acetyl-CoA is a potent inhibitor of the mammalian PanK isoforms and binds at an allosteric site that spans the dimer interface of the PanK proteins. Acetyl-CoA binding keeps the substrate site in an open conformation, thereby disallowing completion of the kinase reaction.
The mammalian PanKs
Humans and mice express four PanK isoforms: PanK1α, PanK1β, PanK2 and PanK3. The isoforms PanK1α and PanK1β arise from alternate initiation exons in the Pank1 gene. PanK1β is the least sensitive to feedback inhibition by acetyl-CoA, with an IC50 of about 5 μM. By contrast, PanK2 has an IC50 of ~0.1 μM and PanK3 has an IC50 of 1 μM for acetyl-CoA. The estimated intracellular concentrations of CoA range from 100 to 400 μM in cytosol and 1 to 4 mM in the mitochondria. PanK2 and PanK3, in particular, would not be active under physiological conditions associated with high acetyl-CoA levels, raising a question about the circumstances under which these PanKs would be functional. The localization of the human PanK2 protein in the intermembrane space of the mitochondria [1] is consistent with the hypothesis that acylcarnitine is a variable metabolite that counteracts inhibition by acetyl-CoA. Long-chain acylcarnitine is formed in the intermembrane mitochondrial space as it intercepts and transfers long-chain acyl groups from CoA in the cytosol to CoA in the interior of mitochondria. Long-chain acylcarnitines and acylethanolamides are activators of PanK2 [2] and PanK3 [3] proteins, thereby enabling regulation by the rise and fall of these metabolites.
The four human and mouse PanK proteins share a homologous C-terminal catalytic domain, but differ in their N-termini [1]. These unique termini direct the isoforms to different subcellular compartments. PanK1α is exclusively nuclear, with preferential association with the granular component of the nucleolus during interphase. PanK1α is also associated with the perichromosomal region in condensing chromosomes during mitosis. The PanK1β and PanK3 isoforms are cytosolic, with a portion of PanK1β associated with clathrin-associated vesicles and recycling endosomes. Human PanK2, known to associate with mitochondria, is specifically localized to the intermembrane space. Human PanK2 is also detected in the nucleus, and has functional nuclear localization and export signals. Nuclear PanK2 traffics from the nucleus to the mitochondria, but not in the other direction, and is absent from the nucleus during the G2 phase of the cell cycle. Localization of human PanK2 in these two compartments is in sharp contrast with mouse PanK2, which is exclusively cytosolic. These data demonstrate that PanK isoforms are differentially compartmentalized allowing them to sense CoA homoeostasis in different cellular compartments and enable interaction with regulatory ligands produced in these same locations.
CoA levels and mammalian tissue functions
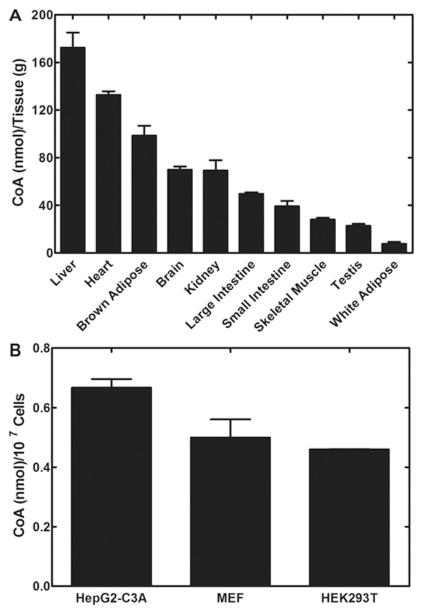
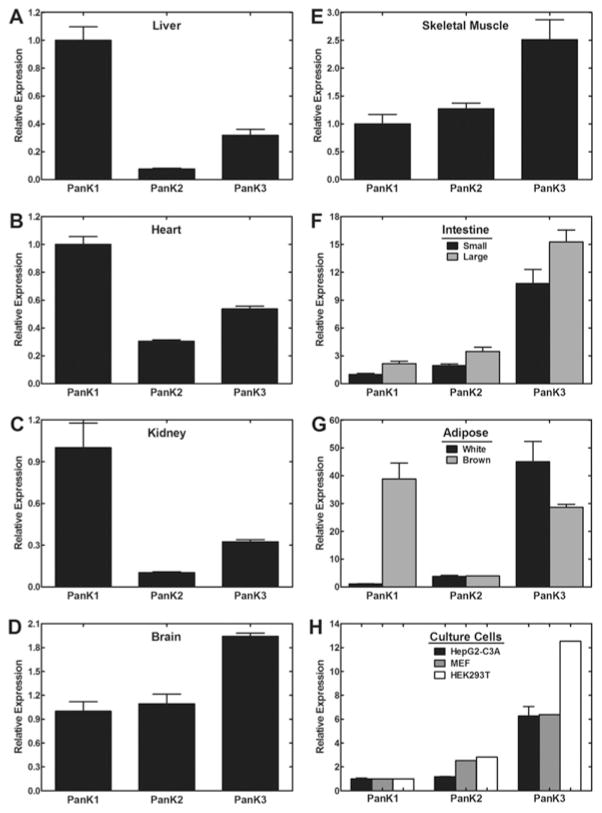
Tissue CoA levels span more than a 10-fold range (Figure 1) and this determination in our laboratory is in general agreement with previously published data [4,5]. Our current methodology for CoA measurement involves tissue extraction and derivatization of CoA [6,7], followed by RP-HPLC (reverse-phase HPLC) with detection at λ393 nm using gradient acetonitrile elution to identify and quantify the CoA. Liver, heart and brown adipose have the highest CoA levels, and these tissues also express high amounts of Pank1 compared with Pank2 and Pank3, as determined by quantitative real-time reverse transcription–PCR (Figure 2). Although kidney and brain have similar CoA levels, kidney expresses a greater amount of Pank1 relative to the other isoforms. These data suggest a correlation between Pank1 expression and CoA function, namely CoA-dependent fatty acid oxidation that, in turn, drives gluconeogenesis as revealed by the Pank1-knockout mouse [8]. Fatty acid oxidation requires an excess of free CoA molecules which act as acceptors for the multiple acetate moieties that are released by the oxidation process. Fatty acid oxidation not only produces acetyl-CoA, but also produces NADH which is a necessary cofactor for gluconeogenesis. Kidney contributes to fasting gluconeogenesis, together with liver, and CoA levels increase in both liver and kidney during fasting. On the other hand, brain maintains a more constant level of CoA, does not readily respond to changes in nutritional status and utilizes the glucose and ketones produced by liver and kidney and other metabolic sources. Glycolysis is not dependent on CoA; however, the entry of the glycolytic end-product, pyruvate, into the citric acid cycle requires CoA. Ketone utilization requires CoA and ketones are a second fuel source for brain cells. The higher expression of Pank2 and Pank3 in brain results in more stringent regulation of CoA synthesis that, in turn, is reflected by more stable CoA levels that support acetyl-CoA formation and ketone utilization.
Figure 1. Mouse tissue steady-state CoA levels.
(A) Wild-type mice (male, three or four per group), 8–12 weeks of age, were fed ad libitum on chow containing 6 % fat. Tissues were removed, snap frozen and extracted for CoA measurement. Data were normalized to tissue wet weight. Animal handling procedures were approved under protocol 323 by the St. Jude Institutional Animal Care and Use Committee. (B) Duplicate adherent cell cultures were harvested at late logarithmic growth and prepared for CoA measurements. Data were normalized to viable cell numbers for each culture.
Figure 2. Mouse Pank gene expression profiles.
Wild-type mice (male, three to four per group), 8–12 weeks of age, were fed ad libitum. Tissues were removed and stored frozen in RNAlater (Ambion). RNA was isolated and prepared for quantitative real-time reverse transcription–PCR. Data were normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) expression and the value for Pank1 transcripts was set at 1.0. Animal handling procedures were approved under protocol 323 by the St. Jude Institutional Animal Care and Use Committee.
Cultured mammalian cells do not maintain high CoA levels and Pank3 is the dominant isoform, as illustrated for HepG2-C3A cells, HEK-293T (human embryonic kidney 293T) cells and MEFs (mouse embryo fibroblasts) (Figure 2). Cultured cells are selected for growth in high glucose medium and rely on glycolysis as a fuel source. It is difficult to demonstrate a reliance on pantothenate for cells cultured in defined media without added pantothenate in the presence of exhaustively dialysed serum. Several passages in pantothenate-deficient media for more than 1 week are required before proliferation is arrested. Finally, the cells cease to divide but retain their viability (S. Reeves and S. Jackowski, unpublished work). Thus investigation of individual PanK functions or CoA-dependent functions, particularly in mitochondria, is compromised by limited CoA/acetyl-CoA availability in cultured cells.
Consequences of reduced CoA
The PanK isoforms in mice have redundant enzymatic activities and reduction of the expression or activity of one isoform can be compensated by the alternate PanKs to some extent, providing a back-up system that ensures maintenance of CoA levels and tissue function under normal conditions. Deletion or inactivation of one of the Pank genes results in a mild phenotype and extreme challenge is often necessary to reveal a metabolic impairment in the global Pank-knockout mice. Fasting for at least 24 h is required to expose impairment of gluconeogenesis in the Pank1-knockout mouse [8]. Hepatic steatosis and accumulation of TAG (triacylglycerol) droplets occurs during fasting as a result of reduced oxidation of fatty acids which are redirected to the TAG storage pool. The Pank2-knockout mouse displays azoospermia that renders the male gender infertile and a ketogenic diet is required to expose locomotor impairment in this mouse model [9]. The Pank1/Pank2 double-knockout mouse cannot survive the ketogenic diet provided by maternal milk [10]. The Pank1/Pank2 double knockouts die as a result of hypoglycaemia and impaired ketone utilization by age 2 weeks postnatal. The Pank1/Pank2 double knockouts also have reduced NADH levels which is reflected by a liver deficiency of oleic acid. Oleic acid is an unsaturated 18-carbon fatty acid with one double bond that arises from stearic acid, a saturated 18-carbon fatty acid without any double bonds. The desaturation reaction requires NADH which is significantly reduced in the livers of Pank1/Pank2 double knockouts.
Results from double knockouts indicate that expression of at least two PanK isoforms is required for mammalian survival. The Pank1/Pank2 double knockouts die at 2 weeks, whereas the Pank1/Pank3 and Pank2/Pank3 double-knockout mice are embryonic lethal [10]. Characterization of the Pank3 single-knockout mouse phenotype is in progress. There is evidence suggesting that expression of genes other than the Pank isoforms can influence the metabolic phenotypes of the knockout mice. When the Pank1/Pank2 double knockouts are backcrossed on to a pure 129SvJ genetic background, the mice do not survive embryogenesis. However, backcrossing on to a pure C57Bl/6 background yields pups which survive to postnatal age 2 weeks, similar to the knockout mice derived on a mixed genetic background that contains genes from both 129SvJ and C57Bl/6 strains [10].
Summary
The activities of the PanK enzymes are governed by multiple mechanisms. Gene expression profiling reveals that tissues and organs that oxidize fatty acids to a greater extent often have higher relative amounts of PanK1. CoA levels are dynamic and although mammals can survive the absence of one isoform, there is a minimum CoA threshold that is necessary to enable responses to changes in nutritional status. The dynamic aspects of CoA synthesis are governed by the PanK isoform activities which respond differentially to intracellular metabolite levels such as acetyl-CoA, acyl-CoA, acylcarnitine and acylethanolamide. We hypothesize that the different subcellular localizations of the PanK isoforms enable these proteins to act as metabolic sensors which modulate their enzyme activities and thus modulate CoA synthesis in response to metabolites produced in situ in the different compartments. CoA plays a major role in mammalian physiology, particularly lipid utilization and energy production from lipid sources. Our current research challenge is to define the extent of PanK influence on metabolic processes in a more detailed manner. PanK isoforms are distributed variably among differentiated tissues and each PanK isoform needs to be studied in the context of all four isoforms and often under challenge to determine its contribution.
Acknowledgments
Funding
Research was supported by the National Institutes of Health [grant number GM062896], a sponsored research agreement with Retrophin, Inc. and the American Lebanese Syrian Associated Charities.
Abbreviations
- PanK
pantothenate kinase
- TAG
triacylglycerol
References
- 1.Alfonso-Pecchio A, Garcia M, Leonardi R, Jackowski S. Compartmentalization of mammalian pantothenate kinases. PLoS ONE. 2012;7:e49509. doi: 10.1371/journal.pone.0049509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leonardi R, Rock CO, Jackowski S, Zhang YM. Activation of human mitochondrial pantothenate kinase 2 by palmitoylcarnitine. Proc Natl Acad Sci USA. 2007;104:1494–1499. doi: 10.1073/pnas.0607621104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leonardi R, Zhang YM, Yun MK, Zhou R, Zeng FY, Lin W, Cui J, Chen T, Rock CO, White SW, Jackowski S. Modulation of pantothenate kinase 3 activity by small molecules that interact with the substrate/allosteric regulatory domain. Chem Biol. 2010;17:892–902. doi: 10.1016/j.chembiol.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allred JB, Guy DG. Determination of coenzyme A and acetyl CoA in tissue extracts. Anal Biochem. 1969;29:293–299. doi: 10.1016/0003-2697(69)90312-1. [DOI] [PubMed] [Google Scholar]
- 5.Shibata K, Nakai T, Fukuwatari T. Simultaneous high-performance liquid chromatography determination of coenzyme A, dephospho-coenzyme A, and acetyl-coenzyme A in normal and pantothenic acid-deficient rats. Anal Biochem. 2012;430:151–155. doi: 10.1016/j.ab.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Minkler PE, Kerner J, Ingalls ST, Hoppel CL. Novel isolation procedure for short-, medium-, and long-chain acyl-coenzyme A esters from tissue. Anal Biochem. 2008;376:275–276. doi: 10.1016/j.ab.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimada K, Mitamura K. Derivatization of thiol-containing compounds. J Chromatogr B Biomed Appl. 1994;659:227–241. doi: 10.1016/0378-4347(93)e0444-u. [DOI] [PubMed] [Google Scholar]
- 8.Leonardi R, Rehg JE, Rock CO, Jackowski S. Pantothenate kinase 1 is required to support the metabolic transition from the fed to the fasted state. PLoS ONE. 2010;5:e11107. doi: 10.1371/journal.pone.0011107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunetti D, Dusi S, Giordano C, Lamperti C, Morbin M, Fugnanesi V, Marchet S, Fagiolari G, Sibon O, Moggio M, et al. Pantethine treatment is effective in recovering the disease phenotype induced by ketogenic diet in a pantothenate kinase-associated neurodegeneration mouse model. Brain. 2014;137:57–68. doi: 10.1093/brain/awt325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia M, Leonardi R, Zhang YM, Rehg JE, Jackowski S. Germline deletion of pantothenate kinases 1 and 2 reveals the key roles for CoA in postnatal metabolism. PLoS ONE. 2012;7:e40871. doi: 10.1371/journal.pone.0040871. [DOI] [PMC free article] [PubMed] [Google Scholar]