Abstract
Background:
Despite significant efforts to understand adverse pregnancy outcome in women receiving Antiretroviral Therapy (ART), ART-related adverse birth outcomes are still poorly understood. We systematically review ART-related adverse birth outcomes among HIV-infected pregnant women; we also review the covariates associated with adverse birth outcomes in the aforementioned group.
Methods:
The main source for our systematic review was electronic bibliographic databases. Databases such as MEDLINE, PubMed, EMBASE and AIDSLINE were searched. Furthermore, search engines such as Google and Google Scholar were specifically searched for gray literature. Methodological quality of available literature was assessed using the Newcastle - Ottawa Quality Assessment Scale & M. Hewitt guideline. We examined a total of 1,124 papers and reviewed the studies using the PICOT criteria which stands for Patient (population), Intervention (or “Exposure”), Comparison, Outcome and Type of study. Finally, 32 methodologically fit studies were retained and included in our review.
Results:
Frequently observed adverse birth outcomes included low birth weight (LBW), Preterm Birth (PB), Small for Gestational Age (SGA), while still birth and congenital anomalies were infrequent. Type of regimen such as Protease Inhibitor (PI) based regimens and timing of initiation of ART are some of the factors associated with adverse pregnancy outcomes. Covariates principally included malnutrition and other co-morbidities such as malaria and HIV.
Conclusions and Public Health Implications:
There is growing evidence in published literature suggesting that ART might be causing adverse birth outcomes among pregnant women in developing countries. There is a need to consider regimen types for HIV-infected pregnant women. There is need to design large cohort studies.
Keywords: Systematic review, Adverse pregnancy outcomes, Antiretroviral Therapy, HAART, Low birth weight, Preterm delivery, Developing countries
Background
Globally, an estimated 35.3 million people were living with HIV in 2012,[1] over 90% of infections in children result from mother-to-child transmission (MTCT), and over 1,600 children are infected through MTCT each day. In parts of Southern Africa, the prevalence of HIV in pregnant women is over 30%.[2] Women in developing countries where fertility rates are between 5-7 children per woman may experience particular spousal and familial pressure to become pregnant, even if they have disclosed their HIV status.[3] The risk of MTCT of HIV is still high in developing countries where there are still deficient standards of healthcare, poor antenatal care, late diagnosis, lack of ART, and poor or haphazard interventions for Prevention of Mother to Child Transmission (PMTCT) of HIV.[4, 5, 6]
The World Health Organization (WHO) recommends triple antiretroviral therapy for all pregnant women with CD4 cells less than 350/mL or who are at clinical stage 3–4 of disease. For those with less advanced disease, two options are recommended. Option A includes short course of Zidovudine during pregnancy and extended infant nevirapine (NVP) prophylaxis. Option B includes maternal 3-drug ART during pregnancy and breastfeeding, with cessation after weaning.[7] Selected PMTCT programs in sub-Saharan Africa are implementing Option B, and ‘‘Option B+’’ which includes a lifelong ART for all pregnant, HIV-infected women, regardless of CD4 cell count or disease stage.[8] ART given to HIV infected pregnant women diminishes the rate of MTCT; this is true for mono-, bi-, or tri-therapy (HAART), with the greatest effects seen in the latter case.[9] Watts and Mofenson reported that as ART is spread out more widely for pregnant women in resource-limited settings, it will be critical to carefully monitor pregnancy outcomes to assess risks and benefits of the different regimens.[10]
Despite the fact that many individual and contextual risk factors to adverse birth outcomes have been identified, ART related causes of adverse birth outcomes are substantially unknown; there are even fewer acknowledged explanations for preterm births. Among the already identified risk factors, some still show mixed evidence, such as the association between preterm births and maternal HIV viral load. It has become very important to evaluate the effects of HAART on both pregnant mothers and their babies. The purpose of this systematic review was to compare perinatal outcomes among HIV infected pregnant women who were on, and not on ART.
Methodology
The main source for this review was electronic bibliographic databases such as MEDLINE, PubMed and EMBASE that cover most areas of health care research. AIDSLINE, the Cochrane Collaboration reports of controlled trials (CENTRAL), and Cumulative Index of Nursing and Allied Health Literature (CINAHL) were also searched. Furthermore, key Search engines, such as Google and Google Scholar, were searched specifically for grey literatures. Adverse perinatal outcomes pre-specified for this review were: spontaneous abortion, fetal anomalies, premature delivery, intrauterine growth retardation (IUGR), LBW, stillbirth, perinatal death, neonatal death, and infant death. Our review only included literature from 1993 to 2013.
Mulrow and colleagues state that systematic reviews use explicit and rigorous methods to identify, critically appraise, and synthesize studies; they seek to assemble and examine high-quality available evidence that pertains to a clinical question at hand.[11]
Study Selection Criteria
Study designs reviewed were principally observational studies (cohort, case-control and cross-sectional). Studies were included if they were quantitative and had metadata pertaining to maternal demography, pregnancy outcome and information regarding the newborn; also, studies had to include an appropriate control or comparison group. Randomized controlled trials are particularly suited to questions of effectiveness, but may be less suitable for considerations of safety or adverse effects thus were not included in this review.[12]
Search Procedure and Keywords
First DARE data base (http://www.library.ucsf.edu) was explored in an attempt to confirm availability of ongoing or already existing systematic reviews and/or meta-analyses related to our topic of interest. Studies were selected and compared following the guidelines outlined in the Hewitt and Cochrane Reviewers Handbook, 2002 edition.[13, 14] Titles of all relevant abstracts collected from electronic and hand searches were entered into EndNote-7 referencing software.
In order to prevent search bias arising from pre-specified adverse effects, we used a broad focused search design (see Table 1).
Table 1.
Keywords and MeSH terms used to retrieve papers*
| ((“pregnancy outcome”[MeSH Terms] OR (“pregnancy”[All Fields] AND “outcome”[All Fields]) OR (perinatal[All Fields] AND outcome[All Fields]) AND (antiretroviral[All Fields] AND (“therapy”[Subheading] OR “therapy”[All Fields] OR “therapeutics”[MeSH Terms] OR “therapeutics”[All Fields])))AND (“developing countries”[MeSH Terms] OR (“developing”[All Fields] AND “countries”[All Fields]) OR “developing countries”[All Fields] OR (“developing”[All Fields] AND “country”[All Fields]) OR “developing country”[All Fields]) |
Note. This review used free text terms, which may impacts on the sensitivity and specify city of the search.
Stage 1: Title and abstract screens. Following a literature search for titles of interest, descriptor terms of all downloaded resources from electronic and hand searches were scrutinized; irrelevant and duplicate papers were discarded. A set of potentially eligible studies was then selected. Subsequently all abstracts were examined for relevance. Elements considered during assessment of relevance included Population(s), Intervention(s), Comparison(s), Outcome(s) and Type of study (PICOT).
Stage 2: Data Abstraction. Data was abstracted using data abstraction format.
Stage 2: Eligibility criteria. Papers were assessed as to whether they met the eligibility criteria as stated (Table 2) by two data collectors supervised by AW.
Table 2.
Inclusion and Exclusion Criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| HIV-positive pregnant women on ART | Exposed or intervention group doesn’t include women who initiate ART |
| HIV-negative pregnant control group or HIV naive pregnant women | Ambiguous inclusion criteria |
| Outcomes are clearly stated and unambiguous | Ambiguous outcome |
| Literature from developing country | Does not specify clearly if women are on ART or not |
| Objectives are clearly formulated | Study is not comparative |
| Sound and appropriate methods are used. | Non English literatures** |
| Conclusion and recommendations are based on study findings | |
For reason stated in the limitation section of this paper
Stage 3: Quality appraisal of papers. Each paper was appraised according to a structured pre-formulated template. Methodological quality was assessed by a predefined set of criteria designed to grade each paper according to Sanderson et al.[15] The quality criteria were as follows: whether the papers included a sample size calculation; an explicit description for testing both HIV-infected and uninfected women, initiation of ART, and type of ART taken, a description of maternal disease stage, the measurement process of outcome and degree of blinding of investigators during data collection, the extent of follow up, and methods used to control for confounding.
Study design considerations
We also considered the study design. For Cohort Studies, we selected papers which matched treatment and control groups, had similar rates of recruitment, refusal, and attrition in the cases/exposed and control groups, that considered the likelihood that some eligible participants might have had the outcome of interest at the time of enrollment and took that into account in their analysis (See Table 3). For Case-control studies, we established that controls were non-cases, that the same inclusion/exclusion criteria were used for both cases and controls, and that studies were cited under cohort studies. Factors that decreased quality of evidence that was looked into were limitations in the design, indirectness of evidence, inconsistency of results, imprecision, publication bias, and study limitations while large magnitude of effect was seen as a strength (see Figure 1).
Table 3.
Methodological quality appraisal and grading of 16 studies included in the systematic review
| First author | Setting | Design | Quality level of a body of evidence | Newcastle-Ottawa Quality Assessment Scale (Total of 9 stars) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Limitations in the design & implementation | Indirectness of Evidence | Unexplained heterogeneity | Imprecision of results | High probability of publication bias | Grade | Selection (Max # ****) | Comparability (Max # **) | Outcome (Max #:***) | |||
| Allan T, 2013 | SSA | Cohort study (Nested over clinical trial) | Nested cohort | No | Yes (INH also included) |
No | No | +++ | ** Nested over clinical trial |
** | *** |
| Kebede B, 2013 | Ethiopia | Cohort (retro-spective) | Selection of controls & cases | No | Yes | No | Unpublished | ++ | ** Classification bias |
* Most confounders were not mentioned |
** |
| Dola CP. 2012 | USA | Retrospective cohort | Yes Small sample size | No | no | Yes Wide CI | No | +++ | *** Small sample size |
** most confounders were not mentioned |
*** |
| Sera Y, 2012 | Uganda | Cohort | No comparison group | Yes | No | No | Yes | ++ | ** Nested over nutritional status study. Classification bias |
** | ** All outcomes not calculated |
| Asavapiri-yanont S, 2011 | Thailand | Retro. cross-sectional | Retro. cross-sectional | No | No | Yes | No | ++ | ** Record review |
Few attempts to control con-founding | ** Wide CI |
| Patrice T, 2010 | Cameroon | Retro. cohort study | Record review | Yes | Yes | No | No | +++ | *** Lab investigation but dilation of cases & controls. |
** | ** Indirectness of Measurement |
| Haeri S, 2009 | USA | Retro. cohort | Yes | No | No | No | No | +++ | ** | ** study controlled for tobacco & alcohol |
** |
| Areechokcha D, 2009 | Thailand | Cohort | Yes | No | Yes | Yes | No | ++ | * Not community represented, inappropriate selection of controls. Only questionnaire were used |
* Few attempts to control con-founding |
** Wide CI |
| Jeniffer C, 2009 | Botswana | Retrospective | Statistical analysis wasn’t satisfactory | No | No | Yes | Unpublished | ++ | *** Definition of controls wasn’t clear |
* No mention was made |
*** |
| Townsend L, 2007 | UK and Ireland | Longitudinal | None | No | No | No | No | ++++ | ** Some women were included more than once |
** Injecting drug use, ethnic origin, maternal age at delivery * clinical status |
*** |
| Ekouevi K, 2008 | Cote d’Ivoire | Repeated cross-sectional | Yes | No | Yes | No | No | +++ | *** Cases & controls weren’t primarily identified. Presence of Lab investigation |
** | *** |
| Szyld G, 2006 | Latin America | Retrospective cohort study | Selection of control groups | No | No | Yes | No | +++ | *** Selection of only those who came for the study |
** | *** |
* Newcastle-Ottawa Assessment scale; a study can be awarded a maximum number of stars within the selection, comparability and outcome categories. A maximum of 4 stars can be awarded for selection, 2 stars for comparability, and 3stars for outcome, a total score of 9 stars. We qualified studies with scores ≥5 to be methodologically fit.
+ Grading was done according to the international GRADE group suggestion; the system classifies quality of evidence (as reflected in confidence in estimates of effects) as high (Grade A ++++), moderate (Grade B++++), or low (Grade C++) according to factors that include the risk of bias, precision of estimates, the consistency of the results, and the directness of the evidence.
Figure 1.
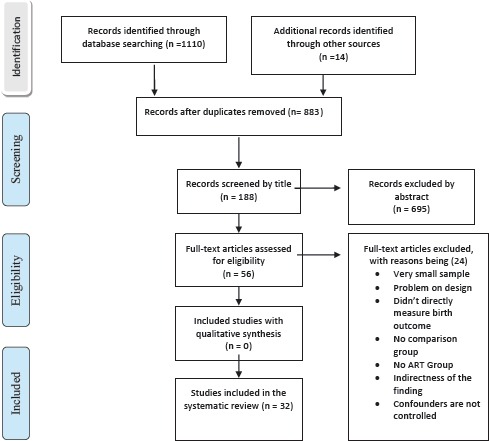
Flow Diagram of the systematic review process, ART and pregnancy outcome, November 2013 (adopted from PRISMA tool)[54
Outcomes and Exposures of Interest
Adverse Effect
An ‘adverse effect’ is an adverse event for which the causal relation between the intervention and the event is at least a reasonable possibility.[14]
Preterm birth (PTB)
was defined as birth before 37+0 weeks gestation.[16] These were categorized as follows: moderately preterm (33+0-36+6weeks), very preterm birth (<32+0weeks) and extremely preterm birth (<28+0weeks).
LBW, SGA and IUGR
LBW was defined as a birth-weight <2500g and Very Low Birth Weight (VLBW) was defined as birth-weight <1500g. SGA, for our purposes, was defined as birth-weight-for-gestational age <10th centile; same as IUGR, which was defined as birth-weight-for gestational age <3rd centile.[17]
Stillbirth and Fetal death
The term “stillbirth” is used inconsistently in the literature we reviewed, which may lead to misclassification of the variable of interest. It is used interchangeably with fetal death. Therefore, for the purpose of this review, it was defined as any 3rd trimester delivery of a demised fetus with ≥1000g birth-weight or ≥28+0 completed weeks and/or ≥ 35cm body length.[18]
Neonatal death and miscarriage
Neonatal death was defined as the death of the infant within 28 days of life and miscarriage was defined as the spontaneous expulsion of the fetus before 28+0 weeks gestation.
Other Exposures
We also considered other exposures of interest. Antiretroviral treatment refers to an intervention for HIV-infected persons to primarily treat AIDS. Additional effects such as a reduced risk of MTCT of HIV are of added value to the intervention.[7] Antiretroviral prophylaxis refers to a short-term intervention to primarily reduce the risk of MTCT. This intervention could be given to an HIV-infected pregnant woman and/or to an uninfected but exposed infant and is not for the treatment of HIV disease.[7]
Results
Low birth weight (LBW) and Preterm birth
PTB, LBW and IUGR are widely acknowledged global causes of perinatal morbidity and mortality.[16, 18] The debate as to the role of maternal HAART as a risk factor for adverse pregnancy outcome is still ongoing.[19, 20] For instance, a 2007 systematic review by Kourtis based on 14 cohort studies reported that ART during pregnancy did not increase the risk of premature delivery, odds ratio (OR)[1.01, 95% (CI) 0.76–1.34]. In subgroup analyses, the use of ARTs containing protease inhibitor (PI) resulted in an OR for premature delivery of 1.24 (95% CI 0.76–2.02), compared to combinations without PI. Compared to therapy initiation in the 2nd trimester and beyond, the initiation of combination therapy before pregnancy or in the 1st trimester showed an OR of 1.71 (95% CI 1.09–2.67) of PTB. However, their review showed a large degree of heterogeneity.[21]
A UK and Ireland based study reported prematurity rate was higher in women on HAART (14.1%) than in women on mono/dual therapy (10.1%) even after adjusting for ethnicity, maternal age, clinical status [AOR = 1.51, 95%(CI), 1.19-1.93]. Delivery at <35 weeks was more strongly associated with HAART [AOR = 2.34; 95% CI, 1.64-3.37]. The effect was the same whether or not HAART included a protease inhibitor. In comparison with exposure to mono/dual therapy, exposure to HAART was associated with LBW standardized for gestational age (P < 0.001), and an increased risk of stillbirth [AOR = 2.27; 95% CI, 0.96-5.41].[22]
However, another cohort study reported the incidence of LBW and preterm birth, respectively, was 9.6% and 7.4%. There was no statistically significant increased risk of LBW [(AOR), 1.5 (95% CI), 0.7-3.2] or preterm birth (AOR, 1.1; 95% CI, 0.5-2.8) among women who received HAART/PI compared to women receiving 1-2 Nucleoside Reverse Transcriptase Inhibiters (NRTI).[23]
A South African based study showed that 27% of HAART-unexposed infants had LBW compared to 23% of early HAART-exposed infants and 19% of late HAART-exposed infants (p = 0.05). In the early HAART group, a higher CD4 cell count was protective against LBW (AOR 0.57 per 50 cells/mm3 increase, 95% CI 0.45-0.71, p < 0.001) and preterm birth (AOR 0.68 per 50 cells/mm3 increase, 95% CI 0.55-0.85, p = 0.001), with early Nevirapine and Efavirenz-based regimens having the strongest associations with preterm birth (AOR 5.4, 95% CI 2.1-13.7, and AOR 5.6, 95% CI 2.1-15.2, respectively). Studies from Cote d’Ivoire and Thailand report similar findings.[24, 25, 26]
A study done in Botswana in a reasonably large number of subjects, 32, 113 women reported that those continuing HAART from before pregnancy had higher odds of pre-term delivery [AOR= 1.2; 95% CI, 1.1-1.4)], SGA (AOR=1.8; 95% CI, 1.6, 2.1) and SB (AOR=1.5; 95% CI, 1.2, 1.8) than those who start later or are on prophylaxis. Among women initiating ART in pregnancy, HAART use (vs Zidovudine) was associated with higher odds of preterm delivery (AOR, 1.4; 95% CI, 1.2, 1.8), SGA (AOR, 1.5; 95% CI, 1.2, 1.9), and SB (AOR, 2.5; 95% CI, 1.6, 3.9).[27] In another study, congenital defects were seen in 7.6% infants on HAART.[28]
Maternal complications
A study from Thailand demonstrated adverse effects, especially anemia, were significantly associated with continuing combined ART in pregnancy (p<0.001). The incidence of low Appearance Pulse-Rate, Grimace, Activity and Respiration (APGAR) scores of the newborn taken in the 1st and 5th minute was 3.6%, and these were associated with initiation of PMTCT during labor (p=0.004).[19] Similarly, a study from Chile identified the following risks: hyperglycemia, lactic acidosis, mitochondrial toxicity, cutaneous rash, hepatitis, hypertension, and premature labor.[29]
Other Outcomes and Covariates
A subgroup review was conducted to assess the influence of possible covariates on adverse pregnancy outcome. Variables such as maternal demography, country, income status of country, alcohol use during pregnancy, smoking, advanced maternal age, teenage pregnancy, ethnicity, number of antenatal care received by the pregnant woman and intravenous drug use, drug related factors such as the type of ART used, time of initiation, and HIV stage i.e. immunological stage (mean CD4+) or clinical stage of HIV were deemed to show impact on adverse pregnancy outcomes.[30] Identified multiple pregnancy (AOR: 8.6; CI: 6.73 – 12.9), presence of opportunistic infection at delivery (AOR: 1.9; CI: 1.1 – 5.7), and 1st trimester exposure to PI based HAART (AOR: 5.4; CI: 3.4 – 7.8) retained a significant association with preterm delivery.
Duration of ART shorter than 4 weeks [HR= 3.6; 95%CI: 2.2–5.8], mothers on regimen during pregnancy (HR: 4.2; 95%CI: 1.6–11), exclusive breast feeding (EBF) (HR= 2.8; 95%CI: 1.5–5.4) or mixed feeding (HR = 6.9; 95%CI: 3.9–12.4) were also associated with adverse pregnancy outcomes.[31, 32] Maternal CD4 counts <200 cells/mm3, maternal body mass index (BMI) < 18.5, maternal anemia and maternal exposure to HAART were factors significantly associated with LBW. Maternal eclampsia and infants whose mothers gained 0.1 kg/week were at increased risk for LBW, preterm delivery, and composite adverse birth outcomes.[33, 34]
The WHO Guidelines (2010) recommend the same group of ART to be prescribed to both pregnant and non-pregnant women with the exception of Efavirenz.[7, 35] Efavirenz has been linked to anencephaly and anophthalmia in monkeys, at doses comparable to those taken by humans as a result Bristol-Myers Squibb Company announced the pregnancy category for SUSTIVA (Efavirenz) has been changed from Category C (risk of fetal harm cannot be ruled out) to Category D (positive evidence of fetal risk).[36, 37, 38]
Nonetheless, emerging data show that other used ART might also have adverse pregnancy outcomes. For example, Amprenavir has been associated with delayed skeletal ossification in rats and Tenofovir is linked to slightly decreased bone porosity among exposed monkeys.[39] If used in pregnant women, Efavirenz can have adverse pregnancy outcomes.
In a study in Tanzania women taking Lopinavir/ritonavir were significantly more likely to give birth prematurely and women on nevirapine were more likely to have still-births. Infant mortality was 2%-4% across the three arms of this study.[40] Dilated cardiomyopathy and mild bradycardia related to Lopinavir/Ritonavir therapy, a boosted protease-inhibitor was reported elsewhere.[41] However, these studies did not employed regression analysis which may limit their internal validity.
Discussion
Maternal HIV infection has been associated with adverse pregnancy outcomes; however, there is conflicting data regarding effect of HAART. While the benefits of HAART for prevention of mother-to-child transmission of HIV (PMTCT) are undisputed, there has been some concerns regarding its possible adverse effects on pregnancy outcomes. There has been an increasing number of studies that suggest higher risk of PB (< 37weeks) and other adverse pregnancy outcomes[19,42,43, 44], others dispute this finding.[45] For example, two papers reported that preterm birth rates were similar to those found in a HIV negative population.[46,47] One study argued ART by itself may not be sufficient for decreasing the burden of adverse birth outcomes in HIV positive women without nutritional care.[33] The potential harm to the fetus from maternal ingestion of a drug not only depends on the drug itself, but on the dose, the gestational age at exposure, the duration of exposure, the interaction with other agents, and to an unknown extent, the genetic makeup of the mother and fetus.[48]
Most studies in this review demonstrated causative associations between combination therapies, especially PI-based therapies, and preterm babies [49, 50]. Furthermore, in developed countries, the increase in the rate of emergency and elective cesarean sections among HIV-infected women was associated to early labor in women who had received HAART.[51] HAART not only reduces viral load but also affects metabolic pathways.[52] The effects antiretroviral therapies have on perinatal outcomes, especially the newer protease inhibitors, has been extensively reported in this review. It had been hypothesized that this may be due to an immunological mechanism, with HAART in pregnancy associated with a reversal of T-Helper (Th) cell 1 (Th1) to cell 2 (Th2).[53] However, further investigation of potential ART mechanisms of action may shed light on these contradictory findings and could inform caregivers on how to improve care given to HIV-infected pregnant women in all settings.
Limitations
In general, the result of systematic reviews needs to be interpreted in the context of the study methodologies. The Cochrane study group warns that observational studies are all prone to bias.[12] It is possible that confounders other than ART exposure may be responsible for observed differences in the reported outcomes. However, we mostly selected studies that stated and accounted for possible confounders. Another likely source of bias in this review may be publication bias. Studies which find no or negative associations may be less likely to be published either because they are not submitted for publication or because journals are less likely to publish them. This phenomenon has been well documented with trials, and there is no reason to believe it is not the case with observational studies also. However gray literature was included in our review to reduce publication bias.[53]
We acknowledge the possibility of selection bias having solely considered observational studies for our review. Scholars, such as Egger and colleagues suggest literature from all languages should be sought to maximise data retrieval and minimise bias[12,14, 15]. However, unavoidable constraints such as a lack of access to translational services did not allow for inclusion of literature in languages other than English into our review.
Even though it is not feasible to say a priori from our results that perinatal outcomes are made worse by a direct effect of ART themselves (HIV could be a cause of adverse perinatal outcomes), the majority of the adverse perinatal outcomes related to ART in this review especially in developing countries had odds ratio of ≥ 1.5. Despite the fact that the beneficial effects of antiretroviral therapy on mother-to-child transmission are unquestionable, monitoring the effect of antiretroviral therapy in pregnancy and newborn baby remains a priority.
Conclusion and Global Health Implications
From this systematic review, a number of conclusions and implication become apparent. There is a relative association between antiretroviral therapy and occurrence of adverse perinatal outcomes in developing countries especially when PI based combinations are used. Although the strength of associations of some studies are weak, and reports are different from setting to setting, there appears to be a real and significant increase in the risk of infant death in developing countries associated with ART use in HIV pregnant mothers.
Our study has significant implications for healthcare providers, policy makers, and public health experts in the field of HIV/AIDS.
For providers, the findings of this review have implications for women infected with HIV who are planning a pregnancy, or who find themselves pregnant. In the preconception phase women need to be given sufficient information about the potential risks for both themselves and their baby, so that they can make an informed choice about whether to become pregnant. Women infected with HIV with an unplanned pregnancy need also to have sufficient information so that they can make an informed decision for earlier intake of ART.
Antenatal surveillance should include fetal growth assessment, nutritional counselling, dietary intake and weight monitoring during pregnancy to improve pregnancy outcomes. There is a potential need to switch Efavirenz and if possible avoidance of PI based therapy. In addition, there is need for proper documentation of birth outcomes and appropriate reporting of the same.
For policy Makers, there is need for strategies to be designed and implemented for early initiation and increased coverage of ART. In addition, nutritional intervention to HIV positive pregnant women on ART and not on ART will be helpful. And for researchers, it is recommended to quantify associations using meta-analysis and understand the pooled effect of ART on pregnancy outcome. Many large well controlled cohort studies are needed in Africa.
Footnotes
Financial Disclosure: None.
Funding Support: None.
Conflicts of Interest: No authors have financial interests that pose a conflict of interest.
References
- 1.World Health Organization (WHO) Global HIV/AIDS response:epidemic update and health sector progress towards universal access:progress report 2014. Geneva, Switzerland: 2011. [Google Scholar]
- 2.Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization (WHO). HIV in pregnancy:A Review. Switzerland, Geneva: UNAIDS; 1998. Contract Number:WHO/RHT/98.24. [Google Scholar]
- 3.Segurado AC, Paiva V. Rights of HIV positive people to sexual and reproductive health Parenthood. Reproductive Health Matters. 2007;15(29):27–45. doi: 10.1016/S0968-8080(07)29032-9. Available at http://www.rhmjournal.org.uk/ [DOI] [PubMed] [Google Scholar]
- 4.Mock PA SN, Bhadrakom C, Siriwasin W, Chotpitayasunondh T, Chearskul S, Young NL, Roongpisuthipong A, Chinayon P, Kalish ML, Parekh B, Mastro TD. Maternal viral load and timing of mother-to-child transmission, Bangkok, Thailand. Bangkok Collaborative perinatal HIV transmission study group. AIDS. 1999;13(3):407–14. doi: 10.1097/00002030-199902250-00014. [DOI] [PubMed] [Google Scholar]
- 5.Bassey EA, Abasiubong F, Ekanem U, Abasiattai AA. Attitude of antenatal attendees to people living with HIV/AIDS in Ugo, South Nigeria. African Health Science. 2007;7(3):238. [PMC free article] [PubMed] [Google Scholar]
- 6.United Nations Childerns Fund (UNICEF) Scaling up Early Infant Diagnosis and Linkages to Care and Treatment. New York, 2008: UNICEF; 2009. [Google Scholar]
- 7.World Health Organization (WHO) Antiretro-viral drugs for treating pregnant women and preventing HIV infection in infants:towards universal access. 2010. Available from: http://whqlibdoc.who.int/publications/2010/9789241599818_eng . [PubMed]
- 8.Schouten E. Making it Happen:Revising national policies to reflect changes in WHO recommendations for preventing vertical transmission of HIV-Malawi. International AIDS Society 2010. Vienna, Austria: 2010. [Google Scholar]
- 9.Colebunders RL, Myer L. Antiretrovirals during pregnancy:a note of caution. The Journal of infectious diseases. 2013;208(4):706–7. doi: 10.1093/infdis/jit215. [DOI] [PubMed] [Google Scholar]
- 10.Watts DH, Mofenson LM. Antiretrovirals in pregnancy:a note of caution. The Journal of infectious diseases. 2012;206(11):1639–41. doi: 10.1093/infdis/jis581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulrow CD, Cook DJ, Davidoff F. Systematic reviews:critical links in the great chain of evidence. In: Mulrow CD, editor. Systematic Reviews Synthesis of Best Evidence For Health Care Decisions. Philadelphia PA: American College of Physicians; 1997. pp. 1–4. [Google Scholar]
- 12.Higgins JPT, Green S e. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006] Chichester, UK: John Wiley & Sons, Ltd: The Cochrane Library; 2006. Available from: http://www.cochrane.org/resources/handbook/hbook.htm . [Google Scholar]
- 13.Hewitt M. Trent Focus for Research and Development in Primary Health Care:Carrying Out a Literature Review. Notthingham, UK: Trent Focus Group; 2002. [Google Scholar]
- 14.Higgins JPT, Green S E. Trent Focus Group. Cochrane Handbook for Systematic Reviews of Interventions 5.2.1. Chichester, UK: The Cochrane Library, John Wiley & Sons, Ltd; [updated September 2010]. Available from: http://www.cochrane.org/resources/handbook/hbook.htm . [Google Scholar]
- 15.Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology:a systematic review and annotated bibliography. International Journal of Epidemiology. 2007;36(3):666–76. doi: 10.1093/ije/dym018. [DOI] [PubMed] [Google Scholar]
- 16.Lawn JE, Kerber K, Enweronu-Laryea C, et al. 3.6 Million Neonatal Deaths-What Is Progressing and What Is Not? Seminars in Perinatology. 2010;34(6):371–86. doi: 10.1053/j.semperi.2010.09.011. doi:10.1053/j.semperi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Fenton T. A new growth chart for preterm babies:Babson and Benda’s chart updated with recent data and a new format. BMC Pediatrics. 2003;3:13. doi: 10.1186/1471-2431-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawn JE, Blencowe H, Pattinson R, Cousens S, Kumar R, Ibiebele I, et al. Stillbirths:Where?When?Why?How to make the data count? Lancet. 2011;377(9775):1448–63. doi: 10.1016/S0140-6736(10)62187-3. [DOI] [PubMed] [Google Scholar]
- 19.Areechokchai D, Bowonwatanuwong C, Phonrat B, Pitisuttithum P, MA. Pregnancy outcomes among HIVinfected women undergoing antiretroviral therapy. Open AIDS Journal. 2009;3:8–13. doi: 10.2174/1874613600903010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brocklehurst P, French R. The association between maternal HIV infection and perinatal outcome:a systematic review of the literature and meta-analysis. British Journal of Obstetetric and Gynaecolology. 1998;105:836–48. doi: 10.1111/j.1471-0528.1998.tb10227.x. [DOI] [PubMed] [Google Scholar]
- 21.Kourtis AP, Schmid CH, Jamieson DJ, Lau J. Use of antiretroviral therapy in pregnant HIV-infected women and the risk of premature delivery:a meta-analysis. AIDS (London, England) 2007;21(5):607–15. doi: 10.1097/QAD.0b013e32802ef2f6. [DOI] [PubMed] [Google Scholar]
- 22.Townsend CL, Cortina-Borja M, Peckham CS, Tookey PA. Antiretroviral therapy and premature delivery in diagnosed HIV-infected women in the United Kingdom and Ireland. AIDS (London, England) 2007;21(8):1019–26. doi: 10.1097/QAD.0b013e328133884b. [DOI] [PubMed] [Google Scholar]
- 23.Szyld EG, Warley EM, Freimanis L, Gonin R, Cahn PE, Calvet GA, et al. Maternal antiretroviral drugs during pregnancy and infant low birth weight and preterm birth. AIDS (London, England) 2006;20(18):2345–53. doi: 10.1097/01.aids.0000253362.01696.9d. [DOI] [PubMed] [Google Scholar]
- 24.van der Merwe K, Hoffman R, Black V, Chersich M, Coovadia A, Rees H. Birth outcomes in South African women receiving highly active antiretroviral therapy:a retrospective observational study. Journal of the International AIDS Society. 2011;14:42. doi: 10.1186/1758-2652-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ekouevi D, Coffie P, Becquet R, Tonwe-Gold B, Horo A, Thiebaut R, et al. Antiretroviral therapy in pregnant women with advanced HIV disease and pregnancy outcomes in Abidjan, Cote d’Ivoire. AIDS (London, England) 2008;22:1815–20. doi: 10.1097/QAD.0b013e32830b8ab9. [DOI] [PubMed] [Google Scholar]
- 26.Asavapiriyanont S, Kasiwat S. Prevalence of low birthweight infants in HIV-infected women delivered in Rajavithi Hospital. Journal of the Medical Association of Thailand =Chotmaihet thangphaet. 2011;94(Suppl 2):S66–70. [PubMed] [Google Scholar]
- 27.Chen JY, Ribaudo HJ, Souda S, Parekh N, Ogwu A, Lockman S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. The Journal of infectious diseases. 2012;206(11):1695–705. doi: 10.1093/infdis/jis553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nielsen-Saines K, Komarow L, Cu-Uvin S, Jourdain G, Klingman KL, Shapiro DE, et al. Infant outcomes after maternal antiretroviral exposure in resource-limited settings. Pediatrics. 2012;129(6):e1525–32. doi: 10.1542/peds.2011-2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abarzua F, Nunez F, Hubinont C, Bernard P, Yombi JC, Vandercam B. [Human immunodeficiency virus (HIV) infection in pregnancy:antiretroviral treatment (ART) and mode of delivery] Revista chilena de infectologia :organo oficial de la Sociedad Chilena de Infectologia. 2005;22(4):327–37. doi: 10.4067/s0716-10182005000600005. [DOI] [PubMed] [Google Scholar]
- 30.Ezechi OC, David AN, Gab-Okafor CV, Ohwodo H, Oladele DA, Kalejaiye OO, et al. Incidence of and socio-biologic risk factors for spontaneous preterm birth in HIV positive Nigerian women. BMC Pregnancy and Childbirth. 2012;12:93. doi: 10.1186/1471-2393-12-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newell M.L, M.J Bunders. “Safety of antiretroviral drugs in pregnancy and breastfeeding for mother and child.”. Current Options in HIV and AIDS. 2013;8(5):504–510. doi: 10.1097/COH.0b013e3283632b88. [DOI] [PubMed] [Google Scholar]
- 32.Tchendjou P, Same-Ekobo C, Nga A, Tejiokem M, Kfutwah A, Nlend AN, et al. Effectiveness of multidrug antiretroviral regimens to prevent mother-to-child transmission of HIV-1 in routine public health services in Cameroon. PloS One. 2010;5(4):e10411. doi: 10.1371/journal.pone.0010411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young S, Murray K, Mwesigwa J, Natureeba P, Osterbauer B, Achan J, et al. Maternal nutritional status predicts adverse birth outcomes among HIV-infected rural Ugandan women receiving combination antiretroviral therapy. PloS One. 2012;7(8):e41934. doi: 10.1371/journal.pone.0041934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kebede B, Andargie G, Gebeyehu A. Birth outcome and correlates of low birth weight and preterm delivery among infants born to HIV-infected women in public hospitals of Northwest Ethiopia. Heath. 2013;5:25–34. [Google Scholar]
- 35.Prestes-Carneiro LE. Antiretroviral therapy, pregnancy, and birth defects:a discussion on the updated data. HIV/AIDS –Research and Palliative Care. 2013;5:181–189. doi: 10.2147/HIV.S15542. Available at http://dx.doi.org/10.2147/HIV.S15542 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bussmann H, Wester CW, Wester CN, Lekoko B, Okezie O, Thomas AM, et al. Pregnancy rates and birth outcomes among women on efavirenz-containing highly active antiretroviral therapy in Botswana. Journal of Acquired Immune Deficiency Syndromes (1999) 2007;45(3):269–73. doi: 10.1097/QAI.0b013e318050d683. [DOI] [PubMed] [Google Scholar]
- 37.Food and Drug Administration (FDA) /United States/:Med watch. Princeton, New Jersey: SUSTIVA Package Insert, Bristol-Myers Squibb Co; [Google Scholar]
- 38.Tarantal AF, Castillo A, Ekert JE, Bischofberger N, RB M. Fetal and maternal outcome after administration of tenofovir to gravid rhesus monkeys (Macaca mulatta) Journal of Acquire Immune Deficiency Syndrome. 2002;29:207–20. doi: 10.1097/00042560-200203010-00001. [DOI] [PubMed] [Google Scholar]
- 39.Toro PL, Katyal M, Carter RJ, Myer L, El-Sadr WM, Nash D, et al. Initiation of antiretroviral therapy among pregnant women in resource-limited countries:CD4+cell count response and program retention. AIDS (London, England) 2010;24(4):515–24. doi: 10.1097/QAD.0b013e3283350ecd. [DOI] [PubMed] [Google Scholar]
- 40.Highleyman Liz. 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention (IAS 2009) Cape Town, South Africa: 2009. Jul 19 - 22, Antiretroviral Therapy for Mothers Improves Pregnancy Outcomes and Reduces Risk of HIV Transmission via Breast-feeding. [Google Scholar]
- 41.McArthur MA, Kalu SU, Foulks AR, Aly AM, Jain SK, Patel JA. Twin preterm neonates with cardiac toxicity related to lopinavir/ritonavir therapy. The Pediatric Infectious Disease Journal. 2009;28(12):1127–9. doi: 10.1097/INF.0b013e3181acd17e. [DOI] [PubMed] [Google Scholar]
- 42.Bansil P, Jamieson DJ, Posner SF, Kourtis AP. Hospitalizations of pregnant HIV-infected women in the United States in the era of highly active antiretroviral therapy (HAART) Journal of Women’s Health. 2007;16(2):159–62. doi: 10.1089/jwh.2006.CDC2. [DOI] [PubMed] [Google Scholar]
- 43.Townsend C, Schulte J, Thorne C, Dominguez KI, Tookey PA, Cortina-Borja M, et al. Antiretroviral therapy and preterm delivery-a pooled analysis of data from the United States and Europe. British Journal of Obstetrics and Gynaecology. 2010;117(11):1399–410. doi: 10.1111/j.1471-0528.2010.02689.x. [DOI] [PubMed] [Google Scholar]
- 44.Morris A, Dobles A, Cu-Uvin S, Zorrilla C, Anderson J, Harwell J, et al. Protease inhibitor use in 233 pregnancies. Journal of Acquired Immune Deficiency Syndromes (1999) 2005;40:30–3. doi: 10.1097/01.qai.0000174651.40782.95. [DOI] [PubMed] [Google Scholar]
- 45.Castetbon K, Ladner J, Leroy V, Chauliac M, Karita E, De Clercq A, et al. Low birthweight in infants born to African HIV-infected women:relationship with maternal body weight during pregnancy:Pregnancy and HIV Study Group (EGE) Journal of Tropical Pediatrics. 1999;45(3):152–7. doi: 10.1093/tropej/45.3.152. [DOI] [PubMed] [Google Scholar]
- 46.Dola CP, Khan R, DeNicola N, Amirgholami M, Benjamin T, Bhuiyan A, et al. Combination antiretroviral therapy with protease inhibitors in HIV-infected pregnancy. Journal of Perinatal Medicine. 2011;40(1):51–5. doi: 10.1515/JPM.2011.111. [DOI] [PubMed] [Google Scholar]
- 47.Ndirangu J, Newell ML, Bland RM, Thorne C. Maternal HIV infection associated with small-for-gestational age infants but not preterm births:evidence from rural South Africa. Human Reproduction (Oxford, England) 2012;27(6):1846–56. doi: 10.1093/humrep/des090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Centers for Disease Control and Prevention. U.S. Public Health Service Task Force recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatan HIV-1 transmission in the United States. U.S. Public Health Service Task Force, editor. MMWR Recommendations and Reports. 2002;51(RR18):1–8. [PubMed] [Google Scholar]
- 49.Coll O, Fiore S, Floridia M, et al. Pregnancy and HIV infection:a European consensus on management. AIDS. 2002;16(Suppl 2):S1–S18. [PubMed] [Google Scholar]
- 50.Powis KM, Kitch D, Ogwu A, Hughes MD, Lockman S, Leidner J, et al. Increased risk of preterm delivery among HIV-infected women randomized to protease versus nucleoside reverse transcriptase inhibitor-based HAART during pregnancy. The Journal of Infectious Diseases. 2011;204(4):506–14. doi: 10.1093/infdis/jir307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siegfried N, van der Merwe L, Brocklehurst P, Sint TT. Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection. The Cochrane Database of Systematic Reviews. 2011;(7):CD003510. doi: 10.1002/14651858.CD003510.pub3. [DOI] [PubMed] [Google Scholar]
- 52.Thorne C, Fiore S, Rudin C. Antiretroviral therapy during pregnancy and the risk of an adverse outcome. New England Journal of Medicine. 2003;348(5):471–2. doi: 10.1056/NEJM200301303480519. author reply -2. [DOI] [PubMed] [Google Scholar]
- 53.Fiore S, Newell ML, Trabattoni D, Thorne C, Gray L, Savasi V, et al. Antiretroviral therapy-associated modulation of Th1 and Th2 immune responses in HIV-infected pregnant women. Journal of Reproductive Immunology. 2006;70(1-2):143–50. doi: 10.1016/j.jri.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 54.Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses:The PRISMA Statement. PLoS Medcine. 2009;6(6) [PMC free article] [PubMed] [Google Scholar]


