Abstract
Osseous tissue defects caused by trauma present a common clinical problem. Although traditional clinical procedures have been successfully employed, several limitations persist with regards to insufficient donor tissue, disease transmission, and inadequate host-implant integration. Therefore, this work aims to address current limitations regarding inadequate host tissue integration through the use of a novel elastomeric material for three-dimensional (3D) printing biomimetic and bioactive scaffolds. A novel thermoplastic polyurethane-based elastomeric composite filament (Gel-Lay) was used to manufacture porous scaffolds. In an effort to render the scaffolds more bioactive, the flexible scaffolds were subsequently incubated in simulated body fluid at various time points and evaluated for enhanced mechanical properties along with the effects on cell adhesion, proliferation, and 3-week osteogenesis. This work is the first reported use of a novel class of flexible elastomeric materials for the manufacture of 3D printed bioactive scaffold fabrication allowing efficient and effective nucleation of hydroxyapatite (HA) leading to increased nanoscale surface roughness while retaining the bulk geometry of the predesigned structure. Scaffolds with interconnected microfibrous filaments of ∼260 μm were created and nucleated in simulated body fluid that facilitated cell adhesion and spreading after only 24 h in culture. The porous structure further allowed efficient nucleation, exchange of nutrients, and metabolic waste removal during new tissue formation. Through the incorporation of osteoconductive HA, human fetal osteoblast adhesion and differentiation were greatly enhanced thus setting the tone for further exploration of this novel material for biomedical and tissue regenerative applications.
Introduction
Large critical sized bone defects (such as craniofacial or spinal bone defects) caused by traumatic injury, cancer, or disease, continue to be challenging to treat owing to the size and complexity of the defects. Surgically implanting osteoconductive scaffolds and/or other forms of osteoinductive bone graft materials to promote osteogenesis is a common approach in promoting large bone tissue growth.1 Common sources of bone grafts include autografts, allografts, and synthetic materials. Autologous and allologous sources are most favored for their good osteoconductivity, biocompatibility, and minimized probability of disease transfection. However, several limitations exist; the amount of available autologous donor tissue is inadequate and donor site morbidity.2,3 In addition, the amount of cadaveric donor tissue available from reserves including bone banks is also in limited supply.4 Therefore, metallic and synthetic materials that closely match the mechanical properties of native tissue while providing morphogenetic cues for enhanced osteoconductivity and resultant host tissue integration come to great clinical need, especially for large bone defect repair.
Current materials in use include metals such as titanium, cobalt, stainless steel, and nonmetallic materials like hydroxyapatite, bioactive glass, or polymers.5 Metallic implants, though widely used as implantable fixtures due to their good mechanical properties, can lead to osteolysis as a result of corrosion.6 While some metallic implants have been modified for enhanced biocompatibility, they have also been found to release toxic ions deleteriously affecting local tissue.7 In an effort to address these concerns, nonmetallic synthetic materials, such as hydroxyapatite, have been employed due to their excellent biocompatibility and manufacturability in the fabrication of porous three-dimensional (3D) structures. Although, most inorganic ceramics exhibit superior mechanical properties to synthetic polymers, they can be difficult to process and manufacture without the encumbrance of commercial equipment. Therefore, synthetic polymers can be readily modified and employed as a scaffold to mimic the mechanical property of bone with high biocompatibility while exhibiting morphogenic factors to promote better host-implant integration.1
With the advent of 3D bioprinting, new polymeric materials are being developed in an effort to extend the applicability of this technology in an effort to construct structurally and mechanically biomimetic scaffolds.8 One of these materials, thermoplastic polyurethane (TPU), has several inherently beneficial properties including elasticity,9 strength, flexibility, and good biocompatibility rendering it a potentially ideal candidate for the fabrication of 3D implantable scaffolds.10 For this purpose, a novel proprietary TPU/polyvinyl alcohol (PVA) composite (Gel-lay, Lay-Filaments™; MatterHackers) was used to manufacture porous 3D printed scaffolds providing desirable elasticity and rigidness allowing the fabrication of high-resolution constructs. In addition to the benefits of a TPU-based 3D printable material, the presence of water soluble PVA contributes to the formation of a secondary microstructure when dissolved rendering the rigid scaffold porous and flexible.11 Owing to these desirable material properties, this material prompted further exploration with regards to surface modification for bone regeneration.
Various surface treatment methods have been employed for increased cell adhesion of TPU implants.12 In particular, submersion of TPU scaffolds within simulated body fluid (SBF) has been found to promote osteogenic apatite formation leading to enhanced bone cell attachment.13 SBF is an ionic solution composed of ion concentrations similar to those found in human blood plasma. It has been shown to induce mineral nucleation resulting in apatite formation upon the materials’ surface thus eliciting enhanced cellular behavior.14 In addition, the formation of an apatite layer on the surface alters the mechanical properties of the bulk material and promotes greater cell adhesion between the material and native bone cells through increased surface roughness, surface area, and focal adhesion points.15 Therefore, porous TPU scaffolds after SBF nucleation can be readily employed for bone tissue regeneration owing to their ability to enhance cell adhesion and proliferation16 and its biodegradability, which is readily absorbed and replaced by native cell populations.17
For this purpose, this work evaluated a novel formulation of TPU/PVA composite filament as a 3D printable biomaterial for tissue-engineered bone scaffold fabrication. This is the first reported use of this unique TPU/PVA proprietary filament for use in bone tissue regeneration. Due to the presence of water soluble PVA, the composite filament exhibits sufficient rigidity for 3D printing where TPU filaments alone are very flexible and difficult to print by fused deposition modeling. In addition to increased material stiffness, subsequent dissolution of the PVA component after 3D printing produces a unique stratified microfilamentous structure along the fiber surface. Subsequently, 3D printed scaffolds can be readily incubated in SBF for mineral nucleation leading to increased mechanical and cytocompatible properties. Therefore, this study is the first to illustrate the great potential of 3D printed Gel-lay scaffolds with SBF nucleation for osseous tissue repair.
Materials and Methods
3D printed TPU/PVA scaffold design, printing, and fabrication
A 35 × 35 × 2.5 mm model was designed in Rhino3D (McNeel North America) and the resultant computer-aided design (CAD) file was prepared for 3D printing by conversion to a computer numerical control file by the open source software package Slic3r. Next, the models were printed using a Solidoodle© table-top fused deposition modeling printer (Solidoodle) with Gel-Lay porous 3D printing filament (Lay-Filaments; MatterHackers).
Dissolution of PVA and SBF nucleation
3D printed TPU/PVA composite scaffolds were immersed in ultrapure water and ultrasonicated at 60°C for three 90 min cycles to fully dissolve the PVA component. Subsequently, scaffolds were washed with distilled water and air-dried at room temperature. TPU scaffolds were nucleated following the protocol detailed by Kokubo and Takadama.14 Briefly, SBF was prepared with 700 mL of ion-exchanged distilled water, 8.035 g NaCl, 0.355 g NaHCO3, 0.225 g KCl, 0.231 g K2HPO4·3H2O, 0.311 g MgCl2·6H2O, 39 mL 1.0 M-HCl, 0.292 g CaCl2, 0.072 g Na2SO4, and 6.118 g Tris with a pH of 7.42 ± 0.0.1 in a polypropylene beaker with a magnetic stir bar at 36.5 ± 1.5°C. 3D printed TPU scaffolds were soaked in SBF for 24, 72, and 120 h nucleation times. The nucleated scaffolds were then removed, blotted dry, and air-dried. Nucleated and non-nucleated scaffolds were trimmed into 5 × 5 mm squares, placed in 96-well cell culture plates, and sterilized under ultraviolet light for 20 min before cell studies.
Scaffold characterization
Scanning electron microscopy (SEM) analysis of 5 × 5 mm nucleated and non-nucleated scaffolds were air-dried overnight and gold sputter-coated before SEM (Zeiss NVision 40 FIB) analysis at an accelerating voltage of 1.5 kV. The compressive modulus of nucleated and non-nucleated 3D printed TPU scaffolds was determined via uniaxial compression testing (n = 5; Applied Test Systems) fitted with a 1 kN load cell at a crosshead speed of 5 mm/min. Load and displacement were used to plot the stress-strain curves and the Young's modulus was calculated from the linear elastic region. Surface charge of the scaffold was measured by drop shape analysis using a contact angle analyzer (DSA4; Kruss).
Osteoblast adhesion and proliferation
Human fetal osteoblasts (hFOBs) (CRL-11372; American Tissue Culture Collection) were cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) supplemented with 10% fetal bovine serum (FBS; Hyclone) and 1% penicillin/streptomycin (Hyclone) under standard cell culture conditions of 37°C, and a 5% CO2/95% humidified air environment. Cells with population numbers of 6–10 were used in the experiments without further characterization. For hFOB adhesion and proliferation, 5 × 5 mm scaffolds were placed in a 96-well cell culture plate and sterilized under UV light for 20 min. The sterilized samples were prewetted with media overnight. A density of 50,000 cells/scaffold and 10,000 cells/scaffold were used for cell adhesion and proliferation experiments, respectively. At predetermined time points, 4 h, 1, 3, and 5 days the scaffolds were rinsed with phosphate-buffered saline (PBS) and the adherent cells were enzymatically lifted with 0.25% Trypsin-EDTA. A 100 μL aliquot of cell suspension was transferred to a fresh 96-well plate and 20 μL of CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay solution (MTS assay; Promega) was added. The well plate was incubated for 1 h then read spectrophotometrically at 490 nm.
Fluorescence microscopy imaging of 24 h osteoblast growth
One scaffold from each nucleation time group was fixed in 10% Formalin for 20 min, rinsed 3× with PBS, and permeabilized with 10% Triton X for 5 min. Fixed samples were double-stained with Texas Red-phalloidin® (cytoskeleton) (Life Technologies) for 20 min, rinsed 3× with PBS followed by staining for 5 min with 4′,6-diamidino-2-phenylindole (DAPI) (Life Technologies). Samples were imaged with a 10× objective (EclipseTi; Nikon Research Corporation of America).
Three-week osteogenesis
For osteogenesis studies, hFOBs were seeded at a density of 100,000 cells/scaffold on non-nucleated, 24, 72, and 120 h nucleated scaffolds in a 96-well cell culture plate. After 24 h, scaffolds were transferred to a 48-well plate and cultured in osteogenic media consisting of DMEM media supplemented 10% FBS, 1% penicillin/streptomycin, 50 μg/mL l-ascorbic acid (Sigma-Aldrich), and 10 mM β-glycerophosphate (Sigma-Aldrich) for 1, 2, and 3 weeks under standard cell culture conditions. Osteoblasts were lysed using distilled water and three freeze-thaw cycles, which removed intracellular and membrane-bound proteins. All lysed samples were stored at −80°C. All biochemical analyses were normalized to cell number with results given as units per cell.
Total DNA content
Total DNA content was determined to directly quantify total cell number for all differentiation biochemical assays. Briefly, a 1:100 mixture of Quant-iT™ PicoGreen® dsDNA Reagent in 1× Tris-EDTA (TE) buffer (10 mM Tris-HCl, 1 mM EDTA, pH 7.5) and lysed sample was added to a 96-well plate and read after 5 min incubation at room temperature with an excitation wavelength of 485 nm and an emission wavelength of 528 nm.
Alkaline phosphatase activity
Alkaline phosphatase (ALP) is an enzyme whose production signifies increased osteoblast differentiation to calcium depositing cells. The ALP activity in the cell lysates prepared above was evaluated by a commercially available Alkaline/Acid Phosphatase Assay kit (Cat#17–128; Upstate). For this purpose, 20 μL of cell lysate was mixed with 5 μL NiCl2, 5 μL bovine serum albumin, 5 μL phosphopeptide stock solution, and 45 μL pNPP ser/thr assay buffer in one well of a 96-well microplate. Then, 25 μL aliquots of above mixtures (in triplicate) were transferred into a new plate and were incubated for 15 min at 37°C. One hundred microliter of Malachite Green solution was added to detect ALP activity. The absorbance of the prepared samples was read on a spectrophotometer at an absorbance wavelength of 650 nm. ALP synthesized by osteoblasts cultured on the substrates of interest to this study was calculated according a standard curve of known concentrations of phosphate versus absorbance run in parallel with the experimental samples.
Calcium deposition
Calcium deposition is one of the most important indicators of bone matrix formation, which was measured using a calcium reagent kit (Pointe Scientific, Inc.). Briefly, after osteoblasts were lysed, the scaffolds were immersed in a 0.6 N HCl solution at 37°C for 24 h. After the prescribed time period, the amount of dissolved calcium present in the acidic supernatant was measured by reacting with the o-cresolphthalein complexone to form a purple-color solution. The light absorbance of these samples was measured at 570 nm spectrophotometrically. Total calcium deposition was calculated from standard curves of known calcium concentrations versus absorbance run in parallel with the experimental samples. Calcium deposition values were normalized to remove the contribution of nucleation and expressed as total mass.
Total collagen content
Collagen is the main organic component of bone. The total collagen content of lysed samples was evaluated via Sircol collagen assay kit (Accurate Chemical & Scientific Corp.,). A 100 μL aliquot of each lysate was added to a 96-well plate and dried overnight on a hotplate at 60°C. One hundred fifty microliter of 0.1% Sirius Red in saturated picric acid was added to each dried lysate sample and incubated for 1 h at room temperature. The samples were then washed four times with 5% acetic acid and then incubated in 0.1 M NaOH for 30 min. A 100 μL aliquot of each sample was transferred to a new 96-well plate and a measurement was taken at 555 nm and total collagen per cell was calculated based on a premade standard curve with known concentrations of collagen.
Human type I collagen enzyme-linked immunosorbent assay
Type I collagen is the predominant collagen type found in mature bone and was measured via type I collagen specific enzyme-linked immunosorbent assay (Fisher Scientific) per manufacturer's protocol. Briefly, 100 μL of lysate was transferred to a 96-well plate precoated with rat immunoglobulin M antibody and incubated at room temperature for 2 h. Aliquots were then decanted and the wells were washed to remove any unbound sample. A secondary type I collagen specific detection antibody was added to the well plate was incubated for an additional 2 h followed by washing with buffer. Finally, streptavidin peroxidase was added to the plates resulting in a color reaction. An acidic stop solution was added to stop the reaction and the plate was read at 490 nm. Type I collagen was calculated based on a premade standard curve with known concentrations of type I collagen.
Statistical analysis
All data presented are in the form of mean value ± standard error of the mean. Student's t-test was used to determine significant differences among the groups with statistical significance considered at p < 0.05.
Results and Discussion
Synthesis and characterization of SBF nucleated 3D printed TPU scaffolds
In this study a new class of 3D printable elastomeric composite filament was evaluated for bone tissue regeneration. The proprietary composite consisted of ∼30 wt% water soluble PVA blended with 70 wt% TPU. In an effort to further enhance the bioactive properties of this new material, scaffolds were subjected to hydroxyapatite nucleation by submersion in SBF. A flow chart illustrating the scope of the current work from scaffold fabrication, nucleation, and cell differentiation studies can be found in Figure 1. First, scaffolds exhibiting a solid bottom and porous interconnecting fiber structure were 3D printed via fused deposition modeling as shown in the CAD model in Figure 2A with corresponding optical images of printed scaffolds before and after nucleation (Fig. 2B, C). Upon printing and complete dissolution of the water soluble PVA component scaffolds were incubated in SBF for hydroxyapatite nucleation. SEM examination of scaffold morphology and nucleation is shown (Fig. 3A) with corresponding data quantifying nucleated Ca2+ as a function of incubation time (Fig. 3B). An interesting microstructural characteristic was noted upon dissolution of PVA as seen via SEM. 3D printed TPU after PVA dissolution displays a microfilamentous surface topography along the long-axis of the extruded filament. It is well known that micro- and nanostructural surface morphology can enhance osteoblast cell attachment and direct new tissue formation,18,19 but, unlike most recent work, which utilizes 3D printing for patternization of scaffolds, the material used in this study upon solubilization of PVA induces microstriations on the surface of the scaffold. This inherent feature of the TPU scaffold may be suitable for applications requiring directionality of cell adhesion as in the case of neural tissue regeneration, which may be an additional application of this novel scaffold. Although this structural feature and the inherent cell adherent properties of the TPU scaffold hold promise, smoothening of the scaffold surface can be seen as a function of nucleation time (Fig. 3A) where the aforementioned microfilamentous structure is lost due to hydroxyapatite formation.
FIG. 1.
Schematic overview of 3D printing, SBF nucleation and osteogenesis of 3D printed TPU/PVA composite scaffolds. 3D, three-dimensional; PVA, polyvinyl alcohol; SBF, simulated body fluid; TPU, thermoplastic polyurethane. Color images available online at www.liebertpub.com/tea
FIG. 2.

CAD models of 3D printed TPU scaffold (A) with corresponding top, bottom and cross-sectional images. (B) Bottom and (C) top optical micrographs of TPU scaffold before (3D printed) and after PVA dissolution and SBF nucleation (Nucleated). CAD, computer-aided design. Color images available online at www.liebertpub.com/tea
FIG. 3.
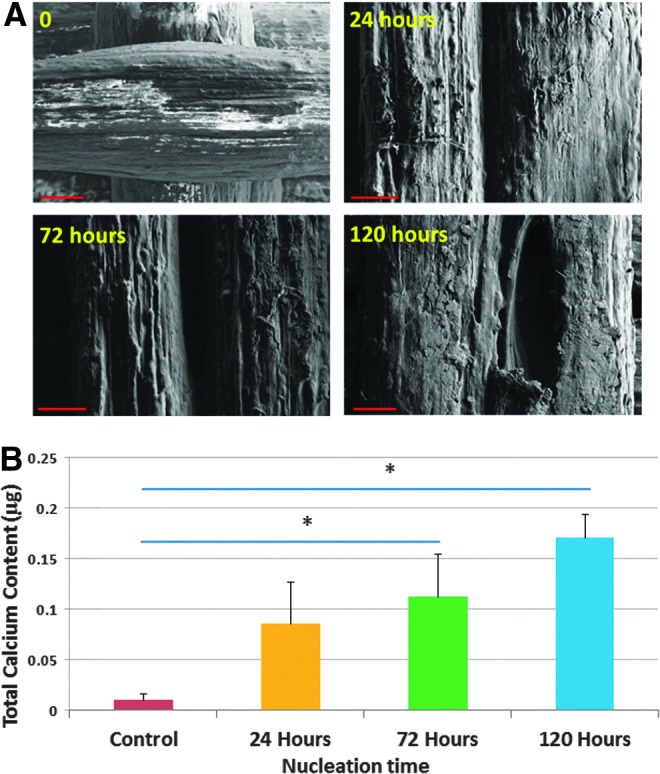
SEM of nucleated TPU 3D printed scaffolds (A) with corresponding quantified Ca2+ as a function of nucleation time in SBF. (B) Total nucleated calcium on TPU scaffolds after various SBF incubation periods. Data are mean ± standard error of the mean, n = 5, *p < 0.05. Scale bar = 100 μm. SEM, scanning electron microscopy. Color images available online at www.liebertpub.com/tea
The effect of nucleation on surface wettability was also evaluated by contact angle analysis and the results are shown in Figure 4. A statistical difference was noted between 72 and 120 h nucleation, which may be attributed to morphological changes in the surface topography and alterations in the surface charge due to apatite formation as a result of instabilities in the SBF solution as described by Zhu et al.20 where slight changes in alkalinity may result in enhanced or inhibited nucleation. Notwithstanding, minimal variation was noted as all samples groups exhibited slight hydrophobic character. Young's modulus of all scaffolds was determined by unconfined uniaxial compression testing (Fig. 5). All 3D printed scaffolds show excellent mechanical properties. Furthermore, SBF nucleation can significantly increase the TPU scaffolds’ modulus when compared to non-nucleated control. Kokubo et al.14,21 have reported SBF nucleation times of implantable materials in the range of several (>3) months with improved results. Therefore, it is postulated that increased incubation time may produce scaffolds exhibiting more physiologically relevant moduli.
FIG. 4.

Contact angle analysis of SBF nucleated TPU scaffolds. Data are mean ± standard error of the mean, n = 5, *p < 0.05. Color images available online at www.liebertpub.com/tea
FIG. 5.

Compressive mechanical property of SBF nucleation on 3D printed TPU scaffold. All nucleated samples showed a significant increase in mechanical properties when compared to non-nucleated control. Data are mean ± standard error of the mean, n = 5, *p < 0.05. Color images available online at www.liebertpub.com/tea
To evaluate the effects of SBF nucleation time on cellular attachment, 4-h hFOB adhesion was examined with results showing a linear correlation between cell attachment and nucleation time where a 10%, 17%, and 31% increase in hFOB adhesion was observed after 24, 72, and 120 h nucleation, respectively (Fig. 6). In addition, fluorescence microscopy analysis revealed excellent cell attachment and spreading after 24 h of culture (Fig. 7) with a high concentration of cell spreading among nucleated samples groups. It is well known that surface topography play a critical role in enhancing cell adhesion as our previous work has illustrated.22–25 Increased surface roughness and altered surface chemistry produces scaffolds with increased surface area and bioactivity for protein adsorption and subsequent cell adhesion. In addition to increased cell adhesions, hFOB proliferation (Fig. 8) also displayed a similar trend with 120 h nucleation outperforming all samples groups. All nucleated sample groups showed increased cell proliferation after 1, 3, and 5 days with 72 and 120 h nucleated samples exhibiting a >30% and >40% increase after 1 and 3 days, respectively. After 5 day culture, a more pronounced increase in cell proliferation was seen with an increase of 46% and 53% for 72 and 120 h nucleation, respectively. Based on these initial findings, all nucleated TPU scaffolds were evaluated for 3-week hFOB osteogenesis.
FIG. 6.

Four-hour hFOB adhesion on nucleated 3D printed TPU scaffolds. Data are mean ± standard error of the mean, N = 3, *p < 0.05 when compared to all other samples. hFOB, human fetal osteoblast. Color images available online at www.liebertpub.com/tea
FIG. 7.

Fluorescence microscopy analysis of 24 h hFOB cell growth on nucleated 3D printed TPU scaffolds. Excellent cell attachment and spreading were observed on all samples including non-nucleated control. Scale bar = 100 μm. Color images available online at www.liebertpub.com/tea
FIG. 8.

hFOB proliferation on SBF nucleated 3D printed bone scaffolds. A significant increase in cell adhesion was observed on 72 and 120 h samples when compared to control with an increase of 34% and 48% after 1 day; a 38% and 47% increase after 3 days; and 46% and 53% increase after 5 days respectively. Data are mean ± standard error of the mean, N = 3, *p < 0.05 when compared to all other samples at respective days; **p < 0.05 when compared to 0 and 24 h nucleated samples; and ***p < 0.05 when compared to controls. Color images available online at www.liebertpub.com/tea
hFOB osteogenesis on 3D printed nucleated TPU
Osteogenesis of hFOBs is an important phase in de novo osseous tissue formation. Several forms of stem cell and mature osteoblast osteoinduction have been employed in an effort to stimulate enhanced tissue formation including mechanical26–28 and chemical stimulation.29–31 Three-week osteogenesis of hFOBs on nucleated 3D printed TPU scaffolds was conducted in this study. At predetermined time points, hFOBs were evaluated for, ALP activity, total extracellular calcium deposition, total collagen and type I collagen. Biochemical analyses of 3-week hFOB osteogenesis are shown in Figures 9–12. In addition, SEM analysis (Fig. 12) was conducted to examine scaffold morphology changes and extracellular matrix deposition after 2 week osteogenic differentiation.
FIG. 9.

Alkaline phosphatase activity on 3D printed bone scaffolds. Seventy-two and 120 h nucleated samples showed a significant increase in alkaline phosphatase activity when compared 24 h and non-nucleated control after 1 (*) and 2 weeks (**). Seventy-two and 120 h nucleation time resulted in a 533% and 443% increase after 1 week and a 219% and 134% increase after 2 weeks, respectively. All nucleated samples showed a dramatic increase in alkaline phosphatase activity after 3 weeks (***) with a >5-fold increase over control. Data are mean ± standard error of the mean, n = 5, p < 0.05. Color images available online at www.liebertpub.com/tea
FIG. 10.

Total calcium deposition on 3D printed bone scaffolds. All nucleated samples showed a significant increase in calcium deposition when compared to non-nucleated control after 2 (**) and 3 weeks (*). After 2 weeks, all nucleated samples exhibited a >5-fold increased and after 3 weeks a greater than 14-fold increase. Data are mean ± standard error of the mean, n = 5, p < 0.05. Color images available online at www.liebertpub.com/tea
FIG. 11.
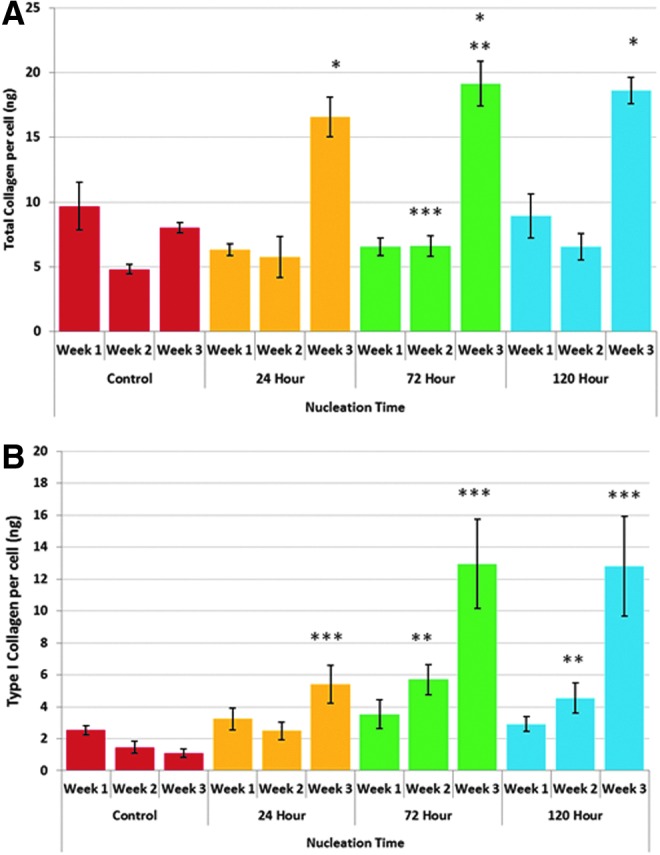
Total collagen (A) and Type I collagen (B) synthesis on 3D printed bone scaffolds. All nucleated samples showed a greater than 100% increase in total collagen when compared to non-nucleated control after 3 weeks (*p < 0.05). **p < 0.05 when compared to 24 h nucleation time after 3 weeks. ***p < 0.05 when compared to control after 2 weeks. Seventy-two and 120 h nucleated samples resulted in a 290% and 211% increase in type I collagen when compared to control (**) after 2 weeks. In addition, all nucleated samples showed a significant increase in type I collagen when compared to non-nucleated control after 3 weeks (***). Twenty-four hours nucleation time resulted in a near fourfold increase with 72 and 120 h nucleation resulting in an 11-fold increase at week 3, respectively. Data are mean ± standard error of the mean, n = 5. Color images available online at www.liebertpub.com/tea
FIG. 12.
SEM images of extracellular matrix deposition on 3D printed thermoplastic bone scaffolds after 2 weeks. All nucleated samples showed noticeable extracellular matrix deposition (red arrow) when compared to non-nucleated control after 2 weeks. (A1–A4) scale bar = 500 μm, (B1–B4) scale bar = 100 μm, (C1–C4) scale bar = 10 μm. Color images available online at www.liebertpub.com/tea
ALP is an early-stage marker of osteogenesis and a precursor to calcium deposition. After 1 week of culture (Fig. 9), 72 and 120 h nucleated samples showed a significant increase, 533% and 443%, respectively, in ALP activity when compared to 24 h and non-nucleated control. After 2 weeks, 72 and 120 h nucleated samples maintained increased ALP activity with a 219% and 134% increase over 24 h and non-nucleated control, respectively. All nucleated samples showed an increase in ALP activity after 3 weeks with a >5-fold increase over control. These results show the effectiveness of SBF nucleation in eventual extracellular calcium deposition.
Extracellular calcium deposition is a late-stage marker for osteogenesis. Deposited extracellular calcium (Fig. 10) on 3D printed thermoplastic TPU composites. All nucleated samples showed a similar trend to ALP activity with a significant increase in extracellular calcium deposition when compared to non-nucleated control after 2 and 3 weeks. After 2 weeks, all nucleated samples exhibited a >5-fold increased and after 3 weeks a greater than 14-fold increase when compared to non-nucleated control. These results further demonstrate that calcium deposition on 3D printed bioactive scaffolds can be further enhanced by SBF nucleation.
In addition to increases in the deposition of the primary inorganic component of bone, increases in total collagen (Fig. 11A) and type I collagen (Fig. 11B) were also observed. Extracellular collagen on nucleated 3D printed TPU scaffolds showed a greater than 100% increase in total collagen when compared to non-nucleated control after 3 weeks. Although no significant difference was observed after 1 week, 72 h nucleated samples did show a 37% increase after 2 weeks. The apparent decrease in total collagen may be attributed to changes in surface energetics as a result of physiochemical interactions between globular proteins and calcium phosphate as described by Znidarsic et al.32 where electrostatic interactions and conformational changes of adsorbed protein alter mineralization. More importantly, type I collagen results are more telling. Seventy-two and 120 h nucleated samples resulted in a 290% and 211% increase when compared to control after 2 weeks. In addition, all nucleated samples showed a significant increase when compared to non-nucleated control after 3 weeks. Twenty-four hours nucleation yielded a near fourfold increase with 72 and 120 h nucleation resulting in an 11-fold increase, respectively. SEM analysis of 2-week differentiation (Fig. 12) illustrated the clear formation of extracellular matrix deposits, which are most evident after 72 and 120 h nucleation. Distinct collagen-like fibrous structures are visible with mineralized nodule formation.
The scope of the work has great significance in the realm of 3D printed implantable bioactive polymeric scaffolds where a dearth of 3D printable cytocompatible material exists for use in bone tissue regeneration. In addition, focused regulatory efforts have begun to lay the groundwork for approval and commercialization of implantable 3D printed bioactive scaffolds with the approval in 2014 of Oxford Performance Materials’ OsteoFab® Patient-Specific Facial Device (OPSFD) composed of a proprietary polymer (polyetherketoneketone [PEKK]) by the Food and Drug Administration.33 Unlike PEKK, the composite material used in this study does not require the use of expensive commercial 3D printing systems thus minimizing cost-constraints associated with the manufacture of patient-specific implants.
Conclusions
The aim of this work was to evaluate a novel 3D printable elastomeric composite for bone tissue regeneration. In addition, SBF nucleation was performed to further enhance cell performance and osseous tissue formation of hFOBs. SBF nucleation and scaffold geometry were well defined with clear nucleation and nanotexturization under SEM examination with increases in elastic modulus with minimal effects on surface charge. hFOB cell adhesion, proliferation, and osteogenesis demonstrate that SBF nucleation can further render the 3D printed scaffolds more bioactive. Additionally, our results are the first to demonstrate the osteogenic potential of this novel class of elastomeric material for use in tissue regeneration. Therefore, SBF nucleation of 3D printed TPU scaffolds provides an excellent tool and promising material for regenerative applications.
Acknowledgments
The authors would like to thank the financial support from NIH Director's New Innovator Award 1DP2EB020549-01. We also appreciate the George Washington University Center for Microscopy and Image Analysis for provided imaging support.
Disclosure Statement
No competing financial interests exist.
References
- 1.Klokkevold P.R., J.S. Advanced implant surgery and bone grafting techniques. In: Carranza T., Newman , ed. Clinical Periodontology, 9th ed. Philadelphia, PA: W.B. Saunders Co., 2002 [Google Scholar]
- 2.Rogers G.F., and Greene A.K. Autogenous bone graft: basic science and clinical implications. J Craniofac Surg 23, 323, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Summers B.N., and Eisenstein S.M. Donor site pain from the ilium. A complication of lumbar spine fusion. J Bone Joint Surg Br 71, 677, 1989 [DOI] [PubMed] [Google Scholar]
- 4.Czitrom A.A. Bone banking in community hospitals. In: Aebi M., Regazzoni P., eds. Bone Transplantation. Berlin, Heidelberg: Springer, 1989, p. 151 [Google Scholar]
- 5.Mok D., Lessard L., Cordoba C., Harris P.G., and Nikolis A. A review of materials currently used in orbital floor reconstruction. Can J Plast Surg 12, 134, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plecko M., Sievert C., Andermatt D., Frigg R., Kronen P., Klein K., Stubinger S., Nuss K., Burki A., Ferguson S., Stoeckle U., and von Rechenberg B. Osseointegration and biocompatibility of different metal implants—a comparative experimental investigation in sheep. BMC Musculoskelet Disord 13, 32, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sansone V., Pagani D., and Melato M. The effects on bone cells of metal ions released from orthopaedic implants. A review. Clin Cases Miner Bone Metab 10, 34, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee M., and Wu B.M. Recent advances in 3D printing of tissue engineering scaffolds. Methods Mol Biol 868, 257, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Lambertz A., Vogels R.R., Binnebosel M., Schob D.S., Kossel K., Klinge U., Neumann U.P., and Klink C.D. Elastic mesh with thermoplastic polyurethane filaments preserves effective porosity of textile implants. J Biomed Mater Res A 103, 2654, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Mi H.Y., Palumbo S., Jing X., Turng L.S., Li W.J., and Peng X.F. Thermoplastic polyurethane/hydroxyapatite electrospun scaffolds for bone tissue engineering: effects of polymer properties and particle size. J Biomed Mater Res B Appl Biomater 102, 1434, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Erfinder, w.s.g.w. Polymergemisch für den 3D Druck zur Erzeugung von Objekten mit Poren-Strukturen Polymer mixture for the pressure for generating 3D objects with pore structures. Parthy, Kai, Patent No. 50823 (DE); 2015
- 12.Henning S., Håvard J.H., Roya S., and Erich W. Fibroblastic response and surface characterization of O 2-plasma-treated thermoplastic polyetherurethane. Biomed Mater 5, 025002, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Mi H.-Y., Jing X., Salick M., Cordie T., Peng X.-F., and Turng L.-S. Morphology, mechanical properties, and mineralization of rigid thermoplastic polyurethane/hydroxyapatite scaffolds for bone tissue applications: effects of fabrication approaches and hydroxyapatite size. J Mater Sci 49, 2324, 2014 [Google Scholar]
- 14.Kokubo T., and Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 27, 2907, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Boone P.S., Zimmerman M.C., Gutteling E., Lee C.K., Parsons J.R., and Langrana N. Bone attachment to hydroxyapatite coated polymers. J Biomed Mater Res 23, 183, 1989 [PubMed] [Google Scholar]
- 16.Dong Z., Li Y., and Zou Q. Degradation and biocompatibility of porous nano-hydroxyapatite/polyurethane composite scaffold for bone tissue engineering. Appl Surf Sci 255, 6087, 2009 [Google Scholar]
- 17.Bergmeister H., Seyidova N., Schreiber C., Strobl M., Grasl C., Walter I., Messner B., Baudis S., Frohlich S., Marchetti-Deschmann M., Griesser M., di Franco M., Krssak M., Liska R., and Schima H. Biodegradable, thermoplastic polyurethane grafts for small diameter vascular replacements. Acta Biomater 11, 104, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Saito T., Teraoka K., and Ota K. Arrayed three-dimensional structures designed to induce and maintain a cell pattern by a topographical effect on cell behavior. Mat Sci Eng C Mater Biol Appl 49, 256, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Mitra J., Tripathi G., Sharma A., and Basu B. Scaffolds for bone tissue engineering: role of surface patterning on osteoblast response. RSC Adv 3, 11073, 2013 [Google Scholar]
- 20.Zhu P., Masuda Y., and Koumoto K. The effect of surface charge on hydroxyapatite nucleation. Biomaterials 25, 3915, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Kokubo T., Hanakawa M., Kawashita M., Minoda M., Beppu T., Miyamoto T., and Nakamura T. Apatite-forming ability of alginate fibers treated with calcium hydroxide solution. J Mater Sci Mater Med 15, 1007, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Castro N., Patel R., and Zhang L. Design of a novel 3D printed bioactive nanocomposite scaffold for improved osteochondral Regeneration. Cell Mol Bioeng 8, 416, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castro N.J., O'Brien C.M., and Zhang L.G. Biomimetic biphasic 3-D nanocomposite scaffold for osteochondral regeneration. Aiche J 60, 432, 2014 [Google Scholar]
- 24.Castro N.J., O'Brien J., and Zhang L.G. Integrating biologically inspired nanomaterials and table-top stereolithography for 3D printed biomimetic osteochondral scaffolds. Nanoscale 7, 14010, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang M., Castro N.J., Li J., Keidar M., and Zhang L.G. Greater osteoblast and mesenchymal stem cell adhesion and proliferation on titanium with hydrothermally treated nanocrystalline hydroxyapatite/magnetically treated carbon nanotubes. J Nanosci Nanotechnol 12, 7692, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Uddin S.M.Z., and Qin Y.X. Enhancement of Osteogenic Differentiation and Proliferation in Human Mesenchymal Stem Cells by a Modified Low Intensity Ultrasound Stimulation under Simulated Microgravity. PLoS One 8, e73914, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sumanasinghe R.D., Osborne J.A., and Loboa E.G. Mesenchymal stem cell-seeded collagen matrices for bone repair: effects of cyclic tensile strain, cell density, and media conditions on matrix contraction in vitro. J Biomed Mater Res A 88, 778, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Wang F.S., Wang C.J., Huang H.J., Chung H., Chen R.F., and Yang K.D. Physical shock wave mediates membrane hyperpolarization and ras activation for osteogenesis in human bone marrow stromal cells. Biochem Biophys Res Commun 287, 648, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Wu W., Ye Z.Y., Zhou Y., and Tan W.S. AICAR, a small chemical molecule, primes osteogenic differentiation of adult mesenchymal stem cells. Int J Artif Organs 34, 1128, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Zhu W., Boachie-Adjei O., Rawlins B.A., Frenkel B., Boskey A.L., Ivashkiv L.B., and Blobel C.P. A novel regulatory role for stromal-derived factor-1 signaling in bone morphogenic protein-2 osteogenic differentiation of mesenchymal C2C12 cells. J Biol Chem 282, 18676, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Notoya K., Nagai H., Oda T., Gotoh M., Hoshino T., Muranishi H., Taketomi S., Sohda T., and Makino H. Enhancement of osteogenesis in vitro and in vivo by a novel osteoblast differentiation promoting compound, TAK-778. J Pharmacol Exp Ther 290, 1054, 1999 [PubMed] [Google Scholar]
- 32.Znidarsic W.J., Chen I.W., and Shastri V.P. Influence of surface charge and protein intermediary layer on the formation of biomimetic calcium phosphate on silica nanoparticles. J Mater Chem 22, 19562, 2012 [Google Scholar]
- 33.Wolff I. A Pathway to Approval for Additive-Made Devices. Manufacturing Engineering: Advanced Manufacturing Media, ManufacturingEngineeringMedia.com, April 2014




