Abstract
Bone is a highly vascularized tissue and efficient bone regeneration requires neovascularization, especially for critical-sized bone defects. We developed a novel hybrid biomaterial comprising nanocalcium sulfate (nCS) and fibrin hydrogel to deliver mesenchymal stem cells (MSCs) and angiogenic factors, vascular endothelial growth factor (VEGF) and fibroblast growth factor 9 (FGF9), to promote neovascularization and bone formation. MSC and growth factor(s)-loaded scaffolds were implanted subcutaneously into mice to examine their angiogenic and osteogenic potential. Micro CT, alkaline phosphatase activity assay, and histological analysis were used to evaluate bone formation, while immunohistochemistry was employed to assess neovessel formation. The presence of fibrin preserved the nCS scaffold structure and promoted de novo bone formation. In addition, the presence of bone morphogenic protein 2-expressing MSC in nCS and fibrin hydrogels improved bone regeneration significantly. While FGF9 alone had no significant effect, the combination FGF9 and VEGF conjugated in fibrin enhanced neovascularization and bone formation more than 4-fold compared to nCS with MSC. Overall, our results suggested that the combination of nCS (to support bone formation) with a fibrin-based VEGF/FGF9 release system (support vascular formation) is an innovative and effective strategy that significantly enhanced ectopic bone formation in vivo.
Introduction
Critical-sized bone defects (CSBDs) are wounds that cannot be spontaneously bridged and result in the formation of fibrous connective tissue rather than bone when left untreated.1 Clinical therapies of CSBDs represent a great challenge for orthopedic and craniomaxillofacial surgeons, because current treatments rely on grafting materials such as autografts, allografts, or xenografts. However, recent advancements in stem cell-based bone repair and regeneration have shown great promise in animal models and clinical studies.
Although the treatment of bone defects using mesenchymal stem cells (MSCs) can effectively promote bone regeneration in human and animal models, the lack of blood vessels in the grafts prevents sufficient nutritional support to the entire bone graft.1,2 Bone is a highly vascularized tissue, but vessel penetration is difficult after bone formation. Therefore, a critical aspect for in vivo bone regeneration is vascularization.1 It is of interest to develop a biomaterial or scaffold capable of promoting angiogenesis as the bone begins to form. Such a scaffold could be used to promote bone healing in larger defects in which vessel formation would be a major limitation.
MSCs have been used extensively to promote bone formation. Recent work from our laboratory demonstrated that bone marrow-derived MSCs (BM-MSCs) engineered to overexpress bone morphogenic protein 2 (BMP2) have greater bone formation potential than native MSCs.3,4 BMPs are potent morphogens that stimulate bone formation in developed tissues5,6 and one member of the family, BMP2, has shown promising results in orthopedic clinical trials. In addition to soluble signals, the mechanical properties of the scaffold have been shown to affect bone formation and surface stiffness has been shown to affect stem cell fate,7–10 with harder/stiffer surfaces promoting osteogenic differentiation of MSCs.9 To this end, our laboratory has recently shown the ability of calcium sulfate nanoparticles (nCS) to promote osteogenic differentiation.3,4 We developed an injectable and porous nanocalcium sulfate/alginate (nCS/A) scaffold and demonstrated that nCS/A is biocompatible and promotes bone regeneration.3,4
In addition to mechanical properties, tuning the angiogenic properties of scaffolds is essential for promoting tissue regeneration, especially for highly vascularized tissues such as bone.1,11–13 Decorating the scaffold with angiogenic factors may be one strategy to enhance angiogenesis. The best-known angiogenic factor is vascular endothelial growth factor (VEGF), a potent mitogen for endothelial cells and a major inducer of angiogenesis, which has been extensively used to promote vascularization in vivo.14–17 However, neovessel formation through the sole use of VEGF is often unstable as neovessels degrade quickly.18 Other factors have been used to stabilize neovessels and promote vessel maturation, thereby complementing the actions of VEGF. One such factor is fibroblast growth factor 9 (FGF9), which was shown to induce proliferation, migration, and spreading of multiple cell types, including glial cells, fibroblasts, and smooth muscle cells (SMCs).19,20 It has been previously shown that when FGF9 and FGF2 (another angiogenic growth factor) were used to induce angiogenesis, the neovessels contained a layer of endothelial cells that were completely enwrapped in SMCs; in addition, vessels were larger in diameter, contractile, had higher flow rates, and remained active for longer than 1 year.18 Therefore, FGF9 promoted angiogenesis not by promoting endothelial proliferation, but rather recruitment, proliferation, and wrapping of SMCs, ultimately stabilizing the neovessels.
Fibrin has been used extensively as a scaffold for tissue regeneration21,22 and controlled delivery of growth factors to accelerate wound healing23–26 and promote vascularization.27–30 In addition, previous studies developed methods to conjugate growth factors into fibrin hydrogels through the action of FXIII and the fibrin-binding peptide, NQEQVSP.31 Previously our group has utilized this method to conjugate growth factors, which are then released to the tissues in a cell-controlled manner, thereby increasing the effectiveness of delivery to the local microenvironment. Specifically, delivery of keratinocyte growth factor improved the time of skin wound healing dramatically, from 2 to 1 week.32 In addition, immobilization of TGF-β1 into fibrin led to prolonged phosphorylation of Smad2, which was accompanied by increased function that is, contractility of SMCs embedded within the hydrogels.33
In this study, we capitalized on previous developments in our laboratories to develop a hybrid biomaterial comprising nCS and fibrin to deliver BMP2-producing MSCs and angiogenic growth factors, VEGF and FGF9, to induce robust angiogenesis and enhance bone regeneration.
Materials and Methods
Production of recombinant fusion proteins
VEGF cloning and protein production
The glutathione-S-transferase (GST)-NQ-VEGF plasmid was generated by subcloning the VEGF-165 gene with N-terminal primer additions to include an NQEQVSP peptide sequence and GGGS peptide linker sequence before the second amino acid in the VEGF-165 gene into a pGEX-2TK plasmid (GE Healthcare). This plasmid encodes for a thrombin-cleavable GST tag followed by the fusion protein (NQ-VEGF). For protein production, bacterial strain Escherichia coli BL21-DE3-pLysis was used, which was kindly provided by Dr. Sriram Neelamegham of the University at Buffalo, SUNY. Bacteria were expanded until optical density (OD) = 0.8, then induced with 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) for protein production for 4–6 h at 37°C and 300 rpm. The bacteria were pelleted at 20,000 g for 30 min. Bacterial pellets were resuspended in a lysis buffer (50 mM Tris, 500 mM NaCl, 1 mM ethylenediaminetetraacetic acid [EDTA], 1 mg/mL lysozyme, and protease inhibitors, pH 8.5) and Triton X-100 was added at 1% before sonication. Sonication consisted of 10 cycles with 70% intensity, 30 s on/30 s off. Sonicated lysates were clarified by ultracentrifugation at 50,000 g for 30 min. Insoluble material consisting mostly of inclusion bodies was subjected to numerous rounds of washing and sonication. The final, washed inclusion body pellet was resuspended in a solubilization buffer (50 mM Tris, 500 mM NaCl, 7 M Urea, 1 M Guanidine-HCl, 1 mM EDTA, 100 mM dithiothreitol [DTT], pH 8.5) before refolding by dialysis. Briefly, solubilized GST-NQ-VEGF was immediately added to a dialysis membrane (SpectraPor-1 6–8 kDa cut-off) and dialyzed in the refolding buffer-1 (50 mM Tris, 500 mM NaCl, 10 mM KCl, 1 mM EDTA, 2 M Urea, 500 mM L-Arginine, 5 mM reduced glutathione, 0.5 mM oxidized glutathione, pH 8.5) for 24 h. The volume of the refolding buffer was 100 × the volume of solubilized GST-NQ-VEGF. Each subsequent day, the refolding buffer was replaced with half the urea concentration of refolding buffer-1 used in the previous day for 3 days. The final dialysis step was performed in phosphate-buffered saline (PBS). Refolding success was determined by homodimer formation as analyzed by 10% SDS-PAGE with and without reducing agent DTT. Properly refolded GST-NQ-VEGF has an apparent molecular weight of 95–110 kDa, which reduces to 55 kDa upon DTT treatment. Refolded GST-NQ-VEGF was then subjected to sequential purification using GST agarose beads (Sigma), thrombin cleavage of GST from NQ-VEGF, and a final purification step by passing cleaved VEGF through according to the manufacturer's instructions.
FGF9 cloning and protein production
The pET28a-NQ-FGF9 plasmid was generated using the pET28a a HiTrap Heparin Column (GE Healthcare) plasmid (Clonetech) and further subcloning the FGF9 gene modified by N-terminal primer extension to include the NQEQVSP peptide sequence followed by a GGGS peptide linker before the second amino acid of the FGF9 gene. Bacterial strain E. coli BL21-DE3-pLysis containing the pET28a-NQ-FGF9 plasmid was then expanded until OD = 0.8, and then induced with 1 mM IPTG for protein production for 4–6 h at 37°C and 300 rpm. The bacteria was pelleted at 20,000 g for 30 min. Bacterial pellets were resuspended in the lysis buffer and Triton X-100 was added at 1% before sonication. Sonication consisted of 10 cycles with 70% intensity, 30 s on/30 s off. Sonicated lysates were clarified by ultracentrifugation at 50,000 g for 30 min. The soluble fraction contained the vast majority of the His-NQ-FGF9, which was then subjected to further purification through Ni+ affinity columns (Histrap; GE), thrombin cleavage of the His-tag, and a final purification step of reapplying the cleaved NQ-FGF9 to the affinity column to bind the free His-tag. NQ-FGF9 has an apparent molecular weight of ∼35 kDa.
Cell culture
Human umbilical vein endothelial cells (HUVECs) were purchased as a pooled donor isolation, maintained in the EGM2 complete medium (Lonza) below 75% confluence, and used between passage 2 and 6. NIH-3T3 fibroblasts were purchased from American Type Culture Collection (ATCC) and maintained in DMEM supplemented with 10% bovine serum (Life Technologies).
Biological activity of recombinant proteins
NQ-VEGF
The biological activity of recombinant NQ-VEGF was assessed using a standard cell proliferation assay. To this end, HUVECs were seeded onto a 96-well plate at a density of 2 × 103 cells per well in the M199 medium (Life Technologies), supplemented with 2% heat-inactivated fetal bovine serum (FBS; Life Technologies) and varying concentrations of recombinant NQ-VEGF or commercial VEGF (Cell Signaling) that was used as control. Concentrations ranged from 0.05 to 100 ng/mL. Cells were allowed to proliferate for 72 h before treatment with 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT; Life Technologies) for 4 h. Then, the medium was carefully removed and replaced with 100 μL of DMSO to solubilize the purple formazan crystals. Absorbance was read at 570 nm using a Biotek Synergy four Spectrophotometer with background absorbance at 650 nm subtracted. A total of three independent biological replicates were assessed.
NQ-FGF9
The biological activity of recombinant NQ-FGF9 was determined using the same methods described in 2.3.1 NQ-VEGF with NIH-3T3 cells instead. A total of three independent biological replicates were assessed.
Release kinetics from fibrin hydrogels
Fibrin hydrogels were polymerized in 96-well plates that were pre-coated with 2% bovine serum albumin (BSA; 50 μL per well) and covered by DMEM/0.1% BSA (100 μL per well). Each hydrogel contained 1 mg/mL fibrinogen, 2.5 U/mL thrombin, 2.5 mm Ca2+, various concentrations of FXIII, and 100 ng/mL of NQ-VEGF or NQ-FGF9. Native VEGF and FGF9 were used as controls, respectively. The medium was collected at the indicated times and stored at −20°C until use. The VEGF or FGF9 concentration of each sample was determined by ELISA according to manufacturer's recommendations (Duoset; R&D systems). A total of three independent biological replicates were assessed per growth factor.
Preparation of BMP2 genetically engineered mouse MSCs (M/B2)
MSCs were isolated from the 6-week-old C57 mice as described before.34,35 Briefly, bone marrow was collected by flushing the diaphysis of long bone with alpha minimum essential medium (alpha-MEM; Life Technologies) and allowed growth to ∼80% confluence in alpha-MEM supplemented with 10% FBS, l-glutamine (2 mM), and penicillin (100 U/mL). Cells were passaged for subsequent passages until passage 4 for preparation of the pure MSC population. The cells used for all experiments were within 10 passages.
BMP2 encoding adenovirus (Ad-BMP2) was produced as previously described.3 MSCs were infected with Ad-BMP2 in a serum-free medium for 4 h and then cultured overnight in the alpha-MEM medium with 2% FBS.4
Scaffold preparation
nCS was produced following the method reported previously.36,37 Briefly, alginate was dissolved in PBS to prepare 10% alginate solution (pH 7.2). The nCS/A disk was formulated by 67.5 mg nCS powder with 75 μL alginate. The disks were allowed to air dry for at least 24 h.
To encapsulate the nCS disk in the various fibrin conditions, nCS disks were placed in 2% BSA-coated 48-well plates directly in the center of the well. Fibrinogen (5 mg/mL) was then added around the nCS disk with immediate addition of the polymerization mix (1 plasma equivalent unit [PEU] FXIII, 50 U/mL thrombin, and 50 mM Ca2+) with mixing to ensure rapid polymerization of the fibrinogen around the nCS.
Scaffold conditions are summarized in Table 1. For the groups of nC/FbM, nC/FbM-V, nC/FbM-F, and nC/FbM-VF, 2 × 106 M/B2 (M) were mixed with fibrin. The scaffold with cells was kept in the complete medium overnight. For the groups of nCM and nCM/Fb, 25 μL cell suspension with 2 × 106 M/B2 was loaded directly to the nCS disk right before the surgery. For the groups of nCM/FbM, nCM/FbM-V, nCM/FbM-F, and nCM/FbM-VF, 1 × 106 M/B2 was loaded during Fb preparation and 1 × 106 M/B2 was loaded into nCS. For growth factor delivery into the Fb, 100 ng/mL of the NQ-VEGF and/or NQ-FGF9 was added during the fibrin encapsulation of the nCS.
Table 1.
Summary of Implant Conditions and Abbreviations
| Group | MSC location | Growth factors |
|---|---|---|
| nC/Fb | None | None |
| nCM | nCS | None |
| nCM/Fb | nCS | None |
| nC/FbM | Fibrin | None |
| nC/FbM-V | Fibrin | NQ-VEGF |
| nC/FbM-F | Fibrin | NQ-FGF9 |
| nC/FbM-VF | Fibrin | NQ-VEGF+ NQ-FGF9 |
| nCM/FbM | nCS+Fibrin | None |
| nCM/FbM-V | nCS+Fibrin | NQ-VEGF |
| nCM/FbM-F | nCS+Fibrin | NQ-FGF9 |
| nCM/FbM-VF | nCS+Fibrin | NQ-VEGF+ NQ-FGF9 |
FGF9, fibroblast growth factor 9; MSC, mesenchymal stem cell; nCS, nanocalcium sulfate; VEGF, vascular endothelial growth factor.
Implantation
Animal procedures were conducted in accordance with the protocol approved by IACUC of the University at Buffalo. A total of 44 C57/BL6 mice (8-week old) were divided into eleven groups (four mice per group) as shown in Table 1. Mice were anesthetized with 1 L/min oxygen and 5% isoflurane by inhalation. The incision sites were shaved and cleaned followed by two longitudinal skin incision of about 0.5–0.6 cm in length. Subcutaneous pockets were formed by blunt dissection and a single transplant was placed into each pocket (n = 8 implants per group). The skin was sutured and carprofen was given for 2 days postsurgery. Eight weeks after implantation, the mice were euthanized by carbon dioxide inhalation and the implants were retrieved.
Micro-CT
The harvested implants were fixed and scanned using a custom-built micro-CT system (n = 4 for each group). The data were analyzed with MicroView 3D Image Viewer & Analysis Tool (GE Medical Systems).
Scanning electron microscopy
The samples were fixed in 2% glutaraldehyde in PBS for 1.5 h at 4°C. The samples were then rinsed with PBS twice and dehydrated in ethanol as follows: 30%, 50%, 70%, 85%, 95%, 100%, and 100% ethanol for 15 min of each. The samples were treated with 100% hexamethyldisilazane and air dried overnight. The scaffolds were sputter coated with carbon and measured by the Hitachi SU70 scanning electron microscope (Hitachi Instruments).
Alkaline phosphatase activity assay
The alkaline phosphatase (ALP) activity assay was performed as previously described with slight changes.38,39 Briefly, cells were induced for osteogenic differentiation for 7 days with an osteogenic medium, which was α-MEM containing 10% FBS, 10 mM β-glycerophosphate (Sigma), 50 μg/mL ascorbic acid (Sigma), and 10−8 M dexamethasone (Sigma). Cells were harvested with a harvest buffer (10 mM Tris-Cl [pH 7.4], 0.2% NP40, and 2 mM PMSF), homogenized, and centrifuged. The supernatants (30 μL) were mixed with 50 μL assay buffer (100 mM glycine and 1 mM MgCl2, pH 10.5) and 20 μL p-nitrophenyl phosphate solution (50 mM in 0.1 M glycine buffer), and incubated at 37°C for 5–10 min. NaOH was used to stop the reaction. The optical density was measured at 405 nm with AD 340 microplate reader (Beckman Coulter). Protein concentration was measured with the BCA protein assay kit (Pierce). The ALP activity was normalized to protein content and expressed as nmol of p-nitrophenol produced per minute per gram of total protein. A total of three independent biological replicates were assessed.
For the implanted samples, the ectopically formed bones were smashed in liquid nitrogen and lysed in 1 mL harvest buffer, followed by homogenization at low power. After centrifugation at 3300 rpm for 15 min, 30 μL supernatant was taken for the ALP activity assay.
Histological analysis and blood vessel analysis
Specimens were fixed and decalcified in 10% EDTA for 1 week. The samples were embedded with paraffin, cut into 5 μm sections, and stained with hematoxylin and eosin. The sections were stained for CD31 and CD144 to visualize the endothelium of newly formed blood vessels. Sections were incubated with primary antibodies for rat anti CD31 (1:100; Santa Cruz Biotechnology) or rabbit anti CD144 (1:500; Cell Signalling) at 4°C overnight, followed by incubation of secondary goat anti-rat or anti-rabbit antibody conjugated with Alexa-Fluor 594 and 488 (1:200; Thermo Fisher Scientific) for 1 h. Hoescht dye was then used (1:200) to visualize the nuclei. Blood vessels, indicated by the staining, were counted from a total of 48 images per group (12 images per n = 4 sections per group) manually at 20 × magnification and normalized to the implant area. Measurement of vessel diameter was performed with the use of AxioVision Software.3,4
Statistical analysis
Statistical analysis was performed with GraphPad Software. Data are reported as mean ± scanning electron microscopy (SEM). Data were analyzed with one-way analysis of variance followed by Tukey's test. A value of p < 0.05 was considered statistically significant.
Results
Preparation of fusion protein NQ-FGF9 and NQ-VEGF
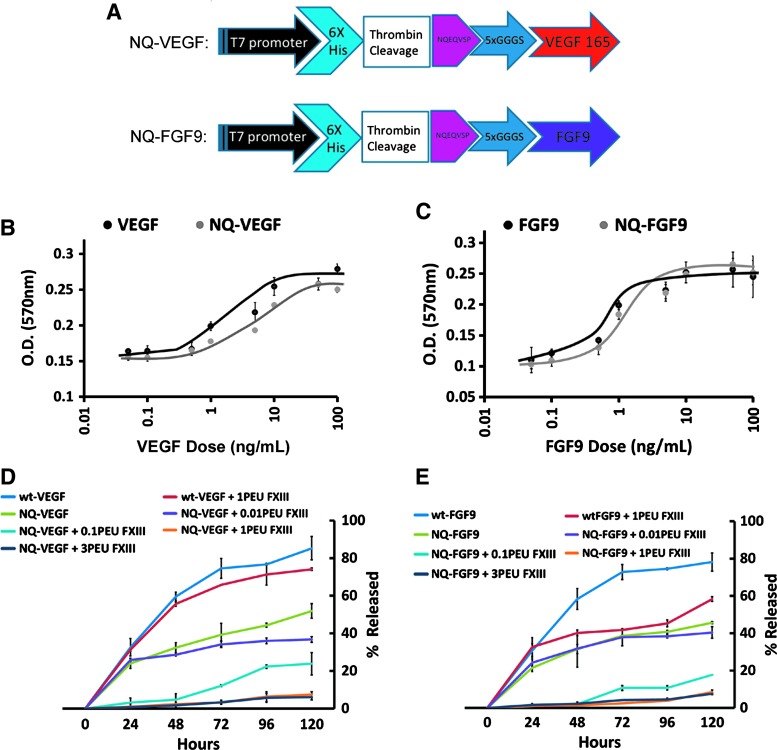
NQ-VEGF was produced as a recombinant histidine-tagged protein with an N-terminal peptide sequence NQEQVSP in E. coli (Fig. 1A). As described in Materials and Methods section, NQ-VEGF expression was induced with IPTG and the protein was harvested as insoluble inclusion bodies followed by dialysis refolding and downstream purification. Typical yields ranged from 5 to 10 mg/L of culture. The biological activity was determined to match that of commercially available VEGF by the HUVEC proliferation assay, with a median effective dose (ED 50) of ∼5 ng/mL (Fig. 1B). FGF9 was produced as a fusion protein (NQ-FGF9) similar to NQ-VEGF (Fig. 1A). NQ-FGF9 expression was also induced with IPTG, but the protein was harvested through His-tag purification of the soluble fraction. Typical NQ-FGF9 yields ranged from 10 to 20 mg/L of culture. The biological activity was determined to match that of commercially available FGF9 by the NIH-3T3 proliferation assay with an ED 50 of ∼1 ng/mL (Fig. 1C).
FIG. 1.
Recombinant VEGF and FGF9 production. (A) Fusion protein NQ-FGF9 and NQ-VEGF plasmid schematic. (B) Human umbilical vein endothelial cell proliferation response is dose dependent on concentration of wt-VEGF and NQ-VEGF as assessed by MTT analysis. (C) NIH-3T3 cell proliferation in response to wt-FGF9 and NQ-FGF9 as assessed by MTT analysis. (D,E) Release kinetics of NQ-VEGF (D) and FGF9 (E) from fibrin hydrogels over 120 h. Statistics, n = 3 independent biological replicates. FGF9, fibroblast growth factor 9; VEGF, vascular endothelial growth factor. Color images available online at www.liebertpub.com/tea
In vitro release kinetics of fusion protein NQ-VEGF and NQ-FGF9
To assess the fibrin-binding capacity of the NQEQVSP leader sequence on both recombinant proteins, the fusion proteins were prepared into 5 mg/mL fibrin hydrogels and the release was assessed every 24 h for 5 days. The peptide sequence NQEQVSP has been previously shown to be covalently incorporated into fibrin hydrogels by the action of FXIII during fibrin polymerization (31). After polymerization, the kinetics of growth factor release in the media was determined over a period of 120 h using ELISA (Fig. 1D, E). Maximum release of 82.3% ± 6.3% and 78.1% ± 4.9% (n = 3) were observed for wt-VEGF and wt-FGF9, respectively (Fig. 1D, E, blue lines). The addition of the NQEQVSP peptide sequence decreased growth factor release in an FXIII concentration-dependent manner. At the maximum concentration of three PEU FXIII, the amount of growth factor release was 6.1% ± 1.5% and 7.5% ± 0.53% (n = 3) for NQ-VEGF and NQ-FGF9, respectively.
In vitro bioactivity of NQ-VEGF and NQ-FGF9
Next, we assessed the effects of NQ-VEGF and NQ-FGF9 on M/B2. As expected, M/B2 proliferation increased in a dose-dependent manner with the addition of NQ-FGF9 (Fig. 2A; black circle), but not induced with NQ-VEGF (gray triangle). The combination of both NQ-VEGF and NQ-FGF9 had the same effect as NQ-FGF9 alone. In addition, we studied the osteogenic differentiation potential of M/B2 upon addition of NQ-FGF9 (Fig. 2B). M/B2 cells have a significantly higher ALP activity (67.6 ± 2.6 U/min/g) compared with wild-type BM-MSCs (M) (8.025 ± 0.8 U/min/g) (n = 3, p < 0.05). NQ-FGF9 had a small inhibitory effect on the osteogenic differentiation of wild-type M cells, but no statistically significant effect on M/B2 cells (n = 3, p > 0.05), even at the very high concentration of 100 ng/mL.
FIG. 2.
Growth factor affect on engineered bone morphogenic protein 2 expressing BM-MSCs. (A) FGF9 and the combination of VEGF and FGF9 induce proliferation in B2/M, whereas VEGF alone does not induce proliferation as assessed by MTT assay, indicating that the proliferation response is mediated by FGF9 alone (n = 3). (B) Osteogenic differentiation as assessed by alkaline phosphatase activity is inhibited in wt-BM-MSCs in response to FGF9, whereas B2-BM-MSC differentiation is not statistically different in FGF9 treated (100 ng/mL) versus nontreated. n = 3 independent biological replicates, *p < 0.01, **p < 0.0001. BM-MSCs, bone marrow-derived mesenchymal stem cells.
Preparation of scaffolds and SEM analysis
To create a scaffold capable of both ectopic bone formation and angiogenesis, we combined the previously established osteogenic inducing material, nanocalcium sulfate (nCS),3,4 with fibrin hydrogels containing the fusion proteins NQ-VEGF and NQ-FGF9, as well as M/B2 cells. This hybrid biomaterial contained a rigid surface for bone formation (nCS) and a soft porous material for angiogenesis and immobilization of the engineered fusion proteins (fibrin, Fb).
Next, we assessed multiple combinations of these hybrid biomaterials that varied in the presence and location of M/B2 cells and the presence of NQ-VEGF, NQ-FGF9, or both within the fibrin. Scaffold preparation was described in Materials and Methods section and is also shown in Figure 3A. The size and shape of nCS disks and fibrin-encapsulated nCS are shown in Figure 3B. A table summarizing the 11 groups with abbreviations used in this study is included as Table 1.
FIG. 3.
Scaffold preparation. (A) Schematic of nCS-fibrin plug preparation, MSC loading, and VEGF/FGF9 conjugating. nC, nanocalcium sulfate; Fb, fibrin; F, FGF9; V, VEGF; M, MSCs. (B) Representative images of nCS disks before and after fibrin encapsulation, and subcutaneous implantation. (C) Scanning electron microscopy view of nanocalcium sulfate (nCS), fibrin (Fb), MSC seeding on fibrin, and nanocalcium sulfate/fibrin mixture (nC/Fb). Color images available online at www.liebertpub.com/tea
SEM analysis was performed on the various parts of the hybrid biomaterial (Fig. 3C). There was a clear distinction between nCS and fibrin composition, and MSCs could easily adhere to the surface of fibrin.
Ectopic bone formation of scaffolds in mice
Implantations were performed in C57BL/6 8-week-old mice in the forelimb subcutaneous pockets as described in Materials and Methods section (Fig. 3B). In total, there were eight hybrid scaffolds per group using four mice (two scaffolds per mouse). Hybrid scaffolds were implanted for a total of 8 weeks before euthanasia and analysis. As shown in X-ray images, ectopic bone formation was evident in all (see arrows), but the nC/Fb group, which is the only group without M/B2 cells (Fig. 4A). Scaffolds containing both VEGF and FGF9 (nC/FbM-VF and nCM/FbM-VF) showed robust ectopic bone formation.
FIG. 4.
X-Ray of 2-month postimplant bone growth. Representative images, arrow indicates newly formed bone. Color images available online at www.liebertpub.com/tea
Furthermore, micro-CT analysis indicated that groups with M/B2 within both the nCS and fibrin components of the hybrid scaffolds (nCM/FbM, nCM/FbM-V, nCM/FbM-F, and nCM/FbM-VF) developed more bone mass than groups containing M/B2 cells only in the fibrin (nC/FbM, nC/FbM-V, nC/FbM-F, and nC/FbM-VF) or only in the nCS (nCM/Fb) component (n = 4 scaffolds) (Fig. 5A, B). In addition, very little bone formation was observed in the completely noncellular control (nCS/Fb) with a bone volume to tissue volume (total implant volume) ratio (BV/TV) of 12.5 ± 2.4 (n = 4).
FIG. 5.
Analysis of explanted tissue. (A) Reconstructed 3D micro-CT images of representative samples. (B) Quantification of bone volume/tissue volume (BVT/TV) from micro-CT result (n = 4). (C) ALP Activity of explanted bone tissue (n = 4). ALP, alkaline phosphatase. Color images available online at www.liebertpub.com/tea
The growth factor varied in their contribution toward new bone growth. Less bone was formed in all groups treated with only FGF9 (BV/TV of 50 ± 4.5 and 117 ± 4.4 for nC/FbM-F and nCM/FbM-F, respectively), compared to the groups treated with only VEGF (BV/TV of 93 ± 23.2 and 161 ± 8.1 for nC/FbM-V and nCM/FbM-V, respectively). However, in the presence of both FGF9 and VEGF, there was significantly more bone formation than in groups with either FGF9 or VEGF (BV/TV of 159 ± 19.4 and 207 ± 9.2 for nC/FbM-VF and nCM/FbM-VF, respectively). Indeed, the highest bone volume was observed when the hybrid scaffold incorporated both VEGF and FGF9 in the fibrin, as well as M/B2 cells in the fibrin and nCS.
This trend was further verified after analyzing the explants for the ALP activity as shown in Figure 5C. The ALP activity further demonstrates the need for M/B2 in both the fibrin and nCS components of the hybrid scaffold. The ALP activity in nC/FbM (no cells in the nCS) was 36.7 ± 5.5 U/min/g protein compared to 60.2 ± 6.5 U/min/g protein (n = 4; p < 0.05) for nCM/FbM (cells were located in both the nCS and Fb). Indeed, all groups in which cells were present in only the Fb (nC/FbM, nC/FbM-V, nC/FbM-F, and nC/FbM-VF) or the nCS (nCM/Fb), regardless of the presence of growth factors, had a significantly lower ALP activity than scaffolds in which the cells were present in both the Fb and nCS (nCM/FbM, nCM/FbM-V, nCM/FbM-F, and nCM/FbM-VF). Furthermore, the addition of FGF9 within the Fb did not increase the ALP activity in constructs containing cells; (ALP of nCM/FbM-F: 61.7 ± 6.6 U/min/g protein; ALP of nCM/FbM: 60.2 ± 6.5 U/min/g protein [n = 4; p > 0.05]). However, the addition of VEGF (nCM/FbM-V: 79.1 ± 9.2 U/min/g protein) significantly improved the ALP activity over nCM/FbM alone (n = 4; p < 0.05). Furthermore, as seen with micro-CT, the combination of both VEGF and FGF9 within the Fb (nCM/FbM-VF) resulted in the highest ALP activity (98.5 ± 4.6 U/min/g protein) over any condition with a single growth factor, suggesting that FGF9 and VEGF improved bone formation in a synergistic manner.
Histological evaluation
Histological assessment of the explanted new bone by hematoxylin/eosin (Fig. 6A) and Goldner's trichrome staining (Fig. 6B) was also performed to further demonstrate ectopic bone formation. As expected, groups with M/B2 cells in both Fb and nCS, as well as both VEGF and FGF9 in the fibrin, resulted in best bone formation (nCM/FbM-VF). This was especially evident in Figure 6B where the dark gray/blue indicates the newly developed bone. In addition, the organization of the new bone was better defined in groups with M/B2 in both the nCS and Fb components (nCM/FbM, nCM/FbM-V, nCM/FbM-F, and nCM/FbM-VF).
FIG. 6.
Histological evaluation of newly formed bone. (A) Hematoxylin and Eosin Staining. (B) Goldner's trichrome staining of histological sections of implants at 8 weeks. Color images available online at www.liebertpub.com/tea
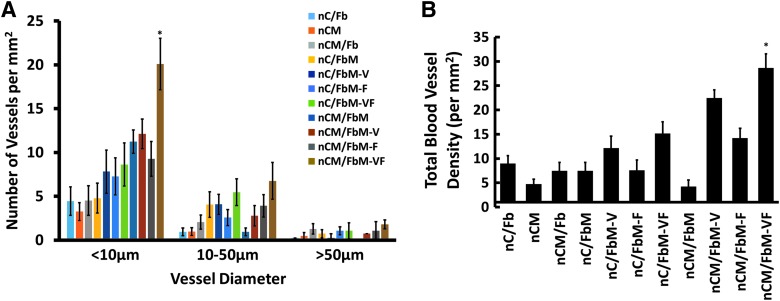
Further histological assessment to evaluate the extent of angiogenesis indicated a strong correlation between the number of blood vessels formed and the size/ALP activity of the newly formed bone (Figs. 7–9). Immunohistochemistry with CD31 and CD144 indicated that the addition of VEGF was crucial for blood vessel development, as expected (Figs. 7–9, nC/FbM-V and nCM/FbM-V). Quantification of the density of blood vessels (Fig. 7) confirmed this observation, with 22.4 ± 1.7 vessels/mm2 in nCM/FbM-V compared with only 4.2 ± 1.5 vessels/mm2 in nCM/FbM (n = 3, p < 0.05). Notably, the combination of both FGF9 and VEGF (nCM/FbM-VF) increased the number of blood vessels even further (28.6 ± 1.3 vessels/mm2) compared with either VEGF (nCM/FbM-V, 22.4 ± 1.7 vessels/mm2) or FGF9 alone (nCM/FbM-F, 14.2 ± 1.1 vessels/mm2). Furthermore, the addition of FGF9 increased the presence of medium-diameter (10–50 μm) microvessels and large-diameter (50 μm+) microvessels compared to VEGF alone (Fig. 7). In addition, gross anatomical images also showed that addition of angiogenic factor(s) was crucial for new vessel formation (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea). Specifically, the same trend observed with bone volume, ALP activity, and blood vessel density was also observed with the number and thickness of the blood vessels surrounding the explants.
FIG. 7.
Quantification of blood vessels within explanted tissues. Each tissue was assessed with anti-CD31 and anti-CD144 antibodies. *Represents statistical difference among all groups assessed. Twelve images were taken to cover the entire tissue section, with n = 4 sections per tissue explant. A total of 48 images were assessed per group and manually counted/measured using AxioVision Software to measure the diameter of vessels. (A) As assessed by vessel diameter measured using AxioVision Software. (B) Total blood vessel counts. Color images available online at www.liebertpub.com/tea
FIG. 8.
Immunohistochemistry of explanted ectopic bone. Representative images of CD31 (red), an endothelial marker and DAPI (blue). color images available online at www.liebertpub.com/tea
FIG. 9.
Immunohistochemistry of explanted ectopic bone. Representative images of CD144 (green), an endothelial marker and DAPI (blue). Color images available online at www.liebertpub.com/tea
Discussion
In this study, we have demonstrated the potential of our hybrid biomaterial for enhanced osteogenesis and angiogenesis for ectopic bone formation. Previously our group has demonstrated that the combination of nCS, BMP2-producing MSCs, and endothelial progenitor cells (EPCs) can promote vascularized bone formation.4 However, EPCs are a very rare cell type in the blood and isolation of these cells is time-consuming and costly.40 In addition, treatment with EPCs required the use of autologous cells to avoid immune rejection, thereby necessitating customized treatments. Therefore, the ability to induce angiogenesis during bone formation with slowly released growth factors is a major improvement. This is the first report demonstrating that the combination of the nCS/A with fibrin hydrogels of VEGF and FGF9, and BMP2-producing MSCs, can significantly promote vascularized bone regeneration.
In this study, nCS was employed as the primary scaffold matrix to promote osteogenesis. Calcium sulfate is a highly biocompatible material that has been used in clinical trials for a long time now. It is one of the simplest synthetic bone-like grafts. Upon dissolution, the calcium ions form calcium phosphate, which then precipitates on the surface of calcium sulfate, thereby providing an osteoblast-friendly environment.41 Previously, we showed that the presence of 10% alginate increased the strength and generated pores of nCS without decreasing cell viability.3 In addition, nCS combined with alginate was shown to promote effective cell proliferation and bone ingrowth.3
Fibrin was also used in this study to promote angiogenesis and as a scaffold for localized delivery of angiogenic factors. Fibrin has been extensively studied as a scaffold and a number of studies have shown that fibrin is a growth factor carrier for tissue regeneration.33,42 In addition, fibrin has also been used to deliver stem cells for bone tissue engineering.43,44 Ideally, incorporation of a vascularized network into engineered bone substitutes to mimic the structure of natural bone tissue is necessary to promote rapid bone regeneration.45 In this study, we demonstrated that growth factor-conjugated fibrin could be used to encapsulate our nCS scaffold to promote angiogenesis. We observed that nCS scaffolds became very soft upon implantation and are often disintegrated. However, fibrin-encapsulated nCS was observed to be much more stable following implantation (data not shown), suggesting that fibrin might have provided a protective layer for nCS scaffold in vivo.
MSCs are the most common stem cells used in bone tissue engineering because of their multilineage differentiation potential and the ability to regulate the local immune response.46,47 In our previous study, we demonstrated the ability of BMP2 genetically engineered MSCs to promote bone regeneration.3 BMP2, the most powerful osteogenic factor, was continuously expressed for at least 21 days in the BMP2 genetically engineered MSCs, which strongly promoted osteogenic differentiation.3 In this study, we found the optimal distribution of BMP2 genetically engineered MSCs within the scaffold. MSCs were incorporated into the fibrin hydrogel, and in some cases also directly loaded into the nCS scaffold. As expected, the group with M/B2 on nCS and fibrin induced the highest level of bone formation (nCM/FbM, nCM/FbM-V, nCM/FbM-F, and nCM/FbM-VF), suggesting that M/B2 might play different roles in nCS and fibrin. Although the mechanism is not currently clear, MSC on nCS might have differentiated into bone cells, while those in the fibrin scaffold might have promoted angiogenesis. More studies tracing MSCs in vivo are required to delineate the role(s) of each MSC population in bone formation in vivo.
To promote angiogenesis during bone formation, we engineered two fusion proteins, VEGF and FGF9, with the fibrin-binding sequence NQEQVSP to enable covalent binding to fibrin during polymerization. This approach has been successful in a wide array of scaffolds for the continual release of active growth factors,25,48 especially VEGF,25,28,49,50 however, this is the first time immobilized FGF9 in fibrin has been used. Previous work in our laboratory has shown that fibrin immobilized TGF-β1-promoted MSC differentiation to SMCs uniformly throughout fibrin rings, eliminating growth factor diffusion limitations.33,42 VEGF has been used to promote angiogenesis in many previous studies, however, neovessels formed only in the presence of VEGF have been shown to be unstable and degrade rapidly in vitro, and at a slower extent in vivo. This is likely due to the lack of pericytes and SMCs, which typically enwrap neovessels within the body.51
Previously, FGF9 was shown to promote long-lasting functional neovessels in vivo when used in conjunction with the angiogenic FGF2.18 Therefore, we hypothesized that FGF9 might also promote bone formation by stabilizing the newly formed blood vessels. Indeed, FGF9 increases bone formation and angiogenesis in the presence of VEGF, but not alone. In addition, groups containing FGF9 alone or in combination with VEGF contained larger diameter microvessels than in conditions with either no growth factors or VEGF alone. Immunostaining showed that explants with scaffold groups containing both VEGF and FGF9 (nC/FbM-VF and nCM/FbM-VF) exhibited the highest number of alpha actin-positive cells (SMCs), as well as the highest number of endothelial cells and vessel density. Consequently, the combination treatment enhanced ectopic bone formation significantly, likely due to improved nutrient delivery through the neovessels. Our findings therefore provide further evidence for the importance of FGF9-mediated neovessel stabilization.
Conclusions
In conclusion, we developed a novel hybrid biomaterial consisting of nCS and fibrin hydrogels. The optimal scaffold contained BMP2-producing MSCs on nCS as well as within fibrin, which was also decorated with immobilized VEGF and FGF9 to induce and stabilize neovessel formation. This type of bone-inducing scaffolds are more advantageous in combination of softer, more malleable materials in the outer surrounding layer and harder, more load-bearing materials in the inner center, which can fit all kinds of critical-sized bone defects, especially for clinical treatment-challenged irregular bone defects, while also markedly promoting large-size bone formation through enhanced angiogenesis.
Supplementary Material
Acknowledgments
We thank Dr. Peter J. Bush for technical assistance with scanning electron microscopy. This study was partially supported by the National Institute of Dental and Craniofacial Research and the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under Award Numbers DE023105 and AR066101 to S.Y., and the Mark Diamond Research Fund of the Graduate Student Association at the University at Buffalo, The State University of New York (Grant No. SU-14-17).
Disclosure Statement
No competing financial interests exist.
References
- 1.Kanczler J.M., and Oreffo R.O.C. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater 15, 100, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Lv J., Xiu P., Tan J., Jia Z., Cai H., and Liu Z. Enhanced angiogenesis and osteogenesis in critical bone defects by the controlled release of BMP-2 and VEGF: implantation of electron beam melting-fabricated porous Ti6Al4V scaffolds incorporating growth factor-doped fibrin glue. Biomed Mater 10, 035013, 2015 [DOI] [PubMed] [Google Scholar]
- 3.He X., Dziak R., Mao K., Genco R., Swihart M., Swithart M., Li C., and Yang S. Integration of a novel injectable nano calcium sulfate/alginate scaffold and BMP2 gene-modified mesenchymal stem cells for bone regeneration. Tissue Eng Part A 19, 508, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He X., Dziak R., Yuan X., Mao K., Genco R., Swihart M., Sarkar D., Li C., Wang C., Lu L., Andreadis S., and Yang S. BMP2 genetically engineered MSCs and EPCs promote vascularized bone regeneration in rat critical-sized calvarial bone defects. PLoS One 8, e60473, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahn S.E., Kim S., Park K.H., Moon S.H., Lee H.J., Kim G.J., Lee Y.J., Cha K.Y., and Chung H.M. Primary bone-derived cells induce osteogenic differentiation without exogenous factors in human embryonic stem cells. Biochem Biophys Res Commun 340, 403, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Snyder A., Fraser S.T., and Baron M.H. Bone morphogenetic proteins in vertebrate hematopoietic development. J Cell Biochem 93, 224, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Tee S.Y., Fu J., Chen C.S., and Janmey P.A. Cell shape and substrate rigidity both regulate cell stiffness. Biophys J 100, L25, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reinhart-King C.A., Dembo M., and Hammer D.A. Cell-cell mechanical communication through compliant substrates. Biophys J 95, 6044, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rowlands A.S., George P.A., and Cooper-White J.J. Directing osteogenic and myogenic differentiation of MSCs: interplay of stiffness and adhesive ligand presentation. Am J Physiol Cell Physiol 295, C1037, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Evans N.D., Minelli C., Gentleman E., LaPointe V., Patankar S.N., Kallivretaki M., Chen X., Roberts C.J., and Stevens M.M. Substrate stiffness affects early differentiation events in embryonic stem cells. Eur Cell Mater 18, 1, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Zhang W., Wang X., Wang S., Zhao J., Xu L., Zhu C., Zeng D., Chen J., Zhang Z., Kaplan D.L., and Jiang X. The use of injectable sonication-induced silk hydrogel for VEGF(165) and BMP-2 delivery for elevation of the maxillary sinus floor. Biomaterials 32, 9415, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y., Fan L., Liu S., Liu W., Zhang H., Zhou T., Wu D., Yang P., Shen L., Chen J., and Jin Y. The promotion of bone regeneration through positive regulation of angiogenic-osteogenic coupling using microRNA-26a. Biomaterials 34, 5048, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Cao L., Wang J., Hou J., Xing W., and Liu C. Vascularization and bone regeneration in a critical sized defect using 2-N,6-O-sulfated chitosan nanoparticles incorporating BMP-2. Biomaterials 35, 684, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Tischer E., Gospodarowicz D., Mitchell R., Silva M., Schilling J., Lau K., Crisp T., Fiddes J.C., and Abraham J.A. Vascular endothelial growth factor: a new member of the platelet-derived growth factor gene family. Biochem Biophys Res Commun 165, 1198, 1989 [DOI] [PubMed] [Google Scholar]
- 15.Risau W. Angiogenesis and endothelial cell function. Arzneimittelforschung 44, 416, 1994 [PubMed] [Google Scholar]
- 16.Tonnesen M.G., Feng X., and Clark R.A. Angiogenesis in wound healing. J Investig Dermatol Symp Proc 5, 40, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Ferrara N. VEGF: an update on biological and therapeutic aspects. Curr Opin Biotechnol 11, 617, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Frontini M.J., Nong Z., Gros R., Drangova M., O'Neil C., Rahman M.N., Akawi O., Yin H., Ellis C.G., and Pickering J.G. Fibroblast growth factor 9 delivery during angiogenesis produces durable, vasoresponsive microvessels wrapped by smooth muscle cells. Nat Biotechnol 29, 421, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Rosenquist T.A., and Martin G.R. Fibroblast growth factor signalling in the hair growth cycle: expression of the fibroblast growth factor receptor and ligand genes in the murine hair follicle. Dev Dyn 205, 379, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Ledoux D., Gannoun-Zaki L., and Barritault D. Interactions of FGFs with target cells. Prog Growth Factor Res 4, 107, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Clark R.A. Fibrin and wound healing. Ann N Y Acad Sci 936, 355, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Clark R.A. Fibrin sealant in wound repair: a systematic survey of the literature. Expert Opin Investig Drugs 9, 2371, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Pandit A.S., Wilson D.J., and Feldman D.S. Fibrin scaffold as an effective vehicle for the delivery of acidic fibroblast growth factor (FGF-1). J Biomater Appl 14, 229, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Breen A., Dockery P., O'Brien T., and Pandit A. Fibrin scaffold promotes adenoviral gene transfer and controlled vector delivery. J Biomed Mater Res Part A 89, 876, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Wong C., Inman E., Spaethe R., and Helgerson S. Fibrin-based biomaterials to deliver human growth factors. Thromb Haemost 89, 573, 2003 [PubMed] [Google Scholar]
- 26.Michlits W., Mittermayr R., Schafer R., Redl H., and Aharinejad S. Fibrin-embedded administration of VEGF plasmid enhances skin flap survival. Wound Repair Regen 15, 360, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Sahni A., Odrljin T., and Francis C.W. Binding of basic fibroblast growth factor to fibrinogen and fibrin. J Biol Chem 273, 7554, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Ehrbar M., Djonov V.G., Schnell C., Tschanz S.A., Martiny-Baron G., Schenk U., Wood J., Burri P.H., Hubbell J.A., and Zisch A.H. Cell-demanded liberation of VEGF121 from fibrin implants induces local and controlled blood vessel growth. Circ Res 94, 1124, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Zisch A.H., Zeisberger S.M., Ehrbar M., Djonov V., Weber C.C., Ziemiecki A., Pasquale E.B., and Hubbell J.A. Engineered fibrin matrices for functional display of cell membrane-bound growth factor-like activities: study of angiogenic signaling by ephrin-B2. Biomaterials 25, 3245, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Pandit A.S., Feldman D.S., Caulfield J., and Thompson A. Stimulation of angiogenesis by FGF-1 delivered through a modified fibrin scaffold. Growth Factors 15, 113, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Schense J.C., and Hubbell J.A. Cross-linking exogenous bifunctional peptides into fibrin gels with factor XIIIa. Bioconjug Chem 10, 75, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Geer D.J., Swartz D.D., and Andreadis S.T. In vivo model of wound healing based on transplanted tissue-engineered skin. Tissue Eng 10, 1006, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Liang M.S., and Andreadis S.T. Engineering fibrin-binding TGF-beta1 for sustained signaling and contractile function of MSC based vascular constructs. Biomaterials 32, 8684, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soleimani M., and Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc 4, 102, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Tan M.L., Shao P., Friedhuber A.M., van Moorst M., Elahy M., Indumathy S., Dunstan D.E., Wei Y., and Dass C.R. The potential role of free chitosan in bone trauma and bone cancer management. Biomaterials 35, 7828, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Park Y., Dziak R., Genco R.J., Swihart M., and Perinpanayagam H. Calcium sulfate based nanoparticles. Google Patents 2010 [Google Scholar]
- 37.Park Y.B., Mohan K., Al-Sanousi A., Almaghrabi B., Genco R.J., Swihart M.T., and Dziak R. Synthesis and characterization of nanocrystalline calcium sulfate for use in osseous regeneration. Biomed Mater 6, 055007, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Wang W., Olson D., Liang G., Franceschi R.T., Li C., Wang B., Wang S.S., and Yang S. Collagen XXIV (Col24α1) promotes osteoblastic differentiation and mineralization through TGF-β/Smads signaling pathway. Int J Biol Sci 8, 1310, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang S., and Wang C. The intraflagellar transport protein IFT80 is required for cilia formation and osteogenesis. Bone 51, 407, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsuda T., Kuwana M., Aomizu T., Yamagishi M., Ohtake H., and Watanabe G. Surface design for in situ capture of endothelial progenitor cells: VEGF-bound surface architecture and behaviors of cultured mononuclear cells. J Biomed Mater Res Part B Appl Biomater 101, 50, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Carinci F., Piattelli A., Stabellini G., Palmieri A., Scapoli L., Laino G., Caputi S., and Pezzetti F. Calcium sulfate: analysis of MG63 osteoblast-like cell response by means of a microarray technology. J Biomed Mater Res Part B Appl Biomater 71, 260, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Liang M.S., Koobatian M., Lei P., Swartz D.D., and Andreadis S.T. Differential and synergistic effects of mechanical stimulation and growth factor presentation on vascular wall function. Biomaterials 34, 7281, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou H., and Xu H.H. The fast release of stem cells from alginate-fibrin microbeads in injectable scaffolds for bone tissue engineering. Biomaterials 32, 7503, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen W., Zhou H., Weir M.D., Bao C., and Xu H.H. Umbilical cord stem cells released from alginate-fibrin microbeads inside macroporous and biofunctionalized calcium phosphate cement for bone regeneration. Acta Biomater 8, 2297, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen L.H., Annabi N., Nikkhah M., Bae H., Binan L., Park S., Kang Y., Yang Y., and Khademhosseini A. Vascularized bone tissue engineering: approaches for potential improvement. Tissue Eng Part B Rev 18, 363, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qin Y., Guan J., and Zhang C. Mesenchymal stem cells: mechanisms and role in bone regeneration. Postgrad Med J 90, 643, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knight M.N., and Hankenson K.D. Mesenchymal stem cells in bone regeneration. Adv Wound Care 2, 306, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Royce S.M., Askari M., and Marra K.G. Incorporation of polymer microspheres within fibrin scaffolds for the controlled delivery of FGF-1. J Biomater Sci Polym Ed 15, 1327, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Shireman P.K., and Greisler H.P. Mitogenicity and release of vascular endothelial growth factor with and without heparin from fibrin glue. J Vasc Surg 31, 936, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Ehrbar M., Metters A., Zammaretti P., Hubbell J.A., and Zisch A.H. Endothelial cell proliferation and progenitor maturation by fibrin-bound VEGF variants with differential susceptibilities to local cellular activity. J Control Release 101, 93, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Armulik A., Abramsson A., and Betsholtz C. Endothelial/pericyte interactions. Circ Res 97, 512, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.