Abstract
Background: Follicular thyroid carcinoma (FTC) comprises 10% of differentiated thyroid cancers. Diagnostic controversy and interobserver variability render the practical diagnosis of FTC difficult. Overall survival rates vary (46–97%). The aims of this study were to review FTC histologically at the authors' tertiary care institution and to evaluate long-term survival and recurrence.
Methods: Diagnostic slides from 66 FTC cases (1965–2007) were reviewed by three pathologists from two institutions (blinded to clinical outcomes), and consensus was obtained. Patient demographics, tumor characteristics, and treatment, survival, and recurrence data were collected. Thyroid cancer–specific and recurrence-free survival were calculated by original and reclassified diagnoses.
Results: Forty-seven cases (71%) were reclassified: 24 (36%) to papillary thyroid carcinoma (PTC), 18 (27%) to follicular adenoma (FA), and five (8%) to poorly differentiated carcinoma (PDC). Nineteen (29%) maintained a diagnosis of FTC. The extent of surgical resection and rates of radioiodine treatment did not differ by reclassification diagnosis. Pre-review FTC-specific survival was 83.5% and 75.1% at 10 and 20 years, respectively. Following contemporary reclassification, FTC-specific survival was 77% and 33.7% at 10 and 20 years, respectively. There were no cancer-specific deaths in the FA or PTC groups.
Conclusions: Over the past 50 years, changes in our understanding of the pathogenesis, histology, and behavior of thyroid carcinoma may partially account for the changes in histologic diagnosis. Elimination of PTC and FA “contaminants” led to decrease in survival following reclassification. Variability in histologic interpretation contributes to diagnostic challenges in follicular lesions. Histologic review of thyroid tumors for research studies is crucial, especially given the ever-changing diagnostic criteria.
Introduction
Follicular thyroid carcinoma (FTC) comprises between 10% and 15% of all differentiated thyroid cancers. This cancer usually presents later in life and is more aggressive than papillary thyroid carcinoma (PTC) (1,2). The diagnosis of FTC in a thyroid neoplasm depends upon pathologic confirmation of follicular cells that lack the nuclear atypia seen in PTC in addition to capsular and/or vascular invasion (3,4). Survival in these cancers may range from 46% to 97%, depending on the degree of capsular or vascular invasion (1,2,5–7).
Despite the theoretically specific definition of FTC, diagnostic controversy and interobserver variability make the diagnosis of FTC difficult. The two major areas of controversy include: (i) the degree of nuclear atypia seen within a follicular lesion, and (ii) what constitutes capsular and/or vascular invasion (4,5,8–10). A sufficient amount of nuclear atypia in a follicular lesion may lead to a diagnosis of follicular variant of papillary thyroid carcinoma (PTC-FV) rather than FTC or follicular adenoma (FA). Similarly, capsular irregularities insufficient for true capsular invasion may lead to a diagnosis of FA rather than FTC. A number of articles demonstrate that universal consensus in the diagnosis of FTC is still lacking, as evidenced by studies analyzing interobserver variability among pathologists (4,8–16). Consensus diagnoses, where all pathologists agree on the same diagnosis, occurred in a minority of cases studied. Furthermore, intraobserver variability (the variation of a single pathologist analyzing the same tumor at different time points) is also high with follicular lesions (15). The diagnosis of FTC can be difficult, even though diagnostic criteria have become more specific over time. Perhaps coincidentally, the frequency with which the diagnosis is made has decreased over time (17).
The authors have previously reported that 16% of patients diagnosed with FTC at their institution died of thyroid cancer within 13 years of diagnosis (2). The aim of the present study was to re-evaluate the pathologic diagnoses of FTC in these patients and in those with a diagnosis of FTC since the publication of the original study. A secondary aim was to see how possible changes in the pathologic diagnosis would impact long-term survival.
Materials and Methods
With IRB approval, all patients diagnosed with FTC or the oncocytic variant of FTC (also known as Hürthle cell carcinoma [HCC]) from 1965 to 2007 were retrospectively identified. All patients had undergone either surgical and/or radioactive iodine (RAI) treatment at the authors' institution. Patients with pathologic diagnoses other than FTC or HCC were excluded. Specifically, diagnoses of “Hürthle cell tumor” not specified as benign or malignant were excluded.
The pathology reports and histology slides from surgical resection specimens were obtained from the pathology department archives. Three pathologists with expertise in head and neck/endocrine pathology from two different institutions independently reviewed the slides (N.A.C., T.A., and P.M.S.). The pathologists were blinded to the clinical outcomes. Consensus diagnosis was achieved if all three pathologists agreed. If there was a discrepancy among pathologists, the diagnosis of the majority (two out of three agreed, or majority consensus) was considered the final diagnosis. If all pathologists disagreed (three different diagnoses, or lack of consensus), another review was conducted until a majority consensus or consensus diagnosis was made. Correlation with original pathology reports was performed to ensure that diagnostic slides were re-evaluated. Tumors were classified according to the current World Health Organization criteria (3). A well-circumscribed or encapsulated, cytologically bland follicular lesion was considered FA in the absence of capsular and vascular invasion. A cytologically bland, encapsulated follicular lesion was considered FTC if there was transcapsular penetration or vascular invasion (4). An infiltrative lesion comprised of papillae and/or follicles with nuclear features of PTC was considered classic PTC. A circumscribed, nodular lesion comprised entirely of follicles but with nuclear features of PTC (enlargement, clearing, overlapping, grooves, membrane irregularities, pseudoinclusions) was considered PTC-FV, regardless of capsular or vascular invasion (8). An invasive neoplasm comprised of follicular cells growing in trabeculae or solid sheets, without nuclear features of PTC, and with one of the following (convoluted nuclei, mitoses ≥3 per 10 high power fields, or tumor necrosis) was considered poorly differentiated carcinoma (PDC) (18).
Additional information was collected, including demographic data, tumor characteristics (size, extrathyroidal extension [ETE], lymph node metastases, distant metastases [DM]), extent of surgical resection, adjuvant RAI treatment, and survival and recurrence data. Time-to-event analysis was then performed using the Kaplan–Meier method. Thyroid cancer–specific survival, overall survival, and recurrence-free survival were calculated for (i) the entire cohort with an original diagnosis of FTC or HCC, (ii) the entire cohort with reclassified diagnoses, and (ii) reclassified FTC patients with and without ETE or DM. Differences in overall survival, thyroid cancer–specific survival, and thyroid cancer recurrence were calculated using the Cox proportional hazards method. All statistical analysis was performed using Stata v13 (StataCorp, College Station, TX).
Results
Basic demographic data and original pathologic classification
There were a total of 108 patients with FTC or HCC during the study time period (1965–2007). Of note, nine cases diagnosed as “Hürthle cell tumor” between 1978 and 1985 (which were included in the previous FTC study) were excluded. Slides from 66 patients were available for review and were included in the final study cohort. There were 55 cases (83%) of FTC and 11 cases (17%) of HCC. The mean age was 45.6 ± 17.2 years (range 14–82 years). There were 45 females (68%), with a mean age of 43.8 ± 16.9 years. There were 21 males (32%), with a mean age of 49.4 ± 17.5 years. Long-term survival data were collected for all 66 patients. The median follow-up time for the entire cohort was 12 years (range 0–48 years).
Retrospective pathologic classification after independent review
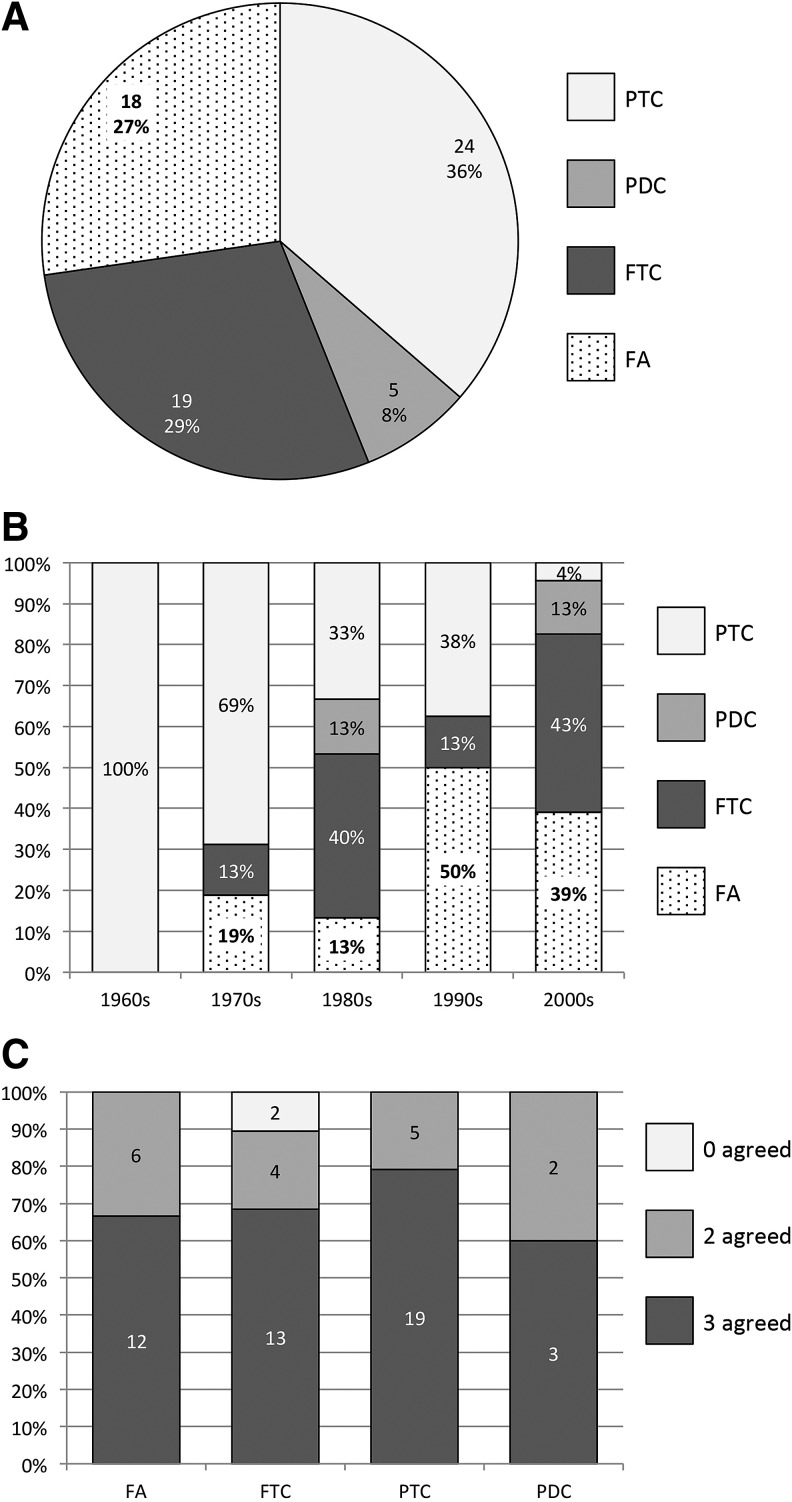
After independent histologic review, 47/66 cases (71%) originally diagnosed as FTC or HCC were reclassified. Twenty-four cases (36%) were reclassified to PTC (21 classical types and three follicular variants). Eighteen cases (27%) were reclassified to benign FA (11 classic and seven oncocytic). Five cases (8%) were reclassified as PDC. Nineteen cases (29%) maintained a diagnosis of FTC (17 classic and two oncocytic; Fig. 1A). Henceforward, all FTC (classic or oncocytic) will be referred to as FTC.
FIG. 1.
FTC reclassification. (A) Distribution of pathologic diagnoses after contemporary review. The majority of cases (n = 47, 71%) were reclassified to diagnoses other than FTC. Nineteen (29%) remained FTC. (B) Distribution of pathologic diagnoses after contemporary review by decade. The proportion of cases reclassified to PTC decreased over time, from 100% (n = 4) in the 1960s to 4% (n = 1) in the 2000s. The proportion of cases reclassified to FA slightly increased over time, from <20% in the 1970s and 1980s to >30% in the 1990s and 2000s. The proportion of cases remaining FTC varied between 0% and 43%. (C) Interobserver variability among three pathologists. Consensus diagnosis (three agreed) was achieved in >60% in all diagnostic categories. Majority consensus (two agreed) was achieved in 20–40% in all categories. Initial lack of consensus occurred in only two cases, in which a majority consensus ultimately revealed FTC. PTC, papillary thyroid carcinoma; PDC, poorly differentiated thyroid carcinoma; FTC, follicular thyroid carcinoma; FA, follicular adenoma.
Seven patients (11%) had regional lymph node metastasis: five PTC, one PDC, and one oncocytic FTC in which the primary carcinoma was not available for review. Nine patients (14%) had DM (five FTC and four PDC) to the lung (three patients), bone (four patients), chest wall (one patient), and lung and kidney (one patient). Five patients (8%) had ETE (all five FTC). Nine patients in the FTC group (5%) had neither DM nor ETE.
Subclassification of FTCs
Nineteen cases (29%) maintained a diagnosis of FTC. One patient had a lymph node metastasis (primary carcinoma not available for review, as above). Five patients had DM, and five patients had ETE. The remaining eight patients had completely intrathyroidal carcinomas and were subclassified as follows: three had capsular invasion only, two had vascular invasion only (fewer than four foci) in which one case had associated thrombosis, and three had both capsular and vascular invasion (fewer than four foci) in which one case had tumor necrosis. The average size was 4.3 cm (range 2.5–8 cm). On average, nine slides were available for review for each case (range 1–17 slides). In some cases, the entire capsule had not been submitted for histologic evaluation, and therefore the findings may not be entirely representative.
Stratification of pathology according to decade of diagnosis
In the 1960s, four cases (100%) were reclassified to PTC. In the 1970s, 11 (69%) were reclassified to PTC, three (19%) were reclassified to FA, and two (13%) remained FTC. In the 1980s, five (33%) were reclassified to PTC, two were reclassified to PDC (13%), two (13%) were reclassified to FA, and six (40%) remained FTC. In the 1990s, three (38%) were reclassified to PTC, four (50%) were reclassified to FA, and one (13%) remained FTC. In the 2000s, one (4%) was reclassified to PTC, three were reclassified to PDC (13%), nine (39%) were reclassified to FA, and 10 (43%) remained FTC (Fig. 1B).
Interobserver variability among three pathologists
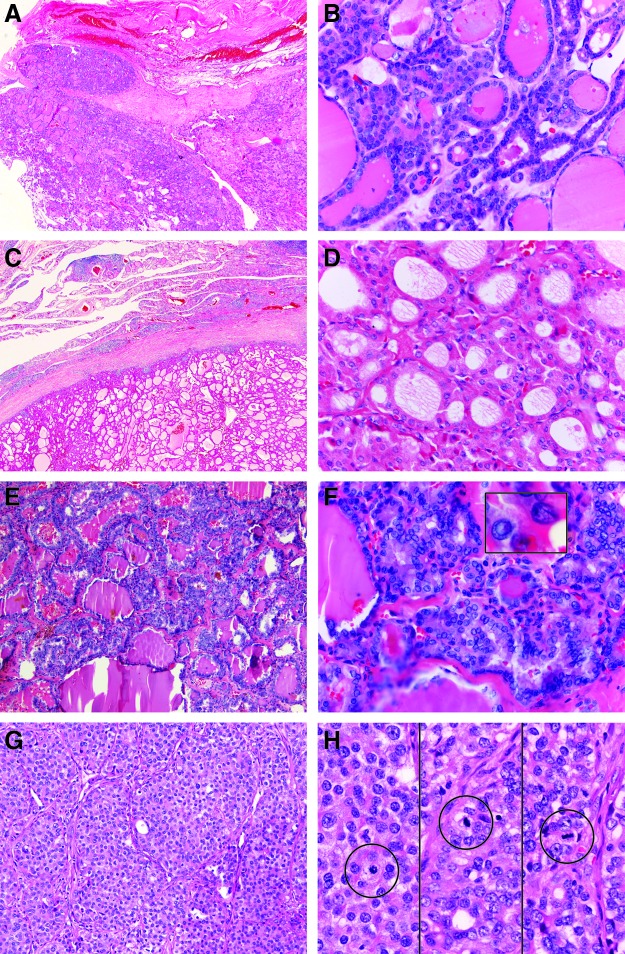
All three pathologists agreed (consensus) in 47 (71%) cases, and two pathologists agreed (majority consensus) in 17 (26%) cases, for an overall consensus of 97%. In only two cases (3%) did all three pathologists initially disagree (lack of consensus). When stratified by final diagnosis, two or three pathologists agreed (consensus or majority consensus) in 100% of FA, PTC, and PDC, and in 89% of FTC (Fig. 1C). Of the six cases with majority consensus of FA, three cases were called PTC-FV by the disagreeing pathologist, and three were called FTC by the disagreeing pathologist. Of the four cases with majority consensus of FTC, one was called atypical FA by the disagreeing pathologist, and three were called PTC-FV by the disagreeing pathologist (all three had either ETE or DM). In the two cases with majority consensus of PDC, one was called oncocytic FTC and one PTC by the disagreeing pathologist. Histologic examples of consensus diagnosis cases are shown (Fig. 2), including FTC, FA (oncocytic), PTC, and PDC.
FIG. 2.
Examples of consensus histology. (A) Low power FTC with mushroom-like invasion through capsule. (B) High power FTC showing small round, dark, bland nuclei. (C) Low power FA (oncocytic) with intact capsule. (D) High power FA (oncocytic) showing eosinophilic cytoplasm and round nuclei with nucleoli. (E) Low power PTC comprised predominantly of follicles. (F) High power PTC showing nuclear atypia, including pseudoinclusion and groove (inset). (G) Low power PDC demonstrating solid to trabecular growth of follicular cells that lack nuclear features of PTC. (H) High power PDC showing numerous mitoses within areas of solid growth (circled). Color images available online at www.liebertpub.com/thy
Lack of consensus occurred in two cases. In both cases, the reasons for disagreement were similar: each pathologist independently diagnosed FTC, PTC-FV, or FA. Upon re-review, two reviewers felt the tumor to be malignant based on invasive growth pattern and ultimately agreed that the nuclear features were not atypical enough for PTC-FV. Therefore, a final diagnosis of FTC was rendered in both cases.
Extent of surgery and adjuvant RAI treatment
Extent of surgical resection was categorized as total thyroidectomy (including patients receiving a total or near total thyroidectomy) versus less than total (including patients receiving a thyroid lobectomy or subtotal thyroidectomy). Twelve (18.2%) of the 66 patients received a less than total thyroidectomy. More than half of these patients (n = 7, 58%) were reclassified as having an adenoma, although the extent of surgical resection did not differ significantly by reclassification diagnosis on chi-square analysis (p = 0.06). Thirty-nine (59%) patients received adjuvant treatment with RAI. Rates of RAI treatment also did not differ significantly by reclassification diagnosis on chi-square analysis (p = 0.17). Of 16 patients reclassified as having an adenoma, eight (50%) received adjuvant RAI.
Survival and recurrence analysis
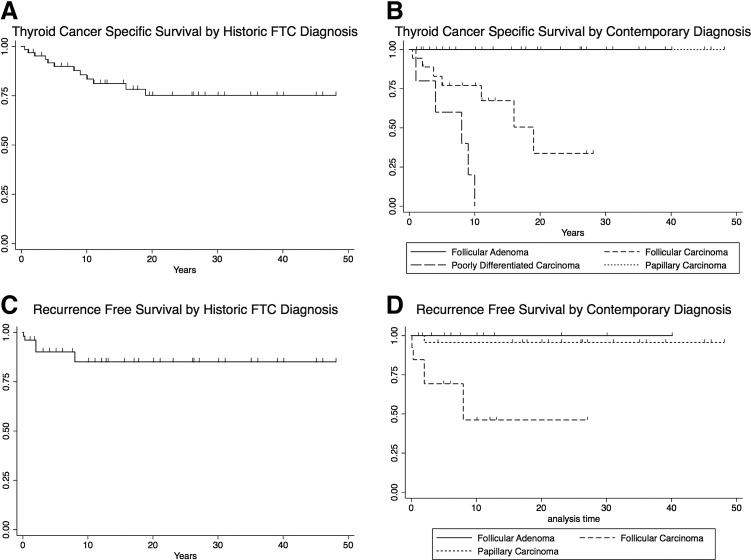
Thyroid cancer–specific survival with a historic diagnosis of FTC (n = 66) was 89.9% at five years, 83.5% at 10 years, and 75.1% at 20 years (Table 1 and Fig. 3A). After contemporary reclassification, thyroid cancer–specific survival in FTC patients (n = 19, 28.8%) was 77.0% at five years, 77.0% at 10 years, and 33.7% at 20 years (hazard ratio [HR] = 4.7, p = 0.01). Patients diagnosed with PDC after contemporary reclassification (n = 5, 7.6%) had a five-year thyroid cancer–specific survival of 60.0%, and none survived to 10 years; all deaths were attributed to thyroid cancer (HR = 19.1, p < 0.01). There were no thyroid cancer–specific deaths in the FA (n = 18, 27.3%) or PTC (n = 24, 36.4%) groups (Fig. 3B).
Table 1.
Thyroid Cancer–Specific and Recurrence-Free Survival at 5, 10, and 20 Years Based on Historic Versus Contemporary Diagnosis of Follicular Carcinoma
| Survival indicator | 5 years | 10 years | 20 years |
|---|---|---|---|
| Thy-Ca-specific survival, historic FTC Dx | 89.9% | 83.5% | 75.1% |
| Thy-Ca-specific survival, contemporary FTC Dx | 77.0% | 77.0% | 33.7% |
| Thy-Ca-specific survival, contemporary FTC Dx, patients with STI or DM | 55.6% | 55.6% | 14.8% |
| Recurrence-free survival, historic FTC Dx | 90.3% | 83.0% | 83.0% |
| Recurrence-free survival, contemporary FTC Dx | 69.2% | 46.2% | 46.2% |
Lowest survivals indicated in bold italics.
Thy-Ca, thyroid cancer; FTC, follicular thyroid carcinoma; Dx, diagnosis; STI, soft tissue invasion; DM, distant metastasis.
FIG. 3.
Thyroid cancer–specific survival and recurrence. (A) Historic thyroid cancer–specific survival for all patients with an initial diagnosis of FTC or HCC between 1965 and 2007. (B) Contemporary thyroid cancer–specific survival after pathology review, stratified by new diagnosis. Median thyroid cancer–specific survival for the FTC group (n = 18) was 19 years ( = 4.7, p = 0.01). Median thyroid cancer–specific survival for the PDC group (n = 5) was eight years (HR = 19.1, p < 0.01). There were no thyroid cancer–specific deaths in the FA or PTC groups. (C) Historic thyroid cancer recurrence for all patients with an initial diagnosis of FTC or HCC between 1965 and 2007. (D) Contemporary thyroid cancer recurrence after pathology review, stratified by new diagnosis. Median time to recurrence for the FTC group (n = 18) was eight years (HR = 22.4, p < 0.01). The single PDC patient that did not present with distant disease recurred after eight years (not shown). There was a single recurrence in the PTC group (n = 24; HR = 0.16, p = 0.09). There were no recurrences in the FA group. HCC, Hürthle cell carcinoma; HR, hazard ratio.
Recurrence data were available and applicable for 57 patients who did not present with DM. Based on the original pathologic diagnosis, 90.3% of patients were recurrence free at five years, 83.0% at 10 years, and 83.0% at 20 years (Table 1 and Fig. 3C). After contemporary reclassification, 69.2% of patients with FTC (n = 14, 25.0%) were recurrence free at five years, 46.2% at 10 years, and 46.2% at 20 years (HR = 22.4, p < 0.01). Of the eight patients with completely intrathyroidal FTCs, only a single recurrence occurred. This patient had an 8 cm carcinoma with both capsular and vascular invasion (fewer than four vessels, no thrombus) and associated tumor necrosis. The first recurrence was two years after original diagnosis. The single patient in the PDC group who did not present with distant disease recurred at eight years. There was a single recurrence in the PTC group (n = 24, 36.4%) at two years (HR = 0.16, p = 0.09). There were no recurrences in the FA group (n = 18, 27.3%; Fig. 3D).
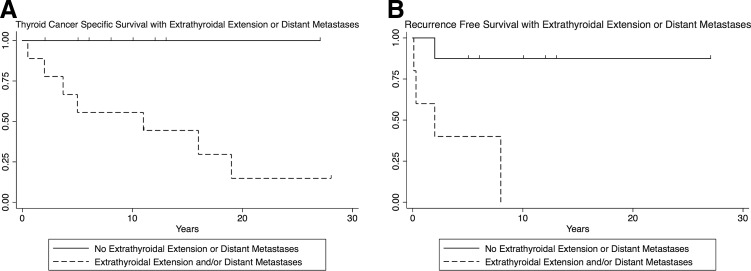
Thyroid cancer–specific survival in reclassified FTC patients with either ETE or DM (n = 10, 15.2%) was 55.6% at five years, 55.6% at 10 years, and 14.8% at 20 years (Table 1). There were no thyroid cancer–specific deaths in FTC patients without ETE or DM (n = 9; Fig. 4A). There was no difference in survival between the ETE and DM groups (p = 0.732). Median time to recurrence was two years in FTC patients with ETE (Cox hazard ratio 35.4, p < 0.01; Fig. 4B). FTC patients with DM were excluded from the recurrence analysis, as these were considered to have persistent disease.
FIG. 4.
Thyroid cancer–specific survival and recurrence stratified by ETE or DM. (A) Thyroid cancer–specific survival with new diagnosis of FTC based on the presence of ETE or DM. Median survival with ETE or DM was 11 years. (B) Recurrence for patients with new diagnosis of FTC based on the presence of ETE or DM. Cox hazard ratio 35.4 ± 29.7, p < 0.01. Median time to recurrence was two years if there was ETE. Patients with DM were excluded from the recurrence analysis—considered persistent disease. ETE, extrathyroidal extension; DM, distant metastasis.
In the nine “Hürthle cell tumor” cases excluded from analysis, none had lymph node metastasis, ETE, DM, recurrence, or thyroid cancer–specific death. Median follow-up was 25 years (range 0.2–41 years).
Discussion
In this study, 66 patients originally diagnosed with FTC between 1965 and 2007 were reviewed, and the effects of histologic reclassification on long-term survival and recurrence were studied. It was found that following contemporary reclassification by three blinded pathologists, the majority (71%) had a diagnosis other than FTC. The most frequent reclassifications were PTC (29%) or FA (27%). It was also shown that long-term thyroid-specific as well as recurrence-free survival decreased in FTC patients after exclusion of non-FTC contaminants (PTCs, FAs, and PDCs). Specifically, 20-year cancer-specific survival decreased from 75.1% to 33.7%, and recurrence-free survival decreased from 83.0% to 46.2%. Our 10-year survival rate (77% after contemporary reclassification) is similar to some studies reporting 67–84% 10-year survival in HCC or FCC patients (7). Other studies report higher survival rates (70–100%), and it is suspected that this phenomenon may in part be due to “contamination” with diagnoses other than FTC (2,19–22). Additionally, it was found that all thyroid-related deaths and most recurrences in the reclassified FTC group occurred in patients with ETE or DM. Finally, it is believed that extent of surgery and adjuvant RAI therapy did not significantly contribute to the change in survival rates, as the rates of subtotal thyroidectomy and RAI therapy did not differ significantly by reclassification diagnosis.
The results of this study highlight the change in understanding of the histology and behavior of thyroid tumors over the past 50 years. One aspect includes the change in understanding of papillary carcinomas with predominantly follicular growth. In this series, the majority of cases reclassified to PTC (n = 21) were small (<2 cm) infiltrative or sclerosing papillary carcinomas with well-developed nuclear features and only focal papillae. Five of these had lymph node metastases. Historically, thyroid carcinoma was classified as papillary (if invasive and demonstrating predominantly papillae), follicular (if invasive and demonstrating predominantly follicles), or mixed (if invasive and demonstrating a mixture of papillae and follicles) (23). Even into the 1980s, the importance of differentiating between papillary and follicular carcinomas was controversial and not well understood. Some studies found no clinical or prognostic difference between the two histologic subtypes when controlling for age and sex, and they recommended a simple diagnosis of “well-differentiated thyroid carcinoma” (24). During this time of controversy, histologic diagnoses were highly dependent upon the opinions and practice preferences of individual pathologists. The PTC-FV was originally recognized as a tumor with a follicular growth pattern, ground-glass nuclei, and a “mode of growth typical of papillary carcinoma, that is heavily infiltrating” (23,25,26). These cases could easily have been diagnosed as either follicular carcinomas or PTC-FVs at that time. In the present study, 21 such tumors were reclassified as classic papillary carcinomas with extensive follicular growth, as it is now recognized that they demonstrate molecular genetics and biologic behaviors similar to classic papillae-rich PTC (25). For consistency in use of the term “follicular variant,” these 21 cases were not classified as infiltrative follicular variants. Instead, the use of the term “follicular variant” was restricted to cases with entirely follicular growth, well-circumscribed borders, and subtle nuclear atypia—in other words, cases now recognized as sharing molecular genetic and behavioral similarities with the FA/carcinoma spectrum of tumors. Using these strict criteria, only three follicular variants were identified, and none had lymph node metastases, recurrence, or thyroid-related death.
Another reason for changing diagnosis relates to the understanding of encapsulated follicular lesions with variable capsular or vascular infiltration. The diagnosis of follicular carcinoma hinges on the identification of complete transcapsular penetration or vascular invasion. The criteria for diagnosis of these features have become increasingly strict. First, focal capsular irregularities or incomplete capsular penetration by tumor cells do not predict malignant behavior. Second, detached tumor cells within vascular spaces or subendothelial bulging of tumor cells into vascular lumina also do not predict malignant behavior (17). Furthermore, according to a recent recommendation issued by the College of American Pathologists, vascular invasion that is not associated with thrombus formation should not be considered definitive evidence of carcinoma (27). It is hypothesized that some cases were diagnosed as FTC due to capsular irregularities and incomplete capsular penetration, which today would be classified as FA. The present study emphasizes the importance of strict criteria for malignant diagnosis. Cases with incomplete or indefinite capsular or vascular invasion were classified as FAs, and none of these patients experienced recurrence or thyroid-related death.
The third reason for changing diagnosis involves the relatively recent Turin guidelines for diagnosis of PDC, published in 2007 (28). All cases reclassified to PDC in this series were originally diagnosed as FTC between 1986 and 2003, prior to the publication of these guidelines. Since then, studies have shown that poorly differentiated cancers have a prognosis intermediate between well-differentiated and undifferentiated (anaplastic) thyroid carcinomas (29). Although there were no undifferentiated carcinomas in the present study, reclassified PDCs did show worse survival than confirmed follicular carcinomas.
The survival and recurrence data support the contemporary reclassification by pathologists blinded to the clinical outcomes. Thyroid cancer–specific death did not occur in any reclassified PTC or FA patient, and recurrence of thyroid disease did not occur in any reclassified FA patient. Use of strict diagnostic criteria for capsular and vascular invasion may more accurately shift equivocal cases into a benign (FA) group, sparing unnecessary cancer diagnoses and additional surgeries. Additionally, there were no thyroid cancer–specific deaths in FTC patients with entirely intrathyroidal growth, supporting the indolent behavior these intrathyroidal FTCs. Of the eight entirely intrathyroidal FTCs, only one experienced recurrence. This was a large (8 cm) tumor with capsular and vascular invasion and tumor necrosis. Adverse outcomes were largely restricted to patients with ETE or DM (T3, T4, or M1 tumors).
Retrospective (albeit blinded) review for the purpose of research also introduces potential bias (30). A reviewer may be more likely to make a definitive benign diagnosis on an equivocal case due to the lack of clinical consequence. Conversely, those cases with obvious extrathyroidal or metastatic disease are less likely to be reclassified, thereby possibly enriching the FTC group with more aggressive tumors.
Additional limitations of this study include those encountered with retrospective analyses. The re-evaluation of these cases depended on the slides available for review. By correlation with gross examination and final diagnosis, attempts were made to ensure that the diagnostic slides were included in the review. Additionally, the overall number of patients was limited (66 total, with only 19 remaining FTCs following reclassification). Case numbers were limited by (i) availability of slides in the pathology archives and (ii) relatively low prevalence of FTC compared to PTC and benign thyroid nodules.
The next step in support of this contemporary reclassification is molecular genetic analysis: a prevalence of BRAF mutation/RET-PTC fusion would be expected in the 21 infiltrative papillary carcinomas versus RAS mutation/PAX8-PPARγ fusion in the 18 FAs, 19 follicular carcinomas, and three PTC-FVs. As the primary aim of this study was histologic classification, molecular analysis will be undertaken separately.
This study elucidates the changes in histologic classification of FTC over the past half-century, and the survival and recurrence data lend credence to the contemporary methods of classification. As diagnostic criteria are changing based on our increasing understanding of the histologic, molecular, and clinical features of thyroid carcinoma, contemporary review is and will be necessary in any retrospective study of this heterogenous disease. Caution should be exercised when retrospectively evaluating FTC in large nationwide databases due to the potential interobserver variability in diagnosis of FTC and the potential “contaminating” diagnoses that may exist in these data sets. A preliminary review of SEER or NCDB slides may be warranted in order to corroborate the present findings. This concept applies not only to thyroid carcinoma, but also to the histopathologic classification of human neoplasia in general. As advanced molecular and genetic techniques in conjunction with critical review of histology allow better classification and subclassification of disease, diagnoses and relevant prognostic information may change over time.
Acknowledgments
Funding provided by internal resources from the University of Chicago Department of Surgery Endocrine Surgery Research Program and the Department of Pathology.
Author Disclosure Statement
The authors have no relevant financial disclosures.
References
- 1.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. 1998. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995. Cancer 83:2638–2648 [DOI] [PubMed] [Google Scholar]
- 2.DeGroot LJ, Kaplan EL, Shukla MS, Salti G, Straus FH. 1995. Morbidity and mortality in follicular thyroid cancer. J Clin Endocrinol Metab 80:2946–2953 [DOI] [PubMed] [Google Scholar]
- 3.Delellis RA, Lloyd RV, Heitz PU, Eng C. (eds) 2004. World Health Organization Classification of Tumors. Pathology and Genetics of Tumours of Endocrine Organs. IARC Press, Lyon, France [Google Scholar]
- 4.Baloch ZW, LiVolsi VA. 2002. Follicular-patterned lesions of the thyroid: the bane of the pathologist. Am J Clin Pathol 117:143–150 [DOI] [PubMed] [Google Scholar]
- 5.O'Neill CJ, Vaughan L, Learoyd DL, Sidhu SB, Delbridge LW, Sywak MS. 2011. Management of follicular thyroid carcinoma should be individualised based on degree of capsular and vascular invasion. Eur J Surg Oncol 37:181–185 [DOI] [PubMed] [Google Scholar]
- 6.D'Avanzo A, Treseler P, Ituarte PH, Wong M, Streja L, Greenspan FS, Siperstein AE, Duh QY, Clark OH. 2004. Follicular thyroid carcinoma: histology and prognosis. Cancer 100:1123–1129 [DOI] [PubMed] [Google Scholar]
- 7.Nagar S, Aschebrook-Kilfoy B, Kaplan EL, Angelos P, Grogan RH. 2013. Hurthle cell carcinoma: an update on survival over the last 35 years. Surgery 154:1263–1271 [DOI] [PubMed] [Google Scholar]
- 8.Chan JKC. 2002. Strict criteria should be applied in the diagnosis of encapsulated follicular variant of papillary thyroid carcinoma. Am J Clin Pathol 117:16–18 [DOI] [PubMed] [Google Scholar]
- 9.Salajegheh A, Petcu EB, Smith RA, Lam AK-Y. 2008. Follicular variant of papillary thyroid carcinoma: a diagnostic challenge for clinicians and pathologists. Postgrad Med J 84:78–82 [DOI] [PubMed] [Google Scholar]
- 10.LiVolsi VA, Baloch ZW. 2004. Follicular neoplasms of the thyroid: view, biases, and experiences. Adv Anat Pathol 11:279. [DOI] [PubMed] [Google Scholar]
- 11.Baloch ZW, LiVolsi VA. 2006. Our approach to follicular-patterned lesions of the thyroid. J Clin Pathol 60:244–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson LD, Wieneke JA, Paal E, Frommelt RA, Adair CF, Heffess CS. 2001. A clinicopathologic study of minimally invasive follicular carcinoma of the thyroid gland with a review of the English literature. Cancer 91:505–524 [DOI] [PubMed] [Google Scholar]
- 13.Hirokawa M, Carney JA, Goellner JR, DeLellis RA, Heffess CS, Katoh R, Tsujimoto M, Kakudo K. 2002. Observer variation of encapsulated follicular lesions of the thyroid gland. Am J Surg Pathol 26:1508. [DOI] [PubMed] [Google Scholar]
- 14.Lloyd RV, Erickson LA, Casey MB, Lam KY, Lohse CM, Asa SL, Chan JK, DeLellis RA, Harach HR, Kakudo K, LiVolsi VA, Rosai J, Sebo TJ, Sobrinho-Simoes M, Wenig BM, Lae ME. 2004. Observer variation in the diagnosis of follicular variant of papillary thyroid carcinoma. Am J Surg Pathol 28:1336–1340 [DOI] [PubMed] [Google Scholar]
- 15.Franc B. 2003. Interobserver and intraobserver reproducibility in the histopathology of follicular thyroid carcinoma. Hum Pathol 34:1092–1100 [DOI] [PubMed] [Google Scholar]
- 16.Elsheikh TM, Asa SL, Chan JK, DeLellis RA, Heffess CS, LiVolsi VA, Wenig BM. 2008. Interobserver and intraobserver variation among experts in the diagnosis of thyroid follicular lesions with borderline nuclear features of papillary carcinoma. Am J Clin Pathol 130:736–744 [DOI] [PubMed] [Google Scholar]
- 17.LiVolsi VA, Asa SL. 1994. The demise of follicular carcinoma of the thyroid gland. Thyroid 4:233–236 [DOI] [PubMed] [Google Scholar]
- 18.Volante M, Landolfi S, Chiusa L, Palestini N, Motta M, Codegone A, Torchio B, Papotti MG. 2004. Poorly differentiated carcinomas of the thyroid with trabecular, insular, and solid patterns: a clinicopathologic study of 183 patients. Cancer 100:950–957 [DOI] [PubMed] [Google Scholar]
- 19.Enomoto K, Enomoto Y, Uchino S, Yamashita H, Noguchi S. 2013. Follicular thyroid cancer in children and adolescents: clinicopathologic features, long-term survival, and risk factors for recurrence. Endocr J 60:629–635 [DOI] [PubMed] [Google Scholar]
- 20.Mazzaferri EL, Jhiang SM. 1994. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med 97:418–428 [DOI] [PubMed] [Google Scholar]
- 21.Lo C-Y, Chan W-F, Lam K-Y, Wan K-Y. 2005. Follicular thyroid carcinoma: the role of histology and staging systems in predicting survival. Ann Surg 242:708–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grogan RH, Kaplan SP, Cao H, Weiss RE, Degroot LJ, Simon CA, Embia OM, Angelos P, Kaplan EL, Schechter RB. 2013. A study of recurrence and death from papillary thyroid cancer with 27 years of median follow-up. Surgery 154:1436–1447 [DOI] [PubMed] [Google Scholar]
- 23.Franssila KO. 1973. Is the differentiation between papillary and follicular thyroid carcinoma valid? Cancer 32:853–864 [DOI] [PubMed] [Google Scholar]
- 24.Donohue JH, Goldfien SD, Miller TR, Abele JS, Clark OH. 1984. Do the prognoses of papillary and follicular thyroid carcinomas differ? Am J Surg 148:168–173 [DOI] [PubMed] [Google Scholar]
- 25.Chem KT, Rosai J. 1977. Follicular variant of thyroid papillary carcinoma: a clinicopathologic study of six cases. Am J Surg Pathol 1:123–130 [DOI] [PubMed] [Google Scholar]
- 26.Lindsay S. 1960. Carcinoma of the Thyroid Gland. A Clinical and Pathologic Study of 293 Patients at the University of California Hospital. Charles C Thomas, Springfield, IL [Google Scholar]
- 27.College of American Pathologists 2014. Protocol for the Examination of Specimens from Patients with Carcinomas of the Thyroid Gland. Seventh edition. Available at: www.cap.org/apps/docs/committees/cancer/cancer_protocols/2014/Thyroid_14Protocol_3100.pdf (accessed April26, 2015)
- 28.Volante M, Collini P, Nikiforov YE, Sakamoto A, Kakudo K, Katoh R, Lloyd RV, LiVolsi VA, Papotti M, Sobrinho-Simoes M, Bussolati G, Rosai J. 2007. Poorly differentiated thyroid carcinoma: the Turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am J Surg Pathol 31:1256–1264 [DOI] [PubMed] [Google Scholar]
- 29.Sanders Jr EM, LiVolsi VA, Brierley J, Shin J, Randolph GW. 2007. An evidence-based review of poorly differentiated thyroid cancer. World J Surg 31:934–945 [DOI] [PubMed] [Google Scholar]
- 30.Duggan MA, Goswami R, Magliocco AM, Burnier M, Sidhu D; Canadian Association of Pathologists (Association Canadienne des Pathologistes) Working Group 2013. Guidelines for the review of pathology in the research context. Surgery 154:111–115 [DOI] [PubMed] [Google Scholar]