Abstract
The objective of the present study (clinicaltrials.gov NCT02026414) was to observe the effects of oral supplementation of a purified and standardized Shilajit extract on skeletal muscle adaptation in adult overweight/class I obese human subjects from the U.S. population. Shilajit is a mineral pitch that oozes out of Himalayan rocks. The study design consisted of a baseline visit, followed by 8 weeks of 250 mg of oral Shilajit supplementation b.i.d., and additional 4 weeks of supplementation with exercise. At each visit, blood samples and muscle biopsies were collected for further analysis. Supplementation was well tolerated without any changes in blood glucose levels and lipid profile after 8 weeks of oral supplementation and the additional 4 weeks of oral supplementation with exercise. In addition, no changes were noted in creatine kinase and serum myoglobin levels after 8 weeks of oral supplementation and the additional 4 weeks of supplementation with exercise. Microarray analysis identified a cluster of 17 extracellular matrix (ECM)-related probe sets that were significantly upregulated in muscles following 8 weeks of oral supplementation compared with the expression at the baseline visit. This cluster included tenascin XB, decorin, myoferlin, collagen, elastin, fibrillin 1, and fibronectin 1. The differential expression of these genes was confirmed using quantitative real-time polymerase chain reaction (RT-PCR). The study provided maiden evidence that oral Shilajit supplementation in adult overweight/class I obese human subjects promoted skeletal muscle adaptation through upregulation of ECM-related genes that control muscle mechanotransduction properties, elasticity, repair, and regeneration.
Key Words: • : adaptation, extracellular matrix, Shilajit, skeletal muscle
Introduction
In recent years, understanding effectiveness of nutritional supplements in enhancing skeletal muscle performance and attenuating muscle injury has gained marked interest.1 Shilajit is a mineral pitch that seeps out of the rocks in the high altitudes of Himalayan Mountains.2 In traditional Ayurvedic medicine, Shilajit has been reported to exhibit adaptogenic and potent anabolic properties.3,4 Furthermore, Shilajit has been used for centuries to treat a number of disorders, including muscle and tendon injuries.2,5 A derivative of Shilajit, fulvic acid (FA) complex, consists of naturally occurring low- and medium-molecular-weight compounds, including oxygenated dibenzo-alpha-pyrones (DBPs) and acylated DBPs.5,6 In albino mice, Shilajit supplementation significantly enhanced physiological energy status in a forced swimming test model.7 DBP, FA, and their derivatives are the principal constituents of Shilajit contributing to these effects.4,8
The skeletal muscle is made up of heterogeneous muscle fibers with distinct metabolic and contractile properties.9 Changes in gene expression is an integral component of skeletal muscle physiological adaptations to exercise and nutritional supplementation.9 Shilajit has been reported to improve physical performance and relieve fatigue with enhanced adenosine triphosphate (ATP) production.4,8,10 The primary aim of the present longitudinal study (the same person's initial visit serves as the baseline for subsequent visits) is to find out the effect of oral Shilajit supplementation and exercise training on human skeletal muscle adaptation in a group of overweight/class I obese human subjects following 12 weeks of study period. This particular cohort was selected for the study because physiological muscle performance is often compromised in this group of subjects.11–13
Materials and Methods
Natreon Inc.'s patented ingredient, PrimaVie® [US 6,969,612; 6,440,436; 6,558,712; 8,894,993; and EP 1 387 614], is a purified and standardized Shilajit extract for nutraceutical use. It is standardized to have not less than 60.3% fulvic acid equivalents with DBP and associated chromoproteins (50% fulvic acid + 10.3% free DBPs and DBPs conjugated with chromoproteins) in Shilajit extract. The test product, PrimaVie Shilajit (PVS) capsules, 250 mg, was supplied by Natreon, Inc., New Brunswick, NJ, USA. PVS is manufactured by a process to reduce the heavy metals to less than 1 ppm of lead, 1 ppm of arsenic, and less than 0.1 ppm of mercury. Quality control is achieved through high-performance liquid chromatography (HPLC) analysis as previously reported.14
Ethics, consent, and permissions
The Western Institutional Review Board (WIRB) approved the study protocols (clinicaltrials.gov NCT02026414) and materials. All subjects provided written informed consent before participation in the study.
Study subjects and experimental design
Overweight/Class I obese, adult human subjects (21–70 years) of both genders with body–mass index (BMI) 25–35 were entitled to participate in this study. They were asked to fast overnight, following which collection of blood samples was done. Any self-reported variations in diet or exercises were recorded. The subjects were excluded from the study if any one of the following medications was used for management/treatment of cardiovascular disease (CVD)-related disorders: steroids (Prednisone, etc.), beta-blockers, hydrochlorothiazide, statins (Crestor, Lipitor, etc.), aspirin, and angiotensin-converting enzyme (ACE) inhibitors. Pregnant females as well as individuals who were therapeutically immunocompromised were also excluded from the study. The experimental study design consisted of four study visits during the 12-week study period: visit 1, baseline visit; visit 2, after 8 weeks of oral supplementation of PVS; visit 3a, additional (following 8 weeks of initial supplementation) 4 weeks of oral supplementation and exercise, sample collection before the final bout of exercise; and visit 3b, same as study visit 3, sample collected 30 min post-final bout of exercise. At each study visit, 50 mL of blood, 5 mm muscle biopsy, and demographic information, including age, gender, weight, BMI, blood pressure, and pulse, were taken (Table 1).
Table 1.
Demographic Characteristics of Study Participants
| Parameters | Values | Baseline | 8 Weeks | 12 Weeks |
|---|---|---|---|---|
| Subjects (n) | 16 | |||
| Age (years) | 35.7 ± 3.4 | |||
| Gender | ||||
| Males | 6 | |||
| Females | 10 | |||
| Body weight (lb.) | 188.5 ± 8.6 | 187.9 ± 8.8 | 187.4 ± 8.9 | |
| Body–mass index, kg/m2 | 28.9 ± 0.6 | 28.8 ± 0.7 | 29.3 ± 0.7 | |
| Blood pressure systolic, mmHg | 114.3 ± 3.1 | 114.8 ± 2.8 | 112.1 ± 2.5 | |
| Blood pressure diastolic, mmHg | 74 ± 1.9 | 71.6 ± 1.5 | 69.8 ± 1.8 | |
| Pulse (min) | 70.1 ± 2.0 | 72.4 ± 1.9 | 70.8 ± 2.2 | |
Values are expressed as mean ± SEM.
Supplementation regimen and compliance
Each subject received 250 mg of PVS capsules twice a day for the first 8 weeks of study period. For the last 4 weeks of the study, subjects took 250 mg of oral PVS supplement twice a day while also completing exercise on a treadmill (70–75% of maximum heart rate for 20 min monitored using Polar FT4, plus 5 min of warm-up and 5 min of cool-down exercises for a total of 30 min a day, 3 days a week). The same exercise regimen was followed during visits 3a and 3b. All subjects participated in the total 12 weeks of study, including one baseline and three follow-up visits. The dose of PVS was chosen based on an earlier human supplementation study.14 The 8-week period of supplementation was selected based on earlier studies on biochemical/genetic adaptations in skeletal muscles.15,16
Safety monitoring
No adverse effect directly related to the dietary supplement was reported by clinical research staff.
Blood sampling and analysis
During each visit, peripheral venous blood was collected in heparinized tubes and transported on ice immediately for analysis. Among lipid profile total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglyceride levels, calculated LDL-C, and non-HDL-C were measured using standard clinical lipid profile,17 and creatine kinase (CK), glucose, and serum myoglobin were measured at the clinical laboratory of Ohio State University Wexner Medical Center.
Muscle biopsy collection
A biopsy was collected by a board-certified physician after application of local anesthetics to the site of biopsy (vastus lateralis) using a 100–120 V, 50–60 Hz, 600VA biopsy machine having 12-gauge SenoRx, stereotactic ultrasound Encor Probe (BARD Encor Ultra, breast biopsy system, Tempe, AZ, USA). Muscle samples were stored in liquid nitrogen for further analysis.
Affymetrix GeneChip® probe array analysis
GeneChip® probe array analysis was performed on RNA extracted from muscle biopsies collected on baseline (visit 1) and visit 2 (8 weeks post-supplementation) using Affymetrix GeneChip Human Transcriptome Array 2.0 as described previously.18,19 Briefly, total RNA was isolated and RNA integrity was interrogated using the Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). Total RNA was linearly amplified, labeled, and fragmented using the GeneChip WT PLUS reagent kit (Affymetrix, Santa Clara, CA, USA). Labeled cRNA targets hybridized to Affymetrix GeneChip Human Transcriptome Array 2.0 (HTA 2.0) for 16 h at 45°C in hybridization oven (Affymetrix model 640) rotating at 60 rpm were washed, stained, and scanned in our own facilities as described earlier.18,19 The expression data have been submitted to the Gene Expression Omnibus (GEO) at NCBI (www.ncbi.nlm.nih.gov/geo/) with the series accession number GSE71219. GCOS (Gene Chip Operating Software; Affymetrix) was employed for data acquisition and image processing. Raw data were analyzed using Genespring GX (Agilent, Santa Clara, CA, USA). Additional processing of data was performed using dChip software (Harvard University).18,19 Arrays were normalized using RMA algorithm in Expression Console and comparisons made in Transcriptome Analysis Console (Affymetrix). Differentially expressed genes were identified using a two-class paired t-test (visit 1 versus visit 2) where the significance level was set at P < .05 with correction for false discovery rate.20 The genes that were significantly upregulated were subjected to functional analysis using DAVID (Database for Annotation, Visualization and Integrated Discovery, NIAID, NIH). Gene ontology (GO) was used to identify broadly gene annotation categories under molecular function and biological process.
Quantification of mRNA expression by real-time polymerase chain reaction
Real-time polymerase chain reaction (PCR) was carried out to validate the extracellular matrix (ECM)-related genes identified using GeneChip probe array analysis. Real-time polymerase chain reaction (RT-PCR) was performed using double-stranded DNA binding dye SYBR Green-I as described previously.21,22 GAPDH was used as a reference housekeeping gene.
Statistical methods
Multivariate linear regression was used to test if all 11 gene expression (ΔΔCT) values were jointly different across adjacent time points. Five comparisons were generated across various time points. The multivariate regression produces estimated differences along with their 95% confidence interval for each gene with a single P-value testing if all 11 ΔΔCT values of the genes were jointly different across adjacent time points. Multivariate normality was checked using standardized normal probability plots. If any values were not normal, then they were transformed using natural logarithms. A new multivariate linear regression model was used to check if patient lipids/glucose/muscle damage marker values were jointly different across adjacent time points. Lipids/glucose/muscle damage marker values were summarized using means and standard deviations for each of the three time points. All analyses were run using Stata 13.1; StataCorp, College Station, TX, USA.
Results
Analysis of glucose, lipid profile, CK, and serum myoglobin levels following oral PVS supplementation
Lipid profile measurements displayed no significant changes in the cholesterol, HDL-C, calculated LDL-C, total cholesterol/HDL, non-HDL–C, and triglycerides following 8 weeks of oral PVS supplementation compared with the baseline levels, suggesting the supplementation was well tolerated (Tables 2 and 3). Additionally, lipid profile levels at the week 12 pre and post-final exercise (visits 3a and 3b) showed no significant changes compared with the baseline levels at 8 weeks (Tables 2 and 3). Moreover, no changes were observed in other variables, including blood glucose and muscle damage markers, including CK and serum myoglobin levels, at all follow-up visits (Tables 2 and 3).
Table 2.
Lipids, Glucose, and Muscle Damage Markers of Baseline, 8 Weeks, and 9–12 Weeks (Pre and Post-Final Exercise)
| Baseline | 8 Weeks | 9–12 Weeks (pre-final exercise) | 9–12 Weeks (post-final exercise) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | N | Mean | SEM | N | Mean | SEM | N | Mean | SEM | N | Mean | SEM |
| Creatine kinase (U/L) (normal value: 30–220) | 16 | 121.50 | 20.12 | 16 | 92.13 | 9.64 | 16 | 118.69 | 18.46 | 16 | 142.19 | 20.28 |
| Glucose (mg/dL) (normal value: 70–99) | 16 | 79.56 | 2.76 | 16 | 80.88 | 3.00 | 16 | 77.50 | 3.88 | 16 | 82.56 | 2.88 |
| Cholesterol (mg/dL) (normal value: <200) | 16 | 175.06 | 7.12 | 16 | 187.38 | 8.61 | 16 | 184.00 | 8.65 | 16 | 184.50 | 8.26 |
| Triglycerides (mg/dL) (normal value: <150) | 16 | 137.44 | 23.74 | 16 | 144.88 | 19.24 | 16 | 137.56 | 27.82 | 16 | 143.75 | 24.03 |
| HDL cholesterol (mg/dL) (normal value: >60) | 16 | 52.06 | 3.05 | 16 | 52.00 | 2.79 | 16 | 55.44 | 3.80 | 16 | 55.31 | 3.67 |
| LDL cholesterol (calculated) (mg/dL) (normal value: <100; optimal) | 16 | 94.86 | 8.17 | 16 | 105.27 | 8.45 | 16 | 105.87 | 7.50 | 16 | 101.13 | 7.19 |
| Total cholesterol/HDL (ratio) (normal value: <4.5: low risk) | 16 | 3.51 | 0.23 | 16 | 3.74 | 0.24 | 16 | 3.46 | 0.21 | 16 | 3.47 | 0.20 |
| Non-HDL cholesterol (mg/dL) (normal value: <130) | 16 | 123.00 | 7.64 | 16 | 135.38 | 8.67 | 16 | 128.56 | 7.56 | 16 | 129.19 | 7.25 |
| Myoglobin (mcg/L) (normal value: ≤90) | 16 | 40.38 | 3.02 | 16 | 40.06 | 2.47 | 15 | 42.40 | 3.26 | 15 | 66.13 | 6.90 |
HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Table 3.
Multivariate Test of All Lipids/Glucose/Muscle Damage Markers (Jointly)
| Comparison | Contrast | Standard Error | Z | P |
|---|---|---|---|---|
| 8 weeks versus baseline | −14.01 | 16.64 | −0.84 | .400 |
| 9–12 weeks (pre-final exercise) versus baseline | −9.78 | 17.20 | −0.57 | .570 |
| 9–12 weeks (pre-final exercise) versus 8 weeks | 4.43 | 16.94 | 0.25 | .803 |
| 9–12 weeks (post-final exercise) versus 8 weeks | 26.80 | 16.94 | 1.58 | .114 |
| 9–12 weeks (pre-final exercise) versus 9–12 weeks (post-final exercise) | 22.57 | 17.49 | 1.29 | .197 |
Transcriptome profiling of skeletal muscle following oral PVS supplementation
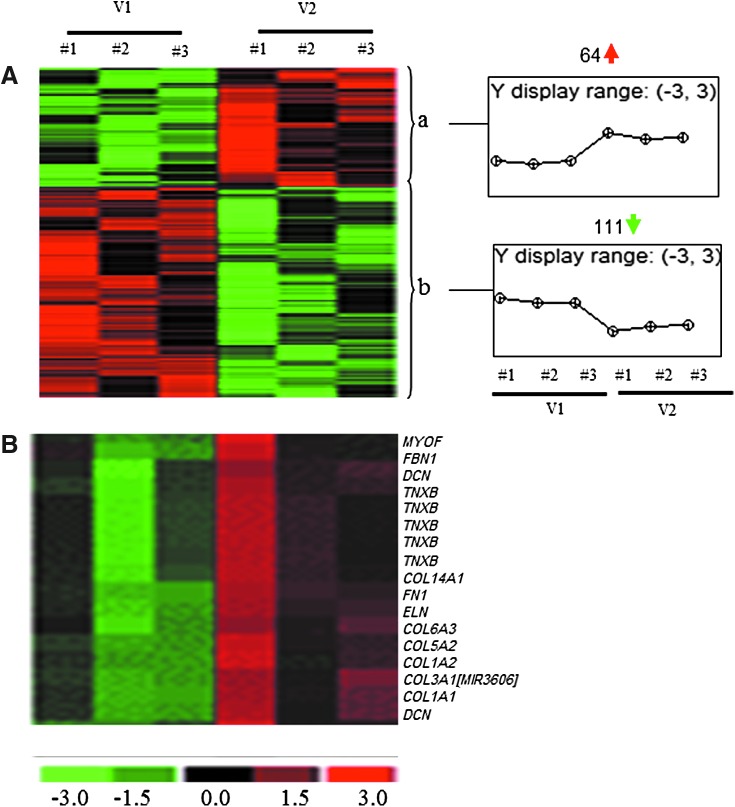
To determine the changes in the transcriptomes of human skeletal muscle in response to oral PVS supplementation, muscle samples were collected during each visit. RNA extraction, target labeling, and GeneChip data analysis were performed using Affymetrix® Human Transcriptome Array 2.0 (HTA 2.0) as described previously.18,19 The HTA 2.0 contains >6.0 million probes covering coding transcripts and exon–exon splice junctions and noncoding transcripts.23 A total of 175 annotated probe sets were differentially (P < .01) regulated following 8-week supplementation compared with baseline visits (Fig. 1A). Top 20 candidates based on fold change (compared with baseline) that was altered (up or downregulated) in the supplementation group have been provided in Tables 4 and 5. Pathway analysis revealed an ECM-related cluster of probe sets that was significantly upregulated in the 8-week supplementation group compared with corresponding baseline visit (Fig 1B). This probe set included ECM genes: tenascin XB (TNXB), decorin (DCN), collagen (COL) (type I, III, V, VI, XIV), fibrillin 1 (FBN1), elastin (ELN), myoferlin (MYOF), and fibronectin 1 (FN1) (Table 6). Among these upregulated genes, TNXB was increased by ∼1.7-fold and DCN was increased by 2.23- and 1.09-fold, respectively (Table 6). COL1A1, COL1A2, and COL3A1 were increased by 4.61-, 5.13-, and 5.18-fold, respectively, and ELN, FBN1, and FN1 were increased by 1.13-, 3.05-, and 3.65-fold, respectively (Table 6).
FIG. 1.
Heat map illustrating cluster of transcripts that were sensitive to PVS supplementation. PVS-sensitive transcripts were subjected to hierarchical clustering. (A) A total of 175 annotated probe sets were differentially (P < .05) regulated following 8 weeks of oral PVS supplementation compared with baseline visits. (B) Pathway analysis revealed an ECM-related cluster of probe sets that was significantly upregulated following 8 weeks of supplementation compared with corresponding baseline visit. COL1A1, collagen type I alpha1; COL1A2, collagen type I alpha 2; COL5A2, collagen type V alpha 2; COL6A3, collagen type VI alpha 3; COL14A1, collagen type XIV alpha 1, DCN, decorin; ECM, extracellular matrix; ELN, elastin; FBN1, fibrillin 1; FN1, fibronectin 1; MYOF, myoferlin; TNXB, tenascin XB; V1, baseline visit; V2, 8 weeks of oral PVS supplementation.
Table 4.
Genes Upregulated in Skeletal Muscles Following Oral PVS Supplementation
| Transcript cluster ID | Gene symbol | Gene description | Fold change | P |
|---|---|---|---|---|
| 18723098 | COL3A1|MIR3606 | Collagen, type III, alpha1|microRNA3606 | 5.18 | .0087 |
| 18811819 | COL1A2 | Collagen, type I, alpha2 | 5.13 | .0077 |
| 18942601 | MMP2 | Matrixmetallopeptidase2 (gelatinaseA, 72 kDa gelatinase, 72 kDa type IV collagenase) | 3.73 | .0100 |
| 18737057 | FN1 | Fibronectin1 | 3.65 | .0085 |
| 18798512 | FNDC1 | Fibronectin type III domain containing 1 | 3.30 | .0006 |
| 18817641 | SFRP4 | Secreted frizzled-related protein 4 | 3.14 | .0031 |
| 18847049 | ASPN | Asporin | 2.32 | .0002 |
| 18830114 | COL14A1 | Collagen, type XIV, alpha1 | 2.08 | .0061 |
| 18864477 | PLXDC2 | Plexin domain containing 2 | 1.83 | .0020 |
| 18907603 | LUM | Lumican | 1.77 | .0017 |
| 18693079 | HMCN1 | Hemicentin1 | 1.71 | .0038 |
| 18915509 | LHFP | Lipoma HMGIC fusion partner | 1.69 | .0015 |
| 18960437 | MFAP4 | Microfibrillar-associated protein4 | 1.67 | .0075 |
| 18717539 | ANTXR1 | Anthrax toxin receptor 1 | 1.61 | .0041 |
| 18936069 | ITGA11 | Integrin, alpha11 | 1.56 | .0058 |
| 18828471 | SULF1 | Sulfatase1 | 1.36 | .0038 |
| 18786580 | EDIL3 | EGF-like repeats and discoid in I-like domains 3 | 1.33 | .0067 |
| 18716082 | LTBP1 | Latent transforming growth factor beta-binding protein 1 | 1.33 | .0005 |
| 18956362 | MRC2 | Mannose receptor, Ctype2 | 1.32 | .0035 |
| 19022949 | RNU5E-1 | RNA, U5E small nuclear 1 | 1.31 | .0053 |
Data presented indicate fold changes in expression of PVS-sensitive genes following 8 weeks of oral PVS supplementation compared with corresponding baseline visits. Transcripts cluster ID, Affymetrix probe identifications. Data correspond to Figure 1A, cluster a (P < .05; FDR <5%).
Table 5.
Genes Downregulated in Skeletal Muscles Following Oral PVS Supplementation
| Transcript cluster ID | Gene symbol | Gene description | Fold change | P |
|---|---|---|---|---|
| 19534964 | SNAR-G2 | Small ILF3/NF90-associated RNA G2 | −1.27 | .0023 |
| 19140074 | MIR4792 | MicroRNA 4792 | −1.24 | .0065 |
| 18729405 | SIX2 | SIX homeobox 2 | −1.23 | .0057 |
| 18928262 | KLF13 | Kruppel-like factor 13 | −1.22 | .0035 |
| 19195587 | MIR4635 | MicroRNA 4635 | −1.21 | .0008 |
| 18820492 | VGF | VGF nerve growth factor inducible | −1.21 | .0095 |
| 19160216 | DRD5 | dopamine receptor D5 | −1.21 | .0003 |
| 18949268 | GCSH | Glycine cleavage system protein H (amino methyl carrier) | −1.20 | .0076 |
| 19113156 | MIR3132 | MicroRNA 3132 | −1.20 | .0006 |
| 18939902 | CLDN9 | Claudin 9 | −1.20 | .0035 |
| 18999582 | IGLV2-33 | Immunoglobulin lambda variable 2-33 (nonfunctional) | −1.19 | .0054 |
| 18885915 | MIR210HG | MIR210 host gene (nonprotein coding) | −1.19 | .0000 |
| 18739218 | IGKV1-37 | Immunoglobulin kappa variable 1–37 (nonfunctional) | −1.18 | .0024 |
| 19043031 | LOC100287934 | LOC100287934 | −1.17 | .0072 |
| 19499270 | MIR4734 | MicroRNA 4734 | −1.17 | .0037 |
| 19222951 | LINC00602 | Long intergenic nonprotein coding RNA 602 | −1.16 | .0008 |
| 18979687 | TICAM1 | Toll-like receptor adaptor molecule 1 | −1.16 | .0031 |
| 19570418 | RN5S497 | RNA, 5S ribosomal 497 | −1.15 | .0027 |
| 18800247 | IER3 | Immediate early response 3 | −1.15 | .0021 |
| 19524368 | SPACA4 | Sperm acrosome associated 4 | −1.14 | .0034 |
| 18889853 | YIF1A | Yip1 interacting factor homolog A (Saccharomyces cerevisiae) | −1.14 | .0003 |
Data presented indicate fold change in expression of PVS-sensitive genes following 8 weeks of oral PVS supplementation compared with corresponding baseline visits. Transcripts cluster ID, Affymetrix probe identifications. Data correspond to Figure 1A, cluster b (P < .05; FDR <5%).
Table 6.
List of ECM-Related Upregulated Genes in Skeletal Muscles Following Oral PVS Supplementation
| Transcript cluster ID | Gene symbol | Gene description | Fold change | P |
|---|---|---|---|---|
| 19015271 | TNXB | Tenascin XB | 1.78 | .0311 |
| 19011964 | TNXB | Tenascin XB | 1.76 | .0467 |
| 18807632 | TNXB | Tenascin XB | 1.75 | .0425 |
| 19018936 | TNXB | Tenascin XB | 1.74 | .0427 |
| 19012977 | TNXB | Tenascin XB | 1.71 | .0430 |
| 18907606 | DCN | Decorin | 2.23 | .0186 |
| 18907622 | DCN | Decorin | 1.09 | .0338 |
| 18874445 | MYOF | Myoferlin | 1.11 | .0207 |
| 18963453 | COL1A1 | Collagen, type I, alpha1 | 4.61 | .0149 |
| 18811819 | COL1A2 | Collagen, type I, alpha2 | 5.13 | .0076 |
| 18723098 | COL3A1/MIR3606 | Collagen, type III, alpha1/microRNA3606 | 5.18 | .0086 |
| 18735673 | COL5A2 | Collagen, type V, alpha2 | 1.62 | .0324 |
| 18738278 | COL6A3 | Collagen, type VI, alpha3 | 2.96 | .0122 |
| 18830114 | COL14A1 | Collagen, type XIV, alpha1 | 2.07 | .0061 |
| 18810809 | ELN | Elastin | 1.13 | .0187 |
| 18934479 | FBN1 | Fibrillin1 | 3.05 | .0254 |
| 18737057 | FN1 | Fibronectin1 | 3.65 | .0084 |
Data presented indicate fold change in expression of ECM-related genes following 8 weeks of oral PVS supplementation compared with corresponding baseline visits. Data correspond to Figure 1B (P < .05; FDR <5%).
ECM, extracellular matrix.
Validation of GeneChip data using RT-PCR
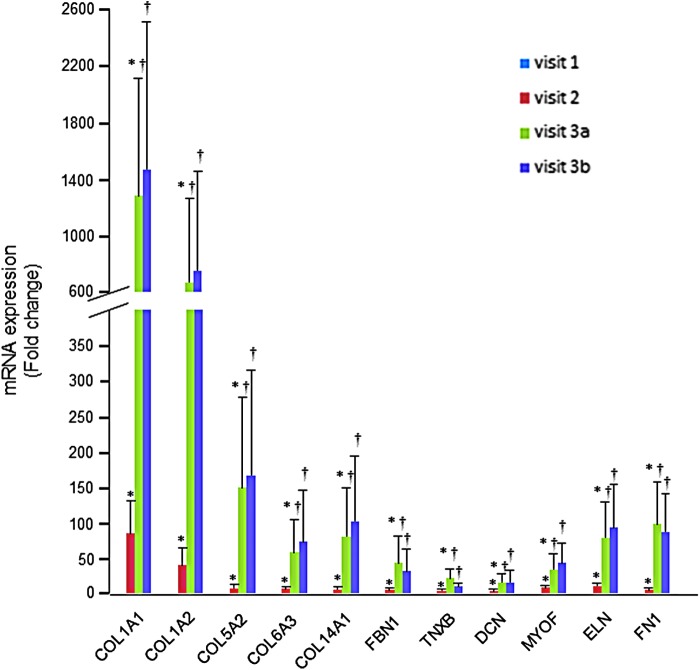
To validate the genes identified using microarray analysis, RT-PCR was performed. Since the above-mentioned ECM-related genes were upregulated, those genes were reexamined. Consistent with microarray results, significant upregulation of collagen and other ECM-associated genes was noted in the muscle samples following 8 weeks of oral PVS supplementation compared with the baseline visit (Fig. 2 and Table 7). Interestingly, additional 4 weeks of oral supplementation with exercise further induced the expression of the microarray-identified genes (Fig. 2 and Table 7).
FIG. 2.
RT-PCR validation of ECM-related genes derived from microarray analysis following oral PVS supplementation. Expression levels of selected collagen genes identified using GeneChip® analyses were independently verified using real-time quantitative (Q) PCR. The effects of oral PVS supplementation (250 mg/b.i.d.) were measured during the course of all visits; V1, baseline; V2, after 8 weeks of oral supplementation; V3a, additional (following 8 weeks of initial supplementation) 4 weeks of oral supplementation and exercise, sample collection before the final stint of exercise; and V3b, same as study visit 3, sample collected 30 min post-final bout of exercise. Data are mean ± SEM (n = 16); *P < .05 compared with the baseline visit and †P < .05 compared with 8 weeks. No significant changes were observed between pre and post 30-min final exercise on week 12. PCR, polymerase chain reaction.
Table 7.
Multivariate Test of All ECM-Related Genes (Jointly)
| Comparison | Contrast | Standard Error | Z | P |
|---|---|---|---|---|
| 8 weeks versus baseline | −3.68 | 1.22 | −3.02 | .003 |
| 9–12 weeks (pre-final exercise) versus baseline | −6.47 | 1.22 | −5.30 | <.001 |
| 9–12 weeks (pre-final exercise) versus 8 weeks | −2.79 | 1.22 | −2.29 | .022 |
| 9–12 weeks (post-final exercise) versus 8 weeks | −2.56 | 1.22 | −2.10 | .035 |
| 9–12 weeks (post-final exercise) versus 9–12 weeks (pre-final exercise) | 0.22 | 1.22 | 0.18 | .855 |
Discussion
Beneficial effects of Shilajit in skeletal muscle adaptation have been highlighted for centuries in ancient Ayurveda medicine.4,5 The underlying mechanisms of such adaptation have not been elucidated. The current study, for the first time, presents a mechanism of action of Shilajit in improving skeletal muscle adaptation in overweight/obese subjects exercising 30 min a day 3 days a week for 4 weeks. ECM plays an essential role in the development, maintenance, and regeneration of skeletal muscles.24,25 Chronic loading of muscles such as physical training leads to augmented synthesis and turnover of ECM.26 Oral supplementation of PVS markedly enhanced ECM-related gene expression in overweight/class I obese human subjects. PVS is one of the very few nutritional supplements that induce skeletal muscle adaptation through upregulation of ECM genes.
During obesity, there is an increase in circulating lipids (free fatty acids, triglycerides) that accumulate in muscle as triacylglycerol as well as fatty acid metabolites such as ceramide, diacylglycerol, and long-chain acyl CoA.27 No alterations in blood lipid profiles of subjects after supplementation suggest that PVS was well tolerated in this class of human subjects. Phosphocreatine (PCr) is one major source of ATP replenishment in tissues with rapidly shifting energy demand.28 The CK reaction mediates this supply, in which creatine and adenosine diphosphate (ADP) are reversibly phosphorylated to PCr and ATP, respectively.28 Functioning as a spatial and temporal buffer of ATP levels, the PCr–CK system requires a high level of total cellular creatine in mammal skeletal muscle.29 So, reduction in CK may disturb ATP formation within skeletal muscle. High intracellular creatine concentrations are achieved by a combination of exogenous dietary intake and endogenous production, followed by cellular uptake of creatine from blood vessels.29 The unaffected levels of serum CK in our study provided evidence for skeletal muscle integrity following oral supplementation. The high amounts of myoglobin in the skeletal and cardiac muscles enable storage and diffusion of oxygen in these tissues.30 The unchanged levels of serum myoglobin and blood glucose on all visits confirmed that PVS was well tolerated and maintained physiological body glucose metabolism, homeostasis, and muscle integrity in the skeletal muscle of overweight/class I obese human subjects.
Skeletal muscle is highly plastic and well known to undergo significant adaptive modifications in response to both endurance and resistance exercise.31–34 Increasing evidences now indicate that in response to nutrition, skeletal muscle undergoes adaptive changes through regulatory processes driven by changes in gene expression and cell signaling.35,36 ECM of skeletal muscle mainly comprises glycoproteins, collagen, and proteoglycans and plays a major role in mechanotransduction, that is, conducting force laterally between fibers and tendons.37–39 In our study, both microarray and RT-PCR results revealed elevated mRNA expression of collagen (type I, III, V, VI, and XIV) in response to oral PVS supplementation. The major structural protein in skeletal muscle ECM, collagen, comprises 1% to 2% of the muscle tissue and represents 6% of the weight of tendinous muscles. The skin comprises mainly collagen type I, which constitutes about 70% of collagen, with type III being 10% and trace amounts of collagen types IV, V, VI, and VII. With strenuous exercise, a rapid increase in the synthesis of collagen in tendons and muscles has been noted in mice and humans.26,40 Increase in the expression of COL1A2, COL3A1, and COL5A1 genes enhances cell proliferation and active remodeling of ECM in tissue repair.41 Exercise-induced ECM synthesis leads to protein degradation through increased matrix metalloproteinase (MMP) activity.26 Microarray data show an increase in MMP-2 gene expression in muscles following oral supplementation, suggesting that the effects of PVS supplementation on skeletal muscle adaptation are comparable with exercise by mediating specific synthesis and degradation of ECM. Growth factors such as TGF-β and IGF-1 are involved in regulation of ECM synthesis in connective tissue.26 The concentration of these growth factors has been shown to increase following exercise. Interestingly, increased expression of IGF1R was noted through microarray analysis. It is plausible that PVS induces ECM gene expression through comparable mechanisms. Further studies are required to determine exact mechanisms of PVS-induced ECM gene expression changes.
Although collagen provides the main structure, other ECM components also play an important role in skeletal muscle adaptation. In our study, both microarray and RT-PCR results revealed elevated mRNA expression of other ECM components including decorin, fibronectin, fibrillin, tenascin XB, myoferlin, and elastin, in response to oral PVS supplementation. Decorin is a small leucine-rich proteoglycan and contributes both to the formation and stabilization of collagen fibers in the perimysium that support muscle fibers assembled with myogenesis.42 Fibronectin plays a major role in synthesizing provisional granulation tissue during the early phases of wound repair.41 Fibrillin, a type of microfibril, is also one of the key structural elements in the ECM of skeletal muscle. Being widely distributed in connective tissues, fibrillins are arranged in tissue-specific architectures.43,44 The other ECM component, tenascin XB, determines the mechanical properties of collagen.45 During the development of muscles, especially during myoblast fusion, myoferlin is highly expressed46 and regulates the reutilizing of vascular endothelial growth factor receptor-2.47 The levels of myoferlin are generally less in adult skeletal muscle and almost lacking in healthy myofibers. An increase in the myoferlin level leads to a buildup of mononuclear myoferlin-positive myoblasts that play a key role in the repair of damaged myofibers, suggesting the importance of this gene in muscle repair and regeneration.46
Conclusions
The current study reports for the first time that oral supplementation of a natural product to overweight/class I obese human subjects resulted in skeletal muscle adaptation through upregulation of ECM-related genes that control muscle mechanotransduction properties, elasticity, repair, and regeneration.
Acknowledgments
Parts of this work were supported by National Institutes of Health awards, GM077185, NR015676, and NR013898, and by a research grant from Natreon, Inc., NJ, USA.
Author Disclosure Statement
The authors declare that PVS and partial research funding were provided by Natreon, Inc., NJ, USA.
References
- 1.Bloomer RJ: The role of nutritional supplements in the prevention and treatment of resistance exercise-induced skeletal muscle injury. Sports Med 2007;37:519–532 [DOI] [PubMed] [Google Scholar]
- 2.Velmurugan C, Vivek B, Wilson E, Bharathi T, Sundaram T: Evaluation of safety profile of black shilajit after 91 days repeated administration in rats. Asian Pac J Trop Biomed 2012;2:210–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhattacharya SK, Bhattacharya A, Chakrabarti A: Adaptogenic activity of Siotone, a polyherbal formulation of Ayurvedic rasayanas. Indian J Exp Biol 2000;38:119–128 [PubMed] [Google Scholar]
- 4.Stohs SJ: Safety and efficacy of shilajit (mumie, moomiyo). Phytother Res 2014;28:475–479 [DOI] [PubMed] [Google Scholar]
- 5.Ghosal S, Reddy JP, Lal VK: Shilajit I: Chemical constituents. J Pharm Sci 1976;65:772–773 [DOI] [PubMed] [Google Scholar]
- 6.Meena H, Pandey HK, Arya MC, Ahmed Z: Shilajit: A panacea for high-altitude problems. Int J Ayurveda Res 2010;1:37–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhattacharya S, Pal D, Gupta AK, Ganguly P, Majumder UK, Ghosal S: Beneficial effect of processed Shilajit on swimming exercise induced impaired energy status of mice. Pharmacologyonline 2009;1:817–825 [Google Scholar]
- 8.Bhattacharyya S, Pal D, Banerjee D, et al. : Shilajit dibenzo-α-pyrones: Mitochondria targeted antioxidants. Pharmacologyonline 2009;2:690–698 [Google Scholar]
- 9.Hargreaves M, Cameron-Smith D: Exercise, diet, and skeletal muscle gene expression. Med Sci Sports Exerc 2002;34:1505–1508 [DOI] [PubMed] [Google Scholar]
- 10.Visser SA: Effect of humic substances on mitochondrial respiration and oxidative phosphorylation. Sci Total Environ 1987;62:347–354 [DOI] [PubMed] [Google Scholar]
- 11.Uusi-Rasi K, Sievanen H, Kannus P, Pasanen M, Kukkonen-Harjula K, Fogelholm M: Influence of weight reduction on muscle performance and bone mass, structure and metabolism in obese premenopausal women. J Musculoskelet Neuronal Interact 2009;9:72–80 [PubMed] [Google Scholar]
- 12.Hilton TN, Tuttle LJ, Bohnert KL, Mueller MJ, Sinacore DR: Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: Association with performance and function. Phys Ther 2008;88:1336–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bollinger LM, Powell JJ, Houmard JA, Witczak CA, Brault JJ: Skeletal muscle myotubes in severe obesity exhibit altered ubiquitin-proteasome and autophagic/lysosomal proteolytic flux. Obesity (Silver Spring) 2015;23:1185–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandit S, Biswas S, Jana U, De RK, Mukhopadhyay SC, Biswas TK: Clinical evaluation of purified Shilajit on testosterone levels in healthy volunteers. Andrologia 2016;48:570–575 [DOI] [PubMed] [Google Scholar]
- 15.Dudley GA, Abraham WM, Terjung RL: Influence of exercise intensity and duration on biochemical adaptations in skeletal muscle. J Appl Physiol Respir Environ Exerc Physiol 1982;53:844–850 [DOI] [PubMed] [Google Scholar]
- 16.Smith GI, Atherton P, Reeds DN, et al. : Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: A randomized controlled trial. Am J Clin Nutr 2011;93:402–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khanna S, Das A, Spieldenner J, Rink C, Roy S: Supplementation of a standardized extract from Phyllanthus emblica improves cardiovascular risk factors and platelet aggregation in overweight/class-1 obese adults. J Med Food 2015;18:415–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roy S, Khanna S, Rink C, Biswas S, Sen CK: Characterization of the acute temporal changes in excisional murine cutaneous wound inflammation by screening of the wound-edge transcriptome. Physiol Genomics 2008;34:162–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roy S, Patel D, Khanna S, et al. : Transcriptome-wide analysis of blood vessels laser captured from human skin and chronic wound-edge tissue. Proc Natl Acad Sci U S A 2007;104:14472–14477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roy S, Biswas S, Khanna S, et al. : Characterization of a preclinical model of chronic ischemic wound. Physiol Genomics 2009;37:211–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roy S, Khanna S, Wallace WA, et al. : Characterization of perceived hyperoxia in isolated primary cardiac fibroblasts and in the reoxygenated heart. J Biol Chem 2003;278:47129–47135 [DOI] [PubMed] [Google Scholar]
- 22.Roy S, Khanna S, Yeh PE, et al. : Wound site neutrophil transcriptome in response to psychological stress in young men. Gene Expr 2005;12:273–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Affymetrix. www.affymetrix.com/catalog/prod760002/AFFY/Human-Transcriptome-Array-2.0#1_1 (accessed on August9, 2015)
- 24.Buck CA, Horwitz AF: Cell surface receptors for extracellular matrix molecules. Annu Rev Cell Biol 1987;3:179–205 [DOI] [PubMed] [Google Scholar]
- 25.Purslow PP: The structure and functional significance of variations in the connective tissue within muscle. Comp Biochem Physiol A Mol Integr Physiol 2002;133:947–966 [DOI] [PubMed] [Google Scholar]
- 26.Kjaer M, Magnusson P, Krogsgaard M, et al. : Extracellular matrix adaptation of tendon and skeletal muscle to exercise. J Anat 2006;208:445–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams JM, 2nd, Pratipanawatr T, Berria R, et al. : Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 2004;53:25–31 [DOI] [PubMed] [Google Scholar]
- 28.O'Connor RS, Steeds CM, Wiseman RW, Pavlath GK: Phosphocreatine as an energy source for actin cytoskeletal rearrangements during myoblast fusion. J Physiol 2008;586(Pt 12):2841–2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wyss M, Kaddurah-Daouk R: Creatine and creatinine metabolism. Physiol Rev 2000;80:1107–1213 [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Tong J, Zweier JR, et al. : Differences in oxygen-dependent nitric oxide metabolism by cytoglobin and myoglobin account for their differing functional roles. FEBS J 2013;280:3621–3631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holloszy JO, Coyle EF: Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol Respir Environ Exerc Physiol 1984;56:831–838 [DOI] [PubMed] [Google Scholar]
- 32.Knuiman P, Hopman MT, Mensink M: Glycogen availability and skeletal muscle adaptations with endurance and resistance exercise. Nutr Metab (Lond) 2015;12:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tesch PA: Skeletal muscle adaptations consequent to long-term heavy resistance exercise. Med Sci Sports Exerc 1988;20(5 Suppl):S132–S134 [DOI] [PubMed] [Google Scholar]
- 34.Rockl KS, Hirshman MF, Brandauer J, Fujii N, Witters LA, Goodyear LJ: Skeletal muscle adaptation to exercise training: AMP-activated protein kinase mediates muscle fiber type shift. Diabetes 2007;56:2062–2069 [DOI] [PubMed] [Google Scholar]
- 35.Hawley JA, Burke LM, Phillips SM, Spriet LL: Nutritional modulation of training-induced skeletal muscle adaptations. J Appl Physiol (1985) 2011;110:834–845 [DOI] [PubMed] [Google Scholar]
- 36.Muoio DM, Koves TR: Skeletal muscle adaptation to fatty acid depends on coordinated actions of the PPARs and PGC1 alpha: Implications for metabolic disease. Appl Physiol Nutr Metab 2007;32:874–883 [DOI] [PubMed] [Google Scholar]
- 37.Fomovsky GM, Thomopoulos S, Holmes JW: Contribution of extracellular matrix to the mechanical properties of the heart. J Mol Cell Cardiol 2010;48:490–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Purslow PP, Trotter JA: The morphology and mechanical properties of endomysium in series-fibred muscles: Variations with muscle length. J Muscle Res Cell Motil 1994;15:299–308 [DOI] [PubMed] [Google Scholar]
- 39.Street SF: Lateral transmission of tension in frog myofibers: A myofibrillar network and transverse cytoskeletal connections are possible transmitters. J Cell Physiol 1983;114:346–364 [DOI] [PubMed] [Google Scholar]
- 40.Miller BF, Olesen JL, Hansen M, et al. : Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol 2005;567(Pt 3):1021–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinez DA, Vailas AC, Vanderby R, Jr., Grindeland RE: Temporal extracellular matrix adaptations in ligament during wound healing and hindlimb unloading. Am J Physiol Regul Integr Comp Physiol 2007;293:R1552–R1560 [DOI] [PubMed] [Google Scholar]
- 42.Nishimura T, Futami E, Taneichi A, Mori T, Hattori A: Decorin expression during development of bovine skeletal muscle and its role in morphogenesis of the intramuscular connective tissue. Cells Tissues Organs 2002;171:199–214 [DOI] [PubMed] [Google Scholar]
- 43.Corson GM, Charbonneau NL, Keene DR, Sakai LY: Differential expression of fibrillin-3 adds to microfibril variety in human and avian, but not rodent, connective tissues. Genomics 2004;83:461–472 [DOI] [PubMed] [Google Scholar]
- 44.Sakai LY, Keene DR, Engvall E: Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J Cell Biol 1986;103(6 Pt 1):2499–2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Margaron Y, Bostan L, Exposito JY, et al. : Tenascin-X increases the stiffness of collagen gels without affecting fibrillogenesis. Biophys Chem 2010;147:87–91 [DOI] [PubMed] [Google Scholar]
- 46.Davis DB, Doherty KR, Delmonte AJ, McNally EM: Calcium-sensitive phospholipid binding properties of normal and mutant ferlin C2 domains. J Biol Chem 2002;277:22883–22888 [DOI] [PubMed] [Google Scholar]
- 47.Bernatchez PN, Sharma A, Kodaman P, Sessa WC: Myoferlin is critical for endocytosis in endothelial cells. Am J Physiol Cell Physiol 2009;297:C484–C492 [DOI] [PMC free article] [PubMed] [Google Scholar]