Abstract
Significance: Acute kidney injury (AKI) and chronic kidney disease (CKD) represent a considerable burden in healthcare. The heme oxygenase (HO) system plays an important role in regulating oxidative stress and is protective in a variety of human and animal models of kidney disease. Preclinical studies of the HO system have led to the development of several clinical trials targeting the enzyme or its products. Recent Advances: Connection of HO, ferritin, and other proteins involved in iron regulation has provided important insight into mechanisms of damage in AKI. Also, HO-1 expression is important in the pathogenesis of hypertension, diabetic kidney disease, and progression to end-stage renal disease. Critical Issues: Despite intriguing discoveries, no drugs targeting the HO system have been translated to the clinic. Meanwhile, treatments for AKI and CKD are urgently needed. Many factors have likely contributed to challenges in clinical translation, including variation in animal models, difficulties in obtaining human tissue, and complexity of the disease processes being studied. Future Directions: The HO system represents a promising avenue of investigation that may lead to targeted therapeutics. Tissue-specific gene modulation, widening the scope of animal studies, and continued clinical research will provide valuable insight into the role HO plays in kidney homeostasis and disease. Antioxid. Redox Signal. 25, 165–183.
Introduction
Heme oxygenase (HO) was first identified in the late 1960s in the microsomal compartment, where liver microsomes converted heme to biliverdin (134, 135). In 1992, Nath et al. discovered that HO-1 is robustly induced in response to acute kidney injury (AKI) and is a protective response (100). Many studies followed supporting the imperative function of HO in mitigating oxidative stress systemically, as well as in kidney health and disease. HO catalyzes the rate-limiting step of heme degradation yielding equimolar amounts of biliverdin, carbon monoxide (CO), and iron (150), and the products are bioactive.
Insult or injury resulting in cellular stress destabilizes intracellular heme proteins and results in the generation of reactive oxygen species (ROS) and lipid peroxidation. The kidney has high metabolic activity and maintains a relatively hypoperfused medulla. Therefore, one may conclude it is highly challenged with oxidizing agents. Extensive evidence exists for the importance of HO in maintaining homeostasis in the presence of oxidative stress, and it is involved in the pathophysiology of many organ systems. Due to this, interest in HO continues to grow rather exponentially (Figs. 1 and 2). This review will update the reader on new findings regarding the role of HO in kidney homeostasis and pathology.
FIG. 1.
Publication count for articles on HO in the MEDLINE database. Publications per year returned from PubMed when searching: [(Heme oxygenase OR Haem oxygenase) OR (HO-1 OR HO-2 OR HO-3 OR HMOX1 OR HMOX2)]. Frequency distribution histogram is shown. HMOX1, human heme oxygenase-1 gene; HMOX2, human heme oxygenase-2 gene; HO, heme oxygenase.
FIG. 2.
Publication count for articles on HO and the kidney in the MEDLINE database. Publications per year returned from PubMed when searching: “heme oxygenase AND kidney,” search term: [(Heme oxygenase OR Haem oxygenase) OR (HO-1 OR HO-2 OR HO-3 OR HMOX1 OR HMOX2)] AND (Kidney[MeSH] OR Kidney[tiab] OR Renal[tiab]). Frequency distribution histogram is shown.
Biochemistry of HO
HO is a microsomal cytoprotective enzyme that is induced in response to injury and cellular stress [reviewed in Refs. (47, 48, 99, 146)]. Importantly, HO mitigates toxicity by degrading heme released from heme-containing proteins, including hemoglobin, myoglobin, and cytochromes, utilizing NADPH and molecular oxygen as cofactors (Fig. 3) (150). The enzymatic mechanism has been elucidated (150), and structural studies provide insight into function (12, 42, 120). Bilirubin is a free radical scavenger that blocks lipid peroxidation (3, 126, 139). CO is a physiologically important vasodilator, acting via cyclic guanosine monophosphate (cGMP) (69, 163). Iron induces ferritin (Ft), which is protective (23). While each product exhibits beneficial properties, potential adverse effects may result if they reach high levels (Fig. 3).
FIG. 3.
HO enzymatic reaction. HO catabolizes the breakdown of heme into CO, iron, and biliverdin, which is further converted to bilirubin by biliverdin reductase. The products of this system are bioactive and play important roles in redox homeostasis, kidney health, and diseases with both beneficial and potential adverse effects. CO, carbon monoxide; HO-1, heme oxygenase-1, ROS, reactive oxygen species.
Three reported isoforms of HO include HO-1, −2, and −3 (2, 86, 93, 94). HO-1 is the highly characterized, inducible form, and its role in kidney health and disease is a focus of this review. The human HO-1 gene is 13 kb in length and its locus is 22q12 (HGNC:5013). It encodes a 288 amino acid monomer with molecular mass of 32 kDa (UniProtKB P09601). Post-translational modifications affecting the size of HO-1 have been reported and are important for cellular function (82). HO-2 is constitutively expressed at low levels in the nervous system, reproductive system, cardiovascular system, kidney, and liver (63, 87, 156, 163). The human HO-2 gene is 36 kb in length, is located on 16p13 (HGNC: 5014), and encodes a 36 kDa monomer consisting of 315 amino acids (UniProtKB: P30519). Three protein isoforms (a, b, and c) have been reported (Entrez Gene: 3163). Recently, endothelial induction of HO-2 was demonstrated in response to vascular stress in an arteriovenous (AV) fistula model (63). The HO-3 gene was found to produce no functional protein and was subsequently identified as a pseudogene (52).
Induction of HO-1
The kidney encounters toxic oxidants, endogenous and exogenous, as well as bound iron from the circulation. Free heme-induced oxidative stress is an important physiologic mechanism of induction of HO-1 [reviewed in Ref. (125)]. In addition, non-heme molecules such as heavy metals, endotoxin, UV radiation, prostaglandins, H2O2, certain growth factors, cytokines, and other stimuli can induce HO-1 in the kidney (8, 125). Ft is coinduced with HO-1 and both play a key role in iron homeostasis and the oxidative stress response (4, 13, 23, 100, 158).
The classical means of induction of renal HO activity is treatment with the heme analog, hemin (78). Tin protoporphyrin (SnPP) and zinc protoporphyrin (ZnPP) have been used to inhibit HO activity (91, 134). Several protoporphyrins modulate HO activity; however, these molecules have off-target effects and have a narrow therapeutic window. More sophisticated recombinant animal models have been developed to study the role of HO-1 in a variety of biological processes (59, 68). HO-1 is induced in animal models of ischemia, hypertension, glomerulonephritis, AKI, and chronic kidney disease (CKD) (4, 5, 61, 100, 141).
Nuclear factor erythroid 2-related factor 2 (Nrf2) is an important regulator of HO-1 transcription in response to oxidative stress. It binds to stress response elements in the gene promoter (7, 8). In mice, the Bach1 transcription factor represses HO-1 expression by competing with Nrf2 binding sites in the HO-1 promoter region. In steady state, Nrf2 is bound to a cytosolic repressor called Kelch-like ECH-associated protein 1 (Keap1), which aids in ubiquitination and proteolytic degradation of Nrf2. During oxidative stress, Keap1 is covalently modified by electrophiles and ROS at its cysteine residues, causing release of Nrf2 and decreased targeting for proteasomal degradation (128). This permits nuclear translocation of Nrf2 and promotes HO-1 transcription in the setting of increased oxidant burden.
Several reports have elucidated the molecular regulation of HO-1 expression, which is robust and complex [reviewed in Refs. (47, 125)]. HO-1 is tightly regulated at the transcriptional level [reviewed in Ref. (125)]. To study in vivo regulation of HO-1 expression, our laboratory generated a humanized HO-1 transgenic mouse model, utilizing a bacterial artificial chromosome (BAC) containing the entire human HO-1 gene, including its regulatory sequences. When crossed with HO-1-deficient mice, the resultant strain globally expressed human HO-1 (humanized BAC [hBAC] mice), but not endogenous murine HO-1 and was able to rescue the phenotype of the HO-1−/− mouse (Fig. 4) (68). The murine transcription factors specificity protein 1 (Sp1), JunB (activator protein-1 family, AP-1), and upstream stimulatory factor (USF)-1 and −2 regulated human HO-1 gene expression in this model (68).
FIG. 4.
Generation of the humanized HO-1 BAC Tg mouse. HO-1−/− males were crossed with HO-1 BAC Tg female mice to generate hBAC. The HO-1 BAC Tg mice express an ∼87 kb BAC DNA containing the entire HO-1 gene with flanking sequences. Progeny expressed human HO-1 and did not express mouse HO-1. These mice offer an opportunity to study regulation and function of the human HO-1 gene in mice. hBAC, humanized bacterial artificial chromosome; Tg, transgenic.
HO-1 expression is regulated differently in humans and mice (Table 1). For example, heat shock induces HO-1 in mice but not in humans (121, 122). In mice, hypoxia and cytokines such as interferon-γ (IFN-γ) are potent inducers of HO-1, whereas they are repressors in humans (71, 121). In humans, a GT-repeat polymorphism in the HO-1 promoter modulates the level of transcription (27, 70, 148). Long polymorphisms, defined as greater than 25–27 repeats (L allele), in this region are associated with lower inducibility of HO-1 and increased risk of disease (14, 27, 30, 107, 148). Significant correlation with disease was identified in the settings of renal transplantation, diabetic kidney disease, CKD progression, AV fistula failure, and others (30, 76, 81, 107, 148). Recent evidence demonstrated microRNAs are involved in the regulation of human HO-1 expression in vitro (15). The promoter sequence of the human HO-1 gene has binding sites for several transcription factors, including nuclear factor-κB (NF-κB), AP-1 and −2, Sp1, USF-1 and −2, and interleukin (IL)-6. These sites include response elements for STATx, c-Rel, hepatocyte factor-1 and −4, and GATA-X (75, 98, 119, 129, 130, 138). In human renal epithelial cells, an intronic enhancer controls HO-1 expression via chromatin looping and Sp1-dependent interaction with the −4.0 kb HO-1 promoter region (36).
Table 1.
Differences Between the Regulation of the Mouse and Human HO-1 Genes
| Feature | Mouse HO-1 gene | Human HO-1 gene |
|---|---|---|
| Promoter elements | E1 and E2 regionsa (all stimuli) | A and B regionsb (limited stimuli) |
| GT repeat region in promoter | Absent | Present |
| Additional regulatory elements | None detected | Internal enhancer |
| Heme response element | E1 and E2 regions | Internal enhancer + A and B regions |
| Cadmium response element | (T/C)GCTGAGTCA (E1 region) | TGCTAGATTT (region A) |
| Response to hyperoxia | E1 region | A and B regions are nonfunctional |
| Responsive to hypoxia | Gene activation | Gene repression |
| Responsive to interferon γ | Gene activation | Gene repression |
| Responsive to heat shock | Gene activation | Nonresponsive |
| Response to oxidized lipids | E1 region | Region between −9.1 and −11.6 kb |
| Response to hyperosmolarity | Gene activation | Gene repression |
Reproduced with permission from Sikorski et al. (125).
E1 and E2 = −4.0 and −10 kb distal promoter elements upstream of the HO-1 gene in mice.
A and B regions = analogous to E1 and E2 in mice. −4.0 and −9 kb distal promoter elements for human HO-1 gene containing stress and cadmium response elements.
HO, heme oxygenase.
Cellular Localization of HO-1
While HO-1 localizes predominantly to the endoplasmic reticulum, expression in the nuclear (18, 82), mitochondrial (17, 21), and secretory (83, 151) subcellular compartments has been reported. In the setting of hypoxia- or heme-mediated oxidative stress in vitro, HO-1 localized to the nucleus and modulated transcription of oxidative stress response genes (82). Cleavage of a hydrophobic C-terminal domain caused HO-1 to localize to the nucleus in cell culture. There, nuclear HO-1 protected the Nrf2 transcription factor from glycogen synthase kinase 3β-mediated phosphorylation and subsequent degradation (18). HO-1 targeted to mitochondria was protective against hypoxia-induced oxidative damage in human embryonic kidney cells. Protection was superior in mitochondrial-targeted HO-1 overexpressing cells compared with cytoplasmic overexpression (21). Bindu et al. found mitochondrial expression of HO-1 in a rat model of gastric mucosal injury, which protected from mitochondrial stress, improved cellular respiratory function, and prevented apoptosis (17). Zager et al. showed that plasma and urinary HO-1 levels positively correlated with renal cortical expression in several models of AKI, suggesting HO-1 as a candidate AKI biomarker (155).
Localization of HO-1 in the Kidney
HO-1 is induced in a variety of kidney substructures in response to injury, including proximal tubules, glomeruli, and renal interstitium, as well as in renal mononuclear phagocytes (RMPs) (4–6, 59, 65, 100). We define RMPs here as tissue-resident and infiltrating macrophages, dendritic cells, and monocytes. In most models of AKI, HO-1 is induced predominantly in renal proximal tubules. HO-1 expression is seen in glomeruli in models of diabetes (6) and in infiltrating macrophages in acute renal transplant rejection (5). Understanding limitations in our approach, we categorized renal pathologies by kidney substructure (Table 2), and implications of expression of HO in each are discussed.
Table 2.
Renal Pathologies Associated with the Heme Oxygenase System
| Kidney substructure affected | Pathology | References |
|---|---|---|
| Glomerular diseases | Diabetic kidney disease | 1, 6, 51, 77, 80, 105, 116 |
| Lupus nephritis | 84, 132 | |
| Nephrotoxic nephritis | 34, 96, 141 | |
| Tubular disease | Polycystic kidney disease | 92, 162 |
| Acute kidney injury | ||
| Ischemia–reperfusion injury | 31, 41, 49, 59, 89, 109, 123, 127, 140 | |
| Rhabdomyolysis | 19, 68, 100, 104, 140, 158 | |
| Sepsis and endotoxin-induced injury | 74, 145 | |
| Cisplatin nephrotoxicity | 4, 68, 124, 159 | |
| Radiocontrast-induced nephropathy | 45 | |
| Gentamicin nephrotoxicity | 4, 133 | |
| Mercuric chloride nephrotoxicity | 101, 149 | |
| Cyclosporine nephrotoxicity | 56, 110, 112, 113 | |
| Interstitial renal disease | Renal transplant rejection | 5, 40, 85, 107, 137, 142 |
| Obstructive uropathy | 32, 65, 67 | |
| Chronic kidney disease | 10, 30, 60, 154 | |
| Vascular disease | Hypertension | 28, 61, 72, 78, 90, 97, 147, 163 |
| Sickle cell nephropathy | 16, 44, 103, 118 | |
HO-2: The Constitutive Isoform of HO
The promoter region of the HO-2 gene contains a glucocorticoid response element, which regulates HO-2 expression in the brain (88, 111). In addition, cellular oxygen tension regulates HO-2 expression in endothelial cells (46, 53). Post-translational modifications, such as casein kinase 2-dependent phosphorylation, also affect HO-2 activity in vitro in hippocampal cells (20).
In 1997, Maines described heme regulatory motifs (HRMs) in HO-2 (87), proposing that the protein might serve as a cellular redox sensor, in addition to catalyzing a regulated step in redox signaling. These HRMs are independent of the enzymatic active site and bind heme with higher affinity (87). Yuan et al. recently substantiated the role of HO-2 and HRMs in redox signaling and demonstrated a role in control of respiratory rate (152). Biochemical and structural NMR studies further supported the conclusion that HRMs in HO-2 allow cellular sensing and response to redox changes (12, 42).
Changes in HO-2 induction occur with age. Increasing age results in a decreased ability to induce renal HO-2 (102). HO-2 knockout mice challenged with heme proteins demonstrated worse renal function, increased histologic damage, and greatly increased elaboration of pro-inflammatory IL-6 compared with wild-type controls (102). Thus, a decreased induction of HO-2 may be related to increased sensitivity to heme protein toxicity in aging mice.
HO-1 and Autophagy
Autophagy is defined as a lysosome-mediated degradation of cellular material, such as proteins and organelles [reviewed in Ref. (25)], a process critical for maintaining cellular homeostasis. Dysregulated, prolonged, or incomplete autophagy results in accumulation of damaged cellular material, eventually leading to cell death. It is a highly complex process starting with the formation of autophagosomes and terminating with fusion to lysosomes (79).
Mammalian markers of autophagy include autophagy proteins Atg5, Atg6 (beclin1, Becn1), Atg7, and Atg8 (LC3, Map1lc3). Oxidative stress can trigger autophagy and lead to cell survival or death [reviewed in Ref. (117)]. A role for HO-1 mediating autophagy in response to oxidative stress has been proposed (Fig. 5). Autophagy and HO-1 induction are protective against injury to renal proximal tubular cells (RPTCs) mediated by cisplatin (CP) (22, 64, 108). Kidneys of HO-1 knockout mice show increased numbers of autophagic vesicles both basally and after CP administration (Fig. 6) (22). In CP-treated primary RPTCs from wild-type mice, Atg5, −6, −7, and −8 were elevated. However, RPTCs from CP-treated HO-1 knockout mice did not exhibit upregulation of these autophagic markers and instead showed increased apoptosis. Overexpression of human HO-1 in hBAC mice reversed maladaptive changes, including low levels of autophagic activity (22). These data collectively suggest that HO-1 induction promotes autophagy and cell survival in CP nephrotoxicity.
FIG. 5.
The role of HO-1 in autophagy. During acute kidney injury, increased ROS cause destabilization of heme proteins resulting in elevated free heme levels in the cell. Autophagy and HO-1 are induced to overcome the oxidative stress and serve as an adaptive response to promote survival and protect from apoptotic cell death (black arrows). In the absence of HO-1 (black dashed arrows), the oxidative environment in the cell is augmented and the cell experiences increased heme load and oxidative stress, thereby leading to dysregulated autophagy and cell death. Experimental overexpression of HO-1 (gray dash-dot arrows) limits heme accumulation, ROS generation, and oxidative stress during injury and delays the onset of autophagy, promoting survival. Reproduced with permission from Bolisetty et al. (22).
FIG. 6.
HO-1−/− mice demonstrate increased autophagic vacuoles. Electron micrographs showing renal proximal tubular cells from HO-1+/+ and HO-1−/− mice kidneys treated with cisplatin or vehicle. Upper panels: kidney cortex. Magnification ×12000; scale bar = 2 μm. Lower panels: kidney cortex at higher power. Magnification ×30000; scale bar = 500 nm. Arrows indicate autophagosomes with double membrane-bound vesicles. Reproduced with permission from Bolisetty et al. (22).
HO Mediation of Vascular Tone: A Role for CO
Early studies demonstrated that heme-induced expression of HO in spontaneously hypertensive rats decreases blood pressure, an effect that is reversed with HO inhibition (90). CO, one of the products of the HO reaction, regulates vascular tone (69). The contribution of CO to relaxation of vascular tone was particularly important in a diabetic kidney disease model, characterized by low renal NO bioavailability (24). CO mediates vasoactive effects through synthesis of cGMP, activation of K+ channels, and blockade of vasoconstrictive endothelin-1. CO production by HO stimulates angiogenesis. Also, it is anti-inflammatory, antiapoptotic, antithrombotic, and exhibits antioxidant properties. Dulak et al. have extensively reviewed the diverse functions of CO in this context (38).
HO in Renal Inflammation
Early studies demonstrated that inflammation in glomeruli induced HO-1 in the renal tubules (96, 141). Hemin treatment reduced glomerular inflammation in a model of nephrotoxic nephritis (NTN) (96). The role of specific cytokines and molecular signals in modulating inflammation will be discussed in a disease-specific context. Recently, an important role for HO activity in the RMP compartment has been demonstrated.
HO in mononuclear phagocyte-mediated renal inflammation
HO-1 is induced in RMPs, in addition to renal parenchymal cells, in the setting of injury (59, 100). RMPs include tissue-resident leukocytes that perform homeostatic functions and those that transmigrate during inflammation or injury. The predominant functions of RMPs are phagocytosis (66), coordination of the immune response, and production of cytokines (58).
In kidney injury, oxidative stress results in destabilization of intracellular heme proteins, organelle damage, and cell death. HO activity is beneficial in oxidative stress due to production of antioxidant bilirubin (57) and vasodilatory CO (55, 69), both of which are anti-inflammatory. In addition, induction of HO-1 is associated with increased Ft levels, which exhibits anti-inflammatory effects as well (23).
RMP trafficking and antigen presentation may be related to HO activity. Global HO-1 knockout animals demonstrated greater homing of tissue-resident RMPs to primary lymphoid organs after injury (59). Also, HO-1 inhibited autophagy, a process necessarily preceding antigen presentation (22). It may be that tissue-resident RMPs contribute to immune tolerance when appropriate, as has been suggested for tissue-resident mononuclear phagocytes (MPs) in the liver (37). MPs support integration of the innate and adaptive immune systems; they contribute to differentiation and activation of lymphocyte subsets, including T lymphocytes and innate lymphoid cells (11). This may be due to effects beyond the elaboration of enzymatic products. Putative signaling mechanisms are linked to redox status (58, 144). Further studies are needed to obtain a full understanding of the role HO plays in kidney inflammation and homeostasis.
HO-1 in Kidney Disease
Glomerular diseases
Diabetic kidney disease
ROS are important in multiple pathways in the pathogenesis of diabetes and its complications (26, 106). On one hand, induction of antioxidant proteins is protective, while blockade or decreased activity worsens damage in diabetic kidneys in animal models and humans (33, 77, 95). In humans, serum bilirubin levels were inversely correlated with progression of kidney disease in type II diabetics in two large-scale clinical trials (115), suggesting bilirubin is a target biomarker for progression of diabetic kidney disease (57).
Streptozotocin (STZ) is a pancreatic islet toxin that is used to induce a model of type I diabetes in animals (136). Within the kidney, HO-1 is induced specifically in the glomeruli in this model (6, 77). Lee et al. provided evidence for functional importance of renal induction of HO-1 in diabetes (77). The authors showed that the enzymatic activity was increased in diabetic rat kidney glomeruli and cultured podocytes stimulated with high glucose. ZnPP, an inhibitor of HO activity, worsened apoptosis of podocytes in vivo and in vitro (77). Inhibition of HO activity also increased albuminuria (77). Induction of HO-1 with hemin abrogated podocyte apoptosis (77). They suggested hypoxia inducible factor (HIF)-1 as an upstream signaling molecule for induction of HO-1 and demonstrated a positive correlation between HIF-1 and HO-1 in diabetic rat glomeruli and glucose-stimulated podocytes (77). In STZ diabetic mice, hemin treatment induced HO-1. In addition to increasing atrial natriuretic peptide (ANP) and adiponectin, treatment caused a decrease in the pro-inflammatory cytokines tumor necrosis factor-α (TNF-α), IL-1β, IL-6, redox response transcription factor NF-κB, and markers of fibrosis (105).
Mouse mesangial cells treated with high glucose demonstrated increased ROS levels, transforming growth factor-β1 (TGF-β1) expression and proliferation (80). Inducing expression of Nrf2 caused a decrease in markers of ROS and oxidant-mediated damage in the presence of high glucose. Upon Nrf2 induction, increased levels of HO-1 and glutamate cysteine ligase, an enzyme involved in antioxidant glutathione synthesis, were observed (80). Upregulation of Nrf2 may provide an avenue for therapeutics for diabetic kidney disease. In a large multicenter trial, patients with type II diabetes and CKD were treated with a potent Nrf2 activator, bardoxolone methyl, or placebo. Although initial results were encouraging, the trial was terminated before completion due to increased risk of cardiovascular events in the treatment group (35).
Oxidative stress plays an important role in early pathological changes in the kidney in STZ diabetic rats (116). Rodriguez et al. demonstrated that the HO system regulates hemodynamics in diabetic rats with hyperglycemia. Treatment with the antioxidant tempol showed similar responses as seen with HO-1 induction (116). Thus, HO-1 may be beneficial in diabetic kidney disease due to its ability to restore the redox health of the tissue. The authors demonstrated low renal cortical NO, vasoconstriction, and hypoperfusion in diseased animals. Induction of HO or treatment with CO reversed these adverse changes by attenuating detrimental vasoconstriction (116).
Hayashi et al. used STZ-induced diabetes in rats and evaluated a number of antioxidant enzymes, including HO-1, catalase, glutathione peroxidase, and zinc–copper superoxide dismutase (51). They showed that HO-1 expression was specifically increased. Insulin and vitamin E treatment reversed induction of HO-1, suggesting that hyperglycemia and oxidative stress were involved. Immunohistochemistry studies provided evidence that podocytes and mesangial cells expressed HO-1 in this model (51).
Akita hyperglycemic mice develop diabetic kidney disease (1). These mice have a heterozygous deletion for the insulin gene and demonstrate induction of renal HO-1. Abdo et al. overexpressed catalase in Akita transgenic mice, decreasing oxidative stress, and found lower levels of Nrf2 expression. Catalase overexpression coincided with lower levels of HO-1 induction, providing evidence that oxidative stress contributes to renal induction of HO-1 in the Akita model of diabetes (1).
These studies collectively demonstrate a protective role for HO in diabetic kidney disease. HO provides this protection via diverse mechanisms. HO-1 induction mitigates oxidative stress-mediated damage, inflammation, vasoconstriction, glomerular histopathologic changes, and decreased kidney function, all of which are important in the pathogenesis of diabetic kidney disease.
Lupus nephritis
Tissue-resident and monocyte-derived MPs are important in the pathogenesis of renal disease in systemic lupus erythematosus (SLE). Significant MP infiltration occurs in lupus nephritis (43, 73), but MPs appear to function poorly because they fail to clear apoptotic fragments. Poor phagocytic function is thought to drive the development of autoreactive anti-double-stranded DNA (anti-dsDNA) antibodies. Patients with SLE show reduced expression of HO-1 in circulating monocytes (54), raising the possibility of a connection between myeloid cell HO-1 expression and lupus nephritis.
Recently, Mackern-Oberti et al. have demonstrated a beneficial role for HO activity in FcγRIIb−/− mice, a model for SLE (84). The FcγR is found on MPs and is important for their phagocytic function. These mice develop proteinuria and renal inflammation that is improved with HO-1 induction or CO treatment (84). The authors showed that HO-1 mRNA transcription was decreased in CD11b+, CD11c+, and CD4+ inflammatory cells in the spleens of FcγRIIb−/− mice relative to controls. CD11b+ cells expanded in the spleens of FcγRIIb−/−, and CO treatment decreased CD11b+ cell numbers and CD11b+ Gr-1+ neutrophils. FoxP3 transcription was reduced and the proportion of CD4+ FoxP3+ Treg cells was reduced in the spleen and inguinal nodes of FcγRIIb−/− mice. This provided evidence for an anti-inflammatory and renoprotective role for HO-1 in a mouse model of lupus nephritis (84).
Takeda et al. used MRL/lpr mice to model SLE. These mice develop impaired kidney function and glomerular injury. Induction of HO-1 with hemin resulted in decreased proteinuria, reduced glomerular immune complex deposition, and increased expression of inducible nitric oxide synthase (iNOS) in the spleen and kidney of diseased mice. Serology showed decreased levels of anti-dsDNA immunoglobulin (132), providing evidence that HO-1 induction changes the immune status and thus the pathogenesis of lupus nephritis.
Nephrotoxic nephritis
NTN is a model of acute glomerulonephritis achieved by intravenous administration of antiglomerular basement membrane antibodies. Vogt et al. used subnephritogenic levels of nephrotoxic serum (NTS) to precondition Sprague Dawley rats before glycerol-induced rhabdomyolysis. NTS pretreatment abrogated the increase in serum creatinine and morphologic damage normally associated with rhabdomyolysis (141). Surprisingly, HO-1 was upregulated in tubules but not glomeruli in this model of glomerulonephritis. NTS treatment also increased Ft levels but failed to increase other antioxidants such as catalase or glutathione peroxidase. Treatment with the HO inhibitor SnPP abolished the beneficial preconditioning effect of NTN (141). The authors suggested elaboration of TNF-α by glomeruli, released into the urinary space or delivered to tubules via peritubular capillaries, as the mediator of HO-1 induction. They therefore postulated a novel mechanism for glomerular–tubulointerstitial communication, such that glomerular inflammation induced an acquired, protective, HO-1-mediated response in the tubules.
Glomerular induction of HO-1 has been shown in acute NTN in rats (96). Positively stained cells appeared to be mononuclear in morphology and coexpressed proinflammatory macrophage markers. In acute NTN, pretreatment with hemin decreased acute inflammation, proteinuria, glomerular microthrombi, and ED1 (macrophage marker)-positive cells (96).
Datta et al. provided evidence for an intersection of iNOS and HO-1 in NTN rats (34). HO-1 and iNOS mRNA were increased in glomeruli of nephritic animals relative to control. Nephritic animals demonstrated a significant increase in urine protein to creatinine ratio that was attenuated by pretreatment with hemin. Induction of HO-1 coincided with decreased iNOS expression and activity (34). The authors suggested that inhibition of iNOS could be attributed to (i) degradation of the heme prosthetic group required by iNOS or (ii) CO product binding to heme iron and interference with iNOS activity. Also, iron, which is liberated by HO-1, is known to downregulate iNOS, and hemin treatment decreased transcription and activity of iNOS in nephritic animals.
Tubular Disease
Polycystic kidney disease
Data suggest polycystic kidneys are in a state of chronic oxidative stress with altered levels of antioxidant enzymes (92). HO-1 mRNA levels were elevated in diseased kidneys from the Han: SPRD-Cy rat and C57BL/6J-cpk mouse indicating the presence of oxidative stress. Also, polycystic kidneys demonstrated a decrease in expression and activity levels of antioxidant enzymes, including extracellular glutathione peroxidase, mitochondrial superoxide dismutase, peroxisomal catalase, and cytosolic glutathione S-transferase (92).
AKI caused by ischemia–reperfusion (IR) or nephrotoxic agents accelerated the progression of cyst formation in mouse models of polycystic kidney disease (PKD) (50, 131). Zhou et al. showed induction of HO using cobalt protoporphyrin (CoPP) reduced cytogenesis in cpk mice, a model of autosomal recessive PKD. There were fewer cysts and smaller kidneys in treated mice compared to controls. In contrast, HO inhibition with SnPP produced the opposite effect, resulting in increased cystogenesis and larger kidneys (162). These data provide evidence that HO-1 modulates disease progression in PDK, possibly by altering the redox balance.
Acute kidney injury
IR injury
Bilateral renal ischemia for 30 min followed by reperfusion increased renal heme content and induced HO-1 expression and HO activity in mice (89). In a similar, uninephrectomized rat model, increased renal heme load appeared to induce HO-1 expression. Rats treated with the HO inhibitor SnPP increased heme levels in the kidney, with worsening renal function and widespread tubular epithelial damage (123).
NF-κB and monocyte chemoattractant protein-1 (MCP-1) are upregulated in oxidative stress that occurs in renal IR injury (IRI). These signaling molecules facilitate recruitment of monocytes and macrophages to the site of ischemic injury. HO-1-deficient mice, when subjected to 10-min bilateral ischemia, exhibited more kidney damage and significantly increased MCP-1 and NF-κB levels, compared with wild-type mice (109, 127). Bone marrow-derived macrophages overexpressing HO-1 localized to the injured kidney when administered intravenously in mice undergoing IRI. Mice adoptively transferred with HO-1 overexpressing MP exhibited improved renal function 24 h after injury compared with controls (41).
Cheng et al. recently showed that adiponectin could protect against IRI-induced renal damage (31). Adiponectin upregulated HO-1 expression via the peroxisome proliferator-activated receptor α (PPARα) pathway. Upregulation of cyclooxygenase 2 was required for adiponectin-mediated induction of HO-1 (31).
Ischemic postconditioning (IPo) is an IR model where the animal is subjected to intervals of ischemia after the primary insult. Guo et al. subjected rats to a bilateral 45-min IRI and showed that the beneficial effect of IPo was due to upregulation of heat shock proteins (HSPs), including HO-1 (49). As expected, IR rats demonstrated severe kidney damage and impaired renal function. There was increased lipid peroxidation, inflammation, and tubular epithelial apoptosis in the kidneys of the IR rats and these were markedly reduced in the IPo group after IRI. Quercetin, a nonspecific HSP inhibitor, significantly repressed the renoprotective effects of IPo (49). In addition, remote ischemic preconditioning has demonstrated HO-1-mediated cardioprotection (160, 161) and improved outcomes in liver IRI (62, 143).
More recently, Hull et al. demonstrated that HO-1-deficient mice are highly susceptible to bilateral ischemia for 10 min when compared to wild type, confirming previous findings by Pittock et al. (109). They used Cre-lox recombinant mice, in which a lysozyme M promoter was utilized to drive the Cre gene, allowing flox-mediated deletion of HO-1 expression in myeloid lineage cells (macrophages, dendritic cells, monocytes, and neutrophils) (HO-1LysM−/−). When these mice were subjected to 25-min bilateral IRI, they exhibited a higher number of renal tubular casts, persistent loss of proximal tubule brush border, increased collagen deposition and fibrosis compared to controls (59). These studies underscore the critical role of HO-1 in the kidney after IRI.
Rhabdomyolysis
Rhabdomyolysis is characterized by widespread necrosis of striated muscle, leading to a massive release of myoglobin into the blood. Exposure of the kidney tubules to heme and heme proteins contributes to AKI in this setting. Nath et al. demonstrated, for the first time, the functional role of HO-1 in a rat model of glycerol-induced rhabdomyolysis. A few hours after glycerol, HO-1 mRNA was increased in the kidneys, accompanied by increased HO activity. SnPP treatment resulted in significantly worse kidney function and kidney damage (100). Prior conditioning of the animals with hemoglobin resulted in induction of HO-1, and these animals were protected from AKI (100). These findings were further confirmed in HO-1 knockout mice that demonstrate 100% mortality following glycerol-induced rhabdomyolysis (68, 104). In contrast, mice that genetically overexpressed HO-1 were highly protected from rhabdomyolysis (68).
In the setting of kidney injury and iron accumulation, investigators have demonstrated subsequent upregulation of Ft coupled to HO-1 induction (4, 100). The importance of Ft in the protective effects of HO-1 was highlighted in recent studies using proximal tubule-specific Ft knockout mice. These mice exhibit significant increase in mortality and worsening of renal injury when compared to control mice subjected to rhabdomylosis and CP-induced AKI (158). More recently, Boddu et al. characterized a leucine-rich repeat kinase 2 (Lrrk2) deletion model of Parkinson's disease in rats, wherein hemoglobin accumulated in the kidney with concomitant induction of HO-1. The Lrrk2 knockout rat model was protected against glycerol-induced rhabdomyolysis. Preconditioning of these rats with endogenous hemoglobin conferred protection against AKI (19).
Sepsis and endotoxin-induced injury
The pathophysiology of AKI associated with sepsis is poorly understood; however, decreased renal blood flow, inflammation and injury of the renal tubular epithelium, and oxidative stress have been implicated in sepsis-associated AKI (9). Endotoxin treatment modeled sepsis in rats and was protective against rhabdomyolysis-induced AKI when given before glycerol (140). Rats treated with endotoxin 24 h before glycerol exhibited HO-1 and Ft induction and improved renal function compared with rats that received glycerol and endotoxin simultaneously. Inhibition of HO resulted in worse renal function in this model (140).
Free heme and HO are important in the pathogenesis of septic shock in a cecal ligation and puncture model of sepsis. Heme levels increased as a result of polymicrobial infection and were associated with tissue damage and reduced immune tolerance (74). Wegiel et al. demonstrated the significance of MP HO-1 in a tissue-specific knockout mouse. They showed beneficial effects of administration of CO on bacterial killing in an animal model of sepsis and primary human macrophages in culture. The Nacht, LRR, and PYD domains-containing protein 3 (NALP3) inflammasome was important for CO-mediated improved bacterial clearance by macrophages (145).
CP nephrotoxicity
Nephrotoxicity secondary to CP treatment is well-established (114). In animals, CP accumulates in the proximal tubules where it causes renal tubular necrosis, cast formation, and loss of the brush border, secondary to oxidative stress (124). In rats, increased kidney heme content resulted from CP nephrotoxicity, and consequently, HO-1 was induced in this model (4). Treatment with the HO inhibitor SnPP resulted in kidney damage (4). Overexpression of HO-1 is protective against CP-induced toxicity (68, 124, 159) [reviewed in Ref. (99)]. Further, CP causes more severe functional and structural renal damage in HO-1 knockout mice compared to wild-type mice (124).
Contrast-induced nephropathy
Contrast-induced nephropathy (CIN) is a common cause of AKI, especially in patients with pre-existing renal disease. Several mechanisms of damage from iodinated contrast administration have been proposed, including (i) decreased renal blood flow, (ii) direct toxicity to renal tubular epithelial cells, and (iii) increased oxidative stress.
Goodman et al. demonstrated that HO-1 induction mitigates AKI from radiocontrast media (45). In this study, uninephrectomized, salt-depleted, and indomethacin-treated, male Sabra rats, a model with pre-existing kidney dysfunction, were given radiocontrast. HO-1 was induced in the renal cortex in this model. Induction of HO-1 with CoPP improved renal function after CIN in rats, whereas treatment with HO inhibitor tin mesoporphyrin (SnMP) led to worsening of renal function. Upregulation of HO-1 was also associated with an increase in antiapoptotic proteins Bcl-2 and Bcl-X in the kidney. The expression of proapoptotic proteins caspase-3 and caspase-9 was reduced (45). These findings illustrated a protective role for HO-1 in CIN. A caveat to these studies is that the model of CIN involved multiple insults, such as reduction of nephron mass, salt-depletion, and indomethacin treatment. Compound insults and radiocontrast cause injury, whereas contrast administration alone fails to cause AKI in rodent models. The requirement for multiple insults may confound results in animal models of CIN.
Gentamicin nephrotoxicity
Gentamicin is an antibiotic that can be nephrotoxic by causing renal tubular epithelial necrosis, primarily in the cortical proximal tubules. Administration of gentamicin in rats caused induction of HO-1 within 6 h, remaining elevated for several days (4). Kidney dysfunction was not abrogated by SnPP inhibition of HO (4). Taye et al. obtained contrary results, demonstrating that ZnPP abolished the renoprotective effect of HO-1 in this model. They found HO induction decreased inflammatory markers in the kidney, conferring protection (133). These studies indicate heterogeneity in the gentamycin model of AKI.
Mercuric chloride nephrotoxicity
Mercuric chloride (HgCl2) causes nephrotoxic AKI in experimental models. HgCl2 increases oxidative stress, depletes antioxidant glutathione, and increases lipid peroxidation in the kidney. Similar to other nephrotoxins, such as CP and gentamicin, HgCl2 caused robust renal induction of HO-1 in rats (101, 149). Preconditioning of rats with hemin resulted in HO-1-mediated protection against HgCl2-induced nephrotoxicity, however, HO activity inhibition with SnPP did not worsen outcomes relative to control (101).
Cyclosporine nephrotoxicity
In cyclosporine (CsA) nephrotoxicity, tubulointerstitial fibrosis occurs secondary to increased production of free radicals, lipid peroxidation, and tissue hypoperfusion. Several reports have demonstrated a protective role for HO-1 in CsA-induced nephrotoxicity (112, 113). CsA nephrotoxicity alone reduced HO-1 levels and HO activity. Rats treated with CsA and the HO inhibitor SnPP exhibited severe renal injury characterized by tubulointerstitial scarring and fibrosis. However, rats treated with CoPP in the setting of CsA nephrotoxicity, showed normal kidney morphology coinciding with HO-1 induction. These data suggested that HO-1 was not induced in response to CsA, but its induction with CoPP was protective in this model of AKI.
In vitro studies using LLC-PK1 cells demonstrated that preincubation with bilirubin, ANP, or cGMP analog, reduced CsA-induced toxicity in a dose-dependent manner and increased cell survival (110). ANP and 8-bromo cGMP induced HO-1 expression and enzyme activity. RPTCs treated with the HO inhibitor SnPP demonstrated heightened sensitivity to CsA-induced cellular toxicity (110).
More recently, mice treated with oleanolic acid (OA), a Nrf2 pathway activator, and CsA were protected from tubulointerstitial fibrosis (56). The CsA + OA-treated mice demonstrated increased nuclear translocation of Nrf2 and HO-1 expression (56). Taken together, these data highlight the beneficial role of HO-1 induction in CsA nephrotoxicity.
Interstitial Renal Disease
Renal transplant rejection
HO-1 is induced in infiltrating MPs in acute renal allograft rejection in rats (5). In this study, the authors demonstrated infiltration of inflammatory cells 5 days post-transplant that costained for ED1, a marker for rat MPs, and HO-1 or iNOS. Infiltration occurred in the peritubular interstitium and glomeruli. This study provided the first description of induction of HO-1 and iNOS, both gas generating systems, in renal transplant rejection.
Magee et al. studied the effects of a peptide inducer of HO-1, RDP1258, in rat renal allograft rejection (85). This peptide was modeled after a region in the HLA-B molecule that was shown to be protective in other models of transplant rejection. Administration of this molecule demonstrated an increased splenic HO activity and reduced activation of inflammatory cells. In rat kidney transplantation, administration of RDP1258 increased survival of transplant recipients (85). This study was limited by a lack of investigation for off-target effects of RDP1258. Therefore, one cannot conclude that improved survival was solely due to HO-1 induction.
In 2002, Tullius et al. found that induction of HO-1 using CoPP improved survival, kidney function, and morphologic characteristics of grafts in a rat renal transplantation model (137). They showed that kidneys from CoPP-treated donor animals could be stored under ischemic conditions for up to 32 h with 60% of recipients surviving 24 weeks. Comparing this to recipients of untreated donors, 40% survival was observed after only 4 h of ischemia and storage. Morphologically, HO-1 induction protected the grafts. The authors demonstrated HO-1 induction increased Bcl-X and decreased TNF-α (137).
Heat preconditioning or CoPP induced expression of HO-1, HSP70, HSP90, and Bcl-X in a rat kidney transplant model. Both treatments improved graft survival and function. Markers of apoptosis and morphologic signs of injury were decreased in animals where HO-1 was induced. These beneficial effects were reversed with SnPP inhibition of HO activity in both heat preconditioned and CoPP-treated animals (142).
GT-repeat length polymorphisms in the HO-1 promoter correlate with renal function post-transplantation in humans (40). Kidney function from donors with at least one short GT-repeat (S allele) was improved compared with donors with long GT-repeats. Results from multivariate analysis, controlling for a variety of parameters, showed that serum creatinine of recipients, who received a graft from S allele carriers, was 0.81 times that of noncarriers (95% confidence interval [CI]: 0.70–0.95, p = 0.01) (40). No difference was found between the two groups for rejection or delayed graft function. This study demonstrated human relevance for HO-1 expression in renal graft function in the setting of transplantation.
Ozaki et al. further demonstrated the importance of HO-1 inducibility in renal allograft function in humans. They examined the HO-1 promoter GT-repeat length of both donors and recipients. Donor GT-repeat length was more important for renal function. Donors containing at least one S allele demonstrated better function over a 3-year follow-up period (107). HO is important for renal allograft function and survival, representing an avenue for therapeutic intervention.
Obstructive uropathy
HO-1 is induced in renal interstitial cells in animal models of obstructive uropathy, for example, in unilateral ureteral obstruction (UUO). Such expression was observed in periglomerular and peritubular interstitial cells (65). Increased α-smooth muscle actin (αSMA) expression and F4/80+ MP infiltration were observed in the interstitium. HO-1 levels peaked 12 h after obstruction and decreased over 7 days (65). These findings indicate damage secondary to UUO in animals is mediated by oxidative stress and is characterized by inflammation and fibrosis. HO-1 is induced in the interstitium presumably in response to increased oxidative stress.
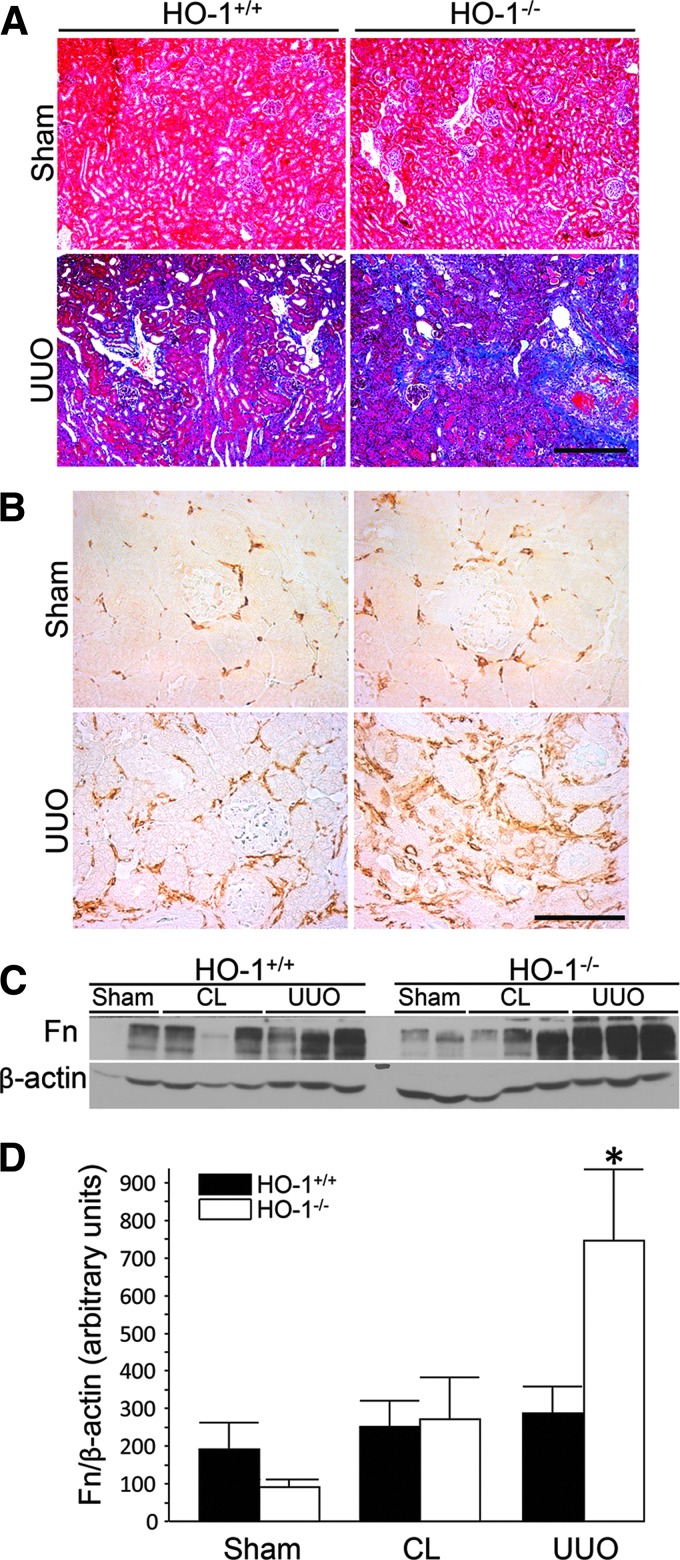
UUO in HO-1 knockout mice confirmed a protective role in renal damage and fibrosis in the setting of obstruction (67). Hydronephrosis was similar in HO-1−/− and control mice, but inflammatory infiltration and cast formation were increased in the knockout mice. Interestingly, the contralateral, unobstructed kidney appeared to display greater inflammatory infiltrate and expressed greater levels of TGF-β compared to sham control in HO-1 deficiency. This observation indicates the possibility of inflammatory crosstalk between the kidneys in this model. UUO caused more severe fibrosis in HO-1 knockout kidneys, and fibrosis was positively correlated with TGF-β and αSMA expression. MP infiltration, defined by F4/80 expression, was greater in HO-1-deficient mice, coinciding with elaboration of profibrotic TGF-β (Fig. 7) (67).
FIG. 7.
Lack of HO-1 augments renal fibrosis and macrophage infiltration following UUO. (A, B) Representative UUO and sham control kidneys of HO-1+/+ and HO-1−/− mice, 7 days following obstruction. (A) Masson's trichrome stain of renal cortex and corticomedullary junction. Scale bar = 400 μm. (B) Immunohistochemistry for F4/80+ cells in the renal cortex. Scale bar = 100 μm. (C) Representative Western blot analysis for Fn in kidney lysates. (D) Densitometry for Fn normalized to β-actin. *p < 0.05 versus all other groups. Reproduced with permission from Kie et al. (67). CL, contralateral, uninjured kidney; Fn, fibronectin; UUO, unilateral ureteral obstruction. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Correa-Costa et al. performed functional studies and investigated the ability of HO activity to reverse fibrosis in UUO (32). HO-1 was induced in rats by pretreatment with hemin. UUO caused proteinuria and increased protein to creatinine ratio that was reduced with HO-1 induction. UUO did not alter glomerular filtration rate, however, it increased mean arterial pressure and kidney hypertrophy. These pathologic changes were reversed with hemin treatment. Morphologically, hemin treatment decreased fibroblast proliferation and inhibited fibrosis at 7 and 14 days postobstruction. HO-1 induction was associated with decreased levels of TGF-β. Also, HO-1 induction decreased expression of IL-1β and IL-6, lymphocyte activation marker IFN-γ, and increased anti-inflammatory IL-10 (32). These investigators also performed experiments where HO-1 was induced 1 week after obstruction to study the ability of HO-1 to reverse functional decline. While not as effective as pretreatment, hemin administration 7 days after UUO improved function and decreased inflammation and fibrosis. Also, kidney hypertrophy was reduced (32). This study provides evidence of functional and morphologic improvement with HO-1 induction in the UUO model of obstructive uropathy.
Chronic kidney disease
AKI is associated with increased risk of CKD (29), however, a causal relationship has not been demonstrated. Poor response to injury may contribute to consequent progression to CKD. Inflammation and repair pathways are dysregulated following unilateral renal IR (154). Several factors implicated in the pathogenesis of CKD (e.g., TGF-β) are activators of HO-1 expression. TGF-β is a cytokine important in kidney tissue repair, regeneration, and fibrosis. TGF-β may beneficially induce HO-1 and conversely, induction of HO-1 has been proposed as a therapeutic approach in mitigating TGF-β-mediated renal disease (157). It is possible that repeated bouts of AKI with poor healing predisposes to the development of CKD. This was best demonstrated in patients with sickle cell disease (SCD) who experience acute renal ischemia during vaso-occlusive events, wherein an association between polymorphisms in the HO-1 gene and the development of CKD has been reported (118).
Zager et al. developed a model of IR-induced AKI that progresses to CKD in mice (154). The protocol for this model involves unilateral IRI and leaving the contralateral kidney intact. Upon infliction of relatively mild IRI, the injured kidney develops progressive histologic damage, inflammation, fibrosis, and functional decline over a 3-week period (148). TNF-α, MCP-1, TGF-β1, and collagen III were upregulated in injured kidneys in these animals. Cortical expression of anti-inflammatory IL-10 spiked postischemia, then dropped to levels below baseline at 1 and 3 weeks. Gene activating histone acetylation was observed at the MCP-1, TGF-β1, and collagen III gene loci (153, 154). As expected, cortical HO-1 was induced in the acute phase and dropped to near baseline levels at 3 weeks (154).
Incorporating toxic levels of adenine into the diet of rats caused tubulointerstitial nephropathy and CKD (10). These animals demonstrated impaired Nrf2 activation and decreased elaboration of its downstream targets, including HO-1, glutamate-cysteine ligase, and catalase. Low Nrf2 activity resulted in increased oxidative stress and activation of the transcription factor NF-κB (10).
Ishima et al. performed a biochemical and in vitro study to learn the effects of uremic toxin indoxyl sulfate (IS) on ROS formation and the role HO-1 plays in this system (60). IS and other uremic toxins are elevated in serum of patients with CKD, and IS has been proposed to play a pathological prooxidant role (39). Induction of HO-1 in HK-2 cells with hemin resulted in an increase in ROS formation that was reversed by inhibition with SnPP (60), suggesting HO-1 induction is a maladaptive response in the progression of CKD. However, this study is limited by lack of experiments in animal models.
In contrast, Chen et al. provided evidence that HO-1 may protect against progression to CKD in patients with coronary artery disease. In a prospective clinical study consisting of a cohort of 386 patients, the authors found that S allele carrier status was associated with a decreased risk of progression (30). Genotypic data were collected from these patients and analyzed for GT-repeat length in the proximal HO-1 promoter. Primary renal endpoints were focused on kidney function, doubling of serum creatinine and end-stage renal disease (ESRD) requiring hemodialysis. Mortality was a secondary endpoint. Adjusted hazard ratios and associated 95% CIs for homozygous L allele individuals (long GT repeats, >27) were 1.99 (1.27–3.14; p = 0.003) for renal endpoints and 1.36 (1.04–1.79; p = 0.03) for mortality (30). This study provides associative evidence that inducibility of HO-1 is protective from progression and beneficial in patients with CKD.
Vascular Disease
Hypertension
A role for HO-1 in the development of hypertensive kidney disease was first shown in spontaneously hypertensive rats (78, 90). Using Doppler flowmetry, Zou et al. found HO-1 modulated renal medullary blood flow (MBF) (163). Treatment with a zinc porphyrin HO inhibitor caused a selective drop in MBF, decreased cGMP concentration from medullary dialysate, and coincided with vasoconstriction and hypoperfusion (163). These findings indicate HO-1 is important in maintaining medullary perfusion under stress, effects that are mediated, at least, in part, via CO derived from the HO-1 catalyzed reaction.
Levere et al. used heme arginate to induce HO-1 in spontaneously hypertensive rats. Systolic blood pressure was decreased with heme arginate treatment, a change that was abrogated following treatment with a zinc porphyrin inhibitor of HO (78). Martasek et al. found that such protection against rising blood pressure was due to elaboration of bilirubin and decreased vasoconstrictive arachidonate metabolites (90).
Use of a subpressor dose of angiotensin II (Ang II) in a model of hypertension showed HO-1 induction protected kidney function and prevented inflammation and fibrosis, but did not change urinary neutrophil gelatinase-associated lipocalin (NGAL) levels (28). SnPP inhibition of HO enzyme activity worsened renal dysfunction, inflammation, injury, and fibrosis. Since CoPP treatment protected against renal injury and kidney dysfunction, but did not reverse hypertension, the authors suggested that HO-1 may mediate renoprotective effects independent of decreasing blood pressure in this model (28).
Recently, Konvalinka et al. used bioinformatics and proteomics in cell culture models to identify, and animal models to verify, candidate biomarkers of renin–angiotensin–aldosterone system (RAAS) activity in the kidney (72). Ang II induced expression of HO-1 in renal cortices. Nrf2, an important transcription factor involved in HO-1 regulation, was increased (72). HO-1 was identified as the top candidate regulated by Ang II activity in the kidney, and it also provided evidence that urinary HO-1 could serve as a marker for renal RAAS-mediated activity.
HO-1 induction is not limited to renal tissue in hypertension (61, 97). In fact, HO-1 may be induced in the vasculature systemically in response to chronic high blood pressure (61). Ishizaka et al. demonstrated increased HO-1 levels in rat aortas of animals infused with Ang II. Whereas basal expression was found in adventitial and medial smooth muscle cells, induction occured in adventitial and endothelial cells (61).
A direct role for HO-1 in the regulation of vascular tone and health was recently demonstrated in a combined animal and human study (147). Compared to wild-type mice, HO-1-deficient mice exhibited increased vascular dysfunction in multiple models, including Ang II-infusion, STZ-induced diabetes, and aging. The authors also evaluated length polymorphisms of the HO-1 promoter region in a cohort of 4937 individuals. HO-1 expression levels in monocytes correlated with flow-mediated dilation and inversely with CD14 in a subset of hypertensive subjects. Importantly, L allele homozygosity in the HO-1 promoter correlated with an increased prevalence of hypertension and reduced survival during a long follow-up period (>7 years) (147).
Sickle cell nephropathy
Cell-free hemoglobin and heme levels are elevated in sickle cell nephropathy (118). During the course of renal vaso-occlusion, intravascular hemolysis occurs and the kidney is exposed to massive amounts of toxic heme. Not surprisingly, Nath et al. found HO-1 was induced in the kidney in mouse models of SCD (103). Human transgenic sickle mice demonstrated increased body weight and kidney hypertrophy relative to control mice. HO activity was increased in the sickle mouse kidney compared with controls. Glutathione peroxidase was decreased and catalase was unchanged, indicating a specific role for HO-1. Use of a prooxidant glutathione synthesis inhibitor caused diffuse sickling throughout the kidney, supporting a pathologic role of redox dysregulation in sickle cell nephropathy. In a patient with SCD, HO-1 was expressed in the tubular epithelium, interstitium, endothelium, and smooth muscle cells of the renal vasculature. Glomerular staining for HO-1 was also observed, however, the authors were uncertain whether the expression was in mesangial or infiltrating inflammatory mononuclear cells (103).
Belcher et al. also found HO-1 was induced systemically in transgenic sickle mice. Pharmacologic HO-1 induction with hemin reduced IR-induced hemostasis in the lungs, liver, spleen, and skin (16). An associated decrease was observed in endothelial activation. In addition, treatment with HO products CO and biliverdin inhibited stasis and endothelial activation (16). Ghosh et al. investigated differential expression of genes in multiple organs of mouse models of SCD. There was selective HO-1 induction in the kidney and liver in both Berkeley and Townes mouse models of SCD (44).
In patients with SCD, Saraf et al. found that L allele homozygosity in the HO-1 promoter was associated with decreased glomerular filtration rate (118). Based on in vitro human RPTC gene expression, the authors found enrichment of single nucleotide polymorphism downstream of HO-1 in sickle patients that was associated with CKD stage (OR = 2.8, p = 0.0003) or ESRD (OR = 9.8, p = 0.0004) (118).
Conclusions
During the past few decades, an important role for the HO-1 enzyme system in the pathophysiology of kidney diseases has been supported by several lines of evidence. (i) HO-1 is induced in the kidney in both animal models and humans with a variety of kidney diseases; (ii) HO-1 expression levels determine the course of kidney disease—deficiency or inhibition of HO-1 in animal models worsens renal structure and function, and increased expression is protective; (iii) plasma and urine levels of HO-1 have been implicated as biomarkers for AKI in both animal models and humans; (iv) polymorphisms in the human HO-1 promoter correlate with HO-1 expression and outcomes in several kidney diseases; (v) many pharmacologic interventions used in preclinical models of kidney disease, including α-melanocyte-stimulating hormone, erythropoietin, IL-10, and NGAL, are potent inducers of HO-1 and mediate their effects, at least, in part, through HO-1 induction; and (vi) several clinical trials targeting the HO-1 pathway in the kidney, heart, and other organ systems are ongoing or in various phases of completion (clinicaltrials.gov: NCT01430156, NCT00483587, NCT02142699, NCT00531856). Therefore, the HO-1 enzyme system represents an important target for intervention in the pathogenesis of acute and CKD.
Abbreviations Used
- αSMA
α-smooth muscle actin
- AKI
acute kidney injury
- Ang II
angiotensin II
- ANP
atrial natriuretic peptide
- AP
activator protein
- AV
arteriovenous
- CdRE
cadmium response element
- cGMP
cyclic guanosine monophosphate
- CI
confidence interval
- CIN
contrast-induced nephropathy
- CKD
chronic kidney disease
- CO
carbon monoxide
- CoPP
cobalt protoporphyrin IX
- CP
cisplatin
- CsA
cyclosporine
- dsDNA
double-stranded DNA
- ESRD
end-stage renal disease
- Fn
fibronectin
- Ft
ferritin
- hBAC
“humanized” bacterial artificial chromosome
- HgCl2
mercuric chloride
- HIF-1
hypoxia inducible factor-1
- HMOX1
human heme oxygenase-1 gene
- HMOX2
human heme oxygenase-2 gene
- HO
heme oxygenase
- HO-1
heme oxygenase-1
- HO-2
heme oxygenase-2
- HRM
heme regulatory motif
- HSPs
heat shock proteins
- IFN-γ
interferon-γ
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- IPo
ischemic postconditioning
- IR
ischemia–reperfusion
- IRI
ischemia–reperfusion injury
- IS
indoxyl sulfate
- Keap1
Kelch-like ECH-associated protein 1
- L allele
long (> 25–27) GT-repeat polymorphism in human HO-1 promoter
- MBF
medullary blood flow
- MCP-1
monocyte chemoattractant protein-1
- MP
mononuclear phagocyte
- NALP3
Nacht, LRR, and PYD domains-containing protein 3
- NF-κB
nuclear factor-κB
- NGAL
neutrophil gelatinase-associated lipocalin
- Nrf2
nuclear factor erythroid 2-related factor 2
- NTN
nephrotoxic nephritis
- NTS
nephrotoxic serum
- OA
oleanolic acid
- PKD
polycystic kidney disease
- PPARα
peroxisome proliferator-activated receptor α
- RAAS
renin–angiotensin–aldosterone system
- RMP
renal mononuclear phagocyte
- ROS
reactive oxygen species
- RPTC
renal proximal tubular cell
- S allele
short (< 25–27) GT-repeat polymorphism in human HO-1 promoter
- SCD
sickle cell disease
- SLE
systemic lupus erythematosus
- SnMP
tin mesoporphyrin
- SnPP
tin protoporphyrin
- Sp1
specificity protein 1
- STZ
streptozotocin
- TGF-β1
transforming growth factor-β1
- TNF-α
tumor necrosis factor-α
- USF
upstream stimulatory factor
- UUO
unilateral ureteral obstruction
- ZnPP
zinc protoporphyrin
Acknowledgments
The authors acknowledge support from NIH grants R01 DK59600 (to A.A.), the core resource of the UAB-UCSD O'Brien Center (P30 DK079337; to A.A.), and partial support from NIGMS MSTP T32GM008361 (to J.M.L.).
References
- 1.Abdo S, Shi Y, Otoukesh A, Ghosh A, Lo CS, Chenier I, Filep JG, Ingelfinger JR, Zhang SL, and Chan JS. Catalase overexpression prevents nuclear factor erythroid 2-related factor 2 stimulation of renal angiotensinogen gene expression, hypertension, and kidney injury in diabetic mice. Diabetes 63: 3483–3496, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham NG, Lin JH, Dunn MW, and Schwartzman ML. Presence of heme oxygenase and NADPH cytochrome P-450 (c) reductase in human corneal epithelium. Invest Ophthalmol Vis Sci 28: 1464–1472, 1987 [PubMed] [Google Scholar]
- 3.Adin CA, Croker BP, and Agarwal A. Protective effects of exogenous bilirubin on ischemia-reperfusion injury in the isolated, perfused rat kidney. Am J Physiol Renal Physiol 288: F778–F784, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Agarwal A, Balla J, Alam J, Croatt AJ, and Nath KA. Induction of heme oxygenase in toxic renal injury: a protective role in cisplatin nephrotoxicity in the rat. Kidney Int 48: 1298–1307, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Agarwal A, Kim Y, Matas AJ, Alam J, and Nath KA. Gas-generating systems in acute renal allograft rejection in the rat. Co-induction of heme oxygenase and nitric oxide synthase. Transplantation 61: 93–98, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Agarwal A. and Nick HS. Renal response to tissue injury: lessons from heme oxygenase-1 gene ablation and expression. J Am Soc Nephrol 11: 965–973, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, and Cook JL. Nrf2, a Cap‘n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem 274: 26071–26078, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Alam J, Wicks C, Stewart D, Gong P, Touchard C, Otterbein S, Choi AM, Burow ME, and Tou J. Mechanism of heme oxygenase-1 gene activation by cadmium in MCF-7 mammary epithelial cells. Role of p38 kinase and Nrf2 transcription factor. J Biol Chem 275: 27694–27702, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Alobaidi R, Basu RK, Goldstein SL, and Bagshaw SM. Sepsis-associated acute kidney injury. Semin Nephrol 35: 2–11, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aminzadeh MA, Nicholas SB, Norris KC, and Vaziri ND. Role of impaired Nrf2 activation in the pathogenesis of oxidative stress and inflammation in chronic tubulo-interstitial nephropathy. Nephrol Dial Transplant 28: 2038–2045, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Annunziato F, Romagnani C, and Romagnani S. The 3 major types of innate and adaptive cell-mediated effector immunity. J Allergy Clin Immunol 135: 626–635, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Bagai I, Sarangi R, Fleischhacker AS, Sharma A, Hoffman BM, Zuiderweg ER, and Ragsdale SW. Spectroscopic studies reveal that the heme regulatory motifs of heme oxygenase-2 are dynamically disordered and exhibit redox-dependent interaction with heme. Biochemistry 54: 2693–2708, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, Eaton JW, and Vercellotti GM. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem 267: 18148–18153, 1992 [PubMed] [Google Scholar]
- 14.Bao W, Song F, Li X, Rong S, Yang W, Wang D, Xu J, Fu J, Zhao Y, and Liu L. Association between heme oxygenase-1 gene promoter polymorphisms and type 2 diabetes mellitus: a HuGE review and meta-analysis. Am J Epidemiol 172: 631–636, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Beckman JD, Chen C, Nguyen J, Thayanithy V, Subramanian S, Steer CJ, and Vercellotti GM. Regulation of heme oxygenase-1 protein expression by miR-377 in combination with miR-217. J Biol Chem 286: 3194–3202, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belcher JD, Mahaseth H, Welch TE, Otterbein LE, Hebbel RP, and Vercellotti GM. Heme oxygenase-1 is a modulator of inflammation and vaso-occlusion in transgenic sickle mice. J Clin Invest 116: 808–816, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bindu S, Pal C, Dey S, Goyal M, Alam A, Iqbal MS, Dutta S, Sarkar S, Kumar R, Maity P, and Bandyopadhyay U. Translocation of heme oxygenase-1 to mitochondria is a novel cytoprotective mechanism against non-steroidal anti-inflammatory drug-induced mitochondrial oxidative stress, apoptosis, and gastric mucosal injury. J Biol Chem 286: 39387–39402, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biswas C, Shah N, Muthu M, La P, Fernando AP, Sengupta S, Yang G, and Dennery PA. Nuclear heme oxygenase-1 (HO-1) modulates subcellular distribution and activation of Nrf2, impacting metabolic and anti-oxidant defenses. J Biol Chem 289: 26882–26894, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boddu R, Hull TD, Bolisetty S, Hu X, Moehle MS, Daher JP, Kamal AI, Joseph R, George JF, Agarwal A, Curtis LM, and West AB. Leucine-rich repeat kinase 2 deficiency is protective in rhabdomyolysis-induced kidney injury. Hum Mol Genet 24: 4078–4093, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boehning D, Moon C, Sharma S, Hurt KJ, Hester LD, Ronnett GV, Shugar D, and Snyder SH. Carbon monoxide neurotransmission activated by CK2 phosphorylation of heme oxygenase-2. Neuron 40: 129–137, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Bolisetty S, Traylor A, Zarjou A, Johnson MS, Benavides GA, Ricart K, Boddu R, Moore RD, Landar A, Barnes S, Darley-Usmar V, and Agarwal A. Mitochondria-targeted heme oxygenase-1 decreases oxidative stress in renal epithelial cells. Am J Physiol Renal Physiol 305: F255–F264, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolisetty S, Traylor AM, Kim J, Joseph R, Ricart K, Landar A, and Agarwal A. Heme oxygenase-1 inhibits renal tubular macroautophagy in acute kidney injury. J Am Soc Nephrol 21: 1702–1712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolisetty S, Zarjou A, Hull TD, Traylor AM, Perianayagam A, Joseph R, Kamal AI, Arosio P, Soares MP, Jeney V, Balla J, George JF, and Agarwal A. Macrophage and epithelial cell H-ferritin expression regulates renal inflammation. Kidney Int 88: 95–108, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Botros FT. and Navar LG. Interaction between endogenously produced carbon monoxide and nitric oxide in regulation of renal afferent arterioles. Am J Physiol Heart Circ Physiol 291: H2772–H2778, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Boya P, Reggiori F, and Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol 15: 713–720, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Brydun A, Watari Y, Yamamoto Y, Okuhara K, Teragawa H, Kono F, Chayama K, Oshima T, and Ozono R. Reduced expression of heme oxygenase-1 in patients with coronary atherosclerosis. Hypertens Res 30: 341–348, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Chandrashekar K, Lopez-Ruiz A, Juncos R, Nath K, Stec DE, Vera T, Liu R, and Juncos LA. The modulatory role of heme oxygenase on subpressor angiotensin II-induced hypertension and renal injury. Int J Hypertens 2012: 392890, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chawla LS, Eggers PW, Star RA, and Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 371: 58–66, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen YH, Kuo KL, Hung SC, Hsu CC, Chen YH, and Tarng DC. Length polymorphism in heme oxygenase-1 and risk of CKD among patients with coronary artery disease. J Am Soc Nephrol 25: 2669–2677, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng CF, Lian WS, Chen SH, Lai PF, Li HF, Lan YF, Cheng WT, and Lin H. Protective effects of adiponectin against renal ischemia-reperfusion injury via prostacyclin-PPARalpha-heme oxygenase-1 signaling pathway. J Cell Physiol 227: 239–249, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Correa-Costa M, Semedo P, Monteiro AP, Silva RC, Pereira RL, Goncalves GM, Marques GD, Cenedeze MA, Faleiros AC, Keller AC, Shimizu MH, Seguro AC, Reis MA, Pacheco-Silva A, and Camara NO. Induction of heme oxygenase-1 can halt and even reverse renal tubule-interstitial fibrosis. PLoS One 5: e14298, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Craven PA, Melhem MF, Phillips SL, and DeRubertis FR. Overexpression of Cu2+/Zn2+ superoxide dismutase protects against early diabetic glomerular injury in transgenic mice. Diabetes 50: 2114–2125, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Datta PK, Koukouritaki SB, Hopp KA, and Lianos EA. Heme oxygenase-1 induction attenuates inducible nitric oxide synthase expression and proteinuria in glomerulonephritis. J Am Soc Nephrol 10: 2540–2550, 1999 [DOI] [PubMed] [Google Scholar]
- 35.de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ, McMurray JJ, Meyer CJ, Parving HH, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Wittes J, Wrolstad D, and Chertow GM. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med 369: 2492–2503, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deshane J, Kim J, Bolisetty S, Hock TD, Hill-Kapturczak N, and Agarwal A. Sp1 regulates chromatin looping between an intronic enhancer and distal promoter of the human heme oxygenase-1 gene in renal cells. J Biol Chem 285: 16476–16486, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doherty DG. Immunity, tolerance and autoimmunity in the liver: a comprehensive review. J Autoimmun 66: 60–75, 2016 [DOI] [PubMed] [Google Scholar]
- 38.Dulak J, Deshane J, Jozkowicz A, and Agarwal A. Heme oxygenase-1 and carbon monoxide in vascular pathobiology: focus on angiogenesis. Circulation 117: 231–241, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duranton F, Cohen G, De Smet R, Rodriguez M, Jankowski J, Vanholder R, and Argiles A. Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol 23: 1258–1270, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Exner M, Bohmig GA, Schillinger M, Regele H, Watschinger B, Horl WH, Raith M, Mannhalter C, and Wagner OF. Donor heme oxygenase-1 genotype is associated with renal allograft function. Transplantation 77: 538–542, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Ferenbach DA, Ramdas V, Spencer N, Marson L, Anegon I, Hughes J, and Kluth DC. Macrophages expressing heme oxygenase-1 improve renal function in ischemia/reperfusion injury. Mol Ther 18: 1706–1713, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fleischhacker AS, Sharma A, Choi M, Spencer AM, Bagai I, Hoffman BM, and Ragsdale SW. The C-terminal heme regulatory motifs of heme oxygenase-2 are redox-regulated heme binding sites. Biochemistry 54: 2709–2718, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frosch M, Vogl T, Waldherr R, Sorg C, Sunderkotter C, and Roth J. Expression of MRP8 and MRP14 by macrophages is a marker for severe forms of glomerulonephritis. J Leukoc Biol 75: 198–206, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Ghosh S, Tan F, Yu T, Li Y, Adisa O, Mosunjac M, and Ofori-Acquah SF. Global gene expression profiling of endothelium exposed to heme reveals an organ-specific induction of cytoprotective enzymes in sickle cell disease. PLoS One 6: e18399, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goodman AI, Olszanecki R, Yang LM, Quan S, Li M, Omura S, Stec DE, and Abraham NG. Heme oxygenase-1 protects against radiocontrast-induced acute kidney injury by regulating anti-apoptotic proteins. Kidney Int 72: 945–953, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Govindaraju V, Teoh H, Hamid Q, Cernacek P, and Ward ME. Interaction between endothelial heme oxygenase-2 and endothelin-1 in altered aortic reactivity after hypoxia in rats. Am J Physiol Heart Circ Physiol 288: H962–H70, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Gozzelino R, Jeney V, and Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol 50: 323–354, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Grochot-Przeczek A, Dulak J, and Jozkowicz A. Haem oxygenase-1: non-canonical roles in physiology and pathology. Clin Sci (Lond) 122: 93–103, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Guo Q, Du X, Zhao Y, Zhang D, Yue L, and Wang Z. Ischemic postconditioning prevents renal ischemia reperfusion injury through the induction of heat shock proteins in rats. Mol Med Rep 10: 2875–2881, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Happe H, Leonhard WN, van der Wal A, van de Water B, Lantinga-van Leeuwen IS, Breuning MH, de Heer E, and Peters DJ. Toxic tubular injury in kidneys from Pkd1-deletion mice accelerates cystogenesis accompanied by dysregulated planar cell polarity and canonical Wnt signaling pathways. Hum Mol Genet 18: 2532–2542, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Hayashi K, Haneda M, Koya D, Maeda S, Isshiki K, and Kikkawa R. Enhancement of glomerular heme oxygenase-1 expression in diabetic rats. Diabetes Res Clin Pract 52: 85–96, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Hayashi S, Omata Y, Sakamoto H, Higashimoto Y, Hara T, Sagara Y, and Noguchi M. Characterization of rat heme oxygenase-3 gene. Implication of processed pseudogenes derived from heme oxygenase-2 gene. Gene 336: 241–250, 2004 [DOI] [PubMed] [Google Scholar]
- 53.He JZ, Ho JJ, Gingerich S, Courtman DW, Marsden PA, and Ward ME. Enhanced translation of heme oxygenase-2 preserves human endothelial cell viability during hypoxia. J Biol Chem 285: 9452–9461, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herrada AA, Llanos C, Mackern-Oberti JP, Carreno LJ, Henriquez C, Gomez RS, Gutierrez MA, Anegon I, Jacobelli SH, and Kalergis AM. Haem oxygenase 1 expression is altered in monocytes from patients with systemic lupus erythematosus. Immunology 136: 414–424, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoetzel A, Dolinay T, Schmidt R, Choi AM, and Ryter SW. Carbon monoxide in sepsis. Antioxid Redox Signal 9: 2013–2026, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Hong YA, Lim JH, Kim MY, Kim EN, Koh ES, Shin SJ, Choi BS, Park CW, Chang YS, and Chung S. Delayed treatment with oleanolic acid attenuates tubulointerstitial fibrosis in chronic cyclosporine nephropathy through Nrf2/HO-1 signaling. J Transl Med 12: 50, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hull TD. and Agarwal A. Bilirubin: a potential biomarker and therapeutic target for diabetic nephropathy. Diabetes 63: 2613–2616, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hull TD, Agarwal A, and George JF. The mononuclear phagocyte system in homeostasis and disease: a role for heme oxygenase-1. Antioxid Redox Signal 20: 1770–1788, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hull TD, Kamal AI, Boddu R, Bolisetty S, Guo L, Tisher CC, Rangarajan S, Chen B, Curtis LM, George JF, and Agarwal A. Heme oxygenase-1 regulates myeloid cell trafficking in AKI. J Am Soc Nephrol 26: 2139–2151, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ishima Y, Narisoko T, Kragh-Hansen U, Kotani S, Nakajima M, Otagiri M, and Maruyama T. Nitration of indoxyl sulfate facilitates its cytotoxicity in human renal proximal tubular cells via expression of heme oxygenase-1. Biochem Biophys Res Commun 465: 481–487, 2015 [DOI] [PubMed] [Google Scholar]
- 61.Ishizaka N, de Leon H, Laursen JB, Fukui T, Wilcox JN, De Keulenaer G, Griendling KK, and Alexander RW. Angiotensin II-induced hypertension increases heme oxygenase-1 expression in rat aorta. Circulation 96: 1923–1929, 1997 [DOI] [PubMed] [Google Scholar]
- 62.Kageyama S, Hata K, Tanaka H, Hirao H, Kubota T, Okamura Y, Iwaisako K, Takada Y, and Uemoto S. Intestinal ischemic preconditioning ameliorates hepatic ischemia/reperfusion injury in rats: role of heme oxygenase 1 in the second window of protection. Liver Transpl 21: 112–122, 2015 [DOI] [PubMed] [Google Scholar]
- 63.Kang L, Hillestad ML, Grande JP, Croatt AJ, Barry MA, Farrugia G, Katusic ZS, and Nath KA. Induction and functional significance of the heme oxygenase system in pathological shear stress in vivo. Am J Physiol Heart Circ Physiol 308: H1402–H1413, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaushal GP, Kaushal V, Herzog C, and Yang C. Autophagy delays apoptosis in renal tubular epithelial cells in cisplatin cytotoxicity. Autophagy 4: 710–712, 2008 [DOI] [PubMed] [Google Scholar]
- 65.Kawada N, Moriyama T, Ando A, Fukunaga M, Miyata T, Kurokawa K, Imai E, and Hori M. Increased oxidative stress in mouse kidneys with unilateral ureteral obstruction. Kidney Int 56: 1004–1013, 1999 [DOI] [PubMed] [Google Scholar]
- 66.Kawakami T, Lichtnekert J, Thompson LJ, Karna P, Bouabe H, Hohl TM, Heinecke JW, Ziegler SF, Nelson PJ, and Duffield JS. Resident renal mononuclear phagocytes comprise five discrete populations with distinct phenotypes and functions. J Immunol 191: 3358–3372, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kie JH, Kapturczak MH, Traylor A, Agarwal A, and Hill-Kapturczak N. Heme oxygenase-1 deficiency promotes epithelial-mesenchymal transition and renal fibrosis. J Am Soc Nephrol 19: 1681–1691, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim J, Zarjou A, Traylor AM, Bolisetty S, Jaimes EA, Hull TD, George JF, Mikhail FM, and Agarwal A. In vivo regulation of the heme oxygenase-1 gene in humanized transgenic mice. Kidney Int 82: 278–291, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim YM, Pae HO, Park JE, Lee YC, Woo JM, Kim NH, Choi YK, Lee BS, Kim SR, and Chung HT. Heme oxygenase in the regulation of vascular biology: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 14: 137–167, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kimpara T, Takeda A, Watanabe K, Itoyama Y, Ikawa S, Watanabe M, Arai H, Sasaki H, Higuchi S, Okita N, Takase S, Saito H, Takahashi K, and Shibahara S. Microsatellite polymorphism in the human heme oxygenase-1 gene promoter and its application in association studies with Alzheimer and Parkinson disease. Hum Genet 100: 145–147, 1997 [DOI] [PubMed] [Google Scholar]
- 71.Kitamuro T, Takahashi K, Ogawa K, Udono-Fujimori R, Takeda K, Furuyama K, Nakayama M, Sun J, Fujita H, Hida W, Hattori T, Shirato K, Igarashi K, and Shibahara S. Bach1 functions as a hypoxia-inducible repressor for the heme oxygenase-1 gene in human cells. J Biol Chem 278: 9125–9133, 2003 [DOI] [PubMed] [Google Scholar]
- 72.Konvalinka A, Zhou J, Dimitromanolakis A, Drabovich AP, Fang F, Gurley S, Coffman T, John R, Zhang SL, Diamandis EP, and Scholey JW. Determination of an angiotensin II-regulated proteome in primary human kidney cells by stable isotope labeling of amino acids in cell culture (SILAC). J Biol Chem 288: 24834–24847, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuroiwa T. and Lee EG. Cellular interactions in the pathogenesis of lupus nephritis: the role of T cells and macrophages in the amplification of the inflammatory process in the kidney. Lupus 7: 597–603, 1998 [DOI] [PubMed] [Google Scholar]
- 74.Larsen R, Gozzelino R, Jeney V, Tokaji L, Bozza FA, Japiassu AM, Bonaparte D, Cavalcante MM, Chora A, Ferreira A, Marguti I, Cardoso S, Sepulveda N, Smith A, and Soares MP. A central role for free heme in the pathogenesis of severe sepsis. Sci Transl Med 2: 51ra71, 2010 [DOI] [PubMed] [Google Scholar]
- 75.Lavrovsky Y, Schwartzman ML, Levere RD, Kappas A, and Abraham NG. Identification of binding sites for transcription factors NF-kappa B and AP-2 in the promoter region of the human heme oxygenase 1 gene. Proc Natl Acad Sci U S A 91: 5987–5991, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee EY, Lee YH, Kim SH, Chung KS, Kwon O, Kim BS, Nam CM, Park CS, Lee BW, Kang ES, Cha BS, and Lee HC. Association between heme oxygenase-1 promoter polymorphisms and the development of albuminuria in type 2 diabetes: a case-control study. Medicine (Baltimore) 94: e1825, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee SC, Han SH, Li JJ, Lee SH, Jung DS, Kwak SJ, Kim SH, Kim DK, Yoo TH, Kim JH, Chang SH, Han DS, and Kang SW. Induction of heme oxygenase-1 protects against podocyte apoptosis under diabetic conditions. Kidney Int 76: 838–848, 2009 [DOI] [PubMed] [Google Scholar]
- 78.Levere RD, Martasek P, Escalante B, Schwartzman ML, and Abraham NG. Effect of heme arginate administration on blood pressure in spontaneously hypertensive rats. J Clin Invest 86: 213–219, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Levine B. and Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest 115: 2679–2688, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li H, Wang F, Zhang L, Cao Y, Liu W, Hao J, Liu Q, and Duan H. Modulation of Nrf2 expression alters high glucose-induced oxidative stress and antioxidant gene expression in mouse mesangial cells. Cell Signal 23: 1625–1632, 2011 [DOI] [PubMed] [Google Scholar]
- 81.Lin CC, Yang WC, Lin SJ, Chen TW, Lee WS, Chang CF, Lee PC, Lee SD, Su TS, Fann CS, and Chung MY. Length polymorphism in heme oxygenase-1 is associated with arteriovenous fistula patency in hemodialysis patients. Kidney Int 69: 165–172, 2006 [DOI] [PubMed] [Google Scholar]
- 82.Lin Q, Weis S, Yang G, Weng YH, Helston R, Rish K, Smith A, Bordner J, Polte T, Gaunitz F, and Dennery PA. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J Biol Chem 282: 20621–20633, 2007 [DOI] [PubMed] [Google Scholar]
- 83.Linnenbaum M, Busker M, Kraehling JR, and Behrends S. Heme oxygenase isoforms differ in their subcellular trafficking during hypoxia and are differentially modulated by cytochrome P450 reductase. PLoS One 7: e35483, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mackern-Oberti JP, Llanos C, Carreno LJ, Riquelme SA, Jacobelli SH, Anegon I, and Kalergis AM. Carbon monoxide exposure improves immune function in lupus-prone mice. Immunology 140: 123–132, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Magee CC, Azuma H, Knoflach A, Denton MD, Chandraker A, Iyer S, Buelow R, and Sayegh M. In vitro and in vivo immunomodulatory effects of RDP1258, a novel synthetic peptide. J Am Soc Nephrol 10: 1997–2005, 1999 [DOI] [PubMed] [Google Scholar]
- 86.Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J 2: 2557–2568, 1988 [PubMed] [Google Scholar]
- 87.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol 37: 517–554, 1997 [DOI] [PubMed] [Google Scholar]
- 88.Maines MD, Eke BC, and Zhao X. Corticosterone promotes increased heme oxygenase-2 protein and transcript expression in the newborn rat brain. Brain Res 722: 83–94, 1996 [DOI] [PubMed] [Google Scholar]
- 89.Maines MD, Mayer RD, Ewing JF, McCoubrey WK., Jr Induction of kidney heme oxygenase-1 (HSP32) mRNA and protein by ischemia/reperfusion: possible role of heme as both promotor of tissue damage and regulator of HSP32. J Pharmacol Exp Ther 264: 457–462, 1993 [PubMed] [Google Scholar]
- 90.Martasek P, Schwartzman ML, Goodman AI, Solangi KB, Levere RD, and Abraham NG. Hemin and L-arginine regulation of blood pressure in spontaneous hypertensive rats. J Am Soc Nephrol 2: 1078–1084, 1991 [DOI] [PubMed] [Google Scholar]
- 91.Martasek P, Solangi K, Goodman AI, Levere RD, Chernick RJ, and Abraham NG. Properties of human kidney heme oxygenase: inhibition by synthetic heme analogues and metalloporphyrins. Biochem Biophys Res Commun 157: 480–487, 1988 [DOI] [PubMed] [Google Scholar]
- 92.Maser RL, Vassmer D, Magenheimer BS, and Calvet JP. Oxidant stress and reduced antioxidant enzyme protection in polycystic kidney disease. J Am Soc Nephrol 13: 991–999, 2002 [DOI] [PubMed] [Google Scholar]
- 93.McCoubrey WK, Jr., Ewing JF, and Maines MD. Human heme oxygenase-2: characterization and expression of a full-length cDNA and evidence suggesting that the two HO-2 transcripts may differ by choice of polyadenylation signal. Arch Biochem Biophys 295: 13–20, 1992 [DOI] [PubMed] [Google Scholar]
- 94.McCoubrey WK, Jr., Huang TJ, and Maines MD. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur J Biochem 247: 725–732, 1997 [DOI] [PubMed] [Google Scholar]
- 95.Mollsten A, Marklund SL, Wessman M, Svensson M, Forsblom C, Parkkonen M, Brismar K, Groop PH, and Dahlquist G. A functional polymorphism in the manganese superoxide dismutase gene and diabetic nephropathy. Diabetes 56: 265–269, 2007 [DOI] [PubMed] [Google Scholar]
- 96.Mosley K, Wembridge DE, Cattell V, and Cook HT. Heme oxygenase is induced in nephrotoxic nephritis and hemin, a stimulator of heme oxygenase synthesis, ameliorates disease. Kidney Int 53: 672–678, 1998 [DOI] [PubMed] [Google Scholar]
- 97.Motterlini R, Gonzales A, Foresti R, Clark JE, Green CJ, and Winslow RM. Heme oxygenase-1-derived carbon monoxide contributes to the suppression of acute hypertensive responses in vivo. Circ Res 83: 568–577, 1998 [DOI] [PubMed] [Google Scholar]
- 98.Muraosa Y, Takahashi K, Yoshizawa M, and Shibahara S. cDNA cloning of a novel protein containing two zinc-finger domains that may function as a transcription factor for the human heme-oxygenase-1 gene. Eur J Biochem 235: 471–479, 1996 [DOI] [PubMed] [Google Scholar]
- 99.Nath KA. Heme oxygenase-1 and acute kidney injury. Curr Opin Nephrol Hypertens 23: 17–24, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt MD, and Rosenberg ME. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest 90: 267–270, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nath KA, Croatt AJ, Likely S, Behrens TW, and Warden D. Renal oxidant injury and oxidant response induced by mercury. Kidney Int 50: 1032–1043, 1996 [DOI] [PubMed] [Google Scholar]
- 102.Nath KA, Grande JP, Farrugia G, Croatt AJ, Belcher JD, Hebbel RP, Vercellotti GM, and Katusic ZS. Age sensitizes the kidney to heme protein-induced acute kidney injury. Am J Physiol Renal Physiol 304: F317–F325, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nath KA, Grande JP, Haggard JJ, Croatt AJ, Katusic ZS, Solovey A, and Hebbel RP. Oxidative stress and induction of heme oxygenase-1 in the kidney in sickle cell disease. Am J Pathol 158: 893–903, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nath KA, Haggard JJ, Croatt AJ, Grande JP, Poss KD, and Alam J. The indispensability of heme oxygenase-1 in protecting against acute heme protein-induced toxicity in vivo. Am J Pathol 156: 1527–1535, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ndisang JF. and Jadhav A. Hemin therapy improves kidney function in male streptozotocin-induced diabetic rats: role of the heme oxygenase/atrial natriuretic peptide/adiponectin axis. Endocrinology 155: 215–229, 2014 [DOI] [PubMed] [Google Scholar]
- 106.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, and Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404: 787–790, 2000 [DOI] [PubMed] [Google Scholar]
- 107.Ozaki KS, Marques GM, Nogueira E, Feitoza RQ, Cenedeze MA, Franco MF, Mazzali M, Soares MP, Pacheco-Silva A, and Camara NO. Improved renal function after kidney transplantation is associated with heme oxygenase-1 polymorphism. Clin Transplant 22: 609–616, 2008 [DOI] [PubMed] [Google Scholar]
- 108.Periyasamy-Thandavan S, Jiang M, Wei Q, Smith R, Yin XM, and Dong Z. Autophagy is cytoprotective during cisplatin injury of renal proximal tubular cells. Kidney Int 74: 631–640, 2008 [DOI] [PubMed] [Google Scholar]
- 109.Pittock ST, Norby SM, Grande JP, Croatt AJ, Bren GD, Badley AD, Caplice NM, Griffin MD, and Nath KA. MCP-1 is up-regulated in unstressed and stressed HO-1 knockout mice: pathophysiologic correlates. Kidney Int 68: 611–622, 2005 [DOI] [PubMed] [Google Scholar]
- 110.Polte T, Hemmerle A, Berndt G, Grosser N, Abate A, and Schroder H. Atrial natriuretic peptide reduces cyclosporin toxicity in renal cells: role of cGMP and heme oxygenase-1. Free Radic Biol Med 32: 56–63, 2002 [DOI] [PubMed] [Google Scholar]
- 111.Raju VS, McCoubrey WK, Jr., and Maines MD. Regulation of heme oxygenase-2 by glucocorticoids in neonatal rat brain: characterization of a functional glucocorticoid response element. Biochim Biophys Acta 1351: 89–104, 1997 [DOI] [PubMed] [Google Scholar]
- 112.Rezzani R, Rodella L, Bianchi R, Goodman AI, and Lianos EA. Protective effects of heme-oxygenase expression in cyclosporine A—induced injury. Curr Neurovasc Res 2: 157–161, 2005 [DOI] [PubMed] [Google Scholar]
- 113.Rezzani R, Rodella L, Buffoli B, Goodman AA, Abraham NG, Lianos EA, and Bianchi R. Change in renal heme oxygenase expression in cyclosporine A-induced injury. J Histochem Cytochem 53: 105–112, 2005 [DOI] [PubMed] [Google Scholar]
- 114.Ries F. and Klastersky J. Nephrotoxicity induced by cancer chemotherapy with special emphasis on cisplatin toxicity. Am J Kidney Dis 8: 368–379, 1986 [DOI] [PubMed] [Google Scholar]
- 115.Riphagen IJ, Deetman PE, Bakker SJ, Navis G, Cooper ME, Lewis JB, de Zeeuw D, Lambers and Heerspink HJ. Bilirubin and progression of nephropathy in type 2 diabetes: a post hoc analysis of RENAAL with independent replication in IDNT. Diabetes 63: 2845–2853, 2014 [DOI] [PubMed] [Google Scholar]
- 116.Rodriguez F, Lopez B, Perez C, Fenoy FJ, Hernandez I, Stec DE, Li Volti G, and Salom MG. Chronic tempol treatment attenuates the renal hemodynamic effects induced by a heme oxygenase inhibitor in streptozotocin diabetic rats. Am J Physiol Regul Integr Comp Physiol 301: R1540–R1548, 2011 [DOI] [PubMed] [Google Scholar]
- 117.Ryter SW. and Choi AM. Regulation of autophagy in oxygen-dependent cellular stress. Curr Pharm Des 19: 2747–2756, 2013 [DOI] [PubMed] [Google Scholar]
- 118.Saraf SL, Zhang X, Shah B, Kanias T, Gudehithlu KP, Kittles R, Machado RF, Arruda JA, Gladwin MT, Singh AK, and Gordeuk VR. Genetic variants and cell-free hemoglobin processing in sickle cell nephropathy. Haematologica 100: 1275–1284, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sato M, Ishizawa S, Yoshida T, and Shibahara S. Interaction of upstream stimulatory factor with the human heme oxygenase gene promoter. Eur J Biochem 188: 231–237, 1990 [DOI] [PubMed] [Google Scholar]
- 120.Schuller DJ, Wilks A, Ortiz de Montellano PR, and Poulos TL. Crystal structure of human heme oxygenase-1. Nat Struct Biol 6: 860–867, 1999 [DOI] [PubMed] [Google Scholar]
- 121.Shibahara S, Kitamuro T, and Takahashi K. Heme degradation and human disease: diversity is the soul of life. Antioxid Redox Signal 4: 593–602, 2002 [DOI] [PubMed] [Google Scholar]
- 122.Shibahara S, Muller RM, and Taguchi H. Transcriptional control of rat heme oxygenase by heat shock. J Biol Chem 262: 12889–12892, 1987 [PubMed] [Google Scholar]
- 123.Shimizu H, Takahashi T, Suzuki T, Yamasaki A, Fujiwara T, Odaka Y, Hirakawa M, Fujita H, and Akagi R. Protective effect of heme oxygenase induction in ischemic acute renal failure. Crit Care Med 28: 809–817, 2000 [DOI] [PubMed] [Google Scholar]
- 124.Shiraishi F, Curtis LM, Truong L, Poss K, Visner GA, Madsen K, Nick HS, and Agarwal A. Heme oxygenase-1 gene ablation or expression modulates cisplatin-induced renal tubular apoptosis. Am J Physiol Renal Physiol 278: F726–F736, 2000 [DOI] [PubMed] [Google Scholar]
- 125.Sikorski EM, Hock T, Hill-Kapturczak N, and Agarwal A. The story so far: molecular regulation of the heme oxygenase-1 gene in renal injury. Am J Physiol Renal Physiol 286: F425–F441, 2004 [DOI] [PubMed] [Google Scholar]
- 126.Stocker R, Yamamoto Y, McDonagh A, Glazer A, and Ames B. Bilirubin is an antioxidant of possible physiological importance. Science 235: 1043–1046, 1987 [DOI] [PubMed] [Google Scholar]
- 127.Sung FL, Zhu TY, Au-Yeung KK, Siow YL, and O K. Enhanced MCP-1 expression during ischemia/reperfusion injury is mediated by oxidative stress and NF-kappaB. Kidney Int 62: 1160–1170, 2002 [DOI] [PubMed] [Google Scholar]
- 128.Suzuki T. and Yamamoto M. Molecular basis of the Keap1-Nrf2 system. Free Radic Biol Med 88: 93–100, 2015 [DOI] [PubMed] [Google Scholar]
- 129.Takahashi S, Matsuura N, Kurokawa T, Takahashi Y, and Miura T. Co-operation of the transcription factor hepatocyte nuclear factor-4 with Sp1 or Sp3 leads to transcriptional activation of the human haem oxygenase-1 gene promoter in a hepatoma cell line. Biochem J 367: 641–652, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Takahashi S, Takahashi Y, Ito K, Nagano T, Shibahara S, and Miura T. Positive and negative regulation of the human heme oxygenase-1 gene expression in cultured cells. Biochim Biophys Acta 1447: 231–235, 1999 [DOI] [PubMed] [Google Scholar]
- 131.Takakura A, Contrino L, Zhou X, Bonventre JV, Sun Y, Humphreys BD, and Zhou J. Renal injury is a third hit promoting rapid development of adult polycystic kidney disease. Hum Mol Genet 18: 2523–2531, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Takeda Y, Takeno M, Iwasaki M, Kobayashi H, Kirino Y, Ueda A, Nagahama K, Aoki I, and Ishigatsubo Y. Chemical induction of HO-1 suppresses lupus nephritis by reducing local iNOS expression and synthesis of anti-dsDNA antibody. Clin Exp Immunol 138: 237–244, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Taye A. and Ibrahim BM. Activation of renal haeme oxygenase-1 alleviates gentamicin-induced acute nephrotoxicity in rats. J Pharm Pharmacol 65: 995–1004, 2013 [DOI] [PubMed] [Google Scholar]
- 134.Tenhunen R, Marver HS, and Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A 61: 748–755, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tenhunen R, Marver HS, and Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem 244: 6388–6394, 1969 [PubMed] [Google Scholar]
- 136.Tesch GH. and Allen TJ. Rodent models of streptozotocin-induced diabetic nephropathy. Nephrology (Carlton) 12: 261–266, 2007 [DOI] [PubMed] [Google Scholar]
- 137.Tullius SG, Nieminen-Kelha M, Buelow R, Reutzel-Selke A, Martins PN, Pratschke J, Bachmann U, Lehmann M, Southard D, Iyer S, Schmidbauer G, Sawitzki B, Reinke P, Neuhaus P, and Volk HD. Inhibition of ischemia/reperfusion injury and chronic graft deterioration by a single-donor treatment with cobalt-protoporphyrin for the induction of heme oxygenase-1. Transplantation 74: 591–598, 2002 [DOI] [PubMed] [Google Scholar]
- 138.Tyrrell RM, Applegate LA, and Tromvoukis Y. The proximal promoter region of the human heme oxygenase gene contains elements involved in stimulation of transcriptional activity by a variety of agents including oxidants. Carcinogenesis 14: 761–765, 1993 [DOI] [PubMed] [Google Scholar]
- 139.Vitek L, Jirsa M, Brodanova M, Kalab M, Marecek Z, Danzig V, Novotny L, and Kotal P. Gilbert syndrome and ischemic heart disease: a protective effect of elevated bilirubin levels. Atherosclerosis 160: 449–456, 2002 [DOI] [PubMed] [Google Scholar]
- 140.Vogt BA, Alam J, Croatt AJ, Vercellotti GM, and Nath KA. Acquired resistance to acute oxidative stress. Possible role of heme oxygenase and ferritin. Lab Invest 72: 474–483, 1995 [PubMed] [Google Scholar]
- 141.Vogt BA, Shanley TP, Croatt A, Alam J, Johnson KJ, and Nath KA. Glomerular inflammation induces resistance to tubular injury in the rat. A novel form of acquired, heme oxygenase-dependent resistance to renal injury. J Clin Invest 98: 2139–2145, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wagner M, Cadetg P, Ruf R, Mazzucchelli L, Ferrari P, and Redaelli CA. Heme oxygenase-1 attenuates ischemia/reperfusion-induced apoptosis and improves survival in rat renal allografts. Kidney Int 63: 1564–1573, 2003 [DOI] [PubMed] [Google Scholar]
- 143.Wang Y, Shen J, Xiong X, Xu Y, Zhang H, Huang C, Tian Y, Jiao C, Wang X, and Li X. Remote ischemic preconditioning protects against liver ischemia-reperfusion injury via heme oxygenase-1-induced autophagy. PLoS One 9: e98834, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wegiel B, Hauser CJ, and Otterbein LE. Heme as a danger molecule in pathogen recognition. Free Radic Biol Med 89: 651–661, 2015 [DOI] [PubMed] [Google Scholar]
- 145.Wegiel B, Larsen R, Gallo D, Chin BY, Harris C, Mannam P, Kaczmarek E, Lee PJ, Zuckerbraun BS, Flavell R, Soares MP, and Otterbein LE. Macrophages sense and kill bacteria through carbon monoxide-dependent inflammasome activation. J Clin Invest 124: 4926–4940, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wegiel B, Nemeth Z, Correa-Costa M, Bulmer AC, and Otterbein LE. Heme oxygenase-1: a metabolic nike. Antioxid Redox Signal 20: 1709–1722, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wenzel P, Rossmann H, Muller C, Kossmann S, Oelze M, Schulz A, Arnold N, Simsek C, Lagrange J, Klemz R, Schonfelder T, Brandt M, Karbach SH, Knorr M, Finger S, Neukirch C, Hauser F, Beutel ME, Kroller-Schon S, Schulz E, Schnabel RB, Lackner K, Wild PS, Zeller T, Daiber A, Blankenberg S, and Munzel T. Heme oxygenase-1 suppresses a pro-inflammatory phenotype in monocytes and determines endothelial function and arterial hypertension in mice and humans. Eur Heart J 36: 3437–3446, 2015 [DOI] [PubMed] [Google Scholar]
- 148.Yamada N, Yamaya M, Okinaga S, Nakayama K, Sekizawa K, Shibahara S, and Sasaki H. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am J Hum Genet 66: 187–195, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yoneya R, Ozasa H, Nagashima Y, Koike Y, Teraoka H, Hagiwara K, and Horikawa S. Hemin pretreatment ameliorates aspects of the nephropathy induced by mercuric chloride in the rat. Toxicol Lett 116: 223–229, 2000 [DOI] [PubMed] [Google Scholar]
- 150.Yoshida T. and Migita CT. Mechanism of heme degradation by heme oxygenase. J Inorg Biochem 82: 33–41, 2000 [DOI] [PubMed] [Google Scholar]
- 151.Yoshida T. and Sato M. Posttranslational and direct integration of heme oxygenase into microsomes. Biochem Biophys Res Commun 163: 1086–1092, 1989 [DOI] [PubMed] [Google Scholar]
- 152.Yuan G, Vasavda C, Peng YJ, Makarenko VV, Raghuraman G, Nanduri J, Gadalla MM, Semenza GL, Kumar GK, Snyder SH, and Prabhakar NR. Protein kinase G-regulated production of H2S governs oxygen sensing. Sci Signal 8: ra37, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zager RA. and Johnson AC. Renal ischemia-reperfusion injury upregulates histone-modifying enzyme systems and alters histone expression at proinflammatory/profibrotic genes. Am J Physiol Renal Physiol 296: F1032–F1041, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zager RA, Johnson AC, and Becker K. Acute unilateral ischemic renal injury induces progressive renal inflammation, lipid accumulation, histone modification, and “end-stage” kidney disease. Am J Physiol Renal Physiol 301: F1334–F1345, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zager RA, Johnson AC, and Becker K. Plasma and urinary heme oxygenase-1 in AKI. J Am Soc Nephrol 23: 1048–1057, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zakhary R, Gaine SP, Dinerman JL, Ruat M, Flavahan NA, and Snyder SH. Heme oxygenase 2: endothelial and neuronal localization and role in endothelium-dependent relaxation. Proc Natl Acad Sci U S A 93: 795–798, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zarjou A. and Agarwal A. Heme oxygenase-1 as a target for TGF-beta in kidney disease. Semin Nephrol 32: 277–286, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zarjou A, Bolisetty S, Joseph R, Traylor A, Apostolov EO, Arosio P, Balla J, Verlander J, Darshan D, Kuhn LC, and Agarwal A. Proximal tubule H-ferritin mediates iron trafficking in acute kidney injury. J Clin Invest 123: 4423–4434, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zarjou A, Kim J, Traylor AM, Sanders PW, Balla J, Agarwal A, and Curtis LM. Paracrine effects of mesenchymal stem cells in cisplatin-induced renal injury require heme oxygenase-1. Am J Physiol Renal Physiol 300: F254–F262, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhou C, Li H, Yao Y, and Li L. Delayed remote ischemic preconditioning produces an additive cardioprotection to sevoflurane postconditioning through an enhanced heme oxygenase 1 level partly via nuclear factor erythroid 2-related factor 2 nuclear translocation. J Cardiovasc Pharmacol Ther 19: 558–566, 2014 [DOI] [PubMed] [Google Scholar]
- 161.Zhou C, Li L, Li H, Gong J, and Fang N. Delayed remote preconditioning induces cardioprotection: role of heme oxygenase-1. J Surg Res 191: 51–57, 2014 [DOI] [PubMed] [Google Scholar]
- 162.Zhou J, Ouyang X, Schoeb TR, Bolisetty S, Cui X, Mrug S, Yoder BK, Johnson MR, Szalai AJ, and Mrug M. Kidney injury accelerates cystogenesis via pathways modulated by heme oxygenase and complement. J Am Soc Nephrol 23: 1161–1171, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zou AP, Billington H, Su N, and Cowley AW., Jr Expression and actions of heme oxygenase in the renal medulla of rats. Hypertension 35: 342–347, 2000 [DOI] [PubMed] [Google Scholar]