Abstract
Tissue engineering approaches using growth factor-functionalized acellular scaffolds to support and guide repair driven by endogenous cells are thought to require a careful balance between cell recruitment and growth factor release kinetics. The objective of this study was to identify a growth factor combination that accelerates progenitor cell migration into self-assembling peptide hydrogels in the context of cartilage defect repair. A novel 3D gel-to-gel migration assay enabled quantification of the chemotactic impact of platelet-derived growth factor-BB (PDGF-BB), heparin-binding insulin-like growth factor-1 (HB-IGF-1), and transforming growth factor-β1 (TGF-β1) on progenitor cells derived from subchondral bovine trabecular bone (bone-marrow progenitor cells, BM-PCs) encapsulated in the peptide hydrogel [KLDL]3. Only the combination of PDGF-BB and TGF-β1 stimulated significant migration of BM-PCs over a 4-day period, measured by confocal microscopy. Both PDGF-BB and TGF-β1 were slowly released from the gel, as measured using their 125I-labeled forms, and they remained significantly present in the gel at 4 days. In the context of augmenting microfracture surgery for cartilage repair, our strategy of delivering chemotactic and proanabolic growth factors in KLD may provide the necessary local stimulus to help increase defect cellularity, providing more cells to generate repair tissue.
Introduction
Acellular scaffolds functionalized with cell stimulatory molecules are widely used in tissue engineering.1–4 This approach relies on endogenous cells to populate the scaffold following surgical implantation. Matching the timescale of release of cell stimulatory molecules from the scaffold with the invasion kinetics of endogenous cells into the scaffold would presumably be important.
For cartilage repair, microfracture surgery is used, in part, to access the endogenous progenitor cell population, but the resulting repair tissue is often mechanically inferior fibrocartilage, and lateral integration with native cartilage can be incomplete.5,6 In vitro and in vivo studies have shown that the addition of cell-free scaffolds functionalized with growth factors (GFs) may help induce chondrogenesis of endogenous progenitor cells, neotissue production by these cells, and subsequent integration of neotissue with host tissue (Fig. 1A).1,7–14
FIG. 1.
Concept and experimental design: (A) The addition of a hydrogel scaffold to augment the standard microfracture approach; (B) 3D gel-to-gel migration assay setup: A KLD hydrogel premixed with GFs was cast at the bottom of a 48-well plate well. A second KLD hydrogel encapsulating 150,000 BM-PCs was cast on top of the first gel, with a layer of green fluorescent beads used to demark the interface between the two gels. Constructs were incubated in migration media for 4 days (see Materials and Methods section), fixed, and BM-PC migration was assessed with confocal microscopy. Color images available online at www.liebertpub.com/tea
One candidate class of scaffolds for augmenting microfracture is self-assembling peptide hydrogels.15,16 These injectable hydrogels assemble upon exposure to physiological pH and ionic strength at concentrations less than 0.5%, allowing ample space for cells to deposit neotissue. The self-assembling sequences KLD and RAD have been shown to foster chondrogenesis of bone marrow progenitor cells and matrix production by primary chondrocytes.13,16,17 Once assembled, acellular KLD has an equilibrium modulus of ∼1 kPa, while chondrocyte-seeded KLD supports the assembly of functional neotissue with a modulus of ∼95 kPa after 4 weeks of culture.16,18 Initial in vivo studies demonstrated KLD to both be biocompatible and show promise toward stimulating repair.1,19
Many proanabolic GFs have been investigated in tissue engineering approaches to stimulate growth, such as insulin-like growth factor-1 (IGF-1) in the case of cartilage tissue,20,21 vascular endothelial growth factor (VEGF) for cardiac tissue,4,22,23 fibroblastic growth factor-2 (FGF-2) and nerve growth factor (NGF) for neural tissue,24 and epidermal growth factor (EGF) for hepatic tissue.24 GFs are also frequently used to stimulate progenitor cell differentiation in vitro, such as transforming growth factor beta-1 (TGF-β1) to stimulate chondrogenesis of mesenchymal progenitor cells.25 When pharmacologically delivered to the body globally, however, some GFs, such as IGF-1, can trigger systemic adverse events.26–28 Local delivery of such GFs by functionalization to scaffolds or particles,24,29 encapsulation in microspheres,20,30 or the development of fusion proteins31 have extended the viability of these GFs as useful tissue engineering tools. The fusion protein heparin-binding insulin-like growth factor-1 (HB-IGF-1), for example, stimulates matrix production by chondrocytes through a single dose delivered through a self-assembling peptide hydrogel scaffold.13,31 Such local delivery allows for lower doses than injection methods, reducing toxicity and costs.
Despite these promising in vitro results with locally delivered anabolic GFs, it is believed that the benefits of these anabolic GFs will not be realized if the GFs diffuse out of the acellular scaffold before endogenous progenitor cell invasion in vivo. We therefore hypothesized that the addition of chemotactic GFs to the scaffold accelerates the migration of endogenous cells into the scaffold, providing potential enhanced benefit from the anabolic GFs.
Previous studies by Ozaki et al., Fiedler et al., and others, using modified Boyden chamber assays, have shown generally that mesenchymal stem cell chemotaxis can be stimulated by platelet-derived growth factor-BB (PDGF-BB), and this effect is enhanced when combined with IGF-1 or TGF-α.32–37 Thus, we further hypothesized that the addition of PDGF-BB to our self-assembling peptide hydrogel scaffold provides a synergistic chemotactic effect with a prochondrogenic member of the TGF-family, TGF-β1, or the proanabolic GF HB-IGF-1.
Building upon the detailed literature characterizing KLD as a suitable environment for progenitor cell chondrogenesis and chondrocyte matrix production, this work focuses on assessing KLD as an environment for GF-induced migration.17,38–41 We hypothesized that the addition of promigratory GFs to KLD would result in increased cell migration into the gel. To test our hypothesis, we developed a novel 3D gel-to-gel migration system and used this system to evaluate the chemotactic impact of GFs on progenitor cells in KLD (Fig. 1B).42 Specifically, using a cell population relevant to the endogenous cells accessed during microfracture, we studied the effects of the GFs PDGF-BB, TGF-β1, and HB-IGF-1 (individually and in combination) for their chemotactic potential in our 3D hydrogel scaffold. Furthermore, we quantified the release kinetics of the candidate GFs from the scaffold to interpret their participation in cell migration.
Materials and Methods
Cell harvest, expansion, and encapsulation
Since the cells accessed in microfracture surgery reside below the microfracture punctures, we isolated progenitor cells isolated from the stroma associated with subchondral trabecular bone. Mesenchymal progenitor cells associated with the marrow stroma found in immature trabecular bone (bone-marrow progenitor cells, BM-PCs) were isolated from the subchondral trabecular bone of 1–2 week-old bovine calf knee joints in a manner modified from Haynesworth et al. and Li et al.43,44 (Fig. 2A) (Research 87, Marlborough, MA).
FIG. 2.
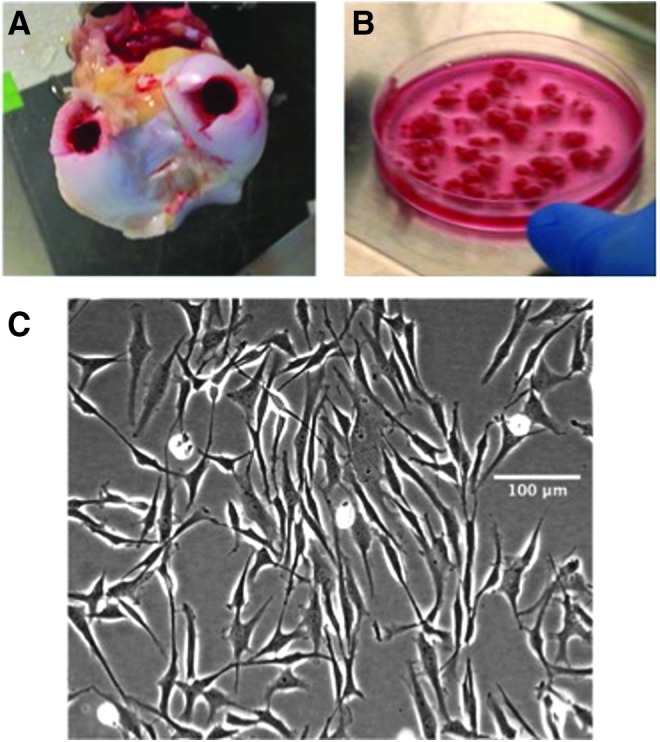
BM-PC isolation: (A) 9 mm osteochondral plugs were harvested from the condyles of 1–2 week old bovine joints, and the cartilage was removed; (B) remaining trabecular bone fragments were rinsed vigorously with media and mechanically disrupted to isolate cells; (C) bright-field microscopy of passage 1 BM-PCs following differential adhesion. Color images available online at www.liebertpub.com/tea
Two 9 mm osteochondral plugs were drilled from the condyle, and the cartilage layer was removed. The remaining trabecular bone explants were finely diced using a scalpel (Fig. 2B) and placed in a primary expansion medium consisting of low-glucose Dulbecco's modified Eagle's medium (DMEM) (Mediatech, Inc., Manassas, VA) with 10% fetal bovine serum (FBS) (GE Healthcare Life Sciences, Hyclone Labs, Logan, UT), 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin (PSA), 10 mM 4-(2-hydroxyethyl)-1-piperzaineethanesulfronic acid (HEPES) (Invitrogen, Carlsbad, CA), and 1 ng/mL basic FGF-2 (R&D Systems, Minneapolis, MN).17 The bone fragments were rinsed vigorously with this medium and vortexed to mechanically drive out the cells.
The resulting cell suspension was filtered through a 70-μm pore strainer and plated in flasks at 2.5 × 105cells/cm2 in the expansion medium for 24 h. The medium was removed and replenished, and cells were cultured until primary colonies reached 80% confluence (Fig. 2C). BM-PC colonies were removed from flasks with 0.05% trypsin in 0.53 mM EDTA (Corning, Corning, NY) and frozen until further use. BM-PCs were passaged 1–2 times by plating with a seeding density of 1 × 103cells/cm2 in an expansion medium containing 5 ng/mL FGF-2 and expanding until 80–90% confluent.
These expanded BM-PCs were encapsulated in 3.5 mg/mL KLD self-assembling peptide hydrogels at 10 × 106cells/mL, as described previously,17 for use in matrix production and migration studies. For matrix production studies, the cell-seeded hydrogels were cultured in a chondrogenic culture medium consisting of high-glucose DMEM, 1% insulin-transferrin-selenium + bovine serum albumin and linoleic acid (ITS+Premix) (BD Biosciences, Bedford, MA), 0.4 mM proline (Sigma-Aldrich, St. Louis, MO), 37.5 μg/mL ascorbate-2-phosphate (A2P) (Wake Chemicals, Richmond, VA), 10 mM HEPES, PSA, 0.1 mM nonessential amino acids (NEAA) (Sigma-Aldrich), 1 mM sodium pyruvate (Invitrogen), 10 ng/mL rhTGF-β1 (Peprotech, Rocky Hill, NJ), and 0.1 μM Dexamethasone (Invitrogen) at 37°C and 5% carbon dioxide, for 22 days.39,45
Analysis of matrix production and cell proliferation
Thorough characterization of the chondrogenic capacity of bone marrow progenitor cells in KLD was previously reported by our laboratory, including both immunohistochemistry of collagen types I and II, as well as qPCR for the temporal evolution of gene expression for collagens types I & II, aggrecan, SOX9, osteocalcin, and PPAR-γ.17 We also measured toluidine blue staining, sGAG production, and hydroxyproline content and confirmed that these measures provided complementary surrogate markers of matrix production by chondrogenic cells.17,38
At day 22 in this study, synthesis rates of sGAG and total protein were assessed by radiolabel incorporation using 5 μCi of 35S-sulfate and 10 μCi of 3H-proline (Perkin Elmer, Waltham, MA), respectively46; total sGAG output was measured by DMMB dye binding, collagen content was measured by hydroxyproline reaction with p-dimethylaminobenzaldehyde, and DNA content was measured by Hoechst dye binding, as described previously.47–49 Histological staining for the spatial appearance of sGAG using toluidine blue was performed as described previously.16,17 N = 6–8 gels per time point.
Gel-to-gel migration assay
We developed a novel gel-to-gel migration assay shown schematically in Figure 1B. TGF-β1 (100 ng/mL), PDGF-BB (100 ng/mL) (Peprotech), or HB-IGF-1 (500 nM), along with all of their combinations, were tested by premixing them in 3.5 mg/mL KLD self-assembling peptide hydrogel. The quantities of GFs added were not high enough to alter the assembly or mechanical properties of the KLD. Gels were cast into wells of a 48-well plate and assembled overnight in low-glucose DMEM, PSA, 10 mM HEPES, and 1% FBS (migration medium). Based on the literature, this low concentration of FBS was chosen to avoid obscuring potential promigratory effects of the GFs being studied, while maintaining cell viability during the course of the 4-day study.50
A thin layer of green fluorescent beads (polystyrene with 2% divinylbenzene, mean diameter 15.45 μm; Bangs Laboratories, Inc., Fishers, IN) was added atop this gel, followed by the casting of a second KLD hydrogel encapsulating 150,000 BM-PCs (2 × 106cells/mL, chosen to optimize imaging and quantification) (Fig. 1B). These green fluorescent beads demarked the interface between the two gels to assist in confocal quantification of migrating cells. Hydrogels were cultured for 4 days at 37°C, then fixed with 4% PFA and stained with propidium iodide in a permeabilization buffer consisting of 0.5% Triton X-100 (Sigma-Aldrich) and 0.5% BSA in PBS.
Multichannel fluorescent confocal microscopy was used to image wells over the depth of the gels in 5 μm steps, and migration was quantified using a modified MATLAB script developed previously.42,51 Briefly, 3D coordinates of cells and beads were calculated using a modified hybrid 2D/3D spot finding algorithm that determines fluorescent spot positions for each 2D image slice and then reassembles them into the initial 3D shape. Variance in the fluorescent bead positions due to the curvature of the surface of the bottom gel was accounted for by comparing cell positions to the mean bead position ± 1–2 standard deviations. For fields of view only containing 1 bead, a standard deviation of 25 μm was used.
PDGF-BB loading efficiency in KLD hydrogels
A radiolabeled form of PDGF-BB (125I-PDGF-BB) (Perkin Elmer) was used to assess its entrapment in KLD, following a method described previously for a similar self-assembling peptide hydrogel, RAD ([RADA]4).13,52 Just before use, free 125I label was first removed from 100 μL of 19 μCi/mL (1.9 μg/mL) stock solution of 125I-PDGF-BB by Sephadex G-25 gel filtration chromatography (GE Healthcare Life Sciences, Marlborough, MA) (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea). The resulting intact, labeled protein fractions were pooled and concentrated in 10,000 MW cutoff Centricon centrifuge filters (EMD Millipore, Billerica, MA) spun at 3000 g at 4°C for 3 h. The buffer was exchanged to a solution containing 10% sucrose (Sigma-Aldrich), 25 mM HEPES, and 0.1% BSA through a subsequent 3000 g spin overnight at 4°C.
The resulting retentate was combined with unlabeled PDGF-BB to a final total mixed species mass of 250 ng, premixed into unassembled KLD to a final peptide concentration of 3.5 mg/mL, and a final PDGF-BB concentration of 335 ng/mL, and 50 μL gels were cast at the bottom of autoclaved low-retention tubes (n = 10). Gel assembly was initiated by the addition of phosphate-buffered saline (PBS) (Sigma-Aldrich) + 1% PSA, and gels were incubated at 37°C at 5% CO2 on a rotary shaker. Three hours following assembly, the baths were removed from the gels, and bath, gels, and tubes were counted by a gamma counter for 2 min. Using similar methods, entrapment of TGF-β1 in KLD was previously characterized by Kopesky et al. using 125I-TGF-β1 (Perkin Elmer),40 and entrapment of HB-IGF-1 in the similar self-assembling peptide hydrogel RAD was previously characterized by Florine et al.13
GF release from KLD hydrogels
Radiolabeled forms of PDGF-BB (125I-PDGF-BB) and HB-IGF-1 (14C-HB-IGF-1) were used to assess their release from KLD. Release of TGF-β1 from KLD was previously reported by our laboratory (Kopesky et al.) using 125I-TGF-β1 (Perkin Elme).40 125I-PDGF-BB: The release of 125I-PDGF-BB from KLD was determined using a method similar to that described previously for 125I-TGF-β1.40 Free 125I label was first removed from 40 μL of 58 μCi/mL (1.9 μg/mL) stock solution of 125I-PDGF-BB by Sephadex G-25 gel filtration chromatography, as in the loading efficiency experiment above. The retentate following buffer exchange was premixed into unassembled KLD to a final peptide concentration of 3.5 mg/mL, and 50 μL gels were cast at the bottom of autoclaved low-retention tubes. Assembly was initiated with PBS + 1% PSA (n = 14). Gels were incubated at 37°C at 5% CO2 on a rotary shaker, and bath aliquots were removed and replenished every 1–2 days for 2 weeks. Aliquots, gels, and tubes were counted by a gamma counter for 2 min following the 2-week incubation. 14C-HB-IGF-1: The release profile of HB-IGF-1 from KLD was determined as described previously for the similar self-assembling peptide hydrogel RAD.13 Briefly, 14C-HB-IGF-1 was mixed with acellular KLD before gel assembly at a final GF concentration of 615 nM, and PBS + 1% PSA was added to trigger self-assembly. Gels were incubated at 37°C, and bath samples were removed and replenished every 24 h for the first 8 days and every 48 h for days 8–16. On day 16, baths were removed, gels were mechanically disrupted, and radiolabeled protein was measured in bath and gel samples by liquid scintillation counting.
Statistical analyses
Values are mean ± SEM. Comparisons use general linear mixed-effects models and Tukey's HSD post hoc test (p < 0.05), (JMP 11, SAS, Inc., Cary, NC).
Results
BM-PC matrix production and cell proliferation
We assessed the matrix production capacity of both passage-1 (P1) BM-PCs and passage-2 (P2) BM-PCs, to determine if four additional population doublings altered their ability to produce matrix. Histological assessment of P2 BM-PCs encapsulated in KLD hydrogels with toluidine blue for sGAG following 22 days of culture showed strong disperse staining, indicative of high proteoglycan content (Fig. 3). Both P1 and P2 BM-PCs continued to proliferate following hydrogel encapsulation (Fig. 4A).
FIG. 3.

Histology: Toluidine blue staining of P2 BM-PCs encapsulated in KLD following 22 days of culture showed strong disperse staining, indicative of high proteoglycan content. Color images available online at www.liebertpub.com/tea
FIG. 4.
Assessment of BM-PC matrix production: P1 or P2 BM-PCs were encapsulated in KLD hydrogels and cultured for 22 days. (A) DNA content per hydrogel as measured by Hoechst dye binding; (B) sGAG content per hydrogel as measured by DMMB, normalized to wet weight; (C) proteoglycan biosynthesis rate as measured by 35S-sulfate incorporation; (D) hydroxyproline content normalized to wet weight. n = 6–8 gels per condition. Horizontal bars indicate statistically significant differences (p < 0.05). Color images available online at www.liebertpub.com/tea
Both populations also showed robust sGAG synthesis and deposition (Fig. 4B, C), along with increasing collagen deposition over the time course of the study (Fig. 4D). Previous studies using bovine BMSCs in KLD scaffolds have shown a similar evolution of sGAG synthesis and deposition, and the sGAG to be associated predominantly with aggrecan production.17 Together these data suggest a chondrogenic potential that is consistent with previous studies using BMSCs derived from cortical bone marrow.17,39
GF-stimulated gel-to-gel BM-PC migration
To assess the ability of BM-PCs to invade a GF-loaded gel, BM-PCs were encapsulated in a second gel atop the first (Fig. 1B). The mean position of the green fluorescent beads in the construct was used to demark the interface between the two gels. Cells that moved beyond one standard deviation past the mean bead position into the bottom gel were considered to have migrated, and the subset of cells that moved beyond two such standard deviations were considered to have migrated strongly (Fig. 5). All 8 combinations of TGF-β1, PDGF-BB, and HB-IGF-1 were tested for their impact on BM-PC migration in KLD hydrogels. The combination of TGF-β1 and PDGF-BB induced statistically significant migration compared to the GF-free control, whereas increases seen with all other GF combinations were not statistically significant (Fig. 6).
FIG. 5.

Example of confocal imaging and quantification of BM-PC migration and fluorescent bead position in the GF-free control (left) and PDGF-BB + TGF-β1 (right) conditions assessed using a spot-finding algorithm38: (A, B) 3D confocal image stack from the perspective of the objective below the well. (C, D) Profile perspective of the 3D confocal image stack. (E, F) Spot-finding algorithm computations of confocal images in (E and F). (G, H) Quantification of cell migration. Cells that migrated beyond one standard deviation (1SD) of the average bead position at the interface were considered to have migrated. The subset of these cells that migrated beyond two standard deviations (2 SD) was considered to have migrated strongly. Color images available online at www.liebertpub.com/tea
FIG. 6.
3D gel-to-gel migration of P1 BM-PCs over 4 days of stimulation by all eight combinations and permutations of TGF-β1, PDGF-BB, and HB-IGF-1: Migration is expressed as the percent of cells migrated (blue bars) or the percent of cells strongly migrated (red bars) (Fig. 5). The combination of TGF-β1 and PDGF-BB induced statistically significant migration compared to the GF-free control. Horizontal lines over bars represent significant differences compared to the GF-free control. * over bars represent significant differences compared to the combination of TGF-β1 and PDGF-BB. # over bars represent significant differences compared to the PDGF-BB alone. Color images available online at www.liebertpub.com/tea
The percent of cells that migrated strongly under the combination treatment of TGF-β1 and PDGF-BB was significantly higher than for TGF-β1 alone, HB-IGF-1 alone, or the combination of PDGF-BB and HB-IGF-1, while the percent of cells that migrated strongly under the PDGF-BB treatment was significantly higher than the combination of PDGF-BB and HB-IGF-1.
Entrapment of 125I-PDGF-BB in acellular KLD
To determine the initial entrapment efficiency of PDGF-BB in unassembled KLD, a mixture of 125I-PDGF-BB and PDGF-BB at a final concentration of 335 ng/mL was added to KLD at a final concentration of 3.5 mg/mL of peptide (see Materials and Methods section). Three hours after initiation of gel assembly, 80% of the PDGF-BB initially loaded was entrapped in the gel.
Release of 14C-HB-IGF-1 and 125I-PDGF-BB from acellular KLD
To assess the release kinetics of HB-IGF-1 and PDGF-BB from KLD, we loaded 14C-HB-IGF-1 and 125I-PDGF-BB into unassembled KLD. We measured the release of the radioactive proteins into the bath over 2 weeks, as well as their final presence in the gel at 2 weeks, by scintillation counting for 14C and gamma counting for 125I (Fig. 7). The release profile of TGF-β1 from KLD, published by Kopesky et al.,40 is included in Figure 7 for comparison.
FIG. 7.

Release kinetics of PDGF-BB (n = 14) and HB-IGF-1 (n = 4) from KLD using their radiolabeled forms: the release of TGF-β1 (n = 6), shown in this study for comparison, was previously published by Kopesky et al.48 By 4 days, when migration studies were terminated (Fig. 6), 42% of PDGF-BB and 84% of TGF-β1 remained in the scaffold, whereas only 14% of HB-IGF-1 was retained. Color images available online at www.liebertpub.com/tea
A first-order exponential of the form 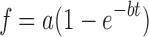 was fit to the release data to obtain a characteristic release time
was fit to the release data to obtain a characteristic release time  for each GF (Table 1). These data show that HB-IGF-1 is largely released from KLD within 4 days, while PDGF-BB is released more slowly and 15% still resides in the gel at 2 weeks. Separate experiments confirmed that PDGF-BB had little or no effect on cell viability, sGAG content or loss, the biosynthesis of sGAG, or total protein in adjacent cartilage tissue (Supplementary Figs. S2 and S3).
for each GF (Table 1). These data show that HB-IGF-1 is largely released from KLD within 4 days, while PDGF-BB is released more slowly and 15% still resides in the gel at 2 weeks. Separate experiments confirmed that PDGF-BB had little or no effect on cell viability, sGAG content or loss, the biosynthesis of sGAG, or total protein in adjacent cartilage tissue (Supplementary Figs. S2 and S3).
Table 1.
Quantification of the Release Kinetics of 14C-HB-IGF-1, 125I-PDGF-BB, and 125I-TGF-β1 from KLD Hydrogels
| Growth factor |
 (days) (days)
|
Release at 4 days | Release at 2 weeks |
|---|---|---|---|
| 14C-HB-IGF-1 | 1.62 | 86% | 95% |
| 125I-PDGF-BB | 3.50 | 58% | 83% |
| 125I-TGF-β1 | 6.20 | 16% | 30% |
The characteristic release time, τ, for each growth factor was determined by fitting a first-order exponential of the form f = a(1-e−bt) to the data shown in Figure 7, where τ = 1/b.
Discussion
Stimulus of tissue regeneration, mediated by acellular scaffolds functionalized with GFs, is thought to hinge on matched kinetics between endogenous cell recruitment into the scaffold and GF retention time in the scaffold. Motivated by an augmented microfracture strategy for cartilage repair, we developed a 3D gel-to-gel migration assay to test the chemotactic effect of GFs on BM-PCs. We demonstrated that the combination of PDGF-BB and TGF-β1 stimulates migration of BM-PCs into and through a 3D self-assembling peptide hydrogel scaffold when delivered as a single dose premixed into the scaffold (Fig. 6). A single premixed dose allows for easy translation to an in vivo surgical setting, but it raises the question of GF retention and release kinetics from the scaffold to bind to cell receptors once cells have entered the scaffold. Entrapment efficiency of PDGF was ∼80%, suggesting that a significant amount of PDGF remained in the gel, consistent with previous results for TGF-β1.40 We measured GF release kinetics and showed the characteristic exponential release time of PDGF-BB from KLD (in the geometry of Fig. 1B) to be 3.5 days, with 58% of the loaded PDGF-BB remaining in the scaffold at the 4-day time point of our 3D gel-to-gel migration studies (Table 1). Previous studies have shown even stronger retention of TGF-β1 in KLD.40 The substantial release of HB-IGF-1 from KLD indicates that a strong gradient of HB-IGF-1 was no longer present in our in vitro system after 4 days. Thus, chemotaxis induced by conditions containing HB-IGF-1 should not be expected in the geometry of Figure 1B, despite the chemotactic properties previously reported for the IGF family of proteins in modified Boyden chamber assays using BMSCs.32,35
The range of release profiles observed between the three GFs reflects how they each interact with the scaffold in a unique manner, although the precise mechanistic differences in scaffold-GF interactions have not been elucidated. There are no known specific binding motifs between any of these three GFs and KLD, although factors that may impact these differences include size, amino acid content and sequence, conformation, net charge, and nonspecific binding. Taken together, these data demonstrate that KLD scaffolds possess GF release kinetics that may effectively stimulate migration of BM-PCs in the scaffold, while retaining a portion of GFs that may subsequently promote cartilage repair by localized BM-PCs in vivo.
Both synergistic and antagonistic interactions between PDGF-BB and TGF-β1 have been shown previously in a variety of cell/tissue systems, including murine embryos,53 human pulmonary smooth muscle cells,54 and murine wound healing.55 In our system, neither PDGF-BB nor TGF-β1 was able to induce statistically significant migration alone. These data are consistent with the murine embryo study by Wang et al., which demonstrated that sclerotome and surrounding mesenchyme cells require PDGF signaling for TGF-β1-mediated migration. The precise mechanism of action that describes how these GFs together induce migration of BM-PCs is not yet known and would require quantification of migratory-associated cell signaling pathways, while beyond the scope of this study, such experiments are underway.
Studies by Steadman et al. and others have theorized that one cause for the formation of fibrocartilage rather than hyaline cartilage following microfracture surgery may be an insufficient quantity of progenitor cells populating the defect in the initial days postsurgery.56–59 Our strategy of GF-mediated acceleration of progenitor cell migration may have the potential to increase the number of cells recruited to a scaffold-filled defect in augmented microfracture surgery, and thereby increases the likelihood of hyaline cartilage production both through higher cellularity and GF-enhanced matrix synthesis.
The data presented here, combined with data from the literature, show that both PDGF-BB and TGF-β1 are retained within KLD at significant levels over the 4-day period during which our migration studies were performed40 (Fig. 7). To induce directed cell migration, cells must be exposed to gradients in chemotactic factors rather than a bolus release.60,61 Our results imply that the release kinetics of PDGF-BB and TGF-β1 from KLD are slow enough to generate a gradient and induce chemotaxis in our in vitro system. Thus, the slow local release of premixed chemotactic GFs from KLD may have the potential to accelerate the population of a defect with progenitor cells in vivo.
Previous studies by Koutsopoulos et al. demonstrated that protein release rates from the self-assembling peptide RAD can be tuned by adjusting the scaffold concentration,62 indicating that the release rates of our chemotactic GFs could be tuned to meet the required kinetics of cell migration in vivo. While the scope of this study is focused on the context of microfracture surgery, the concept of using a self-assembling peptide in combination with chemotactic GFs could be extended to other tissues, such as neural,63 myocardial,64 or hepatic.65
While the complex cell population accessed in microfracture surgery presents a challenge for experimental models, bone marrow-derived mesenchymal progenitor cells are commonly used in vitro to simulate them.66–70 To isolate BM-PCs, bone marrow stroma is typically scooped from the diaphyseal marrow cavity of long bones, aspirated from the iliac crest, or separated from sponge-like network of trabecular bone.43,68 These techniques have also been successfully applied to an array of species, including human, canine, feline, porcine, lapine, and bovine.43,71–77 Regardless of the physiological location of marrow harvested, these techniques all yield progenitor cells capable of undergoing chondrogenesis, whether in pellet culture or 3D scaffolds, such as KLD.17,39,44,66,68,70,77–80 While a phenotypic characterization of the cell population accessed in microfracture or used to simulate them in vitro would be interesting, a complex cell population is an unavoidable product of the surgical technique.
A limitation of this study regarding translation to an in vivo setting concerns the role of TGF-β1. While TGF-β1 has shown promise in combination with PDGF-BB in vitro, its use for in vivo application raises concerns, as active TGF-β1 has been shown to induce fibrosis when injected into animal joints or delivered locally by scaffold, microparticle, or gene vector.1,28,81–83 To avoid an adverse effect of TGF-β1, one possibility is that moderate local activation of latent TGF-β1 found in native synovial fluid by chemical or mechanical forces may provide the necessary cues to stimulate migration if combined with PDGF-BB.84,85 Alternatively, engineering approaches designed to locally deliver exogenous latent rather than active TGF-β1 have shown promising preliminary results in rats.86 A strong local dose of a prochemotactic GF (e.g., PDGF-BB), however, may be sufficient to induce a chemotactic effect similar to that observed in vitro.
An additional limitation of this study is the gel-to-gel experimental setup used in vitro compared to the bone-to-gel geometry in vivo. This difference may result in altered cell migration kinetics, but may also be overcome by tuning the scaffold density or GF dose. Initial rabbit studies with KLD have demonstrated the capacity of the scaffold to support cell migration in vivo, and the work presented in this study suggests that the addition of chemotactic GFs may have the potential to increase cellularity in the defect.1
While the release of HB-IGF-1 from KLD may be too rapid in the geometry of Figure 1B to fully test its chemotactic capacity on BM-PC migration, delivery of HB-IGF-1 from the hydrogel scaffold may still have an important function in cartilage regeneration. HB-IGF-1 has been shown to induce sustained production of aggrecan and collagen in chondrocyte-seeded RAD scaffolds, and is thus a promising candidate stimulant for generating repair tissue. In addition, delivery of HB-IGF-1 from the scaffold to adjacent cartilage explant tissue in vitro has already been shown to stimulate chondrocyte biosynthesis within these explants.13
Thus, HB-IGF-1 has the potential to stimulate repair and integration at the cartilage-scaffold interface. In this context, the KLD scaffold could provide local delivery of GFs such as HB-IGF-1 to the damaged cartilage interface, allowing HB-IGF-1 to serve its intended role as a proanabolic GF acting at the site of damage. Together, the ability of a scaffold to retain certain GFs (e.g., PDGF-BB and TGF-β1), while locally delivering others (e.g., HB-IGF-1), demonstrates the dual role of such a hydrogel scaffold and its capacity to be tailored to a particular tissue repair application.
Conclusions
This study demonstrates that the combination of PDGF-BB + TGF-β1 has the capacity to accelerate progenitor cell migration into a 3D self-assembling peptide scaffold. Delivery of proanabolic GFs such as HB-IGF-1 may also stimulate cell biosynthesis within the scaffold and in adjacent cartilage tissue. We found that multiple growth factors with varying release kinetics can be delivered simultaneously in a single dose through the self-assembling peptide KLD.
This multi-growth factor approach could be used to address multiple tissue regeneration challenges at once, including cell recruitment, tissue production, and integration. In the context of augmenting microfracture surgery for cartilage repair, our strategy of delivering chemotactic and proanabolic growth factors in KLD may provide the necessary local stimulus to help regenerate hyaline-like tissue. An extensive rabbit model is currently being used to test these results in vivo, although it is beyond the scope of this article and will be presented in a detailed follow-up study.
Supplementary Material
Acknowledgments
The research was funded by National Institute of Health (NIAMS) Grant AR060331. HB-IGF-1 was kindly provided by Dr. Richard Lee (Brigham & Women's Hospital, Boston, MA). KLD was kindly donated by 3-D Matrix (Waltham, MA).
Disclosure Statement
James Pancoast and Dr. Richard Lee are founders of ProteoThera, Inc.; Brigham and Women's Hospital has filed for intellectual property on HB-IGF-1, listing them as inventors. James Pancoast is an employee and Richard Lee is a board member and consultant for ProteoThera, Inc. Dr. Grodzinsky has equity in 3-D Matrix, Ltd., Japan.
References
- 1.Miller R.E., Grodzinsky A.J., Vanderploeg E.J., Lee C., Ferris D.J., Barrett M.F., et al. Effect of self-assembling peptide, chondrogenic factors, and bone marrow-derived stromal cells on osteochondral repair. Osteoarthritis Cartilage 18, 1608, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanish W.D., McCormack R., Forriol F., Mohtadi N., Pelet S., Desnoyers J., et al. Novel scaffold-based BST-CarGel treatment results in superior cartilage repair compared with microfracture in a randomized controlled trial. J Bone Joint Surg Am 95, 1640, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Davis M.E. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation 111, 442, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blatchley M.R., and Gerecht S. Acellular implantable and injectable hydrogels for vascular regeneration. Biomed Mater 10, 034001, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Khan I.M., Gilbert S.J., Singhrao S.K., Duance V.C., and Archer C.W. Cartilage integration: evaluation of the reasons for failure of integration during cartilage repair. A review. Eur Cell Mater 16, 26, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Silverman R.P., Bonasser L., Passaretti D., Randolph M.A., and Yaremchuk M.J. Adhesion of tissue-engineered cartilage to native cartilage. Plast Reconstr Surg 105, 1393, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Yu Y., Brouillette M.J., Seol D., Zheng H., Buckwalter J.A., and Martin J.A. Use of recombinant human stromal cell-derived factor 1α-loaded fibrin/hyaluronic Acid hydrogel networks to achieve functional repair of full-thickness bovine articular cartilage via homing of chondrogenic progenitor cells. Arthritis Rheumatol 67, 1274, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher M.B., Henning E.A., Söegaard N.B., Dodge G.R., Steinberg D.R., and Mauck R.L. Maximizing cartilage formation and integration via a trajectory-based tissue engineering approach. Biomaterials 35, 2140, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunter C.J., and Levenston M.E. Maturation and integration of tissue-engineered cartilages within an in vitro defect repair model. Tissue Eng 10, 736, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Tognana E., Chen F., Padera R.F., Leddy H.A., Christensen S., Guilak F., et al. Adjacent tissues (cartilage, bone) affect the functional integration of engineered calf cartilage. Osteoarthritis Cartilage 13, 129, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Vinardell T., Thorpe S.D., Buckley C.T., and Kelly D.J. Chondrogenesis and integration of mesenchymal stem cells within an in vitro cartilage defect repair model. Ann Biomed Eng 37, 2556, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Obradovic B., Martin I., Padera R.F., Treppo S., Freed L.E., Vunjak-and Novakovic G. Integration of engineered cartilage. J Orthop Res 19, 1089, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Florine E.M., Miller R.E., Liebesny P.H., Mroszczyk K.A., Lee R.T., Patwari P., et al. Delivering heparin-binding insulin-like growth factor 1 with self-assembling peptide hydrogels. Tissue Eng Part A 21, 637, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Im G.-I. Endogenous cartilage repair by recruitment of stem cells. Tissue Eng Part B Rev 22, 160, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Caplan M.R., Schwartzfarb E.M., Zhang S., Kamm R.D., and Lauffenburger D.A. Control of self-assembling oligopeptide matrix formation through systematic variation of amino acid sequence. Biomaterials 23, 219, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Kisiday J., Jin M., Kurz B., Hung H., Semino C., Zhang S., et al. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc Natl Acad Sci USA 99, 9996, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kopesky P.W., Vanderploeg E.J., Sandy J.S., Kurz B., and Grodzinsky A.J. Self-assembling peptide hydrogels modulate in vitro chondrogenesis of bovine bone marrow stromal cells. Tissue Eng Part A 16, 465, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kisiday J.D., Jin M., DiMicco M.A., Kurz B., and Grodzinsky A.J. Effects of dynamic compressive loading on chondrocyte biosynthesis in self-assembling peptide scaffolds. J Biomech 37, 595, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Miller R.E., Grodzinsky A.J., Barrett M.F., Hung H.H., Frank E.H., Werpy N.M., et al. Effects of the combination of microfracture and self-assembling peptide filling on the repair of a clinically relevant trochlear defect in an equine model. J Bone Joint Surg 96, 1601, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spiller K.L., Liu Y., Holloway J.L., Maher S.A., Cao Y., Liu W., et al. A novel method for the direct fabrication of growth factor-loaded microspheres within porous nondegradable hydrogels: controlled release for cartilage tissue engineering. J Control Release 157, 39, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Gooch K.J., Blunk T., Courter D.L., Sieminski A.L., Bursac P.M., Vunjak-Novakovic G., et al. IGF-I and mechanical environment interact to modulate engineered cartilage development. Biochem Biophys Res Commun 286, 909, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Zisch A.H., Lutolf M.P., Ehrbar M., Raeber G.P., Rizzi S.C., Davies N., et al. Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. FASEB J 17, 2260, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Sacchi V., Mittermayr R., Hartinger J., Martino M.M., Lorentz K.M., Wolbank S., et al. Long-lasting fibrin matrices ensure stable and functional angiogenesis by highly tunable, sustained delivery of recombinant VEGF164. Proc Natl Acad Sci USA 111, 6952, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Babensee J.E., McIntire L.V., and Mikos A.G. Growth factor delivery for tissue engineering. Pharm Res 17, 497, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Johnstone B., Hering T.M., Caplan A.I., Goldberg V.M., and Yoo J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 238, 265, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Chan J.M., Stampfer M.J., Giovannucci E., Gann P.H., Ma J., Wilkinson P., et al. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science 279, 563, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Jabri N., Schalch D.S., Schwartz S.L., Fischer J.S., Kipnes M.S., Radnik B.J., et al. Adverse effects of recombinant human insulin-like growth factor I in obese insulin-resistant type II diabetic patients. Diabetes 43, 369, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Hulth A., Johnell O., Miyazono K., Lindberg L., Heinegård D., and Heldin C.H. Effect of transforming growth factor‐β and platelet‐derived growth factor‐BB on articular cartilage in rats. J Orthop Res Wiley Online Library 14, 547, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Lee J.M., Ryu J.H., Kim E.A., Jo S., Kim B.-S., Lee H., et al. Adhesive barrier/directional controlled release for cartilage repair by endogenous progenitor cell recruitment. Biomaterials 39, 173, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Gorth D.J., Mauck R.L., Chiaro J.A., Mohanraj B., Hebela N.M., Dodge G.R., et al. IL-1ra delivered from poly(lactic-co-glycolic acid) microspheres attenuates IL-1β-mediated degradation of nucleus pulposus in vitro. Arthritis Res Ther 14, R179, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tokunou T., Miller R., Patwari P., Davis M.E., Segers V.F.M., Grodzinsky A.J., et al. Engineering insulin-like growth factor-1 for local delivery. FASEB J 22, 1886, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozaki Y., Nishimura M., Sekiya K., Suehiro F., Kanawa M., Nikawa H., et al. Comprehensive analysis of chemotactic factors for bone marrow mesenchymal stem cells. Stem Cells Dev 16, 119, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Fielder J., Röderer G., Günther K.-P., and Brenner R.E. BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. J Cell Biochem 87, 305, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Fiedler J.R., Etzel N., and Brenner R.E. To go or not to go: Migration of human mesenchymal progenitor cells stimulated by isoforms of PDGF. J Cell Biochem 93, 990, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Fiedler J., Brill C., Blum W.F., and Brenner R.E. IGF-I and IGF-II stimulate directed cell migration of bone-marrow-derived human mesenchymal progenitor cells. Biochem Biophys Res Commun 345, 1177, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Mishima Y., and Lotz M. Chemotaxis of human articular chondrocytes and mesenchymal stem cells. J Orthop Res 26, 1407, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Ponte A.L., Marais E., Gallay N., Langonné A., Delorme B., Hérault O., et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells 25, 1737, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Kopesky P.W., Lee H.Y., Vanderploeg E.J., Kisiday J.D., Frisbie D.D., Plaas A.H.K., et al. Adult equine bone marrow stromal cells produce a cartilage-like ECM mechanically superior to animal-matched adult chondrocytes. Matrix Biol 29, 427, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kopesky P.W., Vanderploeg E.J., Kisiday J.D., Frisbie D.D., Sandy J.D., and Grodzinsky A.J. Controlled delivery of transforming growth factor β1 by self-assembling peptide hydrogels induces chondrogenesis of bone marrow stromal cells and modulates Smad2/3 signaling. Tissue Eng Part A 17, 83, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kopesky P.W., Byun S., Vanderploeg E.J., Kisiday J.D., Frisbie D.D., and Grodzinsky A.J. Sustained delivery of bioactive TGF-β1 from self-assembling peptide hydrogels induces chondrogenesis of encapsulated bone marrow stromal cells. J Biomed Mater Res 102, 1275, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kisiday J.D., Kopesky P.W., Evans C.H., Grodzinsky A.J., McIlwraith C.W., and Frisbie D.D. Evaluation of adult equine bone marrow- and adipose-derived progenitor cell chondrogenesis in hydrogel cultures. J Orthop Res 26, 322, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Meyer A.S., Hughes-Alford S.K., Kay J.E., Castillo A., Wells A., Gertler F.B., et al. 2D protrusion but not motility predicts growth factor-induced cancer cell migration in 3D collagen. J Cell Biol 197, 721, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haynesworth S.E., Goshima J., Goldberg V.M., and Caplan A.I. Characterization of cells with osteogenic potential from human marrow. Bone 13, 81, 1992 [DOI] [PubMed] [Google Scholar]
- 44.Li W.-J., Tuli R., Okafor C., Derfoul A., Danielson K.G., Hall D.J., et al. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials 26, 599, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Kisiday J.D., Kurz B., DiMicco M.A., and Grodzinsky A.J. Evaluation of medium supplemented with insulin-transferrin-selenium for culture of primary bovine calf chondrocytes in three-dimensional hydrogel scaffolds. Tissue Eng 11, 141, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Sah R.L., Kim Y.J., Doong J.Y., Grodzinsky A.J., Plaas A.H., and Sandy J.D. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res 7, 619, 1989 [DOI] [PubMed] [Google Scholar]
- 47.Kim Y.J., Sah R.L., Doong J.Y., and Grodzinsky A.J. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem 174, 168, 1988 [DOI] [PubMed] [Google Scholar]
- 48.Farndale R.W., Sayers C.A., and Barrett A.J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res 9, 247, 1982 [DOI] [PubMed] [Google Scholar]
- 49.Stegemann H., and Stalder K. Determination of hydroxyproline. Clin Chim Acta 18, 267, 1967 [DOI] [PubMed] [Google Scholar]
- 50.Zhu H., Mitsuhashi N., Klein A., Barsky L.W., Weinberg K., Barr M.L., et al. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells 24, 928, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Santella A., Du Z., Nowotschin S., Hadjantonakis A.-K., and Bao Z. A hybrid blob-slice model for accurate and efficient detection of fluorescence labeled nuclei in 3D. BMC Bioinformatics 11, 580, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsieh P.C.H. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J Clin Invest 116, 237, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y., and Serra R. PDGF mediates TGFβ-induced migration during development of the spinous process. Dev Biol 365, 110, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chan M.C., Hilyard A.C., Wu C., Davis B.N., Hill N.S., Lal A., et al. Molecular basis for antagonism between PDGF and the TGFβ family of signalling pathways by control of miR-24 expression. EMBO J 29, 559, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Plasari G., Calabrese A., Dusserre Y., Gronostajski R.M., Mcnair A., Michalik L., et al. Nuclear factor I-C links platelet-derived growth factor and transforming growth factor 1 signaling to skin wound healing progression. Mol Cell Biol 29, 6006, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steadman J.R., Briggs K.K., Rodrigo J.J., Kocher M.S., Gill T.J., and Rodkey W.G. Outcomes of microfracture for traumatic chondral defects of the knee: Average 11-year follow-up. Arthroscopy 19, 477, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Dhinsa B.S., and Adesida A.B. Current clinical therapies for cartilage repair, their limitation and the role of stem cells. Curr Stem Cell Res Ther 7, 143, 2012 [DOI] [PubMed] [Google Scholar]
- 58.Koga H., Engebretsen L., Brinchmann J.E., Muneta T., and Sekiya I. Mesenchymal stem cell-based therapy for cartilage repair: a review. Knee Surg Sports Traumatol Arthrosc 17, 1289, 2009 [DOI] [PubMed] [Google Scholar]
- 59.Huang H., Zhang X., Hu X., Shao Z., Zhu J., Dai L., et al. A functional biphasic biomaterial homing mesenchymal stem cells for in vivo cartilage regeneration. Biomaterials 35, 9608, 2014 [DOI] [PubMed] [Google Scholar]
- 60.Lauffenburger D.A., and Horwitz A.F. Cell migration: a physically integrated molecular process. Cell 84, 359, 1996 [DOI] [PubMed] [Google Scholar]
- 61.Ridley A.J., Schwartz M.A., Burridge K., Firtel R.A., Ginsberg M.H., Borisy G., et al. Cell migration: integrating signals from front to back. Science 302, 1704, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Koutsopoulos S., Unsworth L.D., Nagai Y., and Zhang S. Controlled release of functional proteins through designer self-assembling peptide nanofiber hydrogel scaffold. Proc Natl Acad Sci USA 106, 4623, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Holmes T.C., de Lacalle S., Su X., Liu G., Rich A., and Zhang S. Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proc Natl Acad Sci USA 97, 6728, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tokunaga M., Liu M-L, Nagai T., Iwanaga K., Matsuura K., Takahashi T., et al. Implantation of cardiac progenitor cells using self-assembling peptide improves cardiac function after myocardial infarction. J Mol Cell Cardiol 49, 972, 2010 [DOI] [PubMed] [Google Scholar]
- 65.Wang S., Nagrath D., Chen P.C., Berthiaume F., and Yarmush M.L. Three-dimensional primary hepatocyte culture in synthetic self-assembling peptide hydrogel. Tissue Eng Part A 14, 227, 2008 [DOI] [PubMed] [Google Scholar]
- 66.Mauck R.L., Yuan X., and Tuan R.S. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage 14, 179, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Terraciano V., Hwang N., Moroni L., Park H.B., Zhang Z., Mizrahi J., et al. Differential response of adult and embryonic mesenchymal progenitor cells to mechanical compression in hydrogels. Stem Cells 25, 2730, 2007 [DOI] [PubMed] [Google Scholar]
- 68.Mouw J.K., Connelly J.T., Wilson C.G., Michael K.E., and Levenston M.E. Dynamic compression regulates the expression and synthesis of chondrocyte-specific matrix molecules in bone marrow stromal cells. Stem Cells 25, 655, 2006 [DOI] [PubMed] [Google Scholar]
- 69.Unterman S.A., Gibson M., Lee J.H., Crist J., Chansakul T., Yang E.C., et al. Hyaluronic acid-binding scaffold for articular cartilage repair. Tissue Eng Part A 18, 2497, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Williams C.G., Kim T.K., Taboas A., Malik A., Manson P., and Elisseeff J. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng 9, 679, 2003 [DOI] [PubMed] [Google Scholar]
- 71.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., et al. Multilineage potential of adult human mesenchymal stem cells. Science 284, 143, 1999 [DOI] [PubMed] [Google Scholar]
- 72.Kadiyala S., Young R.G., Thiede M.A., and Bruder S.P. Culture expanded canine mesenchymal stem cells possess osteochondrogenic potential in vivo and in vitro. Cell Transplant 6, 125, 1997 [DOI] [PubMed] [Google Scholar]
- 73.Martin D.R., Cox N.R., Hathcock T.L., Niemeyer G.P., and Baker H.J. Isolation and characterization of multipotential mesenchymal stem cells from feline bone marrow. Exp Hematol 30, 879, 2002 [DOI] [PubMed] [Google Scholar]
- 74.Ringe J., Kaps C., Schmitt B., Büscher K., Bartel J., Smolian H., et al. Porcine mesenchymal stem cells. Induction of distinct mesenchymal cell lineages. Cell Tissue Res 307, 321, 2002 [DOI] [PubMed] [Google Scholar]
- 75.Fortier L.A., Nixon A.J., Williams J., and Cable C.S. Isolation and chondrocytic differentiation of equine bone marrow-derived mesenchymal stem cells. Am J Vet Res 59, 1182, 1998 [PubMed] [Google Scholar]
- 76.Majumdar M.K., Banks V., Peluso D.P., and Morris E.A. Isolation, characterization, and chondrogenic potential of human bone marrow-derived multipotential stromal cells. J Cell Physiol 185, 98, 2000 [DOI] [PubMed] [Google Scholar]
- 77.Bosnakovski D., Mizuno M., Kim G., Takagi S., Okumura M., and Fujinaga T. Isolation and multilineage differentiation of bovine bone marrow mesenchymal stem cells. Cell Tissue Res 319, 243, 2004 [DOI] [PubMed] [Google Scholar]
- 78.Nöth U., Osyczka A.M., Tuli R., Hickok N.J., Danielson K.G., and Tuan R.S. Multilineage mesenchymal differentiation potential of human trabecular bone-derived cells. J Orthop Res 20, 1060, 2002 [DOI] [PubMed] [Google Scholar]
- 79.Worster A.A., Brower-Toland B.D., Fortier L.A., Bent S.J., Williams J., and Nixon A.J. Chondrocytic differentiation of mesenchymal stem cells sequentially exposed to transforming growth factor-β1 in monolayer and insulin-like growth factor-I in a three-dimensional matrix. J Orthop Res 19, 738, 2006 [DOI] [PubMed] [Google Scholar]
- 80.Ma H.-L., Hung S.-C., Lin S.-Y., Chen Y.-L., and Lo W.-H. Chondrogenesis of human mesenchymal stem cells encapsulated in alginate beads. J Biomed Mater Res 64, 273, 2003 [DOI] [PubMed] [Google Scholar]
- 81.Holland T.A., Bodde E.W.H., Cuijpers V.M.J.I., Baggett L.S., Tabata Y., Mikos A.G., et al. Degradable hydrogel scaffolds for in vivo delivery of single and dual growth factors in cartilage repair. Osteoarthritis Cartilage 15, 187, 2007 [DOI] [PubMed] [Google Scholar]
- 82.Guo X., Park H., Young S., Kretlow J.D., van den Beucken J.J., Baggett L.S., et al. Repair of osteochondral defects with biodegradable hydrogel composites encapsulating marrow mesenchymal stem cells in a rabbit model. Acta Biomaterialia 6, 39, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mi Z., Ghivizzani S.C., Lechman E., Glorioso J.C., Evans C.H., and Robbins P.D. Adverse effects of adenovirus-mediated gene transfer of human transforming growth factor beta 1 into rabbit knees. Arthritis Res Ther 5, R132, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Annes J.P. Making sense of latent TGFβ activation. J Cell Sci 116, 217, 2003 [DOI] [PubMed] [Google Scholar]
- 85.Albro M.B., Cigan A.D., Nims R.J., Yeroushalmi K.J., Oungoulian S.R., Hung C.T., et al. Shearing of synovial fluid activates latent TGF-β. Osteoarthritis Cartilage 20, 1374, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lim E.-H., Sardinha J.P., Myers S., and Stevens M. Latent transforming growth factor-β1 functionalised electrospun scaffolds promote human cartilage differentiation: towards an engineered cartilage construct. Arch Plast Surg 40, 676, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





