Abstract
Vascularization is critical for cell survival within tissue-engineered grafts. Adipose-derived stromal/stem cells (ASCs) are widely used in tissue engineering applications as they are a clinically relevant source of stem cells and endothelial progenitor cells. ASCs have previously been shown to self-assemble into pericyte-stabilized vascular networks in normoxic (20% O2) cultures. This capacity for de novo vascular assembly may accelerate graft vascularization in vivo rather than relying solely on angiogenic ingrowth. However, oxygen depletion within large cell-seeded grafts will be rapid, and it is unclear how this worsening hypoxic environment will impact the vascular assembly of the transplanted cells. The objectives of this study were to determine whether ASC-derived vessels could grow in hypoxia and to assess whether the vessel maturity (i.e., individual cells vs. preformed vessels) influenced this hypoxic response. Utilizing an in vitro vascularization model, ASCs were encapsulated within fibrin gels and cultured in vitro for up to 6 days in either normoxia (20% O2) or hypoxia (0.2% or 2% O2). In a subsequent experiment, vessels were allowed to preform in normoxia for 6 days before an additional 6 days of either normoxia or hypoxia. Viability, vessel growth, pericyte coverage, proliferation, metabolism, and angiogenic factor expression were assessed for each experimental approach. Vessel growth was dramatically inhibited in both moderate and severe hypoxia (47% and 11% total vessel length vs. normoxia, respectively), despite maintaining high cell viability and upregulating endogenous expression of vascular endothelial growth factor in hypoxia. Bromodeoxyuridine labeling indicated significantly reduced proliferation of endothelial cells in hypoxia. In contrast, when vascular networks were allowed to preform for 6 days in normoxia, vessels not only survived but also continued to grow more in hypoxia than those maintained in normoxia. These findings demonstrate that vascular assembly and growth are tightly regulated by oxygen tension and may be differentially affected by hypoxic conditions based on the maturity of the vessels. Understanding this relationship is critical to developing effective approaches to engineer viable tissue-engineered grafts in vivo.
Introduction
Rapid vascularization is essential for the effective application of cell-based tissue engineering strategies as cell survival is critically dependent on adequate supply of oxygen and nutrients. For example, it may take weeks for invading vasculature from the surrounding tissues to fully vascularize clinically sized, centimeter-scaled bone grafts. In the interim, diffusion of oxygen and nutrients into the center of the graft will be slow and countered by high consumption rates by the implanted cells, resulting in diffusion-limited oxygen gradients that can reach near anoxia within hours.1 This ischemic environment is exacerbated if the surrounding tissue has incurred vascular damage due to trauma.
Previous studies have demonstrated that vascularization and blood perfusion can be accelerated by stimulating endothelial cells (ECs) within the graft to form a nascent vascular network that would be capable of anastomosing with host vessels in vivo.2–5 Possible sources of autologous ECs include circulating endothelial progenitor cells and ECs derived from induced pluripotent stem cells6–8; however, their low yields necessitate extensive in vitro expansion. Adipose-derived stromal/stem cells (ASCs) are an abundant, single cell source of stem cells, ECs, and pericytes.9,10 Our group has previously demonstrated that early passage ASCs are inherently heterogeneous, containing a residual subpopulation of endothelial progenitors that can proliferate extensively to grow into densely interconnected vascular networks.11,12 This self-assembly is driven by heterotypic physical and biochemical cell signaling with neighboring ASCs11 and is substantially improved following cell aggregation.12 This heterogeneous self-assembling nature of ASCs makes them an attractive cell source for tissue engineering strategies that require stem cell differentiation or trophic signaling combined with vascular support.
Minimizing ex vivo manipulation and precultivation of cells may be advantageous for clinical translation of cell-based tissue engineering approaches, which puts more emphasis on in situ tissue assembly and vascularization. However, there still remain several unknowns regarding the ability of ASCs to assemble into functional vascular networks within a metabolically challenging environment. Hypoxia is typically a potent stimulus for angiogenesis in vivo through increased expression of vascular endothelial growth factor (VEGF) by hypoxic cells.13 ASCs similarly upregulate angiogenic factors in response to hypoxia,14–17 which can promote EC survival and growth. However, when ECs themselves experience hypoxia, this can inhibit vascular assembly and stability18 and induce apoptosis through increased production of reactive oxygen species.19,20
The current study aims to determine whether ASC-derived vessels can grow in hypoxia and assesses the effects of vessel maturity (i.e., individual cells vs. preformed vessels) on this hypoxic response. We demonstrate that there is a differential response to hypoxia depending on vessel maturity, which has important implications for vascularization strategies that utilize ASCs.
Materials and Methods
ASC isolation and culture
Human subcutaneous adipose tissue was obtained in the form of lipoaspirate from three female Caucasian donors (aged 46–53) undergoing elective surgery and with written informed consent under the approval of the Johns Hopkins Medicine Institutional Review Board. ASCs were isolated as previously described.11 Briefly, tissue was digested with collagenase (1 mg/mL; Worthington Biochemical Corp.) to isolate the stromal vascular fraction of cells. These cells were plated onto tissue culture plastic and were termed passage 0 ASCs when they reached 80–90% confluence. ASCs were used at passage 2 for all experiments. Growth medium consisted of high-glucose DMEM (Gibco) with 10% fetal bovine serum (FBS; Atlanta Biologicals), 1% penicillin/streptomycin (Gibco), and 1 ng/mL basic fibroblast growth factor-2 (FGF-2; PeproTech). All experiments were conducted with cells from three independent donors.
Flow cytometry
Passage 2 ASCs were assessed using flow cytometry for surface expression of mesenchymal (CD73, CD90, CD105) and endothelial markers (CD31, CD34). Briefly, cells were suspended in phosphate-buffered saline (PBS) containing 2% FBS and incubated with monoclonal antibodies for 30 min at 4°C. Cells were analyzed with a BD Accuri C6 flow cytometer. All antibodies were purchased from BD Biosciences.
Cell aggregation using suspension culture
Cells were trypsinized and resuspended at a concentration of 250,000 cells/mL in growth medium containing 0.24% (w/v) methylcellulose (Sigma). The cell suspension was pipetted into 10-cm Petri dishes coated with 2% (w/v) agarose to minimize cellular adherence to the dish. After overnight suspension culture, cellular aggregates were collected with a pipette, and then centrifuged before encapsulation procedures.
Aggregate encapsulation and culture
Cell aggregates were suspended in fibrinogen (8 mg/mL final; Sigma) and thrombin (2 U/mL final; Sigma) at a final cell concentration of 2 × 104 cells/μL. Fibrin gels were formed by pipetting 12 μL of gel solution into 4-mm diameter wells and incubating at 37°C for 30 min to allow complete gelation before adding the medium. Each gel sample was fed with 1 mL of culture medium containing no added growth factors: endothelial basal medium-2 (EBM-2; Lonza), 10% FBS, and 1% penicillin/streptomycin. To assess de novo vascular assembly, freshly encapsulated cells were immediately cultured in either normoxia (20% O2) or hypoxia (2% or 0.2% O2) for 6 days with no media changes to allow for cell-driven nutrient depletion. To assess growth/stability of preformed vessels, encapsulated cells were first cultured in normoxia for 6 days with media changed every other day to establish robust vascular networks. At day 6, these samples were fed once more and then cultured in either normoxia or hypoxia for an additional 6 days with no media changes. Normoxic samples were maintained in a 37°C incubator with 5% CO2, 95% ambient air. Hypoxic samples were placed in a modular incubator chamber (Billups-Rothenberg) that was flushed every other day with premixed gas (0.2% or 2% O2/5% CO2/N2 balance) and placed in a 37°C incubator.
Viability and metabolism assessments
To assess cell viability, samples were incubated with LIVE/DEAD Viability/Cytotoxicity solution (Molecular Probes) for 30 min at 37°C, washed with PBS, and imaged with a confocal microscope. Total DNA content was measured to gage total cell number using the Quant-iT PicoGreen dsDNA Assay (Invitrogen) as previously described.11 Glucose and lactate content in culture media were quantified using the Glucose Assay Kit and L-Lactate Assay Kit I (Eton Bioscience) and subtracted from day 0 values to calculate consumption or production, respectively. These values were normalized to DNA content (pmol/cell).
Enzyme-linked immunosorbent assay
To assess endogenous growth factor production, cells were cultured in 0.5 mL of serum-free medium (EBM-2 + 1% penicillin/streptomycin) for 48 h in either normoxia (20% O2) or hypoxia (0.2% or 2% O2). Supernatant was collected and assayed for VEGF and FGF-2 using human ELISA kits (PeproTech) according to the manufacturer's protocol. Cells were also harvested and assayed for total DNA content to normalize growth factor production to cell number (ng/106 cells).
Bromodeoxyuridine labeling
Cells were incubated with bromodeoxyuridine (BrdU; Sigma) to detect proliferating cells. Briefly, 10 μM BrdU was pipetted into existing culture medium (i.e., the medium was not changed), and samples were quickly returned to their appropriate oxygen environment (less than 5 min of normoxic exposure) for a 20-h incubation. Samples were then washed with PBS and fixed with 3.7% formaldehyde. Incorporated BrdU was detected using whole-mount immunostaining.
Whole-mount immunostaining
Whole-mount immunostaining of fibrin gels was performed as previously described.12 Briefly, samples were fixed with 3.7% formaldehyde for 3 h at 4°C, washed with PBS, and blocked with 5% normal goat serum/0.2% Triton X-100/PBS for 3 h at 4°C. Antibodies were incubated overnight at 4°C, followed by three 1-h washes in PBS with 0.1% Tween. Primary antibodies included mouse anti-human CD31 (4 μg/mL; Sigma) and Cy3-conjugated mouse anti-alpha smooth muscle actin (αSMA, 7 μg/mL; Sigma). One secondary antibody was used: DyLight 488-conjugated goat anti-mouse (1:400; Jackson ImmunoResearch).
Before staining for BrdU, samples were stained for all other antigens and postfixed with 3.7% formaldehyde for 30 min to preserve the stain. Samples were then denatured with 2N HCl/0.5% Triton X-100 for 45 min at room temperature, washed, reblocked, and then incubated with AlexaFluor 647-conjugated mouse anti-BrdU (4 μg/mL; Invitrogen) overnight at 4°C. Cell nuclei were counterstained with 4′-6-diamidino-2-phenylindole (DAPI; Sigma).
Imaging and analysis
Immunostained gels were mounted on glass slides and imaged using a Zeiss LSM 510 confocal microscope (5× and 20× objectives). Confocal z-stacks were z-projected and thresholded for quantification. AngioQuant software21 was used to quantify total vessel length (sum of the lengths of all vessel branches within a gel). ImageJ software (NIH) was used for all other image analysis. Pericyte coverage was defined as αSMA+ area within at least 5 μm of the abluminal face of vessel networks. Briefly, vessel networks were selected in the CD31 channel of thresholded image composites. Selections were enlarged by 5 μm at all edges and applied to the αSMA channel. αSMA+ area fraction within the selected area was measured and displayed as % Pericyte Coverage. BrdU+ nuclei were counted with the Analyze Particles command. BrdU counts from the whole gel indicate overall proliferation within the culture (displayed as Total # BrdU+). To assess proliferation within the vessels only, CD31+ vessel area was selected and applied to the BrdU channel before counting within the selected area. This count was normalized to the CD31+ vessel area to account for differences in vessel density and is displayed as # BrdU+/CD31 (mm2). To approximate the survival/persistence of ECs within the culture, the total number of independent CD31+ units (ranging from solitary cells to small vessels) was counted and divided by the day 0 count. Briefly, whole-gel images were thresholded and assessed with the Analyze Particles command.
Quantitative polymerase chain reaction
Total RNA was isolated using a TRIzol (Invitrogen) extraction method and quantified using a NanoDrop spectrophotometer (Thermo Scientific). Reverse transcription was performed with 1 μg of total RNA using the iScript cDNA Synthesis Kit (BioRAD). Complementary DNA was amplified using SYBR Green PCR Master Mix (Applied Biosystems) and a StepOnePlus Real-Time PCR System (Applied Biosystems). Expression levels were calculated by the comparative CT method using β-Actin as an endogenous reference gene. Primers included the following:
(1) β-Actin [RefSeq: NM_001101]–sense: 5′-AGTTGCGTTACACCCTTTCTTG-3′, antisense: 5′-TCACCTTCACCGTTCCAGTTT-3′
(2) VEGF-A [RefSeq: NM_001025368]–sense: 5′-GCCTTGCCTTGCTGCTCTA-3′, antisense: 5′-GATTCTGCCCTCCTCCTTCTG-3′
(3) VEGFR-2 [RefSeq: NM_002253]–sense: 5′-AGTCTGTGGCATCTGAAGGC-3′, antisense: 5′-ACGGTGGTGTCTGTGTCATC-3′.
Statistical analyses
All experiments were conducted with cells from three independent donors (n = 3 samples per donor). Quantitative data are expressed as mean ± standard error. Statistical analyses were performed using GraphPad Prism 5 software. Statistical significance was determined by one-way ANOVA with Tukey's post-test and is denoted as *p < 0.05, **p < 0.01, and ***p < 0.001.
Results
Cell characterization
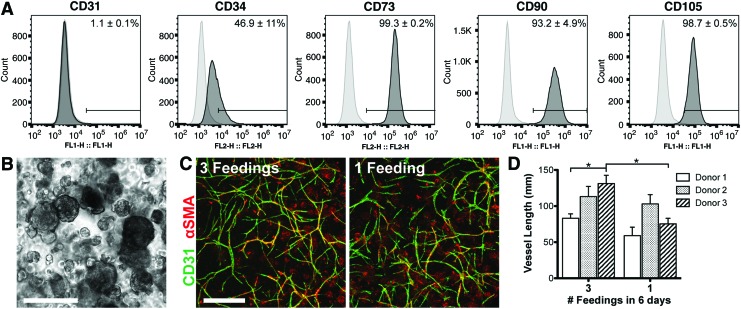
Passage 2 ASCs from multiple donors were predominantly positive for mesenchymal markers, CD73 (99.3% ± 0.2%), CD90 (93.2% ± 4.9%), and CD105 (98.7% ± 0.5%), with very few cells positive for the mature endothelial marker CD31 (1.1% ± 0.1%) (Fig. 1A). Approximately half of the population expressed CD34 (46.9% ± 11%), which is expressed by native ASCs as well as endothelial progenitors.
FIG. 1.
ASC characterization and spontaneous vascular assembly. (A) Flow cytometric analysis of surface markers CD31, CD34, CD73, CD90, and CD105. Percentages are shown as an average of three donors. (B) Morphology of cell aggregates immediately after fibrin encapsulation. (C) Vessels assembled within 6 days of culture in medium containing no added growth factors with routine (three) feedings or a single feeding at the onset of the culture. (D) Quantification of total vascular network length. Scale bars = 200 μm (B), 500 μm (C). ASC, adipose-derived stromal/stem cell. Significance is indicated as *p < 0.05. Color images available online at www.liebertpub.com/tea
Spontaneous self-assembly of vessels
Following overnight suspension culture, ASCs formed aggregates ranging from 50 to 300 μm in diameter (Fig. 1B). ASCs spontaneously self-assembled into vascular networks in 6 days without any exogenously added growth factors (Fig. 1C, D). This was reproduced with cells from multiple donors (Fig. 1D). Vascular assembly still occurred even when the medium was not replenished for the entire culture period, which sets the basis for the vascular assay in subsequent experiments. By only feeding at the start of culture, there will be cell-driven nutrient depletion and waste buildup to simulate the ischemic conditions that arise after in vivo implantation of avascular grafts.
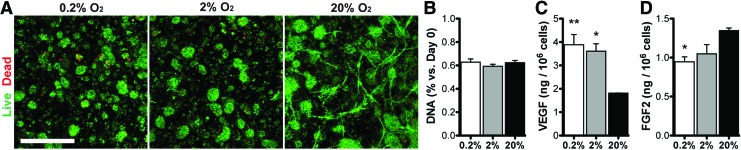
Viability and endogenous growth factor production
ASCs cultured for 6 days in 0.2%, 2%, and 20% O2 demonstrated high viability (Fig. 2A) and similar DNA content (Fig. 2B). Endogenous secretion of the potent angiogenic growth factor, VEGF, was upregulated in hypoxia (Fig. 2C) relative to normoxic conditions, while another angiogenic growth factor, FGF-2, was downregulated at 0.2% and 2% O2 relative to 20% O2 cultures (Fig. 2D).
FIG. 2.
ASC viability and growth factor production. ASCs were fed at day 0 and maintained in 0.2%, 2%, or 20% O2 for 6 days. (A) Live/Dead staining was used to assess viability at day 6. The number of viable (green) cells appeared similar in all groups. (B) The total DNA content normalized to day 0 was also similar in all groups. (C, D) VEGF and FGF-2 production was assessed in all groups using ELISA after 48 h in serum-free medium and normalized to DNA content. Scale bar = 500 μm. Significance indicated versus normoxia (20% O2). FGF-2, fibroblast growth factor-2; VEGF, vascular endothelial growth factor. Significance is indicated as *p < 0.05 or **p < 0.01. Color images available online at www.liebertpub.com/tea
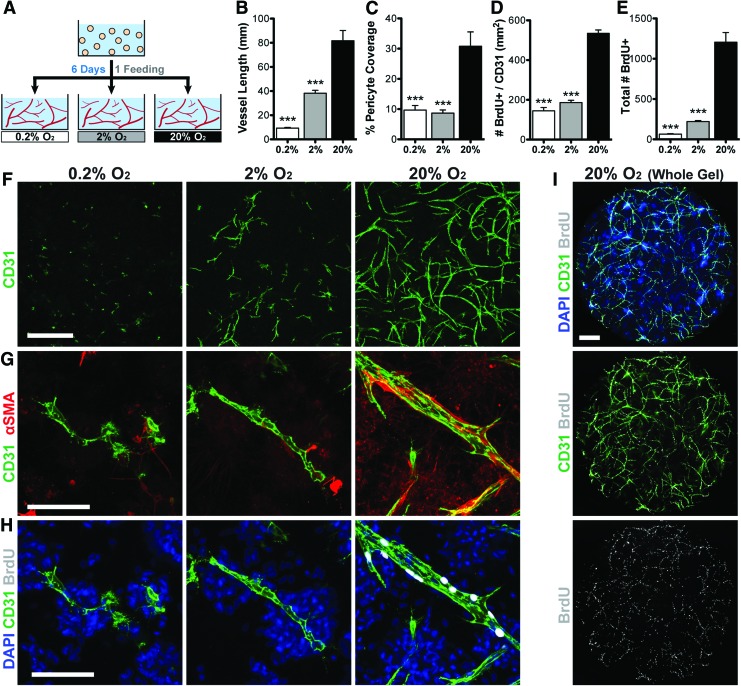
De novo vascular assembly is inhibited by hypoxia
To assess the effects of hypoxia/ischemia on de novo vascular assembly, freshly encapsulated cells were immediately transitioned into the 6-day hypoxia assay (Fig. 3A). Vascular assembly was substantially inhibited in both moderate (2% O2) and severe (0.2% O2) hypoxia (47% and 11% total vessel length vs. normoxia, respectively; Fig. 3B, F). Pericyte coverage of vessels was threefold less in hypoxia (Fig. 3C, G). Vessel proliferation was also significantly lower in hypoxia (Fig. 3H), even when normalized to the vessel area (Fig. 3D). Interestingly, the majority of proliferating cells throughout the culture are vascular cells (62%, 86%, and 90% of BrdU+ nuclei colocalize with CD31 in 0.2%, 2%, and 20% O2, respectively; Fig. 3I), which corresponds to a similar reduction in proliferation within the total population (Fig. 3E). To approximate EC persistence within the cultures, we assessed the total number of spatially distinct CD31+ entities. These numbers were not significantly different in 0.2% or 2% O2 compared with the number of CD31+ cells at day 0 (82.0% and 98.4%, respectively), indicating that the majority of ECs survived in all oxygen conditions. Vessel formation in hypoxia was not affected by the addition of exogenous angiogenic growth factors VEGF and FGF2 (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea), which are known to promote EC survival.
FIG. 3.
Hypoxia inhibits de novo vascular assembly. (A) Schematic of the experimental approach. Vessels were allowed to assemble for 6 days in 0.2%, 2%, or 20% O2. Vascular network length (B, F) and pericyte coverage (C, G) were significantly inhibited in hypoxic conditions relative to normoxia. Proliferation (indicated by BrdU incorporation) was significantly reduced in hypoxia in vascular cells (D, H) and the entire population (E). Vascular cells constituted the majority of proliferating cells within the culture (I). Scale bars = 500 μm (F, I), 100 μm (G, H). Significance indicated versus normoxia (20% O2) as ***p < 0.001. Color images available online at www.liebertpub.com/tea
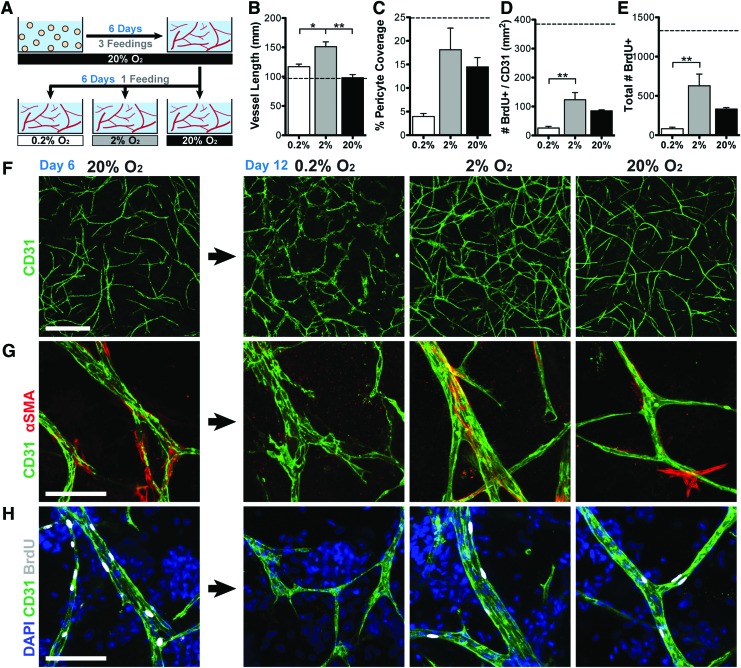
Preformed vessels grow more in hypoxia
To assess the effects of hypoxia/ischemia on preformed vascular networks, ASCs were first allowed to self-assemble into a nascent vascular network for 6 days in normoxia with routine feedings before transitioning into the 6-day hypoxia assay (Fig. 4A). Contrary to the previous experiment, preformed vascular networks survived and continued to grow more in hypoxia than normoxia (1.21-, 1.56-, and 1.01-fold increase in vascular length vs. day 6 for 0.2%, 2%, and 20% O2, respectively; Fig. 4B, F). Vessels also became wider in 2% O2, whereas those in 0.2% O2 developed a highly destabilized morphology. Pericyte coverage (Fig. 4C, G) and cell proliferation (Fig. 4D, E, H) decreased in all groups after continued culture (vs. day 6); however, they remained highest in 2% O2 and lowest in 0.2% O2.
FIG. 4.
Moderate hypoxia (2% O2) enhances the growth and stability of preformed vessels. (A) Schematic of the experimental approach. Vessels were allowed to assemble for 6 days in 20% O2 before being transferred to 0.2%, 2%, or 20% O2 with no subsequent feedings after day 6. (B, F) Vessels grew significantly longer in hypoxia by day 12 versus day 6 (dotted line), with statistically greater length in 2% O2 relative to 0.2% and 20% O2. (C, G) Pericyte coverage decreased in all groups relative to day 6 values (dotted line), but was highest in 2% O2. (D, E, H) Proliferation (indicated by BrdU incorporation) decreased in all groups relative to day 6 values (dotted lines), but remained highest in the 2% O2 group. Scale bars = 500 μm (F), 100 μm (G, H). Significance indicated versus normoxia (20% O2) as *p < 0.05 or **p < 0.01. Color images available online at www.liebertpub.com/tea
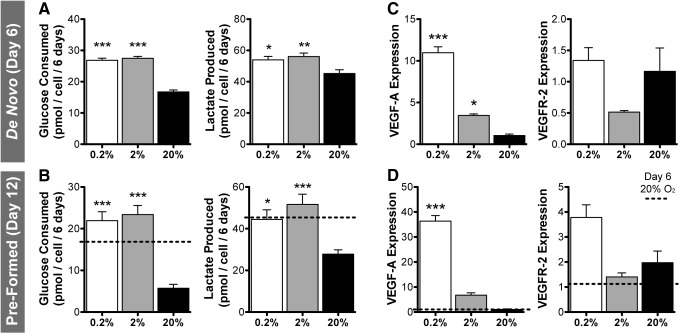
Metabolism and gene expression
ASCs in hypoxia significantly increased glucose consumption and lactate production (Fig. 5A). When cells were precultured in normoxia for 6 days before the onset of hypoxia, there was an even greater glycolytic increase in hypoxia relative to normoxia by day 12 (Fig. 5B); however, basal levels of glucose consumption and lactate production were significantly lower after extended culture (2.9- and 1.6-fold decrease, respectively; day 12 vs. day 6). Expression of VEGF-A mRNA increased substantially after 6 days in hypoxia (Fig. 5C) and was an additional twofold to threefold higher in cells that were precultured for 6 days before hypoxia (Fig. 5D). VEGF receptor-2 (VEGFR-2) mRNA expression was lower in 2% O2, but similar in 0.2% O2 versus normoxia (Fig. 5C). Samples that were precultured before hypoxia demonstrated elevated VEGFR-2 expression at day 12 versus day 6 (end of preculture) and the highest expression in 0.2% O2 (Fig. 5D).
FIG. 5.
Comparison of ASC metabolic activity and gene expression in immediate or delayed hypoxia. Glucose consumption and lactate production increased in 0.2% and 2% O2 relative to 20% O2 after 6 days of immediate hypoxia (de novo vascular assembly) (A) and delayed hypoxia (6 days of preculture in normoxia) (B). By day 12, continued normoxic culture resulted in reduced glucose and lactate metabolism versus day 6 (dotted lines), whereas hypoxic conditions increased glucose consumption. (C) mRNA expression of VEGF-A was substantially upregulated in hypoxia, with highest expression in 0.2% O2. VEGFR-2 expression was reduced in 2% O2, but unchanged in 0.2% O2 relative to normoxia. (D) Changes in VEGF-A and VEGFR-2 expression were even greater following delayed-onset hypoxia. Significance indicated versus normoxia (20% O2) as *p < 0.05, **p < 0.01, or ***p < 0.001. VEGFR-2, VEGF receptor-2.
Discussion
Rapid vascularization is critical for the survival of cell-seeded tissue grafts after implantation as inadequate diffusion of oxygen and nutrients may lead to cell death and tissue necrosis. ASCs, which exhibit spontaneous vascular self-assembly, may be a suitable cell population for providing tissue-engineered grafts with an intrinsic vascular network.12 Our group has previously demonstrated that aggregating ASCs into multicellular spheroids significantly improves their ability to form vascular networks when encapsulated in fibrin gels,12 possibly by facilitating heterotypic cell–cell interactions.11 Other studies have demonstrated that aggregating ASCs may improve their survival and regenerative potential following in vivo implantation.22–25 In this study, we tested whether this capacity for vascular assembly would be impacted by hypoxia, which might be expected following in vivo implantation into a defect site. We demonstrated that hypoxia severely inhibits de novo vascular assembly of ASCs, but promotes further growth of preformed vascular networks.
In this study, we removed all exogenously added growth factors (except for what may be in FBS) and demonstrated that ASCs are still able to rapidly and spontaneously assemble into vascular networks. This finding suggests that endogenous biochemical and physical signals may be instructing this cellular self-assembly. For example, ASCs express detectable levels of angiogenic growth factors, VEGF and FGF-2 (1.81 and 1.35 ng/106 cells/2 days in normoxia, respectively). For this study, growing the cells without exogenous growth factors allows for a more direct assessment of cellular behavior in response to hypoxia.
In addition to low oxygen tension, transplanted cells will also experience cell-driven nutrient depletion and waste buildup, making the environment gradually ischemic. To replicate this environment, cells were only fed at the start of the 6-day simulated implantation period, during which oxygen tension was varied. A direct comparison of multiple media changes versus none shows that vessel network growth is slightly reduced but only significantly in one of the three tested donors.
Viability of ASCs remained high in all oxygen tensions, with no significant difference in cell number even in 0.2% oxygen. The cells may have compensated for reduced oxygen availability by shifting their metabolism more toward anaerobic glycolysis as glucose consumption and lactate production were both significantly higher in hypoxia. Similar hypoxic resiliency and metabolic shifts have been demonstrated in ASCs26 and other cell types.27,28 This finding is promising regarding the ability of ASCs to endure harsh ischemic environments after transplantation.
Hypoxic ASCs increased endogenous expression of the potent angiogenic factor, VEGF. This agrees with previous reports that ASCs increase angiogenic factor production in hypoxia.14–17 Interestingly, expression of another important angiogenic factor, FGF-2, was reduced in hypoxia. Previous studies have demonstrated that ASCs in hypoxia can either upregulate16,29 or downregulate30 endogenous FGF-2 expression, suggesting that the experimental culture conditions (such as spheroid vs. monolayer culture31) can impact this response. Despite the high overall viability and increased VEGF expression, de novo vascular assembly of ASCs was substantially inhibited in hypoxia. This finding is likely due to reduced proliferation of ECs as BrdU incorporation was significantly reduced within vessels. Substantial apoptosis is unlikely as the total number of independent vessel structures (often individual cells in 0.2% O2 and small vessels in 2% O2; Fig. 3F) was not significantly reduced when compared with the number of ECs encapsulated at day 0. This metric for counting vessels and cells alike as just one cell is based on our earlier studies that demonstrated that each vessel arises from an individual EC.11 Pericyte coverage was significantly reduced on hypoxic vessels, which is similar to our previous findings in monolayer ASC cultures.11 Those monolayer experiments, however, demonstrated no significant change in vessel length when grown in hypoxia, which is an interesting observation that warrants further investigation.
Hypoxia is a potent stimulus for angiogenesis in vivo, inducing increased VEGF expression that recruits the sprouting and growth of preformed vessels. Angiogenic vessels may not experience severe hypoxia themselves as they are constantly near a source of blood, only responding to indirect indicators of hypoxic stress. The de novo vascular assembly model presented in this study is fundamentally different from angiogenic sprouting in a number of ways, including the fact that the ECs are immature and are not part of a vessel when the metabolic insult begins. Therefore, we assessed the effects of hypoxia on vessels that were first grown in a normoxic environment with routine media changes to establish healthy vascular network structures. Surprisingly, hypoxia induced increased vascular growth versus normoxia when vessels were preformed. By day 12, vessels demonstrated maximal growth, proliferation, and pericyte coverage in 2% O2. While the vascular networks in 0.2% O2 also increased in length, endothelial morphology indicated destabilization of the vascular structure. This observation may be due to the 36-fold increase in endogenous VEGF-A expression, which can cause pericyte detachment and loosening of EC junctions to prime vessels for sprouting.32
The results of these experiments indicate a differential response of ECs within ASC cultures toward hypoxia, depending on whether they are individual cells or incorporated into a pericyte-stabilized tissue structure. Despite similar global changes in metabolism and growth factor expression in each experimental approach, there were marked differences in EC proliferation and overall vessel growth. Vessel maturity and stabilization likely play a role in this complex system. Pericyte investment promotes EC survival through the expression of VEGF-A and antiapoptotic factors.33 Interestingly, the extended culture period experienced by the preformed vessels resulted in decreased glycolytic activity and proliferation, but increased expression of VEGF-A and VEGFR-2 versus earlier time points. It is possible that the early phase of de novo vascular assembly requires higher proliferation and metabolic activity to initiate the nascent vascular plexus and is therefore dramatically inhibited when the oxygen tension is reduced.
The findings of this study have important implications on vascularization strategies for tissue engineering. The ability of ASCs to self-assemble into vascular structures may be an advantage for their clinical utility as larger grafts will require more rapid vascularization to transport enough oxygen and nutrients for cell survival. However, this study provides preliminary evidence that this vascular ability is itself dependent on oxygen and may require a transient oxygen source to be effective. This may be achieved by preassembly of vascular networks before implantation or through oxygen delivery strategies in situ.34–37 The in vivo tissue environment is certainly much more complex, with inflammation, cellular invasion, and other biological signaling occurring after implantation of a cell-seeded graft. Ongoing in vivo studies are necessary to completely understand the impact of this complex postimplantation environment on the ability of ASCs to functionally vascularize a graft in situ.
Conclusions
This study reveals striking phenomena and provides important insights into the differential responses of ASC-derived vessels to hypoxia. ASCs are a heterogeneous population of stem cells. Subpopulations of ECs and pericytes present within the ASCs can spontaneously self-assemble through tightly orchestrated heterotypic interactions, but this system is remarkably sensitive to changes in oxygen levels. Depending on the stage of tissue assembly and maturity, hypoxia may have disparate effects. We demonstrated that moderate hypoxia has a negative impact on de novo vascular assembly, but it positively impacts the stability and growth of preformed vessels. These novel findings are of importance to the tissue engineering field and may instruct future vascularization strategies.
Supplementary Material
Acknowledgments
This project was funded by the Maryland Stem Cell Research Fund (2014-MSCRFI-0699), NSF CAREER award (CBET 1350554), Johns Hopkins University Center for Musculoskeletal Research, and the American Society for Bone and Mineral Research (2013CEA13) (awarded to W.L.G), as well as the American Heart Association Predoctoral Fellowship (12PRE11780069) (awarded to D.L.H). The authors thank The Wilmer Imaging and Microscopy Core Grant (P30-EY001765) for use of the Zeiss LSM 510 confocal microscope.
Disclosure Statement
No competing financial interests exist.
References
- 1.Ehsan S.M., and George S.C. Nonsteady state oxygen transport in engineered tissue: implications for design. Tissue Eng Part A 19, 1433, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lesman A., Koffler J., Atlas R., Blinder Y.J., Kam Z., and Levenberg S. Engineering vessel-like networks within multicellular fibrin-based constructs. Biomaterials 32, 7856, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Singh S., Wu B.M., and Dunn J.C. Accelerating vascularization in polycaprolactone scaffolds by endothelial progenitor cells. Tissue Eng Part A 17, 1819, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Unger R.E., Ghanaati S., Orth C., Sartoris A., Barbeck M., Halstenberg S., et al. The rapid anastomosis between prevascularized networks on silk fibroin scaffolds generated in vitro with cocultures of human microvascular endothelial and osteoblast cells and the host vasculature. Biomaterials 31, 6959, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Cheng G., Liao S., Kit Wong H., Lacorre D.A., di Tomaso E., Au P., et al. Engineered blood vessel networks connect to host vasculature via wrapping-and-tapping anastomosis. Blood 118, 4740, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertelsen L.B., Bohn A.B., Smith M., Mølgaard B., Møller B., Stødkilde-Jørgensen H., et al. Are endothelial outgrowth cells a potential source for future re-vascularization therapy? Exp Gerontol 58, 132, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Blinder Y.J., Mooney D.J., and Levenberg S. Engineering approaches for inducing blood vessel formation. Curr Opin Chem Eng 3, 56, 2014 [Google Scholar]
- 8.Samuel R., Daheron L., Liao S., Vardam T., Kamoun W.S., Batista A., et al. Generation of functionally competent and durable engineered blood vessels from human induced pluripotent stem cells. Proc Natl Acad Sci U S A 110, 12774, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourin P., Bunnell B.A., Casteilla L., Dominici M., Katz A.J., March K.L., et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 15, 641, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ong W.K., and Sugii S. Adipose-derived stem cells: fatty potentials for therapy. Int J Biochem Cell Biol 45, 1083, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Hutton D.L., Logsdon E.A., Moore E.M., Mac Gabhann F., Gimble J.M., and Grayson W.L. Vascular morphogenesis of adipose-derived stem cells is mediated by heterotypic cell-cell interactions. Tissue Eng Part A 18, 1729, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutton D.L., Moore E.M., Gimble J.M., and Grayson W.L. Platelet-derived growth factor and spatiotemporal cues induce development of vascularized bone tissue by adipose-derived stem cells. Tissue Eng Part A 19, 2076, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirota K., and Semenza G.L. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol Hematol 59, 15, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Hsiao S.T., Dilley R.J., Dusting G.J., and Lim S.Y. Ischemic preconditioning for cell-based therapy and tissue engineering. Pharmacol Ther 142, 141, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Rasmussen J.G., Frobert O., Pilgaard L., Kastrup J., Simonsen U., Zachar V., et al. Prolonged hypoxic culture and trypsinization increase the pro-angiogenic potential of human adipose tissue-derived stem cells. Cytotherapy 13, 318, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Rehman J., Traktuev D., Li J., Merfeld-Clauss S., Temm-Grove C.J., Bovenkerk J.E., et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109, 1292, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Thangarajah H., Vial I.N., Chang E., El-Ftesi S., Januszyk M., Chang E.I., et al. IFATS collection: adipose stromal cells adopt a proangiogenic phenotype under the influence of hypoxia. Stem Cells 27, 266, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Griffith C.K., and George S.C. The effect of hypoxia on in vitro prevascularization of a thick soft tissue. Tissue Eng Part A 15, 2423, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Eguchi R., Suzuki A., Miyakaze S., Kaji K., and Ohta T. Hypoxia induces apoptosis of HUVECs in an in vitro capillary model by activating proapoptotic signal p38 through suppression of ERK1/2. Cell Signal 19, 1121, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Ohta T., Eguchi R., Suzuki A., Miyakaze S., Ayuzawa R., and Kaji K. Hypoxia-induced apoptosis and tube breakdown are regulated by p38 MAPK but not by caspase cascade in an in vitro capillary model composed of human endothelial cells. J Cell Physiol 211, 673, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Niemisto A., Dunmire V., Yli-Harja O., Zhang W., and Shmulevich I. Robust quantification of in vitro angiogenesis through image analysis. IEEE Trans Med Imaging 24, 549, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Amos P.J., Kapur S.K., Stapor P.C., Shang H., Bekiranov S., Khurgel M., et al. Human adipose-derived stromal cells accelerate diabetic wound healing: impact of cell formulation and delivery. Tissue Eng Part A 16, 1595, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng N.C., Chen S.Y., Li J.R., and Young T.H. Short-term spheroid formation enhances the regenerative capacity of adipose-derived stem cells by promoting stemness, angiogenesis, and chemotaxis. Stem Cells Transl Med 2, 584, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng N.C., Wang S., and Young T.H. The influence of spheroid formation of human adipose-derived stem cells on chitosan films on stemness and differentiation capabilities. Biomaterials 33, 1748, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Sart S., Tsai A.C., Li Y., and Ma T. Three-dimensional aggregates of mesenchymal stem cells: cellular mechanisms, biological properties, and applications. Tissue Eng Part B Rev 20, 365, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moriyama H., Moriyama M., Isshi H., Ishihara S., Okura H., Ichinose A., et al. Role of notch signaling in the maintenance of human mesenchymal stem cells under hypoxic conditions. Stem Cells Dev 23, 2211, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denko N.C. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer 8, 705, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Deschepper M., Oudina K., David B., Myrtil V., Collet C., Bensidhoum M., et al. Survival and function of mesenchymal stem cells (MSCs) depend on glucose to overcome exposure to long-term, severe and continuous hypoxia. J Cell Mol Med 15, 1505, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee E.Y., Xia Y., Kim W.S., Kim M.H., Kim T.H., Kim K.J., et al. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen 17, 540, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Hsiao S.T., Lokmic Z., Peshavariya H., Abberton K.M., Dusting G.J., Lim S.Y., et al. Hypoxic conditioning enhances the angiogenic paracrine activity of human adipose-derived stem cells. Stem Cells Dev 22, 1614, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhang S.H., Cho S.W., La W.G., Lee T.J., Yang H.S., Sun A.Y., et al. Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials 32, 2734, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Carmeliet P., and Jain R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 473, 298, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franco M., Roswall P., Cortez E., Hanahan D., and Pietras K. Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood 118, 2906, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benjamin S., Sheyn D., Ben-David S., Oh A., Kallai I., Li N., et al. Oxygenated environment enhances both stem cell survival and osteogenic differentiation. Tissue Eng Part A 19, 748, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Oh S.H., Ward C.L., Atala A., Yoo J.J., and Harrison B.S. Oxygen generating scaffolds for enhancing engineered tissue survival. Biomaterials 30, 757, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Pedraza E., Coronel M.M., Fraker C.A., Ricordi C., and Stabler C.L. Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proc Natl Acad Sci U S A 109, 4245, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cook C.A., Hahn K.C., Morrissette-McAlmon J.B., and Grayson W.L. Oxygen delivery from hyperbarically loaded microtanks extends cell viability in anoxic environments. Biomaterials 52, 376, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.