Abstract
Background:
Endobronchial ultrasound (EBUS)-guided transbronchial needle aspiration (TBNA) requires a dedicated needle for aspiration of mediastinal lesions. There is no data on reuse of these needles.
Methods:
This is a retrospective study of patients who underwent EBUS-TBNA with either new or reused EBUS-TBNA needles. The needles were reused after thorough cleaning with filtered water and organic cleaning solution, disinfection with 2.4% glutaraldehyde solution followed by ethylene oxide sterilization. The yield of EBUS-TBNA was compared between the two groups.
Results:
A total of 500 EBUS-TBNA procedures (351 new, 149 reused needles) were performed. The baseline characteristics were different in the two groups with suspected granulomatous disorders (sarcoidosis or tuberculosis) being significantly more common in the new compared to the reused needle group. Similarly, the median, interquartile range number of lymph node stations sampled, and the total number of passes were significantly higher in the new versus the reused needle group. The diagnostic yield was significantly higher with new needle as compared to reused needle (65.2% vs. 53.7%, P = 0.02). On multivariate logistic regression analysis, clinical suspicion of granulomatous disorders (odds ratio 1.86 [95% confidence interval, 1.20-2.87], P = 0.005) was the only predictor of diagnostic yield, after adjusting for the type of needle (new or reused), total number of passes and the number of lymph node stations sampled. No case of mediastinitis was encountered in either group.
Conclusions:
The yield of EBUS-TBNA might be similar with single reuse of needles as compared to new needles. However, reuse of needle should be performed only when absolutely necessary.
KEY WORDS: Ebus, endobronchial ultrasound, endoscopic ultrasound, eus, lung cancer, sarcoidosis, tbna, tuberculosis
INTRODUCTION
Endobronchial ultrasound (EBUS)-guided transbronchial needle aspiration (TBNA) is widely accepted as the modality of choice for obtaining cytological specimens from mediastinal and hilar lymph nodes.[1,2] It is a useful tool not only for the staging of lung cancer but also for the diagnosis of diseases such as sarcoidosis, tuberculosis (TB), and metastatic malignancy.[3,4,5,6,7] The procedure requires a dedicated TBNA needle for the purpose of aspirating material from the lymph nodes visualized in real time.[8] A proprietary EBUS-TBNA needle designed for use with the respective echobronchoscopes is recommended by the manufacturers. Further, these needles are recommended for a single time use only. The cost of the procedure with a new proprietary EBUS-TBNA needle (Vizishot needle [NA-201SX-4021, Olympus Medical Systems, Japan]) is about 16,250 Indian rupees (US $250) at our center.
It is a common practice at several centers in India to reuse these needles to decrease the costs incurred. Our center caters to a population where a large number of patients belong to the low socioeconomic strata. The vast majority does not have medical insurance, and a proportion of these patients cannot afford the cost of the procedure with a new dedicated EBUS-TBNA needle. Reuse of the EBUS-TBNA needle has the potential to reduce the cost associated with the procedure, as the major cost is due to the needle (about 180 USD). The practice of reusing needle assembly already exists for conventional TBNA (biopsy needle NA-1C-1, Olympus Medical Systems, Japan) where the autoclavable sheaths can be reused, as recommended by the manufacturer. Currently, there is no published data on the yield of EBUS-TBNA with reused needles.
We hypothesized that the yield of EBUS-TBNA would be decreased with reuse of these needles due to deterioration of the needle assembly associated with the procedure. In this study, we compare the yield of the EBUS-TBNA procedure performed with the reused as compared to new needles, in the diagnosis of mediastinal lymphadenopathy.
METHODS
This is a retrospective study of subjects who underwent EBUS-TBNA at the interventional pulmonology suite of this institute between November 2013 and September 2015. The study protocol was approved by the Institute Ethics Committee, and a written informed consent was obtained from all subjects.
Study participants
Consecutive subjects with intrathoracic lymph node enlargement who underwent EBUS during the study period were enrolled. Subjects with any of the following were excluded: Hypoxemia (pulse oximetric saturation <90 mmHg on room air), deranged coagulation profile, pregnancy, or failure to provide informed consent.
Study procedure
All subjects underwent a detailed clinical evaluation, laboratory tests (complete blood count, liver and renal function tests, and coagulation profile), rapid card tests for hepatitis B virus (HBV), hepatitis C virus (HCV), and human immunodeficiency virus (HIV), chest radiography, and computed tomography of the thorax. Subjects were explained the entire EBUS procedure and the requirement of a dedicated needle (Vizishot needle (NA-201SX-4021, Olympus Medical Systems, Japan). They were encouraged to ask questions regarding the procedure. Subjects who could afford the cost of the procedure with a new needle underwent EBUS-TBNA with a new EBUS needle. In the event that the subjects could not afford the cost of the procedure, they were given the option to undergo the procedure with the reused needle. They were clearly explained that the EBUS-TBNA needles would be reused after a thorough sterilization process. However, they were cautioned that they could still incur the risk of contracting infections. If they provided consent for the procedure, they were subjected to the same procedure with the reused needle.
Sterilization procedure
All EBUS-TBNA needles used in subjects with serological evidence of HBV, HCV, or HIV and those used in subjects with suspected TB were discarded. After following the above exclusion criteria, the used EBUS-TBNA needles were thoroughly cleaned, and the channel flushed with filtered soap water followed by plain filtered water. They were then dipped in an organic cleaning solution (3M Rapid) to remove organic matter to allow direct contact of all surfaces with the disinfectant and sterilant. The needles were totally immersed in filtered water for 10 min. The lumen was manually flushed with large volumes (at least 100 mL) of rinse water. Following this, they were submerged in 2.4% glutaraldehyde (CIDEX solution) for 30 min (minimum recommended time is 20 min).[9] The needles were then soaked in and flushed with sterile water. Subsequently, the needles were sent for sterilization with ethylene oxide in a dedicated facility.[10] All the needles were reused only once.
Endobronchial ultrasound
EBUS procedures were performed on an outpatient basis, according to the standard protocol described previously.[11] The subjects were premedicated with atropine (0.6 mg) and promethazine (25 mg) intramuscularly followed by nebulization with 4% lidocaine solution. Two actuations of 10% lignocaine spray were applied to the oropharynx and subjects were then placed in the supine position. The echobronchoscope was inserted via the oral cavity, and 2% lignocaine solution was delivered in an “instil as you proceed” fashion over the vocal cords, carina, and the main bronchi.[12] All the lymph node stations were systematically examined and the lymph node characteristics recorded, as described previously.[4] EBUS-TBNA was performed from 1 to 4 lymph node stations according to the number and size of the lymph nodes, and 2-3 aspirates were obtained from each station. The aspirates were used to prepare slides and a cell block. The aspirates were also sent for Xpert MTB/RIF assay and mycobacterial cultures, as required. Endoscopic ultrasound-guided fine needle aspiration with an echo bronchoscope was performed if the lymph nodes appeared more accessible through the transesophageal route at the discretion of the bronchoscopist, as described earlier.[13,14]
Outcomes
The main outcome was the diagnostic yield of EBUS-TBNA in the two groups (new and the reused needle groups) defined as the proportion of subjects in whom a definitive diagnosis was obtained with EBUS-TBNA. A definitive diagnosis implies that the cytopathological findings point toward a clear diagnosis such as granulomatous disorder (sarcoidosis or TB), metastatic malignancy, or others as opposed to reactive lymphadenopathy. The other outcomes included adequacy of samples (either a definite diagnosis or presence of lymphocytes), complication rate in the two groups and the predictors of diagnostic yield on EBUS-TBNA.
Statistical analysis
Data were analyzed using the commercial statistical package SPSS for MS-Windows (version 22, IBM Inc.,). Data are expressed in a descriptive fashion as mean ± standard deviation (SD) or median with interquartile range. Continuous variables were compared using Student's t-test (or Mann-Whitney U-test) while categorical variables were compared with the Chi-square test (or Fisher's exact test), as applicable. The diagnostic yield of the procedures was calculated by dividing the number of definitive diagnoses obtained by the number of subjects. A multivariate logistic regression analysis was performed to assess the factors predicting the diagnostic yield in subjects undergoing EBUS-TBNA. The results of logistic regression are presented as odds ratio (OR) with 95% confidence intervals (CIs). P <0.05 was considered as statistically significant.
RESULTS
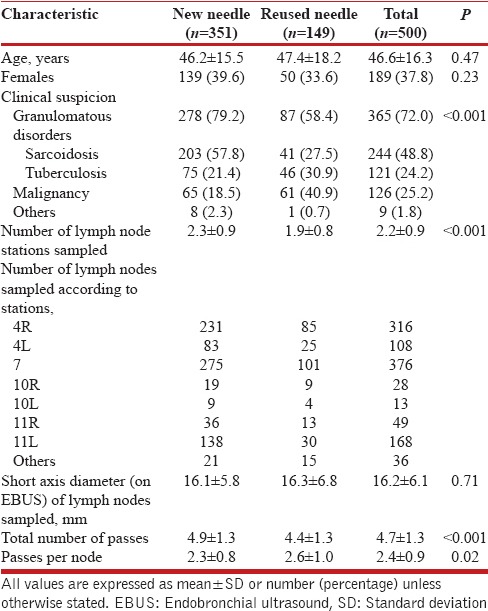
During the study period, a total of 500 EBUS-TBNA procedures were performed; 351 (70.2%) with new and 149 (29.8%) with the reused needle. The mean (SD) age of the subjects (189 [37.8%] women) was 46.6 (16.3) years [Table 1]. The baseline characteristics were different between the two groups. A significantly higher proportion of subjects with suspected granulomatous disorders (sarcoidosis or TB) were present in the new as compared to the reused needle group (79.2% vs. 58.4%, P < 0.001). A total of 1,094 lymph node stations were sampled. Stations 7 and 4R were the most commonly aspirated group of lymph nodes. The mean short axis diameter of the lymph nodes sampled by EBUS-TBNA was similar between the two groups. The mean (SD) number of lymph node stations sampled (2.3 [0.9] vs. 1.9 [0.8], P < 0.001) and the total number of passes (4.9 [1.3] vs. 4.4 [1.3], P < 0.001) were significantly higher in the new needle than the reused needle group [Table 1]; the number of passes per lymph node station were higher in the reused needle group (2.6 [1.0] vs. 2.3 [0.8], P = 0.02).
Table 1.
Baseline characteristics of the study population
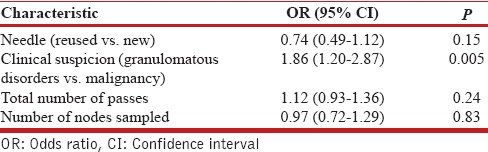
The number of adequate cytological samples obtained were higher with the new (338/351, 95.2%) than with the reused needles (138/149, 92.6%), albeit not statistically different (P = 0.11). The diagnostic yield was significantly higher with the new needle as compared to the reused needle (65.2% vs. 53.7%, P = 0.02, [Table 2]). Among subjects with a definitive diagnosis on EBUS-TBNA, the proportion of subjects found to have granulomatous disorders (sarcoidosis or TB) as opposed to malignancy was higher in the new needle as compared to the reused needle group (82.3% vs. 64.5%, P = 0.002). The complication rate was similar with the two needles [Table 2]; there was no episode of mediastinitis in either group. On multivariate logistic regression analysis, the clinical suspicion of granulomatous disorders was the only predictor of diagnostic yield (OR 1.86 [95% CI, 1.20-2.87], P = 0.005) on EBUS-TBNA. The type of needles (new or reused) was not a significant factor affecting the diagnostic yield [Table 3].
Table 2.
Outcomes of the study
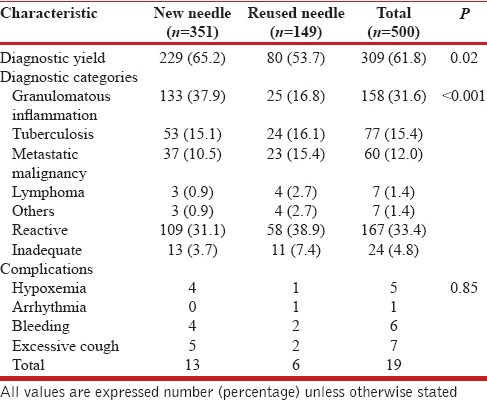
Table 3.
Multivariate logistic regression analysis of patient and endobronchial ultrasound-transbronchial needle aspiration characteristics by diagnostic yield
DISCUSSION
The results of this study suggest that the diagnostic yield and safety of EBUS-TBNA performed with reused versus the new needles is not very different. In this study, a majority (70%) of the patients underwent TBNA with new needles while about 30% of the study subjects were unable to afford the procedure cost.
There were significant differences in the baseline characteristics of the study population such as the clinical diagnosis, the number of lymph node stations sampled and the number of passes. Majority of the patients (58%) undergoing EBUS-TBNA with new needle had a clinical suspicion of sarcoidosis contrary to the reused needle group where malignancy (41%) was the most common clinical suspicion. This probably reflects the socioeconomic distribution of these diseases, with sarcoidosis being more prevalent in the affluent population, thus, patients with suspected sarcoidosis were more likely to afford the cost of the new needles.[15] The unadjusted diagnostic yield was higher with the new as compared to the reused needles. However, to overcome the limitation of uneven baseline characteristics, a multivariate logistic regression analysis was performed to adjust for the effects of baseline differences on the final outcome, i.e., the diagnostic yield.
On the logistic regression analysis, clinical suspicion of granulomatous disorders was the only factor predicting diagnostic yield on EBUS-TBNA. Conversely, a clinical diagnosis of malignant lymphadenopathy predicted a lower diagnostic yield as many of the patients initially suspected to have malignant lymph node enlargement are ultimately diagnosed to have reactive or nonspecific lymphadenopathy after EBUS-TBNA and clinical follow-up.[16] As patients with a clinical diagnosis of malignant lymph node enlargement were significantly higher in the reused needle group, this might have led to the lower yield in this group. The type of needle used (reused or new) did not affect the diagnostic yield in the multivariate analysis, thus demonstrating the equivalent efficacy of the two needles. However, as this is a secondary analysis in a retrospective cohort, it is possible that reused needles are genuinely associated with a lower yield because of the wear and tear of the needle assembly.
EBUS is an expensive procedure. Although cost-effective when compared to mediastinoscopy,[17] a sizeable proportion of patients are still unable to afford EBUS-TBNA in resource-limited settings due to the low per capita income and poor insurance coverage. Many of these patients (about 15-30% at our center) are finally diagnosed with tuberculous lymphadenopathy.[18] In this scenario, they would have access to anti-TB treatment administered free of cost under the national TB program once a definitive diagnosis of TB has been achieved on EBUS-TBNA.[19] The alternative is to subject these patients to conventional TBNA.[20] Although conventional TBNA is a reasonable choice especially with lymph node enlargement at stations 4R and 7,[21] the cost of this procedure (US $125 at our center) is still prohibitive for a proportion of these patients.[22] Further, in our experience, conventional TBNA needles are not suitable for reuse, hence the preference for EBUS-TBNA in these patients.
Despite a reasonable yield, we advise great caution in reusing EBUS-TBNA needles. The manufacturer recommends it for single use only, and no sterilization procedure has been recommended. It is noteworthy that accessories such as guide sheaths used in radial EBUS procedures are reusable as recommended by the manufacturer. By analogy, we sought to determine the feasibility of reuse of the linear EBUS-TBNA needles. We used an exhaustive sterilization procedure with both 2.4% glutaraldehyde and ethylene oxide. Glutaraldehyde (2.4%) is a Food and Drug Administration approved high-level disinfectant for heat-sensitive semi-critical medical devices, with rapid mycobactericidal activity.[9] On the other hand, ethylene oxide is a highly-penetrating sterilizing agent that is compatible with most medical device materials. It has excellent bactericidal, sporicidal, and virucidal activity; and is effective for low-temperature sterilization of heat and moisture sensitive medical devices and instruments without lumen or materials restrictions.[10] Importantly, we reserved the needles as the last resort for compassionate use only in those patients who could not afford the cost of new needles and after a thorough explanation of the pros and cons of such use. In general, we advise against the re-use of these needles, if it can be avoided. In our experience, the bevel of the EBUS-TBNA needles gets blunted after 15-20 passes; thereafter, the puncture through the tracheobronchial wall requires increasingly greater effort making the procedure technically more difficult. Therefore, we suggest that these needles, even for compassionate use, should not be reused more than once.
There are a few limitations of this study. It is a retrospective analysis and thus the results are only hypothesis-generating. However, a prospective randomized trial cannot be performed addressing this issue due to obvious ethical reasons. Thus, we urge pulmonologists involved in reusing EBUS-TBNA needles to publish their experience and follow-up these patients adequately such that this issue is investigated in even greater depth. Furthermore, rapid on-site cytological evaluation was not available in the present study. Finally, prospective follow-up of the patients for development of viral infections is not available, although the elaborate sterilization procedure is known to destroy viruses effectively.
CONCLUSIONS
The reuse of EBUS-TBNA needles after thorough sterilization is feasible, and may provide a diagnostic yield almost comparable to that with new needles. However, it is not advisable to reuse these needles routinely.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Herth FJ, Eberhardt R, Vilmann P, Krasnik M, Ernst A. Real-time endobronchial ultrasound guided transbronchial needle aspiration for sampling mediastinal lymph nodes. Thorax. 2006;61:795–8. doi: 10.1136/thx.2005.047829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yasufuku K, Chiyo M, Sekine Y, Chhajed PN, Shibuya K, Iizasa T, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest. 2004;126:122–8. doi: 10.1378/chest.126.1.122. [DOI] [PubMed] [Google Scholar]
- 3.Gupta D, Dadhwal DS, Agarwal R, Gupta N, Bal A, Aggarwal AN. Endobronchial ultrasound-guided transbronchial needle aspiration vs conventional transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest. 2014;146:547–56. doi: 10.1378/chest.13-2339. [DOI] [PubMed] [Google Scholar]
- 4.Dhooria S, Agarwal R, Aggarwal AN, Bal A, Gupta N, Gupta D. Differentiating tuberculosis from sarcoidosis by sonographic characteristics of lymph nodes on endobronchial ultrasonography: A study of 165 patients. J Thorac Cardiovasc Surg. 2014;148:662–7. doi: 10.1016/j.jtcvs.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal R, Srinivasan A, Aggarwal AN, Gupta D. Efficacy and safety of convex probe EBUS-TBNA in sarcoidosis: A systematic review and meta-analysis. Respir Med. 2012;106:883–92. doi: 10.1016/j.rmed.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Szlubowski A, Kuzdzal J, Kolodziej M, Soja J, Pankowski J, Obrochta A, et al. Endobronchial ultrasound-guided needle aspiration in the non-small cell lung cancer staging. Eur J Cardiothorac Surg. 2009;35:332–5. doi: 10.1016/j.ejcts.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 7.Murgu SD. Diagnosing and staging lung cancer involving the mediastinum. Chest. 2015;147:1401–12. doi: 10.1378/chest.14-1355. [DOI] [PubMed] [Google Scholar]
- 8.Gompelmann D, Eberhardt R, Herth FJ. Endobronchial ultrasound. Endosc Ultrasound. 2012;1:69–74. doi: 10.7178/eus.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Middleton AM, Chadwick MV, Sanderson JL, Gaya H. Comparison of a solution of super-oxidized water (Sterilox) with glutaraldehyde for the disinfection of bronchoscopes, contaminated. J Hosp Infect. 2000;45:278–82. doi: 10.1053/jhin.2000.0772. [DOI] [PubMed] [Google Scholar]
- 10.Mendes GC, Brandão TR, Silva CL. Ethylene oxide sterilization of medical devices: A review. Am J Infect Control. 2007;35:574–81. doi: 10.1016/j.ajic.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Dhooria S, Agarwal R, Aggarwal AN, Gupta N, Gupta D, Behera D. Agreement of mediastinal lymph node size between computed tomography and endobronchial ultrasonography: A study of 617 patients. Ann Thorac Surg. 2015;99:1894–8. doi: 10.1016/j.athoracsur.2015.02.055. [DOI] [PubMed] [Google Scholar]
- 12.Kaur H, Dhooria S, Aggarwal AN, Gupta D, Behera D, Agarwal R. A randomized trial of 1% vs 2% lignocaine by the spray-as-you-go technique for topical anesthesia during flexible bronchoscopy. Chest. 2015;148:739–45. doi: 10.1378/chest.15-0022. [DOI] [PubMed] [Google Scholar]
- 13.Dhooria S, Aggarwal AN, Singh N, Gupta D, Behera D, Gupta N, et al. Endoscopic ultrasound-guided fine-needle aspiration with an echobronchoscope in undiagnosed mediastinal lymphadenopathy: First experience from India. Lung India. 2015;32:6–10. doi: 10.4103/0970-2113.148399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhooria S, Aggarwal AN, Gupta D, Behera D, Agarwal R. Utility and safety of endoscopic ultrasound with bronchoscope-guided fine-needle aspiration in mediastinal lymph node sampling: Systematic review and meta-analysis. Respir Care. 2015;60:1040–50. doi: 10.4187/respcare.03779. [DOI] [PubMed] [Google Scholar]
- 15.Gupta D, Vinay N, Agarwal R, Agarwal AN. Socio-demographic profile of patients with sarcoidosis vis-à-vis tuberculosis. Sarcoidosis Vasc Diffuse Lung Dis. 2013;30:186–93. [PubMed] [Google Scholar]
- 16.Lange TJ, Kunzendorf F, Pfeifer M, Arzt M, Schulz C. Endobronchial ultrasound-guided transbronchial needle aspiration in routine care – plenty of benign results and follow-up tests. Int J Clin Pract. 2012;66:438–45. doi: 10.1111/j.1742-1241.2012.02907.x. [DOI] [PubMed] [Google Scholar]
- 17.Sharples LD, Jackson C, Wheaton E, Griffith G, Annema JT, Dooms C, et al. Clinical effectiveness and cost-effectiveness of endobronchial and endoscopic ultrasound relative to surgical staging in potentially resectable lung cancer: Results from the ASTER randomised controlled trial. Health Technol Assess. 2012;16:1. doi: 10.3310/hta16180. [DOI] [PubMed] [Google Scholar]
- 18.Srinivasan A, Agarwal R, Gupta N, Aggarwal AN, Gupta D. Initial experience with real time endobronchial ultrasound guided transbronchial needle aspiration from a tertiary care hospital in north India. Indian J Med Res. 2013;137:803–7. [PMC free article] [PubMed] [Google Scholar]
- 19.Verma R, Khanna P, Mehta B. Revised national tuberculosis control program in India: The need to strengthen. Int J Prev Med. 2013;4:1–5. [PMC free article] [PubMed] [Google Scholar]
- 20.Punamiya V, Mehta A, Chhajed PN. Bronchoscopic needle aspiration in the diagnosis of mediastinal lymphadenopathy and staging of lung cancer. J Cancer Res Ther. 2010;6:134–41. doi: 10.4103/0973-1482.65231. [DOI] [PubMed] [Google Scholar]
- 21.Khan A, Agarwal R, Aggarwal AN, Gupta N, Bal A, Singh N, et al. Blind transbronchial needle aspiration without an on-site cytopathologist: Experience of 473 procedures. Natl Med J India. 2011;24:136–9. [PubMed] [Google Scholar]
- 22.Agarwal R, Aggarwal AN, Gupta D. Efficacy and safety of conventional transbronchial needle aspiration in sarcoidosis: A systematic review and meta-analysis. Respir Care. 2013;58:683–93. doi: 10.4187/respcare.02101. [DOI] [PubMed] [Google Scholar]


