Abstract
Aims:
To study socioeconomic status (SES) and living conditions (LC) as risk factors for latent tuberculosis infection (LTBI) and their impact on QuantiFERON-TB gold (QFT-G) and tuberculin skin test (TST) outcome for determining a better diagnostic test for LTBI in the malnourished tribal population of Melghat.
Settings and Design:
Six hundred sixty nine participants matching the inclusion criteria were recruited from 10 tribal villages of Melghat region, India.
Subjects and Methods:
Complete information related to various risk factors and test outcome was obtained on 398 participants, which was analyzed as per predefined conceptual framework. Factors were classified based on their relevance either at individual or household level, and subsequently based on the possibility of intervention. Data were partitioned into concordant and discordant sets depending on test agreement.
Results:
In concordant set, the two tests revealed that LTBI was significantly associated with smoking (adjusted odds ratio [aOR]: 2.64 [95% confidence interval [CI]: 1.03–6.79]), tobacco usage (aOR: 2.74 [95% CI: 1.50–4.99]), and malnourishment (aOR: 1.97 [95% CI: 1.12–3.48]) after basic adjustment. Inclusion of latent variable SES and LC in the model has mediating effect on the association of above factors with LTBI. Further, the association of SES and LC with LTBI in concordant set was unaltered in presence of other cofactors. From discordant set, results of QFT-G corroborated with that of concordant set.
Conclusions:
Poor SES and LC can be considered as strong risk factors linked with LTBI as compared to malnourishment, which is often targeted in such communities. Further, our study showed QFT-G test as a reliable tool in screening of LTBI in the tribal population of Melghat, India.
KEY WORDS: QuantiFERON-TB gold, tribal, tuberculin skin test, tuberculosis
INTRODUCTION
An estimated 40% of Indian population harbors Mycobacterium tuberculosis (MTB) infection, making India among the top five countries with high TB incidence cases on a global scale.[1,2] Once infected with MTB, ~30% of the individuals develops latent tuberculosis infection (LTBI) and among these, 5-10% are at lifetime risk of progressing to the active TB.[3] Laboratory tests aiding early and rapid diagnosis along with efficient treatment against LTBI are therefore required to minimize the burden of TB in India.
Available diagnostic tests for LTBI includes tuberculin skin test (TST), which measures the hypersensitivity response to purified protein derivative, and more recently developed interferon gamma (IFN-g) release assays such as QuantiFERON-TB gold (QFT-G) that measures IFN-g by circulating T-cells in the blood.[4,5] The risk of developing LTBI depends upon increased likelihood of exposure to persons with TB disease, clinical conditions, and specific factors associated.[6] Thus, the major challenge apart from its diagnosis remains the identification of associated risk factors that leads to LTBI. Factors like socioeconomic status (SES) and poor living condition (LC) the most important risk factors reported, since they are invariably associated with poverty, malnutrition, hygiene, and illiteracy all of which have confounding effect on outcome of both active and LTBI in high TB endemic regions.[7,8] The identification of associated risk factors with both QFT-G and TST is therefore required for effective monitoring of TB infection, which may also provide valuable information with respect to diagnostic utility of both the available tests.
Our study area, Melghat, is a tribal region located in Maharashtra state of India, with a population of nearly 0.3 million. These tribal population have large families (around 7–8 members per family) who live in small huts (kaccha houses with one or two small rooms) with no ventilation and are exposed to active TB patients in the same house. The tribes have poor hygienic practices, High illiteracy ratess, lack of awareness of diseases and available services, overcrowding in houses, extreme poverty, and take irregular treatments which adversely affects their health. The problems are further aggravated due to lack of primary health care centers in the villages. Various international agencies have also identified Melghat with the highest number of malnutrition cases in India.[9,10] There are studies that have evaluated the QFT-G and TST in the diagnosis of TB (active and latent) in TB endemic zones[11,12] however, in this study the impact of SES, LC, and other risk factors on QFT-G and TST for LTBI diagnosis have been addressed for high burden malnutrition regions of India.
The aim of this study was to asess SES and LC as risk factors for LTBI and their impact on QFT-G and TST test outcome for determining a better diagnostic test for LTBI in the malnourished tribal population of Melghat.
SUBJECTS AND METHODS
Ethics statement
The study was approved by the Ethical Committee of Central India Institute of Medical Sciences (CIIMS), Nagpur and Meditation Addiction Health AIDS Nutrition (MAHAN) Trust, Amravati, Maharashtra, India. All clinical investigations were conducted according to the principles expressed in the declaration of Helsinki. All the participants were given oral explanation of the study, as well as written consents were taken from all of them.
Study design and participant description
We planned a prospective cohort study for a period of 3 years from September 2009 to August 2012 in ten different villages of Melghat region. A total of 993 participants having no symptoms of TB, with normal chest X-ray profile were enrolled from different villages of Melghat based on the information available with Tribal Health Research Centre, Dharni run by MAHAN trust. These participants were screened for eligibility using a set of prespecified inclusion and exclusion criteria. Briefly, participants were recruited from the families having atleast one sputum positive pulmonary tuberculosis patient (index case) living in the same house hold, for atleast 2 months before the start of anti-TB medication, who have high probability of repeated exposure along with high risk of development of TB infection. Bacillus Calmette-Guérin (BCG) vaccination status was assessed based on the examination of BCG scar on left forearm. Other informations like any prior TST, presence of underlying illnesses, and infections experienced in the last 3 months were also recorded. All the individuals, who were on TB treatment or having past history of TB, HIV positive participants, and other with the evidence of immunosuppressive therapy were excluded from the study. Similarly pregnant females and those in lactation period were also excluded from the study.
The information on demographic parameters, behavioral aspects, SES, LC, BCG vaccination status, and exposure to TB patients were obtained through a structured questionnaire. LC primarily targeted basic amenities like housing conditions, electricity, drinking water source, and toiletries.
Based on the reliability of information on above points, 639 participants who matched the inclusion criteria were included in the study [Figure 1]. Out of these, 193 were excluded as they were not willing to provide blood sample. In addition, 41 participants who migrated to other places were also excluded from the analysis. Seven participants were dropped due to incomplete test results. Finally, blood samples of 398 individuals (222 male/176 female) were investigated for the downstream analysis. Written consents were taken from each participant after detailed oral explanation about the study.
Figure 1.

Study flow diagram
Tuberculin skin test and QuantiFERON-TB gold test
TST was performed using Mantoux method as per Revised National Tuberculosis Control Programme (RNTCP) guidelines and read after 48-72 h. QFT-G (Cellestis Limited, Carnegie, Victoria, Australia) test was conducted according to the manufacturer's instructions. Blood was collected directly into two 1 ml heparin containing tubes. One tube contained only heparin as negative control, and the other tube contained overlapping peptides representing the entire sequences of Culture filtrate protein-10 (CFP-10) and Early secretory antigenic target-6 (ESAT-6), and another peptide representing a portion of TB7.7. The tubes were incubated for 20-24 h according to the manufacturer's recommendation. After incubation, plasma was removed and frozen until used for ELISA. The IFN-g values were calculated by subtracting the value of negative control, and the cut-off value of 0.35 IU/mL was selected according to the manufacturer's instruction.
Statistical analysis
Conceptual framework
The study sample (n = 398) was split into two sets. In the concordant set (n = 301), outcome was defined as “LTBI positive by both tests;” while in the discordant set (n = 97), outcomes were “LTBI positive by either QFT-G/TST.” The conceptual framework in Figure 2 portrays various risk factors associated with the outcome. Risk factors were categorized into two viz., individual and household based on their relatedness at personal and family levels, respectively. Further in each level, factors were reclassified as modifiable and nonmodifiable based on the possibility of intervention. At household level, two latent variables were introduced SES and LC representing SES of family and LC of the household. The directly observable variables corresponding to each latent variable are shown in the gray boxes. Individual level nonmodifiable risk factors like age, sex, and history of TB in family were considered as potential confounders of association between individual level/household level modifiable risk factors and test positivity. The postulated conceptual framework would explore relationship between various modifiable factors and test outcomes in the presence of nonmodifiable factors. Further, applying the same protocol in both data sets would ascertain the performance of two tests, provided assumption of homogeneity of samples with respect to baseline characteristics is fulfilled.
Figure 2.
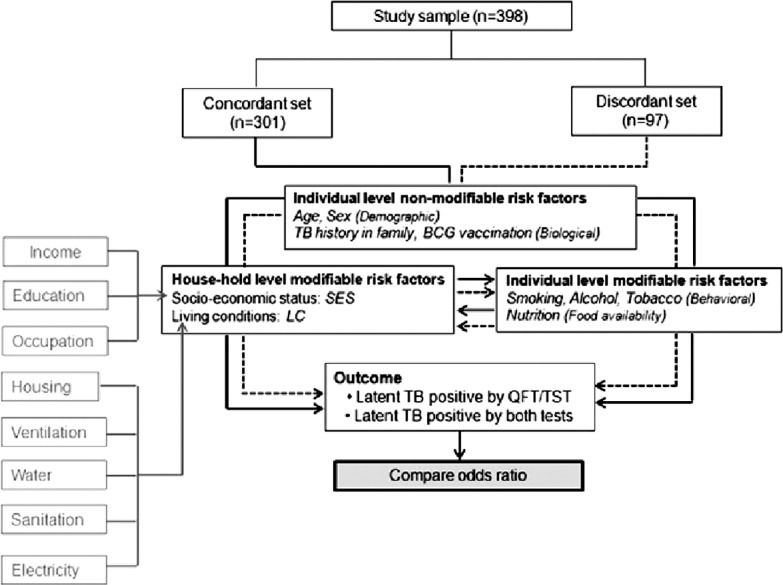
Conceptual framework of the study. Various risk factors for latent tuberculosis were classified according to their effect level (household or individual) and type (modifiable or nonmodifiable)
Data analysis
Data analysis consisted of (a) summarization of population characteristics, (b) assessing homogeneity of baseline characteristics in two data sets, and (c) evaluating risk associated with modifiable factors for test positivity in two sets.
Summarization of the characteristics
The variables describing characteristics of study population were mostly categorical and were summarized in terms of frequencies and percentages. Continuous variable age was transformed to categorical as: Age ≤40 and >40 years. SES of the household was decided according to Kuppuswamy rating system developed for Indian families. Three factors viz., education, occupation, and income were referred to assign particular SES to a household. A latent variable LC was defined as a complex of (i) quality of housing and (ii) available community facilities. It was regarded as an indicator of physical conditions in and around the household. Quality of housing referred to type of houses (concrete or mud) and adequacy of ventilation. The community facilities included water, electricity, and sanitation. An index of LC was obtained by applying categorical principal component analysis to this data. A score was obtained for each household using weights of first principal component, which accounted for 31% of the total variance. Scores were classified using 33% and 67% cut-offs, which were 0.1334 and 1.4546, respectively, to generate tertiles. Three tertile groups representing LC were labeled as very poor, poor, and better. Low scores represented poor status with mud houses, inadequate ventilation, no sanitation, no electricity, and well as the only source of water. On the other hand, high scores represented better living status with concrete housing, adequate ventilation, proper sanitation, electricity, and hand pump along with well as the source of water. During analysis, households with very poor and poor conditions were pooled together due to sample inadequacies, thereby resulting into two groups, that is, poor and better. Accordingly, frequency and percentage of households in each class were obtained as summary statistics.
Homogeneity of sample characteristics in two sets
Subjects were split into concordant and discordant sets as described earlier. In the absence of any gold standard for prediction of LTBI, we adopted splitting strategy to determine which test provides consistent results considering their relationship with different factors. The idea was that in concordant set there is agreement on the LTBI outcome (either positive or negative) by both the tests and hence interpretations of factors association are same for the two tests. In the discordant set with discrepancies in test outcomes, it is expected that interpretations of factor associations for one of the test could be consistent with that of concordant set. However, this comparison would be meaningful only under the assumption of homogeneity of sample characteristics in two sets. If so, the consistent test could be regarded as suitable option for diagnosing LTBI in this population. We ensured the validity of assumption through Categorical Principal Component Analysis, with factors comprising the variable set and the two sets as independent groups.
Risk evaluation
Initially, the effect of risk factors on test positivity was determined in each set using bivariate analysis. Crude estimates of odds ratio (OR) for each factor along with 95% confidence interval (CI) were obtained in both the sets. Only those factors showing statistical significance in atleast one of the sets were retained and considered for multivariate analysis. Variables like age and sex, although showed insignificant effect, were retained in the analysis being biologically relevant. The primary focus of the study was on the modifiable risk factors and their effect on test outcome.
Further, to determine such effects adjusting for the multiple confounders, we used logistic regression with “LTBI positive by both tests” as the outcome in concordant set and “LTBI positive by either QFT-G/or TST” in discordant set. OR for each individual level modifiable risk factor was obtained separately in the presence of combinations of covariates. The model derived using nonmodifiable risk factors (confounders) such as age, sex, and TB history in family was referred as the basic model, while the one with household level modifiable risk factors along with confounders was referred as advanced model. Fitness of each model was evaluated using Hosmer-Lemeshow test. ORs for individual level risk factors were compared with the unadjusted crude estimates and changes were observed. Similar analysis was performed for household level modifiable risk factors, wherein ORs were adjusted with confounders (basic model). At the next stage (advance type I), behavioral factors were added to confounders and the ORs were obtained. In the advance type II model, nutritional factor was also considered along with confounders and the ORs were observed. Apparently in all these models, the interests were to understand (i) how the modifiable risk factors affect the test positivity in presence of one or more cofactors and (ii) the relative performance of diagnostic tests in two data sets. During analysis, a change of <10% in OR was considered as marginal. The statistical significance was tested at 5% level and the analysis was performed using R 2.15 programming package (R Core team 2012).
RESULTS
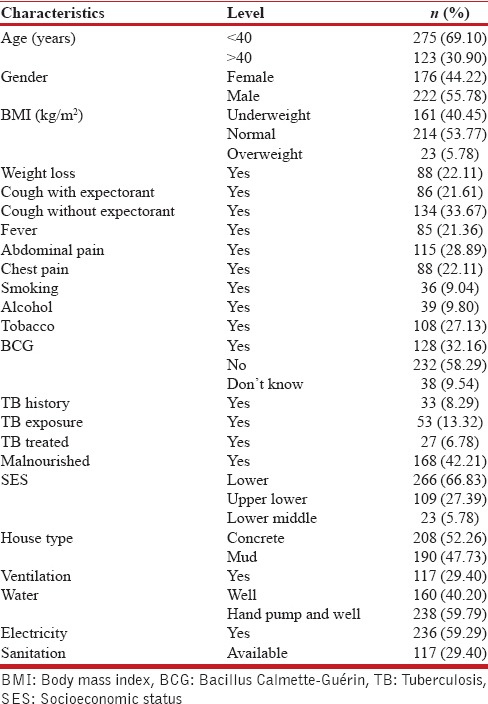
Out of 639 eligible participants, 398 were eventually considered for the study. Data relating to demographic, behavioral, biological, socioeconomic factors, and community services were summarized as shown in Table 1. Majority of the individuals, that is, 275 (69.1%) were below age of 40 years with a minimum age of 18 years, dominated mostly by male population (55.78%). Socioeconomically, 66.83% of the individuals belonged to lower class, while 27.39% belonged to upper lower class as per Kuppuswamy rating system applicable to Indian families. The proportion of malnourished individuals in the region was 42.21%, which was considerably higher compared to other rural set ups within the state.
Table 1.
Summary statistics for the characteristics of study population (n=398)
Bivariate analysis: Effects of individual and household level factors
Association of each factor with test positivity was studied through bivariate analysis in concordant and discordant data sets with results shown in Table 2. In the concordant set (1), smoking (OR: 2·61; 95% CI: 0.99-6·55), tobacco chewing (OR: 2·41 95% CI: 1·36-4·24), and TB history in family (OR: 2·56; 95% CI: 1·36-4.24) significantly doubled the odds in favor of positivity by both tests with P < 0·05. Surprisingly, BCG vaccination also showed increased likelihood of both tests being positive. Malnourishment also showed increased risk of positivity of tests with OR 1·78 (95% CI: 1·04-3·09). The two household level modifiable factors SES and LC showed significantly reduced odds in favor of test positivity with ORs 0·11 (95% CI: 0·04-0·25) and 0·07 (95% CI: 0·03-0·14), respectively, with P < 0·001. In the discordant set, for QFT-G positive outcome (2), ORs indicated similar trends to that of concordant set for eight of the ten factors; although the magnitudes differed. For TST positive outcome (3), the ORs were contradictory to first set and ORs of only two factors matched with those of concordant set.
Table 2.
Bivariate analysis to determine effect of individual and household level risk factors

Multivariate analysis: Effect of modifiable risk factors on test positivity
Individual level modifiable risk factors were assessed by adjusting for nonmodifiable risk factors like age, sex, and TB history in family (basic model) and further augmenting with household level modifiable factors (advanced model) with the results shown in Table 3. In the concordant set, as per basic model (1), the ORs for alcohol and tobacco showed more than 10% change compared to corresponding estimates. In the advanced model (2), a noticeable reduction in the ORs of behavioral and nutritional factors was observed ranging between 32% and 61%. The estimate for alcohol dropped below one (OR: 0·68 [95% CI: 0·24-1·90]); however, statistical significance could not be reached in the revised model.
Table 3.
Effect of individual level modifiable risk factors on test positivity in two data sets
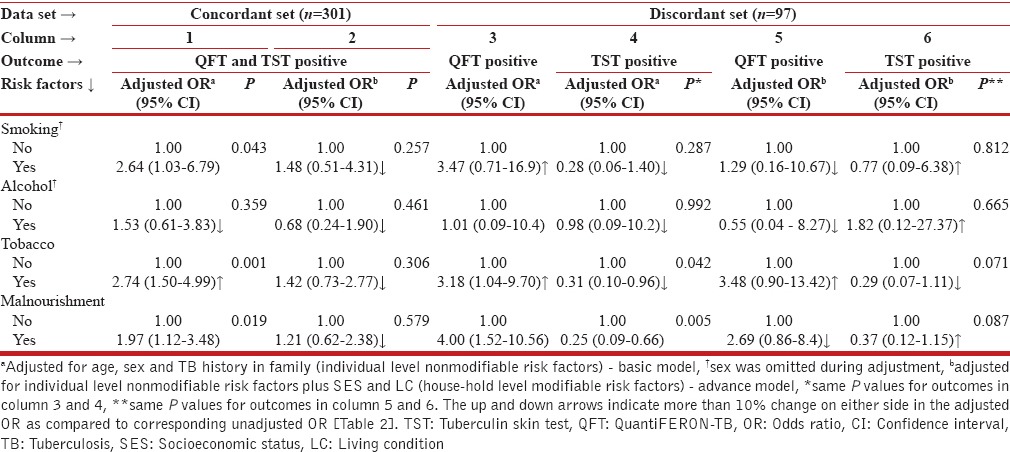
In the discordant set, for QFT-G positive outcome (3), the basic model resulted into increased odds for smoking and tobacco compared to their crude estimates. For malnourishment, OR of 4·00 (95% CI: 1·52–10·56) although significant was smaller than the corresponding crude estimate by 6%. The interpretations for smoking, tobacco, and malnourishment were similar to concordant set, while the effect due to alcohol consumption was unchanged after basic adjustment (OR: 1·01; 95% CI: 0·09–10·4). For the same outcome, in the advanced model (5), none of the factors could attain statistical significance, although the changes in ORs were above 37%. The findings for smoking, alcohol consumption, and malnourishment matched with that of concordant set. Similar analysis was performed for TST positive outcome. With basic adjustment (4), the change in the OR ranged between 8% and 31%, while in the advanced model (6), the change in the ORs were more than 35%.
In the same manner, household level modifiable factors SES and LC were assessed in the presence of other cofactors with the results shown in Table 4. In the concordant set (1), OR corresponding to SES showed significant increase by 27% after adjusting with individual level modifiable and nonmodifiable factors. With other two types of adjustments, changes in ORs for SES were <10%. Despite this, all models ascertained that improved SES reduces the likelihood of test positivity.
Table 4.
Effect of household level modifiable risk factors on test positivity in two data sets
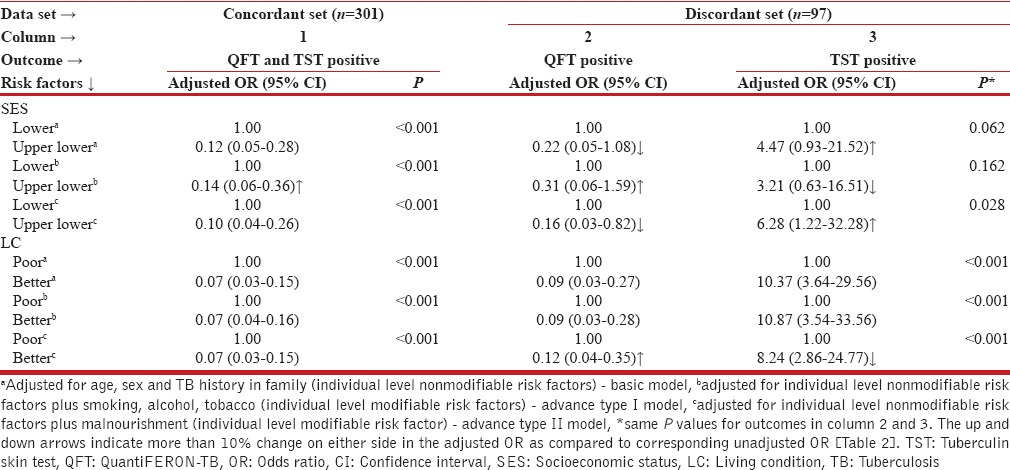
In the discordant set, for QFT-G positive outcome (2), basic model resulted into 15·4% drop in the OR associated with SES, while advanced type I model resulted into 19·2% increase in the OR compared to crude estimate. The OR by including malnourishment in the basic model showed significant effect of SES on the outcome (OR: 0·16; 95% CI: 0·03-0·82; P = 0·028) and gave 38·5% reduction in the OR estimate. For TST positive outcome (3), the advanced type II model showed significant effect of SES on outcome (OR: 6.28; 95% CI: 1·22-32·38; P = 0·028) and the increase with respect to crude estimate was 68·4%. LC was analyzed on similar lines [Table 4]. In concordant set (1), the ORs remained unchanged in all the three models and matched with the crude estimate (OR: 0·07; 95% CI: 0·03-0·14). Further, the small OR suggested that the likelihood of test positivity decreases with the improved LC. This finding was almost coherent with the QFT-G positive outcome in the discordant set, except in advanced type II model, where malnourishment had increased (33·3%) odds (OR: 0·12; 95% CI: 0·04-0·35) compared to crude estimate. For TST positive outcome, the ORs in the basic and advanced type I model were close to the crude estimate.
DISCUSSION
The study primarily aimed at identifying key risk factors for LTBI based on their impacts on the positivity of QFT-G and TST. Key risk factors targeted were SES and LC, apart from demographic and behavioral factors. Earlier, poor LC and nutritional deficiencies have been linked with the prevalence of TB.[13,14]
Upon ascertaining the homogeneity of sample characteristics in two sets, the effects of these factors on test positivity were evaluated in each set. Bivariate analysis without any covariate adjustment revealed that the individual level modifiable risk factors like smoking, alcohol, tobacco consumption, and malnourishment have increased odds in favor of LTBI positivity by both the tests. These factors have previously been reported for decreased immunity, along with risk of progression of TB in other studies.[15,16,17,18] The females of the tribes lack complete healthcare awareness and have increased contact period with TB index cases in the household;[19] hence sex emerged as an important suppressing confounder than age and TB history in family. Smoking and alcohol consumption has been regarded as major risk factors that predispose TB.[20,21] Such habits among the study population significantly influenced test outcome and had a mediating effect on LTBI diagnosis. Malnutrition in tribal population impairs immune functions, which is responsible for host sensitivity to various infectious diseases including TB.[18,22] We observed that malnourishment was significantly influenced by confounders which acted as suppressors by increasing OR as compared to crude estimate. Among the three confounders, effect of TB history in family was pronounced suggesting that malnourished population with family history is more likely to get detected positively by both the tests. In the discordant set association of all the factors except SES and LC were more with QFT-G positivity when compared with TST outcome.
Individual level modifiable risk factors were further adjusted to study the impact of house-hold level modifiable risk factors (SES and LC). Interestingly, it was observed that the combination of these factors had a mediating effect on the modifiable risk factors, indicating their roles in explaining part of the association between individual level factor and test positivity in concordant set. Earlier, we thought malnourishment to be an only and major influencing factor for LTBI in this population. However, in presence of SES and LC, its effect lessened suggesting that malnourishment alone is not the determinant of LTBI. SES and LC had an independent effect modality on LTBI test outcomes.
There is substantial evidence that poverty is a determinant of TB, both at the macroscale and in individual and hierarchical analyses[7,23] however, the association of same with LTBI is a matter of concern. This study revealed the association of SES and LC with the prevalence of LTBI in the tribal population.
With our analytical framework we observed that SES and LC, if improved can prevent or mitigate the prevalence of LTBI in this particular region. We observed less prevalence of LTBI in a village who's SES and LC was better than the other villages enrolled in the study. Poverty continues to be one of the major reasons for TB incidences in India.[24] Low SES status has direct impact on LC of the population. Both factors ultimately may dispose other risk factors which include illiteracy, poor hygiene practices, and poor diet. Malnourishment has been regarded as major risk factor for TB infection. However, factors like SES and LC can be considered as major confounding factors leading to malnourishment in tribals, which further escalates risk of acquiring TB in house hold contacts. Malnourished LTBI population with low SES and poor LC has high risk of conversion into active cases majorly because of the above factors (illiteracy, poor hygiene, and close contacts with TB index cases) and due to their negligence for other diseases. There are considerable chances that tribals diagnosed with active TB and started anti-TB medication may not complete the recommended 6-8 months treatment regime. The major reasons to this include lack of adequate dietary intake (due to poor SES) with treatment due to which patient suffers from other complication like liver disease. Poor SES and LC in patients with active TB may in turn further predispose chances of deterioration and even drug resistance among the tribals.
Our observation is in agreement with various other studies which suggest low SES has important risk factor for TB infection. Studies by Boccia et al. in Zambian population have shown that socioeconomic factors are important in controlling TB.[13] Article by Narasimhan et al. on risk factors for TB suggests low SES as detrimental risk factors which promotes malnutrition, which further increases risk for TB development.[16] Article further states that people with lower SES have a higher likelihood of being exposed to crowded, less ventilated places and follow poor hygienic practices all of which may favor TB development. Oxlade and Murray had also shown that TB control strategies should be targeted to the poorest populations that are at higher risk, and should address the most important determinants of disease like low body mass index.[25] Although the above studies highlight SES and LC as risk factors of active TB; through our present study we have shown that both above factors are important risk factors in latently infected individuals in promoting TB infection. We suggest with improvement in SES and LC, can significantly prevent malnourishment and even the transmission and seroconversion of the participants from LTBI to active diseases can be prevented. The hypothesis is well supported by the ecological studies which have suggested that broad socioeconomic development, rather than the success of TB control programmes, is the main determinant behind the declining trends of TB observed in many regions of the world.[24,26,27]
Apart from assessment of risk factors for LTBI, another major aim was to study the impact of SES and LC and other risk factors on test outcome. In the present study, we further evaluated diagnostic utility of two existing test for LTBI namely QFT-G and TST on the basis of association of risk factors for LTBI positivity in two data sets. Using this approach, QFT-G was found to be more consistent than TST for detecting LTBI through several models. TST for LTBI diagnosis suffers from several limitations which includes inter-reader variability and cross reactivity in individuals having environmental mycobacterial exposure and BCG vaccination. This may provide an alternative reason for higher false positive results with TST.[11] Although metabolic changes and energy response in malnourishment has significant impact on cellular response influencing both T cell dependent tests, through our studies, we however found QFT-G as better test for LTBI diagnosis in malnourished palpation. Improvement in SES and LC among the tribals was significantly associated with increased QFT-G positivity among the tribals.
Although, the present study suggests the possibility of association of SES and LC with the prevalence of LTBI, it has few limitations. Lack of follow-up data from all the participants could be one; nevertheless, statistically significant ORs for factors with reasonably large magnitudes reduce likelihood of chance associations. Another limitation is the noninclusion of variables, especially related to public exposures and migrations. Further, categorization of SES may have some errors due to nonresponse bias about family earnings and occupation. However, considering the evenness of living standards of these households, we believe that the assessment by trained interviewers has certainly minimized the chances of misclassification. Also, we could not measure important covariate like environmental mycobacterial exposure in the tribal population, which might be the reason of false positivity of TST.
CONCLUSION
Low SES and LC are major risk factors that may predispose TB infection in latently infected tribal population. Further, our study showed QFT-G test as a reliable tool in screening of LTBI in the tribal population of Melghat, India. We believe that if policy makers extend their comprehensive and integrated approach of disease control by targeting atleast household level factors, like SES and LC, the prevalence of LTBI in such isolated regions of the country would be much under control.
Financial support and sponsorship
This study was funded by Central India Institute of Medical Sciences. The funders had no role in study design, data collection, analysis and decision to publish.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.World Health Organization. Global Tuberculosis Report: WHO Report. Geneva: World Health Organization; 2012. [Last accessed on 2013 Jan 01]. Available from: http://www.who.int/tb/publications/global_report/en/ [Google Scholar]
- 2.TB INDIA. Revised National TB Control Programme/Annual Status Report. Government of India. 2012. [Last accessed on 2013 Jan 16]. Available from: http://www.tbcindia.nic.in/pdfs/TB .
- 3.Herrera V, Perry S, Parsonnet J, Banaei N. Clinical application and limitations of interferon-gamma release assays for the diagnosis of latent tuberculosis infection. Clin Infect Dis. 2011;52:1031–7. doi: 10.1093/cid/cir068. [DOI] [PubMed] [Google Scholar]
- 4.Lienhardt C, Fielding K, Hane AA, Niang A, Ndao CT, Karam F, et al. Evaluation of the prognostic value of IFN-gamma release assay and tuberculin skin test in household contacts of infectious tuberculosis cases in Senegal. PLoS One. 2010;5:e10508. doi: 10.1371/journal.pone.0010508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fietta A, Meloni F, Cascina A, Morosini M, Marena C, Troupioti P, et al. Comparison of a whole-blood interferon-gamma assay and tuberculin skin testing in patients with active tuberculosis and individuals at high or low risk of Mycobacterium tuberculosis infection. Am J Infect Control. 2003;31:347–53. doi: 10.1016/s0196-6553(02)48240-5. [DOI] [PubMed] [Google Scholar]
- 6.Latent Tuberculosis Infection: A Guide for Primary Health Care Providers. U.S. Department of Health and Human Services Centers for Disease Control and Prevention National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Division of Tuberculosis Elimination Atlanta, Georgia. 2013. [Last accessed on 2013 Jan 16]. Available from: http://www.cdc.gov/tb/publications/LTBI/pdf/TargetedLTBI.pdf .
- 7.Hargreaves JR, Boccia D, Evans CA, Adato M, Petticrew M, Porter JD. The social determinants of tuberculosis: From evidence to action. Am J Public Health. 2011;101:654–62. doi: 10.2105/AJPH.2010.199505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young BN, Rendón A, Rosas-Taraco A, Baker J, Healy M, Gross JM, et al. The effects of socioeconomic status, clinical factors, and genetic ancestry on pulmonary tuberculosis disease in Northeastern Mexico. PLoS One. 2014;9:e94303. doi: 10.1371/journal.pone.0094303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Report MAHAN Trust India. 2011. [Last assessed on 2013 Jan 31]. Available from: http://www.samhita.org/system/datas/3363/original/MAHAN_Melghat._1997-2011_.pdf .
- 10.Singh R, Singh PA. Study on high mortality of children in Melghat region of Amravati (Maharashtra) Stud Tribes Tribals. 2008;6:35–43. [Google Scholar]
- 11.Pai M, Gokhale K, Joshi R, Dogra S, Kalantri S, Mendiratta DK, et al. Mycobacterium tuberculosis infection in health care workers in rural India: Comparison of a whole-blood interferon gamma assay with tuberculin skin testing. JAMA. 2005;293:2746–55. doi: 10.1001/jama.293.22.2746. [DOI] [PubMed] [Google Scholar]
- 12.Mahomed H, Hawkridge T, Verver S, Abrahams D, Geiter L, Hatherill M, et al. The tuberculin skin test versus QuantiFERON TB Gold® in predicting tuberculosis disease in an adolescent cohort study in South Africa. PLoS One. 2011;6:e17984. doi: 10.1371/journal.pone.0017984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boccia D, Hargreaves J, De Stavola BL, Fielding K, Schaap A, Godfrey-Faussett P, et al. The association between household socioeconomic position and prevalent tuberculosis in Zambia: A case-control study. PLoS One. 2011;6:e20824. doi: 10.1371/journal.pone.0020824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dodor E. Evaluation of nutritional status of new tuberculosis patients at the effia-nkwanta regional hospital. Ghana Med J. 2008;42:22–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Lienhardt C, Fielding K, Sillah JS, Bah B, Gustafson P, Warndorff D, et al. Investigation of the risk factors for tuberculosis: A case-control study in three countries in West Africa. Int J Epidemiol. 2005;34:914–23. doi: 10.1093/ije/dyi100. [DOI] [PubMed] [Google Scholar]
- 16.Narasimhan P, Wood J, Macintyre CR, Mathai D. Risk factors for tuberculosis. Pulm Med. 2013;2013:828939. doi: 10.1155/2013/828939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcos A, Nova E, Montero A. Changes in the immune system are conditioned by nutrition. Eur J Clin Nutr. 2003;57(Suppl 1):S66–9. doi: 10.1038/sj.ejcn.1601819. [DOI] [PubMed] [Google Scholar]
- 18.Millet JP, Moreno A, Fina L, del Baño L, Orcau A, de Olalla PG, et al. Factors that influence current tuberculosis epidemiology. Eur Spine J. 2013;22(Suppl 4):539–48. doi: 10.1007/s00586-012-2334-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batra S, Ayaz A, Murtaza A, Ahmad S, Hasan R, Pfau R. Childhood tuberculosis in household contacts of newly diagnosed TB patients. PLoS One. 2012;7:e40880. doi: 10.1371/journal.pone.0040880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horne DJ, Campo M, Ortiz JR, Oren E, Arentz M, Crothers K, et al. Association between smoking and latent tuberculosis in the U.S. population: An analysis of the national health and nutrition examination survey. PLoS One. 2012;7:e49050. doi: 10.1371/journal.pone.0049050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan QH. Epidemiology of pulmonary tuberculosis in rural Aligarh. Indian J Community Med. 2006;31:39–40. [Google Scholar]
- 22.Field CJ, Johnson IR, Schley PD. Nutrients and their role in host resistance to infection. J Leukoc Biol. 2002;71:16–32. [PubMed] [Google Scholar]
- 23.Creswell J, Jaramillo E, Lönnroth K, Weil D, Raviglione M. Tuberculosis and poverty: What is being done. Int J Tuberc Lung Dis. 2011;15:431–2. doi: 10.5588/ijtld.10.0654. [DOI] [PubMed] [Google Scholar]
- 24.Xu L, Gai R, Wang X, Liu Z, Cheng J, Zhou C, et al. Socio-economic factors affecting the success of tuberculosis treatment in six counties of Shandong Province, China. Int J Tuberc Lung Dis. 2010;14:440–6. [PubMed] [Google Scholar]
- 25.Oxlade O, Murray M. Tuberculosis and poverty: why are the poor at greater risk in India? PLoS One. 2012;7:e47533. doi: 10.1371/journal.pone.0047533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dye C, Lönnroth K, Jaramillo E, Williams BG, Raviglione M. Trends in tuberculosis incidence and their determinants in 134 countries. Bull World Health Organ. 2009;87:683–91. doi: 10.2471/BLT.08.058453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obermeyer Z, Abbott-Klafter J, Murray CJ. Has the DOTS strategy improved case finding or treatment success? An empirical assessment. PLoS One. 2008;3:e1721. doi: 10.1371/journal.pone.0001721. [DOI] [PMC free article] [PubMed] [Google Scholar]


