Abstract
Background:
Pulmonary nocardiosis is a rare but a life-threatening infection caused by Nocardia spp. The diagnosis is often missed and delayed resulting in delay in appropriate treatment and thus higher mortality.
Aim:
In this study, we aim to evaluate the clinical spectrum and outcome of patients with pulmonary nocardiosis.
Methods:
A retrospective, 5-year (2009–2014) review of demographic profile, risk factors, clinical manifestations, imaging findings, treatment, and outcome of patients with pulmonary nocardiosis admitted to a tertiary care hospital.
Results:
The median age of the study subjects was 54 years (range, 16–76) and majority of them (75%) were males. The risk factors for pulmonary nocardiosis identified in our study were long-term steroid use (55.6%), chronic lung disease (52.8%), diabetes (27.8%), and solid-organ transplantation (22.2%). All the patients were symptomatic, and the most common symptoms were cough (91.7%), fever (78%), and expectoration (72%). Almost two-third of the patients were initially misdiagnosed and the alternative diagnosis included pulmonary tuberculosis (n = 7), community-acquired pneumonia (n = 5), lung abscess (n = 4), invasive fungal infection (n = 3), lung cancer (n = 2), and Wegener's granulomatosis (n = 2). The most common radiographic features were consolidation (77.8%) and nodules (56%). The mortality rate for indoor patients was 33% despite treatment. Higher mortality rate was observed among those who had brain abscess (100.0%), HIV positivity (100%), need for mechanical ventilation (87.5%), solid-organ transplantation (50%), and elderly (age > 60 years) patients (43%).
Conclusion:
The diagnosis of pulmonary nocardiosis is often missed and delayed resulting in delay in appropriate treatment and thus high mortality. A lower threshold for diagnosing pulmonary nocardiosis needs to be exercised, in chest symptomatic patients with underlying chronic lung diseases or systemic immunosuppression, for the early diagnosis, and treatment of this uncommon but potentially lethal disease. Despite treatment mortality remains high, especially in those with brain abscess, HIV positivity, need for mechanical ventilation, solid-organ transplantation, and elderly.
KEY WORDS: Chronic lung disease, immunosuppression, nocardiosis, pneumonia
INTRODUCTION
Nocardiosis is a localized or disseminated, suppurative disease caused by ubiquitous, soil-borne aerobic actinomycetes of the genus Nocardia. The respiratory tract is the most common portal of entry. Nocardiosis is chiefly regarded as an opportunistic infection, but may occur as a primary one in immunocompetent host in around one-third of the patients.[1] Pulmonary nocardiosis particularly affects individuals with depressed cell-mediated immunity for example those with malignancies, human immunodeficiency virus infection, solid-organ, or hematopoietic stem cell transplantation, and those on long-term treatment with steroids or other immunosuppressants.[2] Besides immunosuppression impaired local pulmonary defenses as in cases of chronic obstructive pulmonary disease (COPD), asthma, bronchiectasis, bronchopulmonary sequestration, tuberculosis, and alveolar proteinosis predispose to pulmonary nocardiosis. The diagnosis of pulmonary nocardiosis is often missed and delayed as there are no pathognomonic signs or symptoms. The clinical spectrum of pulmonary nocardiosis may vary from a subclinical (self-limiting disease) to an acute (fulminant consolidation with respiratory failure), or a chronic process mimicking tuberculosis, or a malignancy or fungal infection. The mortality due to pulmonary nocardiosis is high (14-40%).[3,4,5] The course of the disease is marked by remissions and relapses. Filice G estimated an annual incidence of 0.375 cases per 100,000 persons for nocardiosis across three continents (North America, Europe, and Australia).[6] The incidence of nocardiosis is increasing partly due to better awareness of the disease, enhanced diagnostic tests, and growing number of immunosuppressed hosts.[7]
Experience of pulmonary nocardiosis in the medical literature is limited especially so from the Indian subcontinent. The aim of the present study was to evaluate the risk factors, clinical features, imaging, treatment, and outcome of patients with pulmonary nocardiosis from India.
METHODS
Patients
This retrospective study was carried out at a 1100-bed Tertiary Care Teaching Hospital in North-West India. The cases of nocardiosis were identified by a computerized search of the microbiology laboratory database for the specimens collected over 5 years (January 2009 and December 2014). Thereafter, the demographic characteristics, risk factor such as immunosuppressive treatment and chronic lung disease, clinical manifestations, radiological features, diagnostic methods, concomitant infections, treatment, and outcome were entered on a structured performa after looking at the medical records. Symptomatic subjects with respiratory involvement were included and those with extra pulmonary disease without an evidence of pulmonary involvement were excluded from the study.
Operational definitions used in the study
Colonization
A positive culture of a clinical specimen in an asymptomatic patient.
Definite diagnosis of Nocardiosis: Nocardia spp. isolated from a clinical specimen of a symptomatic patient.
Pulmonary nocardiosis
Nocardia spp. isolated from respiratory samples, (sputum, bronchoalveolar lavage, or fluid obtained from lung aspiration) in a symptomatic patient.
Nocardemia
Isolation of Nocardia spp. from blood cultures.
Disseminated nocardiosis
Nocardia infection at two or more noncontiguous sites and/or nocardaemia.
Chronic lung disease
Patients already diagnosed with COPD, asthma, bronchiectasis, allergic bronchopulmonary aspergillosis, or interstitial lung disease.
Long-term steroid use
Patients taking at least 10 mg of prednisolone (or prednisolone equivalent) per day for more than 1 month before the development of disease.
Airspace disease
Soft opacities with hazy and indistinct margins which tend to respect segmental or lobar boundaries and may contain an air bronchogram.
Air bronchogram
Branching, linear, tubular lucency representing a bronchus, or bronchiole passing through airless lung parenchyma.
Nodular pattern
Multiple round opacities, ranging in diameter from 1 mm to 1 cm that may be very difficult to separate from one another as individual nodules.
Cavitation
A gas-filled space within a zone of pulmonary consolidation or within a mass or nodule.
Microbiological methods
Nocardia spp. were cultured on sheep blood agar (Columbia base), CNA, and chocolate agar incubated aerobically at 35°C, in an atmosphere enriched with 5% CO2. Identification was based on microscopic morphology after gram stain and modified Kinyoun stain.
Statistical analysis
The statistical analyses were performed using SPSS Software, version 17.01 (SPSS, Inc., Chicago, IL, USA). The percentages of patients in each category were calculated for categorical variables. The mean and median were computed (P <</i> 0.05 was considered statistically significant).
RESULTS
During the 5-year study period (2009-2014), 44 patients with evidence of pulmonary nocardiosis either by positive modified acid-fast staining or culture of Nocardia spp. from various clinical specimens were identified. Eight patients who had no clinical evidence of pulmonary disease were excluded from the study. Hence, only 36 patients with clinical evidence of pulmonary disease (with or without other organ involvement) were included for the study purpose. Among these patients, seven (7/36) had concomitant disseminated disease, four had central nervous system involvement, two had skin and soft tissue abscesses, and one had cutaneous and intra-abdominal abscesses.
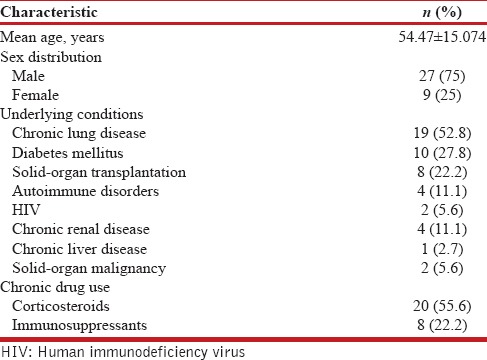
Demographic and underlying diseases of the study subjects
The mean age of the patients was 54.47 ± 15.074 years (range, 16–76) and majority of the patients were male (27/36). The demographic characteristics and underlying risk factors are tabulated in Table 1. All the patients had at least one identifiable risk factor for nocardiosis. The most common predisposing factors identified for pulmonary nocardiosis in our study were long-term steroid use (20/36) and chronic lung disease (19/36).
Table 1.
Demographic and underlying risk factors for nocardiosis
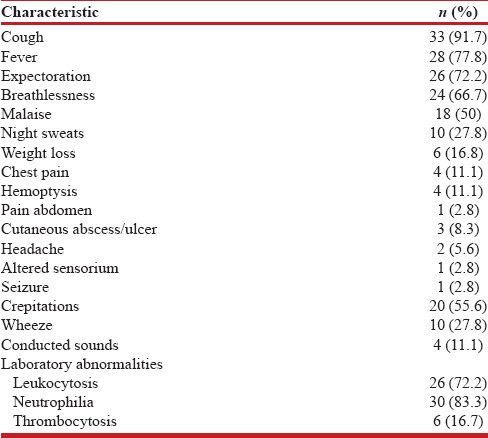
Clinical features and laboratory investigations
Table 2 outlines the clinical features and laboratory abnormalities of the patients with pulmonary nocardiosis. The majority of the patients had a subacute presentation with a mean duration of symptom of 17 days (range, 4-28) prior to hospitalization. Cough (33/36), fever (28/36), and sputum production (26/36) were the most common symptoms. Of the 36 patients, 23 were initially misdiagnosed and the various alternative diagnosis considered initially included pulmonary tuberculosis (n = 7), community-acquired pneumonia (n = 5), lung abscess (n = 4), invasive fungal infection (n = 3), lung cancer (n = 2), and Wegener's granulomatosis (n = 2). Leukocytosis with neutrophilia was present in over 70% of the subjects.
Table 2.
Clinical and laboratory features of the patients with nocardiosis
Radiographic findings
In the present study, unilateral disease was seen in 61% (22/36) of the cases with upper lobes predominance in 70% (25/36). The radiographic findings of the patients are summarized in Table 3. Airspace disease (28/36) and nodules (20/36) were the most common radiographic abnormality. Figures 1–3 shows some of the typical radiographic abnormalities in our patients with pulmonary nocardiosis.
Table 3.
Radiological features of the patients with nocardiosis

Figure 1.
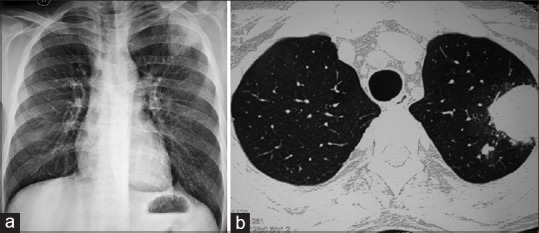
(a) A chest radiograph of a renal allograft recipient infected with Nocardia spp. showing left upper lobe mass lesion. (b) Corresponding computerized tomography revealing mass-like consolidation
Figure 3.
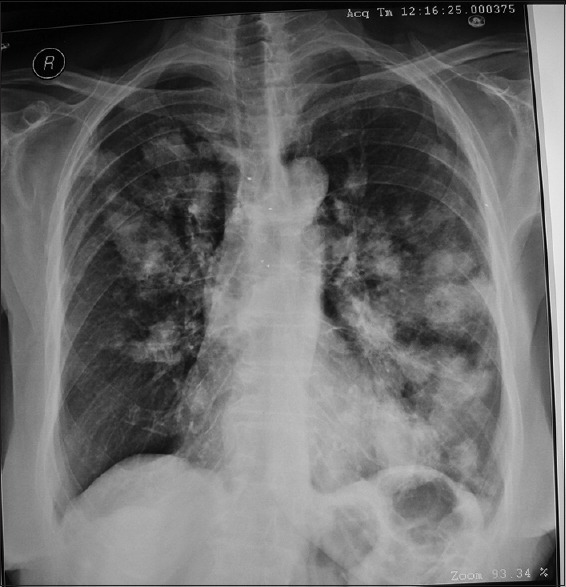
A chest radiograph of a patient with ABPA on long-term oral steroids infected with Nocardia spp. showing bilateral cavitating nodules and masses
Figure 2.
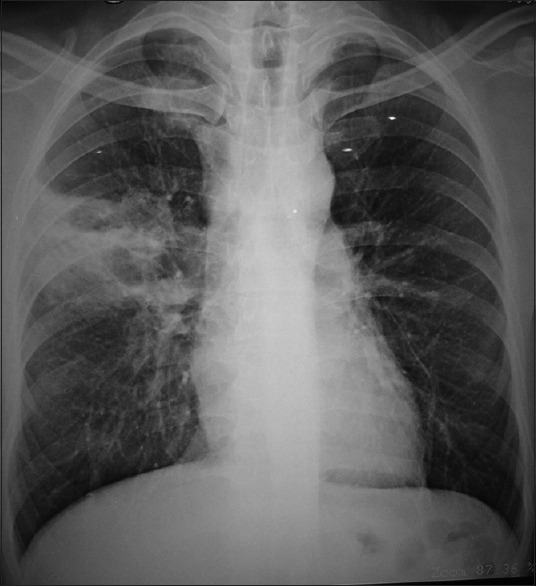
A chest radiograph of a steroid dependent asthma infected with Nocardia spp. showing right upper zone cavitating consolidation
Microbiological identification
The various respiratory specimens for the microbiological diagnosis of pulmonary nocardiosis are listed in Table 4. The diagnostic yield of sputum and endotracheal (ET) tube aspirate specimens were 64% and 62% respectively. Figure 4 demonstrates the classical weakly positive beaded, branching filamentous bacillus on modified acid-fast staining. Bronchoscopic and trans-thoracic needle aspirate specimens were collected in sputum and ET tube aspirate negative subjects. Bronchoscopic specimen and transthoracic needle aspirate had a yield of 90% and 80%, respectively, in sputum or ET aspirate negative patients. Twenty-six patients (72%) had positive cultures for Nocardia spp. and out of these 12 (33%) subjects had negative modified acid-fast staining. Ten patients (28%) had only smear positivity as evidence of Nocardia infection without culture being positive.
Table 4.
Clinical specimen and their diagnostic yield
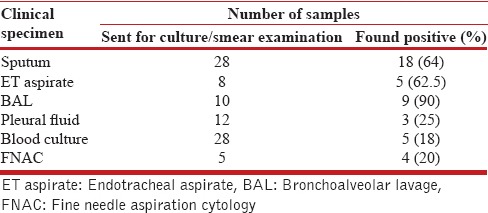
Figure 4.
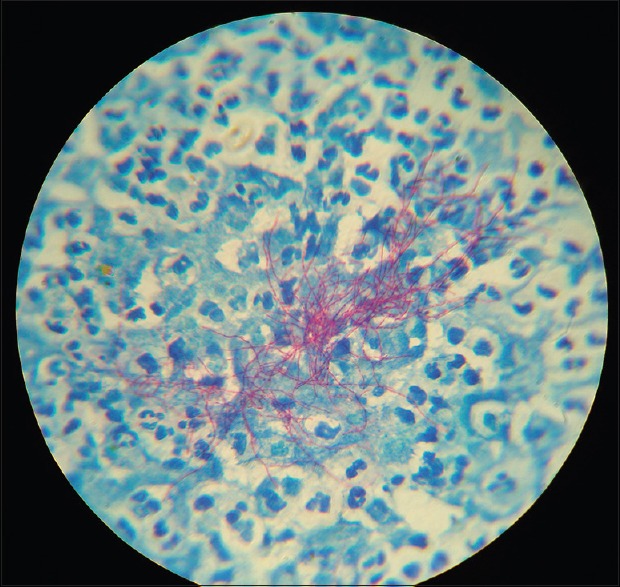
Modified acid-fast staining showing weakly positive beaded, branching filamentous bacillus
Pulmonary coinfection was diagnosed in seven patients that included Klebsiella pneumonia (2), Acinetobacter baumannii (2), Mycobacterium tuberculosis, Pseudomonas aeruginosa, and Aspergillus sp. (one each, respectively). The mean duration from admission to diagnosis was 15.6 ± 12.4 days (median 11 days).
Treatment and outcome
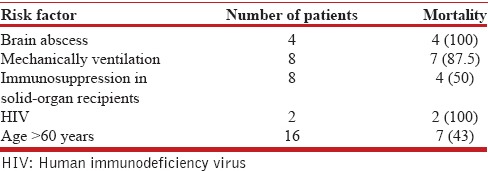
Antibiotics were started empirically, with subsequent modification according to clinical response and antibiotic sensitivity where available. All patients received a combination therapy. The antibiotic treatment regimens that were administered to the patients are listed in Table 5. Adverse events occurred in four patients (severe thrombocytopenia, pancytopenia, severe skin rash, and azotemia in one patient each) during treatment, which resulted in a change of the treatment regimen. Surgical intervention was required in patients with empyema (n = 3) and intra-abdominal abscess (n = 1). The in-hospital mortality rate was 33% (12/36). The mortality was higher among patients with brain abscess (4/4), HIV positive status (2/2), mechanically ventilated patients (7/8), solid-organ transplant recipients (4/8), and those aged >60 years (7/16) [Table 6]. There was no significant difference in mortality between males and females (P = 0.102) and rural and urban (P = 0.238) patients.
Table 5.
Treatment and outcome of the patients

Table 6.
Risk factors for mortality in subjects with pulmonary nocardiosis
DISCUSSION
Pulmonary nocardiosis is rare but a severe infection which may present both as opportunists and as primary pathogens. In our study 18% of Nocardia isolates were colonizers; similar findings have been documented by Minero et al. In their study 13.9% of Nocardia isolates were just colonizers.[8] Most patients in the present study were males, which is consistent with the previously published studies.[9,10,11,12] The male predominance in patients with nocardiosis may be ascribed to the hormonal effects on the virulence or growth of Nocardia.[12]
Nocardiosis is classically regarded as an opportunistic infection mainly, affecting those with altered cellular immunity. A preexisting immunecompromised state, ranging from malignancy, organ and hematopoietic stem cell transplantation, chronic glucocorticoid therapy, and HIV infection, have been reported in 64% cases of nocardiosis.[1] In our study, 66% of the patients had some degree of immunosuppression. The most common reason for immunosuppression in our subset of patients with pulmonary nocardiosis was chronic corticosteroid therapy (20 patients). Chen et al., reported that 60% of their patients with pulmonary nocardiosis were on prolonged corticosteroids.[13]
Chronic lung disease (52%) was the second most common predisposing condition for the development of pulmonary nocardiosis in our cohort of patients. Among this group COPD was the most common underlying respiratory disease followed by asthma and allergic bronchopulmonary aspergillosis. A few studies have shown an increasing number of cases of pulmonary nocardiosis in patients with COPD.[5,14,15] In COPD, impaired local defenses coupled with prolonged corticosteroid treatment facilitate the growth of Nocardia spp. Strikingly, none of our patients had an hematological malignancy, neutropenia, lymphocytopenia, or cytomegalovirus infection. Though, the risk of nocardiosis in transplant patients has decreased with the introduction of cyclosporine and trimethoprim/sulfamethoxazole (TMP-SMX) prophylaxis nevertheless, in our series, eight renal transplant patients developed pulmonary nocardiosis as they were not on routine TMP-SMX prophylaxis.
Most patients had a subacute presentation with a mean duration of symptom of 17 days (range, 4-28) prior to presentation. The clinical recognition of nocardiosis is difficult because of its relatively low incidence and a lack of pathognomonic symptoms, signs, and radiological manifestations. The most common initial diagnosis instead of nocardiosis was pulmonary tuberculosis, community-acquired pneumonia, lung abscess, invasive fungal infection, lung cancer, and Wegener's granulomatosis. A high index of clinical suspicion needs to be exercised to diagnose pulmonary nocardiosis, in chest symptomatic patients with chronic lung diseases, or chronic immunosuppressive therapy. The yield is quite satisfactory even with noninvasive samples such as sputum and ET aspirate.
In our study, most of the patients with pulmonary nocardiosis received an initial combination of at least two or three intravenous antibiotics. After clinical stabilization, the antibiotic regimen was switched to oral TMX-SMX and minocycline or TMX-SMX and linezolid. Pulmonary nocardiosis is associated with a high mortality as in our study (33%). The mortality rate was higher among patients with brain abscess, HIV positive status, with need for mechanical ventilation, solid-organ transplant recipients, and older age. The major limitations of the present study are its retrospective design and non-availability of the microbiological data concerning the identification of species of Nocardia and their antibiotic susceptibility.
Almost two-third of the patients were initially misdiagnosed as tuberculosis, invasive fungal disease, lung abscess, or malignancy. Our data suggests that we must suspect pulmonary nocardiosis in chest symptomatic patients with underlying structural lung diseases or chronic immunosuppressive therapy along with chest radiographic abnormalities especially when the usual microbiological work-up is negative. Invasive procedures such as bronchoscopy and transthoracic needle aspiration do improve the diagnostic yield in sputum negative subjects. Our data further suggest that despite initial combination therapy, the mortality of pulmonary nocardiosis is high especially in those with brain abscess, HIV positive status, those requiring mechanical ventilation, solid-organ transplant recipients, and those of older age.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Beaman BL, Beaman L. Nocardia species: Host-parasite relationships. Clin Microbiol Rev. 1994;7:213–64. doi: 10.1128/cmr.7.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson JW. Nocardiosis: Updates and clinical overview. Mayo Clin Proc. 2012;87:403–7. doi: 10.1016/j.mayocp.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muñoz J, Mirelis B, Aragón LM, Gutiérrez N, Sánchez F, Español M, et al. Clinical and microbiological features of nocardiosis 1997-2003. J Med Microbiol. 2007;56(Pt 4):545–50. doi: 10.1099/jmm.0.46774-0. [DOI] [PubMed] [Google Scholar]
- 4.Chedid MB, Chedid MF, Porto NS, Severo CB, Severo LC. Nocardial infections: Report of 22 cases. Rev Inst Med Trop Sao Paulo. 2007;49:239–46. doi: 10.1590/s0036-46652007000400009. [DOI] [PubMed] [Google Scholar]
- 5.Martínez Tomás R, Menéndez Villanueva R, Reyes Calzada S, Santos Durantez M, Vallés Tarazona JM, Modesto Alapont M, et al. Pulmonary nocardiosis: Risk factors and outcomes. Respirology. 2007;12:394–400. doi: 10.1111/j.1440-1843.2007.01078.x. [DOI] [PubMed] [Google Scholar]
- 6.Filice G. Harrison's Principles of Internal Medicine. 19th ed. New York: McGraw-Hill; 2015. Nocardiosis; pp. 1084–8. [Google Scholar]
- 7.Hannaman MJ, Ertl MJ. Patients with immunodeficiency. Med Clin North Am. 2013;97:1139–59. doi: 10.1016/j.mcna.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Minero MV, Marín M, Cercenado E, Rabadán PM, Bouza E, Muñoz P. Nocardiosis at the turn of the century. Medicine (Baltimore) 2009;88:250–61. doi: 10.1097/MD.0b013e3181afa1c8. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Bellmunt L, Sibila O, Solanes I, Sanchez-Reus F, Plaza V. Pulmonary nocardiosis in patients with COPD: Characteristics and prognostic factors. Arch Bronconeumol. 2012;48:280–5. doi: 10.1016/j.arbres.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Mootsikapun P, Intarapoka B, Liawnoraset W. Nocardiosis in Srinagarind Hospital, Thailand: Review of 70 cases from 1996-2001. Int J Infect Dis. 2005;9:154–8. doi: 10.1016/j.ijid.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Castro JG, Espinoza L. Nocardia species infections in a large county hospital in Miami: 6 years experience. J Infect. 2007;54:358–61. doi: 10.1016/j.jinf.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Matulionyte R, Rohner P, Uçkay I, Lew D, Garbino J. Secular trends of Nocardia infection over 15 years in a tertiary care hospital. J Clin Pathol. 2004;57:807–12. doi: 10.1136/jcp.2004.016923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J, Zhou H, Xu P, Zhang P, Ma S, Zhou J. Clinical and radiographic characteristics of pulmonary nocardiosis: Clues to earlier diagnosis. PLoS One. 2014;9:e90724. doi: 10.1371/journal.pone.0090724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rivière F, Billhot M, Soler C, Vaylet F, Margery J. Pulmonary nocardiosis in immunocompetent patients: Can COPD be the only risk factor? Eur Respir Rev. 2011;20:210–2. doi: 10.1183/09059180.00002211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menéndez R, Cordero PJ, Santos M, Gobernado M, Marco V. Pulmonary infection with Nocardia species: A report of 10 cases and review. Eur Respir J. 1997;10:1542–6. doi: 10.1183/09031936.97.10071542. [DOI] [PubMed] [Google Scholar]


